Submitted:
02 July 2025
Posted:
03 July 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
- Co-administration with complementary bioactive agents;
- Structural modification;
- Encapsulation into nano-sclaed drug delivery systems [20].
2. Methods
3. The Nature of Catechins
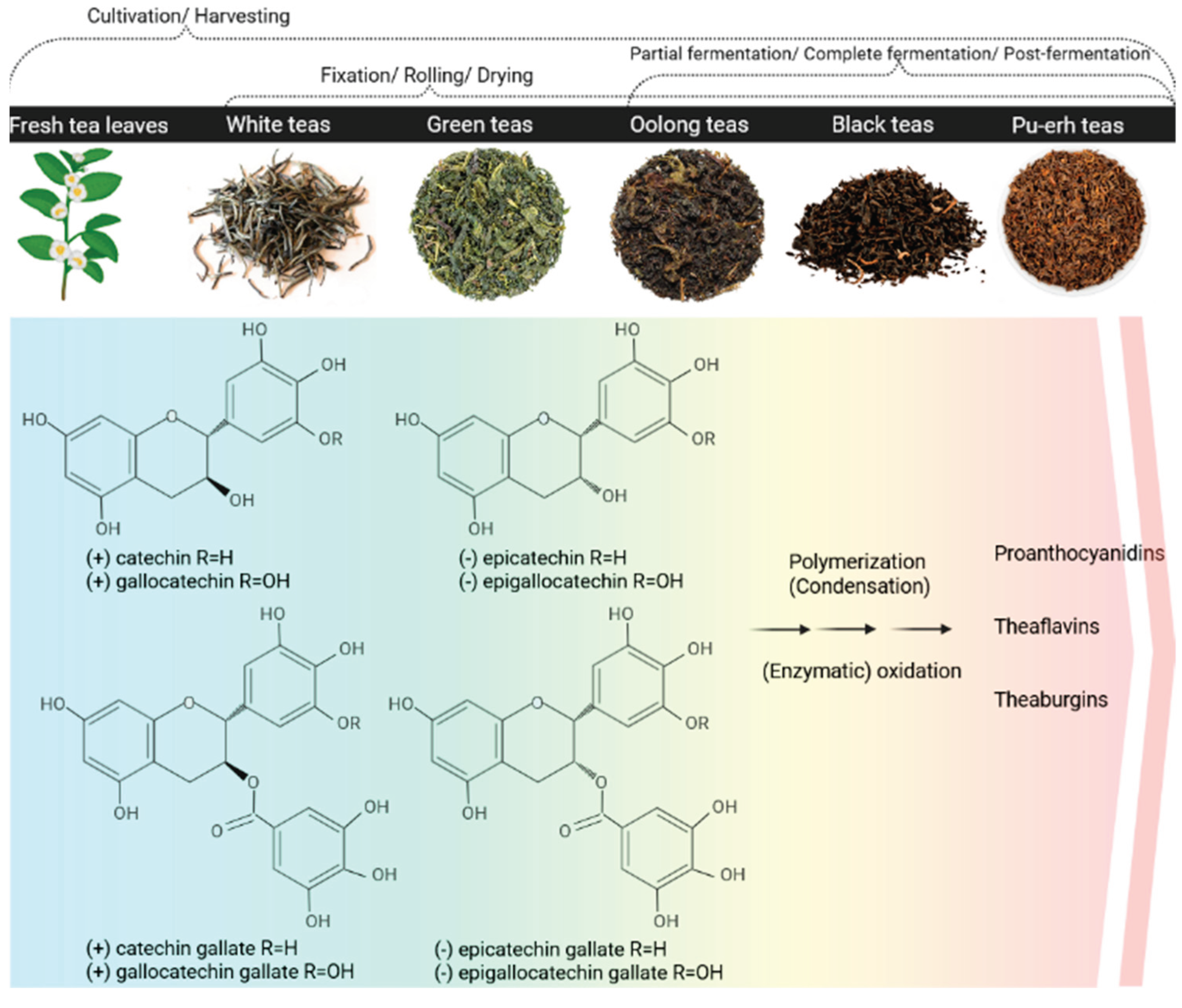
3.1. Natural Sources of Catechins
3.2. Biosynthesis
3.3. Extraction and Processing
3.4. Physicochemical Properties
| GTC | H-bond donor count | H-bond acceptor count | Molecular weight (g/mol) | Solubility data |
|---|---|---|---|---|
| C | 5 | 6 | 290 | In water at 25.6 °C: 2.26 g/L [79] In water: approx. 1.8 g/Lii [80] In PBSi 7.2: approx. 1.6 g/Lii[81] In ethanol: approx. 100 g/Lii [81] |
| EC | 5 | 6 | 290 | Data not found |
| EGC | 6 | 7 | 306 | Data not found |
| ECG | 7 | 10 | 442 | Data not found |
| EGCG | 8 | 11 | 458 | In water at 20 °C: 40 g/L [82] |
4. Pharmacological Activity and Therapeutic Potential of Catechins. Limitations
4.1. Pharmacological Effects of Catechins
- Direct and indirect antioxidant properties
- Anti-inflammatory activity
- Neuroprotective activity
- Anticarcinogenic activity
- Antimicrobial activity
4.2. Limitations
- Low in vitro stability
- Limited bioavailability due to pharmacokinetic constraints
5. Perspectives Offered by Nanotechnology
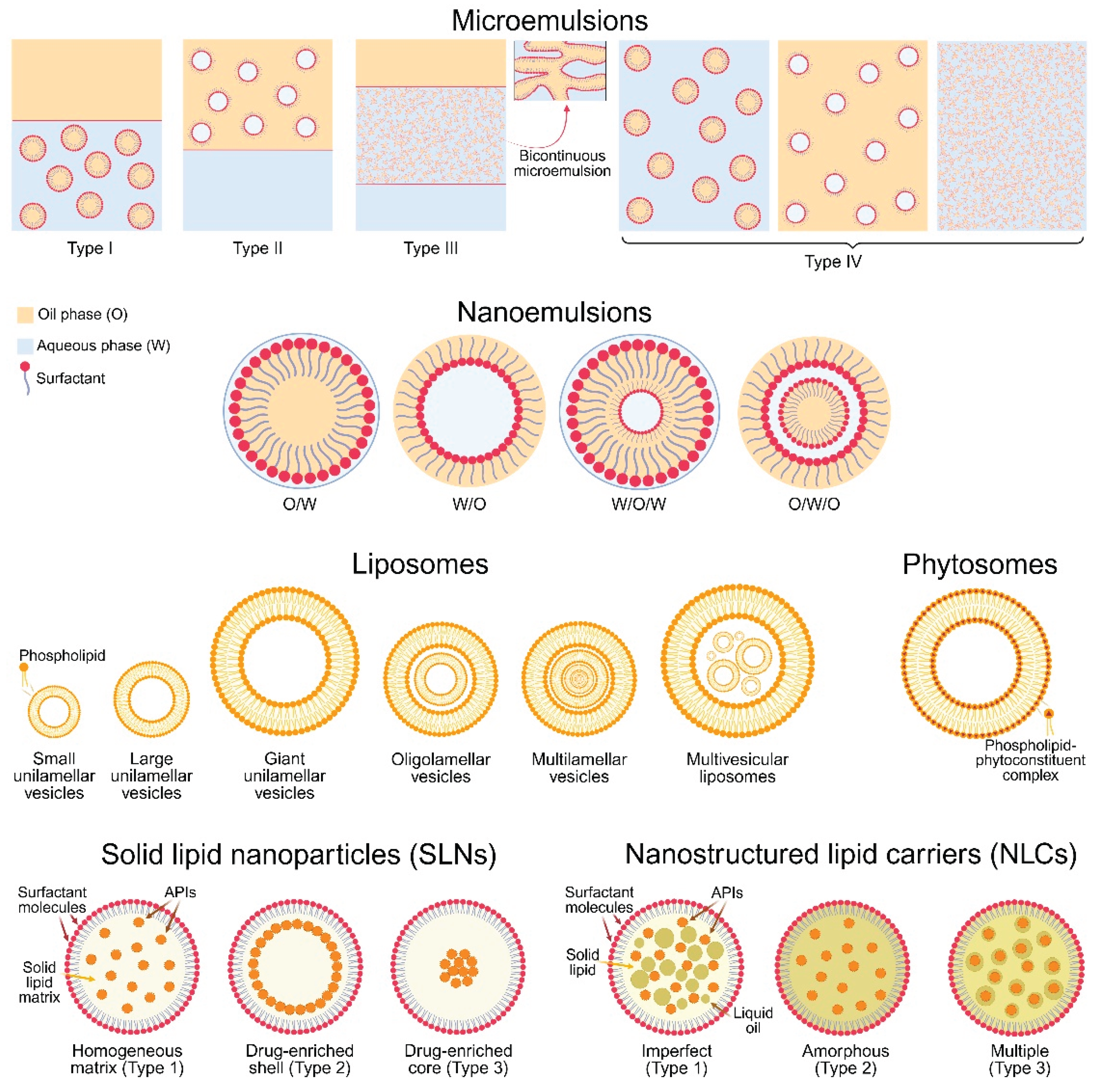
5.1. Microemulsions
5.2. Nanoemulsions
5.3. Liposomes
5.4. Phytosomes
5.5. Solid Lipid Nanoparticles
5.6. Nanostructured Lipid Carriers
5.7. Other Lipid-Based Drug Delivery Systems
6. Lipid-Based Nanotechnologies for Drug Delivery of GTCs in the Search of Improved Anti-Inflammatory and Antioxidant Activity
| Active compound/s | Nano-carrier type | Nano-carrier characteristics | Suggested mechanism/s of action | Scientific result | Reference |
|---|---|---|---|---|---|
| EGCG | NLCs, with or without chitosan coating | Mean size: NLCs: 46.3 nm Chitosan-coated NLCs: 53.5 nm Polydispersity index: NLCs: 0.19 Chitosan-coated NLCs: 0.19 ζ-potential: NLCs: −12.6 mV Chitosan-coated NLCs: +13.3 mV Entrapment efficiency (NLCs and chitosan-coated NLCs): ~99% Loading capacity (NLCs and chitosan-coated NLCs): ~3% |
Both EGCG-loaded NLCs and chitosan-coated NLCs effectively reduced cholesteryl ester accumulation and downregulated MCP-1 expression, suggesting their strong potential to mitigate inflammatory responses and slow or even reverse the progression of atherosclerotic lesions. | Encapsulation of EGCG in NLCs and chitosan-coated NLCs improved EGCG stability; Provided sustained release of EGCG; Increased EGCG cellular bioavailability. |
[222] |
| EGCG | Actively-targeted LNs | Mean size: 106 nm Polydispersity index: 0.19 ζ-potential: −20.6 mV Entrapment efficiency: ~95% Loading capacity: ~10% |
Incorporation of EGCG into CD36-targeted nanoparticles significantly reduced the secretion of inflammatory cytokines (MCP-1, TNF-α, IL-6) from mouse peritoneal macrophages in vitro and decreased atherosclerotic lesion area by ~30% in LDLr−/− mice, compared to native EGCG and non-targeted formulations. | Encapsulation of EGCG in nanoparticles; Improved EGCG stability; Enhancement of the EGCG bioavailability; Enhancement of EGCG targeted delivery. |
[223] |
| EGCG | Cationic LNs, utilizing dimethyldioctadecylammonium bromide (DDAB) or cetyltrimethylammonium bromide (CTAB) | Mean size: DDAB-based LNs: 143.7 nm CTAB-based LNs: 149.1 nm Polydispersity index: DDAB-based LNs: 0.16 CTAB-based LNs: 0.24 ζ-potential: DDAB-based LNs: +25.7 mV CTAB-based LNs: +20.8 mV Entrapment efficiency: n.i. Loading capacity: n.i. |
Cationic EGCG-loaded LNs, formulated with CTAB or DDAB, demonstrated sustained release and effective transcorneal and transscleral permeation, suggesting their strong potential to enhance ocular bioavailability and support the treatment of inflammation- and oxidative stress-related eye disorders. | Encapsulation of EGCG in cationic LNs improved EGCG stability; Increased EGCG bioavailability, when administered onto the ocular mucosa; Enhanced EGCG release and permeation characteristics. |
[224] |
| C | Nanoemulsion (subsequently loaded in Carbopol hydrogel) | Mean size: 98.6 nm Polydispersity index: 0.12 ζ-potential: −27.3 mV Entrapment efficiency: 99.02% Loading capacity: 1.12 % |
C-loaded nanoemulsion based nano-gel, enhanced transdermal permeation, stability, and antioxidant enzyme restoration in UVA-irradiated skin, showing strong potential for topical anti-inflammatory and photoprotective therapy. | Improved C permeation; Enhancement of C bioavailability. |
[225] |
| EGCG | Niosomes | Mean diameter: ~60 nm Polydispersity index: ~0.11 ζ-potential: n.i. Entrapment efficiency: 76.4% Loading capacity: n.i. |
Encapsulation of EGCG in Tween 60/cholesterol-based niosomes significantly improved its chemical stability and antioxidant activity under simulated intestinal conditions, increasing residual EGCG from 3% (free EGCG) to 49% after 2 h. Niosomal EGCG showed ~1.5-fold higher cellular antioxidant activity in HepG2 cells compared to free EGCG, even after digestion. | Enhanced EGCG digestive stability; Enhanced EGCG digestive bioavailability. |
[226] |
| EGCG | Phytosomes | Mean size: 100÷250 nm Polydispersity index: n.i. ζ-potential: n.i. Entrapment efficiency: up to 90% Loading capacity: n.i. |
EGCG-loaded phytosomes significantly reduced carrageenan-induced paw edema in a rat model compared to both free EGCG and green tea extract. The formulation achieved up to 88.2% inhibition of inflammation after four days, with a noticeable reduction in paw volume observed as early as three hours post-administration. Furthermore, the anti-inflammatory response was sustained throughout the study period, highlighting the prolonged efficacy of the phytosomal system. | Improved EGCG stability; Enhancement of EGCG bioavailability; Prolonged retention of EGCG. |
[227] |
| C | Niosomes | Mean size: 204.0 nm Polydispersity value: 0.27 ζ-potential: −41.7 mV Entrapment efficiency: 49.5% Loading capacity: n.i. |
Incorporation of C into niosomes enhanced dermal delivery and antioxidant effects in UVA-irradiated human fibroblasts. Compared to free C, niosome-encapsulated C increased skin deposition by ~5-fold at 24 h, significantly improved cell viability post-UVA exposure (90% vs 79%), reduced lipid peroxidation (levels of malondialdehyde—MDA), and enhanced antioxidant enzyme activities (SOD and GSH-Px). Enhanced cellular uptake via energy-dependent endocytosis was also observed. | Enhanced C physicochemical stability; Improved C dermal deposition; Prolonged C release profile; Increased cellular uptake. |
[228] |
| EGCG | Niosomes | Mean size: 235.4 nm Polydispersity index: 0.27 ζ-potential: −45.2 mV Entrapment efficiency: 53.05% Loading capacity: n.i. |
Incorporation of EGCG into Span 60-based niosomal nanocarriers significantly enhanced dermal penetration and skin deposition (~2-fold vs free EGCG) in full-thickness human skin explants. The formulation provided sustained release over 24 h and protected human dermal fibroblasts from UVA-induced oxidative stress by increasing cell viability, reducing intracellular MDA levels, and enhancing antioxidant enzyme activities (SOD, GSH-Px), compared to free EGCG. | Enhanced EGCG physicochemical stability; Improved EGCG dermal deposition; Prolonged EGCG release profile; Increased EGCG cellular uptake. |
[229] |
| C | Hexosomes, with or without sodium taurocholate (ST) | Mean size: With ST: 158.0 nm Without ST: 160.0 nm Polydispersity index: With ST: 0.14 Without ST: 0.13 ζ-potential: With ST: −54.0 mV Without ST: −29.0 mV Entrapment efficiency: With ST: 99.9% Without ST: 99.5% Loading capacity: n.i. |
Lipid-based hexosomes incorporating ST and loaded with C significantly enhanced in vitro skin penetration and transdermal delivery by overcoming the stratum corneum barrier in pig skin, while preserving strong antioxidant activity (~88% DPPH inhibition), outperforming non-ST-containing hexosomes and vesicles in terms of penetration depth and cargo loading capacity. | Enhanced physicochemical stability of C; Improved C dermal deposition; Prolonged C release profile; Increased cellular uptake of C. |
[230] |
| Green tea extract | Niosomes | Mean size: ~ 300 nm Polydispersity index: n.i. ζ-potential: n.i. Entrapment efficiency: n.i. Loading capacity: n.i. |
Niosomal green tea extract significantly enhanced cellular antioxidant activity in HepG2 cells compared to native green tea extract and led to greater reductions in plasma total and LDL cholesterol (−25.8% vs. −10.9%) in high-fat-fed C57BL/6 mice. The loaded vesicles upregulated hepatic LDL receptor (+274.7%) and CYP7A1 expression, and downregulated HMG-CoA reductase and SREBP2 mRNA expression more effectively than native green tea extract, indicating improved bioavailability and hypocholesterolemic effects via modulation of cholesterol metabolism and enhanced intracellular antioxidant defense. | Enhanced physicochemical stability; Increased cellular uptake. |
[231] |
7. Lipid-Based Nanotechnologies for Drug Delivery of GTCs in the Search of Improved Neuroprotective Activity
8. Lipid-Based Nanotechnologies for Drug Delivery of GTCs in the Search of Improved Anticarcinogenic Activity
9. Lipid-Based Nanotechnologies for Drug Delivery of GTCs in the Search of Improved Antimicrobial Activity
10. Conclusion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ABTS AP-1 APIs BACs Bcl-2 C CTAB DDAB DPPH EC ECG EGC EGCG EGFR ERK FGF2 GLUT1 GRP78 GSH-Px GTCs HIV HMG-CoA HPV IGF1R LNs MAPK MCP-1 MDA MET MIC MRSA NF-κB NLCs Nrf2/ARE O/W O/W/O p27^Kip1 PI3K/Akt RNS ROS sAPP-α SARS-CoV-2 SC-CO2 Sirt1/Nrf2/HO-1 SLE SLNs SOD SREBP2 ST STAT TNF-α VEGFA VEGFR2 W/O W/O/W |
2,2'-Azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) Activator protein 1 Active pharmaceutical ingredients Biologically active compounds B-cell lymphoma 2 (+)-catechin Cetyltrimethylammonium bromide Dimethyldioctadecylammonium bromide 2,2-diphenyl-1-picrylhydrazyl (−)-epicatechin (−)-epicatechin-3-gallate (−)-epigallocatechin (−)-epigallocatechin-3-gallate Epidermal growth factor receptor Extracellular signal-regulated kinase Fibroblast growth factor 2 Glucose transporter type 1 Glucose-regulated protein 78 Glutathione peroxidase Green tea catechins Human immunodeficiency virus 3-hydroxy-3-methylglutaryl coenzyme A Human papilloma virus Insulin-like growth factor 1 receptor Lipid nanoparticles Mitogen-activated protein kinase Monocyte chemoattractant protein-1 Malondialdehyde Mesenchymal-epithelial transition factor Minimum inhibitory concentration Methicillin-resistant staphylococcus aureus Nuclear factor kappa b Nanostructured lipid carriers Nuclear factor erythroid 2-related factor 2/Antioxidant response element Oil-in-water Oil-in-water-in-oil Cyclin-dependent kinase inhibitor 1b Phosphoinositide 3-kinase/Protein kinase b Reactive nitrogen species Reactive oxygen species Soluble amyloid precursor protein alpha Severe acute respiratory syndrome coronavirus 2 Supercritical CO2 extraction Silent information regulator 1/Nuclear factor erythroid 2-related factor 2/Heme oxygenase-1 Solid-liquid extraction Solid lipid nanoparticles Superoxide dismutase Sterol regulatory element-binding protein 2 Sodium taurocholate Signal transducer and activator of transcription Tumor necrosis factor alpha Vascular endothelial growth factor A Vascular endothelial growth factor receptor 2 Water-in-oil Water-in-oil-in-water |
References
- Mathur, S.; Hoskins, C. Drug development: Lessons from nature (Review). Biomed. Rep. 2017, 6, 612–614. [Google Scholar] [CrossRef]
- Najmi, A.; Javed, S.A.; Al Bratty, M.; Alhazmi, H.A. Modern Approaches in the Discovery and Development of Plant-Based Natural Products and Their Analogues as Potential Therapeutic Agents. Molecules 2022, 27, 349. [Google Scholar] [CrossRef]
- Dzobo, K. The Role of Natural Products as Sources of Therapeutic Agents for Innovative Drug Discovery. In Comprehensive Pharmacology; Kenakin, T., Ed.; Elsevier: Amsterdam, Netherlands, 2022; pp. 408–422. ISBN 978-0-12-820876-2. [Google Scholar]
- PAHO/WHO. WHO Global Summit on Traditional Medicine Highlights Scientific Evidence and Integration into Health Systems. Pan American Health Organization. 28 August 2023. Available online: https://www.paho.org/en/news/6-9-2023-who-global-summit-traditional-medicine-highlights-scientific-evidence-and-integration (accessed on 21 April 2025).
- Wang, C.; Han, J.; Pu, Y.; Wang, X. Tea (Camellia sinensis): A Review of Nutritional Composition, Potential Applications, and Omics Research. Appl. Sci. 2022, 12, 5874. [Google Scholar] [CrossRef]
- Verified Market Reports. Botanical and Plant Derivative Drug Market. Available online: https://www.verifiedmarketreports.com/product/botanical-and-plant-derivative-drug-market/ (accessed on 21 April 2025).
- Zhao, T.; Li, C.; Wang, S.; Song, X. Green Tea (Camellia sinensis): A Review of Its Phytochemistry, Pharmacology, and Toxicology. Molecules 2022, 27, 3909. [Google Scholar] [CrossRef] [PubMed]
- Kosugi, Y.; Green Tea Consumption. O-CHA NET. Available online: https://www.o-cha.net/english/teacha/distribution/greentea3.html (accessed on 21 April 2025).
- Wong, M.; Sirisena, S.; Ng, K. Phytochemical Profile of Differently Processed Tea: A Review. J. Food Sci. 2022, 87, 1925–1942. [Google Scholar] [CrossRef] [PubMed]
- Bag, S.; Mondal, A.; Majumder, A.; Banik, A. Tea and Its Phytochemicals: Hidden Health Benefits & Modulation of Signaling Cascade by Phytochemicals. Food Chem. 2022, 371, 131098. [Google Scholar] [CrossRef]
- Engelhardt, U.H. Different Types of Tea: Chemical Composition, Analytical Methods, and Authenticity. In Natural Products in Beverages; Mérillon, J.-M., Rivière, C., Lefèvre, G., Eds.; Springer: Cham, Switzerland, 2025; pp. 39–82. ISBN 978-3-031-38663-3. [Google Scholar]
- Chacko, S.M.; Thambi, P.T.; Kuttan, R.; Nishigaki, I. Beneficial Effects of Green Tea: A Literature Review. Chin. Med. 2010, 5, 13. [Google Scholar] [CrossRef]
- Cabrera, C.; Artacho, R.; Giménez, R. Beneficial Effects of Green Tea—A Review. J. Am. Coll. Nutr. 2006, 25, 79–99. [Google Scholar] [CrossRef]
- Bansal, S.; Syan, N.; Mathur, P.; Choudhary, S. Pharmacological Profile of Green Tea and Its Polyphenols: A Review. Med. Chem. Res. 2012, 21, 3347–3360. [Google Scholar] [CrossRef]
- Mak, J.C. Potential Role of Green Tea Catechins in Various Disease Therapies: Progress and Promise. Clin. Exp. Pharmacol. Physiol. 2012, 39, 265–273. [Google Scholar] [CrossRef]
- Parmar, N.; Rawat, M.; Kumar, J.V. Camellia Sinensis (Green Tea): A Review. Glob. J. Pharmacol. 2012, 6, 52–59. [Google Scholar]
- Radeva-Ilieva, M.; Stoeva, S.; Hvarchanova, N.; Georgiev, K.D. Green Tea: Current Knowledge and Issues. Foods 2025, 14, 745. [Google Scholar] [CrossRef] [PubMed]
- Gusev, P.A.; Andrews, K.W.; Savarala, S.; Tey, P.T.; Han, F.; Oh, L.; Pehrsson, P.R.; Dwyer, J.T.; Betz, J.M.; Kuszak, A.J.; Costello, R.; Saldanha, L.G. Disintegration and Dissolution Testing of Green Tea Dietary Supplements: Application and Evaluation of United States Pharmacopeial Standards. J. Pharm. Sci. 2020, 109, 1933–1942. [Google Scholar] [CrossRef] [PubMed]
- U V, R.; R, S.S.; Kumar K, R.; Narayan Sinha, S. Method Development and Validation for Rapid Identification of Epigallocatechin Gallate Using Ultra-High Performance Liquid Chromatography. PLoS ONE 2020, 15, e0227569. [Google Scholar] [CrossRef]
- Cai, Z.-Y.; Li, X.-M.; Liang, J.-P.; Xiang, L.-P.; Wang, K.-R.; Shi, Y.-L.; Yang, R.; Shi, M.; Ye, J.-H.; Lu, J.-L.; Zheng, X.-Q.; Liang, Y.-R. Bioavailability of Tea Catechins and Its Improvement. Molecules 2018, 23, 2346. [Google Scholar] [CrossRef]
- Eltaib, L. Polymeric Nanoparticles in Targeted Drug Delivery: Unveiling the Impact of Polymer Characterization and Fabrication. Polymers 2025, 17, 833. [Google Scholar] [CrossRef]
- Anselmo, A.C.; Mitragotri, S. A Review of Clinical Translation of Inorganic Nanoparticles. AAPS J. 2015, 17, 1041–1054. [Google Scholar] [CrossRef]
- Eker, F.; Duman, H.; Akdaşçi, E.; Bolat, E.; Sarıtaş, S.; Karav, S.; Witkowska, A.M. A Comprehensive Review of Nanoparticles: From Classification to Application and Toxicity. Molecules 2024, 29, 3482. [Google Scholar] [CrossRef]
- Patel, P.; Garala, K.; Singh, S.; Prajapati, B.G.; Chittasupho, C. Lipid-Based Nanoparticles in Delivering Bioactive Compounds for Improving Therapeutic Efficacy. Pharmaceuticals 2024, 17, 329. [Google Scholar] [CrossRef]
- Chen, B.; Zhang, W.; Lin, C.; Zhang, L. A Comprehensive Review on Beneficial Effects of Catechins on Secondary Mitochondrial Diseases. Int. J. Mol. Sci. 2022, 23, 11569. [Google Scholar] [CrossRef]
- Pedro, A.C.; Maciel, G.M.; Ribeiro, V.R.; Haminiuk, C.W.I. Fundamental and Applied Aspects of Catechins from Different Sources: A Review. Int. J. Food Sci. Technol. 2020, 55, 429–442. [Google Scholar] [CrossRef]
- Kumari, M.; Radha; Kumar, M. ; Zhang, B.; Amarowicz, R.; Puri, S.; Pundir, A.; Rathour, S.; Kumari, N.; Chandran, D.; et al. Acacia catechu (L.f.) Willd.: A Review on Bioactive Compounds and Their Health Promoting Functionalities. Plants 2022, 11, 3091. [Google Scholar] [CrossRef]
- Isemura, M. Catechin in Human Health and Disease. Molecules 2019, 24, 528. [Google Scholar] [CrossRef] [PubMed]
- Gramza, A.; Korczak, J.; Amarowicz, R. Tea Polyphenols - Their Antioxidant Properties and Biological Activity - a Review. Pol. J. Food Nutr. Sci. 2005, 14, 219–235. [Google Scholar]
- Botten, D.; Fugallo, G.; Fraternali, F.; Molteni, C. Structural Properties of Green Tea Catechins. J. Phys. Chem. B 2015, 119, 12860–12867. [Google Scholar] [CrossRef]
- Sivanesan, I.; Gopal, J.; Muthu, M.; Chun, S.; Oh, J.-W. Retrospecting the Antioxidant Activity of Japanese Matcha Green Tea–Lack of Enthusiasm? Appl. Sci. 2021, 11, 5087. [Google Scholar] [CrossRef]
- Nain, C.W.; Mignolet, E.; Herent, M.-F.; Quetin-Leclercq, J.; Debier, C.; Page, M.M.; Larondelle, Y. The Catechins Profile of Green Tea Extracts Affects the Antioxidant Activity and Degradation of Catechins in DHA-Rich Oil. Antioxidants 2022, 11, 1844. [Google Scholar] [CrossRef]
- Stoeva, S.; Ilieva, M.; Zhelev, I.; Georgiev, K. A HPLC-UV Method for Analysis of Total Plant Extract and Catechin Fraction of Bancha Green Tea. Nat.Prod. J. 2023, 13, 90–97. [Google Scholar] [CrossRef]
- Piboolpunthuwong, P.; Changtam, C.; Jularattanaporn, V. The comparison between total phenolic content and antioxidant activity of gyokuro, sencha and matcha from some tea store in Chiang Rai and Bangkok. In Proceedings of the 9th National and International Conference on “Research to Serve Society”, Samutprakarn, Thailand, 1 July 2022; Huachiew Chalermprakiet University: Samutprakarn, Thailand, 2022; pp. 500–509. [Google Scholar]
- Zielinski, A.A.F.; Granato, D.; Alberti, A. Modelling the Extraction of Phenolic Compounds and in Vitro Antioxidant Activity of Mixtures of Green, White and Black Teas (Camellia Sinensis L. Kuntze). J. Food Sci. Technol. 2015, 52, 6966–6977. [Google Scholar] [CrossRef]
- Hinojosa-Nogueira, D.; Pérez-Burillo, S.; Pérez-Burillo, S.; Pastoriza de la Cueva, S.; Rufián-Henares, J.Á. Green and White Teas as Health-Promoting Foods. Food Funct. 2021, 12, 3799–3819. [Google Scholar] [CrossRef]
- Paiva, L.; Rego, C.; Lima, E.; Marcone, M.; Baptista, J. Comparative Analysis of the Polyphenols, Caffeine, and Antioxidant Activities of Green Tea, White Tea, and Flowers from Azorean Camellia sinensis Varieties Affected by Different Harvested and Processing Conditions. Antioxidants 2021, 10, 183. [Google Scholar] [CrossRef] [PubMed]
- Kołodziej, B.; Sugier, D.; Luchowska, K. Changes of Antioxidant Activity and Active Compounds Content in Selected Teas. Foods Raw Mater. 2020, 8, 91–97. [Google Scholar] [CrossRef]
- Nuryana, I.; Andriani, A.; Juanssilfero, A.; Fahrurrozi, F.; Putra, F.J.N. Catechin Contents, Antioxidant and Antibacterial Activities of Different Types of Indonesian Tea (Camellia Sinensis). Ann. Bogor. 2020, 2, 106–113. [Google Scholar] [CrossRef]
- Nur, S.; Aisyah, A.; Fadri, A.; Sapra, A.; Sami, F. Comparative Study of Catechin Levels from Green Tea, Oolong Tea and Black Tea Product with Various Treatments. GSC Biol. Pharm. Sci. 2021, 14, 001–010. [Google Scholar] [CrossRef]
- Chen, M.; Zhu, Y.; Zhang, H.; Wang, J.; Liu, X.; Chen, Z.; Liu, B. Phenolic Compounds and the Biological Effects of Pu-Erh Teas with Long-Term Storage. Int. J. Food Prop. 2016, 20, 1715–1728. [Google Scholar] [CrossRef]
- Gadkari, P.V.; Balaraman, M. Catechins: Sources, Extraction and Encapsulation: A Review. Food Bioprod. Process. 2015, 93, 122–138. [Google Scholar] [CrossRef]
- Nagle, D.; Ferreira, D.; Zhou, Y. Epigallocatechin-3-Gallate (EGCG): Chemical and Biomedical Perspectives. Phytochemistry 2006, 67, 1849–1855. [Google Scholar] [CrossRef]
- Zhu, Y.; Yuzuak, S.; Sun, X.; Xie, D.-Y. Identification and Biosynthesis of Plant Papanridins, a Group of Novel Oligomeric Flavonoids. Mol. Plant 2023, 16, 1773–1793. [Google Scholar] [CrossRef]
- Liu, W.; Feng, Y.; Yu, S.; Fan, Z.; Li, X.; Li, J.; Yin, H. The Flavonoid Biosynthesis Network in Plants. Int. J. Mol. Sci. 2021, 22, 12824. [Google Scholar] [CrossRef]
- Hong, G.; Wang, J.; Zhang, Y.; Hochstetter, D.; Zhang, S.; Pan, Y.; Shi, Y.; Xu, P.; Wang, Y. Biosynthesis of Catechin Components Is Differentially Regulated in Dark-Treated Tea (Camellia Sinensis L.). Plant Physiol. Biochem. 2014, 78, 49–52. [Google Scholar] [CrossRef]
- Yu, D.; Huang, T.; Tian, B.; Zhan, J. Advances in Biosynthesis and Biological Functions of Proanthocyanidins in Horticultural Plants. Foods 2020, 9, 1774. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.-J.; Li, X.-H.; Liu, Z.-W.; Zhuang, J. De Novo Assembly and Transcriptome Characterization: Novel Insights into Catechins Biosynthesis in Camellia Sinensis. BMC Plant Biol. 2014, 14, 277. [Google Scholar] [CrossRef]
- Li, J.; Liu, S.; Chen, P.; Cai, J.; Tang, S.; Yang, W.; Cao, F.; Zheng, P.; Sun, B. Systematic Analysis of the R2R3-MYB Family in Camellia Sinensis: Evidence for Galloylated Catechins Biosynthesis Regulation. Front. Plant Sci. 2022, 12, 782220. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, Y.; Wang, Y.; Wu, L.; He, M.; Mao, Z.; Liu, G.; Wei, K.; Wang, L. Two Shikimate Dehydrogenases Play an Essential Role in the Biosynthesis of Galloylated Catechins in Tea Plants. Hortic. Res. 2025, 12, uhae356. [Google Scholar] [CrossRef]
- Khokhar, S.; Magnusdottir, S.G.M. Total Phenol, Catechin, and Caffeine Contents of Teas Commonly Consumed in the United Kingdom. J. Agric. Food Chem. 2002, 50, 565–570. [Google Scholar] [CrossRef]
- Rusak, G.; Šola, I.; Vujčić Bok, V. Matcha and Sencha Green Tea Extracts with Regard to Their Phenolics Pattern and Antioxidant and Antidiabetic Activity during in Vitro Digestion. J. Food Sci. Technol. 2021, 58, 3568–3578. [Google Scholar] [CrossRef] [PubMed]
- Meyer, B.R.; White, H.M.; McCormack, J.D.; Niemeyer, E.D. Catechin Composition, Phenolic Content, and Antioxidant Properties of Commercially-Available Bagged, Gunpowder, and Matcha Green Teas. Plant Foods Hum. Nutr. 2023, 78, 662–669. [Google Scholar] [CrossRef]
- Fujioka, K.; Iwamoto, T.; Shima, H.; Tomaru, K.; Saito, H.; Ohtsuka, M.; Yoshidome, A.; Kawamura, Y.; Manome, Y. The Powdering Process with a Set of Ceramic Mills for Green Tea Promoted Catechin Extraction and the ROS Inhibition Effect. Molecules 2016, 21, 474. [Google Scholar] [CrossRef]
- Ortiz, J.; Ferruzzi, M.G.; Taylor, L.S.; Mauer, L.J. Interaction of Environmental Moisture with Powdered Green Tea Formulations: Effect on Catechin Chemical Stability. J. Agric. Food Chem. 2008, 56, 7586–7586. [Google Scholar] [CrossRef]
- Ananingsih, V.K.; Sharma, A.; Zhou, W. Green Tea Catechins during Food Processing and Storage: A Review on Stability and Detection. Food Res. Int. 2013, 50, 469–479. [Google Scholar] [CrossRef]
- Cioanca, O.; Lungu, I.-I.; Mita-Baciu, I.; Robu, S.; Burlec, A.F.; Hancianu, M.; Crivoi, F. Extraction and Purification of Catechins from Tea Leaves: An Overview of Methods, Advantages, and Disadvantages. Separations 2024, 11, 171. [Google Scholar] [CrossRef]
- Sang, S.; Lambert, J.D.; Ho, C.-T.; Yang, C.-S. The chemistry and biotransformation of tea constituents. Pharmacol. Res. 2011, 64, 87–99. [Google Scholar] [CrossRef] [PubMed]
- Turkmen, N.; Velioglu, Y.S.; Sari, F.; Polat, G. Effect of Extraction Conditions on Measured Total Polyphenol Contents and Antioxidant and Antibacterial Activities of Black Tea. Molecules 2007, 12, 484–496. [Google Scholar] [CrossRef] [PubMed]
- Devi, S. Catechins. In A Centum of Valuable Plant Bioactives, 1st ed.; Mushtaq, M., Anwar, F., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 525–544. ISBN 978-0-12-822924-8. [Google Scholar]
- Sharif, R.; Ahmad, S.W.; Anjum, H.; Ramzan, N.; Malik, S.R. Effect of Infusion Time and Temperature on Decaffeination of Tea Using Liquid–Liquid Extraction Technique. J. Food Process Eng. 2013, 37, 46–52. [Google Scholar] [CrossRef]
- Liang, H.; Liang, Y.; Dong, J.; Lu, J.; Xu, H.; Wang, H. Decaffeination of Fresh Green Tea Leaf (Camellia Sinensis) by Hot Water Treatment. Food Chem. 2007, 101, 1451–1456. [Google Scholar] [CrossRef]
- Serdar, G.; Demir, E.; Sökmen, M. Sequential Green Extraction of Caffeine and Catechins from Green Tea. Int. J. Second. Metab. 2019, 6, 283–291. [Google Scholar] [CrossRef]
- Choung, M.; Hwang, Y.; Lee, M.; Lee, J.; Kang, S.; Jun, T. Comparison of Extraction and Isolation Efficiency of Catechins and Caffeine from Green Tea Leaves Using Different Solvent Systems. Int. J. Food Sci. Technol. 2013, 49, 1572–1578. [Google Scholar] [CrossRef]
- Ivanova, N.; Ermenlieva, N.; Simeonova, L.; Kolev, I.; Slavov, I.; Karashanova, D.; Andonova, V. Chlorhexidine–Silver Nanoparticle Conjugation Leading to Antimicrobial Synergism but Enhanced Cytotoxicity. Pharmaceutics 2023, 15, 2298. [Google Scholar] [CrossRef]
- Dong, J.-J.; Ye, J.-H.; Lu, J.-L.; Zheng, X.-Q.; Liang, Y.-R. Isolation of Antioxidant Catechins from Green Tea and Its Decaffeination. Food Bioprod. Process. 2011, 89, 62–66. [Google Scholar] [CrossRef]
- Atwi-Ghaddar, S.; Zerwette, L.; Destandau, E.; Lesellier, E. Supercritical Fluid Extraction (SFE) of Polar Compounds from Camellia sinensis Leaves: Use of Ethanol/Water as a Green Polarity Modifier. Molecules 2023, 28, 5485. [Google Scholar] [CrossRef]
- Dutta, S.; Priyadarshini, S.; Moses, J.; Anandharamakrishnan, C. Supercritical Fluid and Ultrasound-assisted Green Extraction Technologies for Catechin Recovery. Chem. Bio. Eng. Reviews 2021, 8, 654–664. [Google Scholar] [CrossRef]
- Zhou, P.; Tang, D.; Zou, J.; Wang, X. An Alternative Strategy for Enhancing Stability and Antimicrobial Activity of Catechins by Natural Deep Eutectic Solvents. LWT 2022, 153, 112558. [Google Scholar] [CrossRef]
- Bajkacz, S.; Adamek, J.; Sobska, A. Application of Deep Eutectic Solvents and Ionic Liquids in the Extraction of Catechins from Tea. Molecules 2020, 25, 3216. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Jiang, Z.; Ma, L.; Huang, Q. Advances in Nanodelivery of Green Tea Catechins to Enhance the Anticancer Activity. Molecules 2021, 26, 3301. [Google Scholar] [CrossRef]
- Jovanović, N.; Miličević, A. A New, Simplified Model for the Estimation of Polyphenol Oxidation Potentials Based on the Number of OH Groups. Arh. Hig. Rada. Toksikol. 2017, 68, 93–98. [Google Scholar] [CrossRef]
- Lambert, J. Cancer Chemopreventive Activity and Bioavailability of Tea and Tea Polyphenols. Mutat. Res. Fundam. Mol. Mech. Mutagen. 2003, 523–524, 201–208. [Google Scholar] [CrossRef]
- Shim, W.; Kim, C.E.; Lee, M.; Lee, S.H.; Park, J.; Do, M.; Yang, J.; Lee, H. Catechin Solubilization by Spontaneous Hydrogen Bonding with Poly(Ethylene Glycol) for Dry Eye Therapeutics. J. Control. Release 2019, 307, 413–422. [Google Scholar] [CrossRef] [PubMed]
- Monsanto, M.; Hooshyar, N.; Meuldijk, J.; Zondervan, E. Modeling and optimization of green tea precipitation for the recovery of catechins. Sep. Purif. Technol. 2014, 129, 129–136. [Google Scholar] [CrossRef]
- Ishizu, T.; Tsutsumi, H.; Sato, T. Mechanism of Creaming Down Based on Chemical Characterization of a Complex of Caffeine and Tea Catechins. Chem. Pharm. Bull. 2016, 64, 676–686. [Google Scholar] [CrossRef]
- Saeki, K.; Hayakawa, S.; Nakano, S.; Ito, S.; Oishi, Y.; Suzuki, Y.; Isemura, M. In Vitro and In Silico Studies of the Molecular Interactions of Epigallocatechin-3-O-gallate (EGCG) with Proteins That Explain the Health Benefits of Green Tea. Molecules 2018, 23, 1295. [Google Scholar] [CrossRef]
- Shi, M.; Huang, L.-Y.; Nie, N.; Ye, J.-H.; Zheng, X.-Q.; Lu, J.-L.; Liang, Y.-R. Binding of Tea Catechins to Rice Bran Protein Isolate: Interaction and Protective Effect during in Vitro Digestion. Food Res. Int. 2017, 93, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Srinivas, K.; King, J.W.; Howard, L.R.; Monrad, J.K. Solubility of Gallic Acid, Catechin, and Protocatechuic Acid in Subcritical Water from (298.75 to 415.85) K. J. Chem. Eng. Data 2010, 55, 3101–3108. [Google Scholar] [CrossRef]
- Selleckchem.com. (+)-Catechin. Available online: https://www.selleckchem.com/products/catechin.html (accessed on 15 May 2025).
- Cayman Chemical. Product Information. (+)-Catechin (hydrate). Available online: https://cdn.caymanchem.com/cdn/insert/70940.pdf (accessed on 15 May 2025).
- Sigma Aldrich. Safety Data Sheet. (-)-Epigallocatechin gallate. Available online: https://www.sigmaaldrich.com/BG/en/sds/sigma/e4143?userType=anonymous (accessed on 15 May 2025).
- Ahmad, R.; Aldholmi, M.; Alqathama, A.; Althomali, E.; Aljishi, F.; Mostafa, A.; Alqarni, A.M.; Shaaban, H. The Effect of Natural Antioxidants, pH, and Green Solvents upon Catechins Stability during Ultrasonic Extraction from Green Tea Leaves (Camellia Sinensis). Ultrason. Sonochem. 2023, 94, 106337. [Google Scholar] [CrossRef]
- Wang, W.; Le, T.; Wang, W.-W.; Yin, J.-F.; Jiang, H.-Y. The Effects of Structure and Oxidative Polymerization on Antioxidant Activity of Catechins and Polymers. Foods 2023, 12, 4207. [Google Scholar] [CrossRef]
- Shan, Z.; Nisar, M.; Li, M.; Zhang, C.; Wan, C. Theaflavin Chemistry and Its Health Benefits. Oxid. Med. Cell Longev. 2021, 2021, 6256618. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Taylor, L.S.; Ferruzzi, M.G.; Mauer, L.J. Kinetic Study of Catechin Stability: Effects of pH, Concentration, and Temperature. J. Agric. Food Chem. 2012, 60, 12531–12539. [Google Scholar] [CrossRef] [PubMed]
- Baranowska, M.; Suliborska, K.; Chrzanowski, W.; Kusznierewicz, B.; Namieśnik, J.; Bartoszek, A. The Relationship between Standard Reduction Potentials of Catechins and Biological Activities Involved in Redox Control. Redox Biol. 2018, 17, 355–366. [Google Scholar] [CrossRef]
- Del Rio, D.; Rodriguez-Mateos, A.; Spencer, J.; Tognolini, M.; Borges, G.; Crozier, A. Dietary (Poly)Phenolics in Human Health: Structures, Bioavailability, and Evidence of Protective Effects against Chronic Diseases. Antioxid. Redox Signal. 2013, 18, 1818–1892. [Google Scholar] [CrossRef]
- Raab, T.; Barron, D.; Vera, F.A.; Crespy, V.; Oliveira, M.; Williamson, G. Catechin Glucosides: Occurrence, Synthesis, and Stability. J. Agric. Food Chem. 2010, 58, 2138–2149. [Google Scholar] [CrossRef]
- Kumamoto, M.; Sonda, T.; Nagayama, K.; Tabata, M. Effects of pH and Metal Ions on Antioxidative Activities of Catechins. Biosci. Biotechnol. Biochem. 2001, 65, 126–132. [Google Scholar] [CrossRef]
- Poaty, B.; Dumarçay, S.; Perrin, D. New Lipophilic Catechin Derivatives by Oxa-Pictet-Spengler Reaction. Eur. Food Res. Technol. 2009, 230, 111–117. [Google Scholar] [CrossRef]
- Koch, W.; Kukuła-Koch, W.; Czop, M.; Helon, P.; Gumbarewicz, E. The Role of Extracting Solvents in the Recovery of Polyphenols from Green Tea and Its Antiradical Activity Supported by Principal Component Analysis. Molecules 2020, 25, 2173. [Google Scholar] [CrossRef]
- Sheng, Y.; Sun, Y.; Tang, Y.; Yu, Y.; Wang, J.; Zheng, F.; Li, Y.; Sun, Y. Catechins: Protective mechanism of antioxidant stress in atherosclerosis. Front Pharmacol. 2023, 14, 1144878. [Google Scholar] [CrossRef]
- Bernatoniene, J.; Kopustinskiene, D.M. The Role of Catechins in Cellular Responses to Oxidative Stress. Molecules 2018, 23, 965. [Google Scholar] [CrossRef] [PubMed]
- Musial, C.; Kuban-Jankowska, A.; Gorska-Ponikowska, M. Beneficial Properties of Green Tea Catechins. Int. J. Mol. Sci. 2020, 21, 1744. [Google Scholar] [CrossRef]
- Tumilaar, S.G.; Hardianto, A.; Dohi, H.; Kurnia, D. A Comprehensive Review of Free Radicals, Oxidative Stress, and Antioxidants: Overview, Clinical Applications, Global Perspectives, Future Directions, and Mechanisms of Antioxidant Activity of Flavonoid Compounds. J. Chem. 2024, 2024, 594386. [Google Scholar] [CrossRef]
- Stoeva, S.; Hvarchanova, N.; Georgiev, K.D.; Radeva-Ilieva, M. Green Tea: Antioxidant vs. Pro-Oxidant Activity. Beverages 2025, 11, 64. [Google Scholar] [CrossRef]
- Rice-evans, C.A.; Miller, N.J.; Bolwell, P.G.; Bramley, P.M.; Pridham, J.B. The relative antioxidant activities of plant-derived polyphenolic flavonoids. Free Radic. Res. 1995, 22, 375–383. [Google Scholar] [CrossRef]
- Simunkova, M.; Barbierikova, Z.; Jomova, K.; Hudecova, L.; Lauro, P.; Alwasel, S.H.; Alhazza, I.; Rhodes, C.J.; Valko, M. Antioxidant vs. Prooxidant Properties of the Flavonoid, Kaempferol, in the Presence of Cu(II) Ions: A ROS-Scavenging Activity, Fenton Reaction and DNA Damage Study. Int. J. Mol. Sci. 2021, 22, 1619. [Google Scholar] [CrossRef]
- Bors, W.; Heller, W.; Michel, C.; Saran, M. Flavonoids as antioxidants: Determination of radical-scavenging efficiencies. Methods Enzymol. 1990, 186, 343–354. [Google Scholar] [CrossRef]
- Fraga, C.G.; Galleano, M.; Verstraeten, S.V.; Oteiza, P.I. Basic biochemical mechanisms behind the health benefits of polyphenols. Mol. Asp. Med. 2010, 31, 435–445. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Zhong, Y.; Duan, Y.; Chen, Q.; Li, F. Antioxidant mechanism of tea polyphenols and its impact on health benefits. Anim. Nutr. 2020, 6, 115–123. [Google Scholar] [CrossRef]
- Farhan, M.; Rizvi, A. Understanding the Prooxidant Action of Plant Polyphenols in the Cellular Microenvironment of Malignant Cells: Role of Copper and Therapeutic Implications. Front. Pharmacol. 2022, 13, 929853. [Google Scholar] [CrossRef] [PubMed]
- Srividhya, R.; Kalaiselvi, P. Neuroprotective potential of epigallo catechin-3-gallate in PC-12 cells. Neurochem. Res. 2013, 38, 486–493. [Google Scholar] [CrossRef] [PubMed]
- Yamagata, K. Protective Effect of Epigallocatechin Gallate on Endothelial Disorders in Atherosclerosis. J. Cardiovasc. Pharmacol. 2020, 75, 292–298. [Google Scholar] [CrossRef]
- Mokra, D.; Joskova, M.; Mokry, J. Therapeutic Effects of Green Tea Polyphenol (‒)-Epigallocatechin-3-Gallate (EGCG) in Relation to Molecular Pathways Controlling Inflammation, Oxidative Stress, and Apoptosis. Int. J. Mol. Sci. 2023, 24, 340. [Google Scholar] [CrossRef]
- Truong, V.L.; Jeong, W.S. Cellular Defensive Mechanisms of Tea Polyphenols: Structure-Activity Relationship. Int. J. Mol. Sci. 2021, 22, 9109. [Google Scholar] [CrossRef]
- Ohishi, T.; Goto, S.; Monira, P.; Isemura, M.; Nakamura, Y. Anti-inflammatory Action of Green Tea. Antiinflamm. Antiallergy Agents Med. Chem. 2016, 15, 74–90. [Google Scholar] [CrossRef]
- Kim, J.M.; Heo, H.J. The roles of catechins in regulation of systemic inflammation. Food Sci. Biotechnol. 2022, 31, 957–970. [Google Scholar] [CrossRef]
- Wu, Y.R.; Choi, H.J.; Kang, Y.G.; Kim, J.K.; Shin, J.W. In Vitro Study on Anti-Inflammatory Effects of Epigallocatechin-3-Gallate-Loaded Nano- and Microscale Particles. Int. J. Nanomed. 2017, 12, 7007–7013. [Google Scholar] [CrossRef]
- Gonçalves, P.B.; Sodero, A.C.R.; Cordeiro, Y. Green Tea Epigallocatechin-3-gallate (EGCG) Targeting Protein Misfolding in Drug Discovery for Neurodegenerative Diseases. Biomolecules 2021, 11, 767. [Google Scholar] [CrossRef] [PubMed]
- Afzal, O.; Dalhat, M.H.; Altamimi, A.S.A.; Rasool, R.; Alzarea, S.I.; Almalki, W.H.; Murtaza, B.N.; Iftikhar, S.; Nadeem, S.; Nadeem, M.S.; Kazmi, I. Green Tea Catechins Attenuate Neurodegenerative Diseases and Cognitive Deficits. Molecules 2022, 27, 7604. [Google Scholar] [CrossRef]
- Lee, J.W.; Lee, Y.K.; Ban, J.O.; Ha, T.Y.; Yun, Y.P.; Han, S.B.; Oh, K.W.; Hong, J.T. Green tea (−)-epigallocatechin-3-gallate inhibits beta-amyloid-induced cognitive dysfunction through modification of secretase activity via inhibition of ERK and NF-κB pathways in mice. J. Nutr. 2009, 139, 1987–1993. [Google Scholar] [CrossRef]
- Frias, I.A.T. Design, Development and Characterization of Innovative Lipid Nanocarrier Based Epigallocatechin Gallate Delivery System for Preventive and Therapeutic Supplementation. Master’s Thesis, University of Porto, 2014.
- Li, S.; Wang, Z.; Liu, G.; Chen, M. Neurodegenerative Diseases and Catechins: (−)-Epigallocatechin-3-gallate Is a Modulator of Chronic Neuroinflammation and Oxidative Stress. Front. Nutr. 2024, 11, 1425839. [Google Scholar] [CrossRef] [PubMed]
- Mandel, S.; Youdim, M.B. Catechin Polyphenols: Neurodegeneration and Neuroprotection in Neurodegenerative Diseases. Free Radic. Biol. Med. 2004, 37, 304–317. [Google Scholar] [CrossRef] [PubMed]
- Katergaris, N.; Dufficy, L.; Roach, P.D.; Naumovski, N. Green tea catechins as neuroprotective agents: Systematic review of the literature in animal pre-clinical trials. Adv. Food Technol. Nutr. Sci. Open J. 2015, 1, 48–57. [Google Scholar] [CrossRef]
- Pervin, M.; Unno, K.; Ohishi, T.; Tanabe, H.; Miyoshi, N.; Nakamura, Y. Beneficial Effects of Green Tea Catechins on Neurodegenerative Diseases. Molecules 2018, 23, 1297. [Google Scholar] [CrossRef]
- Pervin, M.; Unno, K.; Takagaki, A.; Isemura, M.; Nakamura, Y. Function of Green Tea Catechins in the Brain: Epigallocatechin Gallate and Its Metabolites. Int. J. Mol. Sci. 2019, 20, 3630. [Google Scholar] [CrossRef]
- Prasanth, M.I.; Sivamaruthi, B.S.; Chaiyasut, C.; Tencomnao, T. A Review of the Role of Green Tea (Camellia sinensis) in Antiphotoaging, Stress Resistance, Neuroprotection, and Autophagy. Nutrients 2019, 11, 474. [Google Scholar] [CrossRef]
- Khalatbary, A.R.; Khademi, E. The green tea polyphenolic catechin epigallocatechin gallate and neuroprotection. Nutr. Neurosci. 2020, 23, 281–294. [Google Scholar] [CrossRef]
- Sebastiani, G.; Almeida-Toledano, L.; Serra-Delgado, M.; Navarro-Tapia, E.; Sailer, S.; Valverde, O.; Garcia-Algar, O.; Andreu-Fernández, V. Therapeutic Effects of Catechins in Less Common Neurological and Neurodegenerative Disorders. Nutrients 2021, 13, 2232. [Google Scholar] [CrossRef] [PubMed]
- Grabska-Kobyłecka, I.; Szpakowski, P.; Król, A.; Książek-Winiarek, D.; Kobyłecki, A.; Głąbiński, A.; Nowak, D. Polyphenols and Their Impact on the Prevention of Neurodegenerative Diseases and Development. Nutrients 2023, 15, 3454. [Google Scholar] [CrossRef]
- Pervin, M.; Unno, K.; Nakagawa, A.; Takahashi, Y.; Iguchi, K.; Yamamoto, H.; Hoshino, M.; Hara, A.; Takagaki, A.; Nanjo, F.; Minami, A.; Imai, S.; Nakamura, Y. Blood brain barrier permeability of (-)-epigallocatechin gallate, its proliferation-enhancing activity of human neuroblastoma SH-SY5Y cells, and its preventive effect on age-related cognitive dysfunction in mice. Biochem. Biophys. Rep. 2017, 9, 180–186. [Google Scholar] [CrossRef]
- Siegel, R.L.; Kratzer, T.B.; Giaquinto, A.N.; Sung, H.; Jemal, A. Cancer statistics, 2025. CA Cancer J. Clin. 2025, 75, 10–45. [Google Scholar] [CrossRef]
- Chatterjee, N.; Walker, G.C. Mechanisms of DNA damage, repair, and mutagenesis. Environ. Mol. Mutagen. 2017, 58, 235–263. [Google Scholar] [CrossRef] [PubMed]
- Basu, A.K.; Nohmi, T. Chemically-Induced DNA Damage, Mutagenesis, and Cancer. Int. J. Mol. Sci. 2018, 19, 1767. [Google Scholar] [CrossRef] [PubMed]
- Friedberg, E.C. A brief history of the DNA repair field. Cell Res. 2008, 18, 3–7. [Google Scholar] [CrossRef]
- Niida, H.; Nakanishi, M. DNA damage checkpoints in mammals. Mutagenesis 2006, 21, 3–9. [Google Scholar] [CrossRef]
- Yang, C.S.; Wang, X.; Lu, G.; Picinich, S.C. Cancer prevention by tea: animal studies, molecular mechanisms and human relevance. Nat. Rev. Cancer 2009, 9, 429–439. [Google Scholar] [CrossRef]
- Ju, J.; Lu, G.; Lambert, J.D.; Yang, C.S. Inhibition of carcinogenesis by tea constituents. Semin. Cancer Biol. 2007, 17, 395–402. [Google Scholar] [CrossRef]
- Farhan, M. Green Tea Catechins: Nature's Way of Preventing and Treating Cancer. Int. J. Mol. Sci. 2022, 23, 10713. [Google Scholar] [CrossRef] [PubMed]
- Khair, A.M.B.; Maniangat Luke, A.; Patnaik, R.; Testarelli, L. EGCG's anticancer potential unveiled: triggering apoptosis in lung cancer cell lines through in vitro investigation. PeerJ 2025, 13, e19135. [Google Scholar] [CrossRef] [PubMed]
- Tsouh Fokou, P.V.; Kamdem Pone, B.; Appiah-Oppong, R.; Ngouana, V.; Bakarnga-Via, I.; Ntieche Woutouoba, D.; Flore Donfack Donkeng, V.; Tchokouaha Yamthe, L.R.; Fekam Boyom, F.; Arslan Ateşşahin, D.; Sharifi-Rad, J.; Calina, D. An Update on Antitumor Efficacy of Catechins: From Molecular Mechanisms to Clinical Applications. Food Sci. Nutr. 2025, 13, e70169. [Google Scholar] [CrossRef] [PubMed]
- Kciuk, M.; Alam, M.; Ali, N.; Rashid, S.; Głowacka, P.; Sundaraj, R.; Celik, I.; Yahya, E.B.; Dubey, A.; Zerroug, E.; Kontek, R. Epigallocatechin-3-Gallate Therapeutic Potential in Cancer: Mechanism of Action and Clinical Implications. Molecules 2023, 28, 5246. [Google Scholar] [CrossRef]
- Chowdhury, A.; Nandy, S.K.; Sarkar, J.; Chakraborti, T.; Chakraborti, S. Inhibition of Pro-/Active MMP-2 by Green Tea Catechins and Prediction of Their Interaction by Molecular Docking Studies. Mol. Cell. Biochem. 2017, 427, 111–122. [Google Scholar] [CrossRef]
- Sun, X.L.; Xiang, Z.M.; Xie, Y.R.; Zhang, N.; Wang, L.X.; Wu, Y.L.; Zhang, D.Y.; Wang, X.J.; Sheng, J.; Zi, C.T. Dimeric (−)-Epigallocatechin-3-gallate Inhibits the Proliferation of Lung Cancer Cells by Inhibiting the EGFR Signaling Pathway. Chem. Biol. Interact. 2022, 365, 110084. [Google Scholar] [CrossRef]
- Honda, Y.; Takigawa, N.; Ichihara, E.; Ninomiya, T.; Kubo, T.; Ochi, N.; Yasugi, M.; Murakami, T.; Yamane, H.; Tanimoto, M.; Kiura, K. Effects of (−)-Epigallocatechin-3-gallate on EGFR- or Fusion Gene-Driven Lung Cancer Cells. Acta Med. Okayama 2017, 71, 505–512. [Google Scholar] [CrossRef]
- Negri, A.; Naponelli, V.; Rizzi, F.; Bettuzzi, S. Molecular Targets of Epigallocatechin-Gallate (EGCG): A Special Focus on Signal Transduction and Cancer. Nutrients 2018, 10, 1936. [Google Scholar] [CrossRef]
- Gu, J.W.; Makey, K.L.; Tucker, K.B.; Chinchar, E.; Mao, X.; Pei, I.; Thomas, E.Y.; Miele, L. EGCG, a major green tea catechin suppresses breast tumor angiogenesis and growth via inhibiting the activation of HIF-1α and NFκB, and VEGF expression. Vasc. Cell 2013, 5, 9. [Google Scholar] [CrossRef]
- Gopal, J.; Muthu, M.; Paul, D.; Kim, D.; Chun, S. Bactericidal activity of green tea extracts: the importance of catechin containing nano particles. Sci. Rep. 2016, 6, 19710. [Google Scholar] [CrossRef]
- Alkufeidy, R.M.; Altuwijri, L.A.; Aldosari, N.S.; Alsakabi, N.; Dawoud, T.M. Antimicrobial and synergistic properties of green tea catechins against microbial pathogens. J. King Saud Univ. Sci. 2024, 36, 103277. [Google Scholar] [CrossRef]
- Bae, J.; Kim, N.; Shin, Y.; Kim, S.; Kim, Y. Activity of catechins and their applications. Biomed. Dermatol. 2020, 4, 8. [Google Scholar] [CrossRef]
- Wu, M.; Brown, A.C. Applications of Catechins in the Treatment of Bacterial Infections. Pathogens 2021, 10, 546. [Google Scholar] [CrossRef]
- Chang, E.H.; Huang, J.; Lin, Z.; Brown, A.C. Catechin-mediated restructuring of a bacterial toxin inhibits activity. Biochim. Biophys. Acta Gen. Subj. 2019, 1863, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Reygaert, W.C. Green tea catechins: Their use in treating and preventing infectious diseases. Biomed. Res. Int. 2018, 2018, 9105261. [Google Scholar] [CrossRef]
- Schneider-Rayman, M.; Steinberg, D.; Sionov, R.V.; Friedman, M.; Shalish, M. Effect of epigallocatechin gallate on dental biofilm of Streptococcus mutans: An in vitro study. BMC Oral Health. 2021, 21, 447. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.H.; Jang, Y.H.; Kim, Y.S.; Kim, J.; Seong, B.L. Evaluation of green tea extract as a safe personal hygiene against viral infections. J. Biol. Eng. 2018, 12, 1. [Google Scholar] [CrossRef]
- Mhatre, S.; Srivastava, T.; Naik, S.; Patravale, V. Antiviral activity of green tea and black tea polyphenols in prophylaxis and treatment of COVID-19: A review. Phytomedicine 2021, 85, 153286. [Google Scholar] [CrossRef]
- Xu, J.; Xu, Z.; Zheng, W. A Review of the Antiviral Role of Green Tea Catechins. Molecules 2017, 22, 1337. [Google Scholar] [CrossRef]
- Ghosh, R.; Chakraborty, A.; Biswas, A.; Chowdhuri, S. Evaluation of green tea polyphenols as novel corona virus (SARS CoV-2) main protease (Mpro) inhibitors - an in silico docking and molecular dynamics simulation study. J. Biomol. Struct. Dyn. 2021, 39, 4362–4374. [Google Scholar] [CrossRef]
- Miyoshi, N.; Tanabe, H.; Suzuki, T.; Saeki, K.; Hara, Y. Applications of a Standardized Green Tea Catechin Preparation for Viral Warts and Human Papilloma Virus-Related and Unrelated Cancers. Molecules 2020, 25, 2588. [Google Scholar] [CrossRef]
- Food and Drug Administration. Full prescribing information. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2008/021902s017lbl.pdf (accessed on 25 May 2025).
- Baek, N.; Kim, Y.; Duncan, S.; Leitch, K.; O’Keefe, S. (−)-Epigallocatechin Gallate Stability in Ready-To-Drink (RTD) Green Tea Infusions in TiO2 and Oleic-Acid-Modified TiO2 Polylactic Acid Film Packaging Stored under Fluorescent Light during Refrigerated Storage at 4 °C. Foods 2021, 10, 723. [Google Scholar] [CrossRef]
- Wang, R.; Zhou, W.; Jiang, X. Reaction Kinetics of Degradation and Epimerization of Epigallocatechin Gallate (EGCG) in Aqueous System over a Wide Temperature Range. J. Agric. Food Chem. 2008, 56, 2694–2701. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.J.; Lee, S.Y.; Liu, C.I.; Chen, S.H.; Chen, I.Z.; Su, T.C.; Yuann, J.P.; Cheng, C.W.; Huang, S.T.; Liang, J.Y. Catechin Photolysis Suppression by Aluminum Chloride under Alkaline Conditions and Assessment with Liquid Chromatography-Mass Spectrometry. Molecules 2020, 25, 5985. [Google Scholar] [CrossRef] [PubMed]
- Mira, L.; Fernandez, M.T.; Santos, M.; Rocha, R.; Florêncio, M.H.; Jennings, K.R. Interactions of Flavonoids with Iron and Copper Ions: A Mechanism for Their Antioxidant Activity. Free Radic. Res. 2002, 36, 1199–1208. [Google Scholar] [CrossRef]
- Rashidinejad, A.; Boostani, S.; Babazadeh, A.; Rehman, A.; Rezaei, A.; Akbari-Alavijeh, S.; Shaddel, R.; Jafari, S.M. Opportunities and Challenges for the Nanodelivery of Green Tea Catechins in Functional Foods. Food Res. Int. 2021, 142, 110186. [Google Scholar] [CrossRef]
- Peters, C.M.; Green, R.J.; Janle, E.M.; Ferruzzi, M.G. Formulation with Ascorbic Acid and Sucrose Modulates Catechin Bioavailability from Green Tea. Food Res. Int. 2010, 43, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Chen, Y.; Li, R.C. Oral Absorption and Bioavailability of Tea Catechins. Planta Med. 2000, 66, 444–447. [Google Scholar] [CrossRef]
- Cai, Z.-Y.; Li, X.-M.; Liang, J.-P.; Xiang, L.-P.; Wang, K.-R.; Shi, Y.-L.; Yang, R.; Shi, M.; Ye, J.-H.; Lu, J.-L.; Zheng, X.-Q.; Liang, Y.-R. Bioavailability of Tea Catechins and Its Improvement. Molecules 2018, 23, 2346. [Google Scholar] [CrossRef]
- Ezike, T.C.; Okpala, U.S.; Onoja, U.L.; Nwike, C.P.; Ezeako, E.C.; Okpara, O.J.; Okoroafor, C.C.; Eze, S.C.; Kalu, O.L.; Odoh, E.C.; Nwadike, U.G.; Ogbodo, J.O.; Umeh, B.U.; Ossai, E.C.; Nwanguma, B.C. Advances in drug delivery systems, challenges and future directions. Heliyon 2023, 9, e17488. [Google Scholar] [CrossRef]
- Chen, Y.; Tang, Y.; Li, Y.; Rui, Y.; Zhang, P. Enhancing the Efficacy of Active Pharmaceutical Ingredients in Medicinal Plants through Nanoformulations: A Promising Field. Nanomaterials 2024, 14, 1598. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Ma, Y.; Zhang, J.; Kuo, J.C.-T.; Zhang, Z.; Xie, H.; Zhu, J.; Liu, T. Modification of Lipid-Based Nanoparticles: An Efficient Delivery System for Nucleic Acid-Based Immunotherapy. Molecules 2022, 27, 1943. [Google Scholar] [CrossRef] [PubMed]
- Akombaetwa, N.; Ilangala, A.B.; Thom, L.; Memvanga, P.B.; Witika, B.A.; Buya, A.B. Current Advances in Lipid Nanosystems Intended for Topical and Transdermal Drug Delivery Applications. Pharmaceutics 2023, 15, 656. [Google Scholar] [CrossRef]
- Yun, Y.; An, J.; Kim, H.J.; Choi, H.K.; Cho, H.-Y. Recent advances in functional lipid-based nanomedicines as drug carriers for organ-specific delivery. Nanoscale 2025, 17, 7617–7638. [Google Scholar] [CrossRef]
- Priya, S.; Desai, V.M.; Singhvi, G. Surface Modification of Lipid-Based Nanocarriers: A Potential Approach to Enhance Targeted Drug Delivery. ACS Omega 2022, 8, 74–86. [Google Scholar] [CrossRef] [PubMed]
- Hoar, T.; Schulman, J. Transparent Water-in-Oil Dispersions: the Oleopathic Hydro-Micelle. Nature, 1943, 152, 102–103. [Google Scholar] [CrossRef]
- Winsor, P.A. Hydrotropy, solubilisation and related emulsification processes. Trans. Faraday Soc. 1948, 44, 376. [Google Scholar] [CrossRef]
- Zhu, T.; Kang, W.; Yang, H.; Li, Z.; Zhou, B.; He, Y.; Wang, J.; Aidarova, S.; Sarsenbekuly, B. . Advances of microemulsion and its applications for improved oil recovery. Adv. Colloid Interface Sci. 2022, 299, 102527. [Google Scholar] [CrossRef]
- Ait-Touchente, Z.; Zine, N.; Jaffrezic-Renault, N.; Errachid, A.; Lebaz, N.; Fessi, H.; Elaissari, A. Exploring the Versatility of Microemulsions in Cutaneous Drug Delivery: Opportunities and Challenges. Nanomaterials 2023, 13, 1688. [Google Scholar] [CrossRef]
- Anuar, N.A.F.M.; Kormin, F.; Abidin, N.A.Z. . A review on natural-based active compounds delivery system and its potential in food preservative application. IOP Conf. Ser. Earth Environ. Sci. 2019, 269, 012010. [Google Scholar] [CrossRef]
- Shukla, T.; Upmanyu, N.; Agrawal, M.; Saraf, S.; Saraf, S.; Alexander, A. Biomedical applications of microemulsion through dermal and transdermal route. Biomed. Pharmacother. 2018, 108, 1477–1494. [Google Scholar] [CrossRef] [PubMed]
- Froelich, A.; Osmałek, T.; Jadach, B.; Puri, V.; Michniak-Kohn, B. Microemulsion-Based Media in Nose-to-Brain Drug Delivery. Pharmaceutics 2021, 13, 201. [Google Scholar] [CrossRef]
- Lokhande, S.S. Microemulsions as Promising Delivery Systems: A Review. Asian J. Pharm. Res. 2019, 9, 90. [Google Scholar] [CrossRef]
- Suhail, N.; Alzahrani, A.K.; Basha, W.J.; Kizilbash, N.; Zaidi, A.; Ambreen, J.; Khachfe, H.M. . Microemulsions: Unique Properties, Pharmacological Applications, and Targeted Drug Delivery. Front. Nanotechnol. 2021, 3, 754889. [Google Scholar] [CrossRef]
- Nakajima, H.; Tomomasa, S.; Okabe, M. Preparation of nano-emulsions. In Proceedings of the First World Congress on Emulsions, Paris, France; 1993; p. 1-11-162. [Google Scholar]
- Azmi, N.A.N.; Elgharbawy, A.A.M.; Motlagh, S.R.; Samsudin, N.; Salleh, H.M. Nanoemulsions: Factory for Food, Pharmaceutical and Cosmetics. Processes 2019, 7, 617. [Google Scholar] [CrossRef]
- Musakhanian, J.; Osborne, D.W. Understanding Microemulsions and Nanoemulsions in (Trans)Dermal Delivery. AAPS PharmSciTech, 2025, 26, 31. [Google Scholar] [CrossRef]
- Tayeb, H.H.; Felimban, R.; Almaghrabi, S.; Hasaballah, N. Nanoemulsions: Formulation, characterization, biological fate, and potential role against COVID-19 and other viral outbreaks. Colloid Interface Sci. Commun. 2021, 45, 100533. [Google Scholar] [CrossRef]
- Deokar, G.M.; Dhananjay, L.; Manmode, P. Review on Nanoemulsion Drug Delivery System. Int. J. Pharm. Pharm. Res. 2023, 27, 275–296. [Google Scholar]
- Romes, N.B.; Abdul Wahab, R.; Abdul Hamid, M.; Oyewusi, H.A.; Huda, N.; Kobun, R. Thermodynamic stability, in-vitro permeability, and in-silico molecular modeling of the optimal Elaeis guineensis leaves extract water-in-oil nanoemulsion. Sci. Rep, 2021, 11, 20851. [Google Scholar] [CrossRef]
- Souto, E.B.; Cano, A.; Martins-Gomes, C.; Coutinho, T.E.; Zielińska, A.; Silva, A.M. Microemulsions and Nanoemulsions in Skin Drug Delivery. Bioengineering 2022, 9, 158. [Google Scholar] [CrossRef]
- de Oca-Ávalos, J.M.M.; Candal, R.J.; Herrera, M.L. Nanoemulsions: stability and physical properties. Curr. Opin. Food Sci. 2017, 16, 1–6. [Google Scholar] [CrossRef]
- Choi, S.J.; McClements, D.J. Nanoemulsions as delivery systems for lipophilic nutraceuticals: strategies for improving their formulation, stability, functionality and bioavailability. Food Sci Biotechnol. 2020, 29, 149–168. [Google Scholar] [CrossRef] [PubMed]
- Bangham, A.D.; Standish, M.M.; Watkins, J.C. Diffusion of univalent ions across the lamellae of swollen phospholipids. J. Mol. Biol. 1965, 13, 238–252. [Google Scholar] [CrossRef] [PubMed]
- Çetin, M.; Aytekin, E.; Yavuz, B.; Bozdağ-Pehlivan, S. Nanoscience in Targeted Brain Drug Delivery. In Nanotechnology Methods for Neurological Diseases and Brain Tumors; Gürsoy-Özdemir, Y., Bozdağ-Pehlivan, S., Sekerdag, E., Eds. Academic Press: Cambridge, MA, USA, 2017; pp. 117–147. ISBN 978-0-12-803796-6. [Google Scholar]
- Naeini, S.B.M.; Dadashzadeh, S.; Haeri, A.; Mahjoub, M.A.; Javidi, J.; Vatankhah, M. Multivesicular liposomes as a potential drug delivery platform for cancer therapy: A systematic review. J. Drug Deliv. Sci. Technol. 2021, 66, 102842. [Google Scholar] [CrossRef]
- Nsairat, H.; Khater, D.; Sayed, U.; Odeh, F.; Al Bawab, A.; Alshaer, W. Liposomes: structure, composition, types, and clinical applications. Heliyon, 2022, 8, e09394. [Google Scholar] [CrossRef]
- Liu, P.; Chen, G.; Zhang, J. A Review of Liposomes as a Drug Delivery System: Current Status of Approved Products, Regulatory Environments, and Future Perspectives. Molecules 2022, 27, 1372. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.-K. Liposomes for Enhanced Bioavailability of Water-Insoluble Drugs: In Vivo Evidence and Recent Approaches. Pharmaceutics 2020, 12, 264. [Google Scholar] [CrossRef]
- Dymek, M.; Sikora, E. Liposomes as biocompatible and smart delivery systems – the current state. Adv. Colloid Interface Sci. 2022, 309, 102757. [Google Scholar] [CrossRef]
- Eugster, R.; Luciani, P. Liposomes: Bridging the gap from lab to pharmaceuticals. Curr. Opin. Colloid Interface Sci. 2025, 75, 101875. [Google Scholar] [CrossRef]
- Bombardelli, E.; Patri, G.F. Complex compounds of bioflavonoids with phospholipids, their preparation and use, and pharmaceutical and cosmetic compositions containing them. US Patent US5043323A, 27 August 1991. [Google Scholar]
- Alharbi, W.S.; Almughem, F.A.; Almehmady, A.M.; Jarallah, S.J.; Alsharif, W.K.; Alzahrani, N.M.; Alshehri, A.A. Phytosomes as an Emerging Nanotechnology Platform for the Topical Delivery of Bioactive Phytochemicals. Pharmaceutics 2021, 13, 1475. [Google Scholar] [CrossRef]
- Shriram, R.G.; Moin, A.; Alotaibi, H.F.; Khafagy, E.-S.; Al Saqr, A.; Abu Lila, A.S.; Charyulu, R.N. Phytosomes as a Plausible Nano-Delivery System for Enhanced Oral Bioavailability and Improved Hepatoprotective Activity of Silymarin. Pharmaceuticals 2022, 15, 790. [Google Scholar] [CrossRef] [PubMed]
- Sakure, K.; Patel, A.; Pradhan, M.; Badwaik, H.R. Recent Trends and Future Prospects of Phytosomes: A Concise Review. Indian J. Pharm. Sci. 2024, 86. [Google Scholar] [CrossRef]
- Lucks, S.; Müller, R. Medication Vehicles Made of Solid Lipid Particles (Solid Lipid Nanospheres—SLN). European Patent EP0605497A1, 20 March 1996. [Google Scholar]
- Gasco, M.R. Method for Producing Solid Lipid Microspheres Having a Narrow Size Distribution. US Patent US5250236A, 5 October 1993. [Google Scholar]
- Sotirova, Y. Solid Lipid Nanoparticles and Nanostructured Lipid Carriers: Current Perspectives in Wound Care. Scr. Sci. Pharm. 2023; 1–11, https://scholar.google.com/scholar_lookup?title=Solid+Lipid+Nanoparticles+and+Nanostructured+Lipid+ Carriers:+Current+Perspectives+in+Wound+Care&author=Sotirova,+Y.&publication_year=2023&journal= Scr.+Sci.+Pharm.&pages=1%E2%80%9311. [Google Scholar]
- Gugleva, V.; Ivanova, N.; Sotirova, Y.; Andonova, V. Dermal Drug Delivery of Phytochemicals with Phenolic Structure via Lipid-Based Nanotechnologies. Pharmaceuticals 2021, 14, 837. [Google Scholar] [CrossRef]
- Balamurugan, K.; Chintamani, P. Lipid nano particulate drug delivery: An overview of the emerging trend. Pharma Innov. J. 2018, 7, 779–789, https://www.thepharmajournal.com/archives/2018/vol7issue7/PartM/7-6- 166-872.pdf. [Google Scholar]
- Dhiman, N.; Awasthi, R.; Sharma, B.; Kharkwal, H.; Kulkarni, G.T. Lipid Nanoparticles as Carriers for Bioactive Delivery. Front. Chem. 2021, 9, 580118. [Google Scholar] [CrossRef]
- Subroto, E.; Andoyo, R.; Indiarto, R. Solid Lipid Nanoparticles: Review of the Current Research on Encapsulation and Delivery Systems for Active and Antioxidant Compounds. Antioxidants 2023, 12, 633. [Google Scholar] [CrossRef]
- Akanda, M.; Mithu, M.S.H.; Douroumis, D. Solid lipid nanoparticles: An effective lipid-based technology for cancer treatment. J. Drug Deliv. Sci. Technol. 2023, 86, 104709. [Google Scholar] [CrossRef]
- Dangova, M.; Ivanova, N.; Andonova, V. Nanocarriers-Assisted Nose-to-Brain Delivery of Levodopa: Current Progress and Prospects. Appl. Sci. 2025, 15, 331. [Google Scholar] [CrossRef]
- Hussein, H.A.; Khaphi, F.L.; Sivaramakrishnan, R.; Poornima, S.; Abdullah, M.A. Recent developments in sustained-release and targeted drug delivery applications of solid lipid nanoparticles. J. Microencapsul. 2025, 1–31. [Google Scholar] [CrossRef] [PubMed]
- Arabestani, M.R.; Bigham, A.; Kamarehei, F.; Dini, M.; Gorjikhah, F.; Shariati, A.; Hosseini, S.M. Solid lipid nanoparticles and their application in the treatment of bacterial infectious diseases. Biomed. Pharmacother. 2024, 174, 116433. [Google Scholar] [CrossRef]
- Müller, R.H.; Jenning, V.; Mäder, K.; Lippacher, A. Lipid Particles on the Basis of Mixtures of Liquid and Solid Lipids and Method for Producing Same. WIPO (PCT). WO2000067728A2, . https://patents.google. 16 November 8663. [Google Scholar]
- Sotirova, Y.; Ivanova, N.; Ermenlieva, N.; Vilhelmova-Ilieva, N.; Simeonova, L.; Metodiev, M.; Gugleva, V.; Andonova, V. Antimicrobial and Antiherpetic Properties of Nanoencapsulated Hypericum perforatum Extract. Pharmaceuticals 2025, 18, 366. [Google Scholar] [CrossRef] [PubMed]
- Viegas, C.; Patrício, A.B.; Prata, J.M.; Nadhman, A.; Chintamaneni, P.K.; Fonte, P. Solid Lipid Nanoparticles vs. Nanostructured Lipid Carriers: A Comparative Review. Pharmaceutics 2023, 15, 1593. [Google Scholar] [CrossRef]
- Sotirova, Y.; Stoeva, S.; Nikolova, R.; Andonova, V. Nanostructured Lipid Carriers as a Promising Dermal Delivery Platform for St. John’s Wort Extract: Preliminary Studies. J. IMAB 2023, 29, 4911–4919. [Google Scholar] [CrossRef]
- Warkad, S.S.; Awate, S.S.; Kadam, Y.P.; Arote, S.R.; Kadam, R.D.; Galbale, M.M.; Swamy, M.R. An Overview of Nano Structured Lipid Carriers. Int. J. Pharm. Pharm. Res. 2025, 31, 100–117, https://ijppr.humanjournals.com/wp-content/uploads/2023/07/20.Ganesh-Mahadeo-Deokar-Landge- Dhananjay-Priyanka-Manmode.pdf. [Google Scholar]
- Cevc, G. Isothermal lipid phase transitions. Chem. Phys. Lipids 1991, 57, 293–307. [Google Scholar] [CrossRef] [PubMed]
- Matharoo, N.; Mohd, H.; Michniak-Kohn, B. Transferosomes as a transdermal drug delivery system: Dermal kinetics and recent developments. WIREs Nanomed. Nanobiotechnol. 2023, 16, e1918. [Google Scholar] [CrossRef] [PubMed]
- Opatha, S.A.T.; Titapiwatanakun, V.; Chutoprapat, R. Transfersomes: A Promising Nanoencapsulation Technique for Transdermal Drug Delivery. Pharmaceutics 2020, 12, 855. [Google Scholar] [CrossRef]
- Liga, S.; Paul, C.; Moacă, E.-A.; Péter, F. Niosomes: Composition, Formulation Techniques, and Recent Progress as Delivery Systems in Cancer Therapy. Pharmaceutics 2024, 16, 223. [Google Scholar] [CrossRef]
- Momekova, D.B.; Gugleva, V.E.; Petrov, P.D. Nanoarchitectonics of Multifunctional Niosomes for Advanced Drug Delivery. ACS Omega, 2021, 6, 33265–33273. [Google Scholar] [CrossRef]
- Mawazi, S.M.; Ge, Y.; Widodo, R.T. Niosome Preparation Techniques and Structure—An Illustrated Review. Pharmaceutics 2025, 17, 67. [Google Scholar] [CrossRef]
- Hirlekar, R.; Jain, S.; Patel, M.; Garse, H.; Kadam, V. Hexosomes: a novel drug delivery system. Curr. Drug Deliv. 2010, 7, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Fornasier, M.; Krautforst, K.; Kulbacka, J.; Jönsson, P.; Murgia, S.; Bazylińska, U. Cubosomes and hexosomes stabilized by sorbitan monooleate as biocompatible nanoplatforms against skin metastatic human melanoma. J. Colloid Interface Sci. 2025, 677, 842–852. [Google Scholar] [CrossRef]
- Zhang, J.; Nie, S.; Wang, S. Nanoencapsulation enhances epigallocatechin-3-gallate stability and its anti-atherogenic bioactivities in macrophages. J. Agric. Food Chem. 2013, 61, 9200–9209. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Nie, S.; Zu, Y.; Abbasi, M.; Cao, J.; Li, C.; Wu, D.; Labib, S.; Brackee, G.; Shen, C.-L.; . Wang, S. Anti-atherogenic effects of CD36-targeted epigallocatechin gallate-loaded nanoparticles. J. Control. Release 2019, 303, 263–273. [Google Scholar] [CrossRef]
- Fangueiro, J.F.; Calpena, A.C.; Clares, B.; Andreani, T.; Egea, M.A.; Veiga, F.J.; Garcia, M.L.; Silva, A.M.; Souto, E.B. Biopharmaceutical Evaluation of Epigallocatechin Gallate-Loaded Cationic Lipid Nanoparticles (EGCg-LNs): In Vivo, in Vitro and Ex Vivo Studies. Int J Pharm. 2016, 502, 161–169. [Google Scholar] [CrossRef]
- Harwansh, R.K.; Mukherjee, P.K.; Kar, A.; Bahadur, S.; Al-Dhabi, N.A.; Duraipandiyan, V. Enhancement of Photoprotection Potential of Catechin Loaded Nanoemulsion Gel against UVA Induced Oxidative Stress. J. Photochem. Photobiol. B 2016, 160, 318–329. [Google Scholar] [CrossRef] [PubMed]
- Liang, R.; Chen, L.; Yokoyama, W.; Williams, P.A.; Zhong, F. Niosomes Consisting of Tween-60 and Cholesterol Improve the Chemical Stability and Antioxidant Activity of (−)-Epigallocatechin Gallate under Intestinal Tract Conditions. J. Agric. Food Chem. 2016, 64, 9180–9188. [Google Scholar] [CrossRef]
- Shariare, M.H.; Afnan, K.; Iqbal, F.; Altamimi, M.A.; Ahamad, S.R.; Aldughaim, M.S.; Alanazi, F.K.; Kazi, M. Development and Optimization of Epigallocatechin-3-Gallate (EGCG) Nano Phytosome Using Design of Experiment (DoE) and Their In Vivo Anti-Inflammatory Studies. Molecules 2020, 25, 5453. [Google Scholar] [CrossRef]
- Li, D.; Martini, N.; Liu, M.; Falconer, J.R.; Locke, M.; Wu, Z.; Wen, J. Non-Ionic Surfactant Vesicles as a Carrier System for Dermal Delivery of (+)-Catechin and Their Antioxidant Effects. J. Drug Target. 2020, 29, 310–322. [Google Scholar] [CrossRef]
- Li, D.; Martini, N.; Wu, Z.; Chen, S.; Falconer, J.R.; Locke, M.; Zhang, Z.; Wen, J. Niosomal Nanocarriers for Enhanced Dermal Delivery of Epigallocatechin Gallate for Protection against Oxidative Stress of the Skin. Pharmaceutics 2022, 14, 726. [Google Scholar] [CrossRef]
- Fornasier, M.; Pireddu, R.; Del Giudice, A.; Sinico, C.; Nylander, T.; Schillén, K.; Galantini, L.; Murgia, S. Tuning Lipid Structure by Bile Salts: Hexosomes for Topical Administration of Catechin. Colloids Surf. B Biointerfaces 2021, 199, 111564. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Houng, S.-J.; Kim, J.H.; Kim, Y.-R.; Ji, H.G.; Lee, S.-J. Nanoemulsified green tea extract shows improved hypocholesterolemic effects in C57BL/6 mice. J. Nutr. Biochem. 2012, 23, 186–191. [Google Scholar] [CrossRef]
- Rivera, F.; Urbanavicius, J.; Gervaz, E.; Morquio, A.; Dajas, F. Some Aspects of the In Vivo Neuroprotective Capacity of Flavonoids: Bioavailability and Structure-Activity Relationship. Neurotoxicity Res. 2004, 6, 543–553. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.B.; Tsai, M.J.; Wu, P.C.; Tsai, Y.H.; Wu, Y.H.; Fang, J.Y. Elastic Liposomes as Carriers for Oral Delivery and the Brain Distribution of (+)-Catechin. J. Drug Target. 2011, 19, 709–718. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.-Y.; Barro, L.; Tsai, S.-T.; Feng, T.-W.; Wu, X.-Y.; Chao, C.-W.; Yu, R.-S.; Chin, T.-Y.; Hsieh, M.F. Epigallocatechin-3-Gallate-Loaded Liposomes Favor Anti-Inflammation of Microglia Cells and Promote Neuroprotection. Int. J. Mol. Sci. 2021, 22, 3037. [Google Scholar] [CrossRef]
- Al-Najjar, A.H.; Khalifa, M.K.A.; Amin, O.M.; Badawi, N.M. Epigallocatechin-3-gallate loaded proliposomal vesicles for management of traumatic brain injury: In-vitro and in-vivo evaluation. J. Drug Deliv. Sci. Technol. 2024, 97, 105745. [Google Scholar] [CrossRef]
- Nekkanti, V.; Venkatesan, N.; Betageri, G.V. Proliposomes for Oral Delivery: Progress and Challenges. Curr. Pharm. Biotechnol. 2015, 16, 303–312. [Google Scholar] [CrossRef]
- Byeon, J.C.; Lee, S.E.; Kim, T.H.; Ahn, J.B.; Kim, D.H.; Choi, J.S.; Park, J.S. Design of Novel Proliposome Formulation for Antioxidant Peptide, Glutathione with Enhanced Oral Bioavailability and Stability. Drug Deliv. 2019, 26, 216–225. [Google Scholar] [CrossRef]
- Xia, C.; Gu, C.; Liu, G.; Zhao, J.; Wang, S.; Yang, C.; Zhu, Y.; Deng, J.; Xiang, Z.; Yu, M.; Guo, Y.; Wu, Y.; Chen, J. Preparation of a Novel Brain-Targeted EGCG Liposome and Its Antioxidative Neuroprotection. J. Funct. Foods 2023, 111, 105911. [Google Scholar] [CrossRef]
- Kuo, Y.C.; Wang, I.H.; Rajesh, R. Use of Leptin-Conjugated Phosphatidic Acid Liposomes with Resveratrol and Epigallocatechin Gallate to Protect Dopaminergic Neurons Against Apoptosis for Parkinson's Disease Therapy. Acta Biomater. 2021, 119, 360–374. [Google Scholar] [CrossRef]
- Smith, A.; Giunta, B.; Bickford, P.C.; Fountain, M.; Tan, J.; Shytle, R.D. Nanolipidic Particles Improve the Bioavailability and Alpha-Secretase Inducing Ability of Epigallocatechin-3-Gallate (EGCG) for the Treatment of Alzheimer's Disease. Int. J. Pharm. 2010, 389, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Mishra, G.; Awasthi, R.; Mishra, S.K.; Singh, A.K.; Tiwari, A.K.; Singh, S.K.; Nandi, M.K. Development of Epigallocatechin and Ascorbic Acid Dual Delivery Transferosomes for Managing Alzheimer's Disease: In Vitro and In Vivo Studies. ACS Omega 2024, 9, 35463–35474. [Google Scholar] [CrossRef]
- Kaur, H.; Kumar, B.; Chakrabarti, A.; Medhi, B.; Modi, M.; Radotra, B.D.; Aggarwal, R.; Sinha, V.R. A New Therapeutic Approach for Brain Delivery of Epigallocatechin Gallate: Development and Characterization Studies. Curr. Drug Deliv. 2019, 16, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Ganji, S.; Aukunuru, J. Formulation, Excipient Properties, and Pharmacological Activities of Catechin Liposomes. Pharmacogn. Mag. 2008, 4, 13. [Google Scholar]
- Semalty, A.; Semalty, M.; Singh, D.; Rawat, M.S.M. Phyto-Phospholipid Complex of Catechin in Value Added Herbal Drug Delivery. J. Incl. Phenom. Macrocycl. Chem. 2012, 73, 377–386. [Google Scholar] [CrossRef]
- Gadkari, P.V.; Balaraman, M.; Balaraman, M. Extraction of Catechins from Decaffeinated Green Tea for Development of Nanoemulsion Using Palm Oil and Sunflower Oil-Based Lipid Carrier Systems. J. Food Eng. 2015, 147, 14–23. [Google Scholar] [CrossRef]
- Frias, I.; Neves, A.R.; Pinheiro, M.; Reis, S. Design, Development, and Characterization of Lipid Nanocarriers-Based Epigallocatechin Gallate Delivery System for Preventive and Therapeutic Supplementation. Drug Des. Devel. Ther. 2016, 10, 3519–3528. [Google Scholar] [CrossRef] [PubMed]
- Granja, A.; Vieira, A.C.; Chaves, L.L.; Nunes, C.; Neves, A.R.; Pinheiro, M.; Reis, S. Folate-Targeted Nanostructured Lipid Carriers for Enhanced Oral Delivery of Epigallocatechin-3-Gallate. Food Chem. 2017, 237, 803–810. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, N.; Mandal, A.K.A. Pharmacokinetic, Toxicokinetic, and Bioavailability Studies of Epigallocatechin-3-Gallate Loaded Solid Lipid Nanoparticle in Rat Model. Drug Dev. Ind. Pharm. 2019, 45, 1506–1514. [Google Scholar] [CrossRef]
- Shtay, R.; Keppler, J.K.; Schrader, K.; Schwarz, K. Encapsulation of (-)-epigallocatechin-3-gallate (EGCG) in solid lipid nanoparticles for food applications. J. Food Eng. 2019, 244, 91–100. [Google Scholar] [CrossRef]
- Yaneva, Z.; Ivanova, D. Catechins within the Biopolymer Matrix—Design Concepts and Bioactivity Prospects. Antioxidants 2020, 9, 1180. [Google Scholar] [CrossRef] [PubMed]
- Shruthi, P.A.; Pushpadass, H.A.; Magdaline Eljeeva Emerald, F.; Surendra Nath, B.; Laxmana Naik, N. Formulation and Characterization of Catechin-Loaded Proniosomes for Food Fortification. J. Sci. Food Agric. 2021, 101, 2439–2448. [Google Scholar] [CrossRef]
- Gadapa, S.; Battula, S.N.; Mor, S.; Pushpadass, H.A.; Naik, L.N.; Emerald, M.E. Green Tea Catechin Loaded Niosomes: Formulation and Their Characterization for Food Fortification. J. Food Sci. Technol. 2022, 59, 3669–3682. [Google Scholar] [CrossRef] [PubMed]
- Younes, M.; Aggett, P.; Aguilar, F.; Crebelli, R.; Dusemund, B.; Filipič, M.; Frutos, M.J.; Galtier, P.; Gott, D.; Gundert-Remy, U.; Lambré, C.; Leblanc, J.C.; Lillegaard, I.T.; Moldeus, P.; Mortensen, A.; Oskarsson, A.; Stankovic, I.; Waalkens-Berendsen, I.; Woutersen, R.A.; Andrade, R.J.; Fortes, C.; Mosesso, P.; Restani, P.; Arcella, D.; Pizzo, F.; Smeraldi, C.; Wright, M. Scientific Opinion on the safety of green tea catechins. EFSA J. 2018, 16, 5239. [Google Scholar] [CrossRef]
- Dekant, W.; Fujii, K.; Shibata, E.; Morita, O.; Shimotoyodome, A. Safety Assessment of Green Tea-Based Beverages and Dried Green Tea Extracts as Nutritional Supplements. Toxicol. Lett. 2017, 277, 104–108. [Google Scholar] [CrossRef]
- Fang, J.Y.; Lee, W.R.; Shen, S.C.; Huang, Y.L. Effect of Liposome Encapsulation of Tea Catechins on Their Accumulation in Basal Cell Carcinomas. J. Dermatol. Sci. 2006, 42, 101–109. [Google Scholar] [CrossRef]
- Radhakrishnan, R.; Pooja, D.; Kulhari, H.; Gudem, S.; Ravuri, H.G.; Bhargava, S.; Ramakrishna, S. Bombesin Conjugated Solid Lipid Nanoparticles for Improved Delivery of Epigallocatechin Gallate for Breast Cancer Treatment. Chem. Phys. Lipids 2019, 224, 104770. [Google Scholar] [CrossRef]
- Silva, A.M.; Martins-Gomes, C.; Fangueiro, J.F.; Andreani, T.; Souto, E.B. Comparison of Antiproliferative Effect of Epigallocatechin Gallate When Loaded into Cationic Solid Lipid Nanoparticles Against Different Cell Lines. Pharm. Dev. Technol. 2019, 24, 1243–1249. [Google Scholar] [CrossRef]
- de Pace, R.C.; Liu, X.; Sun, M.; Nie, S.; Zhang, J.; Cai, Q.; Gao, W.; Pan, X.; Fan, Z.; Wang, S. Anticancer Activities of (−)-Epigallocatechin-3-Gallate Encapsulated Nanoliposomes in MCF7 Breast Cancer Cells. J. Liposome Res. 2013, 23, 187–196. [Google Scholar] [CrossRef]
- Fang, J.Y.; Hung, C.F.; Hwang, T.L.; Huang, Y.L. Physicochemical Characteristics and In Vivo Deposition of Liposome-Encapsulated Tea Catechins by Topical and Intratumor Administrations. J. Drug Target. 2005, 13, 19–27. [Google Scholar] [CrossRef]
- Song, Q.; Li, D.; Zhou, Y.; Yang, J.; Yang, W.; Zhou, G.; Wen, J. Enhanced Uptake and Transport of (+)-Catechin and (−)-Epigallocatechin Gallate in Niosomal Formulation by Human Intestinal Caco-2 Cells. Int. J. Nanomed. 2014, 9, 2157–2165. [Google Scholar] [CrossRef]
- Radhakrishnan, R.; Kulhari, H.; Pooja, D.; Gudem, S.; Bhargava, S.; Shukla, R.; Sistla, R. Encapsulation of Biophenolic Phytochemical EGCG within Lipid Nanoparticles Enhances Its Stability and Cytotoxicity against Cancer. Chem. Phys. Lipids 2016, 198, 51–60. [Google Scholar] [CrossRef]
- Wu, Z.; Guan, R.; Xiao, C.; Lyu, F.; Cao, G.; Liu, M.; Gao, J. Optimization of Catechin Nanoliposomes and Evaluation of Their Antioxidant Activity and Cytotoxicity. Sci. Adv. Mater. 2016, 9, 697–704. [Google Scholar] [CrossRef]
- Ramadass, S.K.; Anantharaman, N.V.; Subramanian, S.; Sivasubramanian, S.; Madhan, B. Paclitaxel/Epigallocatechin Gallate Coloaded Liposome: A Synergistic Delivery to Control the Invasiveness of MDA-MB-231 Breast Cancer Cells. Colloids Surf. B Biointerfaces 2015, 125, 65–72. [Google Scholar] [CrossRef]
- Jin, M.; Liu, B.; Zhang, Z.; Mu, Y.; Ma, L.; Yao, H.; Wang, D.A. Catechin-Functionalized Cationic Lipopolymer Based Multicomponent Nanomicelles for Lung-Targeting Delivery. Adv. Mater. 2024, 36, e2302985. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.H.; Feng, C.L.; Lai, C.H.; Lin, J.H.; Chen, H.Y. Preparation of epigallocatechin gallate-loaded nanoparticles and characterization of their inhibitory effects on Helicobacter pylori growth in vitro and in vivo. Sci. Technol. Adv. Mater. 2014, 15, 045006. [Google Scholar] [CrossRef] [PubMed]
- Dahiya, S.; Rani, R.; Kumar, S.; Dhingra, D.; Dilbaghi, N. Chitosan-gellan gum bipolymeric nanohydrogels: A potential nanocarrier for the delivery of epigallocatechin gallate. BioNanoSci. 2017, 7, 508–520. [Google Scholar] [CrossRef]
- Gharib, A.; Faezizadeh, Z.; Godarzee, M. Therapeutic efficacy of epigallocatechin gallate-loaded nanoliposomes against burn wound infection by methicillin-resistant Staphylococcus aureus. Skin Pharmacol. Physiol. 2013, 26, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Moreno, A.P.D.; Marcato, P.D.; Silva, L.B.; Salvador, S.L.S.; Arco, M.C.G.D.; Moraes, J.C.B.; Silva, R.S.D.; Rossi, A. Antibacterial Activity of Epigallocatechin-3-gallate (EGCG) Loaded Lipid-chitosan Hybrid Nanoparticle against Planktonic Microorganisms. J. Oleo Sci. 2024, 73, 709–716. [Google Scholar] [CrossRef]
- Zou, L.-Q.; Liu, W.; Liu, W.-L.; Liang, R.-H.; Li, T.; Liu, C.-M.; Cao, Y.-L.; Niu, J.; Liu, Z. Characterization and Bioavailability of Tea Polyphenol Nanoliposome Prepared by Combining an Ethanol Injection Method with Dynamic High-Pressure Microfluidization. J. Agric. Food Chem. 2014, 62, 934–941. [Google Scholar] [CrossRef]
- Manea, A.M.; Andronescu, C.; Meghea, A. Green tea extract loaded into solid lipid nanoparticles. UPB Sci. Bull. Series B 2014, 76, 125–136. [Google Scholar]
- Sinsinwar, S.; Vadivel, V. Development and characterization of catechin-in-cyclodextrin-in-phospholipid liposome to eradicate MRSA-mediated surgical site infection: investigation of their anti-infective efficacy through in vitro and in vivo studies. Int. J. Pharm. 2021, 609, 121130. [Google Scholar] [CrossRef] [PubMed]
| Active compound/s | Nano-carrier type | Nano-carrier characteristics | Suggested mechanism/s of action | Scientific result | Reference |
|---|---|---|---|---|---|
| C, Quercetin, Fisetin | Liposomes | Mean size: n.i. Polydispersity index: n.i. ζ-potential: n.i. Entrapment efficiency: n.i. Loading capacity: n.i. |
Enhancement of in vivo stability; Enhancement of delivery to the brain parenchyma. |
The delivery of the bioactive compound to the brain parenchyma in rats (i.p.) was enhanced (compared to its unformulated form). | [232] |
| C | Liposomes | Mean size: 34.6÷70.3 nm Polydispersity index: 0.13÷0.44 ζ-potential: −15.3÷−18.8 mV Entrapment efficiency: 65.8÷85.6% Loading capacity: n.i. |
Protection of the compound from enzymatic degradation; Enhanced bioavailability and delivery of the compound to the brain; Improved stability upon storage. |
Suppressed release profile in vitro; Improved in vitro stability under simulated intestinal fluid conditions; Blood levels of liposomal C in rats were elevated at a later stage post-administration; 2.9- and 2.7-fold higher accumulation of C in the cerebral cortex and hippocampus, respectively; Increased concentrations of C in the striatum and thalamus. |
[233] |
| EGCG | Liposomes | Mean size: 132.9÷ 161.5 nm Polydispersity index: 0.06÷0.12 ζ-potential: n.i. Entrapment efficiency: 55.4÷76.8% Loading capacity: n.i. |
Reduction in particle size; Enhancement of the stability of EGCG and liposomes; Increased encapsulation efficiency; Improvement in the bioavailability of EGCG. |
Following pre-treatment with EGCG-loaded liposomes, the levels of TNF-α and nitric oxide production in a cellular model of lipopolysaccharide-induced inflammation in BV-2 microglial cells were reduced; Post-treatment with EGCG-loaded liposomes led to symptomatic improvement, suppression of neuroinflammation, and a reduction in TNF-α secretion in a rat model of Parkinson’s disease induced by unilateral injection of lipopolysaccharide into the substantia nigra. |
[234] |
| EGCG | Proliposomal vesicles | Mean size: 150.6 nm Polydispersity index: 0.07 ζ-potential: −71.0 mV Entrapment efficiency: 89.7% Loading capacity: n.i. |
Prolonged in vitro release; Enhancement of the EGCG stability. |
In a rat model of traumatic brain injury, seven-day pre-treatment with EGCG-proliposomes significantly reduced the lipid peroxidation marker MDA and increased antioxidants (glutathione, SOD). EGCG-proliposomes also more effectively activated the Sirt1/Nrf2/HO-1 pathway; Immunohistochemical analysis showed increased HO-1 expression in the cerebral cortex and hippocampus, further confirmed by histopathological analysis. |
[235] |
| EGCG | Glucose-modified liposomes | Mean size: Glucose-modified EGCG liposomes: 158.7 nm Plain EGCG liposomes: 149.6 nm Polydispersity index: Glucose-modified EGCG liposomes: 0.26 Plain EGCG liposomes: 0.24 ζ-potential: Glucose-modified EGCG liposomes: +2.4 mV Plain EGCG liposomes: +2.1 mV Entrapment efficiency: Glucose-modified EGCG liposomes: 73.1% Plain EGCG liposomes: 71.3% Loading capacity: n.i. |
Improved permeability across the blood-brain barrier and increased neuronal cell uptake, mediated by glucose transporter protein 1; Improved stability of both the carriers and the encapsulated EGCG; Improved encapsulation capacity. |
Reduced cytotoxicity and enhanced protection against H₂O₂-induced oxidative stress; Increased cellular uptake and improved permeability across the blood-brain barrier via GLUT1 transporters. |
[238] |
| EGCG, Resveratrol | Leptin-modified liposomes | Mean size: 149.8÷190.4 nm Polydispersity index: n.i. ζ-potential: −18.7÷−41.3 mV Entrapment efficiency: Resveratrol: 54.2% EGCG: 39.5% Loading capacity: n.i. |
Targeted delivery of leptin-modified liposomes to the brain. | The surface modification with leptin enabled the liposomes to bind to HBMECs and SH-SY5Y cells via the leptin receptor, enhancing their ability to cross the blood-brain barrier and be absorbed by the cells; Reductions in the apoptosis-promoting protein Bcl-2-associated X protein and α-synuclein were observed, along with increases in the apoptosis-inhibitory protein B-cell lymphoma 2, tyrosine hydroxylase, and the dopamine transporter. |
[239] |
| EGCG | Nanolipidic particle complexes | Mean size: 30.0÷80.0 nm Polydispersity index: n.i. ζ-potential: n.i. Entrapment efficiency: n.i. Loading capacity: n.i. |
Improved stability and oral bioavailability of EGCG. | Enhanced the neuronal α-secretase activity in vitro by up to 91%; The bioavailability of EGCG was more than two-fold greater after oral administration of the prepared carriers in rats compared to the free form. |
[240] |
| EGCG, Ascorbic acid |
Transferosomes | Mean size: 184.3 nm Polydispersity index: 0.18 ζ-potential: −14.2 mV Entrapment efficiency: EGCG: 82.3% Ascorbic acid: 74.8% Loading capacity: EGCG: 12.5% Ascorbic acid: 15.1% |
Enhanced stability and bioavailability of EGCG; Increased accumulation of EGCG in major organs, including the brain. |
Following intranasal administration in mice, the transferosome-loaded formulation achieved approximately a fivefold increase in long-term brain concentrations of EGCG compared to its free form. In a mouse model of Alzheimer’s disease, intranasal treatment with the transferozomes resulted in enhanced acetylcholinesterase activity, reduced neuroinflammation, and improvements in spatial learning and memory. | [241] |
| EGCG | SLNs | Mean size: 162.4 nm Polydispersity index: n.i. ζ-potential: n.i. Entrapment efficiency: n.i. Loading capacity: n.i. |
Enhanced EGCG bioavailability; Improved delivery of EGCG to the brain. |
Improved brain bioavailability of EGCG; Marked alleviation of memory deficits in a mouse model of cerebral ischemia. |
[242] |
| Active compound/s | Nano-carrier type | Nano-carrier characteristics | Suggested mechanism/s of action | Scientific result | Reference |
|---|---|---|---|---|---|
| EGCG | SLNs | Mean size: 300.2 nm Polydispersity index: 0.418 ζ potential: −18.0 mV Entrapment efficiency: 81.0% Loading capacity: n.i. |
Pharmacokinetic studies in Wistar rats demonstrated higher plasma concentrations of EGCG compared to its free form; Histopathological and toxicological evaluations confirmed the absence of treatment-related adverse effects. |
Improved stability and bioavailability of EGCG upon formulation into SLNs; EGCG-loaded SLNs were demonstrated to be an efficient system for the sustained release of EGCG. |
[248] |
| EGCG | SLNs, conjugated with bombesin | Mean size: 163.4 nm Polydispersity index: 0.34 ζ potential: −25.2 mV Entrapment efficiency: 67.2% Loading capacity: n.i. |
Enhanced stability; Elevated liposome internalization and EGCG accumulation in MCF7 cells; Improved survival and reduced tumor volume in C57BL/6 mice. |
Enhanced bioavailability, solubility, physicochemical stability, and encapsulation capacity of EGCG; Protection of EGCG from degradation under physiological pH conditions while concurrently improving its cytotoxic efficacy and cellular uptake. |
[256] |
| EGCG | Cationic SLNs |
Mean size: ~143.7 nm Polydispersity index: 0.16 ζ potential: +25.7 mV Entrapment efficiency: 96.9% Loading capacity: ~15% |
Slightly enhanced antiproliferative effect in MCF-7 and SV-80 cells; Inherent toxicity of SLNs, attributed to the surfactant utilized. |
Improved stability and protection of EGCG from degradation; Increased cytotoxicity, improved cellular uptake, resulting in better therapeutic efficacy. |
[257] |
| EGCG | Chitosan-coated liposomes | Mean size: 85.0 nm Polydispersity index: 0.35 ζ potential: +16.4 mV Entrapment efficiency: ~90% Loading capacity: ~3% |
Enhanced EGCG stability and prolonged sustained release profile; Increased intracellular accumulation of EGCG in MCF7 breast cancer cells; Augmented induction of apoptosis and inhibition of MCF7 cell proliferation compared to free EGCG. |
The encapsulation protected EGCG from degradation and enabled controlled release; Chitosan coating enhanced cellular uptake by promoting receptor interactions, improving bioavailability; The liposomal structure sustained EGCG release, prolonging therapeutic effect and anticancer activity. |
[258] |
| C, EC, EGCG | Liposomes | Mean size: 135.2÷268.9 nm Polydispersity index: n.i. ζ potential: −66.0÷+68.6 mV Entrapment efficiency: C: 41.9÷75.6% EC: 37.3÷76.9% EGCG: 36.3÷89.7% Loading capacity: n.i. |
Compared to aqueous solutions, liposomal formulations delivered substantially higher amounts of catechins to solid tumors; No significant increase in catechins deposition within the skin was observed following topical liposomal application. |
The incorporation of anionic agents—specifically deoxycholic acid or dicetyl phosphate—enhanced the permeability of the lipid bilayer membranes, thereby facilitating a more rapid release of the encapsulated compounds; Enhanced drug accumulation within tumor tissues was achieved through the optimization of both the vesicle size and the drug release kinetics. |
[259] |
| C, EC, EGCG | Liposomes | Mean size: 131.1÷ 378.2 nm Polydispersity index: n.i. ζ potential: −36.1÷−0.9 mV Entrapment efficiency: C: 39.5÷57.0% EC: 31.9÷64.7% EGCG: 84.6÷99.6% Loading capacity: n.i. |
Significantly increased EGCG tumor uptake, as well as intratumoral retention and distribution; Superior cytotoxicity toward basal cell carcinoma cells than hydroalcoholic solutions, even at lower EGCG doses; Potential for dose reduction and fewer side effects. |
Improved stability of EGCG; Liposomal formulations incorporating deoxycholic acid and 15% ethanol substantially enhanced the intratumoral accumulation of EGCG; The surface charge and bilayer fluidity of the liposomes were identified as critical determinants governing their distribution within tumor tissue. Additionally, increased vesicle size was shown to favor prolonged retention in the tumor microenvironment, likely due to restricted vascular clearance and entrapment within the extracellular matrix. |
[255] |
| C, EGCG |
Niosomes | Mean size: ∼100 nm Polydispersity index: n.i. ζ potential: n.i. Entrapment efficiency: above 40% Loading capacity: n.i. |
Cellular uptake of both C and EGCG was significantly enhanced in Caco-2 cells; Niosomal formulations demonstrated 1.8-fold (C) and 1.4-fold (EGCG) increases in transcellular flux compared to their free counterparts; Encapsulated forms exhibited higher IC₅₀ values, indicating reduced cytotoxicity alongside greater transport efficiency, thereby necessitating dose recalibration in relation to encapsulation efficiency. |
C and EGCG exhibited encapsulation efficiencies exceeding 40%; The nanoscale vesicular architecture facilitated uptake via endocytosis; Encapsulation conferred protection against metabolic degradation; Niosomal formulations circumvented efflux mediated by P-glycoprotein and multidrug resistance-associated protein 2; Enhanced permeability was attributed to surfactant-induced membrane interactions and EDTA-mediated loosening of tight junctions. |
[260] |
| EGCG | SLNs | Mean size: 112.5÷157.4 nm Polydispersity index: 0.13÷0.27 ζ potential: −30.1÷−37.2 mV Entrapment efficiency: 67.2÷89.5% Loading capacity: n.i. |
The in vitro drug release profile demonstrated an initial rapid release of approximately 10% within the first hour, followed by a sustained release reaching 83.9% by 12 hours; In vitro cytotoxicity assays indicated that blank SLNs exhibited high biocompatibility, maintaining over 90% cell viability; EGCG-loaded SLNs caused a significant, dose-dependent reduction in viability of two cancer cell lines: MDA-MB-231 (breast cancer) and DU-145 (prostate cancer); EGCG-loaded SLNs induced markedly greater apoptosis in cancer cells compared to free EGCG treatment. |
EGCG exhibited enhanced stability within the formulation; Controlled drug release is likely mediated through diffusion or erosion of the lipid matrix; Increased cytotoxicity is attributed to improved cellular uptake and selective tumor targeting, facilitated by the enhanced permeation and retention effect that promotes nanoparticle accumulation in tumor tissues. |
[261] |
| C | Liposomes | Mean size: 221.0 nm Polydispersity index: n.i. ζ potential: n.i. Entrapment efficiency: 76% Loading capacity: n.i. |
Demonstrated high stability and efficient encapsulation efficiency; Superior antioxidant performance over free C, with enhanced scavenging of ABTS, hydroxyl, and DPPH radicals in vitro; Exhibited cytotoxicity toward Caco-2 cells only at higher concentrations (≥ 0.025 mg/mL), with increased toxicity observed at 36 hours compared to 24 hours. |
Under the optimized conditions, C was successfully loaded into liposomes, resulting in a stable nanocarrier system with uniform size and well-defined morphology. | [262] |
| Paclitaxel, EGCG | Liposomes | Mean size: 130.5 nm Polydispersity index: 0.42 ζ potential: −36.77 mV Entrapment efficiency: Paclitaxel: 77.1% EGCG: 59.1% Loading capacity: n.i. |
Liposomes successfully co-encapsulated Paclitaxel and EGCG despite their differing solubilities; Paclitaxel-EGCG-loaded liposomes at a 1:5 molar ratio exhibited significantly enhanced cytotoxicity compared to the free drugs or their respective single-drug liposomal formulations; This combination induced a greater extent of apoptosis, with caspase-3 activity increasing approximately 3.9-fold relative to the vehicle control; Treatment with Paclitaxel/EGCG liposomes resulted in a marked reduction (~80%) in the activity of matrix metalloproteinases MMP-2 and MMP-9; Paclitaxel/EGCG liposomes also produced the most pronounced inhibition of tumor cell invasion among all tested formulations. |
The phospholipid bilayer structure of the liposomes facilitated both the efficient co-encapsulation and intracellular delivery of both Paclitaxel and EGCG; The co-loaded liposomes demonstrated synergistic anticancer effects by simultaneously promoting apoptosis, suppressing MMP activity, and limiting tumor cell invasiveness. |
[263] |
| C | C-functionalized cationic lipopolymer based multicomponent nanomicelles | Mean size: n.i. Polydispersity index: n.i. ζ-potential: n.i. Entrapment efficiency: n.i. Loading capacity: n.i |
Improved intracellular delivery and bioavailability of C; Targeted accumulation in pulmonary tissue, rendering the system highly suitable for the treatment of lung-associated diseases; Prolonged systemic circulation of C, thereby enhancing the therapeutic window; Selective cytotoxicity toward cancer cells, validating the chemopreventive efficacy of the delivery system. |
Electrostatic interactions between cationic carriers and cell membranes enhance lung-targeted intracellular uptake; Serum albumin forms stable complexes with lipopolymer, reducing clearance and prolonging circulation; Cationic liposomes accumulate preferentially in pulmonary microvasculature due to surface charge; Functionalized nanomicelles induce ROS production and caspase-3 activation, promoting cancer cell apoptosis. |
[264] |
| Active compound/s | Nano-carrier type | Nano-carrier characteristics | Suggested mechanism/s of action | Scientific result | Reference |
|---|---|---|---|---|---|
| EGCG | Liposomes | Mean size: neutral: 93.2 nm cationic: 93.4 nm anionic: 89.4 nm Polydispersity index: neutral: 0.22 cationic: 0.22 anionic: 0.23 ζ-potential: neutral: −1.2 mV cationic: +18.7 mV anionic: −13.2 mV Entrapment efficiency: neutral: 56% cationic: 78% anionic: 45% Loading capacity: n.i. |
An electrostatic interaction between the outer membrane of MRSA and the cationic liposomes may enhance EGCG entry into the bacterial cell. |
The cationic and neutral EGCG-loaded liposomes are more potent than free EGCG; The cationic EGCG-loaded liposomes led to 100% survival rate in mice (no mice survived in the control group). |
[267] |
| EGCG | Lipid-chitosan hybrid nanoparticles | Mean size: 217.3 nm Polydispersity index: 0.17 ζ-potential: +33.7 mV Entrapment efficiency: 95.9% Loading capacity: n.i. |
Improved antimicrobial activity of EGCG; Mucoadhesive properties of the nanoparticles due to chitosan content that conferred a positive ζ-potential leading to an interaction between the nanoparticle and mucin |
The EGCG-loaded lipid-chitosan hybrid nanoparticles exert higher antibacterial activity compared to free EGCG and lipid-chitosan hybrid nanoparticles alone. | [268] |
| Tea polyphenols | Liposomes |
Mean size: 66.8 nm Polydispersity index: 0.21 ζ-potential: −6.16 mV Entrapment efficiency: 78.5% Loading capacity: 8.5% |
High viscidity of the lipid layer may reduce the diffusion of tea polyphenols into the agar. | The antibacterial activity of GTCs was reduced when they are encapsulated in liposomes. | [269] |
| Green tea extract | SLNs | Mean size: 175÷275 nm Polydispersity index: 0.20÷ ζ-potential: −34.1÷−46.6 mV Entrapment efficiency: n.i. Loading capacity: n.i. |
n.i. | Green tea extract-loaded SLNs showed a good physical stability and exhibited antioxidant and antibacterial activity. | [270] |
| C | C-in-cyclodextrine-in-liposomes | Mean size: 415.0 nm Polydispersity index: 0.54 ζ-potential: −40.6 mV Entrapment efficiency: 98.9% Loading capacity: n.i. |
n.i. | C-in-cyclodextrin-in-phospholipid liposomes showed higher water solubility (25.13%) and in vitro permeability (42.14%) compared to C as well as higher antibacterial activity. | [271] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





