1. Introduction
Achieving content uniformity in pharmaceutical tablet formulations remains one of the most fundamental requirements of product quality and regulatory compliance [
1,
2]. Especially in direct compression processes, where powders are compacted without a granulation step, ensuring homogeneity of the blend is critical for dose accuracy, therapeutic efficacy, and patient safety [
3,
4]. Direct compression is a cost-effective tableting method that requires less processing steps [
5] and is suitable for drugs sensitive to temperature and moisture [
6]. This technique involves producing tablets by compressing a powder mixture of the active ingredient and excipients directly, without prior granulation [
5]. Tablets prepared by direct compression disintegrate into individual API particles rather than granules when exposed to dissolution fluids, resulting in faster dissolution [
7]. The uniform distribution of the active pharmaceutical ingredient (API) within the powder mixture is influenced by several factors, including the physicochemical properties of the components and the blending technique employed. Inhomogeneity in powder mixtures can lead to drug content variability, particularly in low-dose formulations or those involving cohesive or poorly flowing APIs [
8,
9].
Powder blending remains a key unit operation in solid dosage manufacturing [
10]. A wide range of mixing equipment is available, including tumbling mixers, planetary ball mills [
11], and vibrating ball mills [
12], each offering different mechanisms for dispersing ingredients. Previous studies have shown that mixer geometry, agitation speed, and blending time can substantially influence the degree of homogeneity achieved [
8,
13]. In this context, our study compares the performance of three commonly used mixing systems—V-type tumble mixer, planetary ball mill, and vibrating ball mill—on the uniformity and final quality of naproxen sodium tablets produced via direct compression. We evaluated both physical parameters (mass, thickness, friability, hardness) and chemical uniformity using a simplified quantitative method.
Naproxen sodium was selected as a model API due to its well-known analytical behavior and broad pharmaceutical application [
14,
15]. This API is characterized by well-documented poor flowability and low compressibility [
2,
14,
16]. These properties pose significant challenges for formulation via direct compression. It is a nonsteroidal anti-inflammatory drug (NSAID) with favorable UV absorbance properties, which make it particularly suitable for spectrophotometric and chromatographic analyses. The model formulation also included excipients commonly used in tablet production: cellulose, polyvinylpyrrolidone (PVP), magnesium stearate, and calcium carbonate. These components were selected for their standard roles as filler, binder, lubricant, and diluent, respectively, representing a typical direct compression matrix.
The analysis of active pharmaceutical ingredient (API) content is an essential aspect of formulation development, quality assurance, and regulatory procedures. Although HPLC remains an important standard due to its high sensitivity, selectivity, and reproducibility, in simpler, impurity-free matrices, methods that bypass full chromatographic separation are increasingly being employed. For example, the FI-CL technique has demonstrated the ability to detect naproxen at concentrations as low as 3.0 × 10
−8 g/mL without using a column [
17], while the FIA-UV approach has achieved high precision (RSD ≈ 2.2%) and a quantification limit of 5.8 × 10
−7 M [
15]. Amperometric determination using electrodes modified with carbon nanotubes enabled simultaneous analysis of naproxen and paracetamol, achieving throughputs of up to 90 analyses per hour [
18], while employing a short C18 column (10 × 4 mm) allowed for complete separation of naproxen in under 5 minutes [
19].
Advanced chemometric techniques (CLS, PCR, PLS) allow precise resolution of overlapping signals and simultaneous determination of components in complex matrices [
20], while stability studies conducted according to ICH guidelines ensure reliable profiling of degradation products formed under acidic, basic, oxidative, or photolytic conditions [
21]. Additional methods, such as differential thermal analysis (TGA/c-DTA), are used for rapid differentiation between the acidic form and the sodium salt of naproxen in finished formulations [
22], and HPLC solubility studies provide key data for formulation optimization [
23].
From an environmental perspective, research into the electrochemical removal of naproxen from water has shown that modified anodes can achieve higher efficiency with lower energy consumption [
24]. Simple QC procedures based on RP-HPLC enable rapid batch release into production [
25], while more advanced chromatographic strategies allow simultaneous profiling of potential impurities in combination drug products [
26]. Optimization of the mixing process, together with meticulous selection of analytical tools—from rapid bypass HPLC techniques and chemometric algorithms to stability testing and thermal analysis—is crucial for ensuring high API uniformity and effective quality control of pharmaceutical products.
In our research, we completely eliminated the use of a chromatographic column, employing only a bypass setup with UV detection. Thanks to the clear sample matrix and strong light absorption by naproxen, we achieved rapid (< 2 min), precise, and cost-effective API determination, making this method particularly attractive for high-throughput laboratories and facilities with limited resources.
In summary, this study provides an integrated analytical and technological assessment of how different mixing techniques affect tablet quality and API uniformity. It also proposes a simplified but reliable method for naproxen quantification, contributing to the ongoing development of more efficient and accessible pharmaceutical quality control strategies.
2. Results
As shown in
Table 1, nine experimental batches were prepared using three mixing strategies. The first series (V1–V3) was mixed in a V-type blender at 10, 20, and 30 rpm for 20 minutes. The second series (PBM1–PBM3) was blended using a planetary ball mill operating at 200, 300, and 400 rpm for 5 minutes. The third series (VBM1–VBM3) was mixed using a vibratory ball mill for 2, 5, and 10 minutes, respectively. All batches used a consistent powder formulation containing sodium naproxen (API), PVP, magnesium stearate, cellulose, and calcium carbonate.
2.1. Raw Material Properties
The physical characteristics of the raw materials used in this study—sodium naproxen, polyvinylpyrrolidone (PVP), magnesium stearate, cellulose, and calcium carbonate—were assessed through standard powder flow and density tests. The data are summarized in
Table 2 and
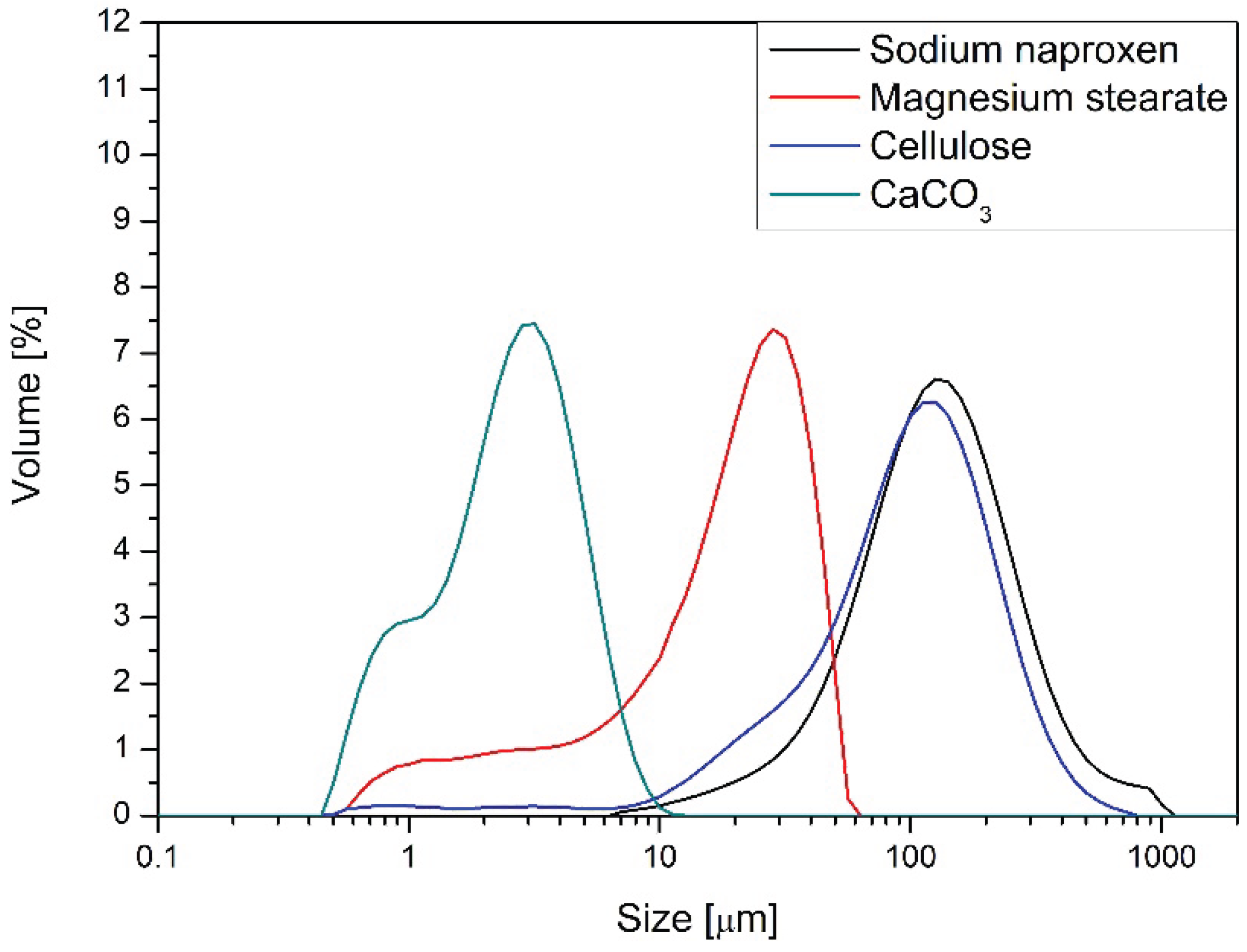
Figure 1.
Sodium naproxen exhibited the poorest flow and packing behavior among all tested materials. Its angle of repose was 46.1°, indicating high cohesiveness and poor flowability. The compressibility index of 45.1% and Hausner ratio of 1.82 reflect significant densification upon tapping, suggesting poor bulk powder behavior during handling or blending. The difference between aerated and packed bulk density was substantial (0.380 vs. 0.692 g/cm3), further confirming poor packing efficiency. These properties classify sodium naproxen as a challenging API for direct compression. Polyvinylpyrrolidone (PVP) showed the most favorable flow and compressibility profile. It had the lowest compressibility index (16.5%) and a Hausner ratio of 1.20, both indicative of excellent flow properties and minimal interparticle friction. Its angle of repose was 39.9°, which also falls within the acceptable range for freely flowing powders. These parameters justify the use of PVP as a suitable binder and carrier in powder blends aimed for direct compression. Magnesium stearate, a common lubricant, presented intermediate flow characteristics. It had a moderate angle of repose (39.7°) and compressibility index of 34.8%, resulting in a Hausner ratio of 1.53. These values suggest fair-to-poor flow and the potential to induce variability in blend homogeneity if not properly dispersed. Cellulose, widely used as a filler and disintegrant, demonstrated reasonable performance. With an angle of repose of 44.2°, compressibility of 18.4%, and a Hausner ratio of 1.23, it showed good bulk flow and compaction characteristics. Its behavior aligns well with its common use in direct compression formulations. Calcium carbonate had the highest bulk density among the excipients (packed: 0.864 g/cm3), but also the highest compressibility index (48.0%) and Hausner ratio (1.92), which indicate poor flow and pronounced packing inefficiency. Its angle of repose (43.1°) confirmed marginally cohesive behavior. The combination of high density and poor flow suggests calcium carbonate may segregate in blends or cause die-filling inconsistencies during compression. Among the analyzed substances, calcium carbonate exhibits the smallest particle size. The particles range from 0.5 μm to 11 μm, with those around 3 μm being the most prevalent. Cellulose shows a particle size distribution similar to that of sodium naproxen, a key component of the tablets. In both naproxen and cellulose, particles approximately 140 μm in size dominate. The particle size distribution of magnesium stearate falls between that of calcium carbonate and the ground forms of sodium naproxen and cellulose. It has the widest range, extending from 0.5 μm to 79 μm, with particles around 25 μm making up the largest fraction. The polyvinylpyrrolidone (PVP) used in the formulation is reported by the manufacturer to have an average particle size close to 20 μm.
Overall, the powder characterization results underscore the importance of understanding the individual flow and packing behaviors of each component. The formulation includes both highly cohesive (naproxen, calcium carbonate) and free-flowing (PVP, cellulose) materials, which presents challenges for uniform blending. These disparities in flowability and compressibility are likely to affect the efficiency of mixing techniques, blend uniformity, and downstream tableting performance.
2.2. Blend Properties
The physical properties of powder blends used for tablet compression were analyzed to evaluate the impact of mixing technology on flow behavior and packing characteristics. The measured parameters include angle of repose, angle of fall, angle of spatula, bulk and tapped densities, compressibility index, and Hausner ratio. The results are summarized in
Table 3.
Blends prepared using the V-type mixer (V1–V3) demonstrated moderate-to-poor flowability. Their angles of repose ranged from 39.6° to 42.7°, and compressibility indices exceeded 43% in all cases, with Hausner ratios between 1.77 and 1.79. These values indicate highly compressible and relatively cohesive powders. Bulk densities ranged from 0.408 to 0.418 g/cm
3, and packed densities approached 0.75 g/cm
3, suggesting limited flow and a strong tendency for densification under tapping. The angle of spatula exceeded 55° in all V-type batches, further confirming their cohesive nature. Blends prepared using the planetary ball mill (PBM1–PBM3) exhibited slightly improved flow behavior and compressibility. Angles of repose ranged from 39.1° to 40.3°, with compressibility indices decreasing progressively from 41.7% (PBM1) to 38.7% (PBM3). The Hausner ratio decreased accordingly from 1.71 to 1.63. These improvements reflect the effect of mechanical energy input, which likely reduced particle size and improved powder packing. The bulk and tapped densities also increased slightly with mixing intensity, indicating better compaction potential. However, the angle of difference and angle of spatula remained comparable to V-type blends, suggesting only modest gains in flow. The vibratory ball mill blends (VBM1–VBM3) achieved the best flow and compressibility profiles. Angles of repose remained in the 39–41° range, but compressibility indices dropped significantly to 33.7–34.5%, with Hausner ratios as low as 1.51. These values indicate improved packing efficiency and reduced cohesiveness, likely due to particle size reduction and enhanced homogenization. The aerated and packed bulk densities for VBM3 reached 0.488 g/cm
3 and 0.739 g/cm
3, respectively—the highest among all tested blends. However, the increasing angle of difference in VBM2 and VBM3 (up to 25.6°) may point to blend instability or segregation tendencies under dynamic conditions. Particle size distribution (PSD) was assessed for the PBM and VBM blends (
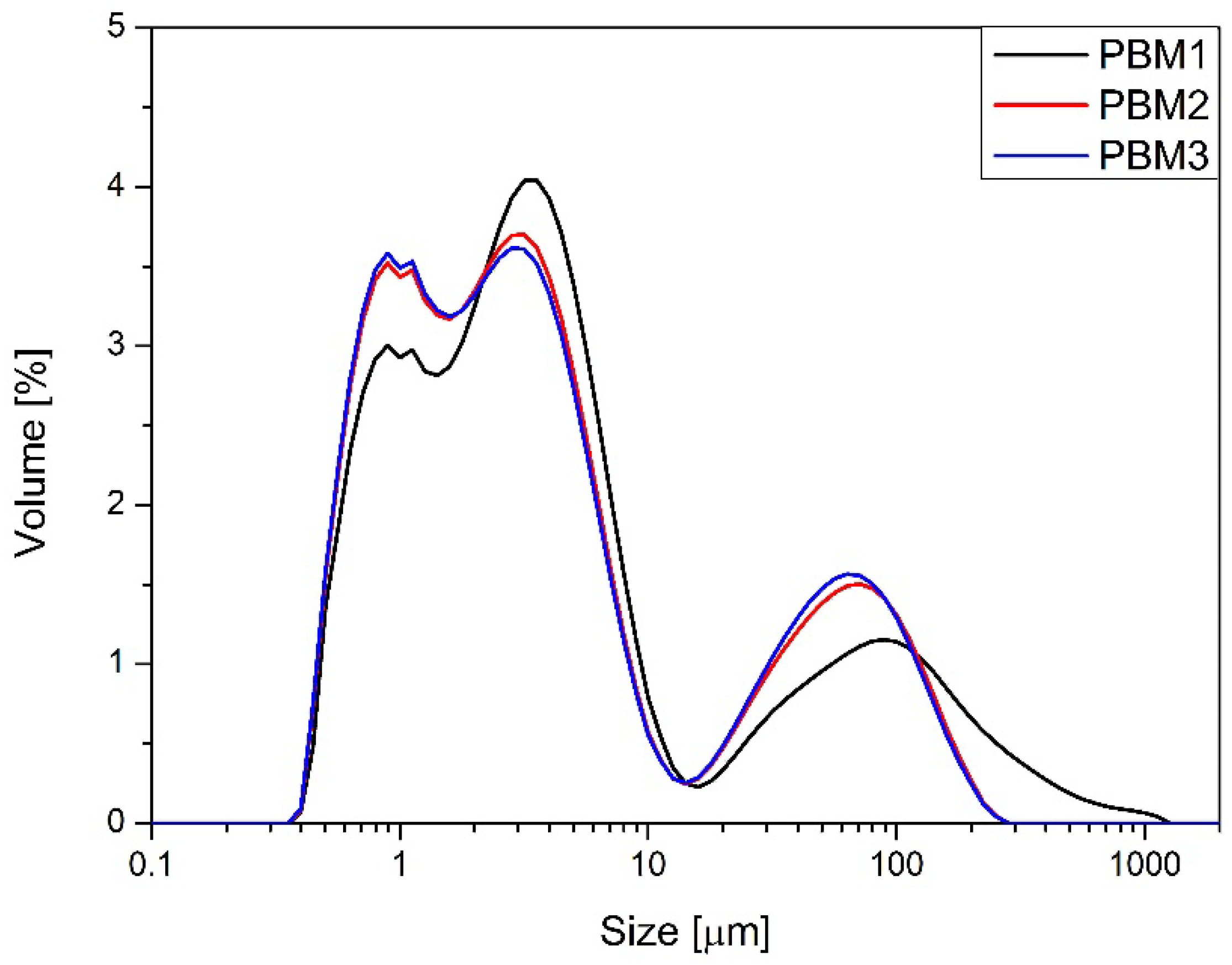
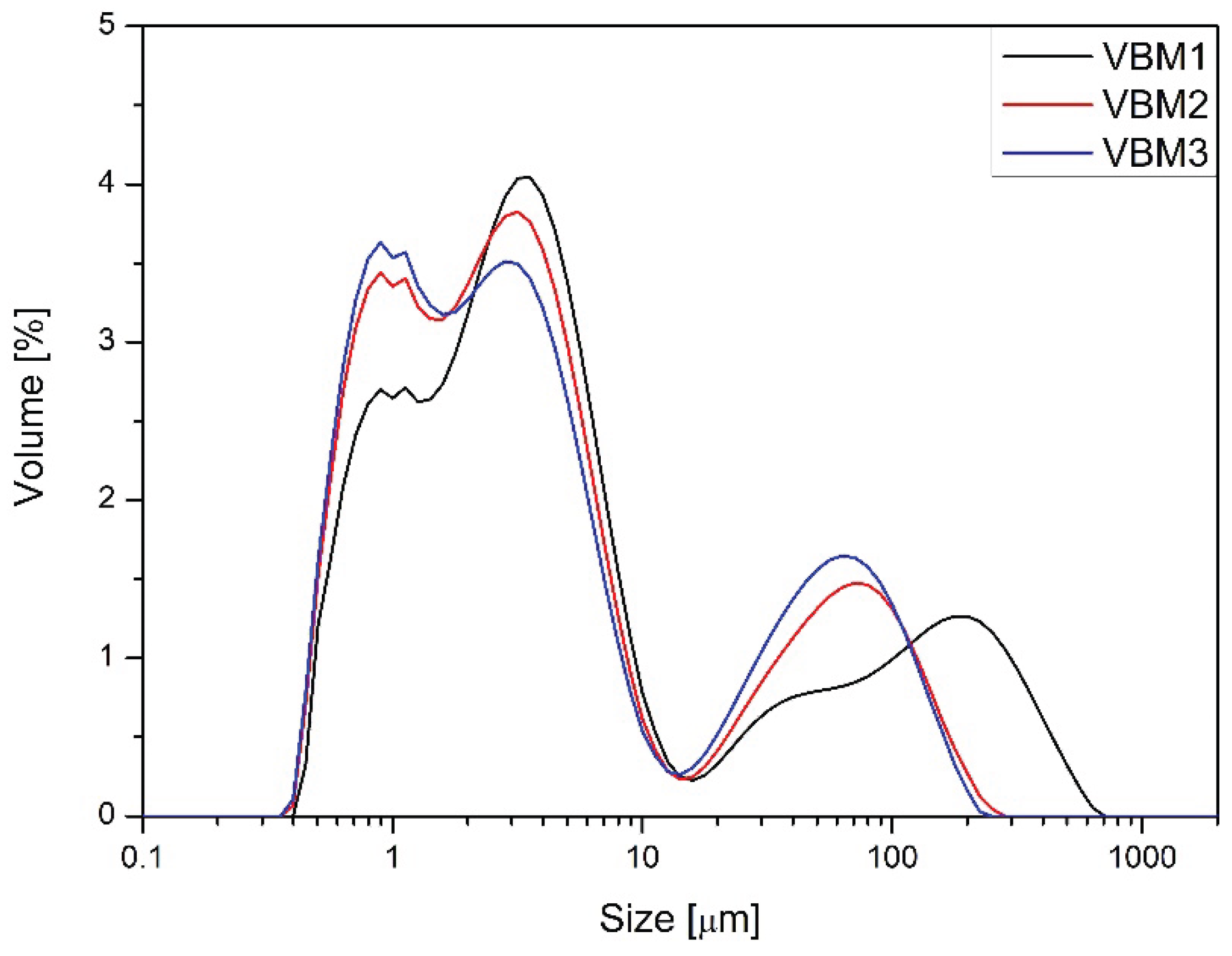
Figure 2 and
Figure 3). All milled samples showed relatively wide PSD profiles, with VBM2 and VBM3 in particular displaying signs of agglomeration and bimodal particle distributions or not homogeneous milling of the components of the mixture. These profiles indicate heterogeneous energy transfer during high-energy mixing, which may lead to local overprocessing or re-agglomeration. PSD was not evaluated for V-type mixer batches, as these underwent no milling or mechanical size reduction. In summary, flow and compressibility characteristics varied markedly depending on the mixing technology used. The V-type mixer produced blends with poor compressibility and flow, yet these were sufficient for tableting due to good component compatibility. Planetary and vibratory ball mills improved bulk behavior but introduced risks of segregation and blend instability, especially at higher energy levels. These findings align with observed differences in tablet mass, thickness, and API content uniformity, further emphasizing the influence of blend quality on downstream performance.
2.3. Tablet Properties
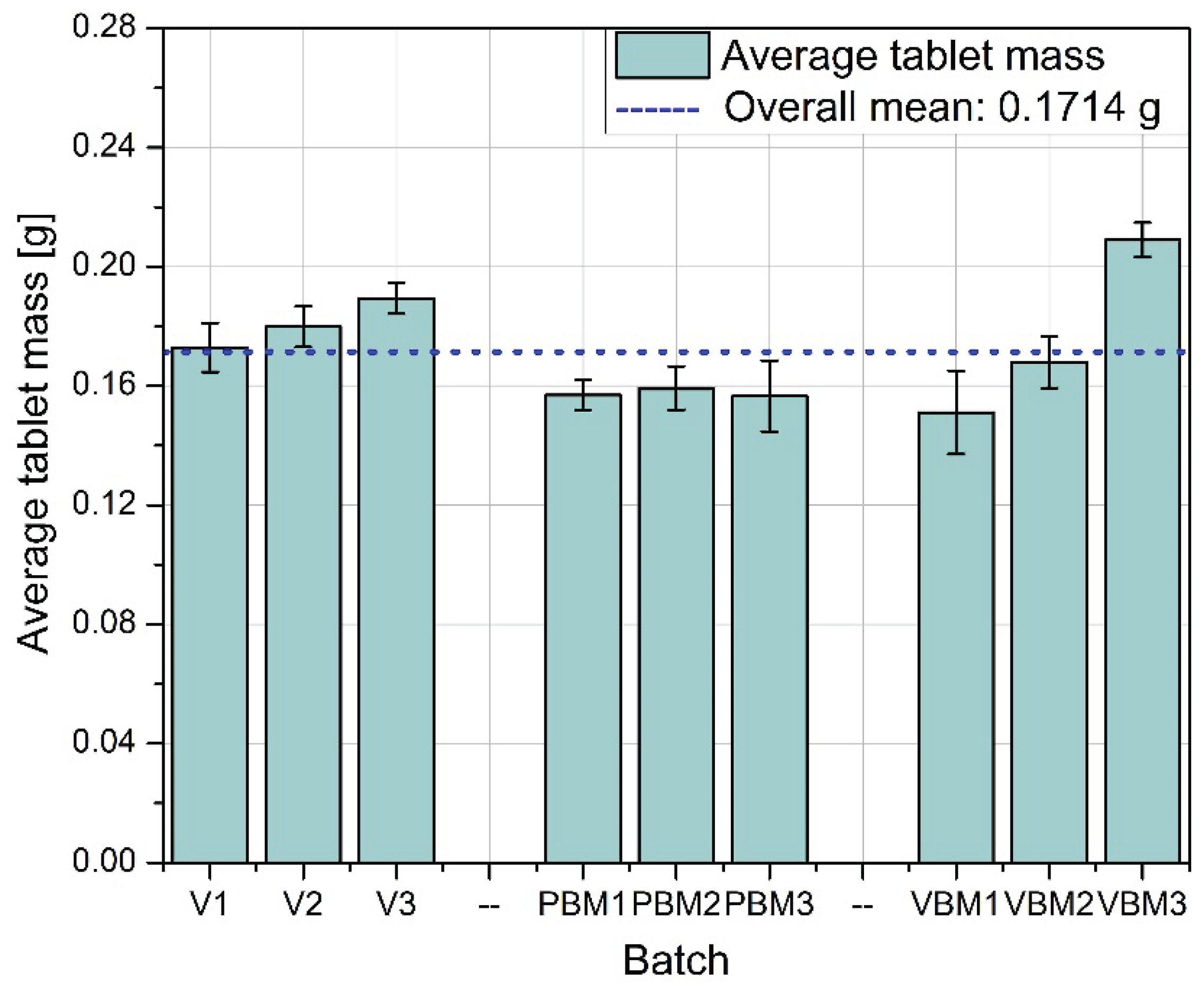
Figure 4 shows the average tablet mass obtained for each of the mixing methods tested. Tablets produced from mixtures prepared in a V-type mixer (V1-V3) have the most stable and close to average weight values, especially in batches V2 and V3. This indicates good homogeneity of the mixture in terms of quantity, which translated into precise powder dosing during tabletting. In the case of PBM (PBM1-PBM3), lower average tablet weights were observed in all batches compared to the V-mixer, which may indicate deteriorated mixture flowability or the effects of excessive powder grinding and segregation. The VBM (VBM1-VBM3) showed differential performance - while VBM1 and VBM2 achieved weights slightly above the global average, VBM3 significantly exceeded it, which may be due to local differences in density or instability in the dispensing process.
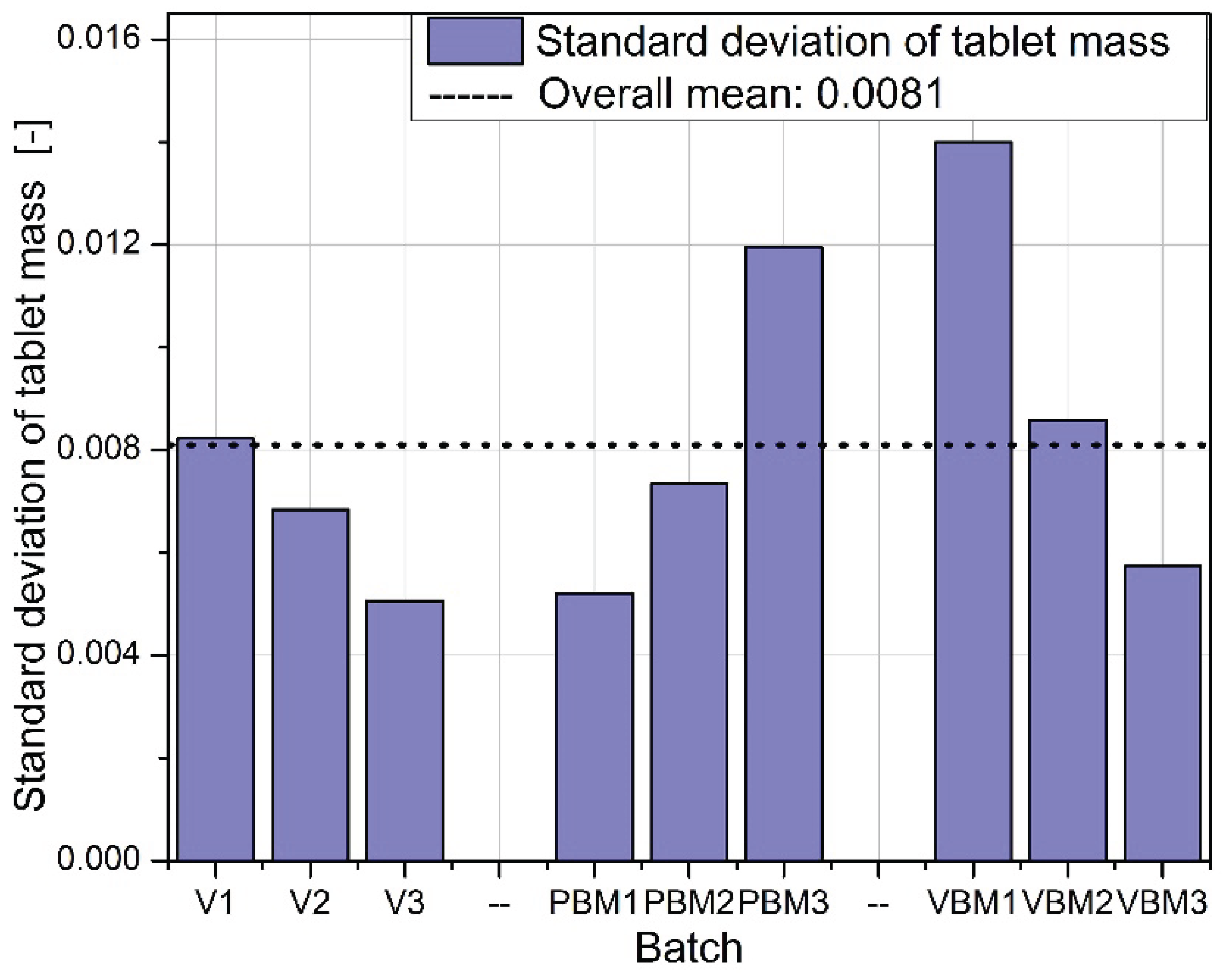
The standard deviations of tablet mass are shown in
Figure 5. The lowest variability was achieved for tablets from batches V2 and V3, confirming the high repeatability of dosing for the V-type mixer. For PBMs, elevated variances are noticeable, especially in the PBM3 batch, indicating a problem with mixture homogeneity or flowability during die filling. VBM2 shows the highest weight variation of all batches tested, suggesting instability in the dosing process or uneven packing of the powder despite good mixing quality.
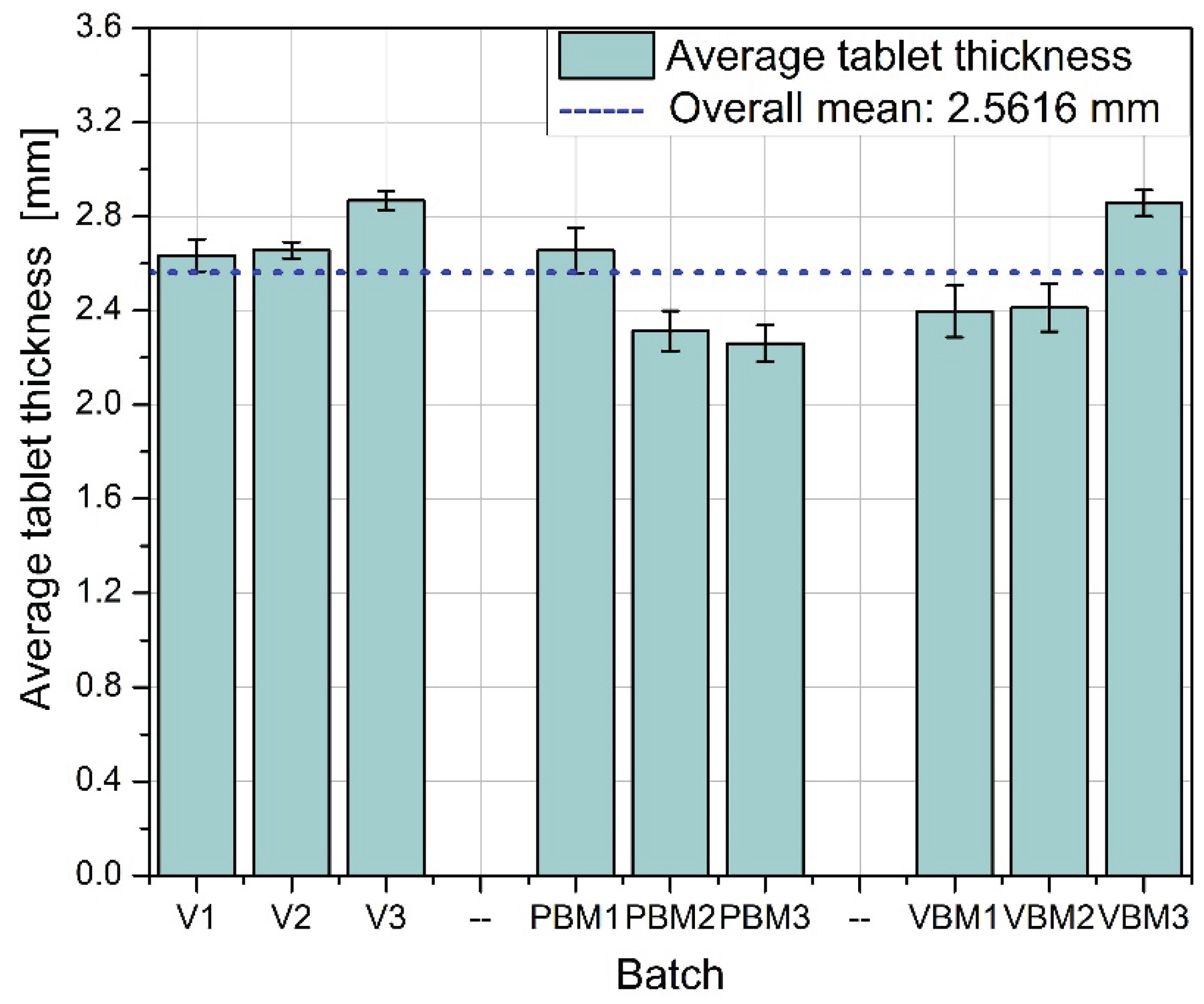
Figure 6 shows the average thickness of tablets in the batches studied. The greatest thickness consistency is again observed in batches V1-V3, indicating good control of the pressing process and uniform compression of the mixture.PBM1 and PBM2 show a slightly lower average thickness, while PBM3 is close to the overall average. The variation in these values may indicate varying compactability of the powder or varying rheological properties of the mixtures depending on the grinding intensity. VBM3 reaches a thickness close to the average, while VBM1 and VBM2 are slightly below this level, suggesting possible differences in the degree of compression between batches.
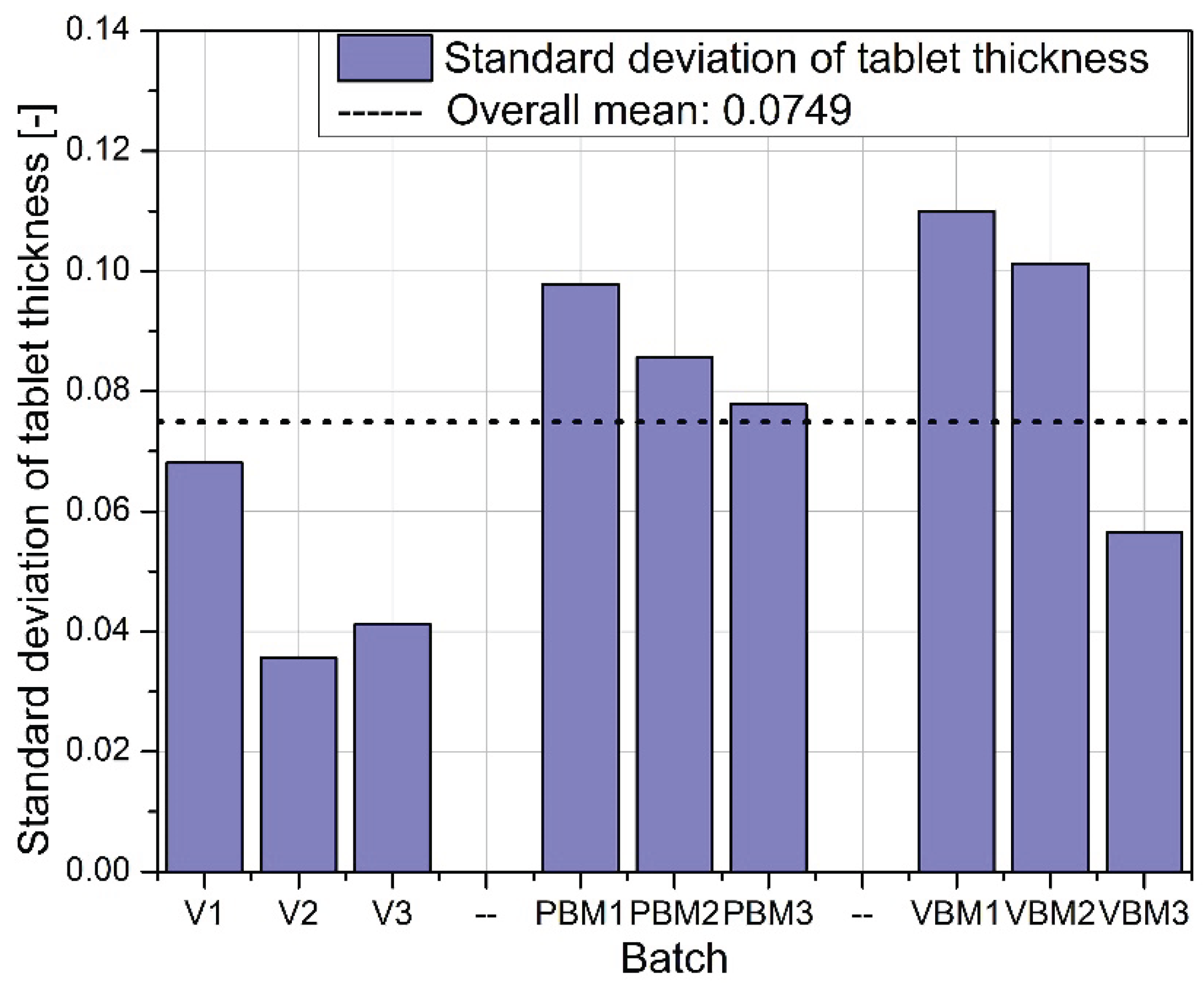
Figure 7 illustrates the variation in tablet thickness, reflecting the stability of the compression process. Batch V2 stands out for having the lowest standard deviation, confirming its exceptional repeatability in terms of thickness and overall tablet quality. PBM3 and VBM2, on the other hand, are characterized by the highest deviations, which may be the result of insufficient particle dispersion or instability in the bulk density of the mixtures during pressing.
The physical uniformity of the tablets (mass, thickness) depends not only on the intensity of mixing, but also on the control of the filling and pressing process, which is particularly important when using high-shear methods of compound preparation. The graphs in
Figure 8,
Figure 9,
Figure 10 and
Figure 11 show the results of hardness and friability measurements of tablets made from mixtures prepared by three different methods: a V-type mixer, a planetary ball mill (PBM) and a vibrating ball mill (VBM). These parameters illustrate the mechanical strength of the finished pharmaceutical forms and their resistance to physical damage.
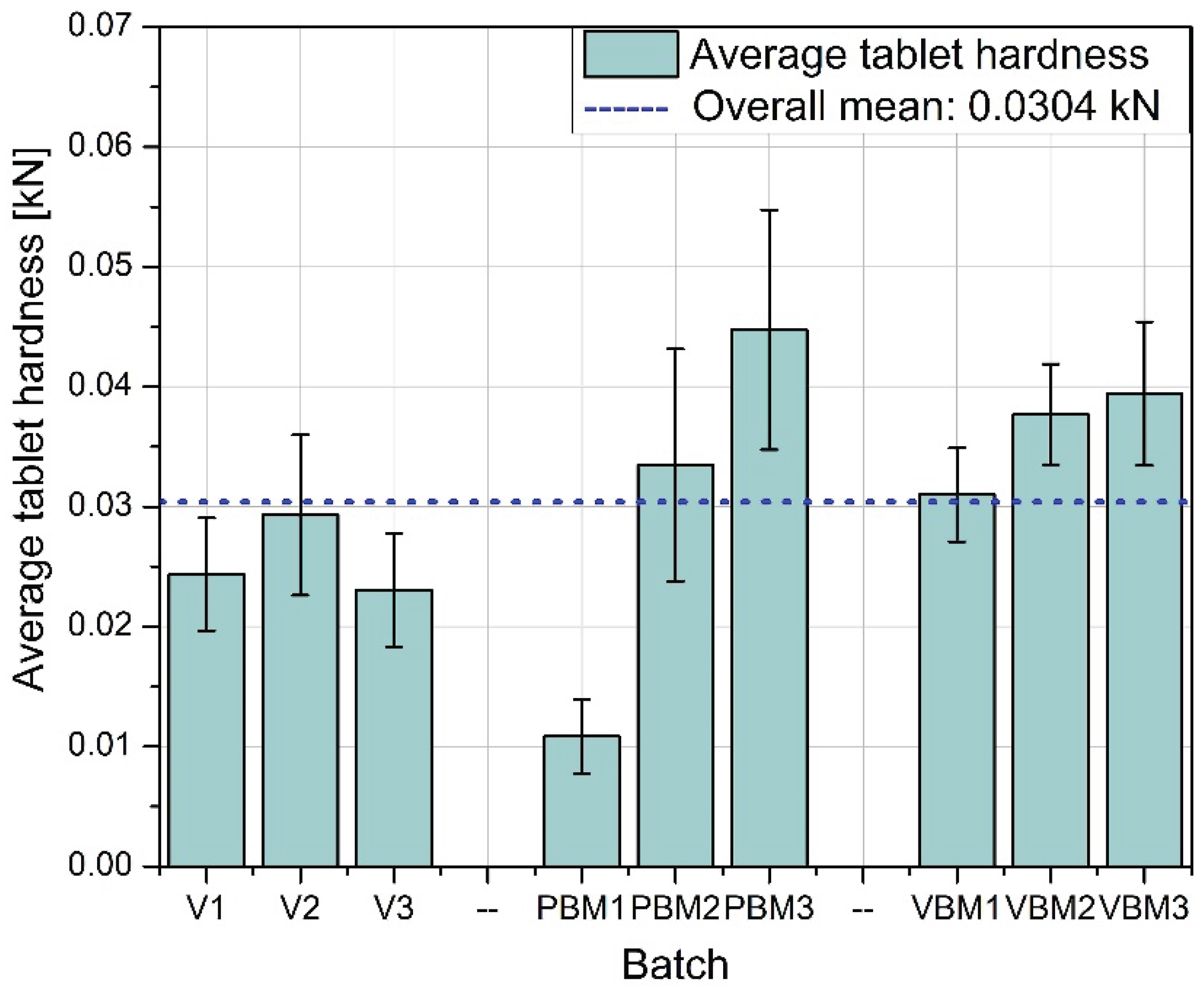
Figure 8 shows the average hardness of the tablets obtained in each of the tested batches. The highest hardness values were achieved for tablets prepared using a vibrating ball mill (VBM1-VBM3), indicating good compactability and strong bonds between particles during pressing. PBM tablets show intermediate values, while the lowest hardness was obtained in tablets from the V-type mixer, suggesting weaker blend cohesion and lower packing density during compression.
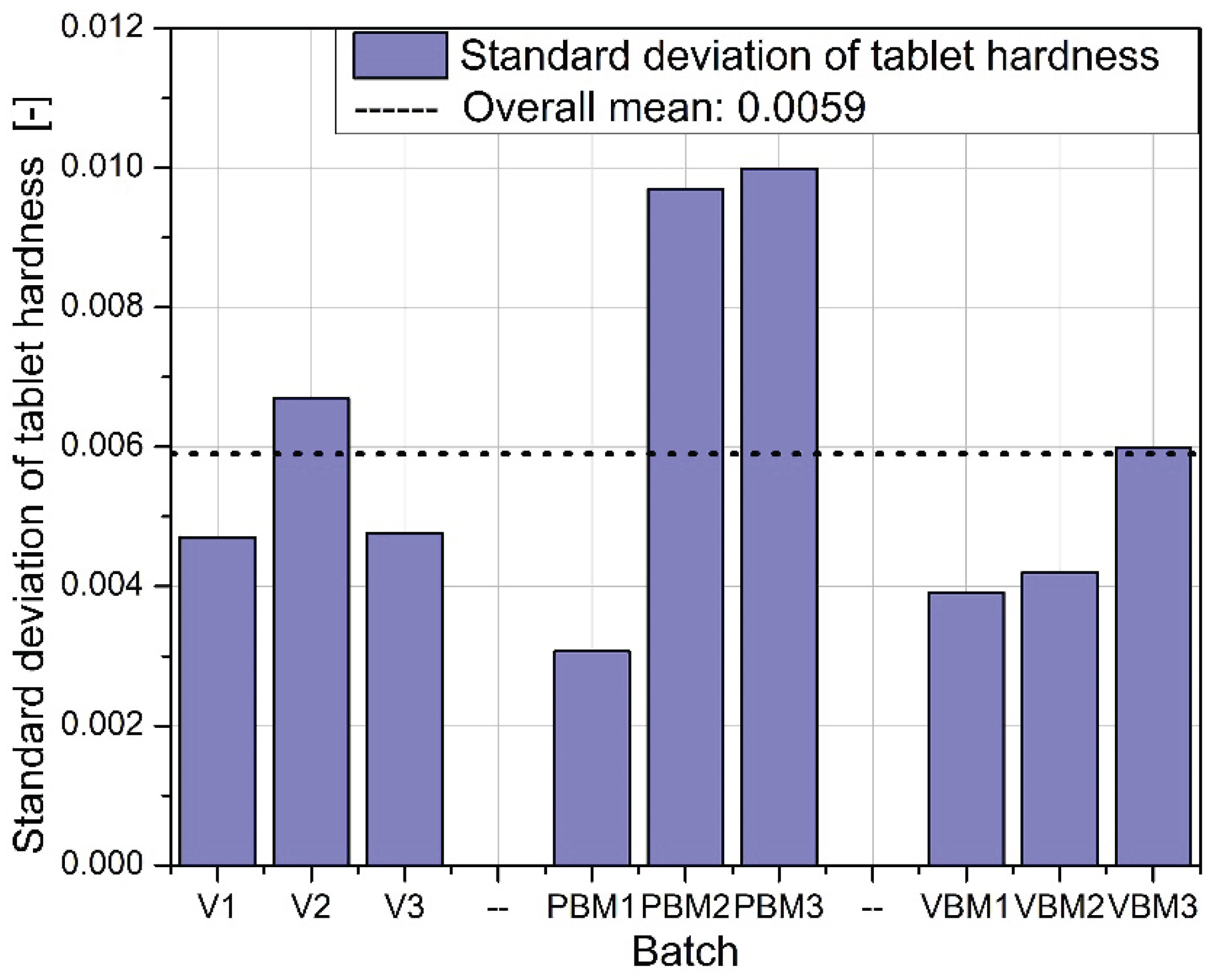
Figure 9 shows the standard deviation of tablet hardness, which provides a measure of the mechanical uniformity of tablets in each batch. The lowest variability is observed for the VBM batches, confirming their highest reproducibility and quality control. For PBM and V, the variability in hardness is moderate, which may be due to the heterogeneous distribution of active and auxiliary ingredients in the powder before tabletting.
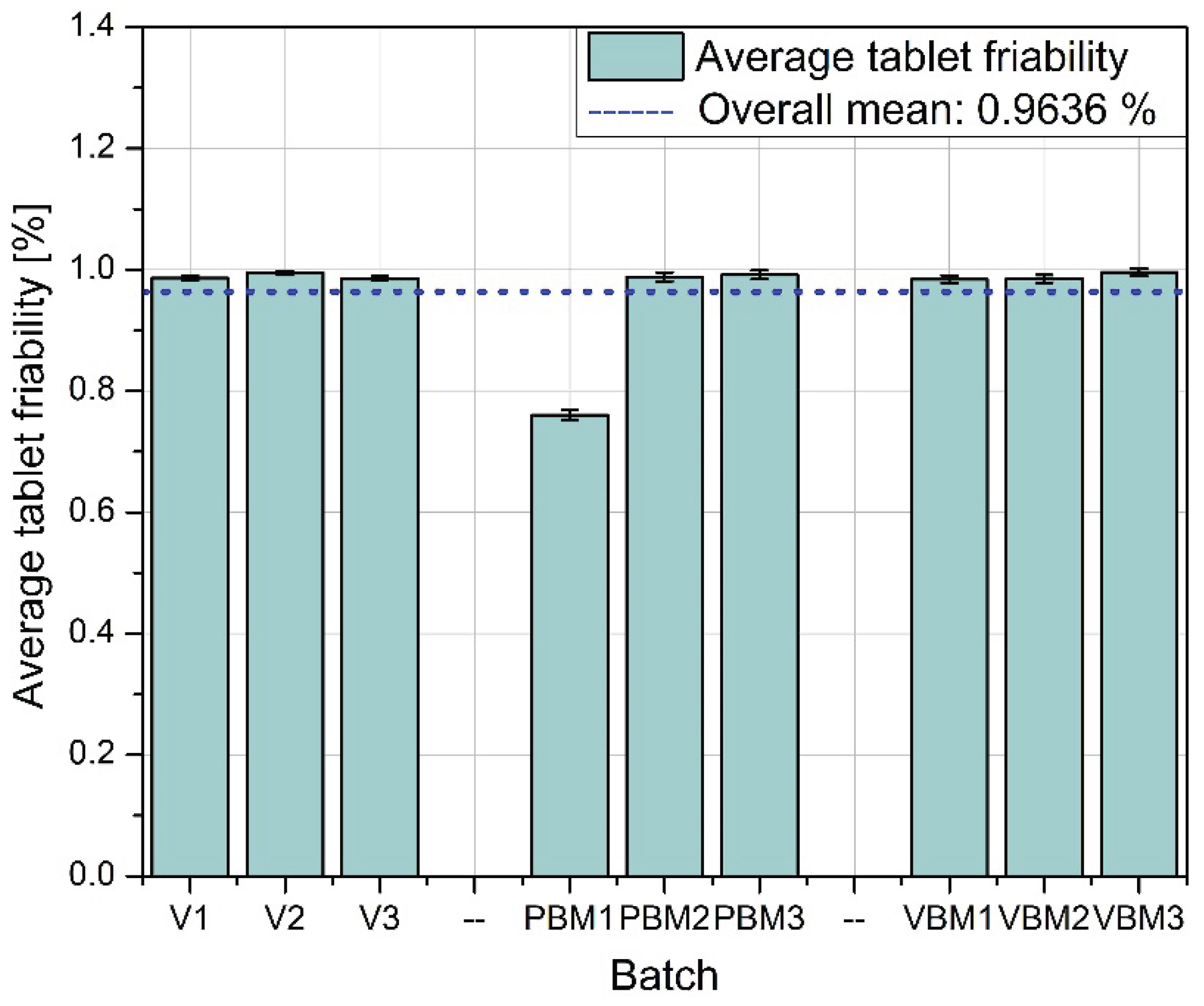
Figure 10 shows the average friability of the tablets, i.e., their susceptibility to weight loss under mechanical loading conditions (e.g., during packaging or transportation). All three methods performed very similarly - the abrasion values remain at low, similar levels, with no clear differences between batches. These results indicate that regardless of the mixing technology used, the resulting tablets have acceptable abrasion resistance, meeting typical pharmacopoeial requirements (<1%).
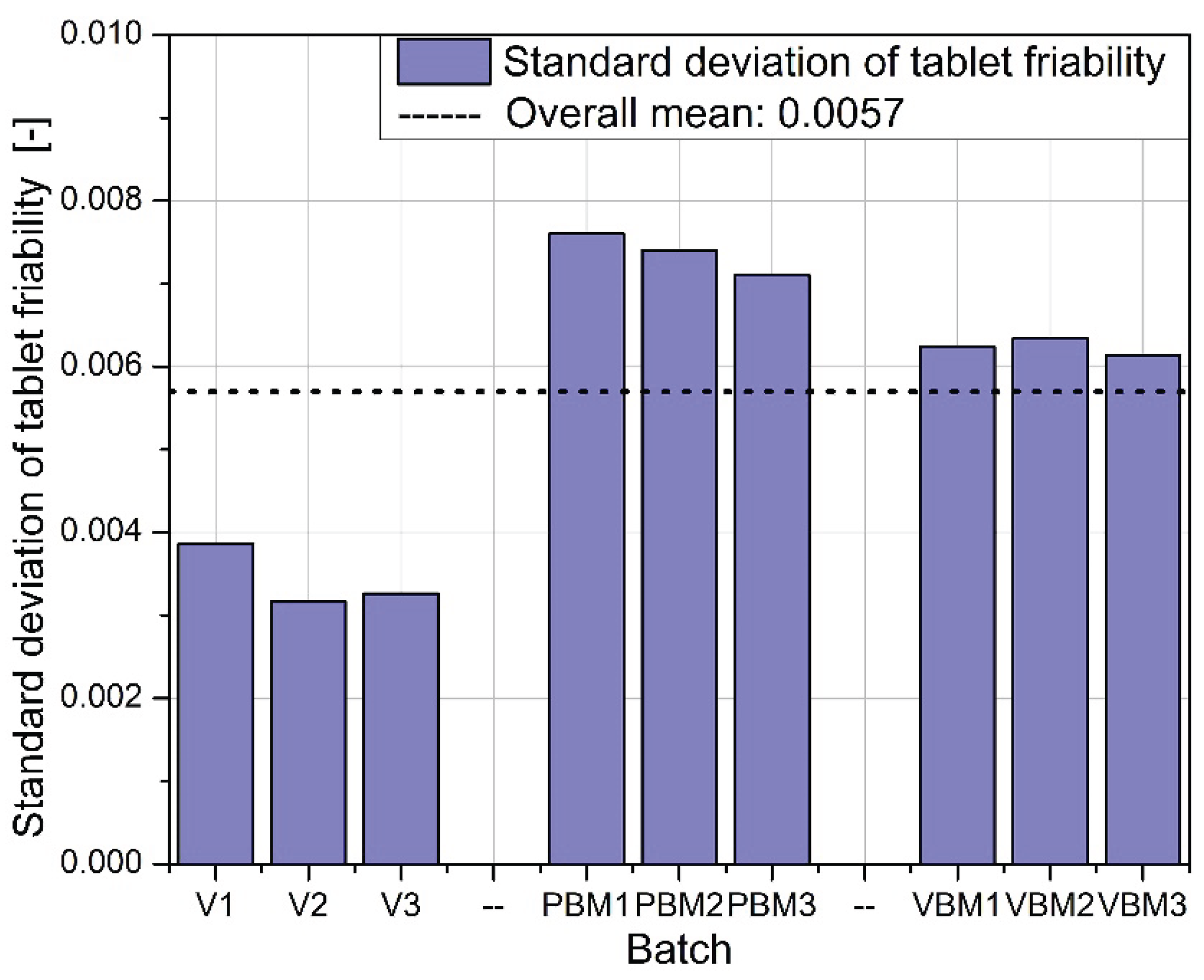
Figure 11 shows the standard deviation of tablet abrasion. As with the mean values, the differences between batches are minimal, and the deviations obtained indicate good repeatability of the results. A slight qualitative advantage can be attributed to VBM, where the deviations are slightly lower, which may be due to the more homogeneous structure of the powder after mixing. A compilation of the results in
Figure 8,
Figure 9,
Figure 10 and
Figure 11 confirms that mixing by the vibratory ball mill (VBM) method leads to the most homogeneous and mechanically robust tablets. Tablets obtained by this method have both the highest hardness and the lowest variation in hardness and friability. The V-type mixer, on the other hand, despite its simplicity and popularity, generates the highest mechanical variability, which may be the result of limited mixing efficiency, especially for powders with poor flow and high cohesion. Average abrasion values remain low in all groups, suggesting that the tabletting parameters used were appropriately chosen and produced forms with good resistance to physical damage, regardless of the mixture preparation method.
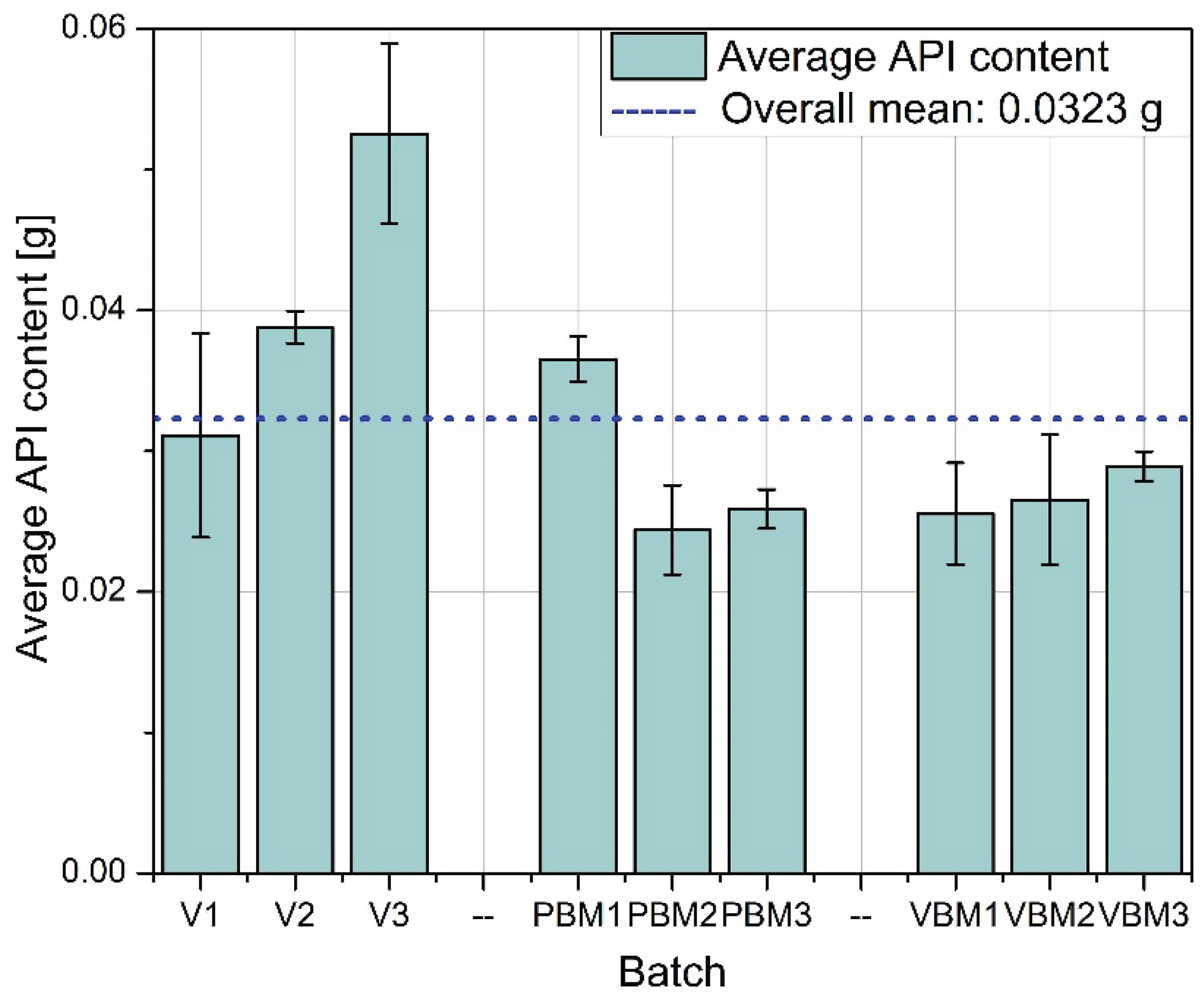
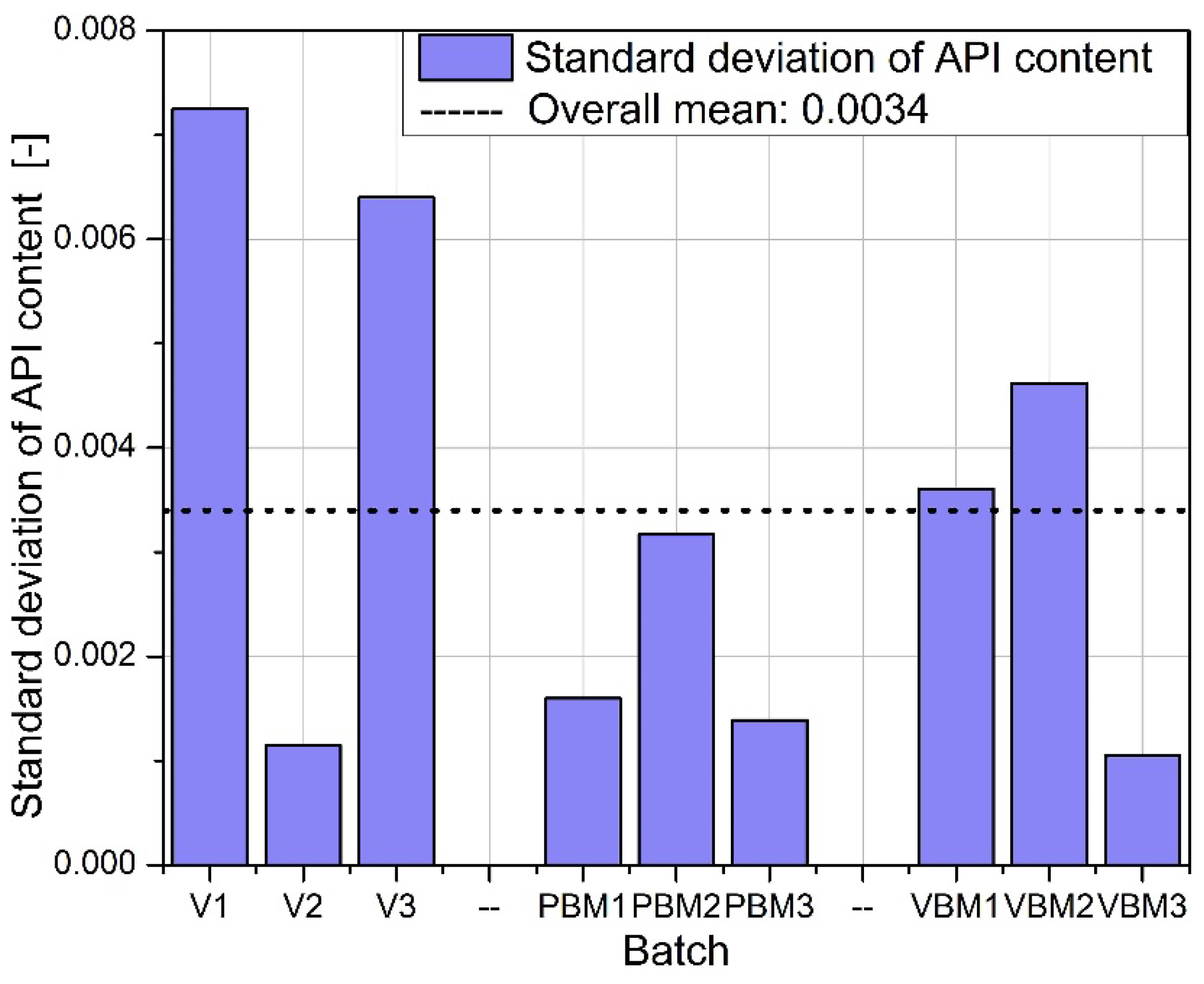
2.4. API Content
Quantitative determination of the Active Pharmaceutical Ingredient (API) in tablet samples, conducted via high-performance liquid chromatography (HPLC), revealed distinct differences in content uniformity across the tested mixing technologies (
Figure 12,
Figure 13 and
Figure 14). The results underscore the influence of blending method on the homogeneity of API distribution in final dosage forms.
Among all groups, tablets produced using the vibratory ball mill (VBM1–VBM3) demonstrated the lowest variability in API content. Individual measurements across these batches were closely clustered, indicating consistent drug distribution within the powder blend and uniformity during the compression process. This outcome aligns with previous findings on improved flow and packing behavior for VBM samples and suggests effective homogenization under vibratory mixing conditions. In contrast, tablets from the V-type mixer (V1–V3) showed the highest variability in API content. The spread of measured concentrations was notably wider compared to other groups, with differences evident both between and within batches. This inconsistency likely results from insufficient blending intensity in the V-type system, particularly in formulations containing poorly flowing or cohesive components. The findings correspond with earlier observations of high compressibility indices and limited powder mobility in these blends. The planetary ball mill samples (PBM1–PBM3) displayed intermediate performance. While some improvement in homogeneity was observed relative to the V-type batches, variability in API content remained pronounced, especially in PBM2 and PBM3. These results may reflect a balance between enhanced mixing efficiency and the onset of segregation effects induced by excessive mechanical energy. Overall, the API content data confirm that mixing technique plays a critical role in determining dosage uniformity. Vibratory milling proved most effective in achieving homogeneous blends, while low-energy V-type mixing was associated with inadequate API distribution. These findings reinforce the need to match blending intensity to formulation characteristics and to control process parameters closely when using high- or low-shear mixing methods.
3. Discussion
The findings of this study clearly demonstrate that the choice of mixing technology has a profound influence on the critical quality attributes (CQAs) of directly compressed naproxen sodium tablets. Specifically, differences were observed in powder blend behavior, tablet mass and thickness uniformity, mechanical strength, and API content homogeneity. These effects stem not only from the energy and motion imparted during mixing, but also from how well each technique accommodates the inherent flow and packing limitations of the component materials.
3.1. Influence of Mixing Method on Powder Blend Properties
Powder blends prepared with the V-type mixer showed the poorest flow and compressibility profiles, as evidenced by their high compressibility indices (>43%), elevated Hausner ratios (~1.78), and angles of repose exceeding 41°. These results are consistent with the well-established limitations of low-shear tumbling mixers when handling cohesive materials such as sodium naproxen or calcium carbonate [
13]. Nevertheless, despite the suboptimal blend characteristics, tablets produced from V-type mixtures exhibited the most consistent performance in terms of mass and thickness, confirming previous observations that low-shear blending, when paired with compatible excipients, can ensure acceptable performance in direct compression formulations [
8]. Planetary and vibratory ball mills significantly improved compressibility indices (as low as 33.7% in VBM3) and reduced Hausner ratios to ~1.51, attributed to increased particle-particle interactions due to size reduction. These findings support literature showing that mechanical energy from high-shear mixers can densify blends and improve apparent flow [
9]. However, this improvement was often offset by increased blend instability, as indicated by higher angles of difference (up to 25.6° in VBM3), suggesting the onset of segregation or uncontrolled particle agglomeration.
3.2. Impact on Tablet Uniformity and Mechanical Properties
V-type mixing yielded tablets with low variability in mass and thickness, which is essential for dosage accuracy and regulatory compliance. These results are supported by previous studies, such as Marianni et al. (2021), who demonstrated that tumbling mixers—despite limited energy input—can produce homogenous blends when component compatibility is high and mixing time is sufficient [
8]. In contrast, tablets produced from high-energy blends, particularly PBM3 and VBM2, exhibited greater variability in both weight and thickness. In contrast, tablets produced from high-energy blends, particularly PBM3 and VBM2, showed greater variability in both weight and thickness. This inconsistency requires careful control of mechanical energy during mixing to prevent mixing, particle abrasion or local over-processing in high-dose formulations. Interestingly, the highest hardness values were obtained from VBM batches, confirming that high-energy mixing enhances compactability. However, this was not always accompanied by improved content uniformity, indicating that improved mechanical properties do not guarantee adequate API distribution.
3.3. API Content Uniformity and Analytical Performance
The greatest differences between mixing methods were observed in the homogeneity of API content. VBM samples consistently showed the lowest RSD values (< 1.5%), while V-type batches sometimes exceeded 3% variability, and excessive energy input in PBM2 and PBM3 led to increased heterogeneity, likely due to particle migration or electrostatic effects. Detection by bypass HPLC proved both rapid and accurate, mirroring the success of other simplified approaches: Şener et al. achieved an RSD of ≈ 2.2% for naproxen using FIA-UV [
15], Stefano et al. demonstrated high-throughput simultaneous detection of naproxen and paracetamol using flow-injection amperometry [
18], and Puttanapitak et al. fully resolved naproxen on a 10 × 4 mm C18 column in less than 5 minutes [
19]. Taken together, these observations underscore that precise control of mixing parameters - and the use of streamlined but robust analytical tools, from bypass HPLC to chemometric signal separation to stability testing and thermal analysis - is essential for achieving high API homogeneity and effective quality control of pharmaceutical products.
3.4. Practical Implications
From a formulation development perspective, this study confirms that low-energy mixers like the V-type provide more predictable physical performance, especially when excipient compatibility is high. Conversely, while vibratory and planetary mills offer improved particle dispersion and flow, their application should be carefully tuned to avoid overprocessing or blend instability. These findings underscore the importance of a case-specific approach to mixer selection, particularly in formulations involving challenging APIs such as sodium naproxen. Furthermore, the implementation of rapid analytical techniques—such as bypass HPLC—may reduce analytical burden in early-stage development or in resource-limited environments, provided that matrix simplicity and signal clarity are assured.
4. Materials and Methods
4.1. Materials
The formulation consisted of sodium naproxen as the active pharmaceutical ingredient and four commonly used excipients, all obtained from recognized suppliers. Specifically, sodium naproxen was sourced from Divi’s Laboratories (India); calcium carbonate from Chmpur (Poland); microcrystalline cellulose from FMC BioPolymer (USA); and both polyvinylpyrrolidone and magnesium stearate from Sigma-Aldrich (USA). All components were of analytical grade purity. The composition of the blend, expressed as mass percentages, included: 20% sodium naproxen, 68% calcium carbonate serving as the primary filler, 7% microcrystalline cellulose functioning as both a filler and disintegrant, 3% polyvinylpyrrolidone acting as a binder, and 2% magnesium stearate employed as a lubricant.
4.2. Methods for Determining the Properties of Raw Materials and Tablet Blends
The properties of both raw powders and formulation blends were assessed using a powder testing instrument compliant with current pharmacopoeial guidelines. All analyses were carried out using the PT-S Powder Tester manufactured by Hosokawa Micron B.V. (Doetinchem, The Netherlands). The scope of the assessment included measurement of the angle of repose, angle of fall, angle of spatula, angle of difference, as well as aerated and tapped bulk densities, Hausner ratio, and compressibility index.
Particle size distribution of the raw materials was analyzed using laser diffraction with a wet dispersion setup. The measurements were performed using a Mastersizer 2000 Hydro MU system from Malvern Instruments (UK), in accordance with the ISO 13320-1:1999 standard.
4.3. Methods of Preparing Tablet Blends and Tablets
Powder blends in the first experimental group were prepared using a V-type tumble mixer with a total chamber volume of 750 cm3, produced by CDK (Poland). Each batch was blended for 20 minutes at rotational speeds of 10, 20, or 30 rpm. The working chamber was loaded to 40% of its maximum capacity to ensure optimal tumbling behavior. The second group of blends was processed using a Mono Pulverisette 6 planetary ball mill (Fritsch, Germany). The milling system featured a 500 cm3 zirconium oxide chamber and was loaded with zirconium oxide balls of 5 mm diameter. The weight ratio of grinding media to powder was maintained at 8:1. Each sample was milled for 5 minutes at rotational speeds of 200, 300, or 400 rpm. For the third group, powder mixtures were processed using a SPEX 8000M high-energy vibratory ball mill (SPEX SamplePrep LLC, USA). The apparatus was fitted with a stainless steel chamber (volume: 50 cm3) and operated with two stainless steel balls of 10 mm diameter. Mixing durations were set to 2.5, 5, and 10 minutes, with the chamber filled to 30% of its total volume.
Tablet compression was carried out on a TDP 0 manual single-punch press (LFA Machines, UK). A 6 mm concave punch set was used for the process, featuring beveled edges and an upper punch equipped with a score line to facilitate tablet splitting. Each unit was formed under a compression force of 3.5 kN.
4.4. Methods for Determining the Properties of Tablets
Tablet weight was determined using an Ohaus Pioneer PX224 analytical balance (Switzerland), ensuring high measurement accuracy. Tablet thickness was assessed using a digital caliper with a resolution of 0.01 mm, with measurements taken from 20 individual tablets per batch. All determinations were performed in quadruplicate to confirm measurement repeatability. Friability was evaluated using a GUNT CE 245 drum mixer (Germany). Approximately 50 g of tablets from each formulation were placed into a 1.15 dm3 rotating drum, which was mounted on shafts rotating at 200 rpm for a 5-minute cycle. After the test, the tablet fragments were passed through a 500 μm mesh sieve. The mass of material retained on the sieve was recorded, and friability was expressed as a percentage of the original tablet mass. Each friability test was conducted in four replicates to ensure statistical robustness. Tablet hardness was measured using a static force method on 20 tablets per batch. The measurements were performed with a hardness tester produced by CDK (Gliwice, Poland).
4.5. Quantitative determination of the Active Pharmaceutical Ingredient (API) in tablets by HPLC
The quantitative assessment of the active pharmaceutical ingredient (API) was conducted on nine randomly selected tablets from each investigated batch. The analytical procedure employed high-performance liquid chromatography (HPLC), utilizing a pre-established calibration curve to quantify the API content. The HPLC system comprised a mobile phase reservoir, a model 1110 pump, an injection valve enabling sample introduction via a syringe into a 20 µl loop, a model 1310 column oven, a bypass-type connector, and a UV detector (model 1410) responsible for capturing the signal corresponding to the presence of the analyte. System operation and data acquisition were managed via D-7000 HSM software (Merck), facilitating control over chromatographic performance and analytical readout.
The decision to employ a bypass connector in place of a chromatographic column stemmed from the unambiguous presence of the target compound and the lack of necessity for component separation, due to the matrix’s simplicity and absence of interfering signals. The analysis was strictly quantitative and based solely on UV detector response. The resulting chromatograms exhibited well-defined single peaks, with a total analysis time of under two minutes per sample.
Initially, standard solutions of sodium naproxen were prepared. Accurately weighed quantities of tablet-forming excipients, each containing incrementally increasing amounts of sodium naproxen, were transferred into 50 ml volumetric flasks, followed by the addition of distilled water to volume. These mixtures were agitated in a shaker for one hour to ensure complete dissolution of sodium naproxen and PVP. Subsequently, the resulting suspensions were filtered through 0.2 µm syringe filters. Analytical samples were then prepared by mixing 0.5 ml of the filtrate with 4.5 ml of methanol, yielding a total volume of 5 ml. These solutions were introduced into the HPLC system via syringe injection. Measurements were carried out at 20 °C. The mobile phase consisted of a methanol-water mixture in a 90:10 v/v ratio, with a flow rate of 1 ml/min. Each determination required approximately 2 minutes.
For each standard, three replicate injections were performed, and peak heights were recorded from the resulting chromatograms. The averaged peak heights were plotted against known concentrations to construct the calibration curve.
For tablet analysis, nine tablets were randomly selected from each batch and individually weighed. Each set was transferred into 100 ml volumetric flasks, to which 50 ml of distilled water was added. These were subjected to shaking for 8 hours at 120 rpm. The resulting suspensions were filtered through 0.2 µm syringe filters, and 0.5 ml of the filtrate was combined with 4.5 ml of methanol, producing 5 ml analytical samples. These were analyzed under the same conditions as the calibration standards. Based on peak areas and the calibration curve equation, the API content in each tablet was calculated.
5. Conclusions
This study provides a comprehensive evaluation of how different powder blending technologies—V-type mixer, planetary ball mill, and vibratory ball mill—affect the uniformity, mechanical performance, and analytical quality of naproxen sodium tablets prepared by direct compression. Despite exhibiting the least favorable flow and compressibility profiles, powder blends produced using the V-type mixer yielded tablets with the most consistent physical dimensions and acceptable API content uniformity, emphasizing the reliability of low-energy tumbling mechanisms for formulations with well-balanced excipient compositions. In contrast, high-energy mixing using planetary and vibratory mills enhanced blend densification and improved flow characteristics, yet introduced variability in tablet mass and API content, particularly at higher mixing intensities. This underlines the importance of optimizing mixing parameters to prevent segregation or overprocessing in energy-intensive systems.
Importantly, the simplified analytical method employed in this study—UV detection with bypass HPLC—proved to be a fast, accurate, and resource-efficient alternative to conventional chromatographic separation. Its successful application to naproxen sodium highlights its potential for broader use in pharmaceutical quality control, especially in settings with limited analytical infrastructure.
Taken together, the results demonstrate that both technological and analytical choices play a critical role in ensuring the quality and reproducibility of solid dosage forms. The findings contribute valuable insights into formulation development strategies and support the integration of streamlined analytical approaches aligned with current trends in pharmaceutical analysis.
Author Contributions
Conceptualization, M.P.; methodology, M.P. and P.R.; software, M.P., R.L.P and WZ; validation, M.P., P.R. and R.L.P.; formal analysis, M.P. and W.Z.; investigation, M.P., P.R. and R.L.P.; resources, M.P.; data curation, M.P.; writing—original draft preparation, M.P.; writing—review and editing, M.P.; visualization, M.P.; supervision, M.P. and W.Z.; project administration, M.P. All authors have read and agreed to the published version of the manuscript.
Funding
Financed by the Minister of Science and Higher Education Republic of Poland within the program “Regional Excellence Initiative”, agreement no. RID/SP/0032/2024/01.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Kudo, Y.; Yasuda, M.; Matsusaka, S. Effect of particle size distribution on flowability of granulated lactose. Adv. Powder Technol. 2020, 31. [CrossRef]
- Przywara, M.; Leszczak, P. Quality by design strategies in fluidized bed granulation: A focus on granules for tablet manufacturing. Adv. Sci. Technol. Res. J. 2025, 429–444, 429–444. [CrossRef]
- Astriani Nurjanah; Nurlelah, N. Formulation and Physical Evaluation of CTM Tablets by Direct Compression Method: A Systematic Literature Review. Eureka Herba Indones. 2023, 4. [CrossRef]
- Sulaiman, T.N.S.; Sulaiman, S. Review: Excipients for Tablet Manufacturing With Direct Compression Method. J. Pharm. Sci. 2020, 3.
- Iqubal, M.K.; Singh, P.K.; Shuaib, M.; Iqubal, A.; Singh, M. Recent Advances in Direct Compression Technique for Pharmaceutical Tablet Formulation. Int. J. Pharm. Res. Dev. 2014, 6.
- Jivraj, M.; Martini, L.G.; Thomson, C.M. An overview of the different excipients useful for the direct compression of tablets. Pharm. Sci. Technol. Today 2000, 3. [CrossRef]
- Gohel, M.C.; Jogani, P.D. A review of co-processed directly compressible excipients. J. Pharm. Pharm. Sci. 2005, 8.
- Marianni, B.; Polonini, H.; Oliveira, M.A.L. Ensuring homogeneity in powder mixtures for pharmaceuticals and dietary supplements: Evaluation of a 3-axis mixing equipment. Pharmaceutics 2021, 13. [CrossRef]
- Asachi, M.; Nourafkan, E.; Hassanpour, A. A review of current techniques for the evaluation of powder mixing. Adv. Powder Technol. 2018, 29. [CrossRef]
- Przywara, M.; Jękot, K.; Jednacz, W. Evaluation of Vibratory Ball Mill Mixing as an Alternative to Wet Granulation in the Manufacturing of Sodium Naproxen Tablets with Dolomite-Based Formulations. Appl. Sci. 2025, 15. [CrossRef]
- Leś, K.; Opaliński, I. Prospective Application of Response Surface Methodology for Predicting High-Energy Mixing Process Conditions towards Fine Powders Flow Improvement. Adv. Sci. Technol. Res. J. 2021, 15, 134–143. [CrossRef]
- Villiers, M.M.; Tiedt, L.R. An analysis of fine grinding and aggregation of poorly soluble drug powders in a vibrating ball mill. Pharmazie 1996, 51.
- Jakubowska, E.; Ciepluch, N. Blend segregation in tablets manufacturing and its effect on drug content uniformity—a review. Pharmaceutics 2021, 13. [CrossRef]
- Di Martino, P.; Malaj, L.; Censi, R.; Martelli, S. Physico-chemical and technological properties of sodium naproxen granules prepared in a high-shear mixer-granulator. J. Pharm. Sci. 2008, 97. [CrossRef]
- Sener, E.; Tuncel, M.; Aboul-Enein, H.Y. Rapid determination of naproxen sodium in pharmaceutical formulations by flow injection analysis (FIA) using UV-detection. J. Liq. Chromatogr. Relat. Technol. 2003, 26. [CrossRef]
- Maghsoodi, M.; Taghizadeh, O.; Martin, G.P.; Nokhodchi, A. Particle design of naproxen-disintegrant agglomerates for direct compression by a crystallo-co-agglomeration technique. Int. J. Pharm. 2008, 351. [CrossRef]
- Wang, L.J.; Tang, Y.H.; Liu, Y.H. Flow injection chemiluminescence determination of loxoprofen and naproxen with the acidic permanganate-sulfite system. J. Pharm. Anal. 2011, 1. [CrossRef]
- Stefano, J.S.; Montes, R.H.O.; Richter, E.M.; Muñoz, R.A.A. Flow-injection analysis with multiple-pulse amperometry for simultaneous determination of paracetamol and naproxen using a homemade flow cell for screen-printed electrodes. In Proceedings of the Journal of the Brazilian Chemical Society; 2014; Vol. 25. [CrossRef]
- Puttanapitak, P.; Chokesinlapasart, S.; Suntornsuk, L.; Phechkrajang, C.; Prutthiwanasan, B. A simple and rapid chromatographic method for determination of naproxen and nabumetone in tablets. Sep. Sci. Plus 2022, 5. [CrossRef]
- Dinç, E.; Özdemir, A.; Aksoy, H.; Üstündaǧ, Ö.; Baleanud, D. Chemometric determination of naproxen sodium and pseudoephedrine hydrochloride in tablets by HPLC. Chem. Pharm. Bull. 2006, 54, 415–421. [CrossRef]
- Pushpa Latha, E.; Sailaja, B. Stability indicating rp-hplc method development and validation for the determination of naproxen sodium in bulk drug and tablet dosage form. Int. J. Pharm. Qual. Assur. 2020, 11, 525–529. [CrossRef]
- Ramos, P.; Raczak, B.K.; Silvestri, D.; Wacławek, S. Application of TGA/c-DTA for Distinguishing between Two Forms of Naproxen in Pharmaceutical Preparations. Pharmaceutics 2023, 15. [CrossRef]
- Kaynak, M.S.; Şahin, S. A new Hplc approach for determination of in-vitro solubility of naproxen sodium. Hacettepe Univ. J. Fac. Pharm. 2008, 28, 49–62.
- Yang, Y.; Xia, Y.; Wei, F.; Teng, G.; Yao, Y. Preparation and characterization of hydrophobic stearic acid-Yb-PbO2 anode and its application on the electrochemical degradation of naproxen sodium. J. Electroanal. Chem. 2020, 868. [CrossRef]
- Tanjin, S.; Islam, F.; Sultan, M.Z.; Rahman, A.; Chowdhury, S.R.; Sharmin, T.; Rashid, M.A. Development and Validation of a Simple RP-HPLC Method for Determination of Naproxen in Pharmaceutical Dosage Forms. Bangladesh Pharm. J. 2015, 16, 137–141. [CrossRef]
- Vittal, S.P.; Rao, S. V.; Ramakrishna, K. Stability indicating rp-hplc method for simultaneous determination of potential impurities of sumatriptan and naproxen sodium in fixed dose combination. Rasayan J. Chem. 2019, 12, 1601–1612. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).