1. Introduction
The bisindole alkaloids caulersin [
1] and caulerpine [
2] have been identified in various marine organisms, including the green algae
Caulerpa racemosa and
Caulerpa serrulata. Chemically, these compounds are characterized by two indole moieties arranged in an antiparallel configuration, connected via seven- and eight-membered rings, respectively. These alkaloids have demonstrated a broad spectrum of biological activities, including antifungal [
3], anticancer [
4], and antiviral properties [
5]. As a result, the development of efficient synthetic methodologies for generating structural analogues has attracted considerable interest. Given the structural features of caulersin and caulerpine, the design and synthesis of related analogues was undertaken. Betti bases obtained through the multicomponent Biginelli reaction were selected as a promising scaffold for this purpose. In this context, benzazoles were employed as imidazole isosteres [
6]. The Biginelli multicomponent reaction offers a highly efficient and environmentally friendly synthetic route, in alignment with the principles of green chemistry [
7].
The red spider mite (
Tetranychus urticae) is the most significant polyphagous species within the
Tetranychidae family, known to affect over 1,100 economically important plant species across a wide range of greenhouse and field crops [
8,
9]. This mite causes characteristic chlorotic spots on leaves by piercing plant tissues and extracting cellular contents [
10]. Under high population densities,
T. urticae can also invade flowers and fruits, significantly reducing stomatal resistance, photosynthetic rate, and transpiration, ultimately leading to decreased crop yields [
11,
12]. The management of phytophagous mites has become increasingly challenging due to the recurrent use of acaricides from the same toxicological class, which has led to the widespread development of resistance [
13]. As a result, current strategies emphasize the use of acaricides with novel modes of action or improved efficacy within existing chemical groups [
14]. Spirodiclofen, a spiroketoenolic compound, has shown high acaricidal activity by inhibiting acetyl-CoA carboxylase, thereby disrupting lipid biosynthesis [
15]. In this context, the synthesis of new chemical entities with enhanced activity and unique mechanisms of action is a key component in the development of next-generation mite control strategies. Recent advances in chemical synthesis have yielded novel derivatives with improved acaricidal properties, and numerous laboratory and field studies have reported their effectiveness in reducing short-term mortality and suppressing
T. urticae population densities.
The use of novel chemical derivatives has been successfully evaluated, demonstrating promising biological activity for the control of
T. urticae. For instance, [
16] reported that newly synthesized phenylpiperazine derivatives exhibited significant acaricidal activity against phytophagous mites. Similarly, other studies have shown that novel diamine compounds and phenyl trifluoroethyl thioether derivatives effectively increased short-term mortality in
T. cinnabarinus under laboratory conditions [
17,
18]
. Field studies have further demonstrated that N-substituted piperazine derivatives can efficiently control phytophagous mites, with effects persisting for up to two to three weeks [
19]. In this context, the present study focuses on the synthesis of Betti base analogues of the alkaloids caulersin and caulerpine via the multicomponent Biginelli reaction and the evaluation of their acaricidal activity against
T. urticae under laboratory conditions
.
2. Materials and Methods
2.1. Molecular Design
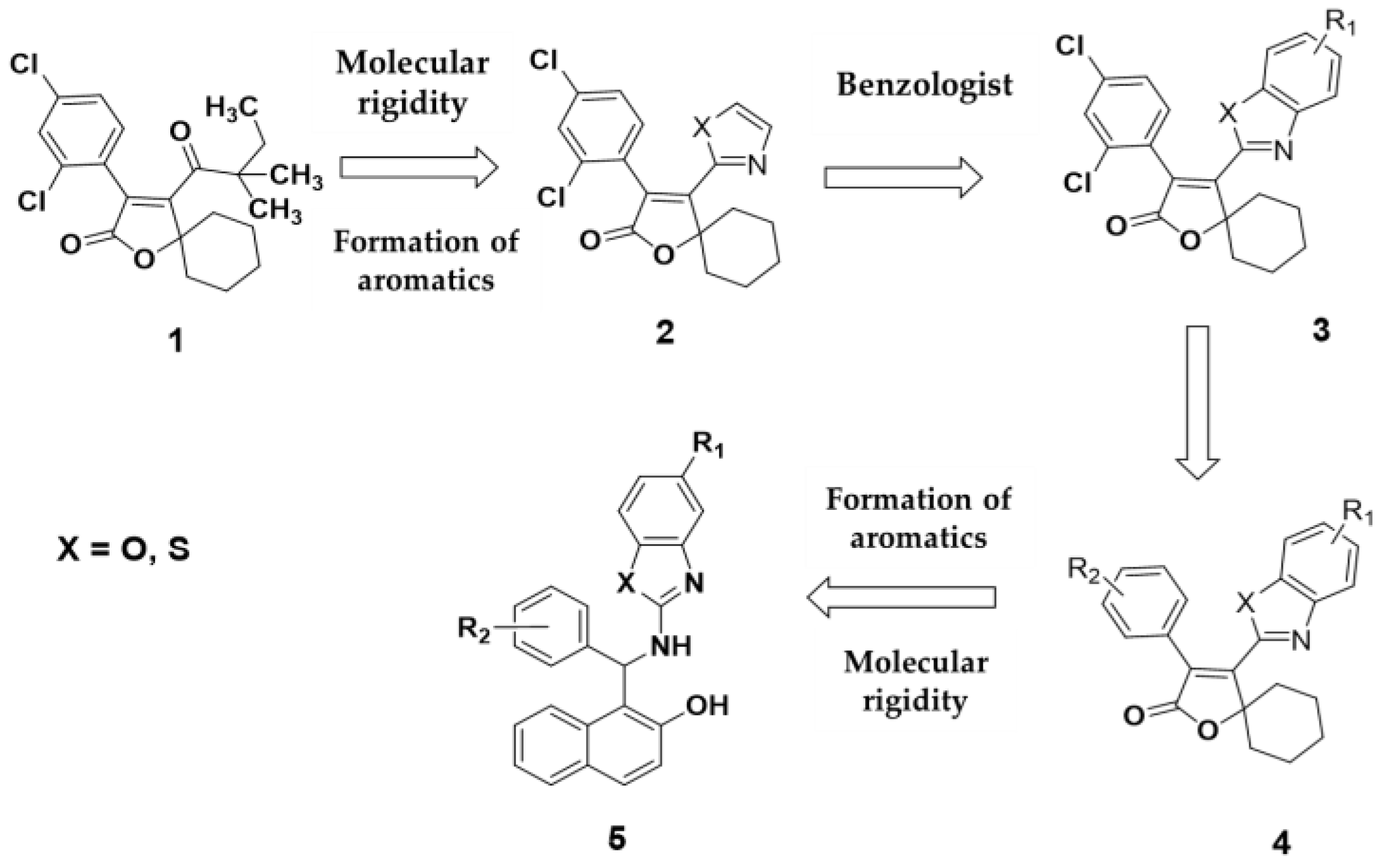
Spirodiclofen
1 has been shown to be an effective acaricide against mites. A key structural feature is the presence of spiroketoenolic rings, comprising a cyclohexyl group attached at the 5-position of a furanone ring. The central scaffold of the molecule is defined by the furanone moiety, with an aromatic ring substituted with two chlorine atoms in a meta configuration, and a 3,3-dimethylpentanone group at position 4. The design of spirodiclofen analogues was based on strategic structural modifications. The first modification targeted the substituent at position 4 of the furanone ring, where an azole fragment (oxazole or thiazole) was introduced to increase molecular rigidity, leading to the design of compound
2. Applying the principle of vinylology, compound
3 was proposed, incorporating benzazole systems (benzoxazoles and benzothiazoles). In parallel, compound 4 retained the phenyl group, but with substituents distinct from the original meta-chlorine configuration. Lastly, compound 5 replaced the spiroketoenol moiety with a 1-(aminomethyl) naphthalen-2-ol group. This study is grounded in the structural inspiration provided by the bisindole alkaloids caulersin and caulerpine (
Figure 1).
2.2. Betti Bases
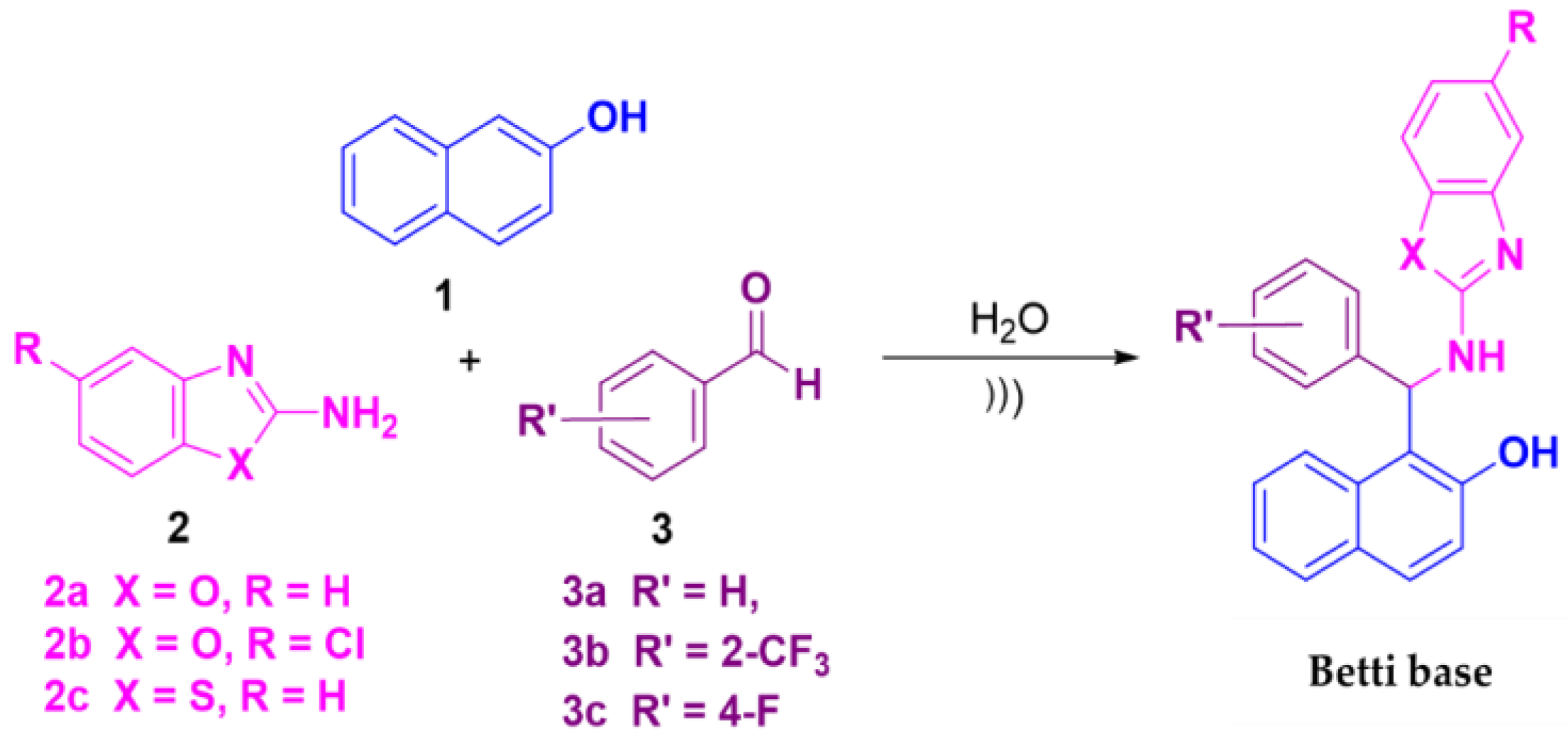
Betti bases were synthesized via a multicomponent Biginelli reaction involving 2-naphthol
1, 2-aminobenzazole derivatives
2 and benzaldehyde derivatives
3 (
Scheme 1). The 2-aminobenzazole derivatives included 2-aminobenzoxazole
2a, 2-amino-5-chlorobenzoxazole
2b and 2-aminobenzothiazole
2c. The aldehydes employed in the synthesis comprised benzaldehyde
3a, 2-trifluoromethylbenzaldehyde
3b and 4-fluorobenzaldehyde
3c. The reaction was performed under green chemistry conditions, using water as the solvent and ultrasound irradiation as a catalyst.
2.3. Establishment of a T. urticae Colony
Tetranychus urticae individuals were originally collected from papaya (Carica papaya L.) plants cultivated in the municipality of Conkal, Yucatán, México. A colony was established on healthy eggplant (Solanum melongena L.) plants. To initiate the colony, mite-infested papaya leaves were clipped and placed on the apical portions of 2-month-old eggplant plants maintained in a greenhouse at the Technological Institute of Conkal. Three weeks after the initial infestation, a stable T. urticae colony containing individuals at various developmental stages was available for use in the bioassays.
2.4. Preparation of the Tested Compounds
For the bioassays, stock solutions of Betti base derivatives were prepared and diluted in a DMSO (dimethyl sulfoxide) solution at a 1:1 ratio (w/v). Subsequently, the mixture was diluted in distilled water to yield a 0.05% (w/v) solution. Similarly, two commercial ketoenol compounds (spirodiclofen and spiromesifen) were utilised at label-recommended doses as controls.
2.5. Evaluation of Mortality of Adults and Nymphs of T. urticae
For adult and nymph mortality assays of T. urticae, the leaf-dip method was employed [21]. Eggplant (S. melongena) leaf discs, 5 cm in diameter, were individually immersed for 5 seconds in 250 mL beakers containing solutions of Betti base analogues of spirodiclofen. After immersion, the discs were air-dried at room temperature for 30 minutes. Each disc was then placed adaxial-side up on moistened cotton wool inside a Petri dish (9 cm in diameter, 1.5 cm deep). The edges of the discs were lined with moist cotton wool to prevent mite escape. Fifteen T. urticae adults or nymphs were transferred onto each disc. Mortality was recorded at 24, 48, and 72 hours post-treatment. Petri dishes were maintained under laboratory conditions at 24 ± 3°C with a photoperiod of 14 hours light and 10 hours dark. Mites were considered dead if they failed to respond to gentle stimulation with a fine brush. Each Petri dish represented one replicate, and ten replicates were conducted for each compound tested.
2.6. Evaluation of Ovicidal Activity
For the egg mortality assay, twenty adults T. urticae females were placed on 5 cm diameter eggplant (S. melongena) leaf discs positioned on moistened cotton wool in Petri dishes (9 cm in diameter). After 24 hours, all adults were removed, leaving the eggs deposited on the leaf surface. Excess eggs were carefully removed to standardize the number to 20 eggs per disc. The leaf discs containing the eggs were gently lifted with forceps and immersed for 5 seconds in the respective Betti base solutions. After treatment, the discs were air-dried at room temperature for 30 minutes and returned to their original Petri dishes. The dishes were maintained at room temperature (24-30 °C) for six days. Egg mortality was assessed at the end of this period, with non-hatched eggs considered dead. Each Petri dish was treated as one replicate, and ten replicates were conducted for each compound tested [22].
2.7. Statistical Analysis
A completely randomized design was used for all experiments. Data were subjected to analysis of variance after checking for normality and homoscedasticity (Shapiro-Wilk test). Effects were considered statistically significant if P < 0.05. All analyses were performed using the Statgraphics statistical package.
3. Results
3.1. Betti Bases
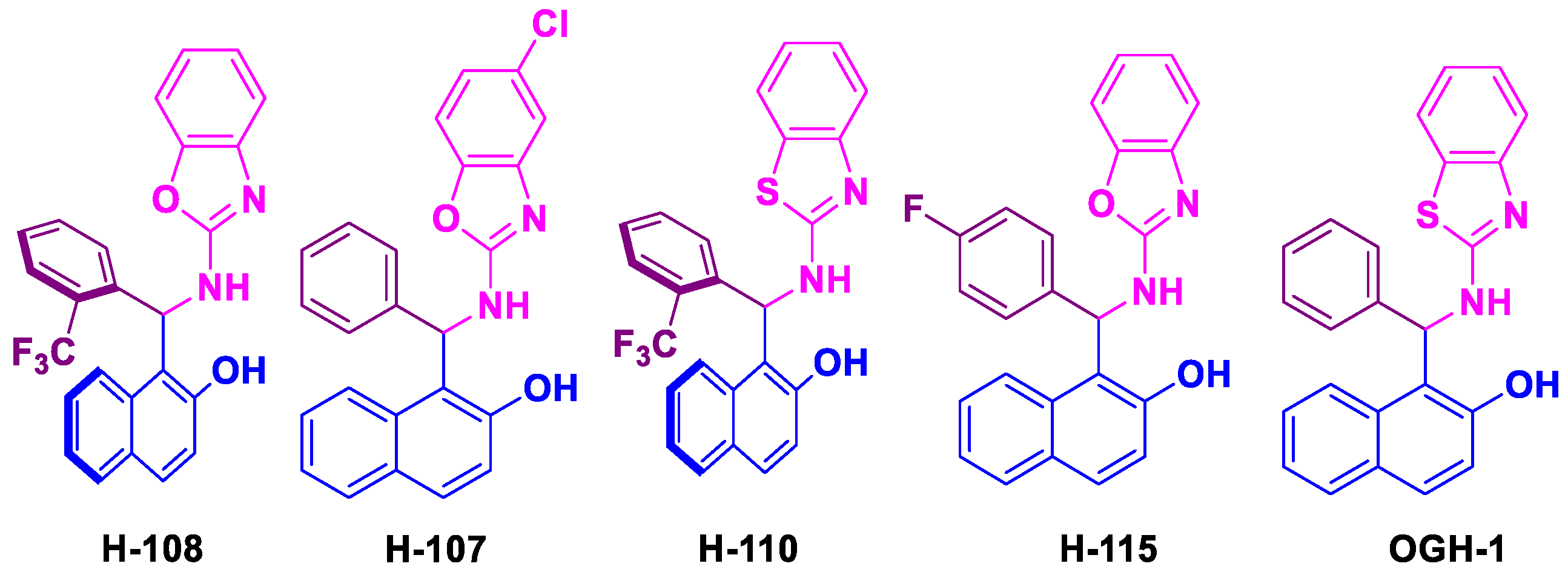
Betti bases were obtained in chemical yields exceeding 80% following purification by recrystallization (
Figure 2).
3.2. Toxicity of Acaricidal Compounds in Adults and Nymphs of T. urticae
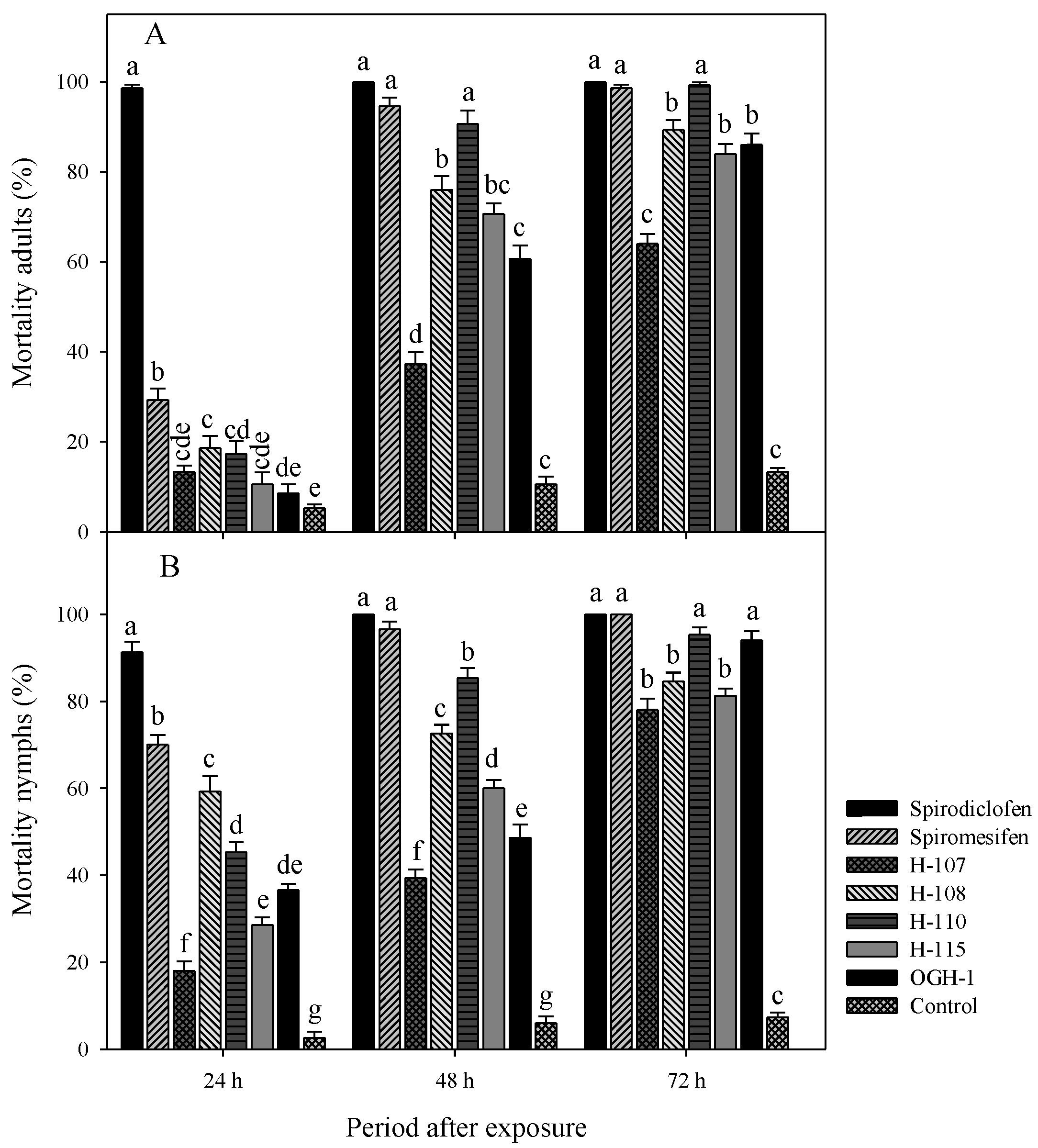
The acaricidal activity of spirodiclofen Betti bases against
T. urticae adults showed statistically significant differences. At 24 hours (F=209.89, gl=7, 72, p < 0.0001), Betti base H-108 exhibited the highest mortality rate (18.6%). At 48 hours (F=155.07, gl=7, 72, p < 0.0001), Betti bases H-108, H-115, and H-110 caused mortality rates ranging from 70% to 90%. By 72 hours post-treatment (F=284.18, gl=7, 72, p < 0.0001), H-108, H-110, H-115, and OGH-1 showed the highest efficacy, with mortality rates between 70% and 99% (
Figure 3A).
Similarly, significant differences were observed in the mortality of nymphs. At 24 hours (F=160.15, gl=7, 72, p < 0.0001), Betti base H-108 caused the highest mortality (59.3%). At 48 hours (F=239.00, gl=7, 72, p < 0.0001), H-108 and H-110 induced mortality rates exceeding 70%. By 72 hours (F=327.57, gl=7, 72, p < 0.0001) the most effective compounds were H-108, H-110, H-115, and OGH-1, achieving mortality rates between 80% and 95% (
Figure 3B).
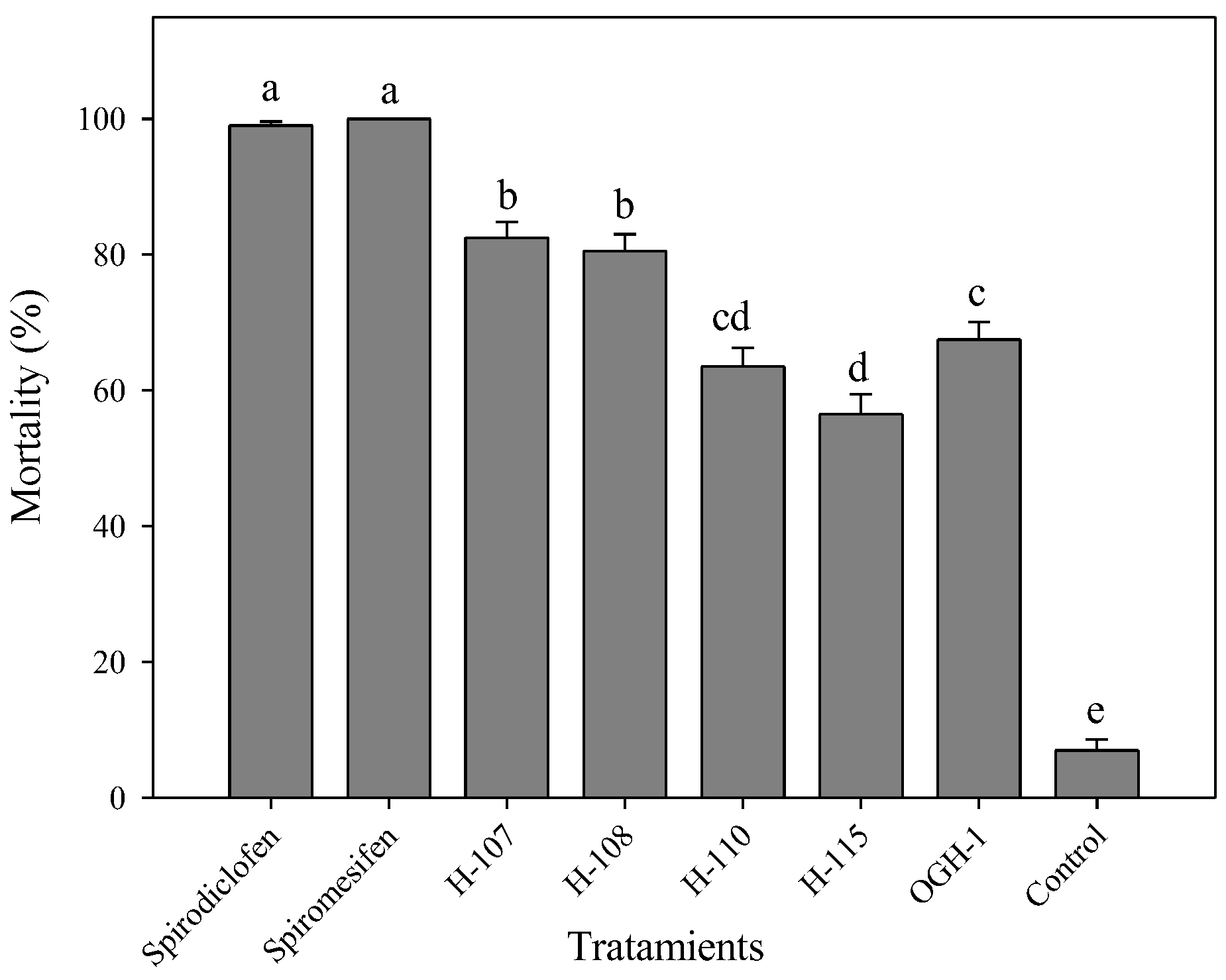
3.3. Ovicidal Activity of Acaricidal Compounds
All spirodiclofen Betti bases exhibited ovicidal activity against
T. urticae eggs, with statistically significant differences among treatments (F=179.68, gl=7, 72,
p < 0.0001). The most effective compounds were H-107 and H-108, both causing egg mortality rates greater than 80%. These were followed by H-110 and OGH-1, which produced approximately 60% mortality. The least effective was H-115, with a mortality rate of 56% (
Figure 4).
4. Discussion
Using a simple and efficient methodology based on a Biginelli-type multicomponent reaction—consistent with the principles of Green Chemistry—we successfully synthesized Betti base analogues of spirodiclofen with high chemical yields. The present study evaluated the acaricidal activity of these analogues against Tetranychus urticae adults, nymphs, and eggs. In adult assays, compounds H-108, H-110, H-115, and OGH-1 exhibited the highest mortality rates, ranging from 70% to 99% at 48- and 72-hours post-application. Similar patterns were observed for nymphs, with the same compounds producing 80% to 95% mortality at 72 hours. In ovicidal evaluations, all compounds demonstrated significant effects, with H-107 and H-108 achieving mortality rates exceeding 80%. The short-term efficacy of acaricidal compounds is a critical factor in the management of phytophagous mites. In this context, the Betti base analogues derived from spirodiclofen represent promising alternatives for mite control, demonstrating high activity within the first 72 hours of application. Recent studies support this finding, highlighting those structural modifications of spirodiclofen derivatives can enhance acaricidal activity and provide rapid control of phytophagous mite populations [23,24].
One of the key attributes of the newly synthesized acaricidal derivatives is their rapid mode of action. In the bioassays conducted on adults and nymphs of T. urticae, spirodiclofen-derived Betti bases H-108, H-110, H-115, and OGH-1 induced mortality rates exceeding 80% within 72 hours post-exposure. While the current literature on novel spirodiclofen derivatives remains limited, recent studies have reported that such compounds can achieve approximately 60% mortality in T. cinnabarinus adults [25]. Additionally, pyridine-spiro derivatives structurally based on spirodiclofen have shown promising acaricidal activity, with mortality rates above 90% in T. urticae after 72 hours of exposure [26]. Our findings underscore the effectiveness of the new Betti base derivatives against T. urticae, which may be attributed to favorable structure-activity relationships and enhanced structural optimization. These characteristics are of particular importance in the context of designing next-generation acaricides to address the growing demand in agricultural pest management.
5. Conclusions
The Betti bases were successfully synthesized using a green chemistry approach via the Biginelli multicomponent reaction, yielding high chemical efficiency. Among these, H-108, H-110, H-115, and OGH-1 demonstrated strong acaricidal effects on adults and nymphs, while H-107 and H-108 were the most effective in ovicidal assays. The observed activity across multiple developmental stages suggests that these compounds may serve as promising candidates for integrated pest management strategies. Future research should focus on the further evaluation of these and other Betti base derivatives, including field trials and mechanistic studies, to fully assess their potential as environmentally friendly acaricides for the control of phytophagous mites.
Author Contributions
Conceptualization, M.C.-B., E.H.-N., and H.P.-X.; methodology, M.C.-B., M.C.E.-C., A.M.H.-G., E.H.-N, and H.P.-X.; Formal analysis, M.C.-B., A.C.-D., E.H.-N., and H.P.-X.; investigation, J.Q.G.-M., M.L.O., and E.H.-N.; resources, E.H.-N., M.J.-R., and E.R.-S.; data curation, M.C.-B., A.C.-D., E.H.-N, and H.P.-X.; writing—original draft preparation, M.C.-B., M.C.E.-C., H.P.-X., E.H.-N, and R.M.-L.; writing: review and editing, E.H.-N., R.M.-L., M.J.-R., and A.M.H.-G.; supervision, E.H.-N., J.Q.G.-M., M.L.O., and E.R.-S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding
Data Availability Statement
The data presented in this study are available upon request from the first and corresponding authors.
Acknowledgments
M.C.-B and H.P-X thanks gratefully acknowledges the support of the Secretariat of Science, Humanities, Technology and Innovation of Mexico (SECIHTI) for Postdoctoral Scholarship 77475 and 592119.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Su, J.Y.; Zhu, Y.; Zeng, L.M.; Xu, X.H. A new bisindole from alga Caulerpa Serrulata. J. Nat. Prod. 1997, 60, 1043–1044. [Google Scholar] [CrossRef]
- Aguilar-Santos, G. Caulerpin, a new red pigment from green Aalgae of the genus Caulerpa. J. Chem. Soc. (C). 1970, 6, 842–843. [Google Scholar] [CrossRef]
- Liu, D.Q.; Mao, S.C.; Yu, X.Q.; Feng, L.H.; Lai, X.P. Caulerchlorin, a novel chlorinated bisindole alkaloid with antifungal activity from the chinese green alga Caulerpa Racemosa. Heterocycles, 2012, 85, 661–666. [Google Scholar] [CrossRef]
- Mehra, R.; Bhushan, S.; Bast, F.; Singh, S. Marine macroalga Caulerpa: role of its Metabolites in modulating cancer signaling. Mol. Biol. Rep. 2019, 46, 3545–3555. [Google Scholar] [CrossRef]
- Esteves, P.O.; De Oliveira, M.C.; De Souza Barros, C.; Cirne-Santos, C.C.; Laneuvlille, V.T.; Palmer, P.IC. Antiviral Effect of Caulerpin Against Chikungunya.” Nat. Prod. Commun. 2019, 14, 1934578X19878295. [CrossRef]
- Mignani, S.; Majoral, J.P.; Desaphy, J.F.; Lentini, G. From riluzole to dexpramipexole via substituted-Benzothiazole derivatives for amyotrophic lateral sclerosis disease treatment: Case studies. Molecules. 2020, 25, 3320. [Google Scholar] [CrossRef]
- Kappe, C.O. Recent Advances in the Biginelli Dihydropyrimidine Synthesis. New Tricks from an Old Dog. Acc.Chem. Res. 2000, 33, 879–888. [Google Scholar] [CrossRef] [PubMed]
- Kumari, S.; Chauhan, U.; Kumari, U.; Nadda, G. Comparative toxicities of novel and conventional acaricides against different stages of Tetranychus urticae Koch (Acarina: Tetranychidae). J. Saudi Soc. Agric. Sci. [CrossRef]
- Adesanya, A.W.; Beauchamp, M.J.; Lavine, M.D.; Lavine, L.C.; Zhu, F.; Walsh, D.B. Physiological resistance alters behavioral response of Tetranychus urticae to acaricides. Sci Rep. 2019, 9, 19308. [Google Scholar] [CrossRef]
- Schmidt-Jeffris, R.; Coffey, J.L.; Miller, G.; Farran, M. 2021. Residual activity of acaricides for controlling spider mites in watermelon and their impacts on resident predatory mites. J. Economic. Entomol. 2021, 114, 818–827. [Google Scholar] [CrossRef]
- Uddin, N.; Alam, Z.; Miah, U.R.; Hossain, M.I.; Mustarin, K.E. Toxicity of pesticides to Tetranychus urticae Koch (Acari: Tetranychidae) and their side effects on Neoseiulus californicus (Acari: Phytoseiidae). Int. J. Acarol. 2015, 41, 688–693. [Google Scholar] [CrossRef]
- Montoya, A.; Galano-Flores, G.; Rodríguez, H.; Franco, A.A.; Zardi, O.Z.; Yamamoto, P.T. Toxicity of acaricides on Tetranychus urticae (Koch) in the laboratory. Rev. Prot. Veg. 2017, 32, 60–67. [Google Scholar]
- Zhao, J.H.; Wang, Z.C.; Ji, M.H.; Cheng, J.L.; Zhu, G.N.; Yu, C.M. Synthesis and bioactivity evaluation of novel spiromesifen derivatives. Pest Manag Sci. 2012, 68, 10–5. [Google Scholar] [CrossRef] [PubMed]
- Van Leeuwen, T.; Tirry, L. , Yamamoto, A.; Nauen, R.; Dermauw, W. The economic importance of acaricides in the control of phytophagous mites and an update on recent acaricide mode of action research. Pest. Biochem. Physiol. 2014, 121, 12–21. [Google Scholar] [CrossRef]
- Badieinia, F.; Khajehali, J.; Nauen, R.; Dermauw, W.; Van Leeuwen, T. Metabolic mechanisms of resistance to spirodiclofen and spiromesifen in Iranian populations of Panonychus ulmi. Crop Prot. 2020, 134, 105166. [Google Scholar] [CrossRef]
- Suzuki, J.; Ootaka, A.; Onoue, S.; Onoue, M. Synthesis and acaricidal activity of phenylpiperazine derivatives. J. Pestic. Sci. 2021, 46, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.Y.; Feng, T.; Liu, Q.; Li, H.T.; Wei, W.; Shi, R.C.; Cao, Y.M.; Liu, S.Z. Design, synthesis, fungicidal and insecticidal activities of novel diamide compounds combining pyrazolyl and polyfluoro-substituted phenyl into alanine or 2-Aminobutyric Acid Skeletons. Molecules. 2023, 28, 561. [Google Scholar] [CrossRef]
- Liu, D.; Zhang, J.; Gao, Y.; Hao, H.; Zhang, C.; Wang, F.; Zhang, L. Synthesis, acaricidal activity, and structure-activity relationships of novel phenyl trifluoroethyl thioether derivatives containing substituted benzyl groups. Pest Manag. Sci. 2024, 80, 544–553. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Xu, Y.; Liu, C.; Guan, A.; Ban, L.; Ding, F.; Peng, W. Intermediate derivatization method in the discovery of new acaricide candidate: synthesis of N-substituted piperazine derivatives and their activity against phytophagous mites. Pest Manag. Sci. 2016, 73, 945–952. [Google Scholar] [CrossRef]
- Cardellicchio, C.; Ciccarella, G.; Naso, F.; Schingaro, E.; Scordari, F. The Betti Base: Absolute configuration and routes to a family of related chiral nonracemic Bases. Tetrahedron-Asymmetry. 1998, 9, 3667–3675. [Google Scholar] [CrossRef]
- Cua-Basulto, M.E.; Ruiz-Sánchez, E.; Chan-Cupul, W.; Reyes-Ramírez, A.; Ballina-Gómez, H.; Hernández Núñez, E.; Martin-Mex, R.; Herrera Gorocica, A.M.; Ruiz-Jiménez, A.L. Effects of botanical acaricides on Tetranychus urticae and compatibility with the predatory mite Amblyseius swirskii. Archiv. Phytopathol. Plant Prot. 2023, 56, 1359–1371. [Google Scholar] [CrossRef]
- Cua-Basulto, M.; Ruiz-Sánchez, E.; Chan-Cupul, W.; Reyes-Ramírez, A.; Ballina-Gómez, H.; Hernández-Núñez, E. Efectos de los acaricidas químicos sobre la mortalidad de la araña de dos manchas Tetranychus urticae koch (Acari: Tetranychidae). Trop. Subtrop. Agroecosyt. 2022, 25, 040. [Google Scholar]
- Ke, S.; Ke, T.; Zhang, Z.; Shi, L.; Huang, D.; Wang, K.; Yang, Z.; Yang, N. Acaricidal activity and structure-activity relationships of Spiro-Butyrolactone derivatives against Tetranychus cinnabarinus. J. Asia-Pac. Entomol. [CrossRef]
- Li, S.; Lv, M.; Li, T.; Hao, M.; Xu, H. Spirodiclofen ether derivatives: semisynthesis, structural elucidation, and pesticidal activities against Tetranychus cinnabarinus Boisduval, Aphis citricola Van der Goot and Mythimna separata Walker. Pest Manag. Sci. 2021, 77, 2395–2402. [Google Scholar] [CrossRef] [PubMed]
- Zhong, L.K.; Yuan, J.; Liu, X.H.; Xu, T.M.; Tan, C.X. Synthesis and acaricidal activity of Aryl-Spirobutyrolactone derivatives against spider mites under greenhouse and field Conditions. Russ. J. Bioorg. Chem. 2021, 47, 221–229. [Google Scholar] [CrossRef]
- Yu, L.; Gua, S.; Wang, Y.; Liao, A.; Zhang, W.; Sun, P.; Wu, J. Design, synthesis, and bioactivity of spiro derivatives containing a pyridine moiety. J. Agric. Food Chem. 2022, 70, 15726–15736. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).