1. Case Presentation:
Our patient was a 54-year-old gentleman with long-standing type 1 diabetes complicated by diabetic nephropathy, neuropathy, and retinopathy as well as hypertension, dyslipidemia, and peripheral vascular disease with remote right great toe amputation.
He underwent simultaneous pancreas and pre-emptive kidney transplantation from a deceased donor following neurological determination of death (NDD). A 12-16 cm section of duodenum was transplanted alongside the donor pancreas. Serostatus was CMV donor seronegative and recipient seronegative, EBV donor seropositive and recipient seropositive. Kidney donor profile index (KDPI) score was 18%. Donor specific antibodies were not detected at time of transplant. Induction of immunosuppression was with rabbit anti-thymocyte globulin (ATG) and methylprednisolone. He received 50 mg of ATG and 250 mg of methylprednisolone IV at the time of surgery followed by a prednisone taper. In total, he received 350 mg (5.8 mg/kg) of rabbit ATG. Mycophenolate sodium (Myfortic®) 720 mg oral q. 12 hours was initiated on post-transplant day (PTD) 1. Tacrolimus (Advagraf®) 10 mg oral daily was initiated on PTD 5 and the dose titrated based on levels.
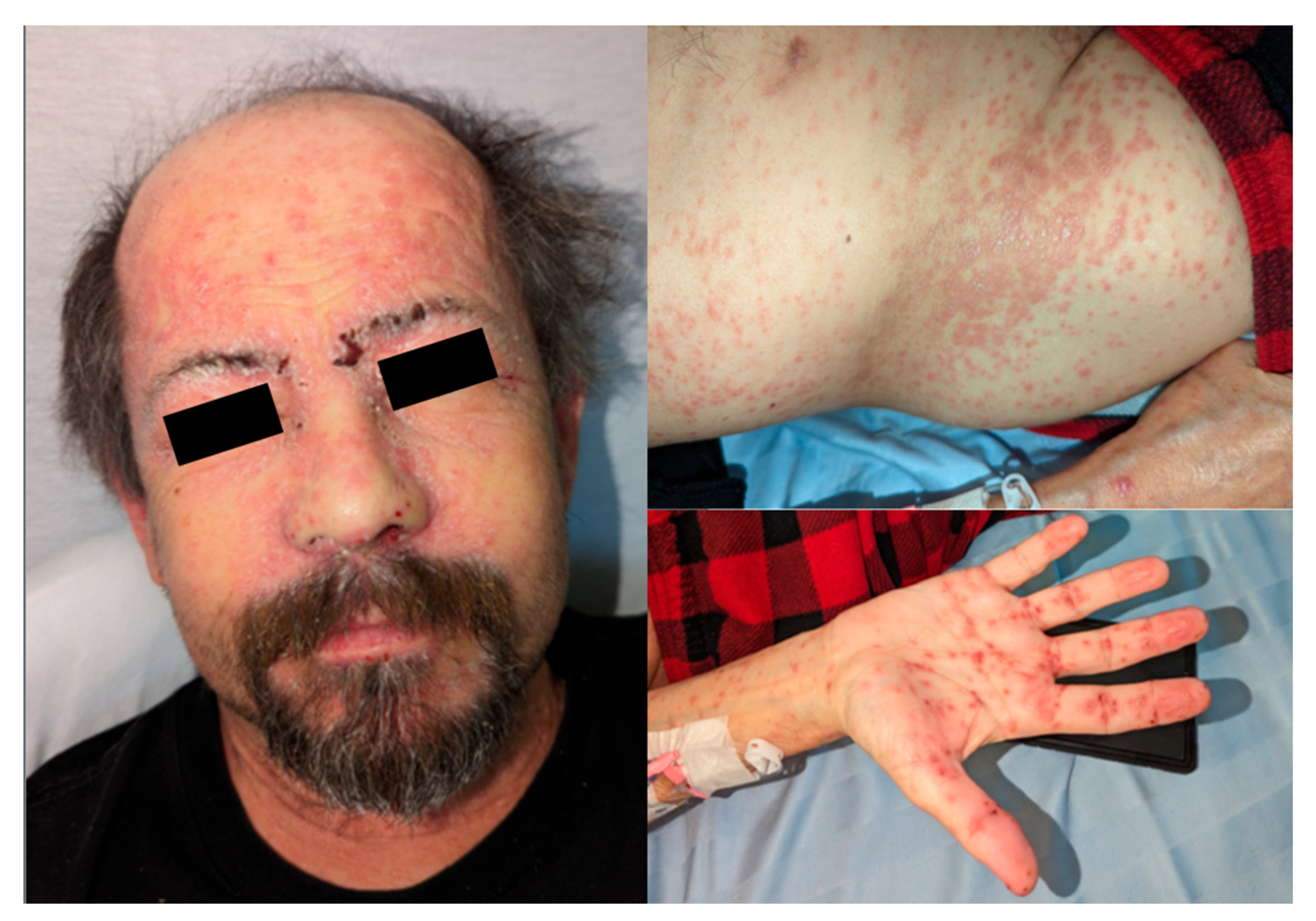
Approximately 3 months post-transplant, the patient developed an erythematous papular eruption, starting on his face and scalp. Over the subsequent 1-2 days, he noted craniocaudal spread of these lesions down the torso and all four extremities including the right palm. There was no oral ulceration or any other mucosal involvement. The lesions were mildly pruritic but not painful and at no point did the patient recall any vesicles or pustules. When the rash did not resolve he notified his surgical team, who suspected disseminated herpes zoster infection and prescribed acyclovir 200 mg oral five times per day for 5 days. He was admitted to hospital on PTD 87 and assessed by the Transplant Infectious Diseases service the following day. Photographs of his lichenoid eruption at that time are shown in
Figure 1. Characteristic lesions were noted to be well-demarcated, non-tender, shiny, reddish-purple polygonal flat-topped papules, some with fine overlying scale. Admission blood work on PTD 87 was notable for elevated transaminases and peripheral eosinophilia, as shown in
Table 1. An extensive infectious workup was also undertaken at the same time, which was non-contributory. Dermatology was also consulted and suspected lichen planus, lichenoid drug eruption, or lichenoid chronic GVHD at that time. A skin punch biopsy was performed on PTD 88, which showed epidermis with focal hyperkeratosis and no parakeratosis, interface lichenoid chronic inflammatory cells with focal vacuolar interface changes and scattered dyskeratotic keratinocytes, with inflammation extending to the upper dermis, overall favoring graft-versus-host disease of the skin. He was started on betamethasone valerate 0.05% topical ointment with significant improvement in his skin lesions.
He was seen in the outpatient Transplant Infectious Diseases clinic on PTD 106 and noted at that time to have worsening liver enzyme elevation and new neutropenia, as shown in
Table 1. Given this, a percutaneous liver biopsy was done on PTD 113. This showed cell dropout and ischemic changes in zone 3 with duct injury and early ductopenia, with these changes felt to be consistent with SOT-GVHD.
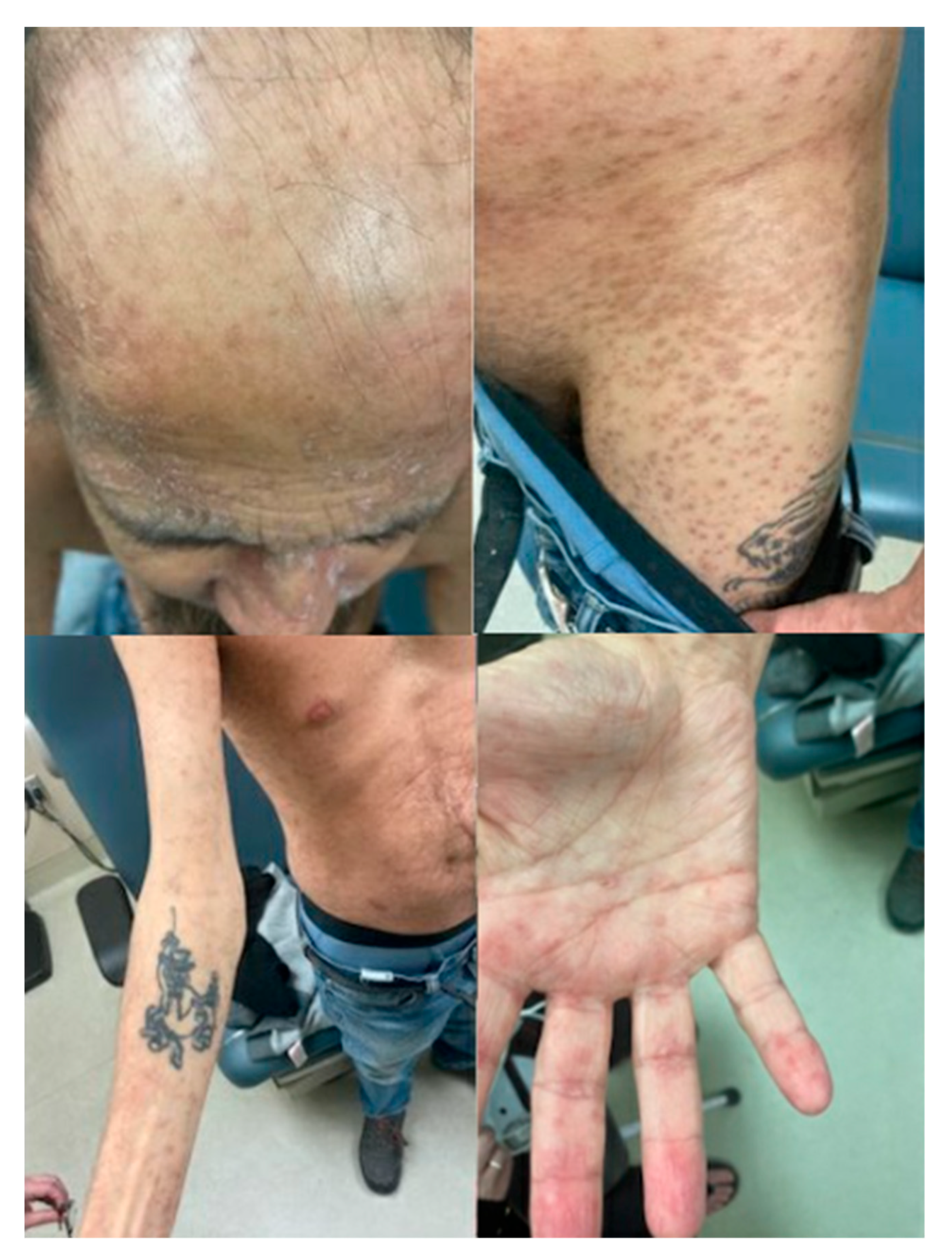
Outpatient follow up with Dermatology took place on PTD 111, at which time he had few active lesions and mainly post-inflammatory hyperpigmentation changes to the previous lesions. The appearance of the skin lesions at that time are shown in
Figure 2. Another skin punch biopsy was performed again showing a superficial vacuolar interface dermatitis with a predominantly lymphohistiocytic inflammatory infiltrate as well as some melanophages in keeping with post-inflammatory hyperpigmentation. Immunofluorescence was negative and unfortunately the sample was lost before microcherism testing could be performed.
A bone marrow biopsy performed on PTD 115 demonstrated normocellular marrow with relative erythroid predominance, immature myeloid cells present with normal blast percentage, rare mature granulocytes, and normal-appearing megakaryocytes. Microchimerism assay was performed using multiplex PCR amplification of Amelogenin locus and 15 micro-satellite markers (D8S1179, D21S11, D7S820, CSF1PO, D3S1358, TH01, D13S317, D16S539, D2S1338, D19S433, vWA, TPOX, D18S51, D5S818 and FGA) to compare the recipient bone marrow aspirate to a banked DNA sample from the organ donor. This showed 43% of the CD3+ T-cells, 2% of the CD19+ B-cells, and <2% of the myeloid cells were of donor origin.
Given all of these findings, a diagnosis of SOT-GVHD was made. On PTD 125, he was started on prednisone 30 mg oral daily (0.5 mg/kg) for 4 weeks. As the mortality from SOT-GVHD is primarily driven by the associated bone marrow suppression and secondary infections, he was also started on granulocyte colony stimulating factor (G-CSF) as well as prophylactic acyclovir 400 mg oral twice daily and levofloxacin 750 mg oral daily. The patient responded very well to this treatment, with significant improvement in his transaminitis and cytopenias by PTD 153, as shown in
Table 1. The G-CSF, acyclovir, and levofloxacin were therefore discontinued on PTD 153, and a slow prednisone taper was also initiated at that time, reducing the daily dose by 5 mg every 14 days until 5 mg oral daily, then to continue on this dose indefinitely.
2. Discussion:
Although a well-known and common complication of hematopoietic stem cell transplants, graft-versus-host-disease (GVHD) was only first reported in an orthotopic liver transplant recipient by Burdick and colleagues in 1988 [
1]. In the solid organ transplant setting, GVHD is thought to occur due to donor-derived immunocompetent lymphocytes within the transplanted organ, which manage to evade the recipient’s immune system and then encounter recipient alloantigens, causing the donor lymphocytes to mount an immune response against the recipient’s tissues [
2,
3]. Thus, the risk of SOT-GVHD is felt to be higher for transplanted organs which contain larger amounts of viable lymphoid tissue such as the small intestine and liver when compared with kidney, heart, and lung [
4]. Pancreas transplant recipients are also at an increased risk of this complication as a small portion of the donor’s duodenum is routinely implanted alongside the donor pancreas [
5], although this risk may be underrecognized by clinicians. Other established risk factors for SOT-GVHD include HLA mismatch, re-transplantation, large graft volume, donor-recipient age and race mismatch, and pre-existing immunodeficiency in the recipient [
4].
SOT-GVHD most commonly occurs in the first 2-12 weeks post-transplant [
6] . Manifestations of SOT-GVHD are varied: cutaneous, gastrointestinal, hepatic, bone marrow, and oropharyngeal mucosal involvement have all been well-described in the literature, and multi-system involvement is common [
4]. Bone marrow involvement is thought to be unique to SOT-GVHD, and hemophagocytic lymphohistiocytosis (HLH) may also be seen [
7]. Fever and other non-specific symptoms such as nausea, vomiting, anorexia, and malaise can also occur [
6]. Cutaneous involvement is reported to be the most common and often earliest presentation [
8], although the rash is often non-specific, with maculopapular, morbilliform, desquamating, and confluent skin eruptions all reported in case series [
9]. Lichenoid eruptions, as was seen with our patient, have been noted to be more common in SOT-GVHD than GVHD following allogeneic stem cell transplant [
10].
The signs and symptoms of SOT-GVHD may be mistaken for more commonly encountered infections or medication side effects, leading to delays in diagnosis [
4]. Biopsies of the affected organs can show characteristic histopathologic findings and remain the gold standard for diagnosis [
11]. Chimerism assays and FISH analysis may confirm the persistence of donor cells and support the diagnosis; these modalities are also being explored for early disease detection and risk stratification [
12].
The optimal management of SOT-GVHD has not yet been determined. Systemic and topical corticosteroids are generally the first line of therapy, with the addition of ATG, alemtuzumab, and ruxolitinib in steroid-refractory cases [
4]. For those with bone marrow involvement not responding to steroids, allogeneic bone marrow transplant may be considered [
4]. Antimicrobial prophylaxis and G-CSF support is recommended for those with bone marrow involvement, noting that mortality in SOT-GVHD is often attributable to secondary infections [
4].
Acknowledgments/Funding
The authors wish to thank the patient for allowing us to share his story.
Disclosures
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
Abbreviations:
ALT: Alanine aminotransferase
AST: Aspartate aminotransferase
ATG: Anti-thymocyte globulin
CD: Clusters of differentiation
CMV: Cytomegalovirus
EBV: Ebstein-Barr virus
FISH: Fluorescence in-situ hybridization
GCSF: Granulocyte colony stimulating factor
GVHD: Graft-versus-host disease
HLH: Hemophagocytic lymphohistiocytosis
IF: Immunofluorescence
IV: Intravenous
KDPI: Kidney donor profile index
NDD: Neurologic determination of death
PTD: Post-transplant day
SOT: Solid organ transplant(ation)
SOT-GHVD: Solid organ transplant graft-versus-host disease
References
- Burdick JF, Vogelsang GB, Smith WJ, et al. Severe Graft-versus-Host Disease in a Liver-Transplant Recipient. New England Journal of Medicine. 1988;318(11):689-691. [CrossRef]
- Starzl TE, Demetris AJ, Murase N, Thomson AW, Trucco M, Ricordi C. Donor cell chimerism permitted by immunosuppressive drugs: a new view of organ transplantation. Immunol Today. 1993;14(6):326-332.
- Zhang Y, Ruiz P. Solid organ transplant-associated acute graft-versus-host disease. Arch Pathol Lab Med. 2010;134(8):1220-1224. [CrossRef]
- Cooper JP, Abkowitz JL. How I diagnose and treat acute graft-versus-host disease after solid organ transplantation. Blood. 2023;141(10):1136-1146. [CrossRef]
- Hampson FA, Freeman SJ, Ertner J, et al. Pancreatic transplantation: surgical technique, normal radiological appearances and complications. Insights Imaging. 2010;1(5-6):339. [CrossRef]
- Smith EP. Hematologic Disorders after Solid Organ Transplantation. Hematology. 2010;2010(1):281-286. Accessed October 25, 2024. http://ashpublications.org/hematology/article-pdf/2010/1/281/1492296/bep00110000281.pdf.
- Newell LF, Dunlap JB, Gatter K, et al. Graft-Versus-Host Disease after Solid Organ Transplant Is Frequently Associated with Bone Marrow Failure and Hemophagocytosis. Biology of Blood and Marrow Transplantation. 2020;26(3):S194. [CrossRef]
- Assi MA, Pulido JS, Peters SG, Mccannel CA, Razonable RR. Graft-vs.-host disease in lung and other solid organ transplant recipients. Clin Transplant. 2007;21(1):1-6. [CrossRef]
- Kim GY, Schmelkin LA, Davis MDP, et al. Dermatologic manifestations of solid organ transplantation–associated graft-versus-host disease: A systematic review. J Am Acad Dermatol. 2018;78(6):1097-1101.e1. [CrossRef]
- Russell AJ, Musiek AC, Staser KW, Rosman IS. Histopathologic and immunophenotypic features of cutaneous solid organ transplant-associated graft-vs-host disease: Comparison with acute hematopoietic cell transplant-associated graft-vs-host disease and cutaneous drug eruption. J Cutan Pathol. 2021;48(12):1480-1488. [CrossRef]
- Zhang Y, Ruiz P. Solid Organ Transplant-Associated Acute Graft-Versus-Host Disease. Am J Clin Pathol. 2010;134(8):1220-1224.
- Ghandorah S, Alsawaf B, Alrefai W, Ghazwani A, Almusabi S. Role of HLA typing and chimerism analysis in post-liver transplantation GVHD in pediatrics: A systematic review. Pediatr Transplant. 2022;26(8):e14381. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).