1. Introduction
Cervical cancer is a preventable disease, with persistent infection by oncogenic human papillomavirus (HPV) types recognized as its necessary cause [
1]. As a result, HPV testing has become central to cervical cancer prevention strategies, gradually replacing cytology in primary screening programs due to its superior sensitivity in detecting high-grade cervical intraepithelial neoplasia (CIN2+) and cancer [
2]. However, the reduced specificity of HPV DNA-based tests, particularly among younger women with transient infections, remains a clinical challenge, often leading to unnecessary colposcopy referrals and overtreatment [
3].
To address this, several triage strategies have been proposed for HPV DNA-positive women. Cytology remains the current standard, but its inherent subjectivity and limited reproducibility constrain its effectiveness. Partial genotyping offers some improvement by identifying high-risk types, yet it does not capture the transcriptional activity that drives disease progression. More recently, additional molecular-based triage strategies, including dual-stained cytology and DNA methylation markers, have been investigated to improve objectivity and reproducibility [
4,
5]. However, some of these approaches depend on cytological infrastructure or have shown reduced specificity in real-world settings [
4,
6].
As self-sampling gains traction in organized screening programs, triage modalities that can be applied to self-collected samples are increasingly important [
7]. This has spurred growing interest in HPV mRNA testing, which detects active transcription of the E6 and E7 viral oncogenes—key drivers of malignant transformation. Unlike HPV DNA detection, which reflects exposure, mRNA testing identifies transcriptionally active, clinically relevant infections and may offer improved risk stratification [
8,
9,
10,
11,
12].
The PreTect HPV-Proofer 7 is a genotype-specific mRNA test targeting E6/E7 transcripts from the seven most oncogenic HPV types (16, 18, 31, 33, 45, 52, and 58), all of which are included in the nonvalent HPV vaccine and account for the majority of cervical cancer cases worldwide. Previous studies have demonstrated that HPV mRNA testing provides higher specificity and positive predictive value (PPV) than cytology or HPV DNA genotyping in triage settings [
13,
14,
15].
Building on prior data from the initial implementation phase, this study provides an expanded analysis of triage performance over five years of routine screening. We evaluated the performance of the 7-type HPV mRNA test as a triage tool for women testing positive for high-risk HPV DNA during primary screening. Sensitivity, specificity, and PPV for CIN2+ were compared to liquid-based cytology with atypical squamous cells of undetermined significance or worse (≥ASC-US), using data from a large population-based screening cohort in Northern Norway. Our findings aim to inform triage strategies that optimize risk stratification, reduce unnecessary procedures, and support personalized management in cervical cancer prevention.
2. Materials and Methods
2.1. Study Design and Population
This quality-assurance cohort study was embedded in the Norwegian Cervical Cancer Screening Programme (NCCSP) and conducted at the Department of Clinical Pathology, University Hospital of North Norway (UNN), Tromsø. The study design, inclusion criteria, and screening procedures have been described in detail in a previous publication covering the initial implementation period from 2019 to 2021 [
14]. The present analysis represents an updated and expanded evaluation, extending the study period through 31 December 2023, with follow-up for histologically confirmed outcomes through 31 October 2024. Eligibility criteria and exclusion parameters remained consistent with those used in the earlier cohort.
2.1.1. Primary HPV DNA Screening
Samples were collected in PreservCyt® solution (ThinPrep®, Hologic) and analyzed using the Cobas 4800 HPV DNA assay (Roche Diagnostics). This assay individually detects HPV genotypes 16 and 18 and reports a pooled result for 12 other high-risk (HR) HPV types.
2.1.2. Triage Procedures
Women who tested positive for high-risk HPV DNA underwent two parallel triage assessments, both performed using residual material from the same PreservCyt® sample:
Liquid-Based Cytology (LBC): Cytological evaluation was conducted according to the 2014 Bethesda system. A result of atypical squamous cells of undetermined significance or worse (≥ASC-US) was considered positive;
7-Type HPV E6/E7 mRNA Assay: The PreTect HPV-Proofer 7 assay (PreTect AS, Norway) detects transcriptionally active infections with HPV genotypes 16, 18, 31, 33, 45, 52, and 58. The assay includes intrinsic sample adequacy controls targeting the mRNA of a housekeeping gene to ensure RNA integrity. RNA was extracted from 1 mL of residual liquid-based cytology sample using the PreTect X protocol, with elution in 80 µL. Amplification was performed using nucleic acid sequence-based amplification (NASBA) and all procedures were performed in accordance with the manufacturer’s instructions and internal SOPs. All mRNA testing was completed within six weeks of sample collection. PreTect Analysis Software (PAS) validated assay performance through positive and negative controls for all targets and provided automated genotype-specific interpretation of results. Samples with less than 1 mL residual volume or invalid intrinsic control results were excluded from analysis.
The PreTect HPV-Proofer 7 assay has also been validated for use on self-collected vaginal specimens, supporting its applicability in decentralized or remote screening settings [
16,
17].
2.2. Follow-Up and Outcome Ascertainment
Clinical management followed the national guidelines in effect at the time of testing. Where indicated, colposcopy-directed biopsies or systematic four-quadrant biopsies of the transformation zone were performed. The primary study endpoint was histologically confirmed cervical intraepithelial neoplasia grade 2 or worse (CIN2+), coded according to World Health Organization (WHO) criteria. Pathologists evaluating histology were blinded to the mRNA test results. Outcome data were collected through 31 October 2024.
2.3. Statistical Analysis
Sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) for CIN2+ detection were calculated for each triage modality (LBC ≥ASC-US and the 7-type HPV mRNA test). Exact 95% confidence intervals (CIs) were calculated using the Clopper–Pearson method. Paired comparisons between test modalities were conducted using McNemar’s test, with p-values < 0.05 considered statistically significant. All analyses were performed using IBM SPSS Statistics for Windows, Version 29.0 (IBM Corp., Armonk, NY, USA, 2022).
2.4. Ethical Approval
This study was approved by the Regional Committee for Medical and Health Research Ethics, North Norway, as a programme evaluation project (REK Nord 203384). In accordance with Norwegian regulations, individual informed consent was not required for quality-assurance studies based on de-identified registry data.
3. Results
3.1. Primary Screening Outcomes and Triage Cohort Formation
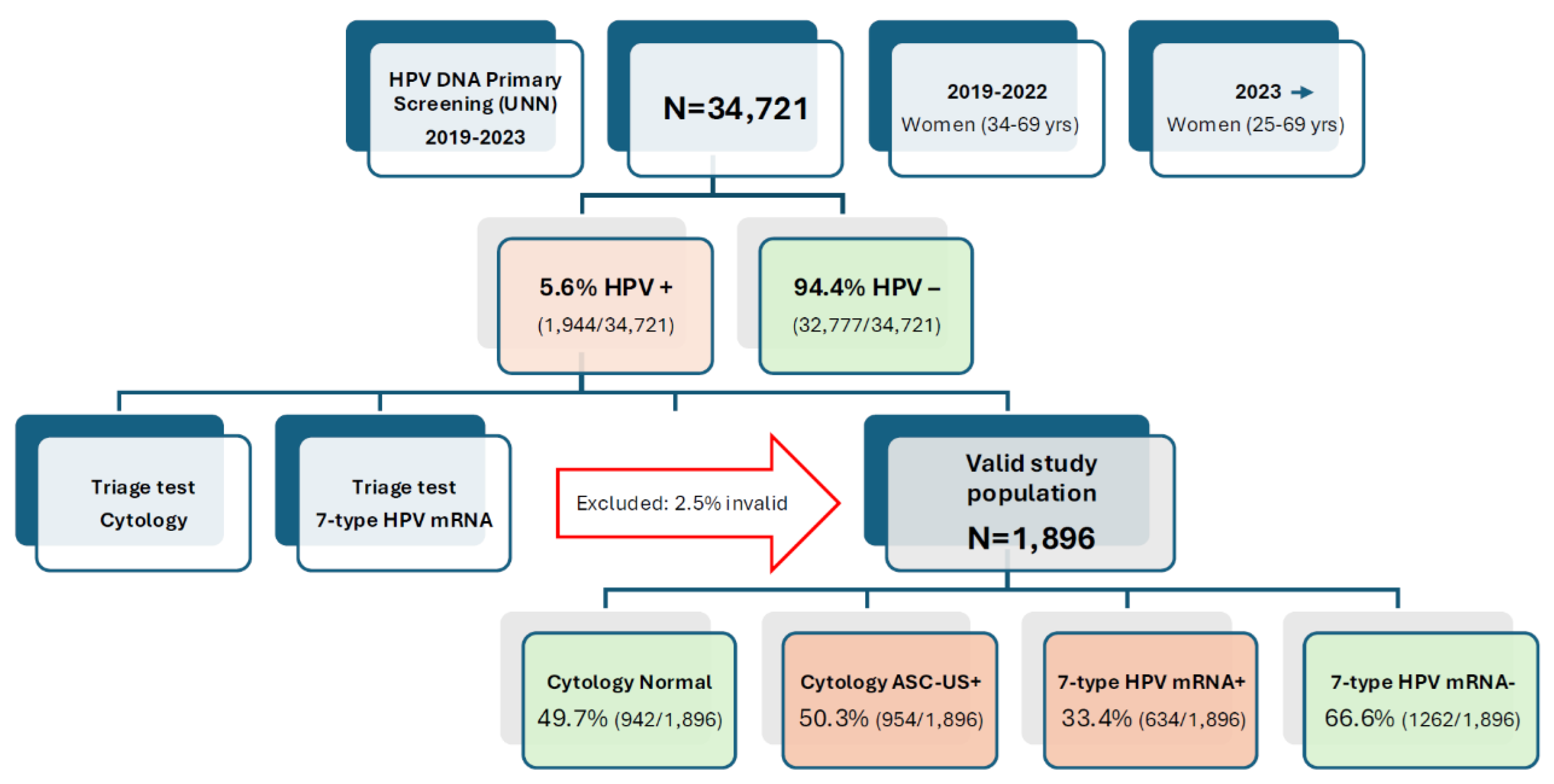
Among 34,721 women screened with the Cobas 4800 HPV DNA assay between January 2019 and December 2023, 1,944 (5.6 %) were HPV DNA-positive. After exclusion of 48 samples with insufficient volume or invalid mRNA internal control, 1,896 women (97.5 %) comprised the analytic triage cohort, doubling the size of our previous interim report (n = 962) and extending follow-up to 31 October 2024 (
Figure 1).
3.2. Comparative Triage Positivity Rates and Impact on Referral Burden
Among the 1,896 HPV DNA-positive women, 50.3% (954/1,896) had abnormal cytology results (≥ASC-US), while 49.7% (942/1,896) were cytology-negative. In contrast, the 7-type HPV mRNA assay was positive in 33.4% (634/1,896) and negative in 66.6% (1,262/1,896) of cases. This reflects a 34% relative reduction in test positivity compared to cytology, corresponding to 320 fewer women being triaged as positive and potentially referred for immediate colposcopy. The lower positivity rate of the mRNA test indicates improved specificity and a reduced risk of over-referral. (
Figure 1).
3.3. CIN2+ Detection Rates and Risk Stratification by Triage Modality
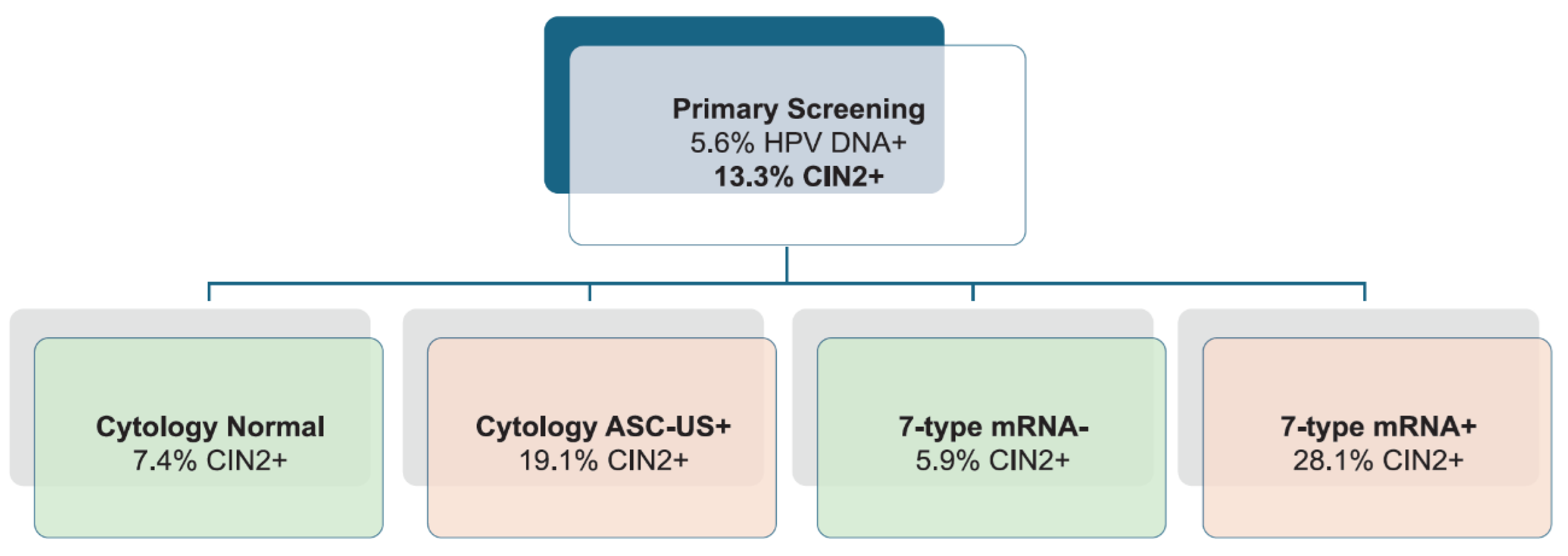
During follow-up, 252 women were diagnosed with CIN2+, yielding a prevalence of 13.3 % (252/1,896), virtually identical to the 13.9 % observed in the earlier analysis. Among women with abnormal cytology (≥ASC-US), 19.1 % (182/954) had CIN2+, compared to 7.4 % (70/942) with normal cytology. For the 7-type HPV mRNA test, 28.1 % (178/634) of mRNA-positive women had CIN2+, whereas only 5.9 % (74/1,262) of mRNA-negative women had CIN2+, demonstrating the improved risk discrimination provided by genotype-specific mRNA triage (
Figure 2).
3.4. Comparative Diagnostic Accuracy of Cytology and HPV mRNA Triage for CIN2+ Detection
The diagnostic accuracy of the two triage strategies for detecting CIN2+ is summarized in
Table 1 and
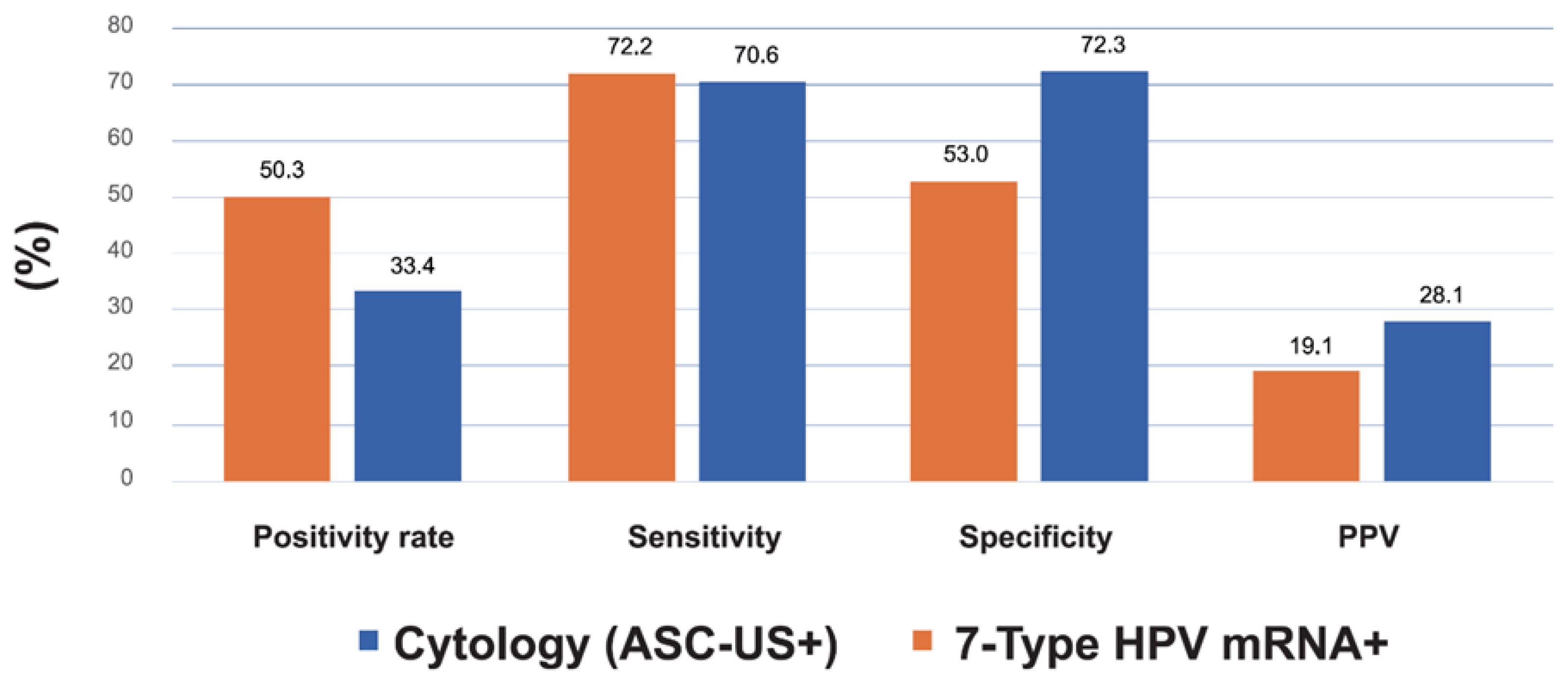
Figure 3. Sensitivity was comparable between cytology (72.2%; 95% CI: 66.3–77.5) and the 7-type HPV mRNA test (70.6%; 95% CI: 64.6–75.9), with a minimal absolute difference of –1.6 percentage points. However, the mRNA test demonstrated substantially higher specificity (72.3% vs. 53.0%), representing a 19.3 percentage point increase and a ~1.4-fold improvement relative to cytology. Positive predictive value (PPV) increased from 19.1% with cytology to 28.1% with mRNA (Δ = +9.0 pp), a 47% relative improvement. Negative predictive value (NPV) was high for both tests, with a slight advantage for mRNA (94.6% vs. 93.0%). These findings highlight the superior risk discrimination achieved with genotype-specific mRNA testing. While statistical comparisons were not included in the table, the clinical implications of these differences—particularly the improved specificity and PPV—support the utility of HPV mRNA triage in reducing unnecessary colposcopies and focusing follow-up on women at highest risk.
Conversely, despite a clinically relevant increase in PPV with the mRNA test, the number of discordant CIN2+ cases was limited, indicating caution in interpreting statistical significance in paired analyses.
3.5. Colposcopy Efficiency: Procedures Required per CIN2+ Case Detected
Applying cytology ≥ASC-US would lead to 5.2 colposcopies per CIN2+ detected (954/182), whereas the mRNA strategy would require 3.6 colposcopies per CIN2+ (634/178), reflecting a 31 % reduction in procedures for comparable disease yield.
3.6. Genotype-Specific Predictive Values
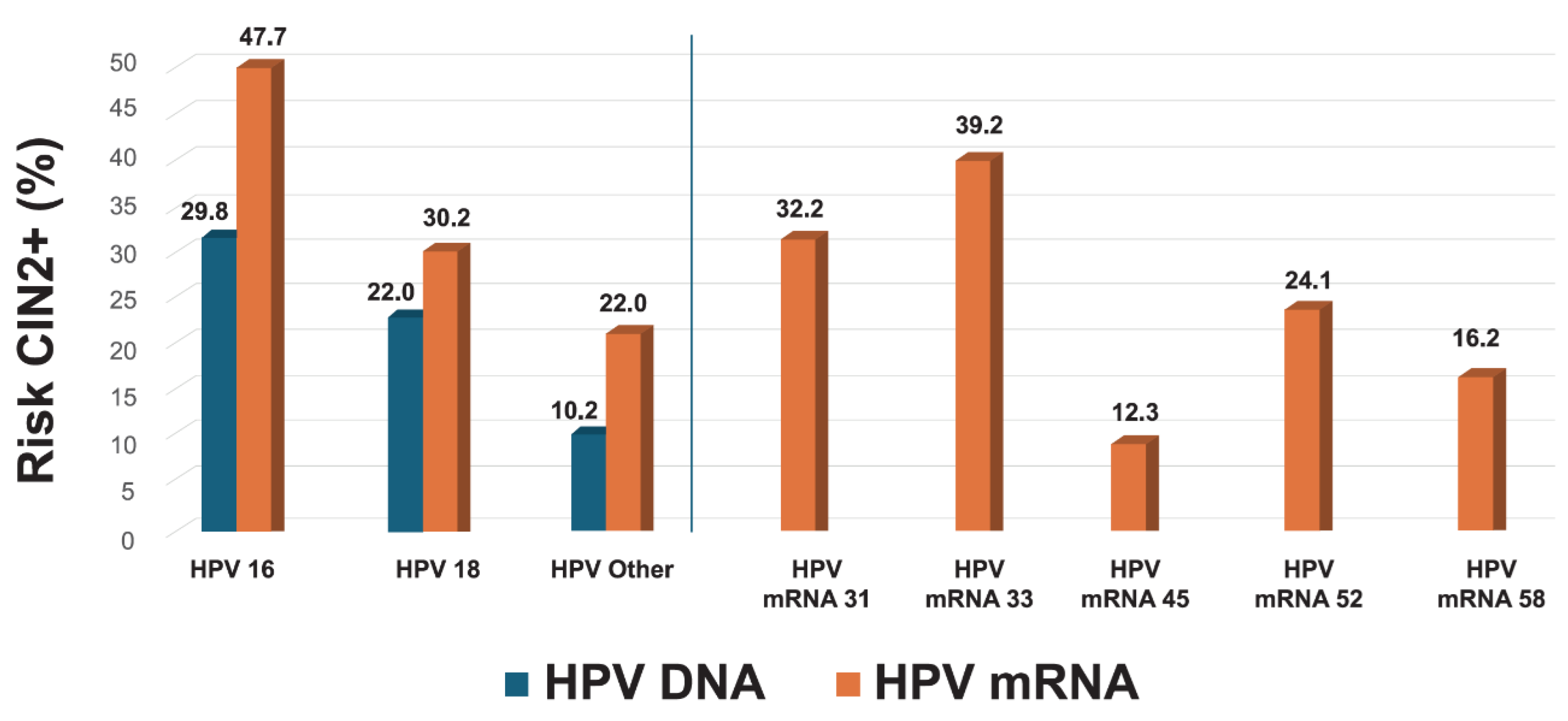
The 7-type HPV mRNA assay provided improved risk stratification compared to HPV DNA genotyping. The positive predictive value (PPV) for CIN2+ was substantially higher for HPV16 mRNA (47.7%) compared to HPV16 DNA (29.8%), and for HPV18 mRNA (30.2%) versus HPV18 DNA (22.0%). Among women with mRNA-positive results for other genotypes (31, 33, 45, 52, 58), the overall PPV was 22.0%, compared to 10.2% for non-16/18 HPV DNA types. Genotype-specific PPVs for the mRNA assay were highest for HPV33 (39.2%), HPV31 (32.2%), HPV52 (24.1%), and HPV45 (12.3%), underscoring the ability of genotype-specific mRNA testing to differentiate clinically meaningful high-grade lesions from transient ones (
Figure 4). The overall PPV across all mRNA-positive samples was 28.1%, reinforcing its utility in triaging HPV DNA-positive women.
In this expanded cohort, 7-type HPV E6/E7 mRNA triage preserved sensitivity, increased specificity by nearly 20 percentage points, and reduced the number of colposcopies required to detect one CIN2+ case from 5.2 to 3.6, confirming and strengthening the advantages previously observed in the interim analysis. This enhanced genotype-specific stratification may be particularly valuable in partially vaccinated populations, where the prevalence of certain HPV types is reduced and residual disease risk is increasingly concentrated in a smaller subset of high-risk genotypes.
3.7. Refining Risk Stratification in HPV16/18 DNA-Positive Women
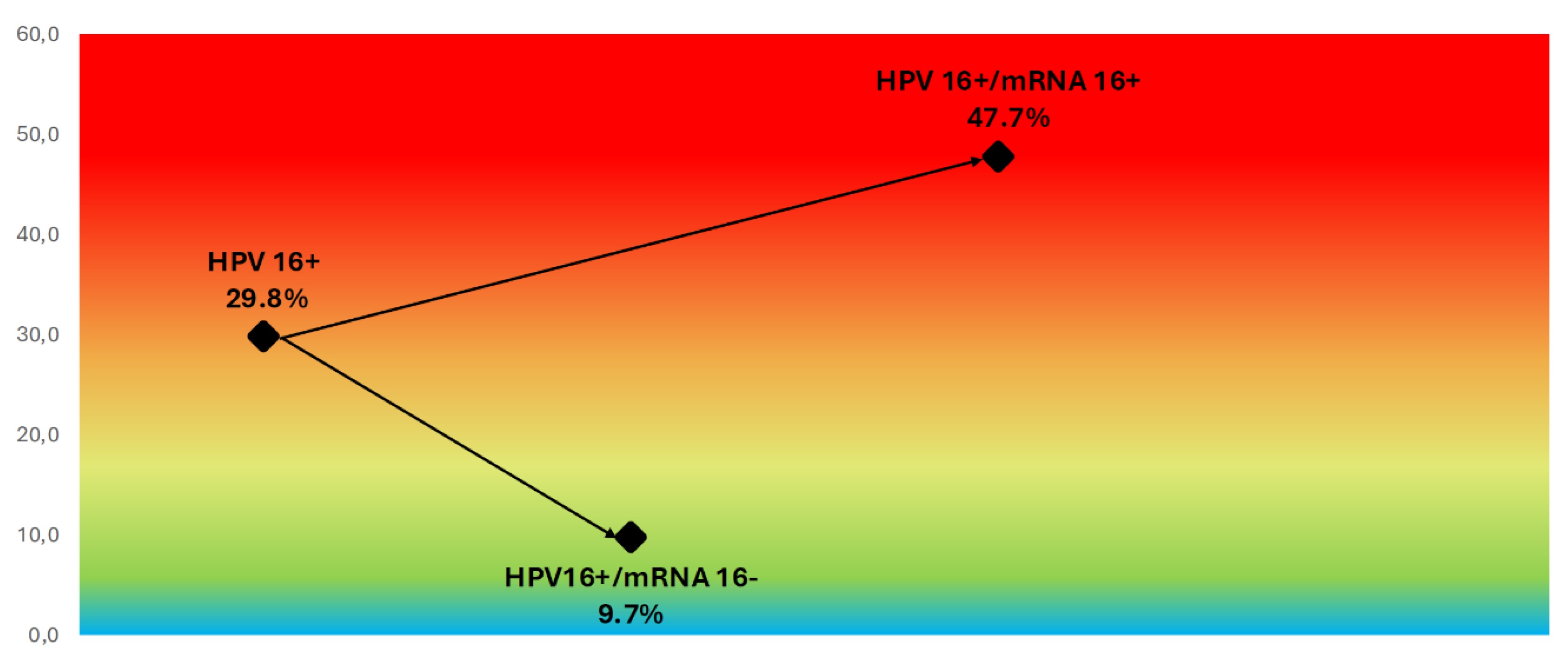
Stratification of HPV DNA-positive women by mRNA status revealed marked differences in CIN2+ risk within genotype groups. Among women positive for HPV DNA type 16, the overall CIN2+ rate was 29.8%, but this increased to 47.7% in those also positive for HPV16 mRNA and dropped to 9.7% in those mRNA-negative (
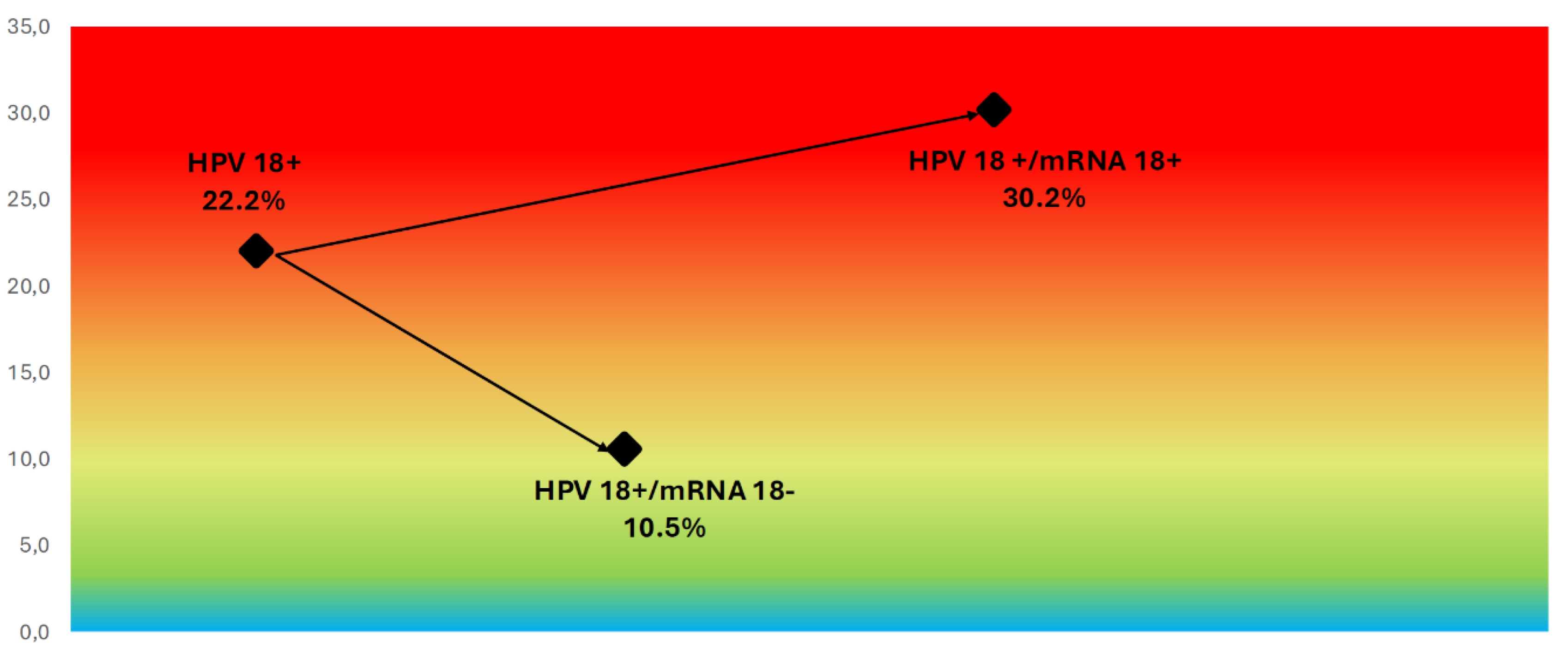
Figure 5). Similarly, for women with HPV18 DNA, the overall CIN2+ rate was 22.2%, with 30.2% in the mRNA-positive group and 10.5% in the mRNA-negative group (
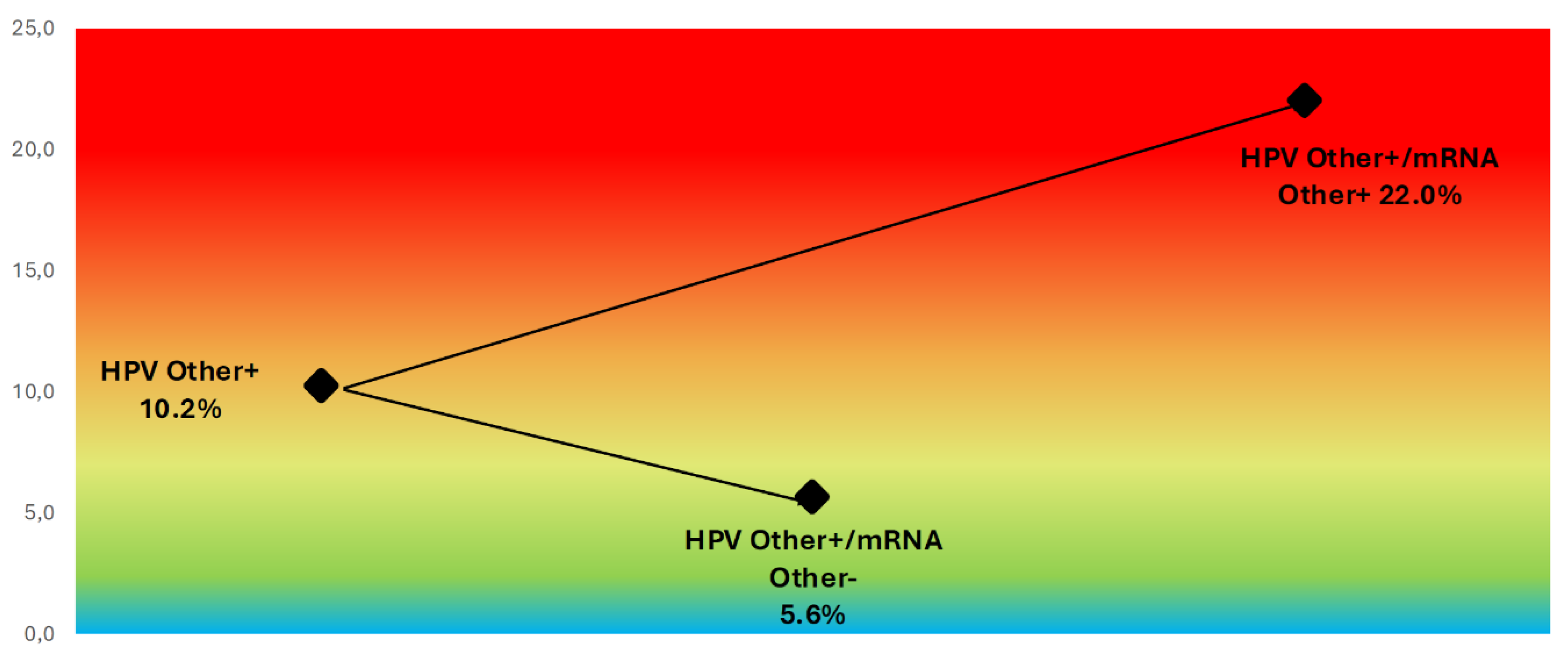
Figure 6). For women with other high-risk HPV DNA types (i.e., non-16/18), the overall CIN2+ rate was 10.2%, but risk increased to 22.0% among those with a positive mRNA result and was only 5.6% in mRNA-negative cases (
Figure 7). These findings demonstrate that genotype-specific HPV mRNA testing refines risk stratification within HPV DNA-positive groups and more accurately identifies women at highest risk of CIN2+.
4. Discussion
4.1. Clinical Utility of Genotype-Specific HPV mRNA Triage
This study demonstrates the clinical utility and substantial advantages of employing the 7-type HPV mRNA E6/E7 assay (PreTect HPV-Proofer`7) for triage of HPV DNA-positive women within a population-based cervical cancer screening program. Our findings confirm that this genotype-specific mRNA test achieves comparable sensitivity to liquid-based cytology while substantially improving specificity and positive predictive value (PPV), thereby addressing a key limitation of HPV DNA-based screening, its relatively low specificity and the consequent risk of overtreatment.
In our cohort, test positivity was substantially lower with HPV mRNA testing (33.4%) compared to cytology (50.3%), resulting in fewer women being referred for colposcopy without compromising sensitivity (70.6% vs 72.2%). This reduction in over-referral aligns with previous reports evaluating HPV mRNA in both primary and delayed triage settings [
14,
18,
19].
By detecting transcriptionally active infections, the mRNA assay offers superior risk discrimination and more effectively identifies women with clinically significant infections while avoiding unnecessary procedures.
4.2. Enhanced Stratification Within HPV DNA 16/18
Importantly, genotype-specific analysis revealed that the mRNA assay considerably improved PPVs for HPV16, HPV18, and other high-risk types, enabling more refined CIN2+ risk stratification among HPV DNA-positive women. This level of detail is particularly valuable in the context of HPV-vaccinated populations, where the prevalence of vaccine-covered genotypes is decreasing and residual disease risk is increasingly concentrated in fewer, non-vaccine HPV types. Moreover, the ability of the mRNA assay to differentiate transcriptionally active, and therefore clinically meaningful, infections is particularly relevant in younger women, who have the highest HPV prevalence and are most likely to be diagnosed with transient CIN2 lesions [
6,
20,
21]. In this group, cytology or DNA-based screening may lead to unnecessary interventions for regressive lesions, resulting in overtreatment and psychological burden. By focusing triage on transcriptionally active infections with known oncogenic potential, genotype-specific mRNA testing may help reduce overtreatment in young women while preserving sensitivity for detecting lesions with true progression risk [
22].
4.3. Negative Predictive Value and Long-Term Safety of mRNA Triage
The observed negative predictive value of the 7-type mRNA test in our study (94.6%) was comparable to that of cytology-based triage (93.0%), supporting the safety of managing mRNA-negative women within current surveillance intervals. Although the assay targets only a subset of the 14 high-risk HPV genotypes detected by primary screening, prior long-term follow-up studies have demonstrated that HPV mRNA-negative women have a persistently low risk of CIN3+ over time. In a Norwegian cohort study involving the earlier 5-type version of the HPV mRNA test, the 10-year cumulative incidence of CIN3+ among mRNA-negative women was just 1.1%, comparable to the risk observed with negative results from extended genotype DNA tests [
23]. These findings reinforce the clinical reliability of a negative HPV mRNA test and support its use as a triage tool with similar follow-up safety expectations as cytology.
4.4. HPV mRNA Versus Other Molecular Triage Strategies
Several molecular triage strategies have been proposed as alternatives to cytology for the management of HPV DNA-positive women, including p16/Ki-67 dual-stained cytology (CINtec® PLUS) and DNA methylation assays. These approaches aim to improve sensitivity and objectivity, particularly in women with equivocal cytology or non-16/18 high-risk HPV infections [
24,
25,
26,
27,
28,
29,
30].
CINtec PLUS has shown increased sensitivity and negative predictive value (NPV) compared to cytology, especially when combined with HPV16/18 genotyping, as demonstrated in studies by McMenamin et al. and Øvestad et al. [
29,
30]. However, reported specificity values have varied. Øvestad et al. found that dual staining led to lower specificity than cytology, implying more false-positive referrals and a higher colposcopy burden. In contrast, McMenamin et al. reported that CINtec PLUS improved specificity over HPV testing in women with equivocal cytology and could reduce referral rates by up to 40%. These inconsistencies highlight that the clinical utility of CINtec PLUS depends significantly on population characteristics, cytology quality, and the operational implementation (standalone vs. with genotyping).
Our findings demonstrate that the 7-type HPV mRNA test achieves a comparable reduction in colposcopy referrals, with test positivity decreasing from 50.3% with cytology to 33.4%—a 34% relative reduction—while also providing substantially higher specificity (72.3% vs. 53.0%) and a 47% relative increase in positive predictive value (28.1% vs. 19.1%). Unlike dual staining, HPV mRNA testing directly detects transcriptionally active oncogenic infections, offering enhanced clinical precision. In addition, genotype-specific results provide more refined risk stratification without the need for additional platforms or cytotechnologist interpretation.
Importantly, HPV mRNA testing is compatible with self-collected vaginal specimens, unlike cytology-based or dual-stained approaches that require clinician-collected samples and microscopy infrastructure. This feature improves feasibility in decentralized settings, reduces logistical barriers to screening, and facilitates broader population reach—particularly in low-resource contexts or programs relying on self-sampling strategies.
Moreover, evidence from Carcea et al. supports the superior clinical relevance of E6/E7 mRNA detection. In a study comparing mRNA testing with HPV DNA and p16/Ki-67 immunostaining for detecting post-treatment CIN2+ recurrence, the mRNA assay achieved the highest sensitivity (100%) and specificity (96.9%), and was the only method significantly correlated with histological severity (ρ = 0.345, p = 0.006) [
26].
Taken together, the clinical performance, operational flexibility, and self-sampling compatibility of the 7-type HPV mRNA assay position it as a highly promising molecular triage tool for organized cervical cancer screening programs.
4.5. Colposcopy Efficiency and Health System Impact
The reduction in the number of colposcopies required per CIN2+ case, from 5.2 with cytology to 3.6 with mRNA testing, illustrates both the clinical efficiency and potential cost-effectiveness of mRNA-based triage. These findings are consistent with results from the initial implementation phase (2019–2021), which reported similar colposcopy efficiency (5.2 vs 3.4) based on a cohort of 962 women [
14]. The current expanded analysis, based on a doubled sample size and extended follow-up through October 2024, reinforces the robustness of these estimates. This consistency across time and population size underscores the reliability of genotype-specific HPV mRNA testing in reducing unnecessary procedures and improving the overall benefit–harm balance of cervical cancer screening.
4.6. Alignment with National Data on HPV Genotype Risk Stratification
Our findings on genotype-specific risk stratification are broadly consistent with earlier population-based data from the Norwegian Cervical Cancer Screening Programme. In the study by Hashim et al. (2020), the estimated CIN3+ risks among HPV DNA-positive women with normal cytology were 19.9% for HPV16, 10.8% for HPV18, and 5.5% for other high-risk HPV types [
31]. In our cohort, using a genotype-specific mRNA triage approach, we observed lower absolute risks within each group—9.7% for mRNA-negative HPV16, 10.5% for mRNA-negative HPV18, and 5.6% for mRNA-negative non-16/18 types—indicating effective de-escalation of risk among women with transcriptionally inactive infections. Conversely, CIN2+ risk among women who were mRNA-positive for HPV16 was 47.7%, compared to 29.8% for HPV16 DNA alone. These differences underscore the enhanced discriminatory capacity of HPV mRNA testing to differentiate clinically relevant infections from transient ones, especially within genotype categories. By more accurately identifying women at low versus high risk, genotype-specific mRNA testing may allow for safer postponement of colposcopy in mRNA-negative women while maintaining robust detection of high-grade lesions among those with transcriptionally active infections. This reinforces the added value of transcriptional activity detection over DNA presence alone in stratifying risk.
4.7. Study Limitations and Need for Further Validation
Several limitations should be acknowledged. First, the follow-up duration may be insufficient to fully capture the long-term safety and predictive value of mRNA-based triage. Second, the lack of stratification by HPV vaccination status limits interpretation in vaccinated populations where genotype prevalence is shifting. Third, while our findings support the safety of standard surveillance intervals for mRNA-negative women, it should be noted that the 7-type assay does not cover all 14 high-risk HPV types identified by the primary DNA screening assay. Although long-term data from a similar 5-type mRNA test indicate durable protection against CIN3+ [
23], further real-world studies evaluating the full 7-type assay over extended follow-up are needed to confirm long-term safety in diverse screening populations.
4.8. Interpretation of Diagnostic Differences and Clinical Relevance
Differences in diagnostic performance between HPV mRNA triage and cytology should be interpreted primarily in terms of clinical relevance. Although the increase in specificity and negative predictive value observed for the HPV mRNA test is substantial, these absolute improvements—while modest—may translate into meaningful clinical benefits in large-scale screening programmes by reducing unnecessary colposcopies. Moreover, the relative increase in positive predictive value (47%) with mRNA triage represents a clinically important gain, indicating more accurate identification of women at highest risk of CIN2+. The interpretation of such differences should therefore prioritize clinical impact rather than relying solely on statistical significance, particularly given the paired nature of the data and the large sample size involved.
In summary, the 7-type HPV mRNA assay significantly improves specificity and PPV over cytology while maintaining high sensitivity. Its applicability to self-collected samples, capacity for genotype-specific risk discrimination, and ability to reduce unnecessary colposcopies support its broader implementation as a precision triage tool in HPV-based cervical cancer screening programs.
5. Conclusions
This study confirms that triage of HPV DNA-positive women using the 7-type HPV mRNA assay (PreTect HPV-Proofer`7) maintains diagnostic sensitivity while markedly improving specificity and positive predictive value compared to cytology-based triage. The use of genotype-specific mRNA detection substantially reduces unnecessary colposcopy referrals, mitigating overtreatment and patient burden. Moreover, validated compatibility with self-collected vaginal specimens supports broader implementation, particularly in screening programs incorporating self-sampling. This approach enables risk-adapted clinical management based on genotype-specific transcriptional activity, efficient allocation of clinical resources, and improved cost-effectiveness, key principles of precision medicine in cervical cancer prevention.
Author Contributions
Conceptualization, S.S., B.M.F. and E.M.; methodology, S.S. and B.M.F.; formal analysis, S.S.; investigation, S.S., M.A., and B.M.F.; resources, S.S., M.A. and B.M.F.; data curation, S.S.; writing—original draft preparation, S.S. and B.M.F.; writing—review and editing, S.S., M.A. and B.M.F.; visualization, S.S. and B.M.F.; supervision, S.S.; project administration, S.S., B.M.F. and E.M.; funding acquisition, S.S., B.M.F. and E.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding. HPV mRNA test kits were provided FOC by PreTect AS.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Regional Committee for Medical and Health Research Ethics (REC North, 203384, 18 December 2020) for studies involving humans.
Informed Consent Statement
Patient consent was waived due to Norwegian regulations which exempt quality assurance studies from written informed consent.
Data Availability Statement
All data presented are available upon request.
Acknowledgments
The authors would like to extend our gratitude to the staff at the department of Clinical Pathology at the University Hospital of North Norway and to all the laboratory staff performing HPV DNA/mRNA testing, cytology and histopathology evaluation for their great work and collaboration during this study.
Conflicts of Interest
S.W.S. and M.A. declare no conflict of interest. B.M.F. is an employee of PreTect AS.
References
- Castellsagué, X. Natural History and Epidemiology of HPV Infection and Cervical Cancer. Gynecol. Oncol. 2008, 110 (Suppl. 2), S4–S7. [CrossRef]
- Dijkstra, M.G.; Snijders, P.J.; Arbyn, M.; Rijkaart, D.C.; Berkhof, J.; Meijer, C.J. Cervical Cancer Screening: On the Way to a Shift from Cytology to Full Molecular Screening. Ann. Oncol. 2014, 25, 927–935. [CrossRef]
- Cuschieri, K.; Cubie, H.; Graham, C.; Rowan, J.; Hardie, A.; Horne, A.; Earle, C.B.; Bailey, A.; Crosbie, E.J.; Kitchener, H. Clinical Performance of RNA and DNA Based HPV Testing in a Colposcopy Setting: Influence of Assay Target, Cut-Off and Age. J. Clin. Virol. 2014, 59, 104–108. [CrossRef]
- Tjalma, W.A.A. Diagnostic Performance of Dual-Staining Cytology for Cervical Cancer Screening: A Systematic Literature Review. Eur. J. Obstet. Gynecol. Reprod. Biol. 2017, 210, 275–280. [CrossRef]
- Verhoef, L.; Bleeker, M.C.G.; Polman, N.; Steenbergen, R.D.M.; Meijer, C.J.L.M.; Melchers, W.J.G.; Bekkers, R.L.; Molijn, A.C.; Quint, W.G.; van Kemenade, F.J.; Berkhof, J.; Heideman, D.A.M. Performance of DNA Methylation Analysis of ASCL1, LHX8, ST6GALNAC5, GHSR, ZIC1 and SST for the Triage of HPV-Positive Women: Results from a Dutch Primary HPV-Based Screening Cohort. Int. J. Cancer 2021, 149, 2136–2144. [CrossRef]
- Wentzensen, Nicolas MD, PhD; Silver, Michelle I. PhD. Biomarkers for Cervical Cancer Prevention Programs: The Long and Winding Road From Discovery to Clinical Use. Journal of Lower Genital Tract Disease 20(3):p 191-194, July 2016. |. [CrossRef]
- Cuschieri, K.; Ronco, G.; Lorincz, A.; Smith, L.; Ogilvie, G.; Mirabello, L.; Carozzi, F.; Cubie, H.; Wentzensen, N.; Snijders, P.; Arbyn, M.; Monsonego, J.; Franceschi, S. Eurogin Roadmap 2017: Triage Strategies for the Management of HPV-Positive Women in Cervical Screening Programs. Int. J. Cancer 2018, 143, 735–745. [CrossRef]
- Cattani, P.; Zannoni, G.F.; Ricci, C.; D'Onghia, S.; Trivellizzi, I.N.; Di Franco, A.; Vellone, V.G.; Durante, M.; Fadda, G.; Scambia, G.; Capelli, G.; De Vincenzo, R. Clinical Performance of Human Papillomavirus E6 and E7 mRNA Testing for High-Grade Lesions of the Cervix. J. Clin. Microbiol. 2009, 47, 3895–3901. [CrossRef]
- Derbie, A.; Mekonnen, D.; Woldeamanuel, Y.; Van Ostade, X.; Abebe, T. HPV E6/E7 mRNA Test for the Detection of High Grade Cervical Intraepithelial Neoplasia (CIN2+): A Systematic Review. Infect. Agents Cancer 2020, 15, 9. [CrossRef]
- Benevolo, M.; Vocaturo, A.; Caraceni, D.; French, D.; Rosini, S.; Zappacosta, R.; Terrenato, I.; Ciccocioppo, L.; Frega, A.; Giorgi Rossi, P. Sensitivity, Specificity, and Clinical Value of Human Papillomavirus (HPV) E6/E7 mRNA Assay as a Triage Test for Cervical Cytology and HPV DNA Test. J. Clin. Microbiol. 2011, 49, 2643–2650. [CrossRef]
- Origoni, M.; Cristoforoni, P.; Carminati, G.; Stefani, C.; Costa, S.; Sandri, M.T.; Mariani, L.; Preti, M. E6/E7 mRNA Testing for Human Papilloma Virus-Induced High-Grade Cervical Intraepithelial Disease (CIN2/CIN3): A Promising Perspective. Ecancermedicalscience 2015, 9, 533. [CrossRef]
- Frega, A.; Lorenzon, L.; Giovagnoli, M.R.; De Sanctis, L.; Fabiano, V.; Lukic, A.; Moscarini, M.; Torrisi, M.R.; French, D. Prognostic Implication of High-Risk Human Papillomavirus E6 and E7 mRNA in Patients with Intraepithelial Lesions of the Cervix in Relationship to Age. Int. J. Immunopathol. Pharmacol. 2011, 24, 461–470. [CrossRef]
- Ratnam, S.; Coutlee, F.; Fontaine, D.; Bentley, J.; Escott, N.; Ghatage, P.; Gadag, V.; Holloway, G.; Bartellas, E.; Kum, N.; Giede, C.; Lear, A. Clinical Performance of the PreTect HPV-Proofer E6/E7 mRNA Assay in Comparison with That of the Hybrid Capture 2 Test for Identification of Women at Risk of Cervical Cancer. J. Clin. Microbiol. 2010, 48, 2779–2785. [CrossRef]
- Sørbye, S.W.; Falang, B.M.; Antonsen, M. Performance of a 7-Type HPV mRNA Test in Triage of HPV DNA Primary Screen Positive Women Compared to Liquid-Based Cytology. J. Mol. Pathol. 2023, 4, 69–80. [CrossRef]
- Sørbye, S.W.; Fismen, S.; Gutteberg, T.; Mortensen, E.S. Triage of Women with Minor Cervical Lesions: Data Suggesting a “Test and Treat” Approach for HPV E6/E7 mRNA Testing. PLoS ONE 2010, 5, e12724. [CrossRef]
- Flores, C.E.A.; Falang, B.M.; Gómez-Laguna, L.; Gutiérrez, G.G.; León, J.M.O.; Uribe, M.; Cruz, O.; Sørbye, S.W. Enhancing Cervical Cancer Screening with 7-Type HPV mRNA E6/E7 Testing on Self-Collected Samples: Multicentric Insights from Mexico. Cancers 2024, 16, 2485. [CrossRef]
- Aranda Flores, C.E., Gomez Gutierrez, G., Ortiz Leon, J.M. et al. Self-collected versus clinician-collected cervical samples for the detection of HPV infections by 14-type DNA and 7-type mRNA tests. BMC Infect Dis 21, 504 (2021). [CrossRef]
- Westre, B.; Giske, A.; Guttormsen, H.; Sørbye, S.W.; Skjeldestad, F.E. 5-Type HPV mRNA versus 14-Type HPV DNA Test: Test Performance, Over-Diagnosis and Overtreatment in Triage of Women with Minor Cervical Lesions. BMC Clin. Pathol. 2016, 16, 9. [CrossRef]
- Sørbye, S.W.; Fismen, S.; Gutteberg, T.J.; Mortensen, E.S.; Skjeldestad, F.E. HPV mRNA Testing in Triage of Women with ASC-US Cytology May Reduce the Time for CIN2+ Diagnosis Compared with Repeat Cytology. Curr. Pharm. Des. 2013, 19, 1401–1405. [CrossRef]
- Rad, A.; Sørbye, S.W.; Brenn, T.; Tiwari, S.; Løchen, M.L.; Skjeldestad, F.E. 13-Type HPV DNA Test versus 5-Type HPV mRNA Test in Triage of Women Aged 25–33 Years with Minor Cytological Abnormalities—6 Years of Follow-Up. Int. J. Environ. Res. Public Health 2023, 20, 4119. [CrossRef]
- Tainio, K.; Athanasiou, A.; Tikkinen, K.A.O.; Aaltonen, R.; Cárdenas Hernándes, J.; Glazer-Livson, S.; Jakobsson, M.; Joronen, K.; Kiviharju, M.; Louvanto, K.; et al. Clinical Course of Untreated Cervical Intraepithelial Neoplasia Grade 2 under Active Surveillance: Systematic Review and Meta-Analysis. BMJ 2018, 360, k499. [CrossRef]
- St-Martin, G.; Holst Thamsborg, L.; Andersen, B.; Christensen, J.; Ejersbo, D.; Jochumsen, K.; Lynge, E. Management of Low-Grade Cervical Cytology in Young Women: Cohort Study from Denmark. Acta Oncol. 2021, 60, 444–451. [CrossRef]
- Rad, A.; Sørbye, S.W.; Tiwari, S.; Løchen, M.-L.; Skjeldestad, F.E. Risk of Intraepithelial Neoplasia Grade 3 or Worse (CIN3+) among Women Examined by a 5-Type HPV mRNA Test during 2003 and 2004, Followed through 2015. Cancers 2023,15, 3106. [CrossRef]
- Wentzensen, N.; Clarke, M.A.; Bremer, R.; Poitras, N.; Tokugawa, D.; Goldhoff, P.E.; Castle, P.E.; Schiffman, M.; Kingery, J.D.; Grewal, K.K.; et al. Clinical Evaluation of Human Papillomavirus Screening with p16/Ki-67 Dual Stain Triage in a Large Organized Cervical Cancer Screening Program. JAMA Intern. Med. 2019, 179, 881–888. [CrossRef]
- De Strooper, L.M.; Meijer, C.J.; Berkhof, J.; Hesselink, A.T.; Snijders, P.J.; Steenbergen, R.D.; Heideman, D.A. Methylation Analysis of the FAM19A4 Gene in Cervical Scrapes Is Highly Efficient in Detecting Cervical Carcinomas and Advanced CIN2/3 Lesions. Cancer Prev. Res. 2014, 7, 1251–1257. [CrossRef]
- Carcea, F.; Vavoulidis, E.; Petousis, S.; Papandreou, P.; Margioula Siarkou, C.; Nasioutziki, M.; Papanikolaou, A.; Dinas, K.; Daniilidis, A. Diagnostic Performance of HPV E6/E7 mRNA Testing Towards HPV-DNA Testing and p16/Ki67 Immunostaining as a Biomarker of High-Risk HPV Recurrence in Greek Women Surgically Treated for Their Cervical Lesions. J. Obstet. Gynaecol. Res. 2021, 47, 3082–3090. [CrossRef]
- Wright, T.C., Jr.; Behrens, C.M.; Ranger-Moore, J.; Rehm, S.; Sharma, A.; Stoler, M.H.; Ridder, R. Triaging HPV-Positive Women with p16/Ki-67 Dual-Stained Cytology: Results from a Sub-Study Nested into the ATHENA Trial. Gynecol. Oncol. 2017, 144, 51–56. [CrossRef]
- Wentzensen, N.; Fetterman, B.; Castle, P.E.; Schiffman, M.; Wood, S.N.; Stiemerling, E.; Tokugawa, D.; Bodelon, C.; Poitras, N.; Lorey, T.; Kinney, W. p16/Ki-67 Dual Stain Cytology for Detection of Cervical Precancer in HPV-Positive Women. J. Natl. Cancer Inst. 2015, 107, djv257. [CrossRef]
- McMenamin M, McKenna M, McDowell A. Clinical Utility of CINtec PLUS Triage in Equivocal Cervical Cytology and Human Papillomavirus Primary Screening. Am J Clin Pathol. 2018 Oct 24;150(6):512-521. PMID: 30169728. [CrossRef]
- Øvestad, I.T.; Dalen, I.; Andersland, M.S.; Vintermyr, O.K.; Moltu, P.; Berland, J.M.; Janssen, E.A.M.; Haugland, H.K. Triaging HPV-Positive Cervical Samples with p16 and Ki-67 Dual Stained Cytology within an Organized Screening Program—A Prospective Observational Study from Western Norway. Int. J. Mol. Sci. 2023, 24, 7158. [CrossRef]
- Hashim, D.; Engesæter, B.; Baadstrand Skare, G.; et al. Real-World Data on Cervical Cancer Risk Stratification by Cytology and HPV Genotype to Inform the Management of HPV-Positive Women in Routine Cervical Screening. Br. J. Cancer 2020, 122, 1715–1723. [CrossRef]
Figure 1.
Flowchart of Study Population and Triage Cohort Formation. Flow diagram outlining the inclusion of women from primary HPV DNA screening through triage and histological follow-up. Among 34,721 women screened between 2019 and 2023, 1,944 (5.6%) tested positive for high-risk HPV DNA. After excluding samples with insufficient volume or invalid mRNA internal control, 1,896 women were included in the analytic cohort. All underwent cytology and 7-type HPV mRNA testing, with follow-up for histologically confirmed CIN2+ through October 2024.
Figure 1.
Flowchart of Study Population and Triage Cohort Formation. Flow diagram outlining the inclusion of women from primary HPV DNA screening through triage and histological follow-up. Among 34,721 women screened between 2019 and 2023, 1,944 (5.6%) tested positive for high-risk HPV DNA. After excluding samples with insufficient volume or invalid mRNA internal control, 1,896 women were included in the analytic cohort. All underwent cytology and 7-type HPV mRNA testing, with follow-up for histologically confirmed CIN2+ through October 2024.
Figure 2.
CIN2+ Prevalence Stratified by Cytology and HPV mRNA Triage Results. CIN2+ prevalence among HPV DNA-positive women stratified by triage test result. Among women with abnormal cytology (≥ASC-US), 19.1% had CIN2+, compared to 7.4% with normal cytology. For the 7-type HPV mRNA test, CIN2+ was detected in 28.1% of mRNA-positive women and 5.9% of mRNA-negative women. These findings demonstrate improved risk discrimination with genotype-specific mRNA triage.
Figure 2.
CIN2+ Prevalence Stratified by Cytology and HPV mRNA Triage Results. CIN2+ prevalence among HPV DNA-positive women stratified by triage test result. Among women with abnormal cytology (≥ASC-US), 19.1% had CIN2+, compared to 7.4% with normal cytology. For the 7-type HPV mRNA test, CIN2+ was detected in 28.1% of mRNA-positive women and 5.9% of mRNA-negative women. These findings demonstrate improved risk discrimination with genotype-specific mRNA triage.
Figure 3.
Comparative Performance Metrics for CIN2+ Detection: Cytology vs. 7-Type HPV mRNA Triage. Bar graph comparing sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) for CIN2+ detection using cytology (≥ASC-US) versus the 7-type HPV E6/E7 mRNA assay. Values are based on the cohort of 1,896 HPV DNA-positive women with histological follow-up.
Figure 3.
Comparative Performance Metrics for CIN2+ Detection: Cytology vs. 7-Type HPV mRNA Triage. Bar graph comparing sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) for CIN2+ detection using cytology (≥ASC-US) versus the 7-type HPV E6/E7 mRNA assay. Values are based on the cohort of 1,896 HPV DNA-positive women with histological follow-up.
Figure 4.
Genotype-Specific Risk Stratification for CIN2+: Comparison of HPV DNA and mRNA Testing. Positive predictive values (PPVs) for CIN2+ are shown by genotype, comparing HPV DNA and mRNA detection. The figure presents DNA-based PPVs for HPV16, HPV18, and a pooled group of 12 high-risk HPV types, alongside mRNA-based PPVs for HPV16, HPV18, and a pooled group of five non-16/18 genotypes (HPV31, 33, 45, 52, 58). Individual mRNA-based PPVs are also shown for each of the seven genotypes included in the assay. This analysis illustrates the superior risk discrimination provided by genotype-specific mRNA detection compared to DNA-based stratification.
Figure 4.
Genotype-Specific Risk Stratification for CIN2+: Comparison of HPV DNA and mRNA Testing. Positive predictive values (PPVs) for CIN2+ are shown by genotype, comparing HPV DNA and mRNA detection. The figure presents DNA-based PPVs for HPV16, HPV18, and a pooled group of 12 high-risk HPV types, alongside mRNA-based PPVs for HPV16, HPV18, and a pooled group of five non-16/18 genotypes (HPV31, 33, 45, 52, 58). Individual mRNA-based PPVs are also shown for each of the seven genotypes included in the assay. This analysis illustrates the superior risk discrimination provided by genotype-specific mRNA detection compared to DNA-based stratification.
Figure 5.
CIN2+ Risk Stratification in HPV16 DNA-Positive Women Based on mRNA Status. Color-gradient risk map illustrating CIN2+ prevalence among HPV16 DNA-positive women. Risk increases from 9.7% in mRNA-negative cases to 47.7% in mRNA-positive cases, demonstrating the enhanced stratification achieved by detecting transcriptionally active HPV16.
Figure 5.
CIN2+ Risk Stratification in HPV16 DNA-Positive Women Based on mRNA Status. Color-gradient risk map illustrating CIN2+ prevalence among HPV16 DNA-positive women. Risk increases from 9.7% in mRNA-negative cases to 47.7% in mRNA-positive cases, demonstrating the enhanced stratification achieved by detecting transcriptionally active HPV16.
Figure 6.
CIN2+ Risk Stratification in HPV18 DNA-Positive Women Based on mRNA Status. Color-gradient risk map showing CIN2+ prevalence among HPV18 DNA-positive women, stratified by mRNA status. Risk increased from 10.5% in mRNA-negative cases to 30.2% in mRNA-positive cases, highlighting the additional discriminatory value of transcriptional activity detection for HPV18 infections.
Figure 6.
CIN2+ Risk Stratification in HPV18 DNA-Positive Women Based on mRNA Status. Color-gradient risk map showing CIN2+ prevalence among HPV18 DNA-positive women, stratified by mRNA status. Risk increased from 10.5% in mRNA-negative cases to 30.2% in mRNA-positive cases, highlighting the additional discriminatory value of transcriptional activity detection for HPV18 infections.
Figure 7.
CIN2+ Risk Stratification in Non-16/18 HPV DNA-Positive Women Based on mRNA Status. Color-gradient risk map showing CIN2+ prevalence among women positive for high-risk HPV DNA genotypes other than 16 and 18. Risk was 5.6% in mRNA-negative cases and 22.0% in mRNA-positive cases, demonstrating the ability of genotype-specific mRNA testing to refine risk estimates even within pooled non-16/18 infections.
Figure 7.
CIN2+ Risk Stratification in Non-16/18 HPV DNA-Positive Women Based on mRNA Status. Color-gradient risk map showing CIN2+ prevalence among women positive for high-risk HPV DNA genotypes other than 16 and 18. Risk was 5.6% in mRNA-negative cases and 22.0% in mRNA-positive cases, demonstrating the ability of genotype-specific mRNA testing to refine risk estimates even within pooled non-16/18 infections.
Table 1.
Comparative Diagnostic Performance of Cytology and 7-Type HPV mRNA Triage for CIN2+ Detection.
Table 1.
Comparative Diagnostic Performance of Cytology and 7-Type HPV mRNA Triage for CIN2+ Detection.
Metric
value |
Cytology ≥ASC-US % (95 % CI) |
7-type HPV mRNA % (95 % CI) |
Δ (mRNA – Cyt)
pp* |
| Sensitivity |
72.2 (66.3-77.5) |
70.6 (64.6-75.9) |
–1.6 |
| Specificity |
53.0 (50.3-55.7) |
72.3 (69.8-74.7) |
+19.3 |
| PPV |
19.1 (16.4-22.0) |
28.1 (24.9-31.6) |
+9.0 |
| NPV |
93.0 (91.1-94.6) |
94.6 (92.8-96.0) |
+1.6 |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).