Submitted:
27 June 2025
Posted:
30 June 2025
You are already at the latest version
Abstract
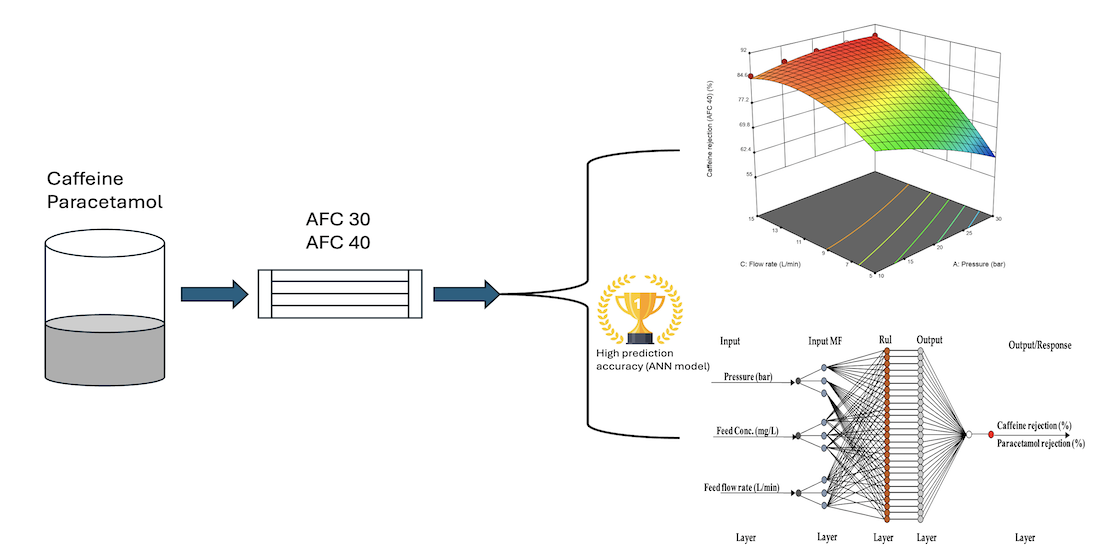
Keywords:
1. Introduction
2. Materials and Methods
2.1. Feed Solution Preparation
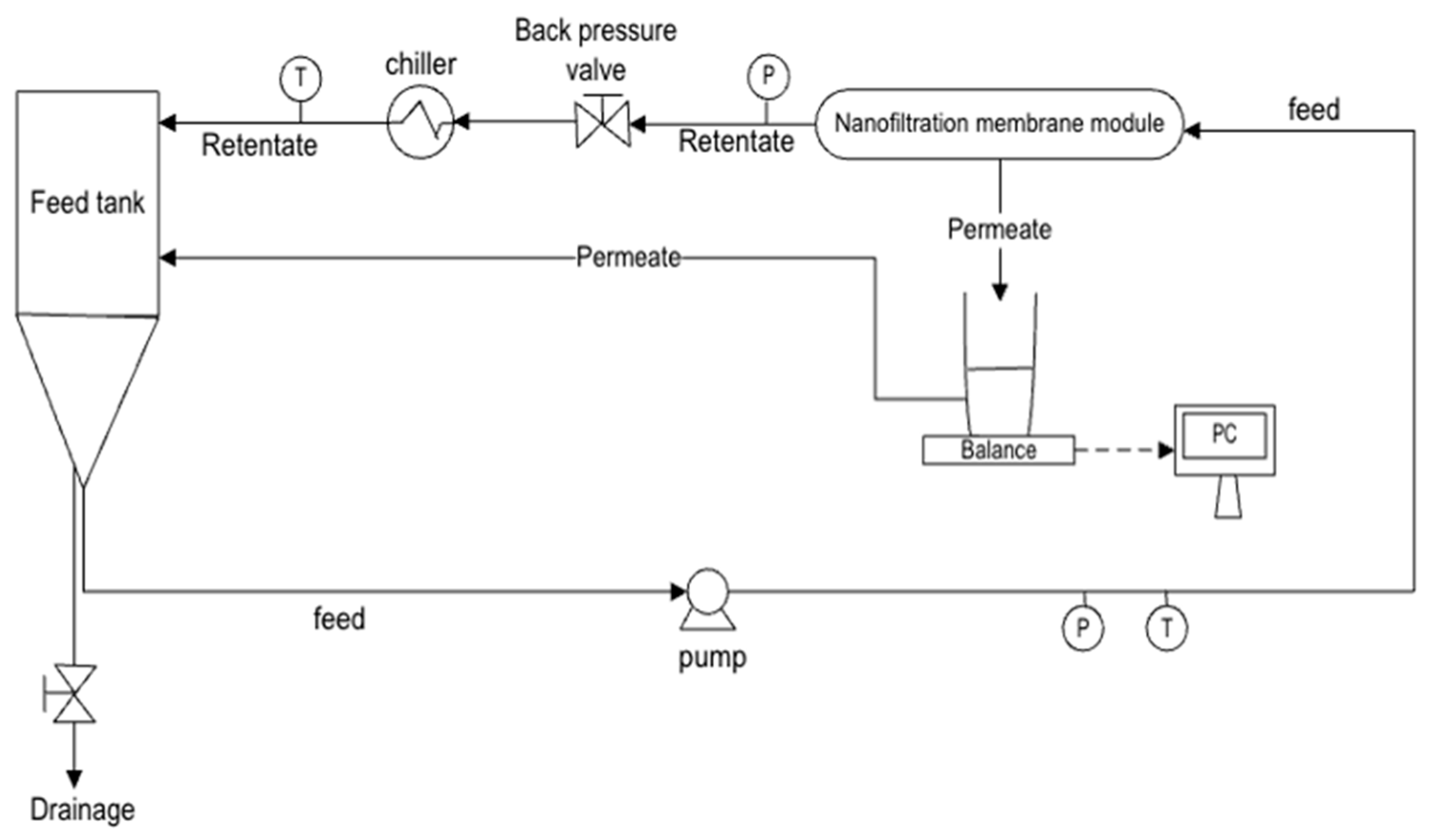
2.2. Experimental Set-Up and Procedures
2.3. Pharmaceuticals Rejection Tests
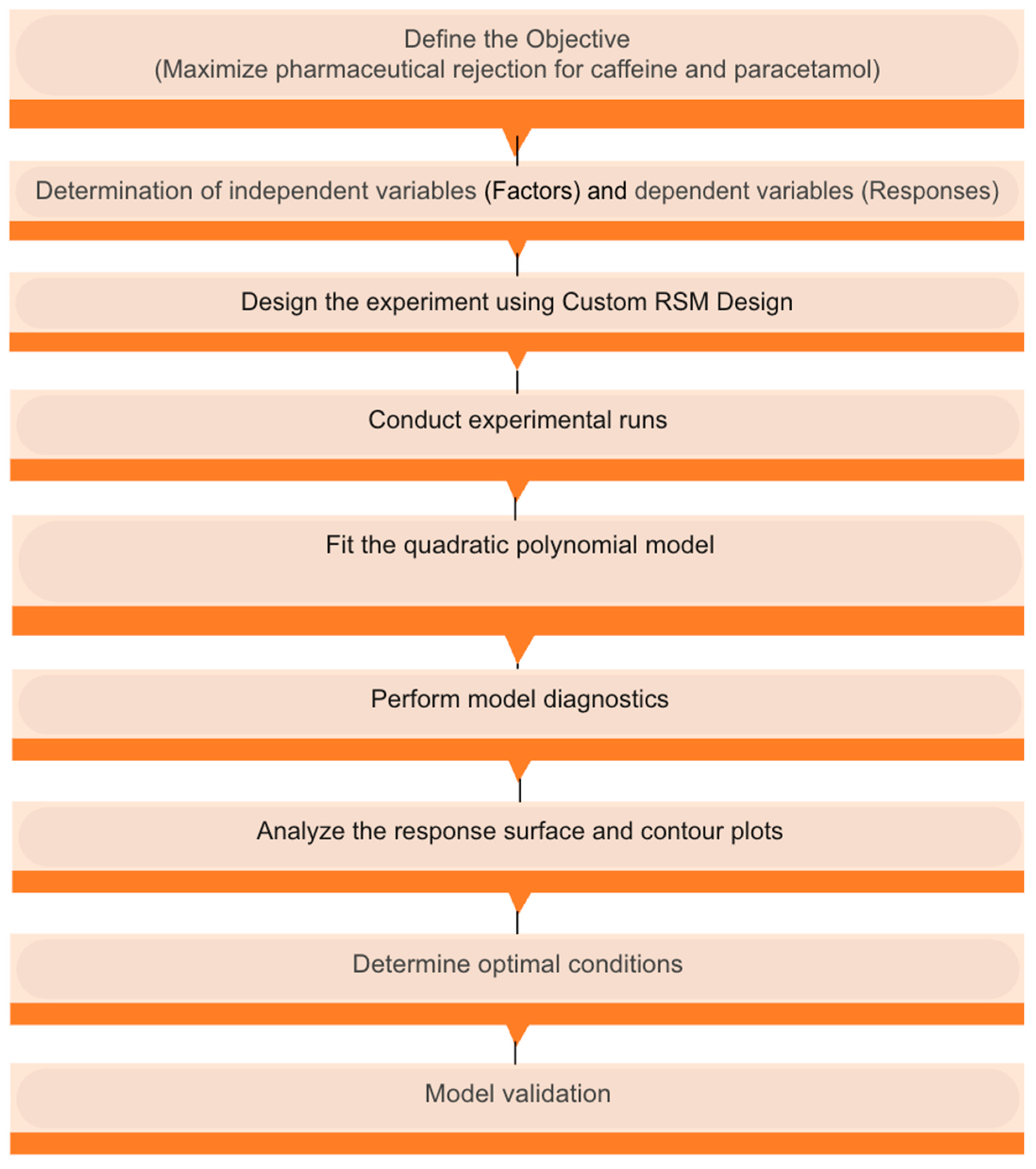
2.4. Experimental Design
2.4.1. Modelling of NF Membranes Using RSM
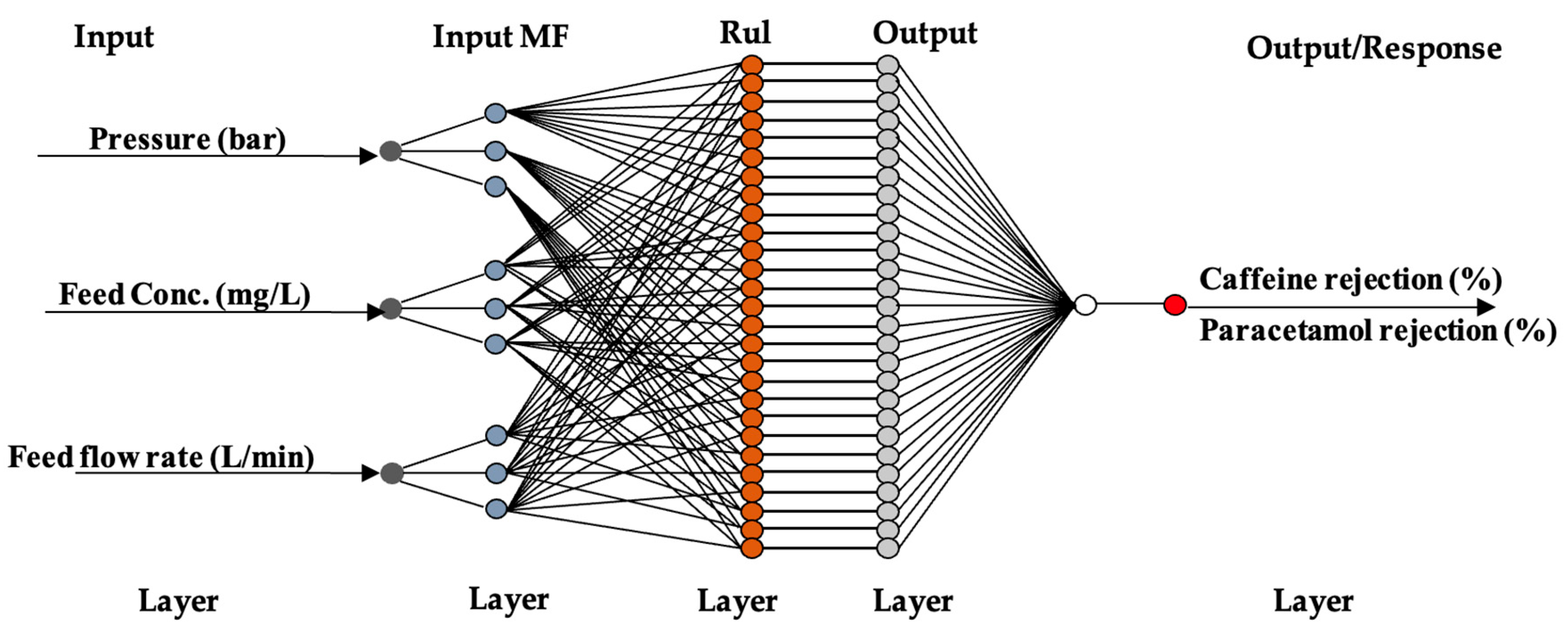
2.4.2. Modelling of NF Membranes Using ANN
3. Results and Discussion
3.1. Development and Validation
3.1.1. ANOVA for Reduced Quadratic Model
3.1.2. Regression Equation
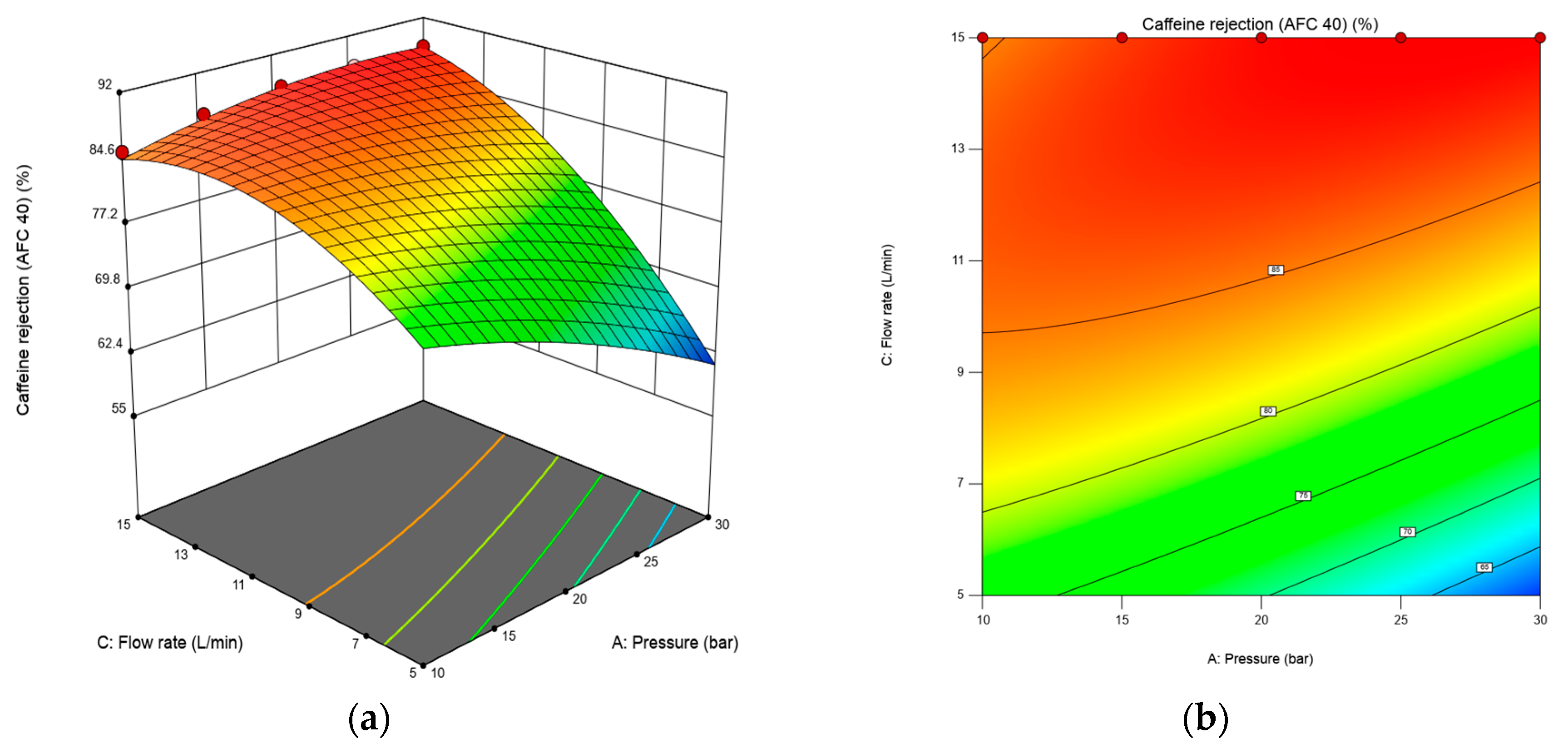
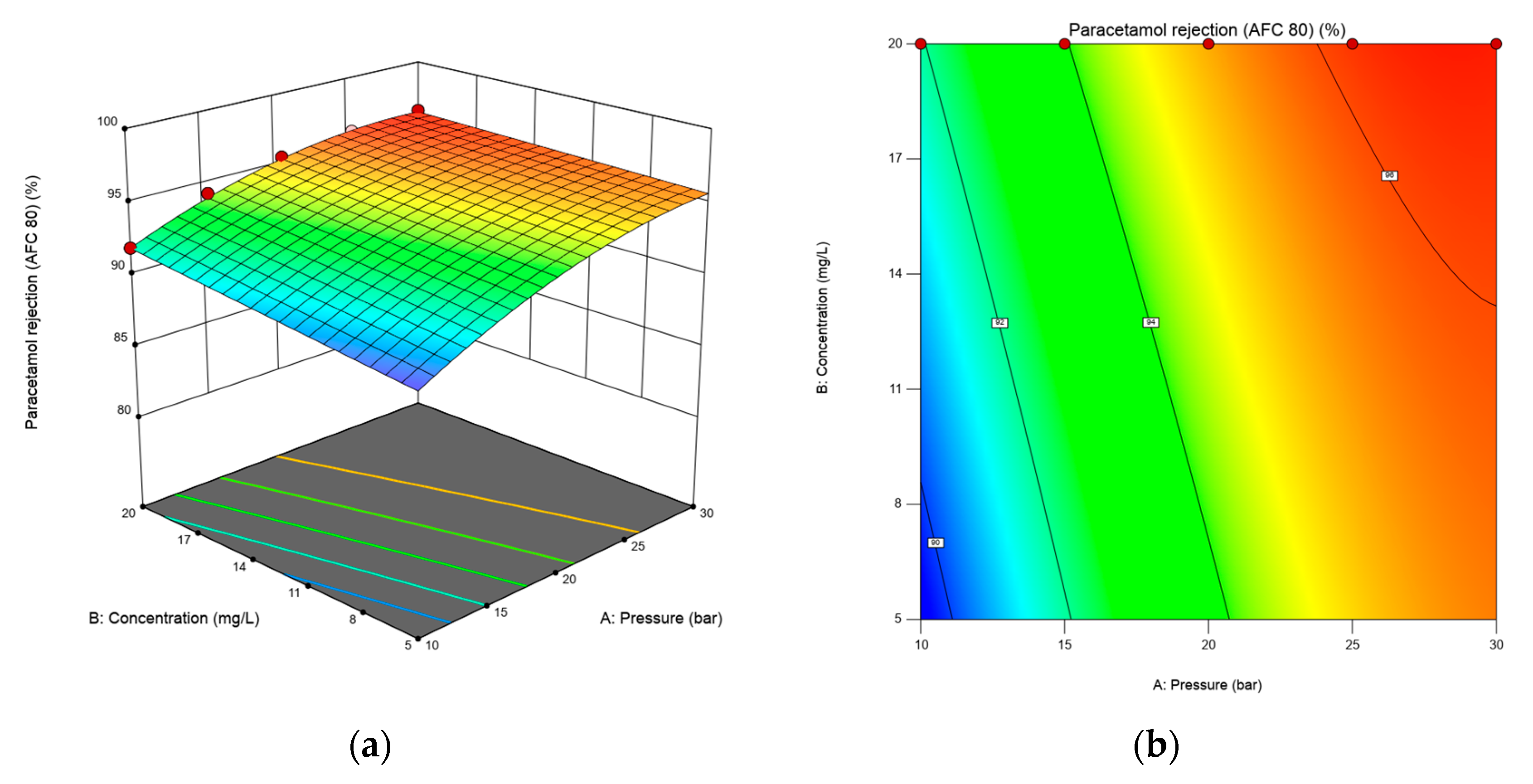
3.1.3. Effects of Transmembrane Pressure, Feed Concentration and Flow Rate on Caffeine and Paracetamol Rejection Using RSM Plots.
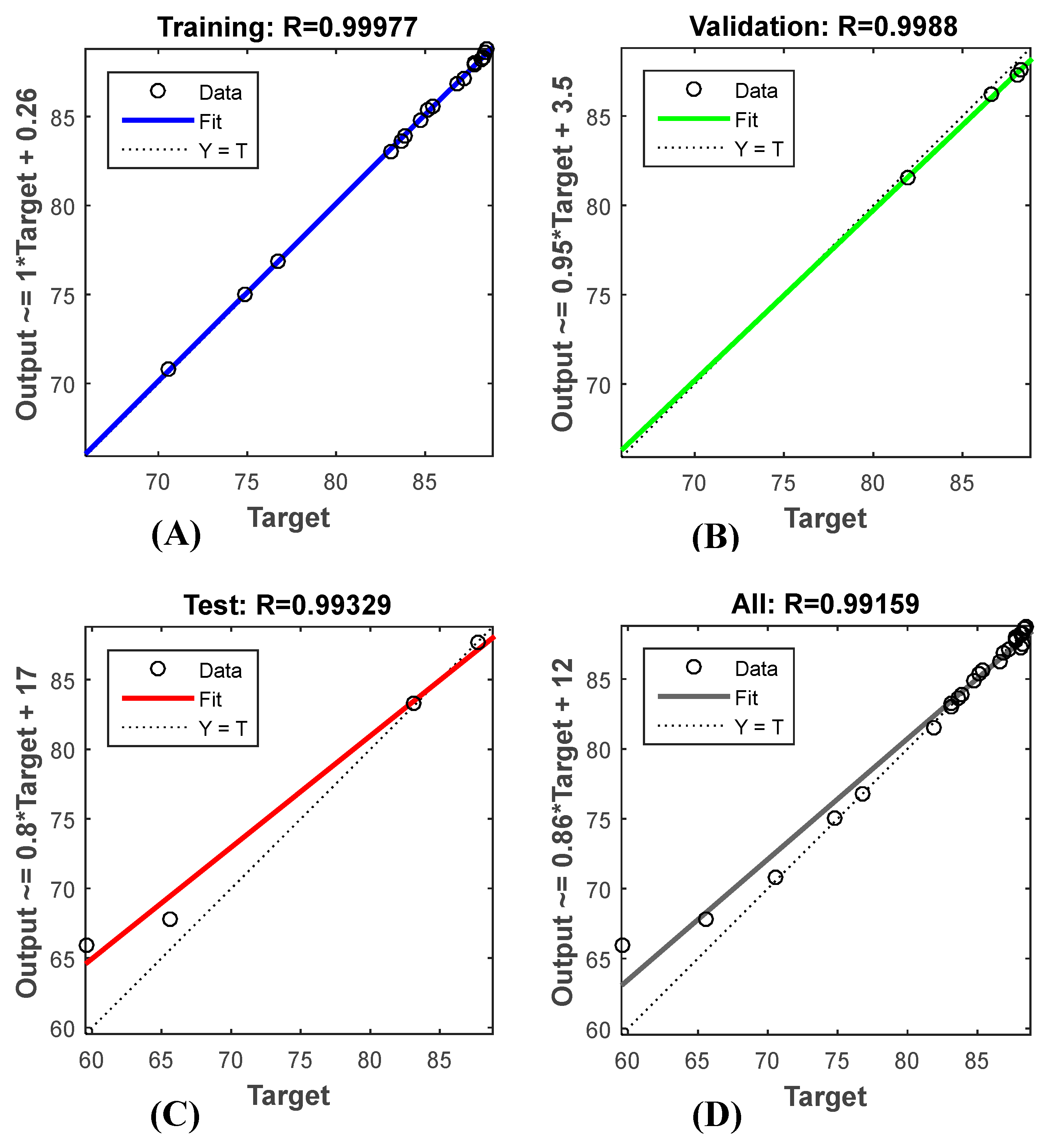
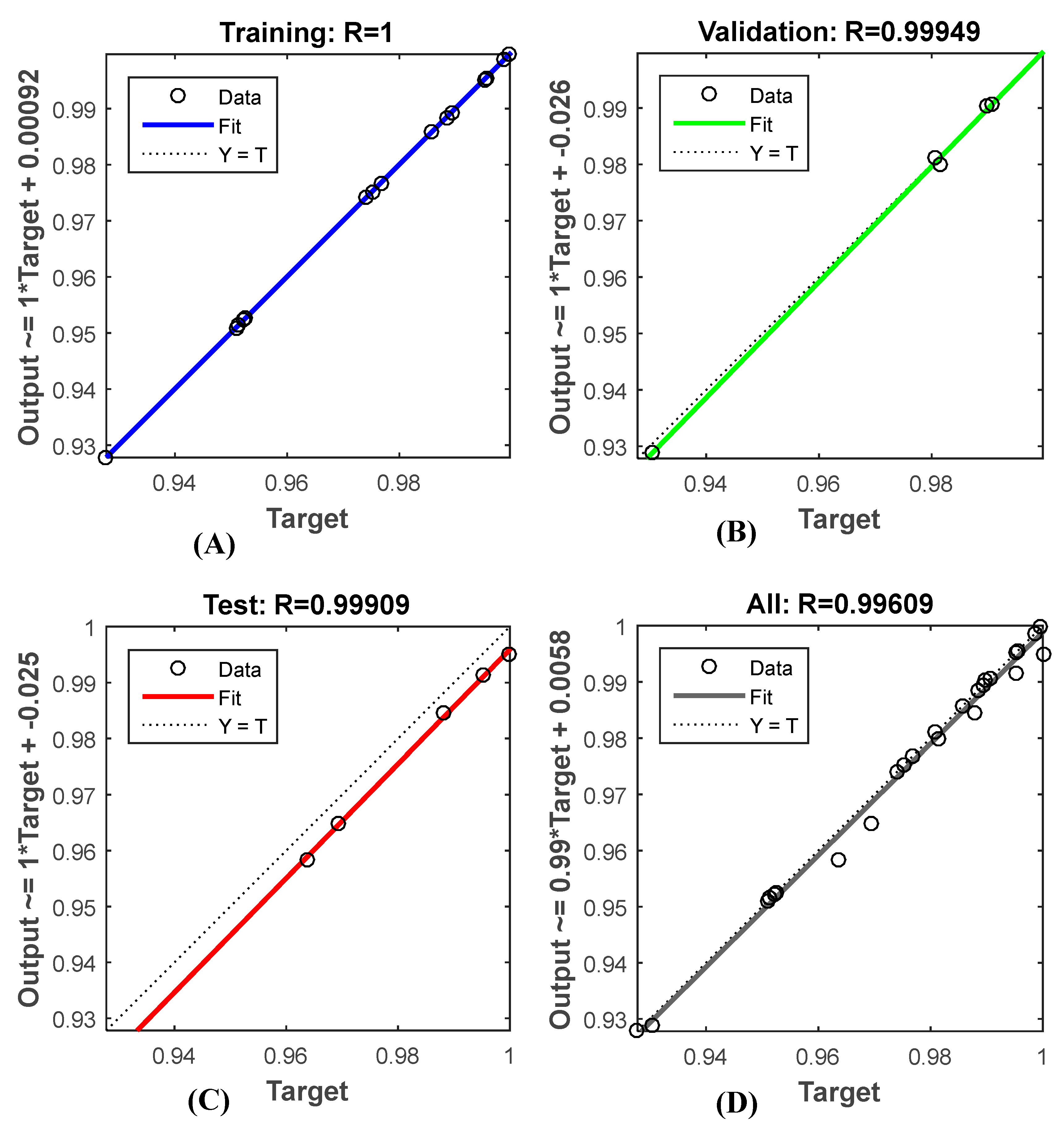
3.2. Predictive Modelling Using ANN
3.2.1. Training, Testing, and Validation of the Model ANN
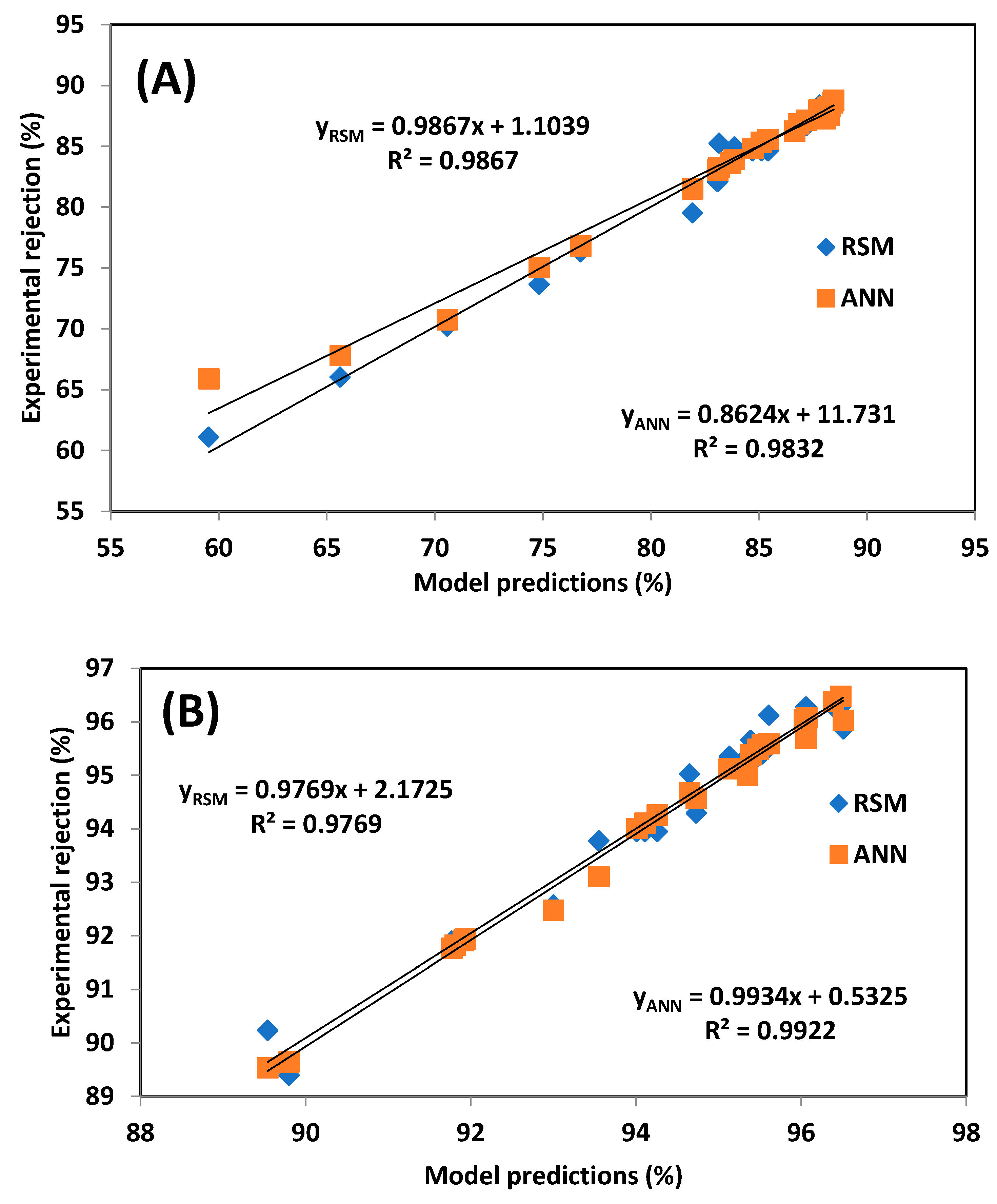
3.3. Comparative Study of the RSM and ANN Models
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgements
Conflicts of Interest
References
- Wilkinson, J.L.; Boxall, A.B.A.; Kolpin, D.W.; Leung, K.M.Y.; Lai, R.W.S.; Galbán-Malagón, C.; Adell, A.D.; Mondon, J.; Metian, M.; Marchant, R.A.; et al. Pharmaceutical pollution of the world’s rivers. Proc Natl Acad Sci U S A 2022, 119. [Google Scholar] [CrossRef] [PubMed]
- Majumder, A.; Gupta, B.; Gupta, A.K. Pharmaceutically active compounds in aqueous environment: A status, toxicity and insights of remediation. Environmental Research 2019, 176, 108542. [Google Scholar] [CrossRef]
- Guo, Y.; Qi, P.S.; Liu, Y.Z. A Review on Advanced Treatment of Pharmaceutical Wastewater. IOP Conference Series: Earth and Environmental Science 2017, 63, 012025. [Google Scholar] [CrossRef]
- Obotey Ezugbe, E.; Rathilal, S. Membrane Technologies in Wastewater Treatment: A Review. Membranes (Basel) 2020, 10. [Google Scholar] [CrossRef] [PubMed]
- Khayet, M.; Seman, M.N.A.; Hilal, N. Response surface modeling and optimization of composite nanofiltration modified membranes. Journal of Membrane Science 2010, 349, 113–122. [Google Scholar] [CrossRef]
- Hilal, N.; Al-Zoubi, H.; Mohammad, A.W.; Darwish, N.A. Nanofiltration of highly concentrated salt solutions up to seawater salinity. Desalination 2005, 184, 315–326. [Google Scholar] [CrossRef]
- Anike, O.; Cuhorka, J.; Ezeogu, N.; Mikulášek, P. Separation of Antibiotics Using Two Commercial Nanofiltration Membranes—Experimental Study and Modelling. Membranes 2024, 14, 248. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration, Don’t Overuse Acetaminophen, FDA. Available online: https://www.fda.gov/consumers/consumer-updates/dont-overuse-acetaminophen (accessed on October 24).
- Ivanova, D.; Tzvetkov, G.; Kaneva, N. Degradation of Paracetamol in Distilled and Drinking Water via Ag/ZnO Photocatalysis under UV and Natural Sunlight. Water 2023, 15, 3549. [Google Scholar] [CrossRef]
- Emami, M.R.S.; Amiri, M.K.; Zaferani, S.P.G. Removal efficiency optimization of Pb2+ in a nanofiltration process by MLP-ANN and RSM. Korean Journal of Chemical Engineering 2021, 38, 316–325. [Google Scholar] [CrossRef]
- Alghooneh, A.; Razavi, S.M.A.; Mousavi, S.M. Nanofiltration treatment of tomato paste processing wastewater: process modeling and optimization using response surface methodology. Desalination and Water Treatment 2016, 57, 9609–9621. [Google Scholar] [CrossRef]
- Mojarrad, M.; Noroozi, A.; Zeinivand, A.; Kazemzadeh, P. Response surface methodology for optimization of simultaneous Cr (VI) and as (V) removal from contaminated water by nanofiltration process. Environmental Progress & Sustainable Energy 2018, 37, 434–443. [Google Scholar]
- Ghaemi, N.; Madaeni, S.S.; Daraei, P.; Rajabi, H.; Shojaeimehr, T.; Rahimpour, F.; Shirvani, B. PES mixed matrix nanofiltration membrane embedded with polymer wrapped MWCNT: Fabrication and performance optimization in dye removal by RSM. Journal of Hazardous Materials 2015, 298, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, A.; K, A.; Nair, A.; Ram, S.; Agarwal, S.; Ali, J.; Singh, R.; Garg, M.C. Response surface methodology and artificial neural network modelling for the performance evaluation of pilot-scale hybrid nanofiltration (NF) & reverse osmosis (RO) membrane system for the treatment of brackish ground water. Journal of Environmental Management 2021, 278, 111497. [Google Scholar] [PubMed]
- Abadikhah, H.; Zokaee Ashtiani, F.; Fouladitajar, A. Nanofiltration of oily wastewater containing salt; experimental studies and optimization using response surface methodology. Desalination and Water Treatment 2015, 56, 2783–2796. [Google Scholar] [CrossRef]
- Gasemloo, S.; Khosravi, M.; Sohrabi, M.R.; Dastmalchi, S.; Gharbani, P. Response surface methodology (RSM) modeling to improve removal of Cr (VI) ions from tannery wastewater using sulfated carboxymethyl cellulose nanofilter. Journal of Cleaner Production 2019, 208, 736–742. [Google Scholar] [CrossRef]
- Rajkumar, K.; Muthukumar, M. Response surface optimization of electro-oxidation process for the treatment of C.I. Reactive Yellow 186 dye: reaction pathways. Applied Water Science 2017, 7, 637–652. [Google Scholar] [CrossRef]
- Koç, B.; Kaymak-Ertekİn, F. Response surface methodology and food processing applications. GIDA - Journal of Food 2010, 35, 63–70. [Google Scholar]
- Aydar, A.Y. Utilization of response surface methodology in optimization of extraction of plant materials. Statistical approaches with emphasis on design of experiments applied to chemical processes 2018, 157–169. [Google Scholar]
- Jomy, J.; Prabhu, D. Custom design approach of design of experiment as a tool for the optimisation of parameters for corrosion inhibition study of (+)-arabinogalactan on AISI 5140 steel. Corrosion Engineering, Science and Technology 2024, 0, 1478422X241272083. [Google Scholar] [CrossRef]
- Peydayesh, M.; Bagheri, M.; Mohammadi, T.; Bakhtiari, O. Fabrication optimization of polyethersulfone (PES)/polyvinylpyrrolidone (PVP) nanofiltration membranes using Box–Behnken response surface method. RSC Advances 2017, 7, 24995–25008. [Google Scholar] [CrossRef]
- Okonkwo, C.P.; Ajiwe, V.I.E.; Obiadi, M.C.; Okwu, M.O.; Ayogu, J.I. Production of biodiesel from the novel non-edible seed of Chrysobalanus icaco using natural heterogeneous catalyst: Modeling and prediction using Artificial Neural Network. Journal of Cleaner Production 2023, 385, 135631. [Google Scholar] [CrossRef]
- Okwu, M.O.; Tartibu, L.K.; Samuel, O.D.; Omoregbee, H.O.; Ivbanikaro, A.E. Predictive Ability of Response Surface Methodology (RSM) and Artificial Neural Network (ANN) to Approximate Biogas Yield in a Modular Biodigester. Cham, 2021; pp. 202-215.
- Al-Abri, M.; Hilal, N. Artificial neural network simulation of combined humic substance coagulation and membrane filtration. Chemical Engineering Journal 2008, 141, 27–34. [Google Scholar] [CrossRef]
- Ibrahim, M.; Haider, A.; Lim, J.W.; Mainali, B.; Aslam, M.; Kumar, M.; Shahid, M.K. Artificial neural network modeling for the prediction, estimation, and treatment of diverse wastewaters: A comprehensive review and future perspective. Chemosphere 2024, 362, 142860. [Google Scholar] [CrossRef] [PubMed]
- Addar, F.Z.; Farah, M.; Belfaquir, M.; Tahaikt, M.; Taky, M.; Elmidaoui, A. Comparison of response surface method and artificial neural network in predicting fluoride removal by nanofiltration. Desalination and Water Treatment 2023, 297, 215–226. [Google Scholar]
- Aydiner, C.; Demir, I.; Yildiz, E. Modeling of flux decline in crossflow microfiltration using neural networks: the case of phosphate removal. Journal of Membrane Science 2005, 248, 53–62. [Google Scholar] [CrossRef]
- Samuel, O.D.; Okwu, M.O. Comparison of Response Surface Methodology (RSM) and Artificial Neural Network (ANN) in modelling of waste coconut oil ethyl esters production. Energy Sources, Part A: Recovery, Utilization, and Environmental Effects 2019, 41, 1049–1061. [Google Scholar] [CrossRef]
- Silva, G.F.; Camargo, F.L.; Ferreira, A.L.O. Application of response surface methodology for optimization of biodiesel production by transesterification of soybean oil with ethanol. Fuel Processing Technology 2011, 92, 407–413. [Google Scholar] [CrossRef]
- Onu, C.E.; Nwabanne, J.T.; Ohale, P.E.; Asadu, C.O. Comparative analysis of RSM, ANN and ANFIS and the mechanistic modeling in eriochrome black-T dye adsorption using modified clay. South African Journal of Chemical Engineering 2021, 36, 24–42. [Google Scholar] [CrossRef]
- Addar, F.; Qaid, S.; Zeggar, H.; El Hajji, H.; Tahaikt, M.; Elmidaoui, A.; Taky, M. Ultrafiltration of Moroccan Valencia orange juice: juice quality, optimization by custom designs and membranefouling. Sustainability, Agri, Food and Environmental Research-DISCONTINUED 2023, 11. [Google Scholar] [CrossRef]
- Zheng, Y.; Wang, A. Removal of heavy metals using polyvinyl alcohol semi-IPN poly (acrylic acid)/tourmaline composite optimized with response surface methodology. Chemical Engineering Journal 2010, 162, 186–193. [Google Scholar] [CrossRef]
- Gherasim, C.-V.; Mikulášek, P. Influence of operating variables on the removal of heavy metal ions from aqueous solutions by nanofiltration. Desalination 2014, 343, 67–74. [Google Scholar] [CrossRef]
- Fang, J.; Deng, B. Arsenic Rejection by Nanofiltration Membranes: Effect of Operating Parameters and Model Analysis. Environmental Engineering Science 2014, 31, 496–506. [Google Scholar] [CrossRef]
- Ghorbani, A.; Bayati, B.; Drioli, E.; Macedonio, F.; Kikhavani, T.; Frappa, M. Modeling of Nanofiltration Process Using DSPM-DE Model for Purification of Amine Solution. Membranes 2021, 11, 230. [Google Scholar] [CrossRef]
- Garcia-Ivars, J.; Martella, L.; Massella, M.; Carbonell-Alcaina, C.; Alcaina-Miranda, M.-I.; Iborra-Clar, M.-I. Nanofiltration as tertiary treatment method for removing trace pharmaceutically active compounds in wastewater from wastewater treatment plants. Water Research 2017, 125, 360–373. [Google Scholar] [CrossRef] [PubMed]
- Onu, C.E.; Ohale, P.E.; Ekwueme, B.N.; Obiora-Okafo, I.A.; Okey-Onyesolu, C.F.; Onu, C.P.; Ezema, C.A.; Onu, O.O. Modeling, optimization, and adsorptive studies of bromocresol green dye removal using acid functionalized corn cob. Cleaner Chemical Engineering 2022, 4, 100067. [Google Scholar] [CrossRef]
- Arulsudar, N.; Subramanian, N.; Murthy, R. Comparison of artificial neural network and multiple linear regression in the optimization of formulation parameters of leuprolide acetate loaded liposomes. J Pharm Pharm Sci 2005, 8, 243–258. [Google Scholar]
- Fetimi, A.; Dâas, A.; Benguerba, Y.; Merouani, S.; Hamachi, M.; Kebiche-Senhadji, O.; Hamdaoui, O. Optimization and prediction of safranin-O cationic dye removal from aqueous solution by emulsion liquid membrane (ELM) using artificial neural network-particle swarm optimization (ANN-PSO) hybrid model and response surface methodology (RSM). Journal of Environmental Chemical Engineering 2021, 9, 105837. [Google Scholar] [CrossRef]
- Addar, F.; Farah, M.; Tahaikt, M.; Taky, M.; Fatima Zahra, A. Desalination and Water Treatment Comparison of response surface method and artificial neural network in predicting fluoride removal by nanofiltration. 2023.
- Ohale, P.E.; Onu, C.E.; Nwabanne, J.T.; Aniagor, C.O.; Okey-Onyesolu, C.F.; Ohale, N.J. A comparative optimization and modeling of ammonia–nitrogen adsorption from abattoir wastewater using a novel iron-functionalized crab shell. Applied Water Science 2022, 12, 193. [Google Scholar] [CrossRef]
| Factor or Independent variable | Level 1 | Level 2 | Level 3 | Level 4 | Level 5 |
|---|---|---|---|---|---|
| X1 (Pressure, bar) | 10 | 15 | 20 | 25 | 30 |
| X2 (Feed concentration, mg/L) | 10 | 15 | 20 | - | - |
| X3 (Feed flow rate, L/min) | 5 | 10 | 15 | - | - |
| Run | Independent variables | Caffeine Rejection (AFC 40) |
Paracetamol Rejection (AFC 80) |
Model Residuals | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (AFC 40) | (AFC 80) | |||||||||||||
| X1 [bar] | X2 [mg/L] |
X3 [L/min] | Y Exp. [%] |
YRSM [%] |
YANN [%] |
YExp. [%] |
YRSM [%] |
YANN [%] |
RSM [-] |
ANN [-] |
RSM [-] |
ANN [-] |
||
| 1 | 10 | 20 | 15 | 84.71 | 84.62 | 84.83 | 91.91 | 91.92 | 91.91 | 0.0897 | -0.1195 | -0.0143 | 0.0017 | |
| 2 | 15 | 20 | 15 | 86.65 | 86.69 | 86.26 | 94.01 | 93.95 | 94.01 | -0.0390 | 0.3919 | 0.0576 | -0.0018 | |
| 3 | 20 | 20 | 15 | 87.77 | 88.01 | 87.73 | 95.35 | 95.36 | 95.01 | -0.2369 | 0.0412 | -0.0051 | 0.3413 | |
| 4 | 25 | 20 | 15 | 88.16 | 88.57 | 88.21 | 96.04 | 96.13 | 96.04 | -0.4139 | -0.0512 | -0.0923 | -0.0025 | |
| 5 | 30 | 20 | 15 | 88.29 | 88.39 | 88.29 | 96.48 | 96.28 | 96.48 | -0.1000 | 0.0031 | 0.1958 | -0.0005 | |
| 6 | 10 | 10 | 15 | 85.42 | 84.62 | 85.57 | 89.54 | 90.24 | 89.54 | 0.7997 | -0.1503 | -0.6993 | -0.004 | |
| 7 | 15 | 10 | 15 | 87.19 | 86.69 | 87.15 | 93.00 | 92.58 | 92.48 | 0.5010 | 0.0358 | 0.4154 | 0.5196 | |
| 8 | 20 | 10 | 15 | 88.06 | 88.01 | 87.28 | 94.73 | 94.30 | 94.57 | 0.0531 | 0.7786 | 0.4254 | 0.1625 | |
| 9 | 25 | 10 | 15 | 88.23 | 88.57 | 87.54 | 95.53 | 95.40 | 95.58 | -0.3439 | 0.6924 | 0.1309 | -0.0504 | |
| 10 | 30 | 10 | 15 | 88.45 | 88.39 | 88.80 | 96.51 | 95.87 | 96.03 | 0.0600 | -0.3528 | 0.6418 | 0.4831 | |
| 11 | 10 | 5 | 15 | 85.11 | 84.62 | 85.34 | 89.80 | 89.40 | 89.65 | 0.4897 | -0.2261 | 0.4032 | 0.1533 | |
| 12 | 15 | 5 | 15 | 86.83 | 86.69 | 86.85 | 91.77 | 91.90 | 91.77 | 0.1410 | -0.0179 | -0.1308 | 0.0007 | |
| 13 | 20 | 5 | 15 | 87.77 | 88.01 | 87.98 | 93.55 | 93.78 | 93.11 | -0.2369 | -0.2084 | -0.2293 | 0.4361 | |
| 14 | 25 | 5 | 15 | 88.40 | 88.57 | 88.59 | 94.65 | 95.03 | 94.68 | -0.1739 | -0.185 | -0.3825 | -0.0326 | |
| 15 | 30 | 5 | 15 | 87.80 | 88.39 | 87.91 | 95.39 | 95.66 | 95.39 | -0.5900 | -0.111 | -0.2702 | 0.0018 | |
| 16 | 10 | 20 | 10 | 83.15 | 85.26 | 83.25 | 91.93 | 91.92 | 91.94 | -2.11 | -0.1032 | 0.0057 | -0.0122 | |
| 17 | 15 | 20 | 10 | 83.86 | 84.95 | 83.92 | 94.26 | 93.95 | 94.26 | -1.09 | -0.0642 | 0.3076 | -0.0047 | |
| 18 | 20 | 20 | 10 | 83.68 | 83.89 | 83.63 | 95.48 | 95.36 | 95.49 | -0.2109 | 0.0502 | 0.1249 | -0.0056 | |
| 19 | 25 | 20 | 10 | 83.09 | 82.08 | 83.05 | 96.07 | 96.13 | 96.08 | 1.01 | 0.0375 | -0.0623 | -0.0057 | |
| 20 | 30 | 20 | 10 | 81.92 | 79.52 | 81.51 | 96.39 | 96.28 | 96.39 | 2.40 | 0.4122 | 0.1058 | 0.0003 | |
| 21 | 10 | 20 | 5 | 76.76 | 76.33 | 76.82 | 91.81 | 91.92 | 91.83 | 0.4255 | -0.0613 | -0.1143 | -0.0205 | |
| 22 | 15 | 20 | 5 | 74.83 | 73.65 | 75.05 | 94.11 | 93.95 | 94.11 | 1.18 | -0.2214 | 0.1576 | -0.0002 | |
| 23 | 20 | 20 | 5 | 70.58 | 70.22 | 70.75 | 95.13 | 95.36 | 95.13 | 0.3631 | -0.1731 | -0.2251 | -0.0012 | |
| 24 | 25 | 20 | 5 | 65.62 | 66.03 | 67.81 | 95.61 | 96.13 | 95.60 | -0.4117 | -2.1909 | -0.5223 | 0.0113 | |
| 25 | 30 | 20 | 5 | 59.54 | 61.10 | 65.91 | 96.06 | 96.28 | 95.69 | -1.56 | -6.3702 | -0.2242 | 0.3688 | |
| Source | Sum of Squares | df | Mean Square | F-value | p-value | |
|---|---|---|---|---|---|---|
| Model | 1389.46 | 5 | 277.89 | 282.59 | < 0.0001 | significant |
| A-Pressure [X₁] | 82.21 | 1 | 82.21 | 83.60 | < 0.0001 | |
| C-Flow rate [X3] | 1186.82 | 1 | 1186.82 | 1206.90 | < 0.0001 | |
| AC | 180.66 | 1 | 180.66 | 183.72 | < 0.0001 | |
| A² | 9.87 | 1 | 9.87 | 10.03 | 0.0051 | |
| C² | 85.65 | 1 | 85.65 | 87.10 | < 0.0001 | |
| Residual | 18.68 | 19 | 0.9834 | < 0.0001 | ||
| Cor Total | 1408.14 | 24 | ||||
| R2 | 0.9867 | |||||
| Adjusted R2 | 0.9832 | |||||
| Predicted R2 | 0.9645 | |||||
| Adequate Precision | 58.5621 |
| Source | Sum of Squares | df | Mean Square | F-value | p-value | |
|---|---|---|---|---|---|---|
| Model | 97.84 | 4 | 24.46 | 211.73 | < 0.0001 | significant |
| A-Pressure [X₁] | 76.25 | 1 | 76.25 | 660.04 | < 0.0001 | |
| B-Concentration [X2] | 11.04 | 1 | 11.04 | 95.52 | < 0.0001 | |
| AB | 2.01 | 1 | 2.01 | 17.42 | 0.0005 | |
| A² | 6.85 | 1 | 6.85 | 59.25 | < 0.0001 | |
| Residual | 2.31 | 20 | 0.1155 | |||
| Cor Total | 100.15 | 24 | ||||
| R2 | 0.9769 | |||||
| Adjusted R2 | 0.9723 | |||||
| Predicted R2 | 0.9590 | |||||
| Adequate Precision | 45.3104 |
| Error Function | AFC 40 membrane | AFC 80 membrane | ||
| RSM | ANN | RSM | ANN | |
| R2 | 0.9867 | 0.9832 | 0.9769 | 0.9922 |
| RMSE | 0.8654 | 1.3700 | 0.3041 | 0.1999 |
| MPSED (%) | 0.0125 | 0.0512 | 0.0011 | 0.0004 |
| HYBRID (%) | 1.0893 | 3.5254 | 0.1124 | 0.0480 |
| AAD (%) | 0.7610 | 0.7720 | 0.2534 | 0.1101 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





