1. Introduction
Diabetes mellitus (DM) is a persistent metabolic condition. Hyperglycemia, caused by impaired insulin secretion, action, or both, is a condition in which the body fails to generate or utilise insulin effectively. This leads to elevated blood sugar levels, glycosuria, excessive thirst, increased appetite, and slow weight loss [
1]. Estimates place the prevalence of diabetes in the 20–79 age group at 10.5%, and in the 75–79 age group at 24.0% in 2021. Low- and middle-income countries have 432.7 million people with diabetes. The prevalence of diabetes was 360 million in urban regions and 176.6 million in rural areas. It is estimated that the urban population with diabetes will reach 596.5 million in 2045. The Middle East and North Africa region showed the highest prevalence (18.1%), while the lowest was in the Africa region (5.3%) [
2].
Diabetes mellitus is commonly accompanied by dreadful co-morbid conditions like nephropathy, which can lead to renal failure; retinopathy can cause vision loss, and peripheral neuropathy can cause foot ulcers., lower extremity gangrene, amputations, stupor, coma, and other symptoms. Diabetes also causes end-stage organ damage and failure, including damage to and failure of the heart, kidneys, eyes, nerves, and blood vessels. The prevalence of cardiovascular and cerebrovascular disorders is rising. Diabetes with cardiovascular problems is to blame for the high morbidity and mortality rate [
3]. Diabetes (
Dhayābītus) is also a concept in the Unani medical system. Ibn Sina (Avicenna) [980-1037 AD] described the indications and symptoms of diabetes, including polyuria, polydipsia, and physical and mental weakness. Ibn Sina also listed two particular diabetes consequences, namely erectile dysfunction and gangrene [
4].
There are several side effects associated with the medications used to treat DM. Some drugs, such as biguanide and sulfonylureas, are commonly used to manage DM to reduce hyperglycemia. However, such treatments have adverse effects and are also very expensive. Long-term use of certain medications may result in irreversible damage [
5].
Unani medicines are beneficial in the management of DM. Unani physicians mentioned safe and valuable medications; that have been used successfully for centuries. A wide variety of drugs with antidiabetic effects and effective in treating diabetes (
Dhayābītus) (NUMC: G-2) have been listed in Unani literature. Unani physicians have used several essential single medications in the treatment of DM, i.e., Jamun (
Syzygium cumini (L.) Skeels)
, Amla (
Emblica officinalis Gaertn.)
, Gilo (
Tinospora cordifolia (Willd.) Miers)
, Neem (
Azadirachta indica A. Juss.)
, Tukhm Kahu (
Lactuca sativa L.)
, Mastagi (
Pistacia lentiscus L.)
, Tukhm Khurfa (
Portulaca oleracea L.), Kalonji (
Nigella sativa L.)
, Tukhm Hayat (
Withania coagulans (Stocks) Dunal), Tukhm Karela (
Momordica charantia L.)
, Kachnar (
Bauhinia variegata L.)
, Lahsan (
Allium sativum L.)
, and Halela zard (
Terminalia chebula Retz.) [
6].
In addition to single medications, Unani classical literature describes various multi-ingredient combinations formulations for Diabetes (
Dhayābītus) (NUMC: G-2), like
Qurs Dhayābītus, Qurs Dhayābītus Kafoori, Qurs Kafoor, Qurs Tabasheer Kafoori, Qurs Kushta Baiza Murgh, Kushta Zamarrud, Safoof Dhayābītus, and Dulabi [
7,
8].
The medical industry is still grappling with controlling diabetes mellitus without causing side effects because conventional diabetes medications have several adverse effects. The traditional medicines used to treat diabetes do not fully meet the need; new anti-diabetic pharmaceuticals are urgently needed to address this issue. Given this urgency, a Compound Unani Formulation (CUF) has been selected to evaluate its antidiabetic potential. The formulation has six ingredients, i.e.,
Maghz Khasta-e-Jamun (kernel of the seeds of
Eugenia jambolana L.) [10 parts], Maghz Khasta-e-Anb (kernel of the seeds of
Mangifera indica L.) [10 parts], Gudmaar booti (
Gymnema sylvestre R. Br.) [10 parts], Post Kekar (bark of
Acacia arabica L.) [10 parts], Zanjabeel (rhizome of
Zingiber officinale Rosc.) [3 parts] and Marjān Sokhta (
Corralium rubrum) [3 parts]. Eminent Unani physicians prescribe it as a Mujarrabat therapy (proven classical regimen) for DM control [
9]. The fine powder of these six ingredients were used in a dose of 3 gm twice a day.
However, the CUF has been used clinically for so many years successfully; it was chosen to analyze scientific parameters for establishing its reverse pharmacology. This study aimed to assess the antidiabetic effects in Nicotinamide-Streptozotocin (Nic-STZ) induced diabetic Rats. The selection for combination therapy (Nic-STZ) is based on the finding by Szkudelski T, 2012 to create a model with moderate hyperglycemia which mimics type-2 diabetes [
10].
2. Materials and methods
2.1. Plant Ingredients
All the ingredients of the formulation were purchased from commercial sources in Hyderabad. The Botanist confirmed the identity of the raw samples. The representative specimens were preserved for reference and record (SMPU/CRI-Hyd-14084, 14085, 14086, 14087, 14088, and 14089) for Maghz Khasta-e-Jamun, Maghz Khasta-e-Anb, Gudmaar booti, Post Kekar, Zanjabeel and Marjaan Sokhta, respectively.
2.2. TLC and HPTLC Fingerprinting
The ethanolic extract was used for TLC examination on pre-coated silica gel plates as per the standard method [
11].
2.3. HPTLC Instrument and Method Conditions
Desaga Sarstedt Gruppe (Germany) manufactured instrument; 20x10 cm development chamber; stationary phase 60 F254 aluminium plates with pre-coated silica gel (Merck, Germany); HPLC grade solvents were utilized; Toluene: ethyl acetate (8:2) v/v mobile phase was used; UV at 366 nm, 254 nm, and 580 nm after derivatization as reported earlier [
12].
2.3. Animals
In this experiment, Wistar rats (180-200 g; age: 8-10 weeks) were used. The ICMR-National Institute of Nutrition Hyderabad provided the animals. Standard laboratory conditions were maintained (12/12 hrs light/dark, Temp: 22°C±3°C, RH: 30-70%). Three animals of the same sex were housed in laboratory cages with corncob bedding. A standard rodent chow diet and unlimited access to potable drinking water were provided. The experimental approach adhered to the requirements set by CPCSEA and received approval from the Institutional Animal Ethics Committee of the Institute with Registration No. CRIUM/IAEC/2018/02/P01.
2.4. Experimental Procedure
The animal dose of CUF was determined by allometric dose conversion for rats based on body surface area [
13]. CUF has a dosing range of 3 g twice daily, according to Unani literature. The animal dose was determined to be 600 mg/kg body weight. Furthermore, the impact of the test drug at different doses was examined using a second dose of 1200 mg/kg, which is twice the therapeutic equivalent dose. The powdered crude drug was made using a grinder in the GMP-certified pharmacy and stored. The test medication’s fine powder was mixed with a 0.3% aqueous suspension of carboxyl methylcellulose (CMC). Low and high dosages were made by mixing 10 ml of 0.3 % CMC with 600 mg of CUF powder and 20 ml of 0.3 % CMC with 1200 mg of CUF powder, respectively. Streptozotocin was dissolved in ice-cold citrate buffer and given intraperitoneally at a dose of 65 mg/kg, 15 min. after that Nicotinamide (120 mg/kg) was administration intraperitoneally. Every day, a new test dose suspension was prepared. The suspension was administered through a stainless-steel feeding cannula after being homogenized for a minute by vigorous shaking.
2.5. Oral Glucose Tolerance Test (OGTT)
The OGTT was performed to determine how much glucose was used in the peripheral tissues. Rats were fasted for 24 hours before being given the drug. Rats were randomised into four groups (n=6) as follows:
Vehicle control group: The animals were treated with 0.3% CMC suspension orally.
Positive control group: The animals were administered glibenclamide (10 mg/kg) orally.
Low Dose: The animals were administered CUF powder (600 mg/kg) orally.
High Dose: The animals were administered CUF powder (1200 mg/kg) orally.
After the CUF powder and glibenclamide treatments, glucose (2 gm/kg) was given orally 30 minutes later. Blood samples were taken at 0 minutes (before glucose injection), 30, 60, and 120 minutes after glucose administration to measure blood glucose levels [
14].
2.6. Test for Nicotinamide Streptozotocin-Induced Diabetes
A total of 30 male rats were randomly allocated into 5 groups (n=6). Prior to commencing the trial, all animals had an overnight fasting period. During the experiment, the body weight of rats was measured and diabetes was induced by injecting Nicotinamide (120 mg/kg; i.p.) and Streptozotocin (65 mg/kg; i.p.) within 15 minutes. After 10 days of inductions, blood samples were withdrawn from the retro-orbital plexus, and fasting blood glucose (FBG) was estimated in all experimental animals using a glucometer (ACCU-CHEK active). Rats found with steady hyperglycemia (FBG~200 mg/dL) were taken for further study, and the following dose regimen was given [
15]:
Vehicle control: The animals were administered with 0.3% CMC suspension orally from the 10th day onward for 28 days.
Streptozotocin Control: Rats were administered with 0.3% CMC suspension orally from the 10th day onward for 28 days.
Positive Control: The animals were treated with glibenclamide (10 mg/kg) orally from the 10th day onward for 28 days.
Low dose: The animals were administered CUF powder (600 mg/kg) orally from the 10th day onward for 28 days.
High dose: The animals were administered CUF powder (1200 mg/kg) orally from the 10th day onward for 28 days.
2.7. Blood Collection for Biochemical Assays
After completion of the experiment (day 28), 2-3 ml of blood was collected from the retro-orbital plexus under isoflurane inhalation (EZ anaesthetic system) for biochemical estimation.
2.8. Histopathological Examination
Immediately after the collection of blood samples, rats were euthanised under carbon dioxide and subjected to gross necropsy. Liver, kidney, and pancreas were isolated and processed for histological examination.
2.9. DPPH Scavenging Activity
The scavenging behaviour of DPPH was assessed by a procedure which was already shown to have been changed slightly [
1]. Aliquoting of 80μl of 0.1 mM DPPH was quickly mixed into a test plate with 20μl of different concentrated test extracts (3.75-960 μg / ml) were added to the reaction mix followed by allowing the plate for 30 min in dark. Later the OD readings were taken at 517nm by using Tecan Multimode Microplate reader. The scavenging activity percentage (%) was determined by
% scavenging = [(Absorbance of control-Absorbance of sample) / (Absorbance of control)] x 100 [
16].
2.10. ABTS Scavenging Activity
The ABTS assay was performed as described earlier with few modifications. The ABTS•+ formation was generated by preparing 7 mM ABTS in distilled water and oxidized using 2.45 mM of potassium sulphate by coalescing the same quantity of the two stock solutions, and permitting for a 16 hours room temperature reaction of a stock blue green mixture maintaining in dark room. 1 mL ABTS •+ stock solution with 60 ml of 100 per cent methanol was then prepared as working solution. Test extracts of 20μl from indicated concentrations (3.75-960 μg / ml) were added to 180μl of ABTS•+ work reagent in a 96- micro well plate followed by incubating the plate at room temperature for 2 hours in a dark condition. Absorption was calculated at 734 nm in Tecan Multimode Microplate reader. In this study. The % of ABTS was calculated by:
% scavenging = [(Absorbance of control-Absorbance of sample) / (Absorbance of control)] x 100 [
17].
2.11. FRAP Scavenging Activity
The FRAP (Ferric reducing antioxidant power) activity was performed as described earlier with few modifications [
18] using Tecan Multimode Microplate Reader. Ascorbic acid was taken as positive control.
% scavenging = [(Absorbance of control-Absorbance of sample) / (Absorbance of control)] x 100 [
18]].
2.12. Statistical Analysis
For statistical analysis purposes, GraphPad Prism (Version 5.0) was used. One-way analysis of variance (ANOVA) followed by Tukey’s test was performed. The results were reported as Mean of six observations ± SEM. A significance level of p<0.05 was considered statistically significant.
3. Results
3.1. TLC and HPTLC Fingerprinting
TLC plate detection at UV 366 nm reveals eleven spots with Rf values of 0.03, 0.11, 0.14, 0.19 (red), 0.20 (pinkish red), 0.49, 0.51, 0.57, 0.63, 0.71, 0.79 (red); and after derivatization with vanillin sulphuric acid reveals seven spots with Rf values of 0.07, 0.09, 0.34, 0.47, 0.63, 0.96, 0.99 (All grey). The acquired HPTLC densitogram at 366 and 580 were also attached (data not presented).
3.2. Oral Glucose Tolerance Test (OGTT)
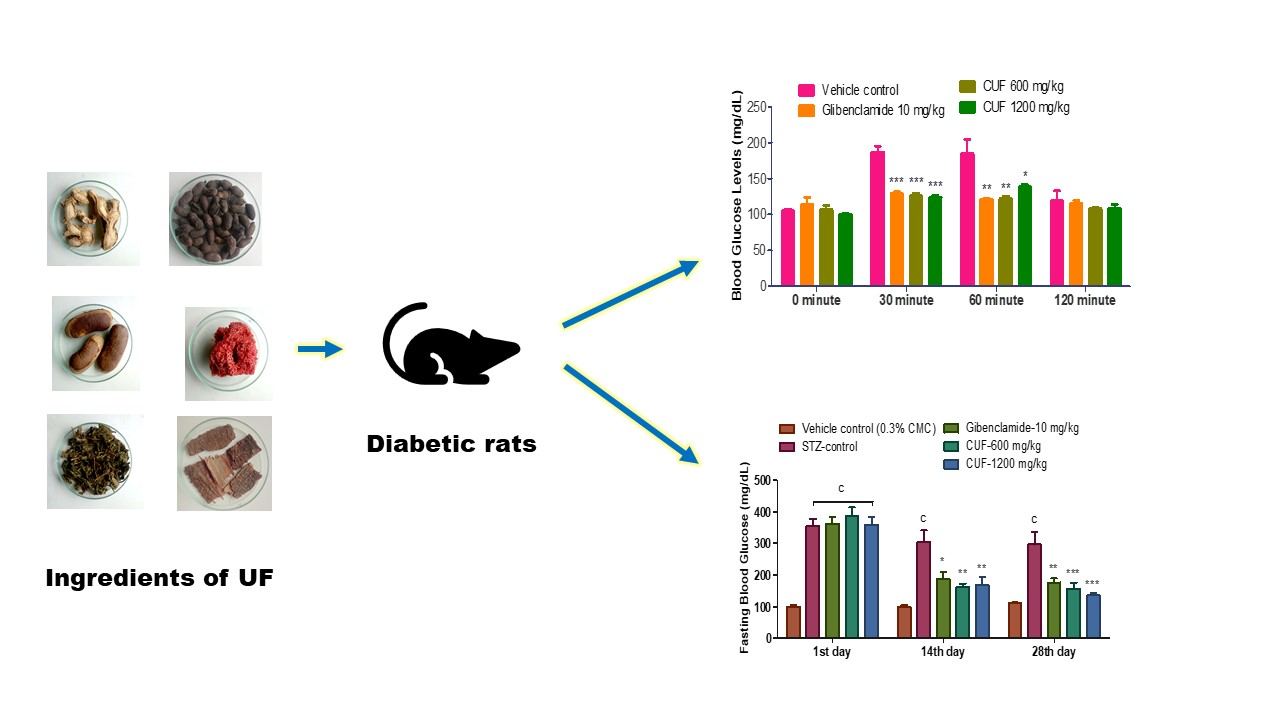
Compared to vehicle control animals, animals treated with CUF (600 mg/kg), CUF (1200mg/kg), and glibenclamide demonstrated a significant drip (p˂0.001) in blood glucose levels at 30 minutes and a significant decline (p˂0.01) at 60 minutes after oral glucose administration. CUF had the most significant effect (600 mg/kg and 1200 mg/kg) 30 minutes after oral glucose treatment (p˂0.001) (
Figure 1).
3.3. Fasting Blood Glucose (FBG) in Nic-Stz induced Diabetic Rats
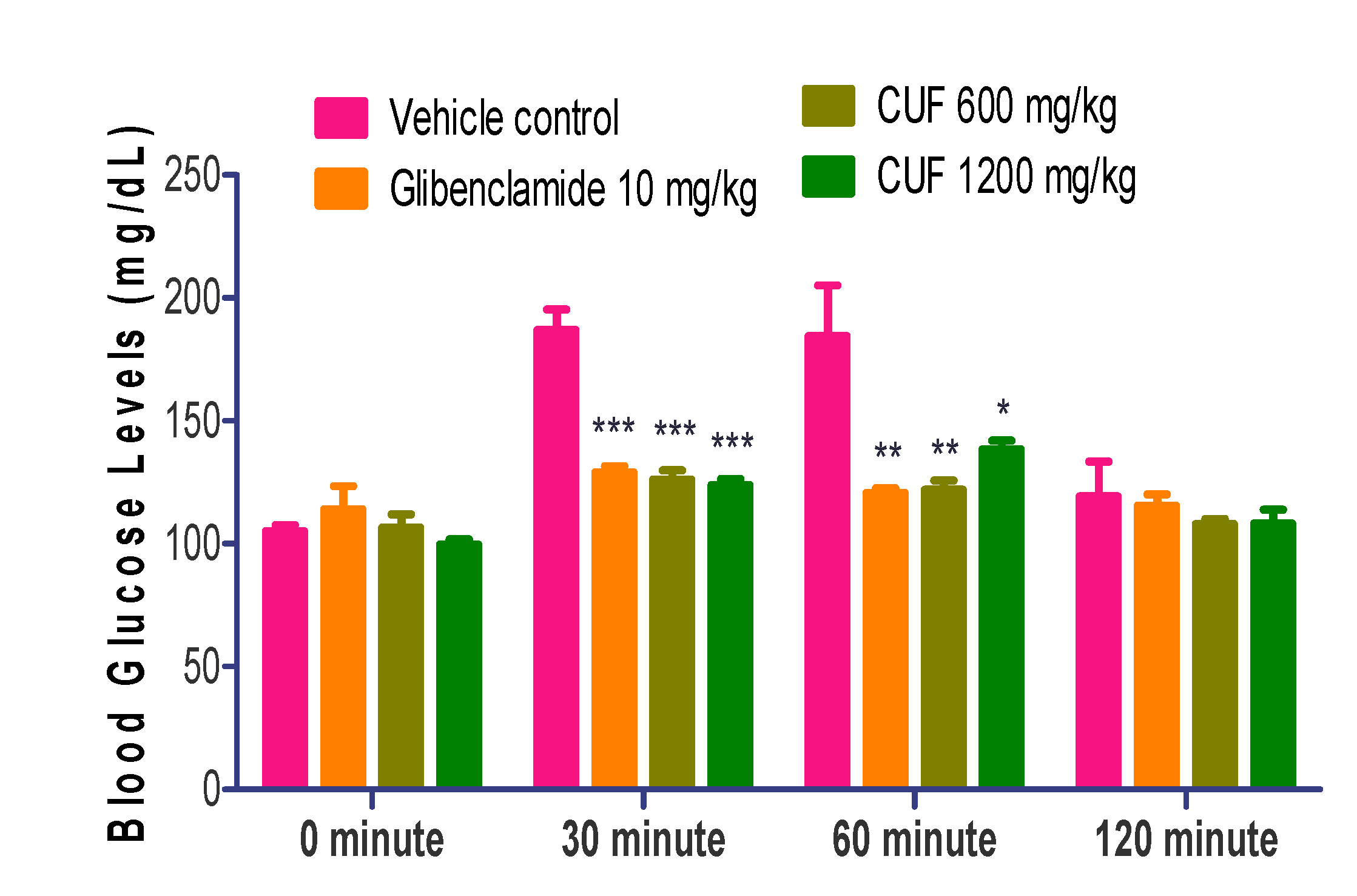
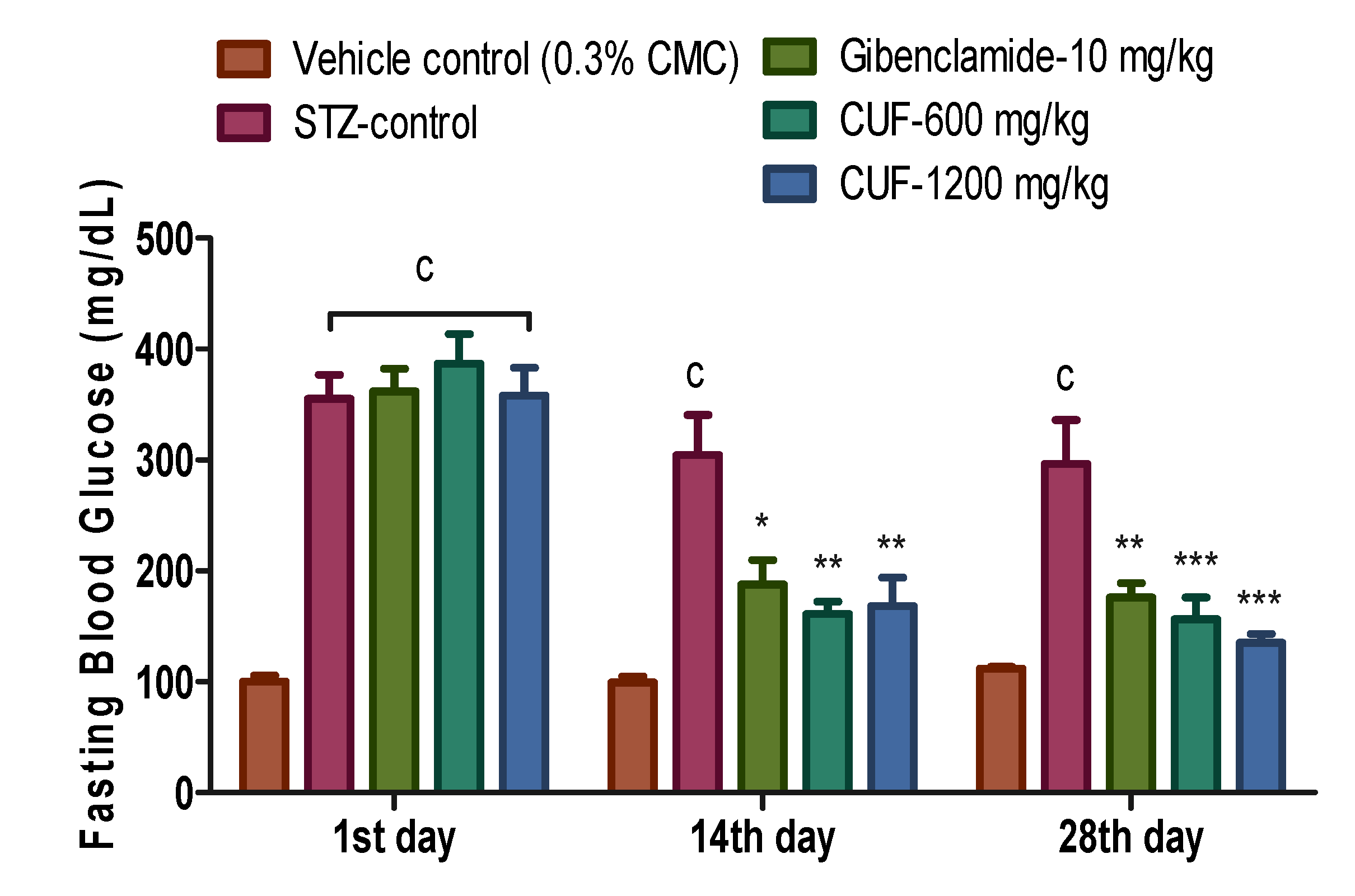
When compared to the vehicle control, the difference in FBG values at the commencement of treatment (Day-1) was statistically significant (p<0.001). On the 14th day (304.30±36.41 vs. 99.17±5.57 of control) and the 28th day of the trial (296.50±39.70 vs. 111.80±1.70 of control), FBG values in the streptozotocin control group remained substantially higher (p<0.001) than those in the vehicle control group.
Compared to the control group treated with streptozotocin, the administration of low dose CUF (600 mg/kg) and high dose CUF (1200 mg/kg) resulted in a substantial reduction in FBG levels (p<0.01). On the 14th day, the value of FBG in the glibenclamide 10 mg/kg group drops significantly (p<0.05) as compared to the streptozotocin control group. On the 28
thday of therapy, the value of FBG in the CUF-600 mg/kg and CUF-1200 mg/kg dropped even further, with a highly significant difference (p<0.001) in comparison with streptozotocin control group. On the 28th day, the value of FBG in the positive control drops significantly compared to streptozotocin control group (p<0.01). On days 14 and 28, FBG values in the glibenclamide and CUF (600 and 1200 mg/kg) groups were statistically comparable to vehicle control group (
Figure 2).
3.4. Renal Function Parameters
Serum creatinine level was statistically comparable in all groups. The serum creatinine level dropped slightly with the CUF-600 mg/kg, but it was not statistically significant. All of the results were within the typical physiological range for rats. In all Nic-Stz treated groups, there was a statistically significant rise (p<0.001) in blood urea in comparison with vehicle control group. Serum uric acid levels increased significantly in streptozotocin control (p<0.05), positive control (p<0.01), CUF-600 mg/kg (p<0.01), and CUF-1200 mg/kg (p<0.001) vs. vehicle control (data not presented).
3.5. Liver Function Parameters
The value of AST decreased in the streptozotocin control and CUF-600 mg/kg but increased in the CUF-1200 mg/kg and positive control groups; however, it was not statistically significant. The value of ALT increased in all treatment groups, with the most substantial increase occurring in the positive control group, while the difference was not statistically significant. In all therapy groups, total bilirubin levels were significantly higher. The increase in total bilirubin level in the streptozotocin control group (p<0.05), positive control (p<0.001), low dose of CUF (600 mg/kg) (p<0.05), and high dose of CUF (1200 mg/kg) (p<0.01) was statistically significant. Though the observed values in all the treatment groups were under normal limits. In all treatment groups, the value of ALP increased significantly. Positive control showed the most significant rise, followed by streptozotocin control, whereas high dose CUF (1200mg/kg) showed greater values than low dose CUF (600 mg/kg). In the positive control, streptozotocin control, and high dose of CUF (1200 mg/kg), the increase in ALP levels was very significant (p<0.001), while it was significant (p<0.05) in the low dose of CUF (600 mg/kg) (data not presented).
3.6. Serum Total Protein, Albumin, and Globulin
When comparing the vehicle control to the other groups, there were no notable changes in the overall protein levels, albumin, globulin, or the ratio of albumin to globulin. Total protein, albumin, and globulin levels are within normal ranges in all groups (data not presented).
3.7. Lipid Profile
Except for a substantial reduction in total cholesterol in the CUF-1200 mg/kg group (p<0.05), no difference was observed in any lipid parameter in any group compared to the vehicle control (data not presented).
3.8. Body Weight
Before the induction of diabetes, the baseline body weight of all groups was comparable. A constant drop in body weight in diabetic rats was recorded after inducing diabetes (Nic-Stz). On day 10 (i.e., the first day of treatment), there was a significant reduction in body weight in all Nic-Stz treated groups vs. vehicle control (p<0.001). In diabetic rats, treatment with glibenclamide-10 mg/kg or CUF (600 and 1200 mg/kg) for four weeks did not significantly gain body weight compared to the vehicle control. On the 1st, 14th, and 28th days of treatment, a significant difference in body weight (p<0.001) between all groups (STZ control, positive control, CUF 600, and CUF 1200 mg/ kg) was noted compared to the vehicle control (
Table 1).
3.9. Relative Organ Weight
Induction of diabetes caused a significant increase in relative organ weight (ROW) of the liver in all the Nic-Stz treated groups with respect to the vehicle control group (p<0.001) in streptozotocin, positive control, and a high dose of CUF (1200 mg/kg) and (p<0.05) in low dose CUF (600 mg/kg). Though there is a reduction in ROW in low dose CUF (600 mg/kg), none of the treatments (glibenclamide or CUF) reverses the increased ROW of liver comparable to vehicle control animals. Similarly, ROW of the right and the left kidney was significantly increased in all the Nic-Stz treated groups vs. vehicle control group (p<0.05 to p<0.001), as presented (data not presented).
3.10. Histopathological Examination
Sinusoidal hemorrhages in the liver were found in one animal from a high dose, CUF (1200mg/kg), and one Streptozotocin control. Foci of inflammation were seen in the periportal/ centrilobular region of the liver in one vehicle control animal, and two glibenclamide treated animals. However, there were no inflammatory alterations in the liver in the Low dose (CUF-600mg/kg), high dose (CUF-1200mg/kg), and the Streptozotocin control group (data not presented).
In kidney, tubular hemorrhages were observed in one animal from vehicle control and Streptozotocin control. At the same time, low dose CUF (600mg/kg), high dose CUF (1200mg/kg), and glibenclamide-treated animals did not show vascular changes in kidneys. Foci of tubular / glomerulus inflammation were observed in two animals from Streptozotocin control [2/4] and two animals from low dose CUF (600mg/kg) [2/4], but vehicle control, high dose CUF (1200mg/kg), and glibenclamide treated did not show inflammatory changes in kidneys (data not presented).
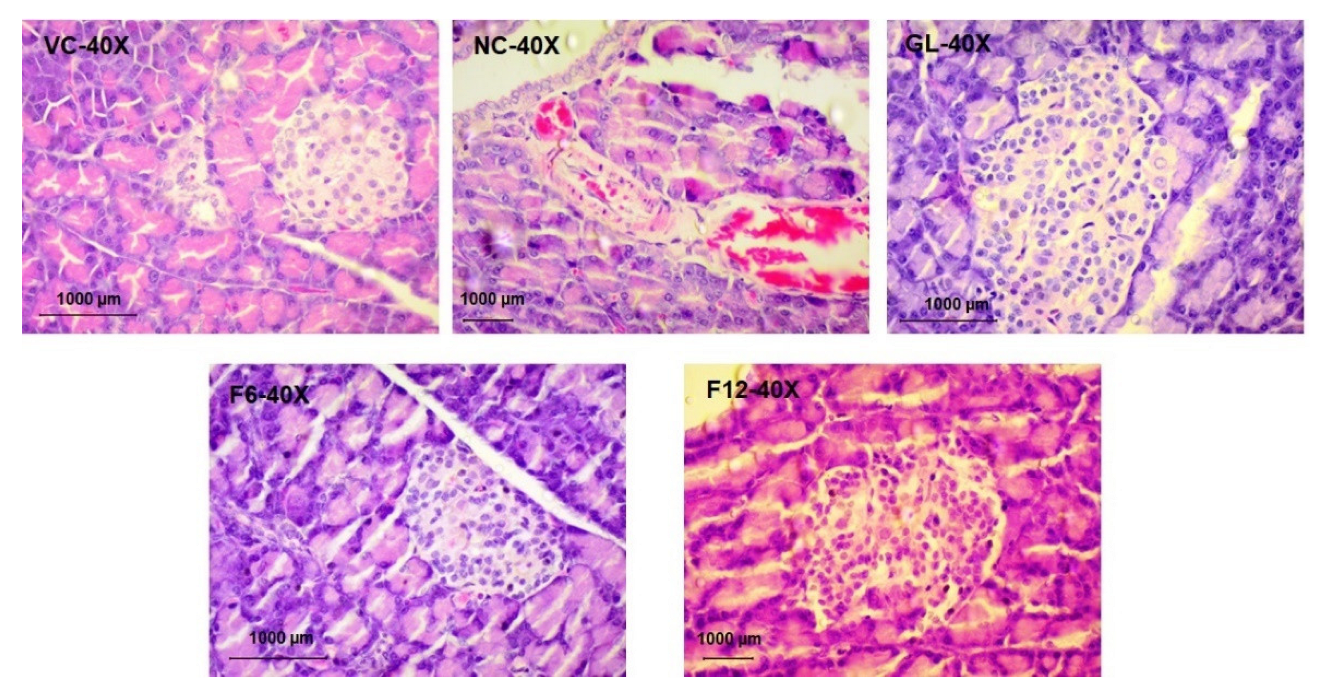
In pancreas, one animal from the glibenclamide treated group showed acinar cell degeneration and peri ductular fibrosis. In contrast, the pancreas of the low dose CUF (600mg/kg), high dose CUF (1200mg/kg), vehicle control, and Streptozotocin control showed no alterations (
Figure 3).
Based on the above findings, the treatment group (CUF-1200mg/kg) showed less reactive changes and a positive response when compared with vehicle control, Streptozotocin control, and Positive control (data not presented).
3.11. Antioxidant Activity of CUF
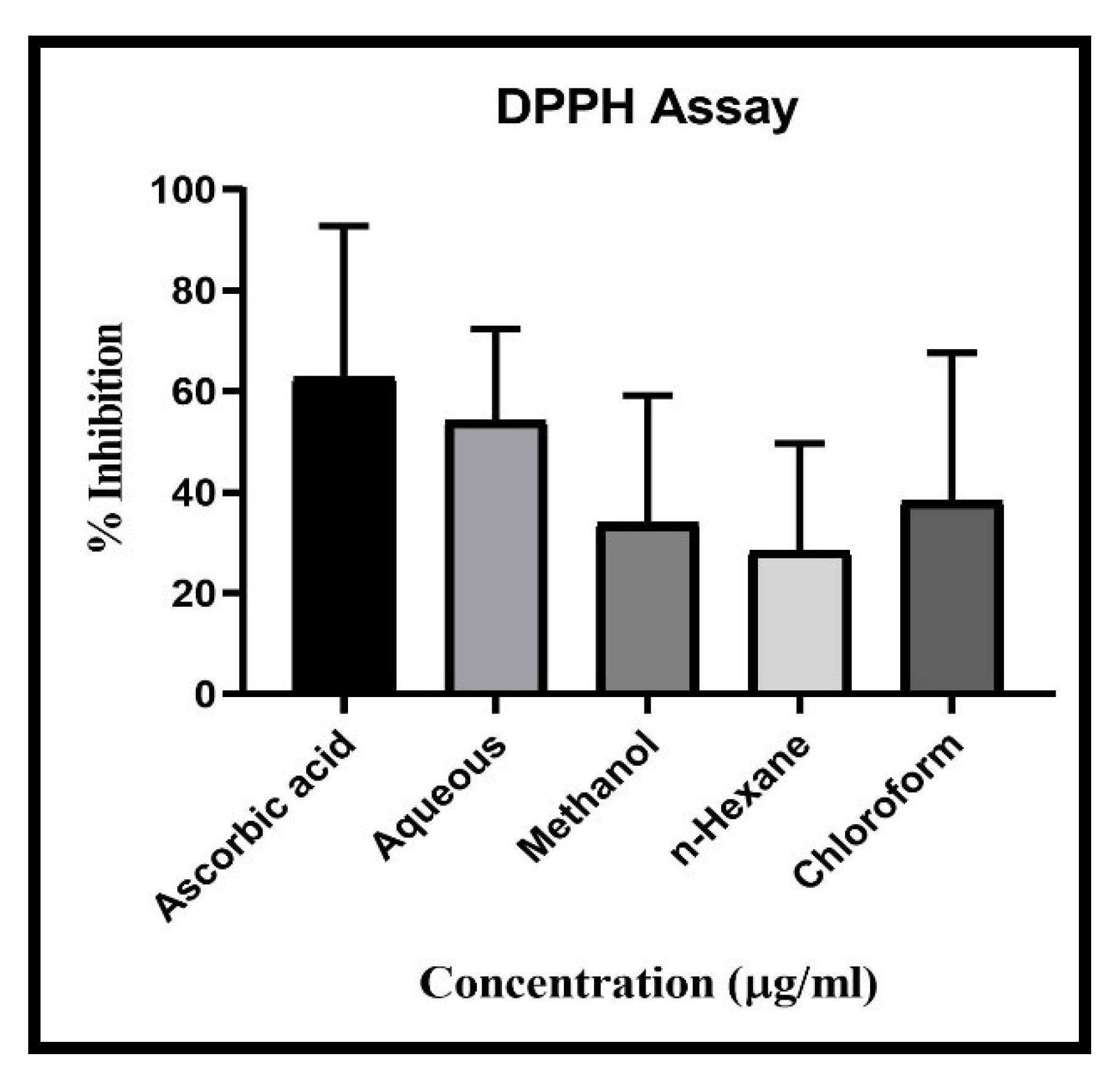
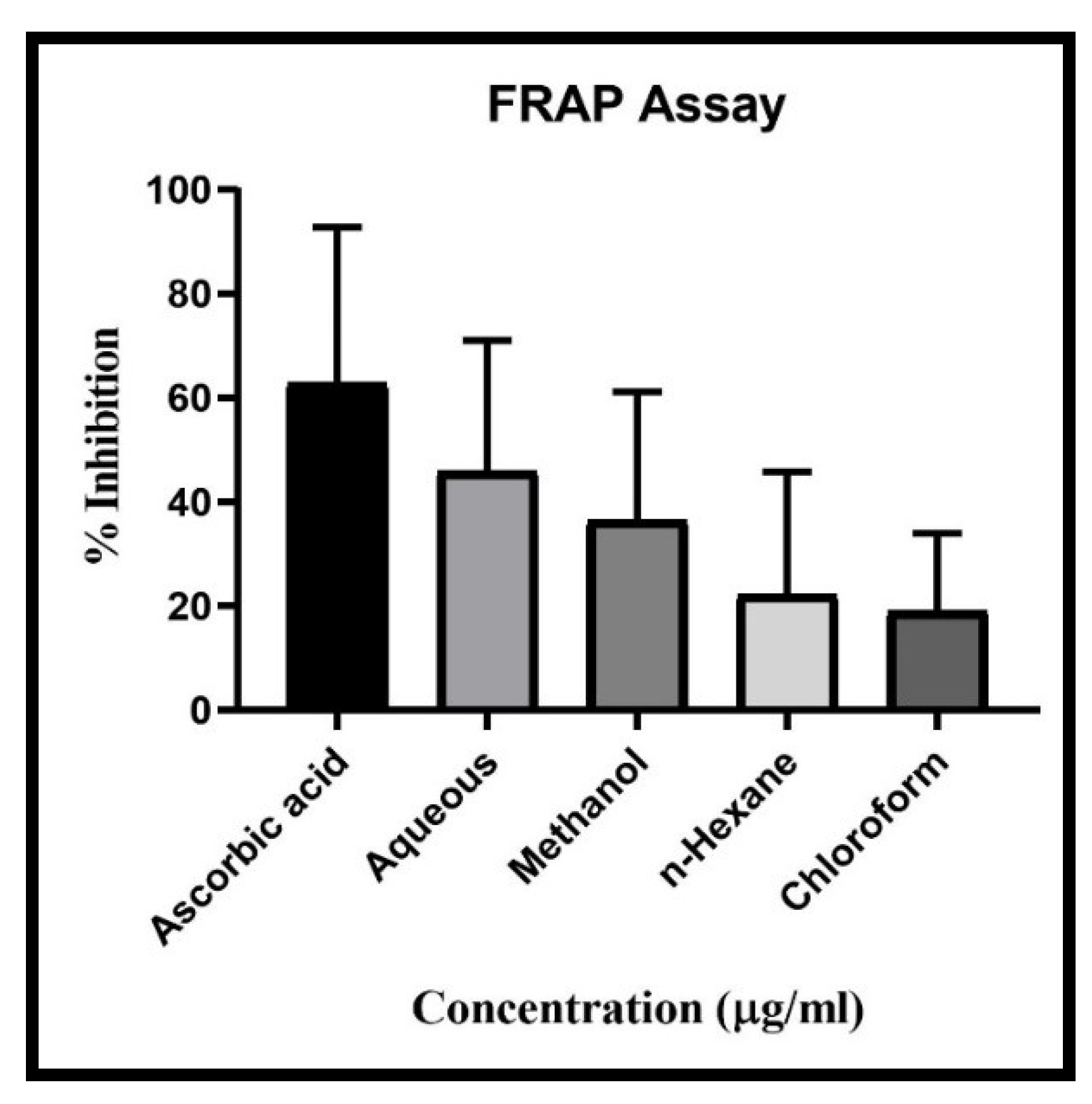
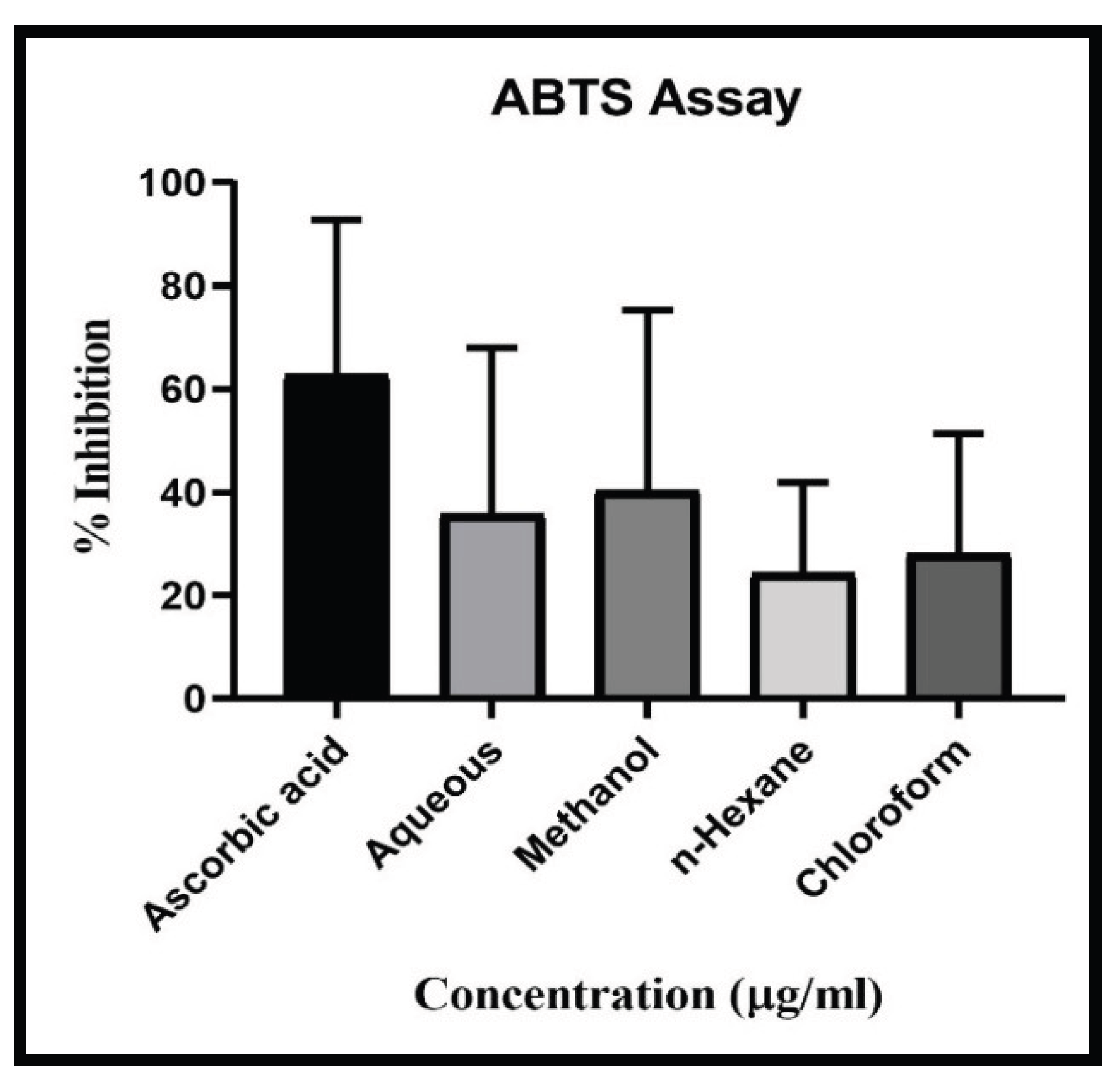
The CUF showed antioxidant activity in all extract of Aqueous, Methanolic, chloroform and n-Hexane along with standard ascorbic acid. Result showed highest DPPH Scavenging activity in aqueous extract with IC50 value 28.3 µg/ml, followed by chloroform extract with IC50 49.11 µg/ml, Methanolic extract with IC50 93.01µg/ml and lowest scavenging activity found in n-Hexane extract with IC50 158.61 µg/ml compared with standard Ascorbic acid (IC50 13.17 µg/ml) (
Figure 4). Results showed descending scavenging activity in FRAP test as Aqueous (IC50 13.17 µg/ml), Methanolic (IC50 82.19 µg/ml), n-Hexane (IC50 169.60 µg/ml) and chloroform (IC50 310.39 µg/ml) (
Figure 5). In case of ABTS test the results are as Methanolic (IC50 67.66 µg/ml), Aqueous (IC50 86.6 µg/ml), chloroform (IC50 153.24 µg/ml), and n-Hexane (IC50 205.77 µg/ml) (
Figure 6). In all three-scavenging assay the Aqueous extract showed promising antioxidant activity.
4. Discussion
There are several time-tested formulations in Unani medicine for managing diabetes without producing severe side effects and can be used safely for a long duration. In the case of a single herb or mineral, it is easy to postulate the theory for their action based on secondary metabolites. Still, in formulations, it is just impractical due to the presence of several active chemical constituents. The Unani system of Medicine is based on Holism, where the whole body is treated as a single unit. The basic principle is to strengthen the body’s immunity to assist the body in maintaining homeostasis. In case of a disease condition, the drugs which help body defence mechanisms and help correct the derangement of humour are used. Antidiabetic effects of herbal medicines may be exerted by diverse mechanisms including sensitizing pancreatic β-cells that secrete insulin or altering the way that glucose is used, such as by postponing stomach emptying or slowing down the intestinal absorption of glucose [
19].
Activating pancreatic β-cells, inhibiting other hormones that elevate blood sugar, and increasing insulin receptor sensitivity and affinity are the fundamental methods for reducing blood sugar. Nevertheless, there are a number of other factors at play, including reducing the release of glycogen, increasing the organs’ ability to use glucose, eliminating free radicals, stopping lipid peroxidation, and addressing anomalies in the metabolism of proteins and lipids [
20]. Regulation of insulin signalling pathways, translocation of the GLUT-4 (Glucose transporter type 4) receptor, and/or activation of the Peroxisome Proliferator-Activated Receptor Gamma are among the mechanisms of action of herbal or natural medicines in diabetes. The anti-inflammatory and immunomodulatory activity also help indirectly to correct hyperglycemia [
21].
In the present study, CUF effectively inhibited the fasting blood glucose at both dose levels on the 14th day and 28th day of treatment. The reduction in the FBG values was much better when compared with the positive control (glibenclamide 10 mg/kg bw). The results have shown the dose-dependent effect of CUF on FBG. CUF may have enhanced glucose absorption into peripheral tissues of diabetic rats at both dose levels, as indicated by OGTT data. CUF significantly lowered blood glucose levels 30 and 60-minute time points after an oral glucose load and was comparable to the positive control (glibenclamide 10 mg/kg). The findings show that insulin sensitivity could be improved by enhancing insulin receptors, receptor substrates, glucose transporters, or enzymes involved in glucose phosphorylation [
22,
23]. The results of the OGTT test demonstrated that CUF administration lowered glucose levels via the insulin-sensitized glucose absorption mechanism [
24].
The renal indicators were within normal limits, suggesting that the CUF had no adverse effects on kidney tissue at either dosage. The findings show that CUF may be useful in treating diabetes-related dyslipidemia. The CUF-induced weight loss was similar to that of glibenclamide 10 mg/kg group.
The blood glucose, glycosylated haemoglobin, and other glycosylated proteins in plasma are reported to be significantly lowered by
G. sylvestre leaf extract. It acts as a strong antidiabetic medication by restoring blood glucose homeostasis. Increased insulin release from the pancreas and pancreatic islet cell regeneration has been hypothesized as mechanisms of the hypoglycemic impact of gymnemic acid [
25]. Gymnemic acids can also reduce blood sugar levels by delaying glucose absorption in the blood and preventing sugar absorption in the gut [
26].
G. sylvestre has been shown to have antidiabetic and sugar-inactivating activities in several investigations [
27].
The alcoholic extract of
E. jambolana seed has hypoglycemic properties and lowers glycosuria. It also replenishes the glycogen content of the hepatic and skeletal muscles, which has been changed. The seed extract also showed a drop in fasting and peak blood glucose levels and an increase in glycogen production, indicating that it has anti-diabetic properties [
28]. The seed extract inhibits the enzyme α-glucosidase, which is how it works as an antidiabetic medication [
29].
In STZ-induced diabetic rats,
Z. officinale juice lowered FBS levels while increasing serum insulin levels. In diabetic rats, it also lowers blood cholesterol and triglycerides. Serotonin (5-HT) receptors play a crucial role in glycaemic management in this case [
30]. Gingerol increases cell-mediated glucose absorption by improving insulin sensitivity, which is particularly important for glycaemic management [
31].
The
M. indica seed kernel extract has demonstrated high antioxidant action. By modifying cell-growth regulators, seed extract was found to be effective in fighting oxidative stress-induced cellular injury in the mouse liver, validating its hepatoprotective action [
32]. Ethanolic extract of mango seeds revealed significant anti-inflammatory and antibacterial action in acute, subacute, and chronic cases of inflammation [
33]. Acetone and methanolic extracts of
A. arabica were found to have antiviral action against the Hepatitis C Virus in the liver [
34]. The bark of
A. arabica showed blood glucose lowering effect and histological evidence of its therapeutic activity in the pancreas [
35].
CUF showed antioxidant activity in aqueous, methanolic, chloroform and n-Hexane extracts in dose dependent manner (3.75-240 μg/ml). Result showed lowest scavenging activity in aqueous extract as per DPPH and FRAP assay, while lowest scavenging activity was shown in methanolic extract in ABTS assay. CUF has shown antioxidant activity in dose dependant manner as per our study.
The condition of hyperglycemia results in the excessive generation of free radicals, specifically reactive oxygen species and reactive nitrogen species, hence exacerbating oxidative stress. Numerous studies have indicated that oxidative stress significantly contributes to the advancement of diabetes, specifically in relation to pancreatic β-cell malfunction, insulin resistance, and metabolic problems associated with diabetes. Hence, the therapeutic strategy that focuses on targeting oxidative stress has exhibited considerable potential in the treatment of diabetes and its related complications. In comparison to synthetic antioxidants like vitamin C/E supplementation and superoxide dismutase, which exhibit poor solubility, permeability, and stability, natural form of drugs may produce better effects [
36].
Recent research indicates that the redox modification of certain crucial proteins, including IκB kinase β (IKKβ), protein kinase C (PKC) and Kelch-like ECH-associated protein 1 (Keap1), is associated with the development and advancement of diabetes and the resulting damage to diabetic end-organs [
37,
38]. Oxidative stress triggers the activation of the IκB kinase β (IKKβ/NF-kB) pathway, potentially leading to inflammation and pancreatic β-cell dysfunction linked to vascular complications [
39]. Hyperglycemia stimulates the production of diacylglycerol (DAG), resulting in an excessive increase in PKC activation [
40]. The activation of PKC results in endothelial dysfunction, increase vascular permeability, and suppression of angiogenesis [
41].
According to the study conducted by Zhong et al., it was observed that tert-butylhydro-quinone (tbhq), a phenolic antioxidant, exerts inhibitory effects on the development of diabetic retinopathy through the alteration of Keap1 cysteine residues [
42]. Antioxidants like curcumin, and vitamin E are reported to inhibit cellular PKC activity [
43]. The presence of sulforaphane in cruciferous vegetables has been observed to exhibit reno-protective properties in rats with streptozotocin-induced diabetes [
44]. Curcumin has the ability to alter the cysteine residues of Keap1 and decrease the likelihood of developing type 2 diabetes mellitus in individuals with prediabetes [
45].
Controlling oxidative stress is certainly helpful for the management of diabetes and its complications. Our findings suggest that natural antioxidants may be potential therapeutic strategy for diabetes and diabetic complications. The CUF has exhibited antidiabetic activity in addition to its antioxidant capabilities, hence providing additional advantages for the efficient control of T2DM.
The observed antidiabetic efficacy of CUF in this study may be ascribed to the combined and synergistic impacts of its many constituents. All groups exhibited substantial weight reduction in the present study, however, rats treated with CUF demonstrated superior performance compared to the positive control. During the 28-day therapy with the formulation, there was a significant decrease in BGL compared to glibenclamide. This suggests that the formulation may be a more effective treatment for enhancing the secretory activity of insulin-producing β-cells. The adverse effects on the pancreas, liver, and kidney were minimal and may be due to some secondary metabolites present in the formulation or some other conditions. The observed favourable effect on liver histology may be attributed to the hepatoprotective activity of components such as M. indica and C. rubrum. The CUF has demonstrated notable antihyperglycemic properties and has been found to be efficacious in mitigating nephrotoxicity. The CUF has demonstrated both antidiabetic and antioxidant properties, without producing undesirable side effects, although the mechanism of action is unclear. Natural drugs possess significant potential as effective antidiabetic agents. However, the precise mechanism of action CUF is unclear.