Submitted:
25 June 2025
Posted:
26 June 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Understanding Lithium Dendrites in Solid-State Lithium Batteries (SSLBs)
2.1. Mechanism of Dendrite Formation
2.2. Impact on Battery Performance and Safety
3. Dendrite Suppression Strategies
3.1. Material Innovations
3.1.1. Sulfide-Based Electrolytes
3.1.2. Oxide-Based Electrolytes
3.1.3. Polymer-Based Electrolytes
3.2. Interface Engineering Techniques
3.2.1. Surface Coatings
3.2.2. Artificial Interlayers
- (i)
- Lithiophilicity: Materials with high affinity for lithium, such as silver (Ag) or tin (Sn), can facilitate uniform nucleation and growth of lithium, leading to smoother deposition. For example, a study demonstrated that a rational layer-by-layer strategy using a lithiophilic and electron-blocking multilayer can substantially enhance the performance and stability of lithium-metal solid-state batteries by effectively blocking electron leakage and maintaining low electronic conductivity even at high temperatures [58].
- (ii)
- Ion Conductivity: Incorporating ion-conductive polymers or ceramics into the interlayer can enhance lithium-ion transport across the interface. For example, constructing a Li-rich artificial SEI layer in an alloy–polymer composite electrolyte has been shown to achieve high ionic conductivity for all-solid-state lithium metal batteries [59].
- (iii)
- Mechanical Compliance: Flexible interlayers can accommodate volume changes during cycling, maintaining intimate contact between the anode and SSE. In situ construction of a flexible interlayer has been reported to enhance the durability of solid-state lithium metal batteries [60].
3.2.3. Electrolyte–Electrode Modifications
- (i)
- Electron-Blocking Interlayers
- (ii)
- Mechanical Reinforcement
- (iii)
- Uniform Current Distribution
3.3. Mechanical Design Approaches for Dendrite Suppression
3.3.1. Nanomaterials and Composite Structures for Reinforcement and Ion Transport
3.3.2. Self-Healing Materials and Adaptive Interfaces
3.3.3. Gradient and Multilayer Electrolytes
3.3.4.3. D-Structured Anodes and Current Collectors
3.4. Advanced Characterization Techniques
3.5. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AFM | Atomic Force Microscopy |
| ALD | Atomic Layer Deposition |
| ASSLBs | All-Solid-State Lithium Batteries |
| DFT | Density Functional Theory |
| EIS | Electrochemical Impedance Spectroscopy |
| ETEM | Environmental Transmission Electron Microscopy |
| FEC | Fluoroethylene Carbonate |
| GCSE | Gradient Composite Solid Electrolyte |
| GO | Graphene Oxide |
| LLZO | Li₇La₃Zr₂O₁₂ |
| LATP | Li–Al–Ti–P oxide |
| LFP | LiFePO₄ |
| LiTFSI | Lithium bis(trifluoromethanesulfonyl)imide |
| LLZTO | Li₆.₄La₃Zr₁.₄Ta₀.₆O₁₂ |
| LIBs | Lithium-Ion Batteries |
| LiPON | Lithium Phosphorus Oxynitride |
| MRI | Magnetic Resonance Imaging |
| MPa | Megapascal |
| MoS₂ | Molybdenum Disulfide |
| NCM811 | LiNi₀.₈Co₀.₁Mn₀.₁O₂ |
| NMR | Nuclear Magnetic Resonance |
| PEO | Polyethylene Oxide |
| PSE | Polymer-Based Solid Electrolytes |
| PVDF-TrFE | Poly(vinylidene fluoride-co-trifluoroethylene) |
| rGO | Reduced Graphene Oxide |
| SEI | Solid Electrolyte Interphase |
| SEM | Scanning Electron Microscopy |
| SHE | Standard Hydrogen Electrode |
| SSE | Solid-State Electrolyte |
| SSLBs | Solid-State Lithium Batteries |
| TEM | Transmission Electron Microscopy |
| ToF-SIMS | Time-of-Flight Secondary Ion Mass Spectrometry |
| XPS | X-ray Photoelectron Spectroscopy |
References
- Albertus, P.; Babinec, S.; Litzelman, S.; Newman, A. Status and Challenges in Enabling the Lithium Metal Electrode for High-Energy and Low-Cost Rechargeable Batteries. Nat Energy 2017, 3, 16–21. [CrossRef]
- Janek, J.; Zeier, W.G. A Solid Future for Battery Development. Nat Energy 2016, 1, 16141. [CrossRef]
- Goodenough, J.B.; Singh, P. Review—Solid Electrolytes in Rechargeable Electrochemical Cells. J. Electrochem. Soc. 2015, 162, A2387–A2392. [CrossRef]
- Famprikis, T.; Canepa, P.; Dawson, J.A.; Islam, M.S.; Masquelier, C. Fundamentals of Inorganic Solid-State Electrolytes for Batteries. Nat. Mater. 2019, 18, 1278–1291. [CrossRef]
- Kerman, K.; Luntz, A.; Viswanathan, V.; Chiang, Y.-M.; Chen, Z. Review—Practical Challenges Hindering the Development of Solid State Li Ion Batteries. J. Electrochem. Soc. 2017, 164, A1731–A1744. [CrossRef]
- Nzereogu, P.U.; Oyesanya, A.; Ogba, S.N.; Ayanwunmi, S.O.; Sobajo, M.S.; Chimsunum, V.C.; Ayanwunmi, V.O.; Amoo, M.O.; Adefemi, O.T.; Chukwudi, C.C. Solid-State Lithium-Ion Battery Electrolytes: Revolutionizing Energy Density and Safety. Hybrid Advances 2025, 8, 100339. [CrossRef]
- Li, Y.; Kim, C.; Cho, Y.; Musgrove, A.L.; Parker, G.D.; Su, Y.-F.; Sacci, R.L.; Yu, X.-Y.; Zawodzinski, T.; Nanda, J.; et al. Promising Performance of Sulfide Catholytes Compared to Halide Alternatives in NMC811 Cathodes for Sheet-Type Sulfide Solid-State Batteries. Energy Storage Materials 2025, 80, 104385. [CrossRef]
- Maurel, A.; Armand, M.; Grugeon, S.; Fleutot, B.; Davoisne, C.; Tortajada, H.; Courty, M.; Panier, S.; Dupont, L. Poly(Ethylene Oxide)−LiTFSI Solid Polymer Electrolyte Filaments for Fused Deposition Modeling Three-Dimensional Printing. J. Electrochem. Soc. 2020, 167, 070536. [CrossRef]
- Mindemark, J.; Lacey, M.J.; Bowden, T.; Brandell, D. Beyond PEO—Alternative Host Materials for Li + -Conducting Solid Polymer Electrolytes. Progress in Polymer Science 2018, 81, 114–143. [CrossRef]
- Zhu, Y.; He, X.; Mo, Y. Https://Doi.Org/10.1039/C8EE0. J. Mater. Chem. A 2016, 4, 3253–3266. [CrossRef]
- Zhang, Z.; Shao, Y.; Lotsch, B.; Hu, Y.-S.; Li, H.; Janek, J.; Nazar, L.F.; Nan, C.-W.; Maier, J.; Armand, M.; et al. New Horizons for Inorganic Solid State Ion Conductors. Energy Environ. Sci. 2018, 11, 1945–1976. [CrossRef]
- Wang, C.; Fu, K.; Kammampata, S.P.; McOwen, D.W.; Samson, A.J.; Zhang, L.; Hitz, G.T.; Nolan, A.M.; Wachsman, E.D.; Mo, Y.; et al. Garnet-Type Solid-State Electrolytes: Materials, Interfaces, and Batteries. Chem. Rev. 2020, 120, 4257–4300. [CrossRef]
- Burton, M.; Narayanan, S.; Jagger, B.; Olbrich, L.F.; Dhir, S.; Shibata, M.; Lain, M.J.; Astbury, R.; Butcher, N.; Copley, M.; et al. Techno-Economic Assessment of Thin Lithium Metal Anodes for Solid-State Batteries. Nat Energy 2024, 10, 135–147. [CrossRef]
- Machín, A.; Morant, C.; Márquez, F. Advancements and Challenges in Solid-State Battery Technology: An In-Depth Review of Solid Electrolytes and Anode Innovations. Batteries 2024, 10, 29. [CrossRef]
- Ningappa, N.G.; Vishweswariah, K.; Bouguern, M.D.; M R, A.K.; Amine, K.; Zaghib, K. Mechanistic Insights and Materials Strategies for Dendrite-Free Metal Anodes in Alkali and Zinc Batteries. Nano Energy 2025, 141, 111144. [CrossRef]
- Zhang, B.; Yuan, B.; Yan, X.; Han, X.; Zhang, J.; Tan, H.; Wang, C.; Yan, P.; Gao, H.; Liu, Y. Atomic Mechanism of Lithium Dendrite Penetration in Solid Electrolytes. Nat Commun 2025, 16, 1906. [CrossRef]
- An, Y.; Hu, T.; Pang, Q.; Xu, S. Observing Li Nucleation at Li Metal-Solid Electrolyte Interface in All-Solid-State Batteries 2024.
- Guo, W.; Wang, A.; He, X.; Lei, Y.; Li, Y.; Zhou, Z.; Li, C.; Lv, X.; Wang, H.; Shen, F.; et al. Unveiling the Mechanism of Lithium Dendrite Infiltration into Solid State Electrolyte through the Coupling of Electrochemical and In-Situ Optical Characterization. Electrochimica Acta 2024, 508, 145294. [CrossRef]
- Liu, H.; Chen, Y.; Chien, P.-H.; Amouzandeh, G.; Hou, D.; Truong, E.; Oyekunle, I.P.; Bhagu, J.; Holder, S.W.; Xiong, H.; et al. Dendrite Formation in Solid-State Batteries Arising from Lithium Plating and Electrolyte Reduction. Nat. Mater. 2025, 24, 581–588. [CrossRef]
- Yildirim, C.; Flatscher, F.; Ganschow, S.; Lassnig, A.; Gammer, C.; Todt, J.; Keckes, J.; Rettenwander, D. Understanding the Origin of Lithium Dendrite Branching in Li6.5La3Zr1.5Ta0.5O12 Solid-State Electrolyte via Microscopy Measurements. Nat Commun 2024, 15, 8207. [CrossRef]
- Wang, Y.; Richards, W.D.; Ong, S.P.; Miara, L.J.; Kim, J.C.; Mo, Y.; Ceder, G. Design Principles for Solid-State Lithium Superionic Conductors. Nature Mater 2015, 14, 1026–1031. [CrossRef]
- Zhao, J.; Feng, X.; Pang, Q.; Fowler, M.; Lian, Y.; Ouyang, M.; Burke, A.F. Battery Safety: Machine Learning-Based Prognostics. Progress in Energy and Combustion Science 2024, 102, 101142. [CrossRef]
- Misiani, A.N.; Oni, B.A. A Review on Challenges in Low Temperature Lithium-Ion Cells and Future Prospects. Applied Energy 2025, 393, 125987. [CrossRef]
- Reisecker, V.; Flatscher, F.; Porz, L.; Fincher, C.; Todt, J.; Hanghofer, I.; Hennige, V.; Linares-Moreau, M.; Falcaro, P.; Ganschow, S.; et al. Effect of Pulse-Current-Based Protocols on the Lithium Dendrite Formation and Evolution in All-Solid-State Batteries. Nat Commun 2023, 14, 2432. [CrossRef]
- Du, H.; Wang, Y.; Kang, Y.; Zhao, Y.; Tian, Y.; Wang, X.; Tan, Y.; Liang, Z.; Wozny, J.; Li, T.; et al. Side Reactions/Changes in Lithium-Ion Batteries: Mechanisms and Strategies for Creating Safer and Better Batteries. Advanced Materials 2024, 36, 2401482. [CrossRef]
- Krauskopf, T.; Richter, F.H.; Zeier, W.G.; Janek, J. Physicochemical Concepts of the Lithium Metal Anode in Solid-State Batteries. Chem. Rev. 2020, 120, 7745–7794. [CrossRef]
- Gao, Y.; Wang, D.; Li, Y.C.; Yu, Z.; Mallouk, T.E.; Wang, D. Salt-Based Organic–Inorganic Nanocomposites: Towards A Stable Lithium Metal/Li10 GeP2 S12 Solid Electrolyte Interface. Angew Chem Int Ed 2018, 57, 13608–13612. [CrossRef]
- Yao, M.; Shi, J.; Luo, A.; Zhang, Z.; Zhu, G.; Xu, H.; Xu, J.; Jiang, L.; Jiang, K. Advances in Sulfide Solid–State Electrolytes for Lithium Batteries. Energy Storage Materials 2025, 75, 104018. [CrossRef]
- Xu, C.; Chen, L.; Wu, F. Unveiling the Power of Sulfide Solid Electrolytes for Next-Generation All-Solid-State Lithium Batteries. Next Materials 2025, 6, 100428. [CrossRef]
- Man, B.; Zeng, Y.; Liu, Q.; Chen, Y.; Li, X.; Luo, W.; Zhang, Z.; He, C.; Jie, M.; Liu, S. A Comprehensive Review of Sulfide Solid-State Electrolytes: Properties, Synthesis, Applications, and Challenges. Crystals 2025, 15, 492. [CrossRef]
- Apelt, S.; Höhne, S.; Mehner, E.; Böhm, C.; Malanin, M.; Eichhorn, K.; Jehnichen, D.; Uhlmann, P.; Bergmann, U. Poly(Vinylidene Fluoride-co-trifluoroethylene) Thin Films after Dip- and Spin-Coating. Macro Materials & Eng 2022, 307, 2200296. [CrossRef]
- Liu, S.; Zhou, L.; Han, J.; Wen, K.; Guan, S.; Xue, C.; Zhang, Z.; Xu, B.; Lin, Y.; Shen, Y.; et al. Super Long-Cycling All-Solid-State Battery with Thin Li6 PS5 Cl-Based Electrolyte. Advanced Energy Materials 2022, 12, 2200660. [CrossRef]
- Liu, M.; Hong, J.J.; Sebti, E.; Zhou, K.; Wang, S.; Feng, S.; Pennebaker, T.; Hui, Z.; Miao, Q.; Lu, E.; et al. Surface Molecular Engineering to Enable Processing of Sulfide Solid Electrolytes in Humid Ambient Air 2024.
- Kwon, O.; Kim, S.Y.; Hwang, J.; Han, J.; Yu, S.; Yim, T.; Oh, S.H. Design Principles for Moisture-Tolerant Sulfide-Based Solid Electrolytes and Associated Effect on the Electrochemical Performance of All-Solid-State Battery. J. Electrochem. Sci. Technol 2024, 15, 437–458. [CrossRef]
- Umair, M.; Zhou, S.; Li, W.; Rana, H.T.H.; Yang, J.; Cheng, L.; Li, M.; Yu, S.; Wei, J. Oxide Solid Electrolytes in Solid-State Batteries. Batteries & Supercaps 2024, e202400667. [CrossRef]
- Gonzalez Puente, P.M.; Song, S.; Cao, S.; Rannalter, L.Z.; Pan, Z.; Xiang, X.; Shen, Q.; Chen, F. Garnet-Type Solid Electrolyte: Advances of Ionic Transport Performance and Its Application in All-Solid-State Batteries. J Adv Ceram 2021, 10, 933–972. [CrossRef]
- Neises, J.; Scheld, W.S.; Seok, A.-R.; Lobe, S.; Finsterbusch, M.; Uhlenbruck, S.; Schmechel, R.; Benson, N. Study of Thermal Material Properties for Ta- and Al-Substituted Li7 La3 Zr2 O12 (LLZO) Solid-State Electrolyte in Dependency of Temperature and Grain Size. J. Mater. Chem. A 2022, 10, 12177–12186. [CrossRef]
- Ni, J.E.; Case, E.D.; Sakamoto, J.S.; Rangasamy, E.; Wolfenstine, J.B. Room Temperature Elastic Moduli and Vickers Hardness of Hot-Pressed LLZO Cubic Garnet. J Mater Sci 2012, 47, 7978–7985. [CrossRef]
- Wolfenstine, J.; Allen, J.L.; Sakamoto, J.; Siegel, D.J.; Choe, H. Mechanical Behavior of Li-Ion-Conducting Crystalline Oxide-Based Solid Electrolytes: A Brief Review. Ionics 2018, 24, 1271–1276. [CrossRef]
- Ding, H.; Wang, M.; Shan, X.; Yang, G.; Tian, M. Advancements in Active Filler-Contained Polymer Solid-State Electrolytes for Lithium-Metal Batteries: A Concise Review. Supramolecular Materials 2025, 4, 100097. [CrossRef]
- Irfan, M.; Yang, Z.; Su, J.; Zhang, W. Polymer-Based Solid-State Electrolytes. In ACS Symposium Series; Gupta, R.K., Ed.; American Chemical Society: Washington, DC, 2022; Vol. 1413, pp. 201–232 ISBN 978-0-8412-9768-5.
- Lee, T.K.; Zaini, N.F.M.; Mobarak, N.N.; Hassan, N.H.; Noor, S.A.M.; Mamat, S.; Loh, K.S.; KuBulat, K.H.; Su’ait, M.S.; Ahmad, A. PEO Based Polymer Electrolyte Comprised of Epoxidized Natural Rubber Material (ENR50) for Li-Ion Polymer Battery Application. Electrochimica Acta 2019, 316, 283–291. [CrossRef]
- Xue, Z.; He, D.; Xie, X. Poly(Ethylene Oxide)-Based Electrolytes for Lithium-Ion Batteries. J. Mater. Chem. A 2015, 3, 19218–19253. [CrossRef]
- Appetecchi, G.B.; Hassoun, J.; Scrosati, B.; Croce, F.; Cassel, F.; Salomon, M. Hot-Pressed, Solvent-Free, Nanocomposite, PEO-Based Electrolyte Membranes. Journal of Power Sources 2003, 124, 246–253. [CrossRef]
- Wu, Z.; Wang, Y.; Du, W.; Shen, K.; Chen, B.; Pan, H.; Wu, Y.; Lu, Y. Controlled Radical Polymerization-Derived Solid-State Polymer Electrolytes for Lithium Batteries. EnergyChem 2025, 100160. [CrossRef]
- Croce, F.; Appetecchi, G.B.; Persi, L.; Scrosati, B. Nanocomposite Polymer Electrolytes for Lithium Batteries. Nature 1998, 394, 456–458. [CrossRef]
- Armand, M.; Tarascon, J.-M. Building Better Batteries. Nature 2008, 451, 652–657. [CrossRef]
- Yoon, J.H.; Cho, W.-J.; Kang, T.H.; Lee, M.; Yi, G.-R. Nanostructured Polymer Electrolytes for Lithium-Ion Batteries. Macromol. Res. 2021, 29, 509–518. [CrossRef]
- Luo, S.; Liu, X.; Gao, L.; Deng, N.; Sun, X.; Li, Y.; Zeng, Q.; Wang, H.; Cheng, B.; Kang, W. A Review on Modified Polymer Composite Electrolytes for Solid-State Lithium Batteries. Sustainable Energy Fuels 2022, 6, 5019–5044. [CrossRef]
- Chae, W.; Kim, B.; Ryoo, W.S.; Earmme, T. A Brief Review of Gel Polymer Electrolytes Using In Situ Polymerization for Lithium-Ion Polymer Batteries. Polymers 2023, 15, 803. [CrossRef]
- Chattopadhyay, J.; Pathak, T.S.; Santos, D.M.F. Applications of Polymer Electrolytes in Lithium-Ion Batteries: A Review. Polymers 2023, 15, 3907. [CrossRef]
- Li, Y.; Canepa, P.; Gorai, P. Role of Electronic Passivation in Stabilizing the Lithium- Li x PO y N z Solid-Electrolyte Interphase. PRX Energy 2022, 1, 023004. [CrossRef]
- Cao, D.; Sun, X.; Li, Q.; Natan, A.; Xiang, P.; Zhu, H. Lithium Dendrite in All-Solid-State Batteries: Growth Mechanisms, Suppression Strategies, and Characterizations. Matter 2020, 3, 57–94. [CrossRef]
- Cheng, D.; Wynn, T.A.; Wang, X.; Wang, S.; Zhang, M.; Shimizu, R.; Bai, S.; Nguyen, H.; Fang, C.; Kim, M.; et al. Unveiling the Stable Nature of the Solid Electrolyte Interphase between Lithium Metal and LiPON via Cryogenic Electron Microscopy. 2020. [CrossRef]
- LaCoste, J.; Li, Z.; Xu, Y.; He, Z.; Matherne, D.; Zakutayev, A.; Fei, L. Investigating the Effects of Lithium Phosphorous Oxynitride Coating on Blended Solid Polymer Electrolytes. ACS Appl. Mater. Interfaces 2020, 12, 40749–40758. [CrossRef]
- Klorman, J.A.; Guo, Q.; Lau, K.C. First-Principles Study of Amorphous Al2O3 ALD Coating in Li-S Battery Electrode Design. Energies 2022, 15, 390. [CrossRef]
- Zhou, H.; Yu, S.; Liu, H.; Liu, P. Protective Coatings for Lithium Metal Anodes: Recent Progress and Future Perspectives. Journal of Power Sources 2020, 450, 227632. [CrossRef]
- Lee, S.; Lee, K.; Kim, S.; Yoon, K.; Han, S.; Lee, M.H.; Ko, Y.; Noh, J.H.; Kim, W.; Kang, K. Design of a Lithiophilic and Electron-Blocking Interlayer for Dendrite-Free Lithium-Metal Solid-State Batteries. Sci. Adv. 2022, 8, eabq0153. [CrossRef]
- Wan, J.; Liu, X.; Diemant, T.; Wan, M.; Passerini, S.; Paillard, E. Single-Ion Conducting Interlayers for Improved Lithium Metal Plating. Energy Storage Materials 2023, 63, 103029. [CrossRef]
- Ci, N.; Zhang, L.; Li, J.; Li, D.; Cheng, J.; Sun, Q.; Xi, Z.; Xu, Z.; Zhao, G.; Ci, L. In Situ Construction of a Flexible Interlayer for Durable Solid-State Lithium Metal Batteries. Carbon 2022, 187, 13–21. [CrossRef]
- You, X.; Chen, N.; Xie, G.; Xu, S.; Buriak, J.; Sang, L. Dual-Component Interlayer Enables Uniform Lithium Deposition and Dendrite Suppression for Solid-State Batteries 2024.
- Sang, J.; Tang, B.; Pan, K.; He, Y.-B.; Zhou, Z. Current Status and Enhancement Strategies for All-Solid-State Lithium Batteries. Acc. Mater. Res. 2023, 4, 472–483. [CrossRef]
- Zhang, Y.; Wang, J. Stabilizing the Cathode-Electrolyte Interphase for Superior Li-Ion Batteries. Green Chemical Engineering 2025, S2666952825000470. [CrossRef]
- Nogales, P.M.; Lee, S.; Yang, S.; Yang, I.; Choi, S.H.; Park, S.-M.; Lee, J.H.; Kim, C.J.; An, J.-C.; Jeong, S.-K. Stabilizing the Solid Electrolyte Interphase of SiOx Negative Electrodes: The Role of Fluoroethylene Carbonate in Enhancing Electrochemical Performance. Batteries 2024, 10, 385. [CrossRef]
- Park, S.K.; Copic, D.; Zhao, T.Z.; Rutkowska, A.; Wen, B.; Sanders, K.; He, R.; Kim, H.-K.; De Volder, M. 3D Porous Cu-Composites for Stable Li-Metal Battery Anodes. ACS Nano 2023, 17, 14658–14666. [CrossRef]
- Sastre, J.; Futscher, M.H.; Pompizi, L.; Aribia, A.; Priebe, A.; Overbeck, J.; Stiefel, M.; Tiwari, A.N.; Romanyuk, Y.E. Blocking Lithium Dendrite Growth in Solid-State Batteries with an Ultrathin Amorphous Li-La-Zr-O Solid Electrolyte. Commun Mater 2021, 2, 76. [CrossRef]
- Du, C.; Li, Z.; Fang, Z.; Fang, X.; Ji, X.; Zhao, Z.; Liu, D.; Li, R.; Xiang, X.; Yang, H. Constructing a Three-Dimensional Continuous Grain Boundary with Lithium Ion Conductivity and Electron Blocking Property in LLZO to Suppress Lithium Dendrites. Journal of Alloys and Compounds 2024, 1003, 175769. [CrossRef]
- Huang, J.; Li, C.; Jiang, D.; Gao, J.; Cheng, L.; Li, G.; Luo, H.; Xu, Z.; Shin, D.; Wang, Y.; et al. Solid-State Electrolytes for Lithium Metal Batteries: State-of-the-Art and Perspectives. Adv Funct Materials 2025, 35, 2411171. [CrossRef]
- Pervez, S.A.; Madinehei, M.; Moghimian, N. Graphene in Solid-State Batteries: An Overview. Nanomaterials 2022, 12, 2310. [CrossRef]
- Yang, T.; Zheng, J.; Cheng, Q.; Hu, Y.-Y.; Chan, C.K. Composite Polymer Electrolytes with Li7 La3 Zr2 O12 Garnet-Type Nanowires as Ceramic Fillers: Mechanism of Conductivity Enhancement and Role of Doping and Morphology. ACS Appl. Mater. Interfaces 2017, 9, 21773–21780. [CrossRef]
- Zhang, Y.; Bao, W.; Hu, Z.; Wang, Y.; Jiang, J.; Huo, S.; Fan, W.; Chen, W.; Jing, X.; Long, X. Poly(Ionic Liquid)-Functionalized Graphene Oxide Towards Ambient Temperature Operation of All-Solid-State Peo-Based Polymer Electrolyte Lithium Metal Batteries. SSRN Journal 2022. [CrossRef]
- Otabil, A.; Kharbatli, A.-R.; Siddique, S.K.; Liao, K.; Schiffer, A. Recent Developments in the Incorporation of 1D/2D Nanofillers in Polymer Derived Ceramics—a Review. Adv Compos Hybrid Mater 2025, 8, 267. [CrossRef]
- Athanasiou, C.E.; Jin, M.Y.; Ramirez, C.; Padture, N.P.; Sheldon, B.W. High-Toughness Inorganic Solid Electrolytes via the Use of Reduced Graphene Oxide. Matter 2020, 3, 212–229. [CrossRef]
- Safian, M.T.; Umar, K.; Mohamad Ibrahim, M.N. Synthesis and Scalability of Graphene and Its Derivatives: A Journey towards Sustainable and Commercial Material. Journal of Cleaner Production 2021, 318, 128603. [CrossRef]
- Soldano, C.; Mahmood, A.; Dujardin, E. Production, Properties and Potential of Graphene. Carbon 2010, 48, 2127–2150. [CrossRef]
- Cha, E.; Kim, D.K.; Choi, W. Advances of 2D MoS2 for High-Energy Lithium Metal Batteries. Front. Energy Res. 2021, 9. [CrossRef]
- Fu, J.; Yu, P.; Zhang, N.; Ren, G.; Zheng, S.; Huang, W.; Long, X.; Li, H.; Liu, X. In Situ Formation of a Bifunctional Interlayer Enabled by a Conversion Reaction to Initiatively Prevent Lithium Dendrites in a Garnet Solid Electrolyte. Energy Environ. Sci. 2019, 12, 1404–1412. [CrossRef]
- He, Y.; Wang, C.; Zhang, R.; Zou, P.; Chen, Z.; Bak, S.-M.; Trask, S.E.; Du, Y.; Lin, R.; Hu, E.; et al. A Self-Healing Plastic Ceramic Electrolyte by an Aprotic Dynamic Polymer Network for Lithium Metal Batteries. Nat Commun 2024, 15. [CrossRef]
- Chen, J.; Gao, Y.; Shi, L.; Yu, W.; Sun, Z.; Zhou, Y.; Liu, S.; Mao, H.; Zhang, D.; Lu, T.; et al. Phase-Locked Constructing Dynamic Supramolecular Ionic Conductive Elastomers with Superior Toughness, Autonomous Self-Healing and Recyclability. Nat Commun 2022, 13. [CrossRef]
- Liu, F.-Q.; Wang, W.-P.; Yin, Y.-X.; Zhang, S.-F.; Shi, J.-L.; Wang, L.; Zhang, X.-D.; Zheng, Y.; Zhou, J.-J.; Li, L.; et al. Upgrading Traditional Liquid Electrolyte via in Situ Gelation for Future Lithium Metal Batteries. Sci. Adv. 2018, 4. [CrossRef]
- Le Mong, A.; Kim, D. Self-Healable, Super Li-Ion Conductive, and Flexible Quasi-Solid Electrolyte for Long-Term Safe Lithium Sulfur Batteries. J. Mater. Chem. A 2023, 11, 6503–6521. [CrossRef]
- He, Y.; Wang, C.; Lin, R.; Hu, E.; Trask, S.E.; Li, J.; Xin, H.L. A Self-Healing, Flowable, Yet Solid Electrolyte Suppresses Li-Metal Morphological Instabilities. Advanced Materials 2024, 36. [CrossRef]
- Zhang, X.; Zhao, H.; Wang, N.; Xiao, Y.; Liang, S.; Yang, J.; Huang, X. Gradual Gradient Distribution Composite Solid Electrolyte for Solid-State Lithium Metal Batteries with Ameliorated Electrochemical Performance. Journal of Colloid and Interface Science 2024, 658, 836–845. [CrossRef]
- Adany Putra Afauly, R.; Faiz Habibi, M.; Arief Budiman, B. Review on Manufacturing Methods of Functionally Graded Material of Solid-State Batteries. In Proceedings of the 2021 3rd International Symposium on Material and Electrical Engineering Conference (ISMEE); IEEE: Bandung, Indonesia, November 10 2021; pp. 236–241.
- Deng, J.; Ren, X.; Lin, H.; Hu, L.; Bai, Y.; Yu, X.; Mo, J.; Zhang, Q.; Kang, F.; Li, B. Functionally Gradient Materials for Sustainable and High-Energy Rechargeable Lithium Batteries: Design Principles, Progress, and Perspectives. Journal of Energy Chemistry 2024, 99, 426–449. [CrossRef]
- Kim, J.; Lee, J.; Yun, J.; Choi, S.H.; Han, S.A.; Moon, J.; Kim, J.H.; Lee, J.; Park, M. Functionality of Dual-Phase Lithium Storage in a Porous Carbon Host for Lithium-Metal Anode. Adv Funct Materials 2020, 30. [CrossRef]
- Chen, L.; Liu, H.; Li, M.; Zhou, S.; Mo, F.; Yu, S.; Wei, J. Boosting the Performance of Lithium Metal Anodes with Three-Dimensional Lithium Hosts: Recent Progress and Future Perspectives. Batteries 2023, 9, 391. [CrossRef]
- Pathak, R.; Chen, K.; Wu, F.; Mane, A.U.; Bugga, R.V.; Elam, J.W.; Qiao, Q.; Zhou, Y. Advanced Strategies for the Development of Porous Carbon as a Li Host/Current Collector for Lithium Metal Batteries. Energy Storage Materials 2021, 41, 448–465. [CrossRef]
- Zhang, Y.; Liu, B.; Hitz, E.; Luo, W.; Yao, Y.; Li, Y.; Dai, J.; Chen, C.; Wang, Y.; Yang, C.; et al. A Carbon-Based 3D Current Collector with Surface Protection for Li Metal Anode. Nano Res. 2017, 10, 1356–1365. [CrossRef]
- Xu, Q.; Yang, X.; Rao, M.; Lin, D.; Yan, K.; Du, R.; Xu, J.; Zhang, Y.; Ye, D.; Yang, S.; et al. High Energy Density Lithium Metal Batteries Enabled by a Porous Graphene/MgF2 Framework. Energy Storage Materials 2020, 26, 73–82. [CrossRef]
- Lee, J.; Won, E.-S.; Kim, D.-M.; Kim, H.; Kwon, B.; Park, K.; Jo, S.; Lee, S.; Lee, J.-W.; Lee, K.T. Three-Dimensional Porous Frameworks for Li Metal Batteries: Superconformal versus Conformal Li Growth. ACS Appl. Mater. Interfaces 2021, 13, 33056–33065. [CrossRef]
- Zhao, X.; Xia, S.; Zhang, X.; Pang, Y.; Xu, F.; Yang, J.; Sun, L.; Zheng, S. Highly Lithiophilic Copper-Reinforced Scaffold Enables Stable Li Metal Anode. ACS Appl. Mater. Interfaces 2021, 13, 20240–20250. [CrossRef]
- Chen, H.; Pei, A.; Wan, J.; Lin, D.; Vilá, R.; Wang, H.; Mackanic, D.; Steinrück, H.-G.; Huang, W.; Li, Y.; et al. Tortuosity Effects in Lithium-Metal Host Anodes. Joule 2020, 4, 938–952. [CrossRef]
- Yan, X.; Lin, L.; Chen, Q.; Xie, Q.; Qu, B.; Wang, L.; Peng, D. Multifunctional Roles of Carbon-based Hosts for Li-metal Anodes: A Review. Carbon Energy 2021, 3, 303–329. [CrossRef]
- Nkosi, F.P.; Valvo, M.; Mindemark, J.; Dzulkurnain, N.A.; Hernández, G.; Mahun, A.; Abbrent, S.; Brus, J.; Kobera, L.; Edström, K. Garnet-Poly(ε-Caprolactone- Co -Trimethylene Carbonate) Polymer-in-Ceramic Composite Electrolyte for All-Solid-State Lithium-Ion Batteries. ACS Appl. Energy Mater. 2021, 4, 2531–2542. [CrossRef]
- Rizvi, S.; Aladhyani, I.; Ding, Y.; Zhang, Q. Recent Advances in Doping Na3Zr2Si2PO12 (NASICON) Solid-State Electrolyte for Sodium-Ion Batteries. Nano Energy 2024, 129, 110009. [CrossRef]
- Sayed, S.Y.; Reese, C.; Kim, Y.; Sachdev, A.K. 3D Current Collectors for Lithium Metal Anodes: A Review on Concepts of Performance. International Materials Reviews 2025, 70, 139–204. [CrossRef]
- Vilá, R.A.; Boyle, D.T.; Dai, A.; Zhang, W.; Sayavong, P.; Ye, Y.; Yang, Y.; Dionne, J.A.; Cui, Y. LiH Formation and Its Impact on Li Batteries Revealed by Cryogenic Electron Microscopy. Sci. Adv. 2023, 9, eadf3609. [CrossRef]
- Zhao, L.; Feng, M.; Wu, C.; Guo, L.; Chen, Z.; Risal, S.; Ai, Q.; Lou, J.; Fan, Z.; Qi, Y.; et al. Imaging the Evolution of Lithium-Solid Electrolyte Interface Using Operando Scanning Electron Microscopy. Nat Commun 2025, 16, 4283. [CrossRef]
- Gao, H.; Ai, X.; Wang, H.; Li, W.; Wei, P.; Cheng, Y.; Gui, S.; Yang, H.; Yang, Y.; Wang, M.-S. Visualizing the Failure of Solid Electrolyte under GPa-Level Interface Stress Induced by Lithium Eruption. Nat Commun 2022, 13, 5050. [CrossRef]
- Zhang, L.; Yang, T.; Du, C.; Liu, Q.; Tang, Y.; Zhao, J.; Wang, B.; Chen, T.; Sun, Y.; Jia, P.; et al. Lithium Whisker Growth and Stress Generation in an in Situ Atomic Force Microscope–Environmental Transmission Electron Microscope Set-Up. Nat. Nanotechnol. 2020, 15, 94–98. [CrossRef]
- Sarah Zaccarine In-Situ XPS: Investigating Stable Interfaces for Improved Solid-State Battery Performance.
- Huo, H.; Jiang, M.; Mogwitz, B.; Sann, J.; Yusim, Y.; Zuo, T.; Moryson, Y.; Minnmann, P.; Richter, F.H.; Veer Singh, C.; et al. Interface Design Enabling Stable Polymer/Thiophosphate Electrolyte Separators for Dendrite-Free Lithium Metal Batteries. Angew Chem Int Ed 2023, 62, e202218044. [CrossRef]
- Zhu, C.; Fuchs, T.; Weber, S.A.L.; Richter, Felix.H.; Glasser, G.; Weber, F.; Butt, H.-J.; Janek, J.; Berger, R. Understanding the Evolution of Lithium Dendrites at Li6.25Al0.25La3Zr2O12 Grain Boundaries via Operando Microscopy Techniques. Nat Commun 2023, 14, 1300. [CrossRef]
- Lu, Y.; Zhao, C.-Z.; Hu, J.-K.; Sun, S.; Yuan, H.; Fu, Z.-H.; Chen, X.; Huang, J.-Q.; Ouyang, M.; Zhang, Q. The Void Formation Behaviors in Working Solid-State Li Metal Batteries. Sci. Adv. 2022, 8, eadd0510. [CrossRef]
- Thomas, C.; Zhang, W.; Chancey, M.R.; Di Michiel, M.; Garman, K.; Wang, Y.; Harris, S.; Finegan, D.P.; Wang, Y.; Ban, C. Cell Reports Physical Science 2025, 6, 102544. [CrossRef]
- Hu, J.; Sun, Z.; Gao, Y.; Li, P.; Wu, Y.; Chen, S.; Wang, R.; Li, N.; Yang, W.; Shen, Y.; et al. 3D Stress Mapping Reveals the Origin of Lithium-Deposition Heterogeneity in Solid-State Lithium-Metal Batteries. Cell Reports Physical Science 2022, 3, 100938. [CrossRef]
- Sun, H.; Celadon, A.; Cloutier, S.G.; Al-Haddad, K.; Sun, S.; Zhang, G. Lithium Dendrites in All-solid-state Batteries: From Formation to Suppression. Battery Energy 2024, 3, 20230062. [CrossRef]
- Liang, Z.; Xiang, Y.; Wang, K.; Zhu, J.; Jin, Y.; Wang, H.; Zheng, B.; Chen, Z.; Tao, M.; Liu, X.; et al. Understanding the Failure Process of Sulfide-Based All-Solid-State Lithium Batteries via Operando Nuclear Magnetic Resonance Spectroscopy. Nat Commun 2023, 14, 259. [CrossRef]
- Maity, A.; Svirinovsky-Arbeli, A.; Buganim, Y.; Oppenheim, C.; Leskes, M. Tracking Dendrites and Solid Electrolyte Interphase Formation with Dynamic Nuclear Polarization—NMR Spectroscopy. Nat Commun 2024, 15, 9956. [CrossRef]
- Drvarič Talian, S.; Kapun, G.; Moškon, J.; Dominko, R.; Gaberšček, M. Operando Impedance Spectroscopy with Combined Dynamic Measurements and Overvoltage Analysis in Lithium Metal Batteries. Nat Commun 2025, 16, 2030. [CrossRef]
- Li, Z.; Fu, J.; Guo, X. How to Commercialize Solid-State Batteries: A Perspective from Solid Electrolytes. NSO 2023, 2, 20220036. [CrossRef]
- Wan, H.; Wang, Z.; Liu, S.; Zhang, B.; He, X.; Zhang, W.; Wang, C. Critical Interphase Overpotential as a Lithium Dendrite-Suppression Criterion for All-Solid-State Lithium Battery Design. Nat Energy 2023, 8, 473–481. [CrossRef]
- Kato, Y.; Hori, S.; Kanno, R. Li10 GeP2 S12 -Type Superionic Conductors: Synthesis, Structure, and Ionic Transportation. Advanced Energy Materials 2020, 10, 2002153. [CrossRef]
- Yuan, C.; Sheldon, B.W.; Xu, J. Heterogeneous Reinforcements to Mitigate Li Penetration through Solid Electrolytes in All-Solid-State Batteries. Advanced Energy Materials 2022, 12, 2201804. [CrossRef]
- Hu, B.; Zhang, S.; Ning, Z.; Spencer-Jolly, D.; Melvin, D.L.R.; Gao, X.; Perera, J.; Pu, S.D.; Rees, G.J.; Wang, L.; et al. Deflecting Lithium Dendritic Cracks in Multi-Layered Solid Electrolytes. Joule 2024, 8, 2623–2638. [CrossRef]
- Ning, Z.; Li, G.; Melvin, D.L.R.; Chen, Y.; Bu, J.; Spencer-Jolly, D.; Liu, J.; Hu, B.; Gao, X.; Perera, J.; et al. Dendrite Initiation and Propagation in Lithium Metal Solid-State Batteries. Nature 2023, 618, 287–293. [CrossRef]
- Wang, Z.-X.; Lu, Y.; Zhao, C.-Z.; Huang, W.-Z.; Huang, X.-Y.; Kong, W.-J.; Li, L.-X.; Wang, Z.-Y.; Yuan, H.; Huang, J.-Q.; et al. Suppressing Li Voids in All-Solid-State Lithium Metal Batteries through Li Diffusion Regulation. Joule 2024, 8, 2794–2810. [CrossRef]
- Tao, J.; Chen, Y.; Bhardwaj, A.; Wen, L.; Li, J.; Kolosov, O.V.; Lin, Y.; Hong, Z.; Huang, Z.; Mathur, S. Combating Li Metal Deposits in All-Solid-State Battery via the Piezoelectric and Ferroelectric Effects. Proc. Natl. Acad. Sci. U.S.A. 2022, 119, e2211059119. [CrossRef]
- Shan, J.; Gu, R.; Xu, J.; Gong, S.; Guo, S.; Xu, Q.; Shi, P.; Min, Y. Heterojunction Ferroelectric Materials Enhance Ion Transport and Fast Charging of Polymer Solid Electrolytes for Lithium Metal Batteries. Advanced Energy Materials 2025, 15, 2405220. [CrossRef]
- Sung, J.; Heo, J.; Kim, D.-H.; Jo, S.; Ha, Y.-C.; Kim, D.; Ahn, S.; Park, J.-W. Recent Advances in All-Solid-State Batteries for Commercialization. Mater. Chem. Front. 2024, 8, 1861–1887. [CrossRef]
- Avadanii, D.; Ganschow, S.; Stypa, M.; Müller, S.; Lang, S.; Kramer, D.; Kirchlechner, C.; Mönig, R. Disconnected Lithium Metal Damages Solid-State Electrolytes. ACS Energy Lett. 2025, 10, 2061–2067. [CrossRef]
- Antony Jose, S.; Gallant, A.; Gomez, P.L.; Jaggers, Z.; Johansson, E.; LaPierre, Z.; Menezes, P.L. Solid-State Lithium Batteries: Advances, Challenges, and Future Perspectives. Batteries 2025, 11, 90. [CrossRef]
- Nikodimos, Y.; Huang, C.-J.; Taklu, B.W.; Su, W.-N.; Hwang, B.J. Chemical Stability of Sulfide Solid-State Electrolytes: Stability toward Humid Air and Compatibility with Solvents and Binders. Energy Environ. Sci. 2022, 15, 991–1033. [CrossRef]
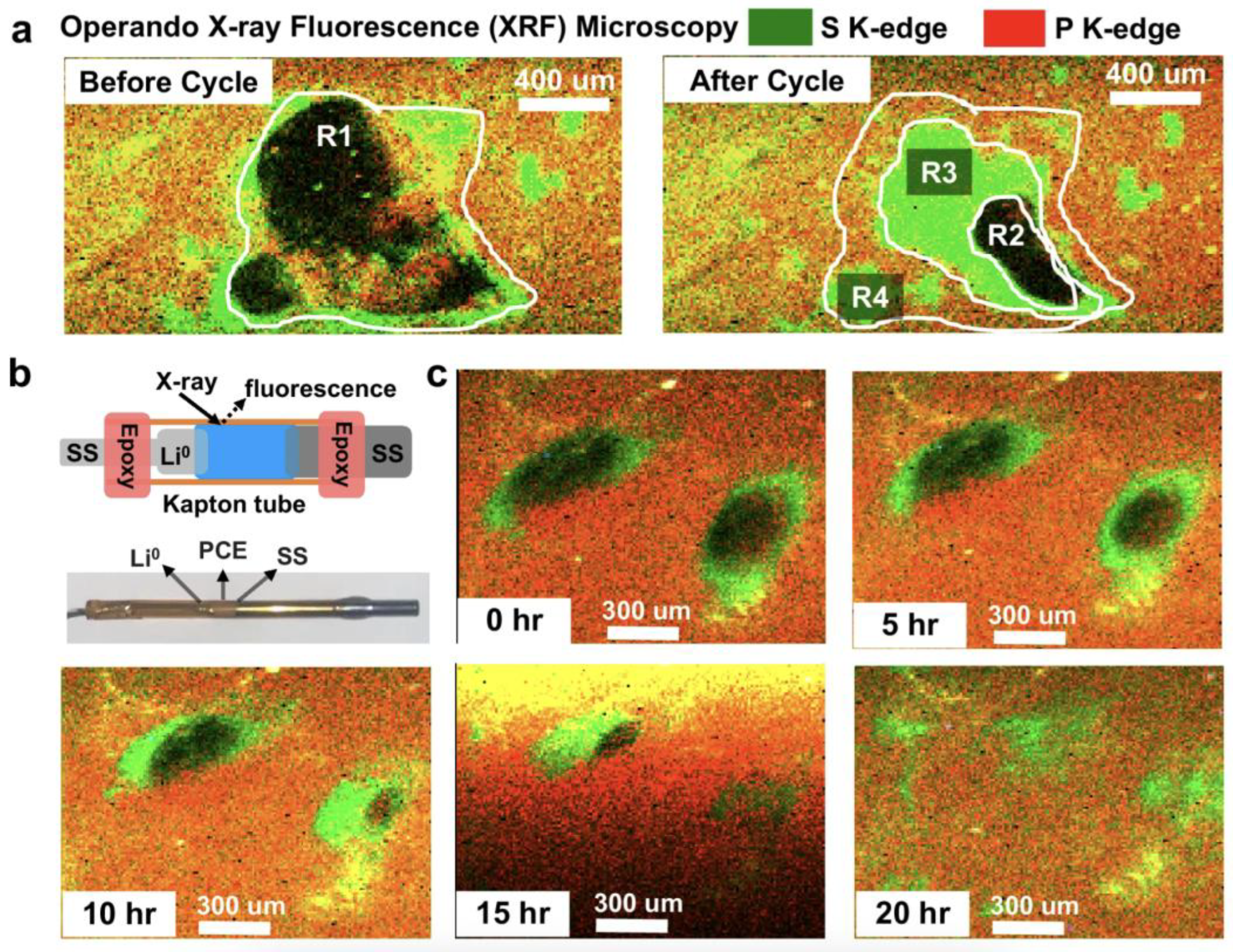
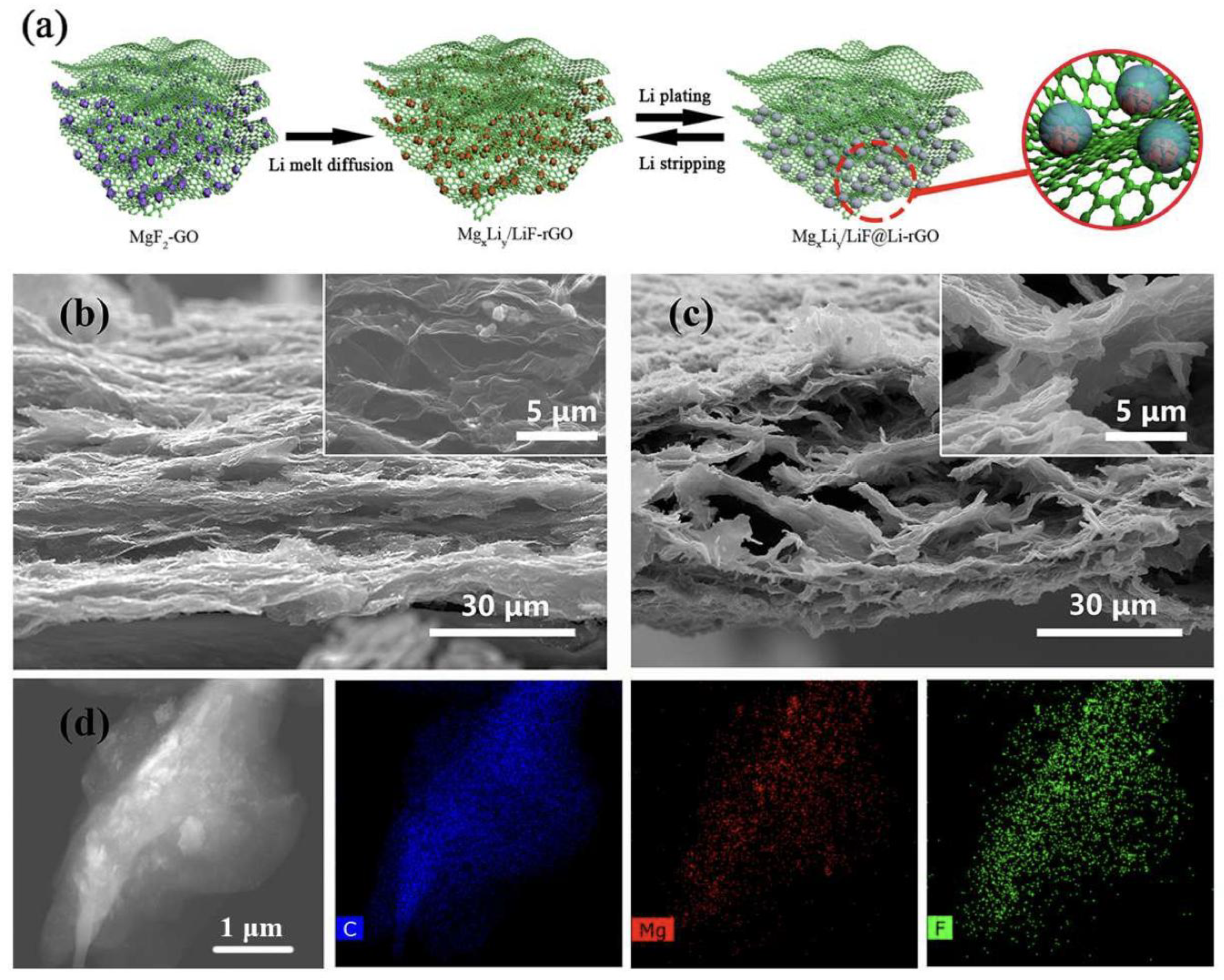

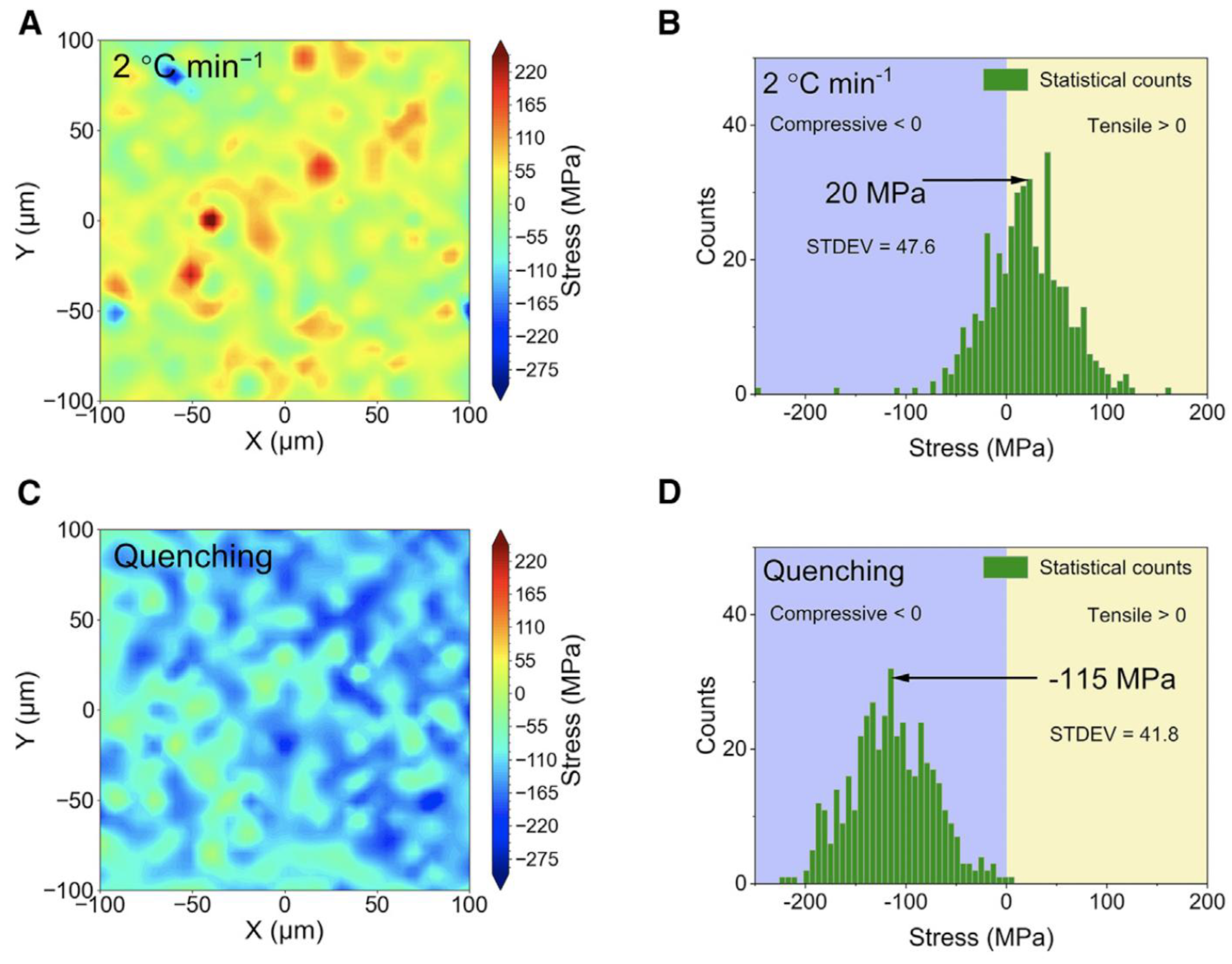
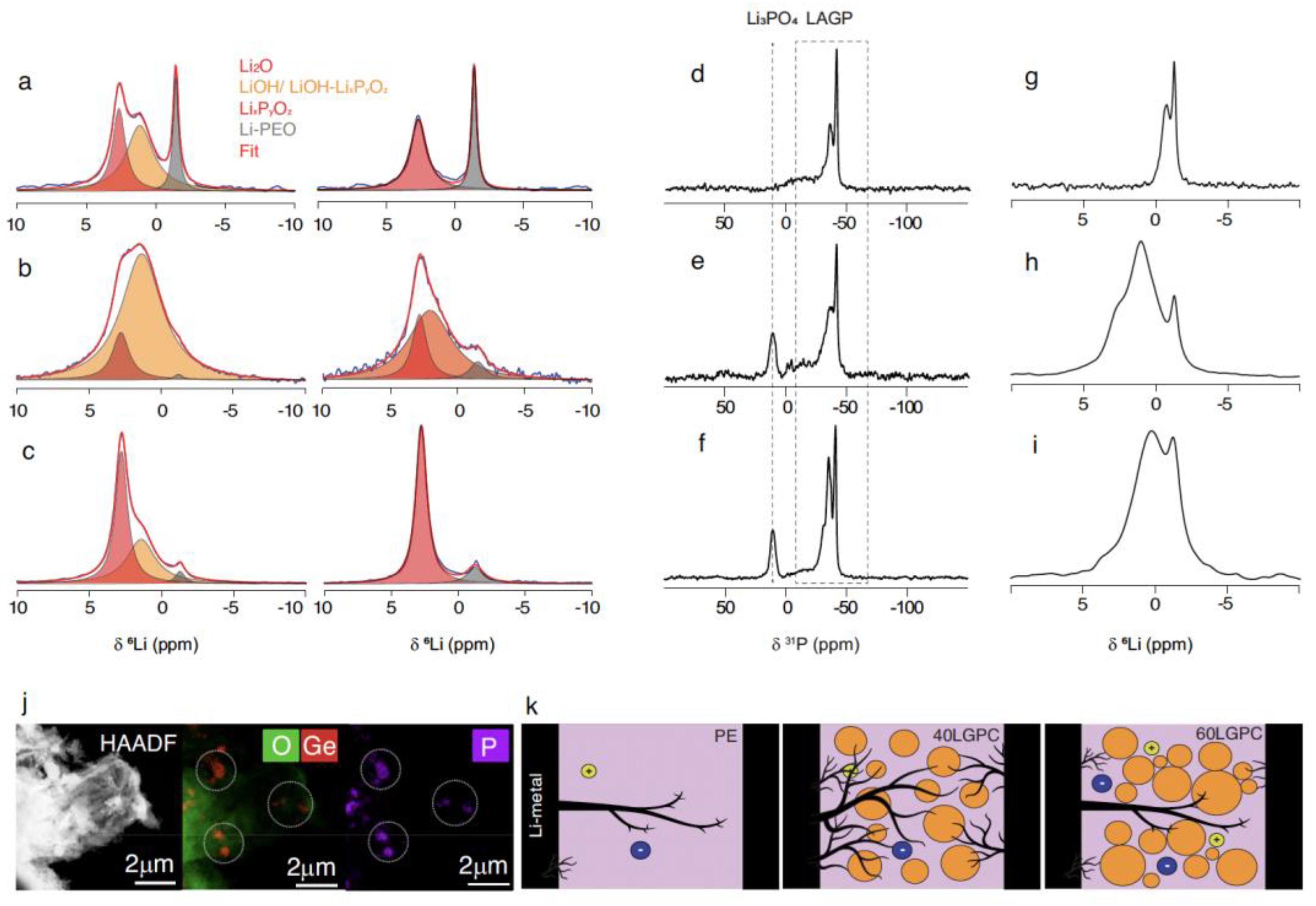
| Strategy | Main Advantage | Key Challenge | References |
|---|---|---|---|
| Interface Coatings | Enhances chemical stability at Li/SSE interface | Maintaining ionic conductivity across coating layer | [57] |
| Electron-Blocking Interlayers | Suppresses electron flow, blocks dendrite growth | Complex fabrication and interface compatibility | [58] |
| Polymer-Ceramic Composite Electrolytes | Combines mechanical flexibility and ionic conductivity | Achieving uniform dispersion and phase stability | [95] |
| Doping of SSEs | Improves ionic conductivity and structural robustness | Controlling dopant homogeneity and effects | [96] |
| 3D-Host Architectures | Reduces local current density, directs Li deposition | Fabrication scalability and maintaining conductivity | [97] |
| LiH Mitigation | Avoids insulating phases that degrade performance | Complete elimination of hydrogen sources is difficult | [98] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





