Submitted:
30 November 2025
Posted:
02 December 2025
You are already at the latest version
Abstract
Keywords:
I. Introduction
II. Material and Methods
Ingenuity Pathway Analysis Software
III. Results and Outcomes, All the Figures and Tables Are Provided as Supplementary Materials
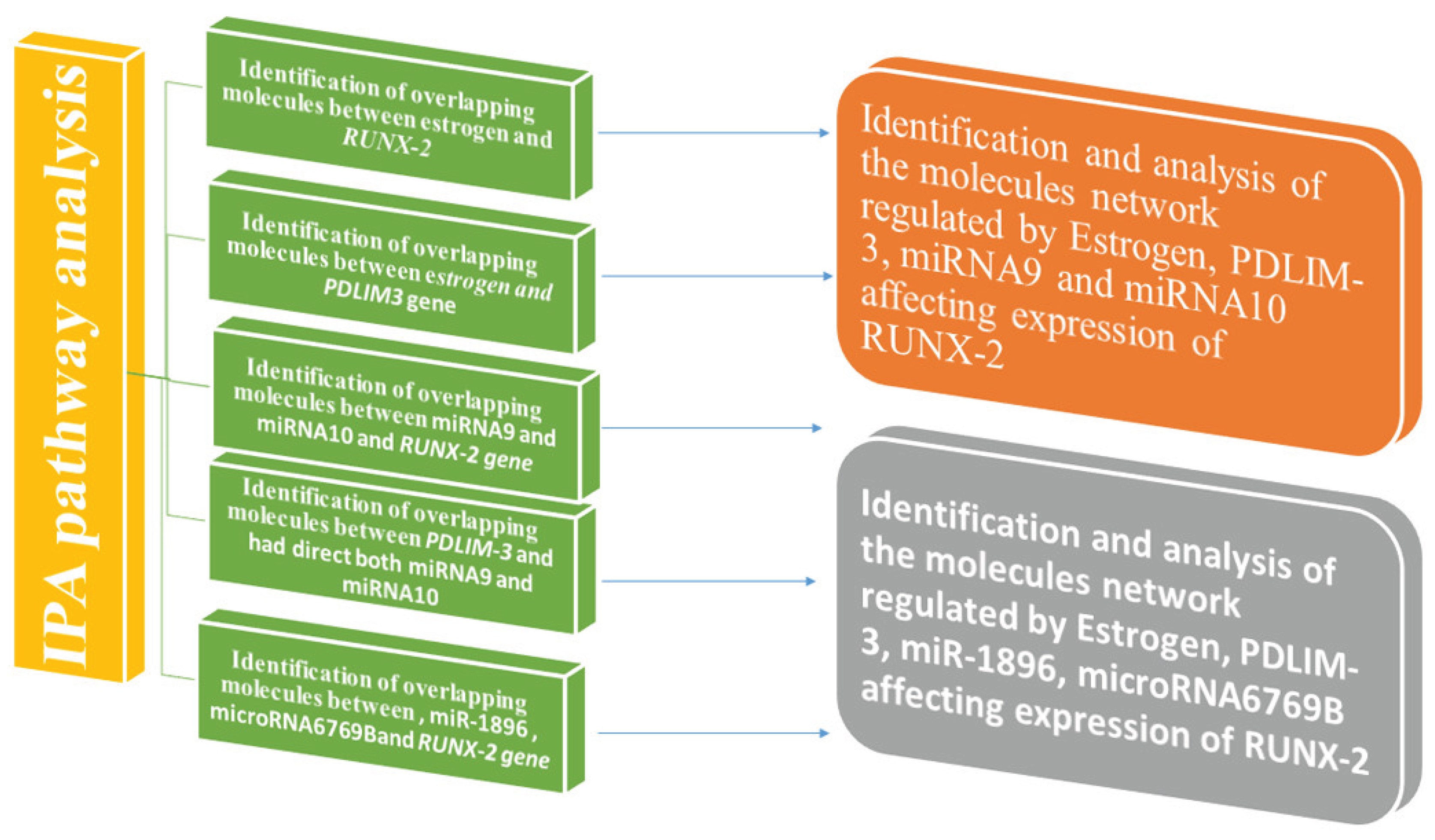
1. Molecular Pathway Analysis of Molecules Mediating the Relationship Between Estrogen and PDLIM3
2. Molecular Pathway Analysis of Molecules Mediating the Relationship Between Estrogen and Affect Expression of RUNX-2
3. Molecular Pathway Analysis of Molecules Mediating the Relationship Between miRNA9 and Directly Affecting Expression of the RUNX-2 Gene
4. Molecular Pathway Analysis of Molecules Mediating the Relationship Between miRNA10 and Directly Affecting Expression of the RUNX-2 Gene
5. Molecular Pathway Analysis of Molecules Mediating the Relationship Between PDLIM-3 and Had Direct Effects on Expression of miRNA9
6. Molecular Pathway Analysis of Molecules Mediating the Relationship Between PDLIM-3 and Had Direct Effects on Expression of miRNA10
7. Identification and Analysis of the Molecule Network Regulated by Estrogen, PDLIM-3, miRNA9, and miRNA10 affects the expression of RUNX-2.
8. Identification and Analysis of the Molecules Network Regulated by Estrogen, PDLIM-3, miR-1896, microRNA6769B affects the expression of RUNX-2.
IV. Constraints
V. Discussion and Conclusion
VI. Conclusion
VII. Figure Legends
- (a)
-
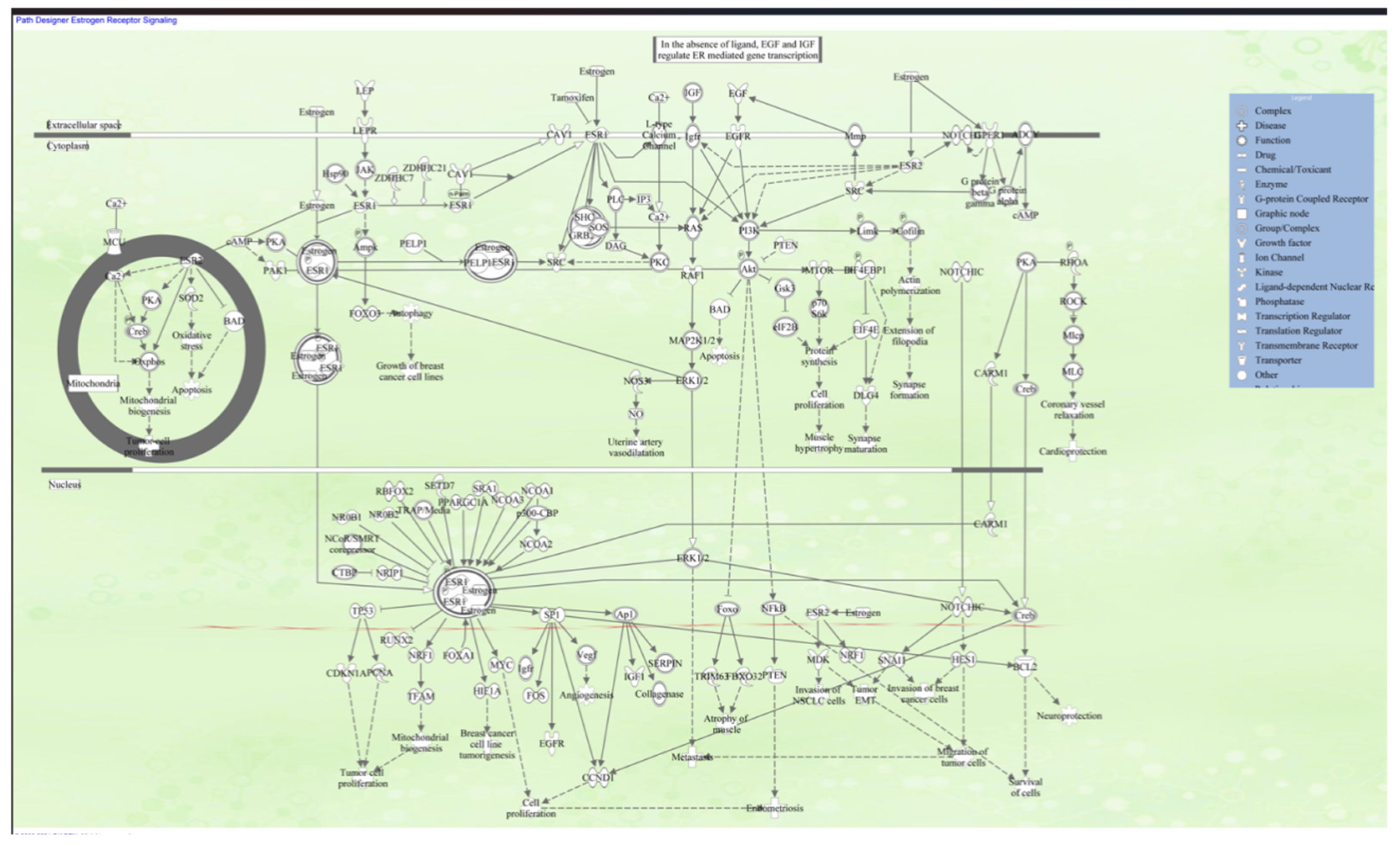
Figure 1. Diagram of Estrogen Receptor Signaling Pathway.This figure, produced with QIAGEN’s Ingenuity Pathway Analysis (IPA), depicts the principal molecular connections and regulatory mechanisms of estrogen receptor signaling. It encompasses fundamental elements such estrogen receptors (ERα and ERβ), heat shock proteins (HSPs), and downstream signaling molecules, emphasizing mechanisms such as gene expression, cellular proliferation, and apoptosis.
- (b)
-
Figure 2. Workflow for Bioinformatics Data Mining and Analysis.schematic depiction of the data analysis process employing QIAGEN’s Ingenuity Pathway Analysis (IPA) technologies. The method illustrates the utilization of the “Grow,” “Connect,” “Pathway Explorer,” and “Molecule Activity Predictor” (MAP) tools for constructing and analyzing biological networks, then comparing them to canonical pathways in the QIAGEN Knowledge Base (QKB).
- (c)
-
Figure 3. Molecular Network Connecting Estrogen Signaling to PDLIM3 Expression.connection map produced with the IPA “MAP” tool, illustrating the direct relationships between estrogen and the PDLIM3 gene through 10 principal intermediary molecules, such as PCNA, CDK4, ESR1, and ESR2. This network indicates that estrogen modulates PDLIM3 via pathways associated with cell cycle progression and gonadotropin signaling.
- (d)
-
Figure 4. Comprehensive Network of Estrogen-Regulated Pathways Modulating RUNX2 Expression.The “MAP” software and QKB found 75 unique molecular pathways by which estrogen regulates RUNX2 expression. This network includes several molecules, such as transcription regulators, kinases, and cytokines, highlighting estrogen’s multifaceted role as a principal regulator of osteogenesis.
- (e)
-
Figure 5. Regulatory Network of miRNA-9 Targeting RUNX2.The molecular network illustrates eight pathways by which miRNA-9 directly influences RUNX2 expression, encompassing transcription regulators and other molecular families. This designates miR-9 as a precise epigenetic modulator of the osteogenic master regulator.
- (f)
-
Figure 6. Extensive Regulatory Network of miRNA-10 Targeting RUNX2.The study revealed 34 pathways regulated by miRNA-10 that directly affect RUNX2 expression. The extensive network associated with miR-10, in contrast to miR-9, indicates that miR-10 functions as a superior integrator, orchestrating several signals to culminate in RUNX2 activation.
- (g)
-
Figure 7. Regulation of miRNA-9 Expression Mediated by PDLIM3.Network analysis identifies 49 pathways directly regulated by PDLIM3 that influence the expression of miRNA-9. This establishes a molecular connection, demonstrating how the structural protein PDLIM3 operates as a signaling hub to regulate epigenetic processes through miR-9.
- (h)
-
Figure 8. PDLIM3 as a Principal Upstream Regulator of miRNA-10 Expression.significant network of 98 pathways demonstrates direct regulation of miRNA-10 expression by PDLIM3. This signifies the most comprehensive connectivity in our investigation, pinpointing the control of miR-10 as a primary downstream function of PDLIM3 in the estrogen signaling pathway.
- (i)
-
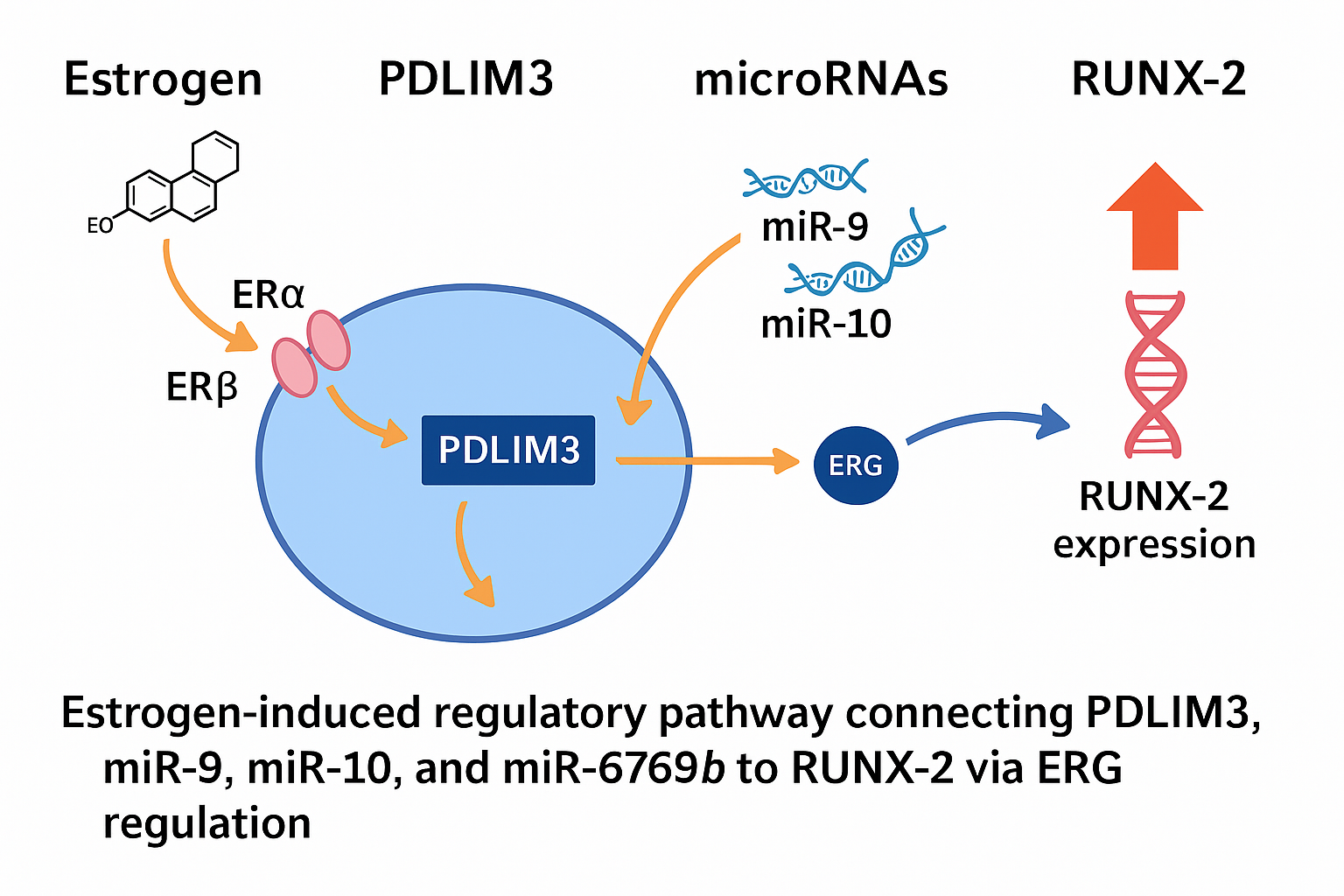
Figure 9. Integrated Molecular Network of the Estrogen-PDLIM3-miRNA-RUNX2 Axis.fundamental network comprising 39 molecules that are directly or indirectly implicated in RUNX2 expression, as modulated by the interaction between estrogen, PDLIM3, miRNA-9, and miRNA-10. This integrated map vividly delineates the novel multi-layered regulatory pathway from estrogen receptor stimulation to the transcriptional regulation of RUNX2.
- (j)
-
Figure 10. Innovative Network Incorporating miR-6769b in Estrogen-Driven Osteogenesis.The analysis revealed a network of 45 molecules that regulate RUNX2 expression, influenced by estrogen, PDLIM3, miR-1896, and the recently identified microRNA-6769B. This substantially broadens the suggested regulatory framework by incorporating miR-6769b as a new pivotal component.
Acknowledgments
Conflicts of Interest
Abbreviations
| Abbreviation | Definition |
| AKT | Protein kinase B; serine/threonine kinase in the PI3K/AKT signaling pathway regulating survival and metabolism. |
| ALP | Actin-associated LIM protein; structural and signaling protein encoded by PDLIM3, localized in cardiac and skeletal muscle Z-discs. |
| AP-1 | Activator protein 1; transcription factor complex (Fos/Jun) regulating proliferation and apoptosis. |
| BAX | Bcl-2–associated X protein; pro-apoptotic member of the Bcl-2 family. |
| BCL-2 | B-cell lymphoma 2; anti-apoptotic protein that promotes cell survival. |
| CBFα1 | Core-binding factor subunit alpha-1; alternative name for RUNX2 transcription factor. |
| CCD | Cleidocranial dysplasia; hereditary skeletal disorder caused by mutations in RUNX2. |
| CDK | Cyclin-dependent kinase; enzyme family that regulates cell-cycle progression. |
| CDK4 | Cyclin-dependent kinase 4; G1 phase cell-cycle regulator. |
| CREB | cAMP response element-binding protein; transcription factor that regulates metabolism, survival, and plasticity. |
| CV | Coefficient of variation; measure of relative variability in gene expression or stability. |
| DCM | Dilated cardiomyopathy; disorder characterized by dilation and impaired contraction of cardiac chambers. |
| E1 | Estrone; endogenous estrogen. |
| E2 | 17β-estradiol; the most potent and biologically active endogenous estrogen. |
| E3 | Estriol; estrogen predominant during pregnancy. |
| E4 | Estetrol; fetal liver–derived estrogen present during pregnancy. |
| ECM | Extracellular matrix; structural network of proteins and polysaccharides surrounding cells. |
| EGFR | Epidermal growth factor receptor; receptor tyrosine kinase regulating proliferation and survival. |
| ER | Estrogen receptor; ligand-activated transcription factor mediating estrogen actions. |
| ERα / ERβ | Estrogen receptor alpha / beta; two main nuclear estrogen receptor isoforms. |
| ERGs | Estrogen-responsive genes; genes whose transcription is modulated by estrogen receptor signaling. |
| EREs | Estrogen response elements; DNA sequences bound by ER complexes to regulate transcription. |
| ERK | Extracellular signal-regulated kinase; MAPK family kinase involved in proliferation and differentiation. |
| ESR1 / ESR2 | Estrogen receptor 1 / 2; gene symbols encoding ERα and ERβ, respectively. |
| FSH | Follicle-stimulating hormone; gonadotropin regulating follicle development and spermatogenesis. |
| Fos | Proto-oncogene c-Fos; component of the AP-1 transcription factor complex. |
| GPER1 | G protein-coupled estrogen receptor 1; membrane-bound estrogen receptor mediating rapid non-genomic signaling. |
| GR | Glucocorticoid receptor; nuclear receptor for glucocorticoid hormones. |
| GSK3β | Glycogen synthase kinase 3 beta; serine/threonine kinase involved in metabolism and Wnt/MAPK signaling. |
| HRT | Hormone replacement therapy; clinical use of hormones (e.g., estrogens) to treat menopausal or deficiency symptoms. |
| HSPs | Heat shock proteins; molecular chaperones that stabilize and refold proteins, also associated with steroid receptors. |
| ID | Inhibitor of differentiation; family of helix-loop-helix transcriptional regulators. |
| ID3 | Inhibitor of DNA-binding protein 3; an ID family member downregulated by miR-10a-3p during osteogenic differentiation. |
| IGF-1 | Insulin-like growth factor 1; peptide growth factor important for growth and metabolism. |
| IPA | Ingenuity Pathway Analysis; QIAGEN bioinformatics software for pathway and network modeling. |
| IRS-1 | Insulin receptor substrate 1; adaptor protein transmitting insulin/IGF-1 receptor signaling. |
| JNK | c-Jun N-terminal kinase; stress-activated protein kinase within the MAPK family. |
| LH | Luteinizing hormone; gonadotropin regulating ovulation and gonadal steroid production. |
| MAP | Molecule Activity Predictor; Ingenuity Pathway Analysis (IPA) tool predicting activation/inhibition effects within networks. |
| MAPK / MAPK-1 | Mitogen-activated protein kinase / MAPK-1; serine/threonine kinases mediating downstream signaling (ERK2 often referred to as MAPK-1). |
| MDSCs | Myeloid-derived suppressor cells; immune-suppressive myeloid cell population modulating tumor and inflammatory responses. |
| MEK | Mitogen-activated protein kinase kinase; dual-specificity kinase upstream of ERK in the MAPK cascade. |
| miR / miRNA | MicroRNA; small (~19–25 nt) non-coding RNA that regulates gene expression post-transcriptionally. |
| miR-9 | MicroRNA-9; miRNA regulating neurogenesis, osteogenesis, and immune functions. |
| miR-10 / miRNA10 / miR-10a | MicroRNA-10; family associated with differentiation, apoptosis, and cancer; miR-10a is a specific isoform. |
| miR-1896 | MicroRNA-1896; miRNA implicated in indirect regulation of RUNX2 via shared seed sequences. |
| miR-6769b / microRNA6769B | MicroRNA-6769b; newly implicated epigenetic regulator in RUNX2-related signaling. |
| mTOR | Mechanistic target of rapamycin; central kinase controlling growth and metabolism. |
| NF-κB | Nuclear factor kappa-B; transcription factor regulating inflammatory and immune responses. |
| OPN | Osteopontin; extracellular matrix phosphoprotein involved in bone remodeling and mineralization. |
| PCNA | Proliferating cell nuclear antigen; sliding clamp protein and marker of DNA replication and repair. |
| PDLIM3 | PDZ and LIM domain protein 3; actin-associated structural and signaling protein (ALP), here identified as an estrogen-regulated intermediary upstream of RUNX2. |
| PI3K | Phosphoinositide 3-kinase; lipid kinase that generates PIP3 and activates AKT signaling. |
| PTEN | Phosphatase and tensin homolog; tumor suppressor and negative regulator of PI3K/AKT signaling. |
| QKB | QIAGEN Knowledge Base; curated database underpinning Ingenuity Pathway Analysis. |
| Ras | Rat sarcoma; small GTP-binding protein family regulating proliferation and survival pathways. |
| RUNX2 / RUNX-2 | Runt-related transcription factor 2; master transcription factor for osteoblast differentiation and skeletal morphogenesis. |
| SRC | Steroid receptor coactivator; transcriptional co-regulator that enhances nuclear receptor–mediated gene expression. |
| STAT | Signal transducer and activator of transcription; transcription factor family that mediates cytokine and growth factor signaling. |
| VEGF | Vascular endothelial growth factor; central regulator of angiogenesis and vascular permeability. |
References
- Huether, S.E.; McCance, K.L. Understanding pathophysiology, 7th ed.; Elsevier Health Sciences, 2019; p. 767. ISBN 978-0-32-367281-8. [Google Scholar]
- Delgado, B.J.; Lopez-Ojeda, W. Estrogen. In StatPearls [Internet]; StatPearls Publishing, 2023; Available online: https://www.ncbi.nlm.nih.gov/books/NBK555922/.
- Sier, J.H.; Thumser, A.E.; Plant, N.J. Linking physiologically-based pharmacokinetic and genome-scale metabolic networks to understand estradiol biology. BMC Systems Biology 2017, 11, 93. [Google Scholar] [CrossRef] [PubMed]
- Shaban, N.Z.; Talaat, I.; Elrashidy, F.; Hegazy, A.; Sultan, A. Therapeutic role of Punica granatum (pomegranate) seed oil extract on bone turnover and resorption induced in ovariectomized rats. The Journal of Nutrition, Health & Aging 2017, 21, 1299–1306. [Google Scholar] [CrossRef]
- Thompson, P.A.; Ambrosone, C. Molecular epidemiology of genetic polymorphisms in estrogen-metabolizing enzymes in human breast cancer. Journal of the National Cancer Institute Monographs 2000, 27, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, N.; Silveyra, P. Estrogen receptor signaling mechanisms. Advances in Protein Chemistry and Structural Biology 2019, 116, 135–170. [Google Scholar] [CrossRef]
- Le Dily, F.; Beato, M. Signaling by steroid hormones in the 3D nuclear space. International Journal of Molecular Sciences 2018, 19, 306. [Google Scholar] [CrossRef]
- Komori, T. RUNX2, an inducer of osteoblast and chondrocyte differentiation. Histochemistry and Cell Biology 2019, 151, 1–11. [Google Scholar] [CrossRef]
- Lucero, M.J.; Vega, O.A.; Osorio, M.M.; Tapia, J.C.; Antonelli, M.; Stein, G.S.; Galindo, M.A. The cancer-related transcription factor RUNX2 modulates cell proliferation in human osteosarcoma cell lines. Journal of Cellular Physiology 2013, 228, 714–723. [Google Scholar] [CrossRef]
- Lin, T.C. RUNX2 and cancer. International Journal of Molecular Sciences 2023, 24, 7001. [Google Scholar] [CrossRef]
- Hu, X.; Chen, M.; Ruan, Q.; Shi, C.; Pan, J.; Luo, L. Comprehensive analysis of PDLIM3 expression profile, prognostic value, and correlations with immune infiltrates in gastric cancer. Journal of Immunology Research 2022, 2022, 2039447. [Google Scholar] [CrossRef]
- Nishi, K.; Fu, W.; Kiyama, R. Novel estrogen-responsive genes (ERGs) for the evaluation of estrogenic activity. PLoS ONE 2022, 17, e0272003. [Google Scholar] [CrossRef]
- Ranganathan, K.; Sivasankar, V. MicroRNAs: Biology and clinical applications. Journal of Oral and Maxillofacial Pathology: JOMFP 2014, 18, 229–234. [Google Scholar] [CrossRef]
- Luo, H.; Gao, H.; Liu, F.; Qiu, B. Regulation of RUNX2 by microRNA-9 and microRNA-10 modulates the osteogenic differentiation of mesenchymal stem cells. International Journal of Molecular Medicine 2017, 39, 1046–1052. [Google Scholar] [CrossRef] [PubMed]
- Tamkovich, S.; Borisova, A.; Shevela, A.; Chernyavskiy, A. ; Chernyshovа; A Exosomal microRNA: Diagnostic potential and role in breast cancer dissemination. Molecules 2025, 30, 3858. [Google Scholar] [CrossRef]
- Tian, J.; Rui, K.; Tang, X.; Ma, J.; Wang, Y.; Tian, X.; et al. MicroRNA-9 regulates the differentiation and function of myeloid-derived suppressor cells via targeting Runx1. Journal of Immunology 2015, 195, 1301–1311. [Google Scholar] [CrossRef]
- Jiang, M.; Zhang, W.; Zhang, R.; Liu, P.; Ye, Y.; Yu, W.; Guo, X.; Yu, J. Cancer exosome-derived miR-9 and miR-181a promote the development of early-stage MDSCs via interfering with SOCS3 and PIAS3 respectively in breast cancer. Oncogene 2020, 39, 4681–4694. [Google Scholar] [CrossRef]
- Mu, N.; Gu, J.; Huang, T.; Zhang, C.; Shu, Z.; Li, M.; et al. A novel NF-κB/YY1/microRNA-10a regulatory circuit in fibroblast-like synoviocytes regulates inflammation in rheumatoid arthritis. Scientific Reports 2016, 6, 20059. [Google Scholar] [CrossRef]
- Xu, D.; Huang, C.C.; Kachaochana, A.; Morgan, G.A.; Bonaldo, M.F.; Soares, M.B.; et al. MicroRNA-10a regulation of proinflammatory mediators: An important component of untreated juvenile dermatomyositis. Journal of Rheumatology 2016, 43, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Stadthagen, G.; Tehler, D.; Høyland-Kroghsbo, N.M.; Wen, J.; Krogh, A.; Jensen, K.T.; et al. Loss of miR-10a activates lpo and collaborates with activated Wnt signaling in inducing intestinal neoplasia in female mice. PLoS Genetics 2013, 9, e1003913. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Zhang, H.; Gu, W.; Wu, H.; Chen, Y.; Zhou, W.; et al. The microRNA-10a/ID3/RUNX2 axis modulates the development of ossification of posterior longitudinal ligament. Scientific Reports 2018, 8, 12577. [Google Scholar] [CrossRef]
- Bishir, M.; Rengifo, T.; Huang, W.; Kim, R.J.; Chidambaram, S.B.; Chang, S.L. Network meta-analysis on alcohol-mediated modulation of Alzheimer’s disease in the diseases of inflammation including COVID-19. NeuroImmune Pharmacology and Therapeutics 2023, 2, 267–281. [Google Scholar] [CrossRef]
- Rengifo, T.; Bishir, M.; Huang, W.; Snyder, M.; Chang, S.L. Network meta-analysis of the molecular mechanisms and signaling pathways underlying alcohol-induced thymic atrophy. Alcohol: Clinical and Experimental Research 2024, 48, 795–809. [Google Scholar] [CrossRef]
- Zhang, J.; Bishir, M.; Barbhuiya, S.; Chang, S.L. Meta-analysis of the mechanisms underlying COVID-19 modulation of Parkinson’s disease. International Journal of Molecular Sciences 2023, 24, 13554. [Google Scholar] [CrossRef]
- Otto, F.; Thornell, A.P.; Crompton, T.; Denzel, A.; Gilmour, K.C.; Rosewell, I.; et al. Cbfa1, a candidate gene for cleidocranial dysplasia syndrome, is essential for osteoblast differentiation and bone development. Cell 1997, 89, 765–771. [Google Scholar] [CrossRef] [PubMed]
- Krcmery, J.; Klaska, V.; Cimler, R.; Kocianova, E. Loss of the cytoskeletal protein α-actinin-2 leads to dilated cardiomyopathy and skeletal muscle dysfunction. Proceedings of the National Academy of Sciences 2010, 107, 2345–2350. [Google Scholar] [CrossRef]
- Yao, Q.; Wu, X.; Tao, C.; Zhang, Y.; Wang, Q.; Li, Y. Osteoarthritis: Pathogenic signaling pathways and therapeutic targets. Signal Transduction and Targeted Therapy 2023, 8, 56. [Google Scholar] [CrossRef]
- Zhou, L.; Zhang, H.; Zhang, Z.; Li, H.; Sun, Y. MiR-9 promotes osteoblast differentiation and suppresses osteoclastogenesis via targeting DKK1. Gene 2015, 564, 137–144. [Google Scholar] [CrossRef]
- Wu, D.; Chen, W.; Zhang, Y.; Zhang, J.; Li, H. MiR-10a promotes osteogenesis and suppresses adipogenesis in human mesenchymal stem cells. Aging Cell 2014, 13, 333–341. [Google Scholar] [CrossRef]
- Sun, G.; Yu, R.; Wang, X.; Zhang, X. miR-9 regulates neural stem cell proliferation and differentiation by targeting TLX and REST. Developmental Cell 2013, 20, 897–909. [Google Scholar] [CrossRef]
- Wang, H.; Qiu, X.; Zhang, X.; Li, F.; Chen, J. miR-10a regulates T cell activation and promotes immunosuppression in ovarian cancer. Cell Death & Disease 2015, 6, e1915. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, H.; Li, S. miR-6769b-5p targets CCND1 to regulate proliferation in cadmium-exposed placental trophoblasts. Environmental Toxicology and Pharmacology 2022, 91, 103752. [Google Scholar] [CrossRef]
- NET Research Team. Exosome-mediated regulation of bone remodeling by miRNAs including miR-6769b. NET Archives. 2019. Available online: https://www.netarchives.org/exosome-bone-miRNA.
- ResearchGate. Network meta-analysis of estrogen’s direct and indirect influence on signaling pathways controlling RUNX2 through novel ERG genes. 2025. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




