Submitted:
25 June 2025
Posted:
26 June 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Computational Details
3. Results and Discussion
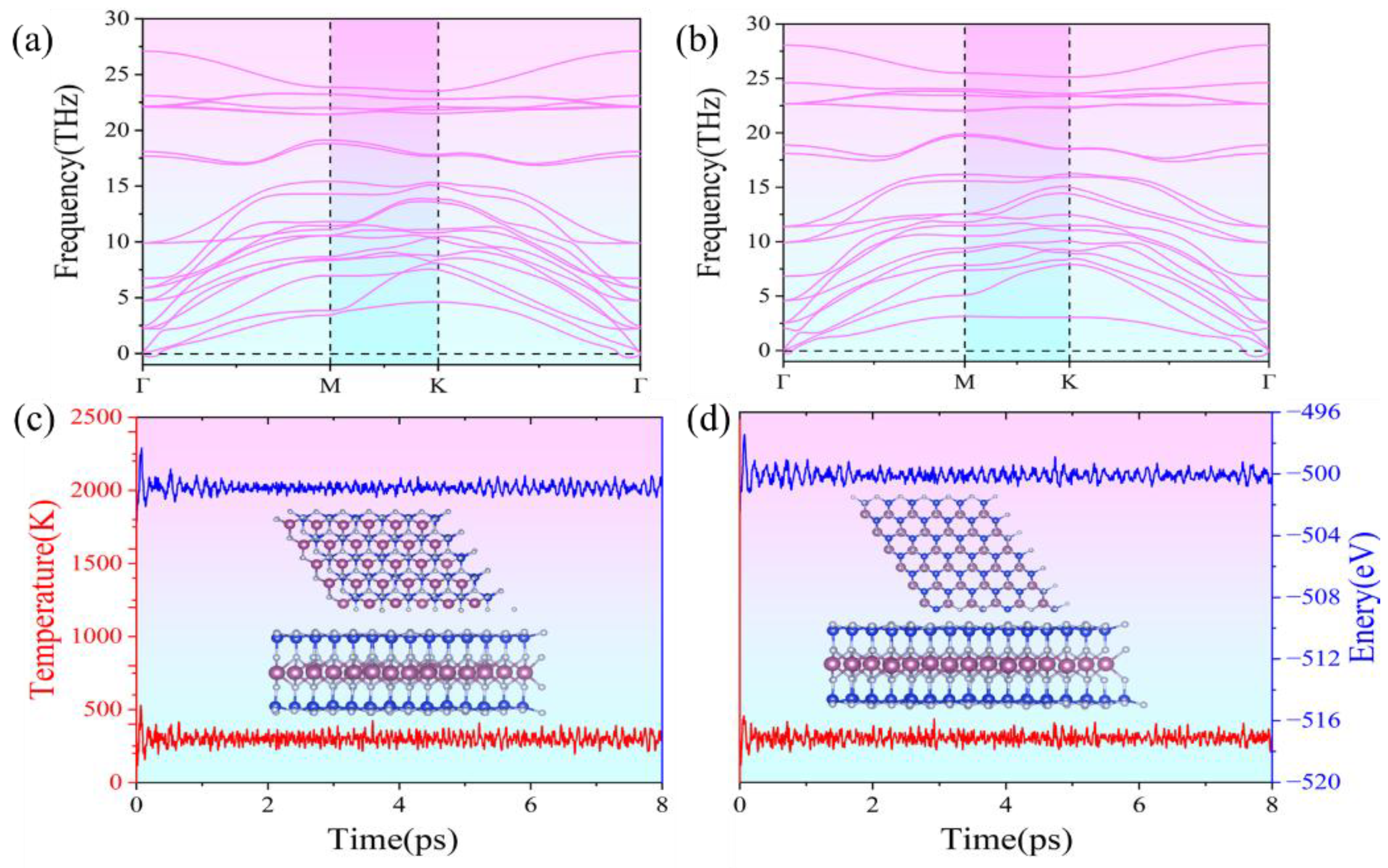
3.1. Structure and Stability
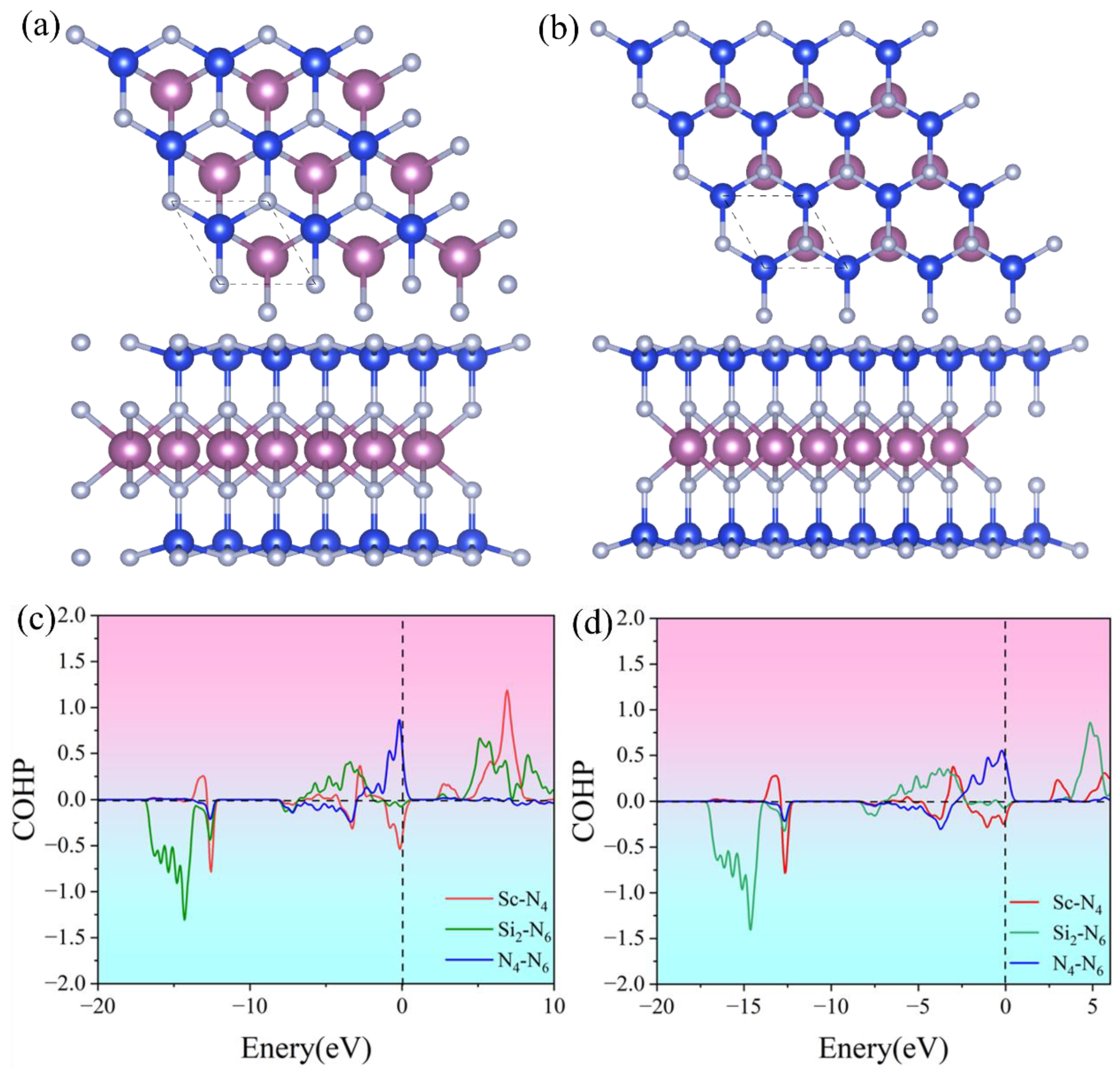
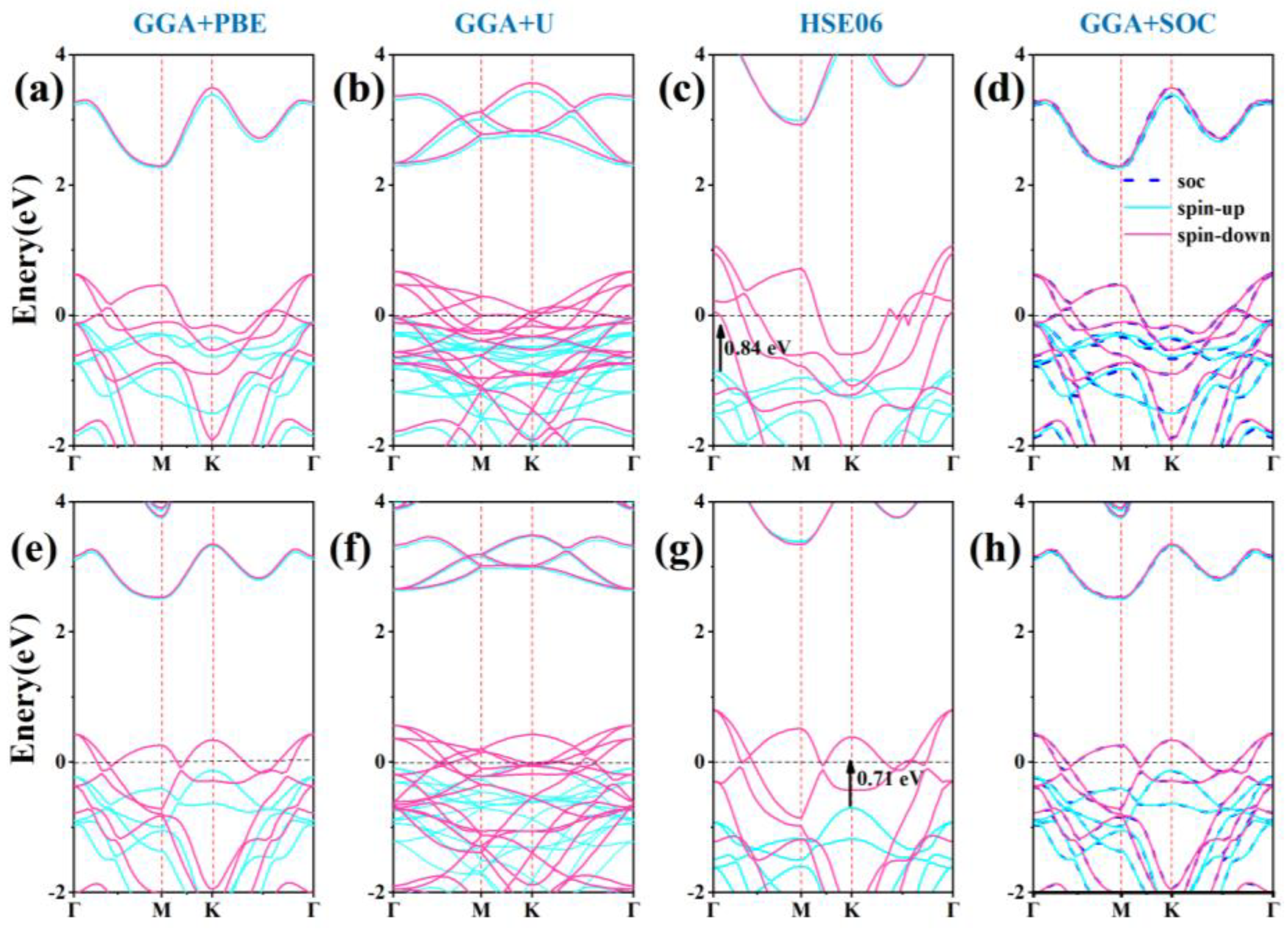
3.2. Electronic Behavior
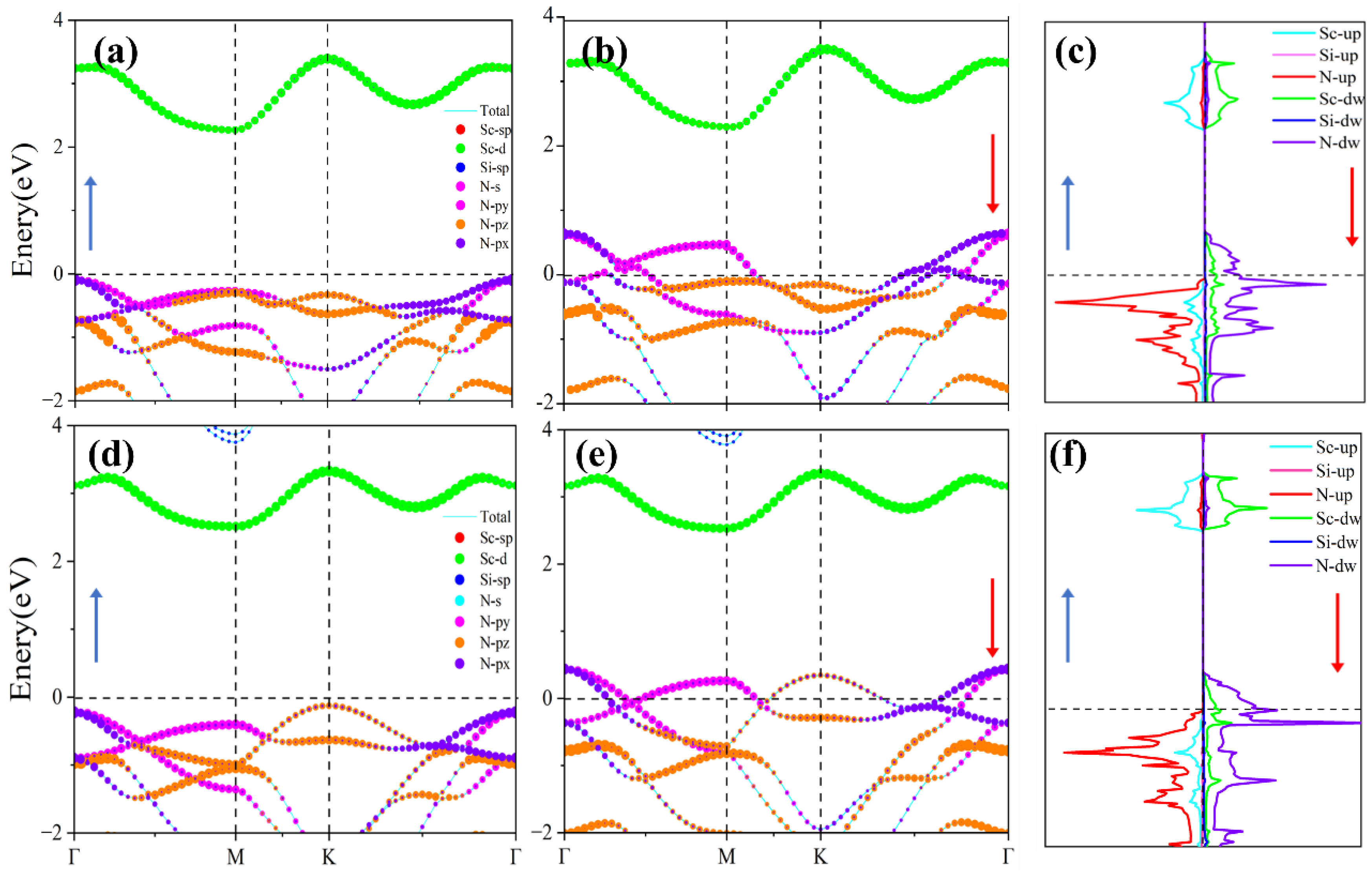
3.3. Magnetic Properties
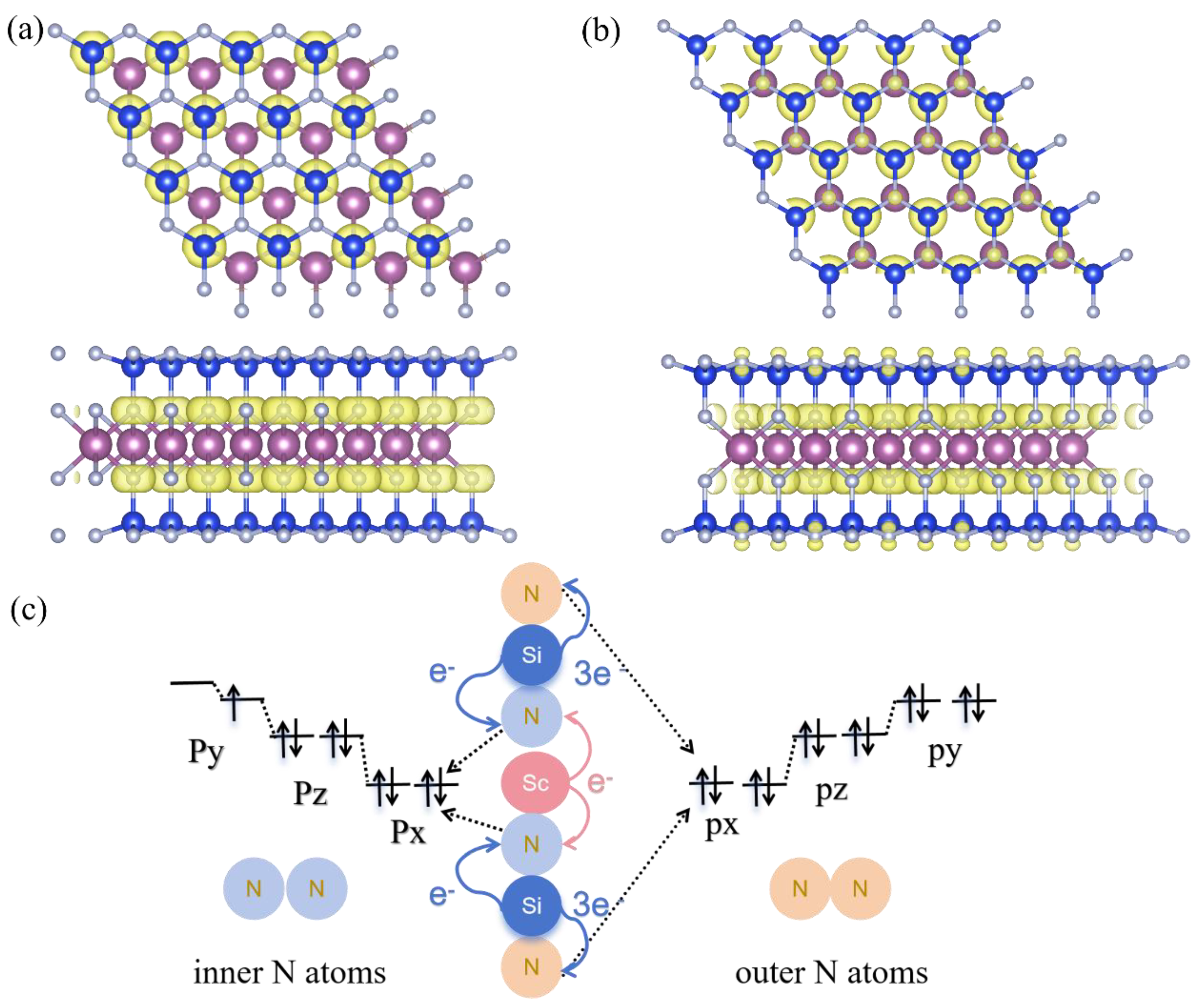
3.4. Optical Properties
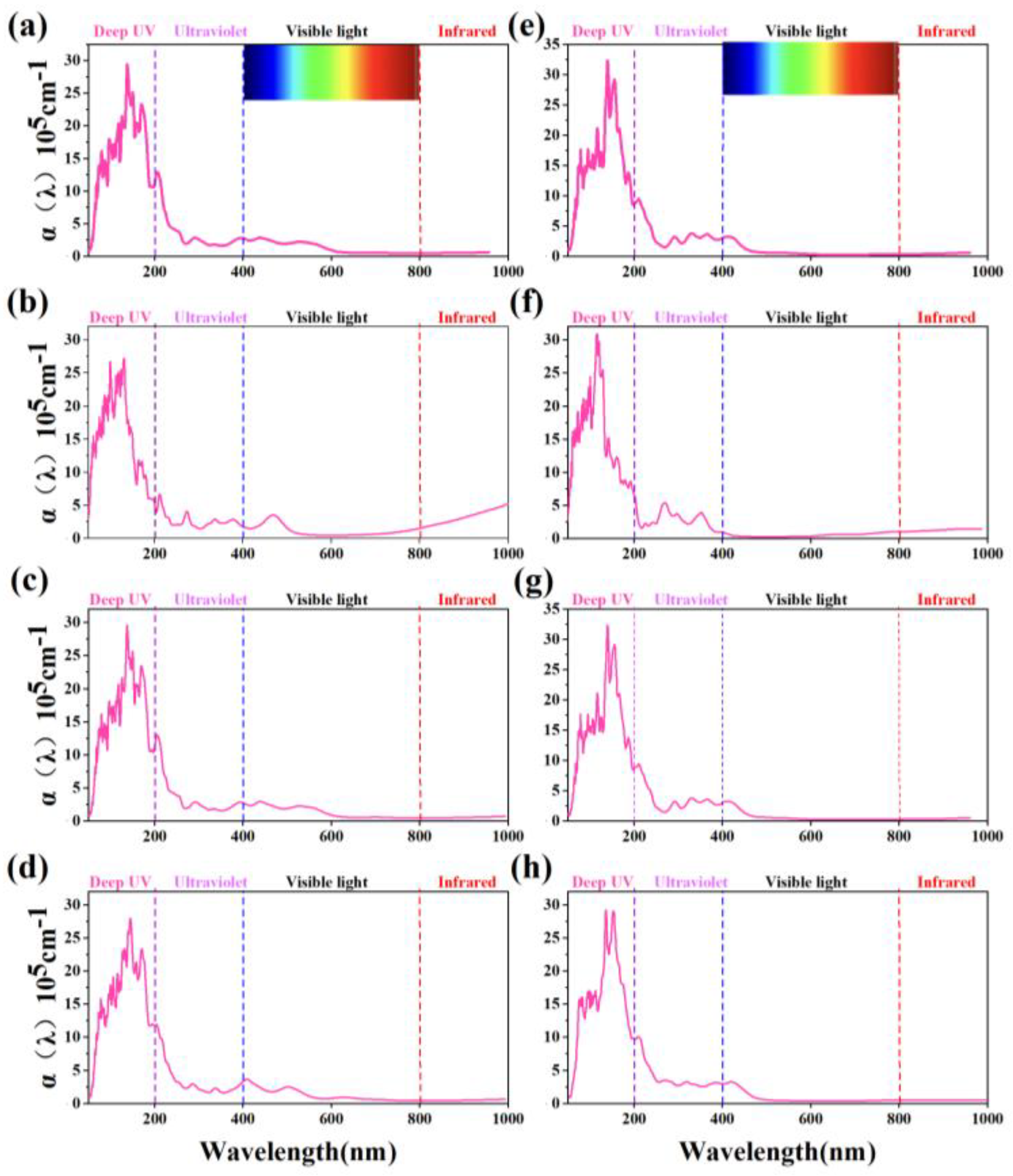
3.5. Regulation of Electronic and Optical Properties
3.5.1. Strain Effects
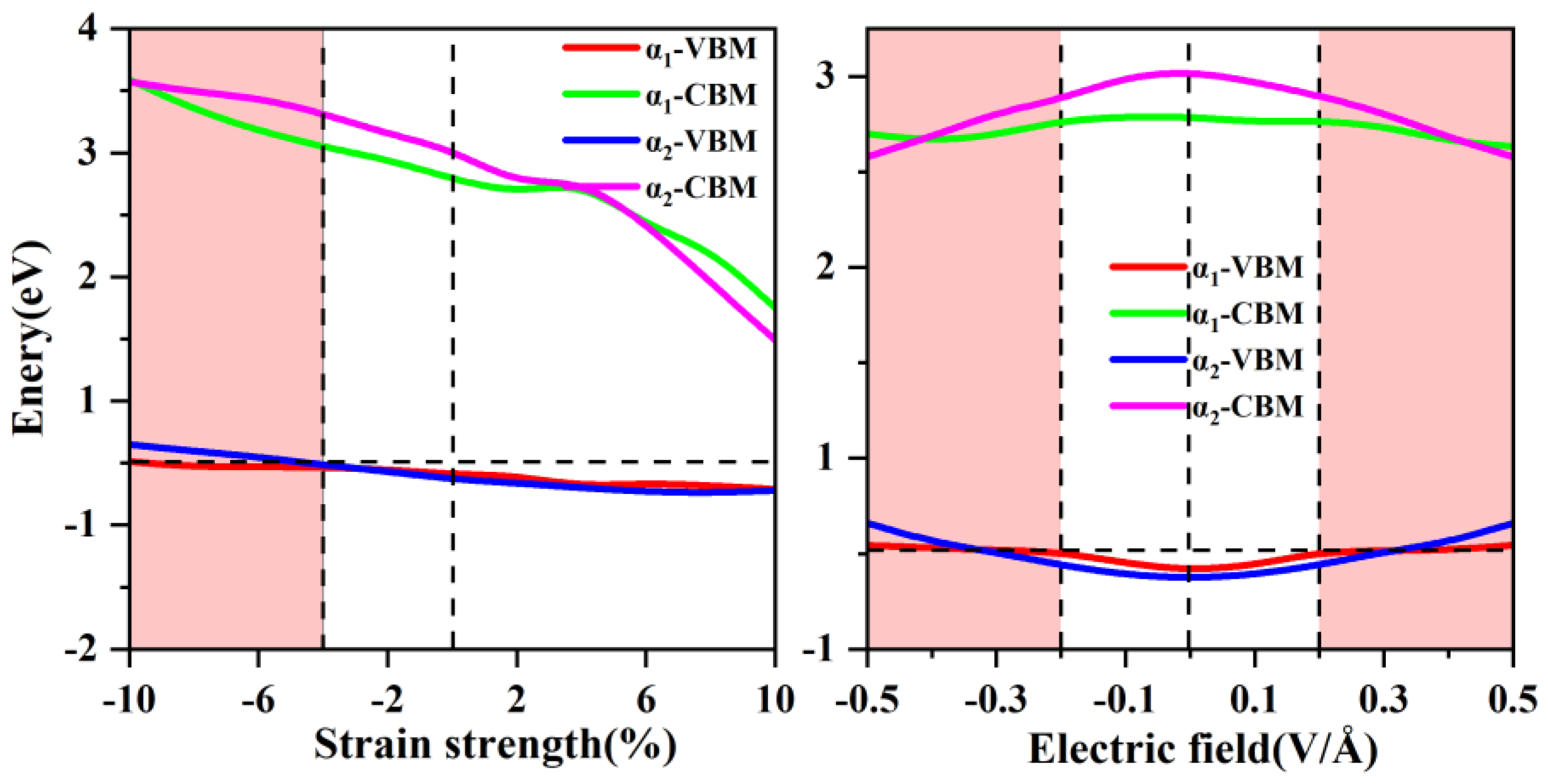
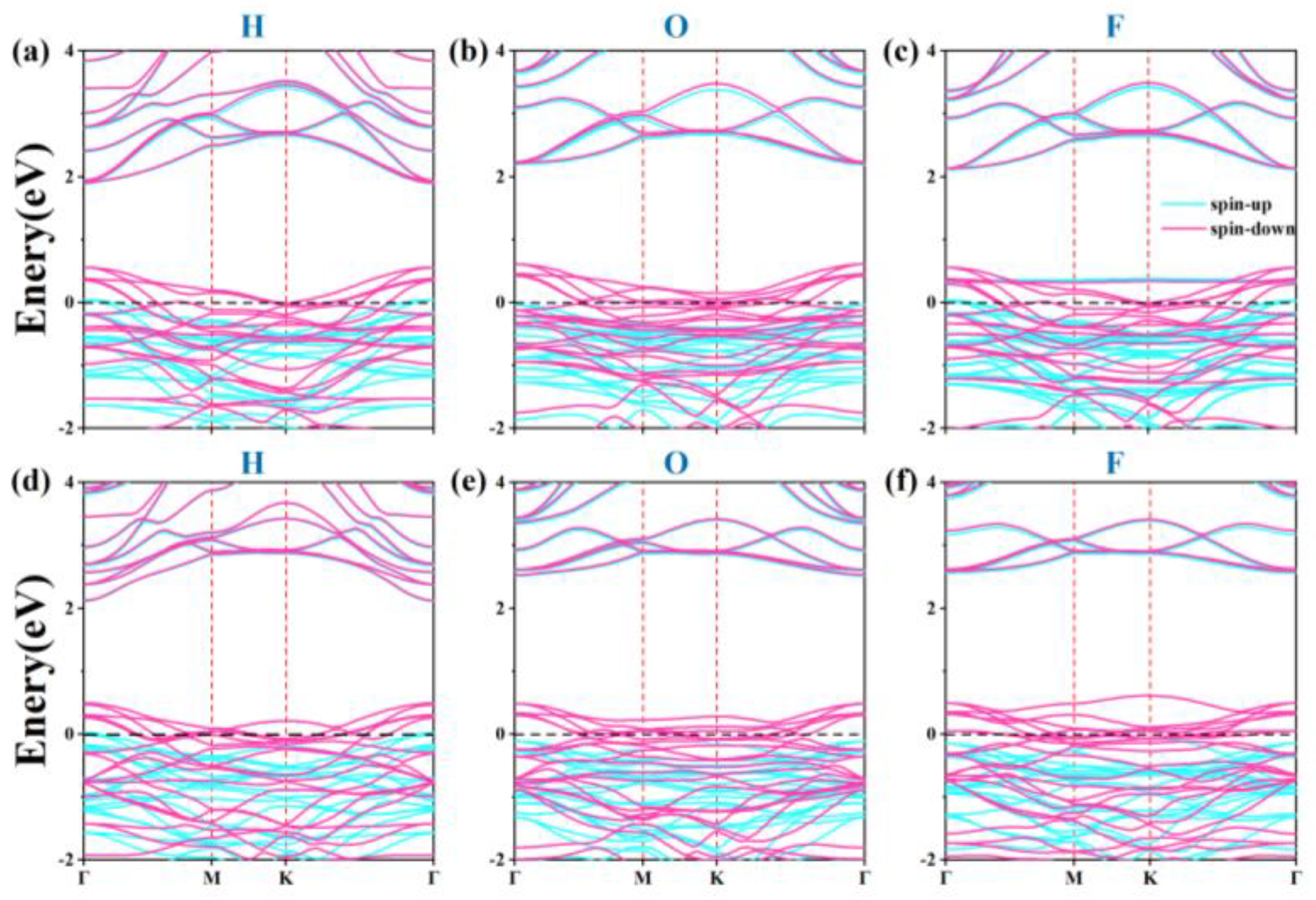
3.5.2. Atoms Adsorption
3.5.3. Electric Field Control
3.5.4. Mechanism of Regulating Electronic and Optical Properties
4. Conclusions
Supplementary Materials
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kou, L.; Frauenheim, T.; Chen, C. Phosphorene as a Superior Gas Sensor: Selective Adsorption and Distinct I – V Response. J Phys Chem Lett 2014, 5, 2675–2681. [CrossRef]
- Das, S.; Zhang, W.; Demarteau, M.; Hoffmann, A.; Dubey, M.; Roelofs, A. Tunable Transport Gap in Phosphorene. Nano Lett 2014, 14, 5733–5739. [CrossRef]
- Balandin, A.A.; Ghosh, S.; Bao, W.; Calizo, I.; Teweldebrhan, D.; Miao, F.; Lau, C.N. Superior Thermal Conductivity of Single-Layer Graphene. Nano Lett 2008, 8, 902–907. [CrossRef]
- King, A.; Johnson, G.; Engelberg, D.; Ludwig, W.; Marrow, J. Observations of Intergranular Stress Corrosion Cracking in a Grain-Mapped Polycrystal. Science (1979) 2008, 321, 382–385. [CrossRef]
- Geim, A. K.; Novoselov, K.S. The Rise of Graphene. Nat Mater 2007, 6, 183–191. [CrossRef]
- Pacilé, D.; Meyer, J.C.; Girit, Ç.Ö.; Zettl, A. The Two-Dimensional Phase of Boron Nitride: Few-Atomic-Layer Sheets and Suspended Membranes. Appl Phys Lett 2008, 92, 133107. [CrossRef]
- Yadav, V.K.; Mir, S.H.; Singh, J.K. A Computational Study of Structural, Electronic and Carrier Mobility of Boron and Phosphorus/Nitrogen Co-Doped Graphene. Physica B Condens Matter 2019, 571, 291–295. [CrossRef]
- Jiang, X.; Liu, Q.; Xing, J.; Liu, N.; Guo, Y.; Liu, Z.; Zhao, J. Recent Progress on 2D Magnets: Fundamental Mechanism, Structural Design and Modification. Appl Phys Rev 2021, 8, 31305. [CrossRef]
- Gupta, S.; Chakraborty, S.; Pakhira, S.; Barreteau, C.; Crivello, J.-C.; Bandyopadhyay, B.; Greneche, J.M.; Alleno, E.; Mazumdar, C. Coexisting Structural Disorder and Robust Spin-Polarization in Half-Metallic FeMnVAl. Phys Rev B 2022, 106, 115148. [CrossRef]
- Wu, Q.; Zhang, Y.; Zhou, Q.; Wang, J.; Zeng, X.C. Transition-Metal Dihydride Monolayers: A New Family of Two-Dimensional Ferromagnetic Materials with Intrinsic Room-Temperature Half-Metallicity. Journal of Physical Chemistry Letters 2018, 9, 4260–4266. [CrossRef]
- Zhu, M.X.; Ji, W.X.; Zhu, H.W.; Cao, Q.; Zhang, B.M. Symmetry-Protected Two-Dimensional Half-Semi-Metal NiVS6As2 Monolayer. J Electron Mater 2024, 53, 6250–6257. [CrossRef]
- Yan, B.; Felser, C. Topological Materials: Weyl Semimetals. Annu Rev Condens Matter Phys 2017, 8, 337–354. [CrossRef]
- Griffin, S.M.; Neaton, J.B. Prediction of a New Class of Half-Metallic Ferromagnets from First Principles. Phys Rev Mater 2017, 1, 044401. [CrossRef]
- Li, B.G.; Zheng, Y.F.; Cui, H.; Wang, P.; Zhou, T.W.; Wang, D.D.; Chen, H.; Yuan, H.K. First-Principles Investigation of a New 2D Magnetic Crystal: Ferromagnetic Ordering and Intrinsic Half-Metallicity. J Chem Phys 2020, 152, 244704. [CrossRef]
- Dihingia, K.D.; Saikia, S.; Yedukondalu, N.; Saha, S.; Sastry, G.N. 2D-Double Transition Metal MXenes for Spintronics Applications: Surface Functionalization Induced Ferromagnetic Half-Metallic Complexes. J Mater Chem C Mater 2022, 10, 17886–17898. [CrossRef]
- Tao, W.-L.; Lan, J.-Q.; Hu, C.-E.; Chen, X.-R.; Geng, H.-Y. Biaxial Strain Tuned Electronic Structure, Lattice Thermal Conductivity and Thermoelectric Properties of MgI2 Monolayer. Mater Sci Semicond Process 2022, 148, 106791. [CrossRef]
- Qi, W.; Abdugopur, H.; Xu, W.; Gao, M.; Hushur, A.; Zhang, H. Pressure-Induced Phase Transition toward High Symmetry in Zero-Strain Li2TiO3. Physical Chemistry Chemical Physics 2023, 25, 14918–14927. [CrossRef]
- Wang, Y.; Li, A.; Cheng, C. Ultrathin Co(OH)2 Nanosheets@Nitrogen-Doped Carbon Arrays as Efficient Air Cathodes for Rechargeable–Air Batteries. Small 2021, 17, 2101720. [CrossRef]
- Huang, H.M.; Cao, M.L.; Jiang, Z.Y.; Xiong, Y.C.; Zhang, X.; Luo, S.J.; Laref, A. High Spin Polarization in Formamidinium Transition Metal Iodides: First Principles Prediction of Novel Half-Metals and Spin Gapless Semiconductors. Physical Chemistry Chemical Physics 2019, 21, 16213–16222. [CrossRef]
- Maymoun, M.; Oukahou, S.; Bahou, Y.; Hasnaoui, A.; Sbiaai, K. Strain- and Electric Field-Enhanced Optical Properties of the Penta-Siligraphene Monolayer. New Journal of Chemistry 2022, 46, 13905–13917. [CrossRef]
- Huang, L.; Li, J. Tunable Electronic Structure of Black Phosphorus/Blue Phosphorus van Der Waals p-n Heterostructure. Appl Phys Lett 2016, 108, 083101. [CrossRef]
- Wu, Q.; Cao, L.; Ang, Y.S.; Ang, L.K. Semiconductor-to-Metal Transition in Bilayer MoSi2N4 and WSi2N4 with Strain and Electric Field. Appl Phys Lett 2021, 118, 1–5. [CrossRef]
- Friák, M.; Schindlmayr, A.; Scheffler, M. Ab Initio Study of the Half-Metal to Metal Transition in Strained Magnetite. New J Phys 2007, 9, 5–5. [CrossRef]
- Nayak, A.P.; Bhattacharyya, S.; Zhu, J.; Liu, J.; Wu, X.; Pandey, T.; Jin, C.; Singh, A.K.; Akinwande, D.; Lin, J.-F. Pressure-Induced Semiconducting to Metallic Transition in Multilayered Molybdenum Disulphide. Nat Commun 2014, 5, 3731. [CrossRef]
- Bafekry, A.; Faraji, M.; Fadlallah, M.M.; Bagheri Khatibani, A.; abdolahzadeh Ziabari, A.; Ghergherehchi, M.; Nedaei, Sh.; Shayesteh, S.F.; Gogova, D. Tunable Electronic and Magnetic Properties of MoSi2N4 Monolayer via Vacancy Defects, Atomic Adsorption and Atomic Doping. Appl Surf Sci 2021, 559, 149862. [CrossRef]
- Siwal, S.S.; Saini, A.K.; Rarotra, S.; Zhang, Q.; Thakur, V.K. Recent Advancements in Transparent Carbon Nanotube Films: Chemistry and Imminent Challenges. J Nanostructure Chem 2021, 11, 93–130.
- Li, Y.; Liu, J.; Zhang, P.; Jing, Q.; Liu, X.; Zhang, J.; Xiao, N.; Yu, L.; Niu, P. Electrical Transport Properties of EuTe under High Pressure. J Mater Chem C Mater 2021, 9, 17371–17381. [CrossRef]
- Hong, Y.-L.; Liu, Z.; Wang, L.; Zhou, T.; Ma, W.; Xu, C.; Feng, S.; Chen, L.; Chen, M.-L.; Sun, D.-M.; et al. Chemical Vapor Deposition of Layered Two-Dimensional MoSi2N4 Materials. Science (1979) 2020, 369, 670–674. [CrossRef]
- Mortazavi, B.; Javvaji, B.; Shojaei, F.; Rabczuk, T.; Shapeev, A. V; Zhuang, X. Exceptional Piezoelectricity, High Thermal Conductivity and Stiffness and Promising Photocatalysis in Two-Dimensional MoSi2N4 Family Confirmed by First-Principles;
- Pedroza-Rojas, B.; Sanchez-Castillo, A.; Ponce-Pérez, R. Structural, Electronic, and Magnetic Properties of the van Der Waals ScSi2N4/VSi2N4 Heterostructure: A First-Principles Study. ACS Omega 2025. [CrossRef]
- Huang, H.; Zhao, W.; Yang, M.; Xue, S.; He, Z.; Laref, A. Half-Metallic Behavior and Anisotropy of Two-Dimensional MoSi2N4/ScSi2N4 Heterojunction. J Magn Magn Mater 2024, 610. [CrossRef]
- Zhao, Z.; Wang, X.; Mi, W. Ferroelectric Polarization Tailored Spin Polarized Electronic Structure and Magnetic Anisotropy in Two-Dimensional ScSi2N4/CuInP2S6 Multiferroic Heterostructures. J Phys D Appl Phys 2023, 56. [CrossRef]
- Cohen, A.J.; Mori-Sánchez, P.; Yang, W. Challenges for Density Functional Theory. Chem Rev 2012, 112, 289–320. [CrossRef]
- Allouche, A.R. Gabedita - A Graphical User Interface for Computational Chemistry Softwares. J Comput Chem 2011, 32, 174–182. [CrossRef]
- Blöchl, P.E. Projector Augmented-Wave Method. Phys Rev B 1994, 50, 17953–17979. [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys Rev Lett 1996, 77, 3865–3868. [CrossRef]
- Paier, J.; Hirschl, R.; Marsman, M.; Kresse, G. The Perdew-Burke-Ernzerhof Exchange-Correlation Functional Applied to the G2-1 Test Set Using a Plane-Wave Basis Set. Journal of Chemical Physics 2005, 122. [CrossRef]
- Togo, A.; Tanaka, I. First Principles Phonon Calculations in Materials Science. Scr Mater 2015, 108, 1–5. [CrossRef]
- Martyna, G.J.; Klein, M.L.; Tuckerman, M. Nosé-Hoover Chains: The Canonical Ensemble via Continuous Dynamics. J Chem Phys 1992, 97, 2635–2643. [CrossRef]
- Wang, V.; Xu, N.; Liu, J.C.; Tang, G.; Geng, W.-T. VASPKIT: A User-Friendly Interface Facilitating High-Throughput Computing and Analysis Using VASP Code. 2019. [CrossRef]
- Wang, L.; Shi, Y.; Liu, M.; Zhang, A.; Hong, Y.-L.; Li, R.; Gao, Q.; Chen, M.; Ren, W.; Cheng, H.-M.; et al. Intercalated Architecture of MA2Z4 Family Layered van Der Waals Materials with Emerging Topological, Magnetic and Superconducting Properties. Nat Commun 2021, 12, 2361. [CrossRef]
- Li, S.; Wu, W.; Feng, X.; Guan, S.; Feng, W.; Yao, Y.; Yang, S.A. Valley-Dependent Properties of Monolayer MoSi2N4, WSi2N4 and MoSi2As4. Phys Rev B 2020, 102, 235435. [CrossRef]
- Bafekry, A.; Faraji, M.; Hoat, D.M.; Shahrokhi, M.; Fadlallah, M.M.; Shojaei, F.; Feghhi, S.A.H.; Ghergherehchi, M.; Gogova, D. MoSi2N4 Single-Layer: A Novel Two-Dimensional Material with Outstanding Mechanical, Thermal, Electronic, Optical, and Photocatalytic Properties. J Phys D Appl Phys 2021, 54, 155303. [CrossRef]
- Kang, L.; Lin, Z. Second Harmonic Generation of MoSi2N4 Layer. Phys Rev B 2021, 103, 195404. [CrossRef]
- Cao, L.; Zhou, G.; Wang, Q.; Ang, L.K.; Ang, Y.S. Two-Dimensional van Der Waals Electrical Contact to Monolayer MoSi2N4. Appl Phys Lett 2021, 118, 013106. [CrossRef]
- Yin, Y.; Gong, Q.; Yi, M.; Guo, W. Emerging Versatile Two-Dimensional MoSi2N4 Family. Adv Funct Mater 2023, 33, 2214050. [CrossRef]
- Wang, L.G.; Sun, J.X.; Yang, W.; Tian, R.G. Analytic Equation of State and Thermodynamic Properties for α-, β-, and γ-Si 3 N 4 Based on Analytic Mean Field Approach. Acta Phys Pol A 2008, 114, 807–818. [CrossRef]
- Bader, R.F.W. Atoms in Molecules. Acc Chem Res 1985, 18, 9–15. [CrossRef]
- Han, F.; Yan, X.; Li, F.; Yu, H.; Li, W.; Zhong, X.; Bergara, A.; Yang, G. Prediction of Monolayer FeP4 with Intrinsic Half-Metal Ferrimagnetism above Room Temperature. Phys Rev B 2023, 107, 024414. [CrossRef]
- Bai, K.; Cui, Z.; Li, E.; Ding, Y.; Zheng, J.; Liu, C.; Zheng, Y. Adsorption of Gas Molecules on Group III Atoms Adsorbed G-C3N4: A First-Principles Study. Vacuum 2020, 175, 109293. [CrossRef]
- Wang, Z.; Lou, H.; Han, F.; Yan, X.; Liu, Y.; Yang, G. An Antiferromagnetic Semiconducting FeCN2 Monolayer with a Large Magnetic Anisotropy and Strong Magnetic Coupling. Physical Chemistry Chemical Physics 2023, 25, 21521–21527. [CrossRef]
- Waller, I. Dynamical Theory of Crystal Lattices by M. Born and K. Huang. Acta Crystallogr 1956, 9, 837–838. [CrossRef]
- Zhang, S.; Zhou, J.; Wang, Q.; Chen, X.; Kawazoe, Y.; Jena, P. Penta-Graphene: A New Carbon Allotrope. Proc Natl Acad Sci U S A 2015, 112, 2372–2377. [CrossRef]
- Papageorgiou, D.G.; Kinloch, I.A.; Young, R.J. Mechanical Properties of Graphene and Graphene-Based Nanocomposites. Prog Mater Sci 2017, 90, 75–127.
- Cooper, R.C.; Lee, C.; Marianetti, C.A.; Wei, X.; Hone, J.; Kysar, J.W. Nonlinear Elastic Behavior of Two-Dimensional Molybdenum Disulfide. Phys Rev B 2013, 87, 035423. [CrossRef]
- Yu, Q.; Huang, H.; Zhao, W.; Xue, S.; Tong, R.; Chen, J.; Hu, Y.; Laref, A.; Luo, S. Electronic and Half-Metallic Properties of Novel Two-Dimensional YSi2N4 Monolayer by Theoretical Exploration. Mater Sci Semicond Process 2024, 169, 107862. [CrossRef]
- He, J.; Ma, S.; Lyu, P.; Nachtigall, P. Unusual Dirac Half-Metallicity with Intrinsic Ferromagnetism in Vanadium Trihalide Monolayers. J Mater Chem C Mater 2016, 4, 2518–2526. [CrossRef]
- Liu, Z.; Liu, J.; Zhao, J. YN2 Monolayer: Novel p-State Dirac Half Metal for High-Speed Spintronics. Nano Res 2017, 10, 1972–1979. [CrossRef]
- Liu, J.; Liu, Z.; Song, T.; Cui, X. Computational Search for Two-Dimensional Intrinsic Half-Metals in Transition-Metal Dinitrides. J Mater Chem C Mater 2017, 5, 727–732. [CrossRef]
- Gao, G.; Ding, G.; Li, J.; Yao, K.; Wu, M.; Qian, M. Monolayer MXenes: Promising Half-Metals and Spin Gapless Semiconductors. Nanoscale 2016, 8, 8986–8994. [CrossRef]
- Ulises Reveles, J.; Khanna, S.N. Nearly-Free-Electron Gas in a Silicon Cage. Phys Rev B 2005, 72, 165413. [CrossRef]
- Liu, Z.; Wang, X.; Cai, J.; Zhu, H. Room-Temperature Ordered Spin Structures in Cluster-Assembled Single V@Si 12 Sheets. The Journal of Physical Chemistry C 2015, 119, 1517–1523. [CrossRef]
- Cui, Z.; Yang, K.; Ren, K.; Zhang, S.; Wang, L. Adsorption of Metal Atoms on MoSi2N4 Monolayer: A First Principles Study. Mater Sci Semicond Process 2022, 152, 107072. [CrossRef]
- Bafekry, A.; Faraji, M.; Fadlallah, M.M.; Abdolahzadeh Ziabari, A.; Bagheri Khatibani, A.; Feghhi, S.A.H.; Ghergherehchi, M.; Gogova, D. Adsorption of Habitat and Industry-Relevant Molecules on the MoSi2N4 Monolayer. Appl Surf Sci 2021, 564, 150326. [CrossRef]
- Samy, O.; Zeng, S.; Birowosuto, M.D.; El Moutaouakil, A. A Review on MoS2 Properties, Synthesis, Sensing Applications and Challenges. Crystals (Basel) 2021, 11. [CrossRef]
- Xue, S.; Huang, H.; Zhao, W.; Yu, Q.; Yang, J.; Tong, R.; Hu, Y.; Laref, A.; Luo, S. Theoretical Exploration of Promising Optoelectronic Two-Dimensional Materials MSi2N4 (M=Cr, Mo, W). Vacuum 2024, 219, 112757. [CrossRef]
- Lv, P.; Tang, G.; Yang, C.; Deng, J.; Liu, Y.; Wang, X.; Wang, X.; Hong, J. Half-Metallicity in Two-Dimensional Co2Se3 Monolayer with Superior Flexibility. 2d Mater 2018, 5, 045026. [CrossRef]
- Han, F.; Yan, X.; Li, F.; Yu, H.; Li, W.; Zhong, X.; Bergara, A.; Yang, G. Prediction of Monolayer FeP4 with Intrinsic Half-Metal Ferrimagnetism above Room Temperature. Phys Rev B 2023, 107, 024414. [CrossRef]
- Guo, S.-D.; Mu, W.-Q.; Zhu, Y.-T.; Chen, X.-Q. Coexistence of Intrinsic Piezoelectricity and Ferromagnetism Induced by Small Biaxial Strain in Septuple-Atomic-Layer VSi2P4. Physical Chemistry Chemical Physics 2020, 22, 28359–28364. [CrossRef]
- Bafekry, A.; Faraji, M.; Fadlallah, M.M.; Bagheri Khatibani, A.; abdolahzadeh Ziabari, A.; Ghergherehchi, M.; Nedaei, Sh.; Shayesteh, S.F.; Gogova, D. Tunable Electronic and Magnetic Properties of MoSi2N4 Monolayer via Vacancy Defects, Atomic Adsorption and Atomic Doping. Appl Surf Sci 2021, 559, 149862. [CrossRef]
- Chen, R.; Chen, D.; Zhang, W. First-Principles Calculations to Investigate Stability, Electronic and Optical Properties of Fluorinated MoSi2N4 Monolayer. Results Phys 2021, 30, 104864. [CrossRef]
- Yuan, G.; Cheng, Z.; Cheng, Y.; Duan, W.; Lv, H.; Liu, Z.; Han, C.; Ma, X. Highly Sensitive Band Alignment of the Graphene/MoSi2N4 Heterojunction via an External Electric Field. ACS Appl Electron Mater 2022, 4, 2897–2905. [CrossRef]
- Bafekry, A.; Stampfl, C.; Naseri, M.; Fadlallah, M.M.; Faraji, M.; Ghergherehchi, M.; Gogova, D.; Feghhi, S.A.H. Effect of Electric Field and Vertical Strain on the Electro-Optical Properties of the MoSi2N4 Bilayer: A First-Principles Calculation. J Appl Phys 2021, 129, 155103. [CrossRef]
- Wu, Q.; Cao, L.; Ang, Y.S.; Ang, L.K. Semiconductor-to-Metal Transition in Bilayer MoSi2N4 and WSi2N4 with Strain and Electric Field. Appl Phys Lett 2021, 118, 1–5. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




