Submitted:
24 June 2025
Posted:
25 June 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results and Discussion
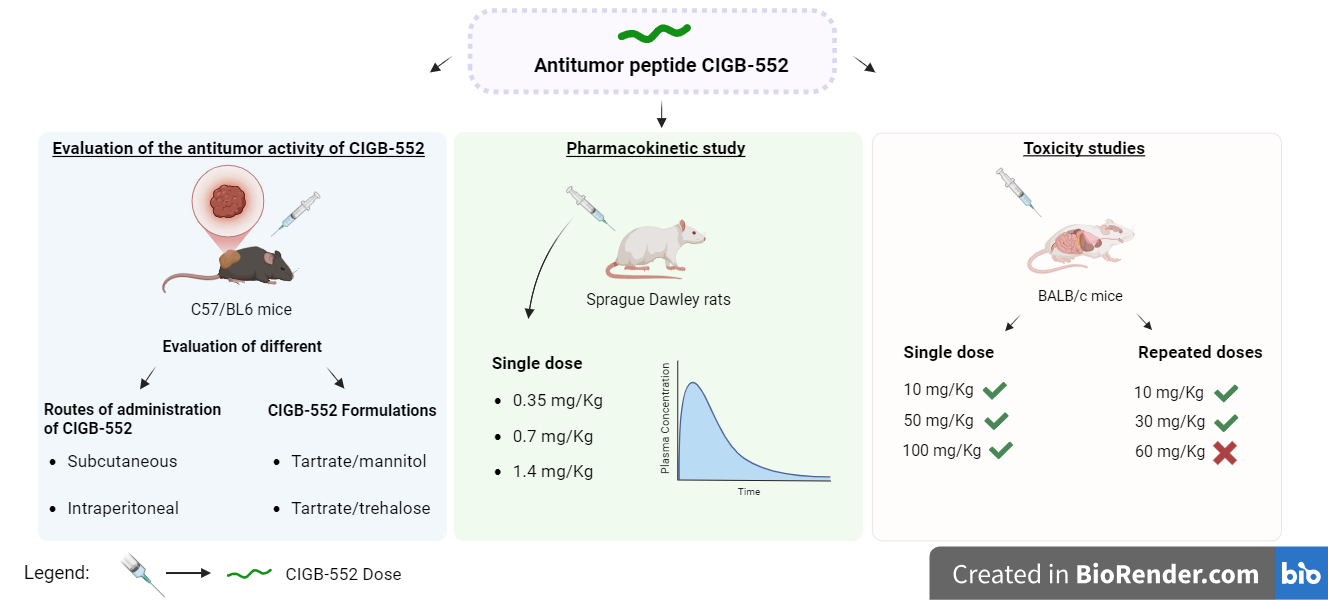
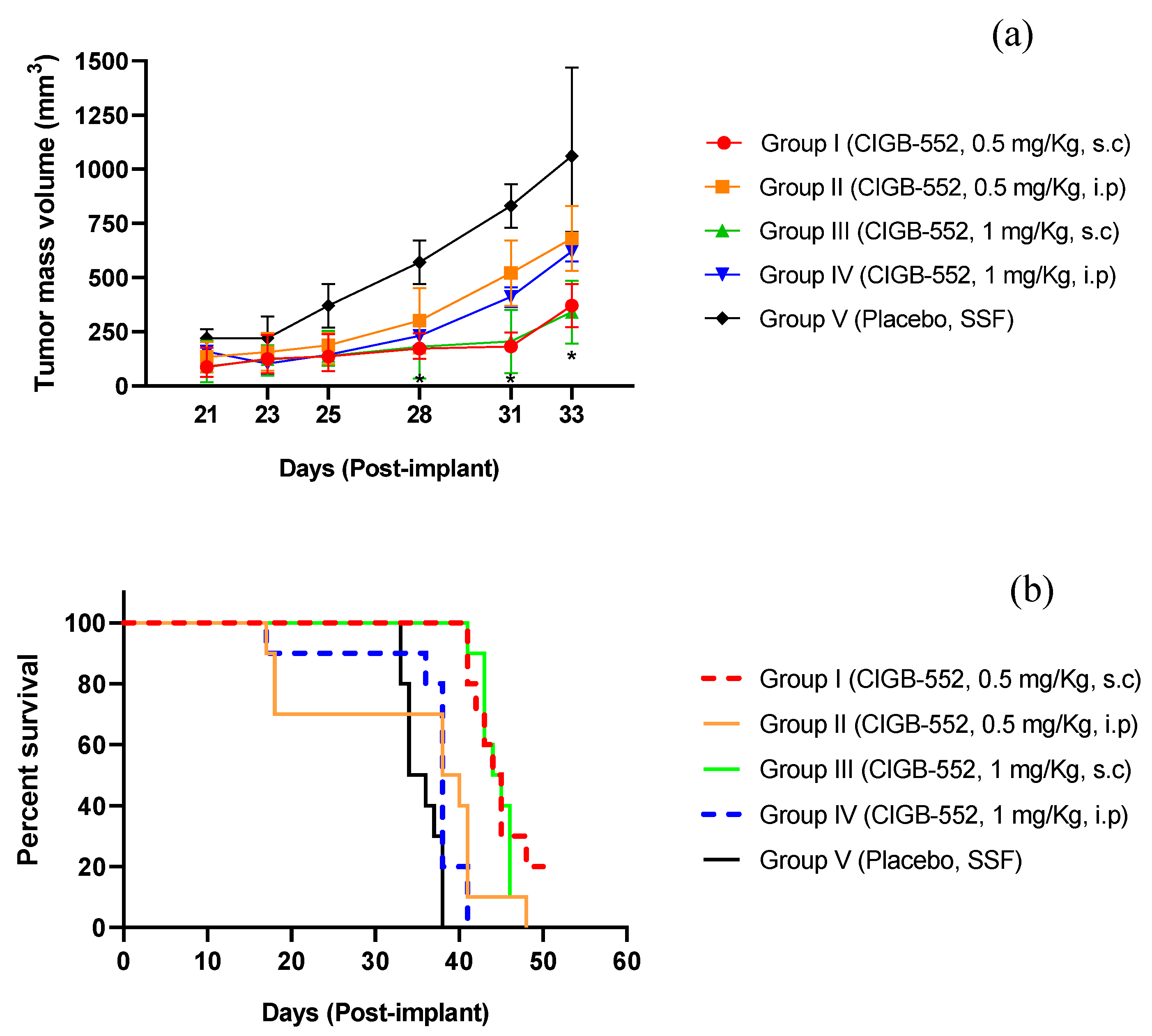
2.1. Evaluation of Different Routes of Administration of the CIGB-552 Peptide in the TC-1 Tumor Model
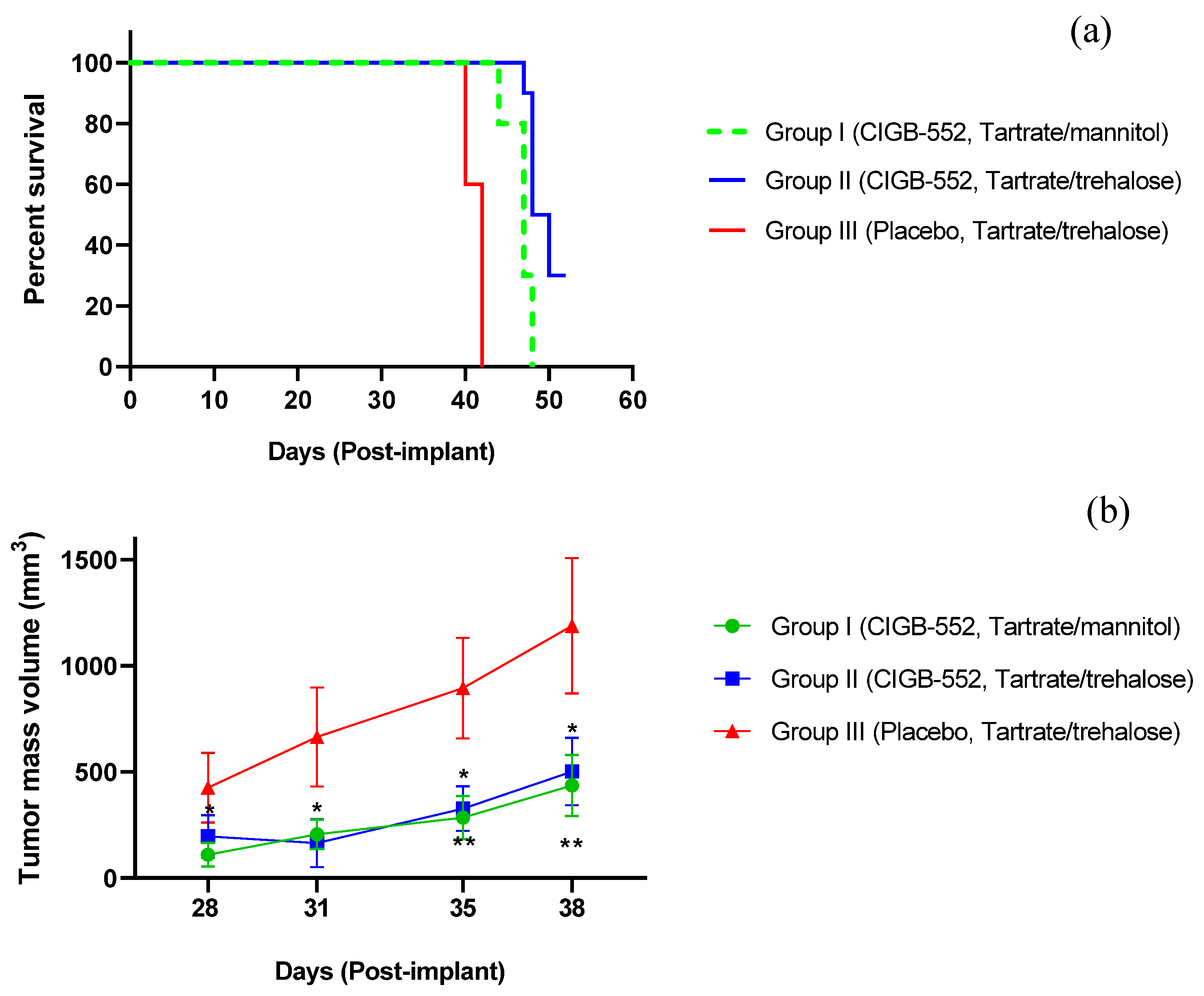
2.2. Evaluation of Different Routes of Administration of the CIGB-552 Peptide in the TC-1 Tumor Model
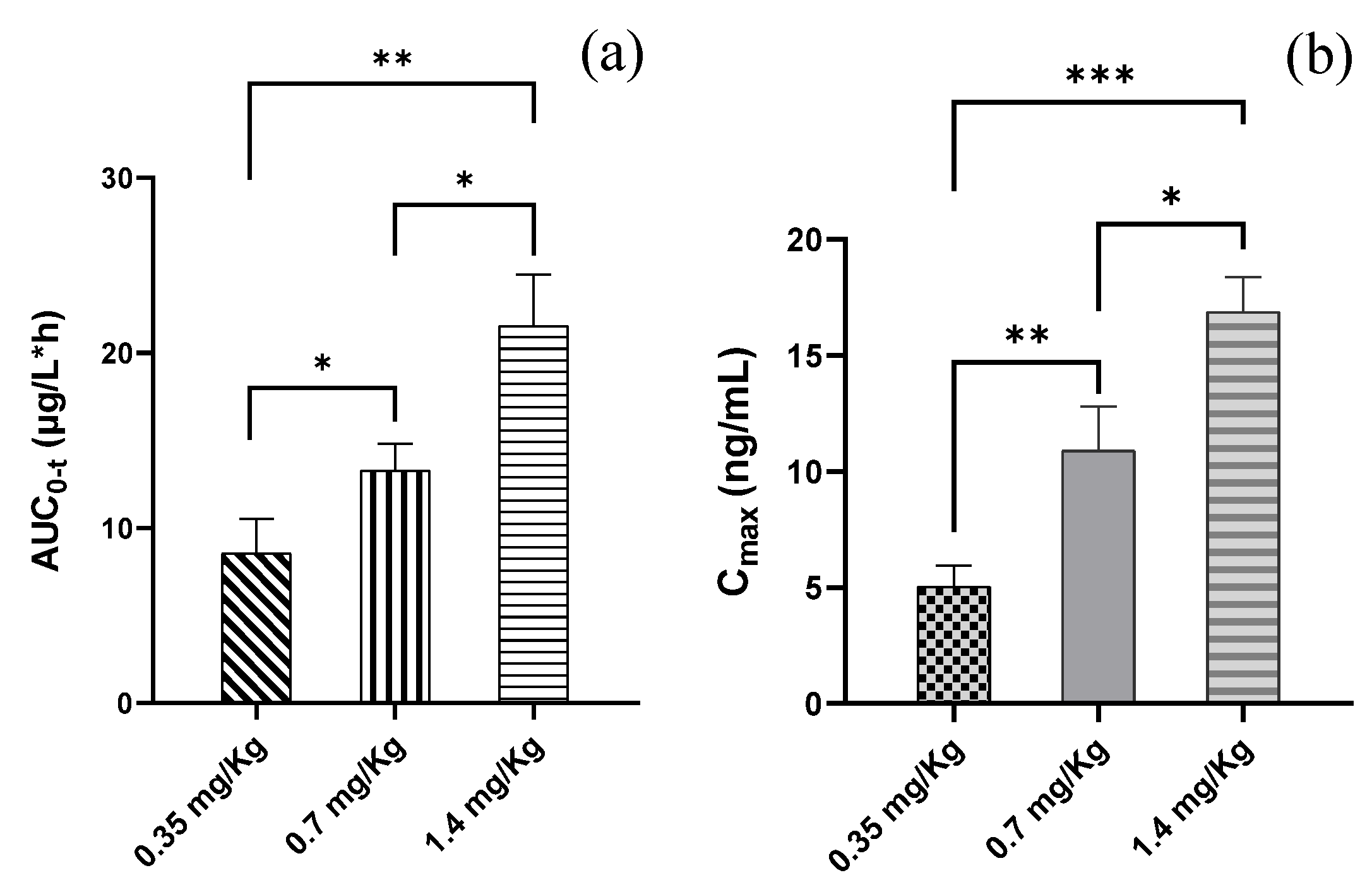
2.3. Pharmacokinetics of the CIGB-552 Peptide
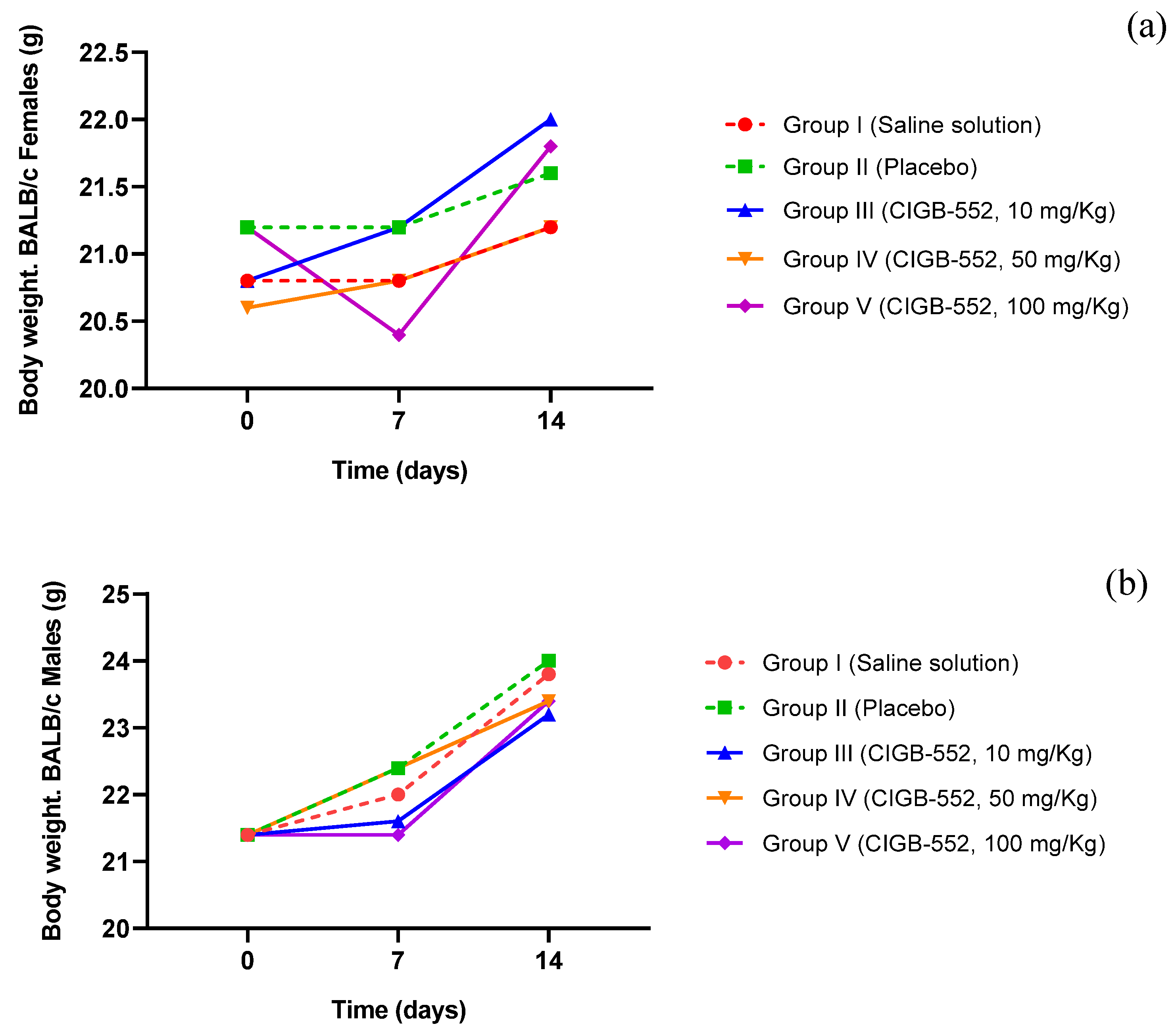
2.4. Acute Toxicity of the CIGB-552 Peptide in BALB/c Mice
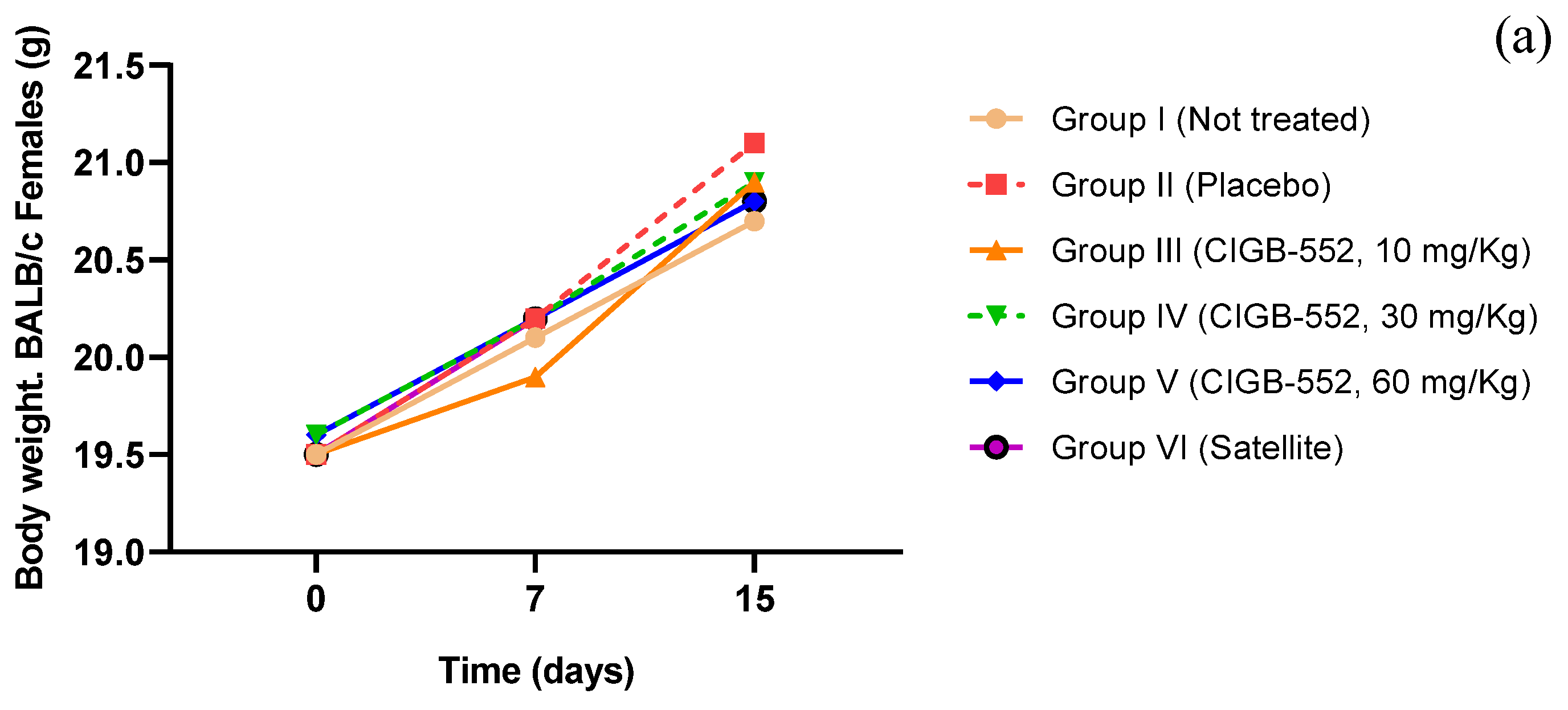
2.5. Toxicity of the CIGB-552 Peptide Administered in Repeated Doses in BALB/c Mice
3. Materials and Methods
3.1. Synthesis of CIGB-552 Peptide
3.2. Animals
3.3. Evaluation of Different Routes of Administration of the CIGB-552 Peptide in the TC-1 Tumor Model in C57/BL6 Mice
3.4. Evaluation of Different Formulations of the CIGB-552 Peptide in the TC-1 Tumor Model in C57/BL6 Mice
3.5. ELISA Protocol for the Quantification of the CIGB-552 Peptide
3.6. Experimental Design of the Pharmacokinetic Study of CIGB-552 in Sprague Dawley Rats
3.7. Acute Toxicity of the CIGB-552 Peptide in BALB/c Mice
3.8. Toxicity of the CIGB-552 Peptide Administered in Repeated Doses in BALB/c Mice
3.9. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AUC0-∞ | Area under plasma concentration-time curve from time zero to infinity |
| AUC0-t | Area under plasma concentration-time curve from time zero to the last measurable time point |
| BSA | Bovine serum albumin |
| Cl/F | Total plasma clearance |
| Cmax | Maximum observed plasma concentration |
| COMMD1 | MURR1 domain-containing protein 1 of copper metabolism |
| ELISA | Enzyme-linked immunosorbent assay |
| HIF-1 | Hypoxia-induced factor 1 |
| HRP | Horseradish peroxidase |
| LALF | Limulus anti-LPS factor |
| MRT | Mean residence time |
| MTD | Maximum tolerable dose |
| NF-κB | Nuclear factor kappa light chain enhancer of activated B cells |
| RP-HPLC | Reversed-phase high-performance liquid chromatography |
| t1/2 | Terminal half-life |
| Tmax | Time to peak |
| Vz/F | Volume of distribution at steady state |
| λz | Elimination rate constant |
References
- Blanco-Míguez, Aitor et al. “From amino acid sequence to bioactivity: The biomedical potential of antitumor peptides.” Protein science: a publication of the Protein Society vol. 25,6 (2016): 1084-95. [CrossRef]
- Marqus, Susan et al. “Evaluation of the use of therapeutic peptides for cancer treatment.” Journal of biomedical science vol. 24,1 21. 21 Mar. 2017. [CrossRef]
- Vallespi, Maribel G et al. “Identification of a novel antitumor peptide based on the screening of an Ala-library derived from the LALF (32-51) region.” Journal of peptide science: an official publication of the European Peptide Society vol. 16,1 (2010): 40-7. [CrossRef]
- Dolcet, Xavier et al. “NF-kB in development and progression of human cancer.” Virchows Archiv: an international journal of pathology vol. 446,5 (2005): 475-82. [CrossRef]
- van de Sluis, Bart et al. “COMMD1 disrupts HIF-1alpha/beta dimerization and inhibits human tumor cell invasion.” The Journal of clinical investigation vol. 120,6 (2010): 2119-30. [CrossRef]
- Khan, Abad et al. “Editorial: The practical implication of clinical pharmacokinetics in drug development, pharmaceutical analysis, and clinical research.” Frontiers in pharmacology vol. 14 1252030. 25 Jul. 2023. [CrossRef]
- Vallespi, Maribel G et al. “Antitumor efficacy, pharmacokinetic and biodistribution studies of the anticancer peptide CIGB-552 in mouse models.” Journal of peptide science: an official publication of the European Peptide Society vol. 20,11 (2014): 850-9. [CrossRef]
- Gomez Rodriguez, Yolanda et al. “Synergic effect of anticancer peptide CIGB-552 and Cisplatin in lung cancer models.” Molecular biology reports vol. 49,4 (2022): 3197-3212. [CrossRef]
- Diao, Lei, and Bernd Meibohm. “Pharmacokinetics and pharmacokinetic-pharmacodynamic correlations of therapeutic peptides.” Clinical pharmacokinetics vol. 52,10 (2013): 855-68. [CrossRef]
- Ibraheem, D et al. “Administration strategies for proteins and peptides.” International journal of pharmaceutics vol. 477,1-2 (2014): 578-89. [CrossRef]
- Vallespi, Maribel G et al. “A first-in-class, first-in-human, phase I trial of CIGB-552, a synthetic peptide targeting COMMD1 to inhibit the oncogenic activity of NF-κB in patients with advanced solid tumors.” International journal of cancer vol. 149,6 (2021): 1313-1321. [CrossRef]
- Koenitz, Laura et al. “Pharmacokinetic differences between subcutaneous injection and intradermal microneedle delivery of protein therapeutics.” European journal of pharmaceutics and biopharmaceutics: official journal of Arbeitsgemeinschaft fur Pharmazeutische Verfahrenstechnik e.V vol. 204 (2024): 114517. [CrossRef]
- Reagan-Shaw, Shannon et al. “Dose translation from animal to human studies revisited.” FASEB journal: official publication of the Federation of American Societies for Experimental Biology vol. 22,3 (2008): 659-61. [CrossRef]
- Cabrales, Ania et al. “Pharmacokinetic study of Growth Hormone-Releasing Peptide 6 (GHRP-6) in nine male healthy volunteers.” European journal of pharmaceutical sciences: official journal of the European Federation for Pharmaceutical Sciences vol. 48,1-2 (2013): 40-6. [CrossRef]
- Pognan, Francois et al. “The evolving role of investigative toxicology in the pharmaceutical industry.” Nature reviews. Drug discovery vol. 22,4 (2023): 317-335. [CrossRef]
- Ruberte, Jesús et al. “Bridging mouse and human anatomies; a knowledge-based approach to comparative anatomy for disease model phenotyping.” Mammalian genome: official journal of the International Mammalian Genome Society vol. 34,3 (2023): 389-407. [CrossRef]
- Greaves P. Histopathology of Preclinical Toxicity Studies. Interpretation and relevance in Grug Safety Evaluation. Second Edition. ELSEVIER. 2000.
- Vallespi, Maribel G et al. “The first report of cases of pet dogs with naturally occurring cancer treated with the antitumor peptide CIGB-552.” Research in veterinary science vol. 114 (2017): 502-510. [CrossRef]
- CPMP/ICH/286/95 (2009) No-clinical safety studies for the conduct of Human clinical trials for pharmaceuticals. ICH M3 (M). https://www.ich.org/page/multidisciplinary-guidelines.
- Tanaka, Tomoyuki et al. “Treatment of lung cancer using clinically relevant oral doses of the cyclooxygenase-2 inhibitor rofecoxib: potential value as adjuvant therapy after surgery.” Annals of surgery vol. 241,1 (2005): 168-78. [CrossRef]
- Gómez Hernández, Nivaldo Angel et al. “A sandwich ELISA for the quantification of the anticancer peptide CIGB-552 in human plasma.” Analytical biochemistry vol. 698 (2025): 115725. [CrossRef]
- Gómez Y, González M, Hernández D, Aragón H, Vallespi MG, et al. (2018) Monoclonal Antibodies for the CIGB-552 Antitumor Synthetic Peptide Quantification. J Vet Sci Ani Husb 6(5): 503.
- European Medicines Agency. ICH guideline M10 on bioanalytical method validation and study sample analysis, 2022. Available: https://www.ema.europa.eu/en/documents/scientific-guideline/ich-guideline-m10-bioanalytical-method-validation-step-5_en.pdf.
- Zhang, Yong et al. “PKSolver: An add-in program for pharmacokinetic and pharmacodynamic data analysis in Microsoft Excel.” Computer methods and programs in biomedicine vol. 99,3 (2010): 306-14. [CrossRef]
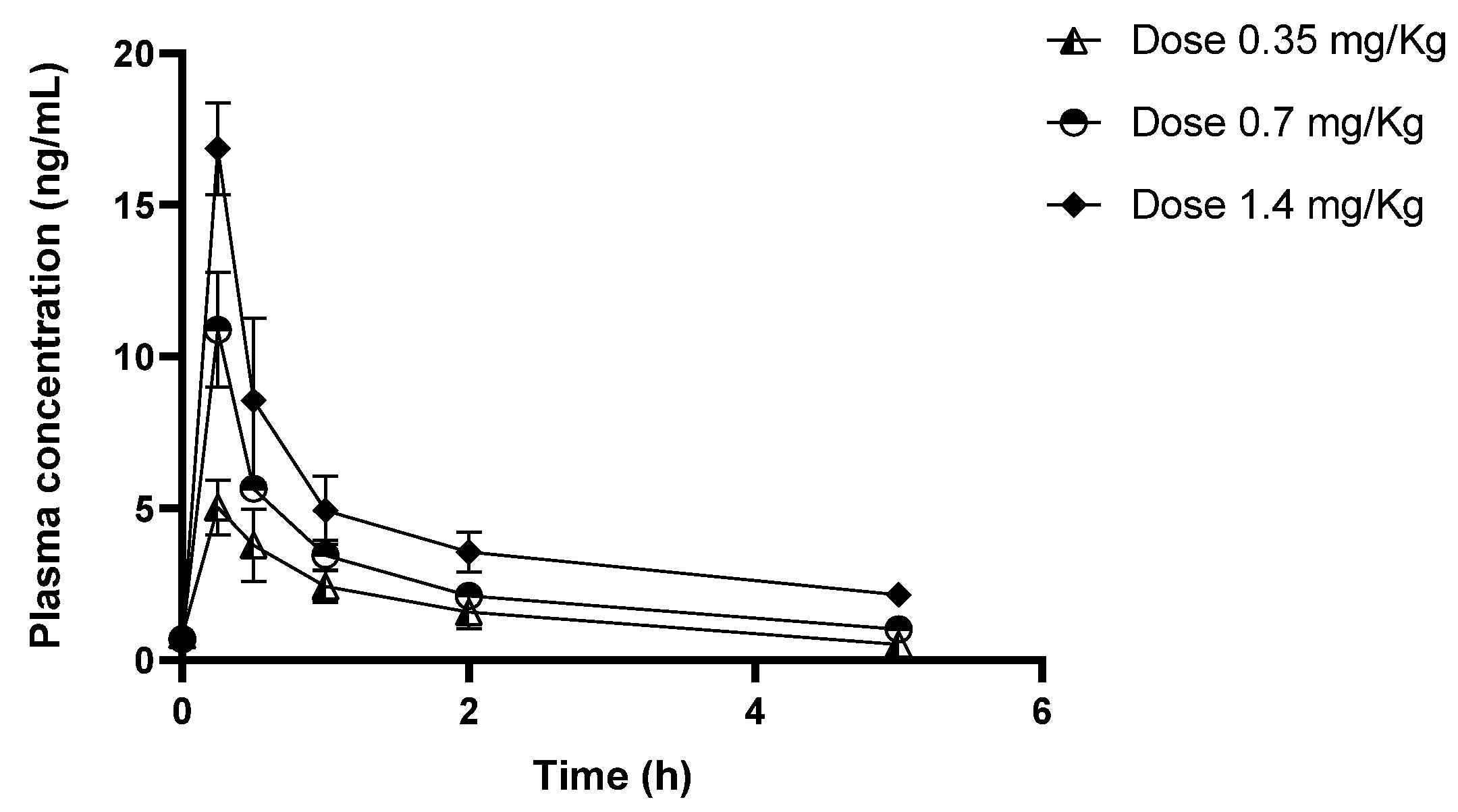
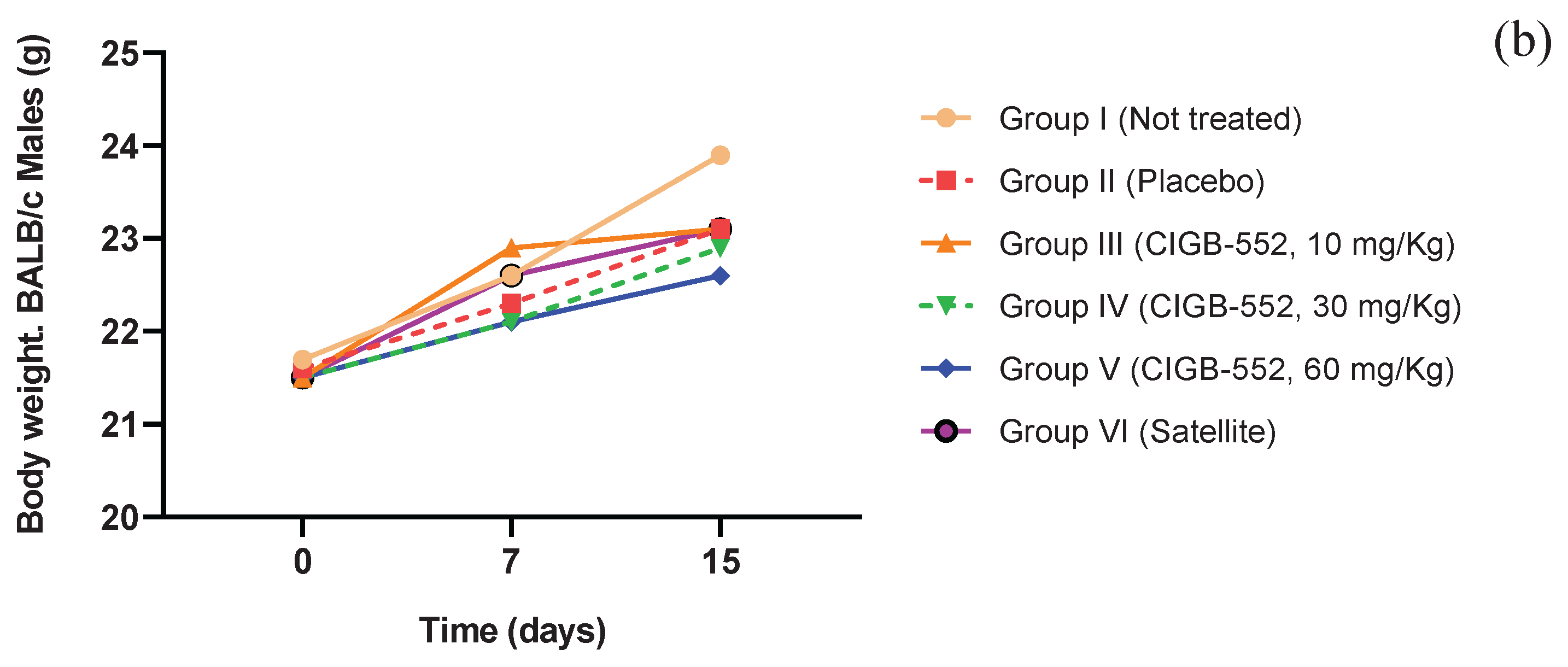
| Time (h) | Plasma concentration of CIGB-552 (ng/mL) | ||
|---|---|---|---|
| Group I (0.35 mg/Kg) |
Group II (0.7 mg/Kg) |
Group III (1.4 mg/Kg) |
|
| 0 | 0.81 ± 0.32 | 0.69 ± 0.04 | 0.64 ± 0.03 |
| 0.25 | 5.03 ± 0.90 | 10.88 ± 1.89 | 16.85 ± 1.51 |
| 0.5 | 3.79 ± 1.19 | 5.64 ± 0.4 | 8.55 ± 2.70 |
| 1 | 2.42 ± 0.52 | 3.46 ± 0.49 | 4.93 ± 1.13 |
| 2 | 1.58 ± 0.55 | 2.11 ± 0.30 | 3.56 ± 0.65 |
| 5 | 0.51 ± 0.09 | 1.02 ± 0.14 | 2.15 ± 0.29 |
| PK parameters | Group I (0.35 mg/Kg) |
Group II (0.7 mg/Kg) |
Group III (1.4 mg/Kg) |
|---|---|---|---|
| ʎz(1/h) | 0.41 ± 0.09 | 0.34 ± 0.06 | 0.20 ± 0.10 |
| t1/2 (h) | 1.76 ± 0.36 | 2.26 ± 0.44 | 4.62 ± 3.20 |
| Tmax (h) | 0.25 ± 0.00 | 0.25 ± 0.00 | 0.25 ± 0.00 |
| Cmax (µg/L) | 5.03 ± 0.91 | 10.86 ± 1.90 | 16.86 ± 1.51 |
| AUC0-t (µg/L*h) | 8.56 ± 1.98 | 11.91 ± 1.53 | 21.56 ± 2.91 |
| AUC0-∞ (µg/L*h) | 9.90 ± 1.81 | 16.98 ± 1.92 | 36.80 ± 9.75 |
| MRT (h) | 2.41 ± 0.50 | 2.90 ± 0.51 | 6.09 ± 4.48 |
| Vz/F (L) | 22.50 ± 8.58 | 30.88 ± 6.87 | 56.65 ± 23.61 |
| Cl/F (L/h) | 8.69 ± 1.72 | 9.23 ± 1.07 | 9.52 ± 2.21 |
| Groups | Liver | Spleen | GLM | |||
| HE | CG | HE | CG | |||
| I(Saline solution) | 5/10 | 10/10 | 10/10 | 10/10 | ||
| II (Placebo) | 1/10 | 8/10 | 9/10 | 10/10 | ||
| III(CIGB-552, 10 mg/Kg) | 4/10 | 8/10 | 9/10 | 10/10 | ||
| IV(CIGB-552, 50 mg/Kg) | 6/10 | 10/10 | 10/10 | 10/10 | ||
| V(CIGB-552, 100 mg/Kg) | 4/10 | 8/10 | 9/10 | 10/10 | ||
| Groups | Liver | Spleen | GLM | Applicationsite | ||||
| NF | HE | CG | HE | PALS | CG | II | ||
| I (Not treated) | 0/20 | 14/20 | 16/20 | 20/20 | 16/20 | 19/20 | 0/20 | |
| II (Placebo) | 1/20 | 14/20 | 18/20 | 20/20 | 18/20 | 20/20 | 0/20 | |
| III (CIGB-552, 10 mg/Kg) | 0/20 | 15/20 | 15/20 | 20/20 | 15/20 | 20/20 | 4/20 | |
| IV (CIGB-552, 30 mg/Kg) | 0/20 | 16/20 | 19/20 | 20/20 | 19/20 | 20/20 | 3/20 | |
| V (CIGB-552, 60 mg/Kg) | 1/20 | 10/20 | 17/20 | 20/20 | 17/20 | 20/20 | 7/20 | |
| VI (CIGB-552, 60 mg/Kg) | 0/15 | 10/15 | 15/15 | 15/15 | 15/15 | 15/15 | 0/15 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





