Abstract
Background: Endometrial cancer (EC) is recognized as one of the leading invasive gynecological malignancies globally. Glutamine metabolism is closely correlated with cancer development through various processes. In this study, we aimed to investigate the effects of erianin, a derivate from traditional Chinese medicinal herb, on EC therapy and glutamine metabolism. Methods: Human endometrial (EC) cell lines HEC-1A and Ishikawa were treated with erianin, and cell viability and migration were measured by cell counting kit 8 (CCK-8) and Transwell assay. The glutamine metabolism was determined by analyzing the levels of intracellular glutamine, α-ketoglutaric acid (α-KG) and ATP. A xenograft tumor model was established to check the in vivo effects of erianin. The changes of PI3K/AKT signaling were analyzed by western blotting assay. Results: Treatment with erianin notably suppressed the in vitro and in vivo growth of EC cells, simultaneously reduced the levels of glutamic acid, α-KG and ATP, suggesting the repressed glutamine metabolism. Moreover, activation of ERK signaling could abolish these anti-growth and anti-metabolism effects of erianin on EC. ConclusionS: Erianin suppressed the glutamine metabolism and growth of EC through the ERK signaling pathway.
Introduction
Erianin, a quintessential alkaloid derived from the traditional Chinese medicinal herb Sophora flavescens, has been a subject of considerable interest due to its profound impact on the field of oncology. This natural compound, with a quinolizidine framework, has been celebrated for its multifaceted pharmacological profiles, which include anti-inflammatory, antioxidant, and notably, antitumor capabilities[
1,
2]. The role of erianin in cancer therapy is multi-pronged, encompassing the inhibition of cancer cell proliferation, the induction of cell cycle arrest and apoptosis, the suppression of angiogenesis, and the modulation of the tumor microenvironment. Clinical studies and experimental models have corroborated erianin's antitumor efficacy across a spectrum of cancers, including hepatocellular carcinoma, lung cancer, breast cancer, and colorectal cancer[
3,
4,
5,
6]. It has been observed to not only curb the proliferation of cancer cells but also to attenuate their invasiveness and metastatic potential, thereby presenting a promising therapeutic strategy in cancer management[
7,
8]. Despite its promising effects, the clinical application of erianin in cancer treatment remains in the investigative phase, a deeper comprehension of the pharmacological mechanisms of erianin is anticipated to broaden its horizons in cancer therapy.
Cancer cells utilize metabolic reprogramming to regulate metabolism, providing sufficient energy and materials, and maintaining redox status and rapid cell proliferation[
9,
10]. Dysregulation of glucose metabolism is a hallmark of metabolic reprogramming in many cancer cells, leading to an increased rate of glucose consumption compared to normal cells, with most glucose being secreted as lactate rather than being oxidized in the tricarboxylic acid (TCA) cycle[
11]. As a result, in rapidly proliferating cancer cells, the dysregulation of glucose metabolism cannot produce enough ATP or the intermediates needed for biosynthesis[
12]. Another source of energy is amino acids, particularly glutamine, which is the most abundant amino acid in mammals. Compared to other amino acids, cancer cells are more dependent on glutamine and upregulate glutamine metabolism to meet the bioenergetic and biosynthetic demands of continuous growth[
13]. The main purpose of glutamine metabolism is to provide a carbon source for energy production during cell proliferation. Glutamine enters the cell through the transporter alanine-serine-cysteine transporter 2 (ASCT2) and is catabolized to glutamate in the mitochondria by glutaminase (GLS)[
14]. Glutamate is then converted to α-ketoglutarate (α-KG), primarily by glutamate dehydrogenase 1 (GLUD1), to generate ATP in the TCA cycle.[
15] Another function of glutamine is to provide a nitrogen source for the synthesis of nucleotides and other non-essential amino acids. Additionally, glutamate can produce glutathione, an antioxidant that serves as a redox buffer against oxidative stressors[
16,
17].
The connection between erianin’s antitumor effects with glutamine metabolism remains unclear. Here, in this work, we aimed to study the effects of erianin on endometrial cancer (EC) and to explore the involvement of glutamine metabolism.
Materials and Methods
Cell Culture
The human endometrial (EC) cell lines HEC-1A and Ishikawa were sourced from the China Center for Type Culture Collection, located in Hubei, China. These cells were maintained in a controlled environment with an atmosphere of 5% carbon dioxide at a temperature of 37 degrees Celsius, following the specific growth conditions recommended for these cell types.
Cell Proliferation
Cell proliferation and viability were examined using the Cell Counting Kit-8 (CCK-8) assay (Dojindo, Japan). After treatment with different Erianin concentrations for 24 hours, CCK-8 reagent was added into each well and incubated for another 2 hours. The optical density at 450 nm was measured.
Cell Migration
In the migration assay, a quantity of 5 × 10^4 Ishikawa and HEC-1A cells were plated into the upper compartments of Transwell plates. Both the upper and lower chambers were filled with media containing an equal concentration of P4. Post a 48-hour incubation period, cells that had migrated through the membrane were stained using a 0.1% solution of Crystal Violet. The stained cells were then visualized and documented using a microscope.
Western Blot Assay
Protein extraction was performed using a RIPA lysis buffer (Beyotime, China). A quantity of 30 μg of protein was subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) on a 10% gel, followed by transfer to a nitrocellulose (NC) membrane from Millipore, U.S.A. The membranes were then blocked with a 5% skim milk solution before being incubated with specific primary antibodies (Proteintech, China) including anti-AKT, anti-pAKT, anti-PI3K and anti- β-actin overnight at 4°C. Afterward, the membranes were incubated with a horseradish peroxidase (HRP)-conjugated secondary antibody (Proteintech, China) for 1 hour at room temperature. The immunoreactive bands were detected and visualized using an enhanced chemiluminescence (ECL) detection kit (Thermo, USA).
Xenograft TUMOR model
Four-week-old female BALB/c nude mice were employed for the in vivo experiments. In the subcutaneous tumor transplantation model, a dose of 1 × 106 Ishikawa cells was inoculated into the dorsal region of the mice (five mice per experimental group). Tumor growth was monitored weekly using a caliper, and the volume was determined with the formula: tumor volume = (length × width2) / 2. All animal research was carried out following the approval of the Ethics Committee of Li Huili Hospital Affiliated to Ningbo University and was in compliance with the National Institutes of Health (NIH) Guidelines for the Care and Use of Laboratory Animals.
Statistics
Data were expressed in terms of the mean ± standard deviation (SD). Statistical significance was assessed using either a two-tailed t-test for two groups or a one-way ANOVA with a Bonferroni post-hoc test for multiple comparisons. GraphPad 9.0 software (GraphPad, Inc., USA) was used for statistical analysis. A p-value of less than 0.05 was considered the threshold for statistical significance.
Results
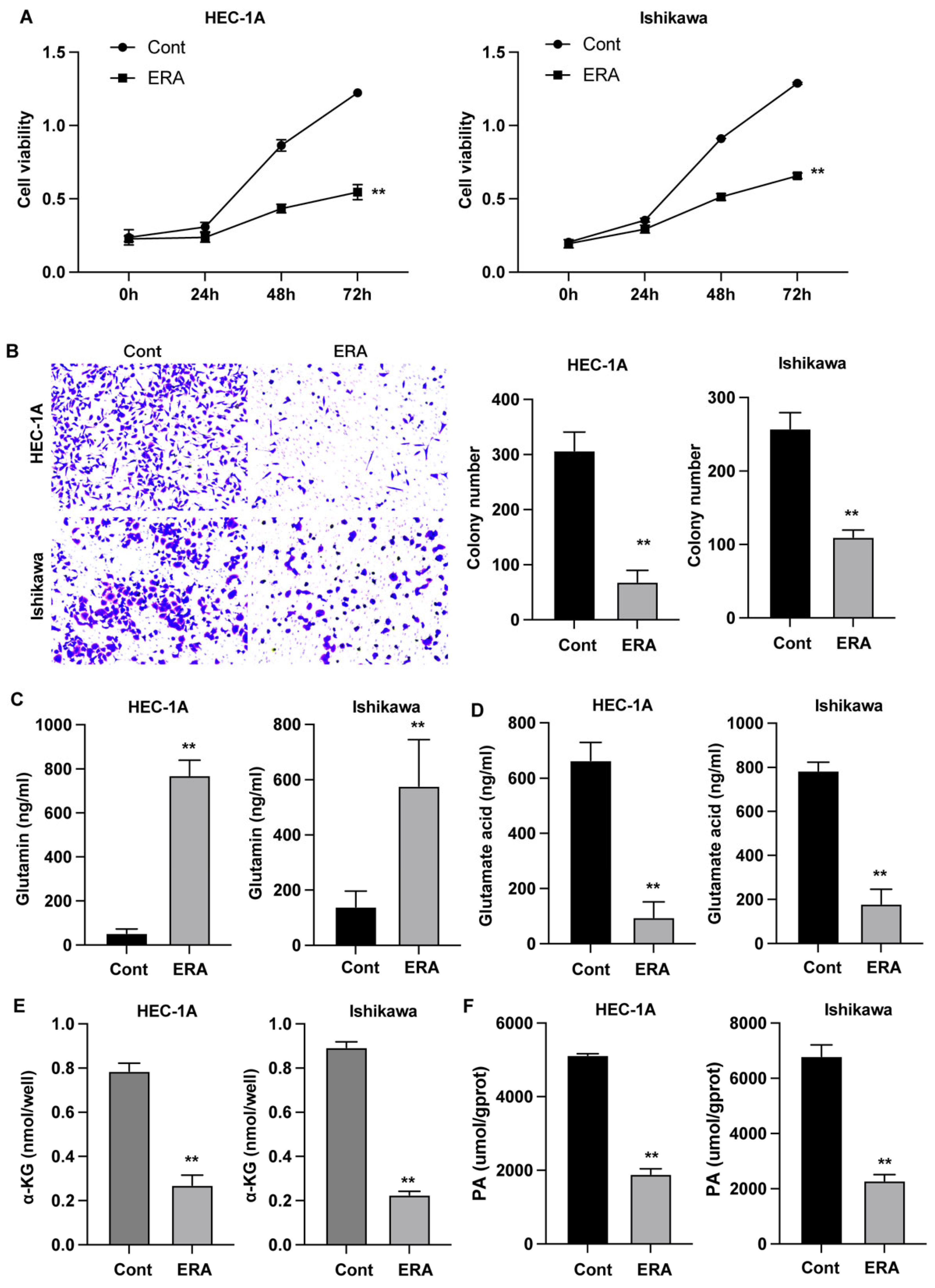
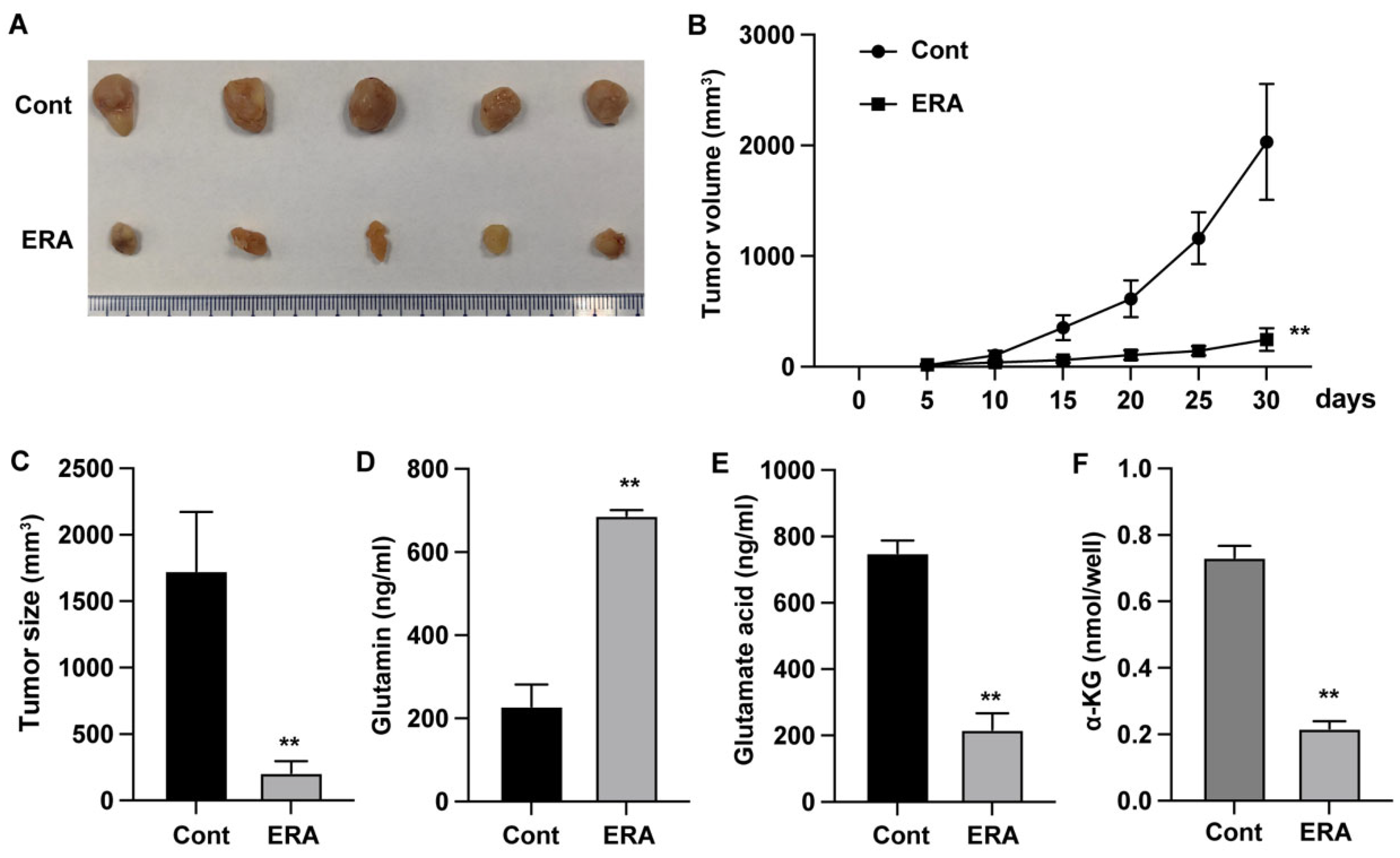
Erianin Modulates Glutamine Metabolism and Growth of Endometrial Carcinoma In Vivo
We next examined the in vivo effects of erianin by establishing the xenograft tumor model. Erianin treatment notably repressed the tumor growth and tumor size (
Figure 2A-C). Similar with the in vitro results, the treatment with erianin also significantly elevated glutamine level (
Figure 2D) and reduced the glutamic acid (
Figure 2E) and α-KG (
Figure 2F) levels of tumor tissues.
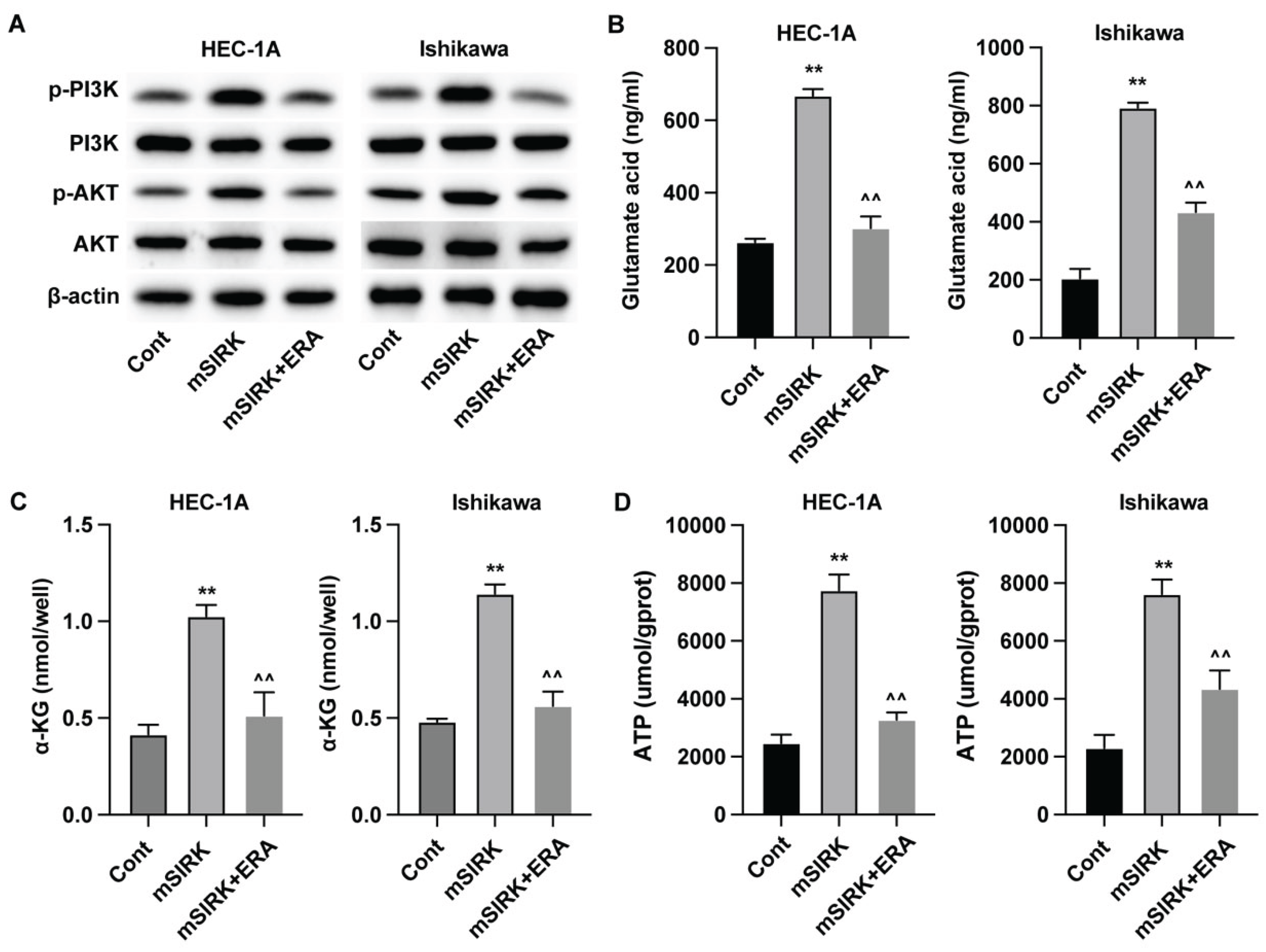
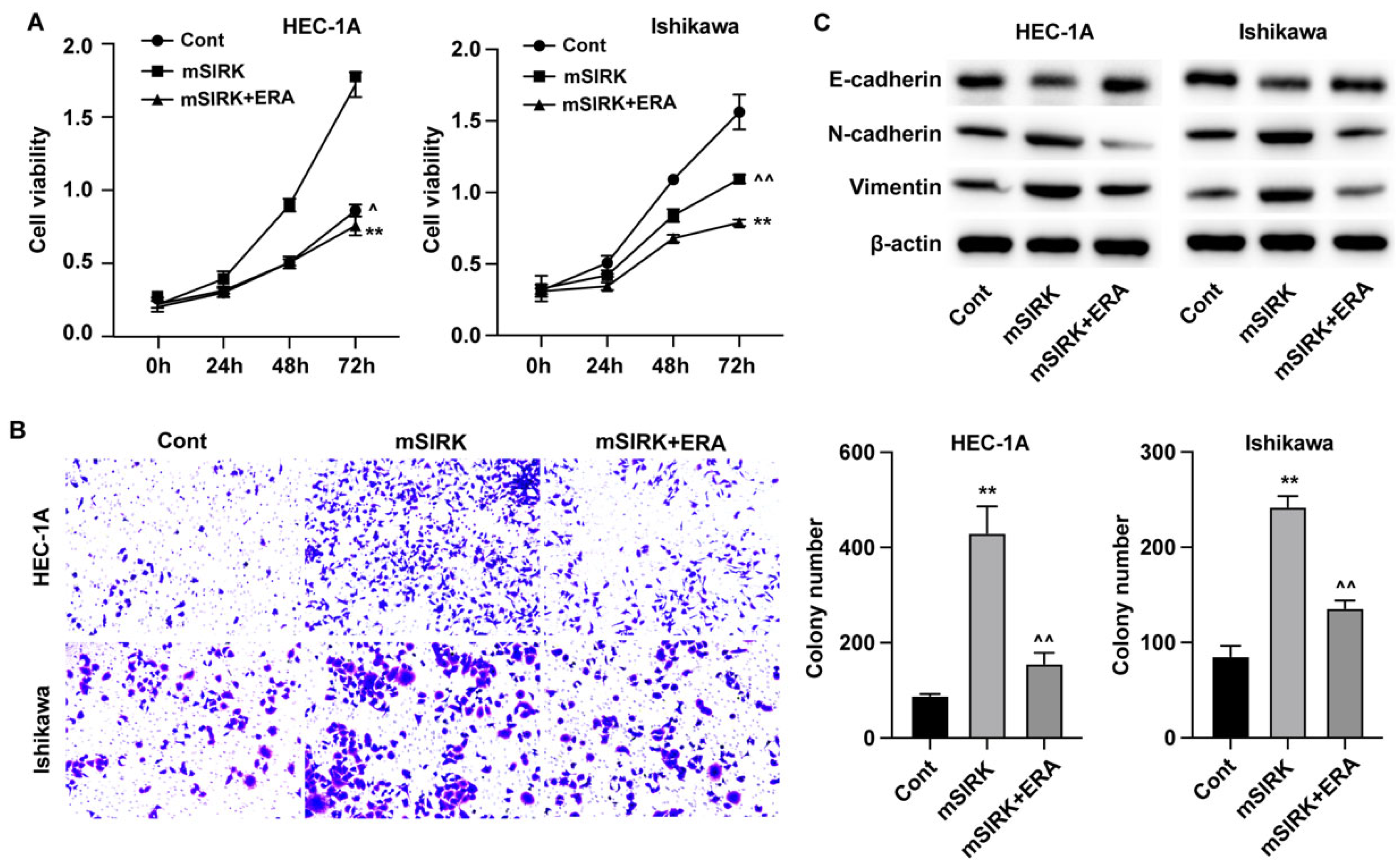
Erianin Represses Proliferation And Migration of Endometrial Cancer Cells Through ERK Signaling Pathway
Subsequently, the results from CCK-8 assay indicated that ERK activator enhanced the in vitro growth of EC cells, and administration of erianin abolished this elevated cell growth (
Figure 4A). The results from Transwell assay also showed that erianin treatment could suppress the ERK-activated migration of EC cells (
Figure 4B). Besides, the levels of migration biomarkers N-cadherin and Vimentin were upregulated, and E-cadherin was downregulated after treatment with ERK activator, which was reversed by erianin (
Figure 4C).
Discussion
Endometrial cancer (EC) is recognized as one of the leading invasive gynecological malignancies globally. It is noteworthy that a significant subset of individuals diagnosed with EC are under the age of 40. With the trend of postponing childbirth becoming more prevalent among women, a considerable percentage of EC patients within this younger demographic express a desire to preserve their fertility, with estimates suggesting that approximately 70% of these patients have such needs[
18]. This underscores the importance of developing treatment strategies that take into account the fertility preservation needs of this particular group of patients. In this study, we observed that erianin could repress the in vitro and in vivo growth of EC cells via regulating the glutamine metabolism.
Cancer is marked by diverse metabolic activities, with tumor cells frequently repurposing TCA cycle precursors for the assembly of proteins, lipids, and nucleic acids. When the levels of TCA cycle intermediates are depleted, it challenges the mitochondria's ability to function effectively. To counteract this metabolic shift, tumor cells employ various strategies to make up for the shortfall[
19,
20]. Glutamine, in particular, is converted into α-ketoglutarate, fueling the TCA cycle and offering energy to the cell. It also fulfills the cellular needs for nitrogen and carbon in the synthesis of purines, pyrimidines, and the hexosamine pathway. The heightened consumption of glutamine by tumor cells aids in the replenishment of TCA cycle components, which is essential for energy production, biomolecule synthesis, and the preservation of redox balance. Research indicates that the metabolic processing of glutamine is instrumental in enhancing the viability of endometrial cancer (EC) cells[
21]. Once inside the cell via its transporters, glutamine is cleaved by GLS into glutamate and ammonia, with the glutamate subsequently transformed into α-ketoglutarate by GDH, thus feeding into the TCA cycle[
22]. Notably, the ERK signaling pathway plays a pivotal role in glutamine metabolism, a process central to cellular energy homeostasis and biosynthesis. ERK, a component of the MAPK cascade, modulates the expression of enzymes involved in glutamine utilization, such as glutaminase, thereby regulating its conversion to glutamate and entry into the tricarboxylic acid (TCA) cycle[
23]. This regulation is critical for adjusting cellular respiration and supporting the anabolic demands of rapidly proliferating cells, such as cancer cells. The ERK pathway also intersects with glutamine metabolism by influencing the activity of transcription factors that control the expression of genes related to glutamine synthesis and transport[
24,
25].
Previous studies have demonstrated that erianin interacts with and influences a variety of signaling pathways such as MAPK, PI3K/Akt, and JNK, thereby exerting suppressive effects on the survival and proliferation of cancer cells[
26,
27,
28].Consistent with the previously identified involvement of ERK in glutamine metabolism, in our mechanism study, we determined that activation of ERK signaling abolished the effects of erianin targeting the glutamine metabolism and EC growth. However, further experiments are necessary to study other potential molecular mechanisms involved in the anti-tumor effects of erianin in EC.
Conclusions
In this study, we demonstrated that erianin treatment could repress the growth and metabolism of EC cells and identified that ERK signaling is involved in this process. Our findings may provide novel evidence for application of erianin as an effective therapy for cancers.
References
- Yan L, Z Zhang, Y Liu, et al., Anticancer Activity of Erianin: Cancer-Specific Target Prediction Based on Network Pharmacology. Frontiers in molecular biosciences. 2022. 9: p. 862932. [CrossRef]
- Yang Z, R Liu, M Qiu, et al., The roles of ERIANIN in tumor and innate immunity and its' perspectives in immunotherapy. Frontiers in immunology. 2023. 14: p. 1170754. [CrossRef]
- Dong H, M Wang, C Chang, et al., Erianin inhibits the oncogenic properties of hepatocellular carcinoma via inducing DNA damage and aberrant mitosis. Biochem Pharmacol. 2020. 182: p. 114266. [CrossRef]
- Li M, S Kang, X Deng, et al., Erianin inhibits the progression of triple-negative breast cancer by suppressing SRC-mediated cholesterol metabolism. Cancer Cell Int. 2024. 24(1): p. 166. [CrossRef]
- Shen H, Z Geng, X Nie, et al., Erianin Induces Ferroptosis of Renal Cancer Stem Cells via Promoting ALOX12/P53 mRNA N6-methyladenosine Modification. J Cancer. 2023. 14(3): p. 367-378. [CrossRef]
- Zhang HQ, XF Xie, GM Li, et al., Erianin inhibits human lung cancer cell growth via PI3K/Akt/mTOR pathway in vitro and in vivo. Phytotherapy research : PTR. 2021. 35(8): p. 4511-4525. [CrossRef]
- Miao Q, WQ Deng, WY Lyu, et al., Erianin inhibits the growth and metastasis through autophagy-dependent ferroptosis in KRAS(G13D) colorectal cancer. Free Radic Biol Med. 2023. 204: p. 301-312. [CrossRef]
- Chen P, Q Wu, J Feng, et al., Erianin, a novel dibenzyl compound in Dendrobium extract, inhibits lung cancer cell growth and migration via calcium/calmodulin-dependent ferroptosis. Signal Transduct Target Ther. 2020. 5(1): p. 51. [CrossRef]
- Park JH, WY Pyun, and HW Park, Cancer Metabolism: Phenotype, Signaling and Therapeutic Targets. Cells. 2020. 9(10).
- Stine ZE, ZT Schug, JM Salvino, et al., Targeting cancer metabolism in the era of precision oncology. Nat Rev Drug Discov. 2022. 21(2): p. 141-162. [CrossRef]
- Hay N, Reprogramming glucose metabolism in cancer: can it be exploited for cancer therapy? Nat Rev Cancer. 2016. 16(10): p. 635-649. [CrossRef]
- Mossmann D, S Park, and MN Hall, mTOR signalling and cellular metabolism are mutual determinants in cancer. Nat Rev Cancer. 2018. 18(12): p. 744-757. [CrossRef]
- Zhu L, X Zhu, and Y Wu, Effects of Glucose Metabolism, Lipid Metabolism, and Glutamine Metabolism on Tumor Microenvironment and Clinical Implications. Biomolecules. 2022. 12(4). [CrossRef]
- Cluntun AA, MJ Lukey, RA Cerione, et al., Glutamine Metabolism in Cancer: Understanding the Heterogeneity. Trends Cancer. 2017. 3(3): p. 169-180. [CrossRef]
- Altman BJ, ZE Stine, and CV Dang, From Krebs to clinic: glutamine metabolism to cancer therapy. Nat Rev Cancer. 2016. 16(10): p. 619-634. [CrossRef]
- Jin J, JK Byun, YK Choi, et al., Targeting glutamine metabolism as a therapeutic strategy for cancer. Experimental & molecular medicine. 2023. 55(4): p. 706-715. [CrossRef]
- Yang WH, Y Qiu, O Stamatatos, et al., Enhancing the Efficacy of Glutamine Metabolism Inhibitors in Cancer Therapy. Trends Cancer. 2021. 7(8): p. 790-804. [CrossRef]
- Koskas M, E Azria, F Walker, et al., Progestin treatment of atypical hyperplasia and well-differentiated adenocarcinoma of the endometrium to preserve fertility. Anticancer Res. 2012. 32(3): p. 1037-1043.
- Jeong SM, S Hwang, K Park, et al., Enhanced mitochondrial glutamine anaplerosis suppresses pancreatic cancer growth through autophagy inhibition. Sci Rep. 2016. 6: p. 30767. [CrossRef]
- Owen OE, SC Kalhan, and RW Hanson, The key role of anaplerosis and cataplerosis for citric acid cycle function. J Biol Chem. 2002. 277(34): p. 30409-30412. [CrossRef]
- Zhou WJ, J Zhang, HL Yang, et al., Estrogen inhibits autophagy and promotes growth of endometrial cancer by promoting glutamine metabolism. Cell Commun Signal. 2019. 17(1): p. 99. [CrossRef]
- Still ER and MO Yuneva, Hopefully devoted to Q: targeting glutamine addiction in cancer. Br J Cancer. 2017. 116(11): p. 1375-1381.
- Recouvreux MV, MR Moldenhauer, KMO Galenkamp, et al., Glutamine depletion regulates Slug to promote EMT and metastasis in pancreatic cancer. The Journal of experimental medicine. 2020. 217(9). [CrossRef]
- Lu H, H Yin, L Qu, et al., Ginsenoside Rk1 regulates glutamine metabolism in hepatocellular carcinoma through inhibition of the ERK/c-Myc pathway. Food Funct. 2022. 13(7): p. 3793-3811. [CrossRef]
- Ma G, Y Liang, Y Chen, et al., Glutamine Deprivation Induces PD-L1 Expression via Activation of EGFR/ERK/c-Jun Signaling in Renal Cancer. Molecular cancer research : MCR. 2020. 18(2): p. 324-339. [CrossRef]
- Liu YT, MJ Hsieh, JT Lin, et al., Erianin induces cell apoptosis through ERK pathway in human nasopharyngeal carcinoma. Biomed Pharmacother. 2019. 111: p. 262-269. [CrossRef]
- Wang P, X Jia, B Lu, et al., Erianin suppresses constitutive activation of MAPK signaling pathway by inhibition of CRAF and MEK1/2. Signal Transduct Target Ther. 2023. 8(1): p. 96.
- Mo C, D Shetti, and K Wei, Erianin Inhibits Proliferation and Induces Apoptosis of HaCaT Cells via ROS-Mediated JNK/c-Jun and AKT/mTOR Signaling Pathways. Molecules. 2019. 24(15). [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).