Submitted:
17 June 2025
Posted:
18 June 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
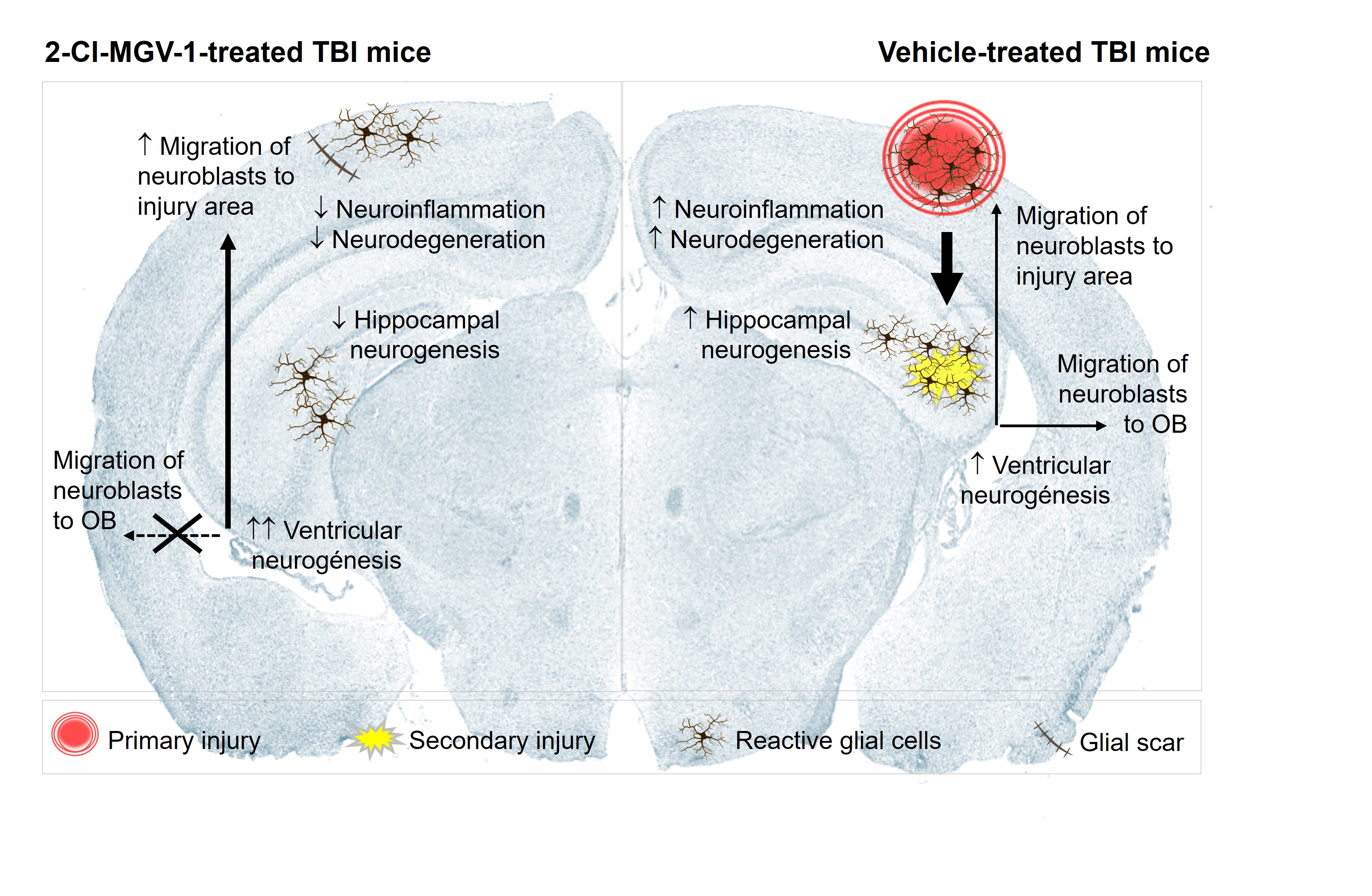
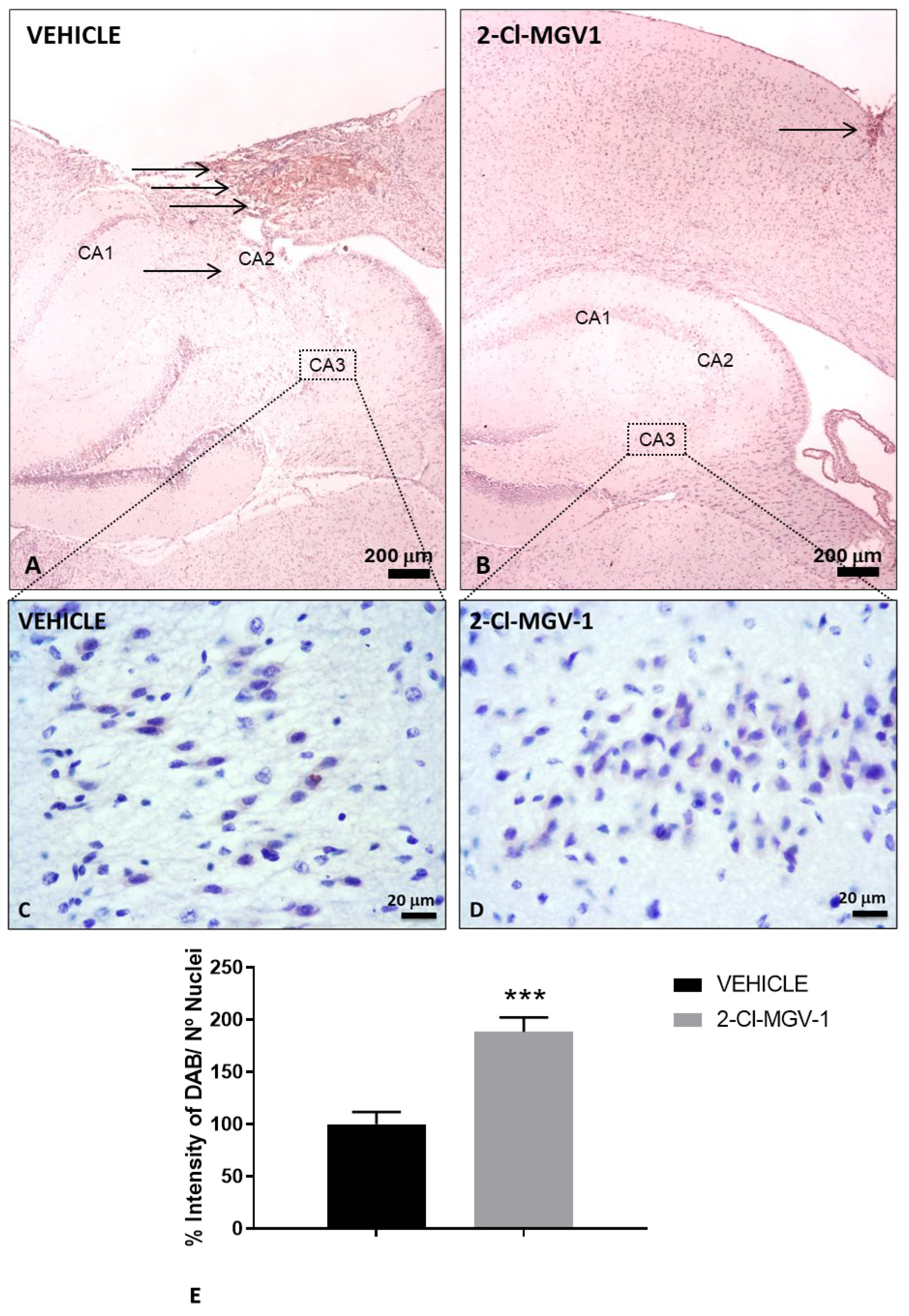
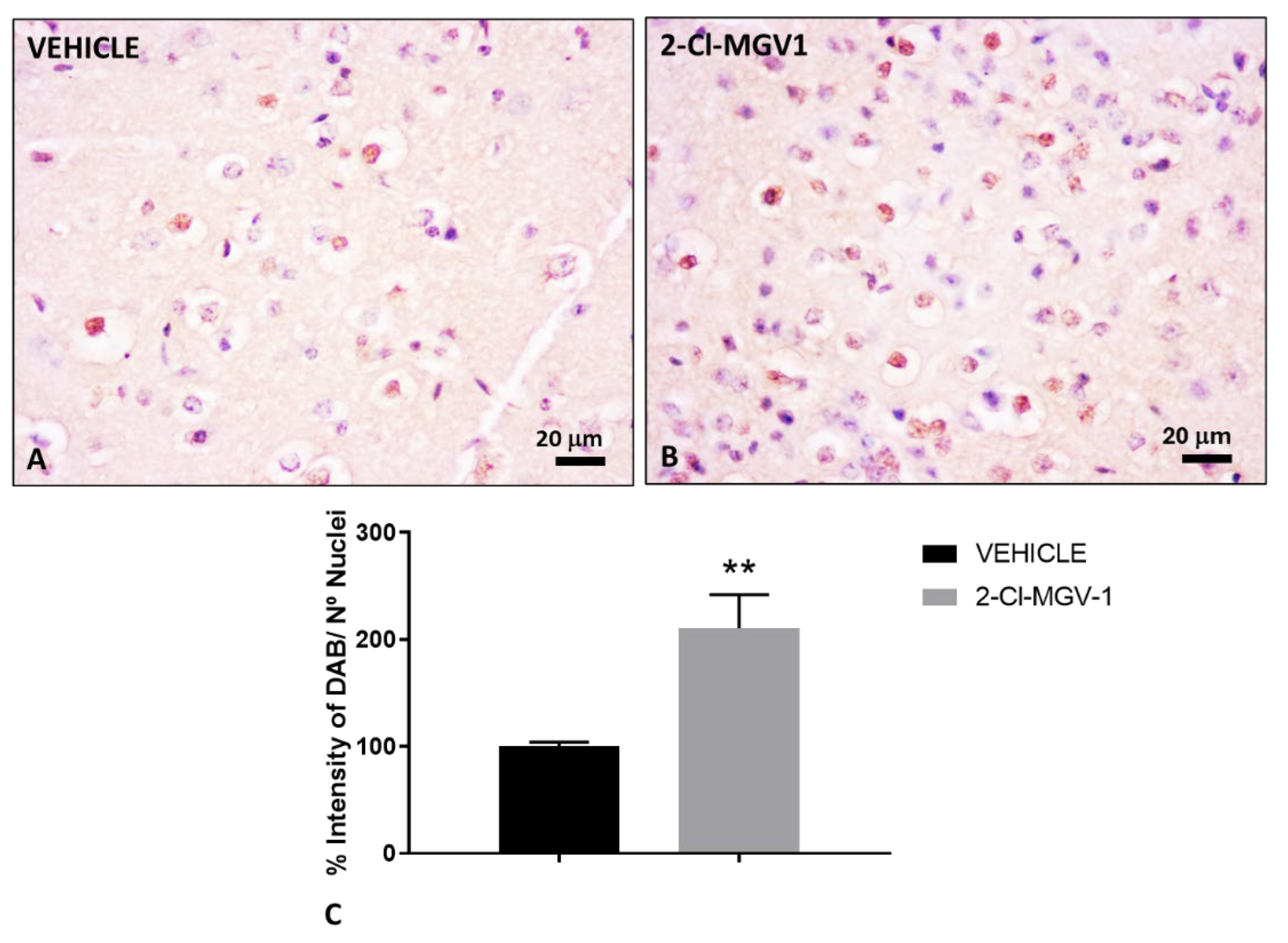
2.1. The TSPO Ligand 2-Cl-MGV-1 Promotes Neural Tissue Regeneration Following TBI
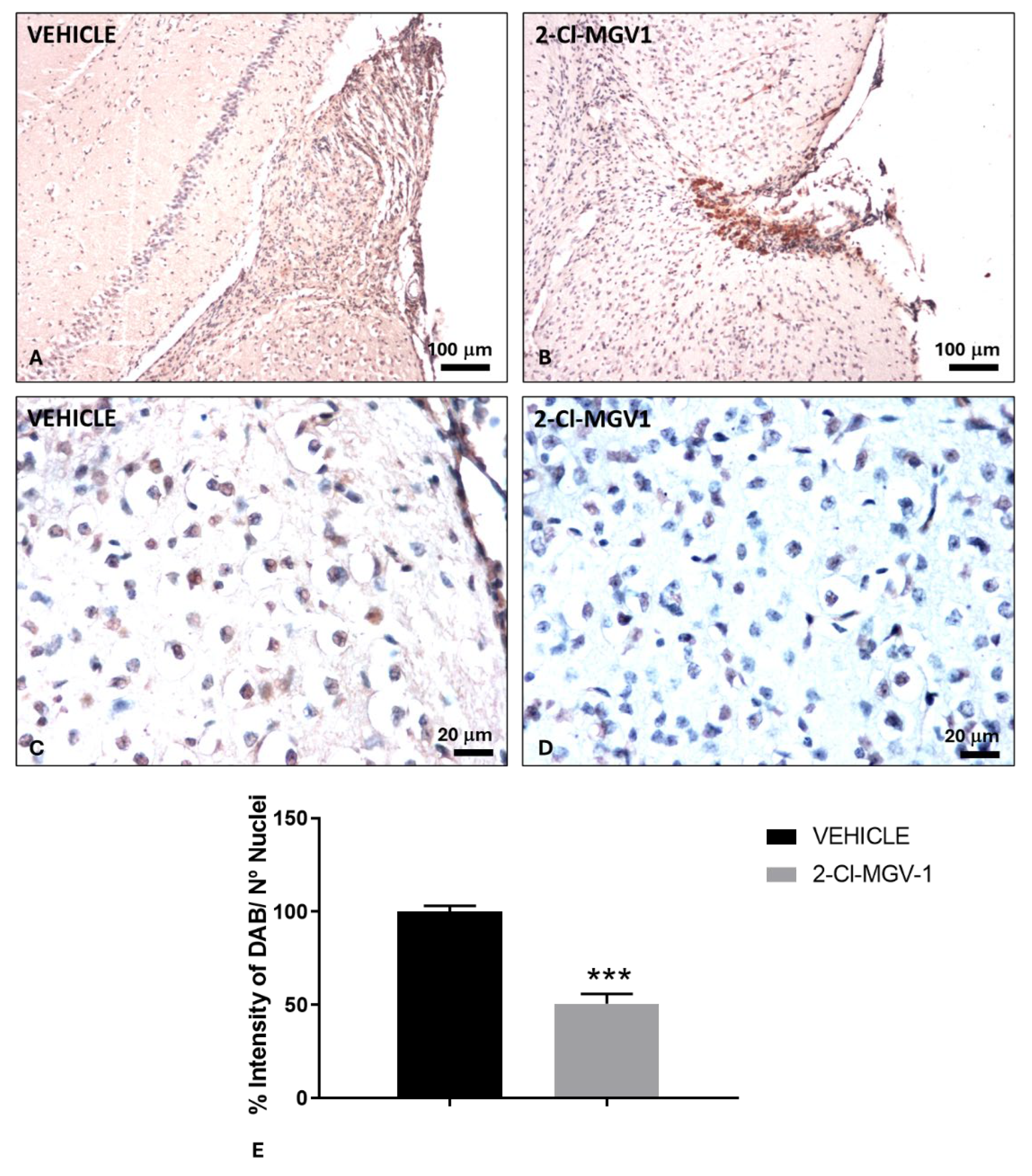
2.2. The TSPO Ligand 2-Cl-MGV-1 Reduces Neuroinflammation Following TBI
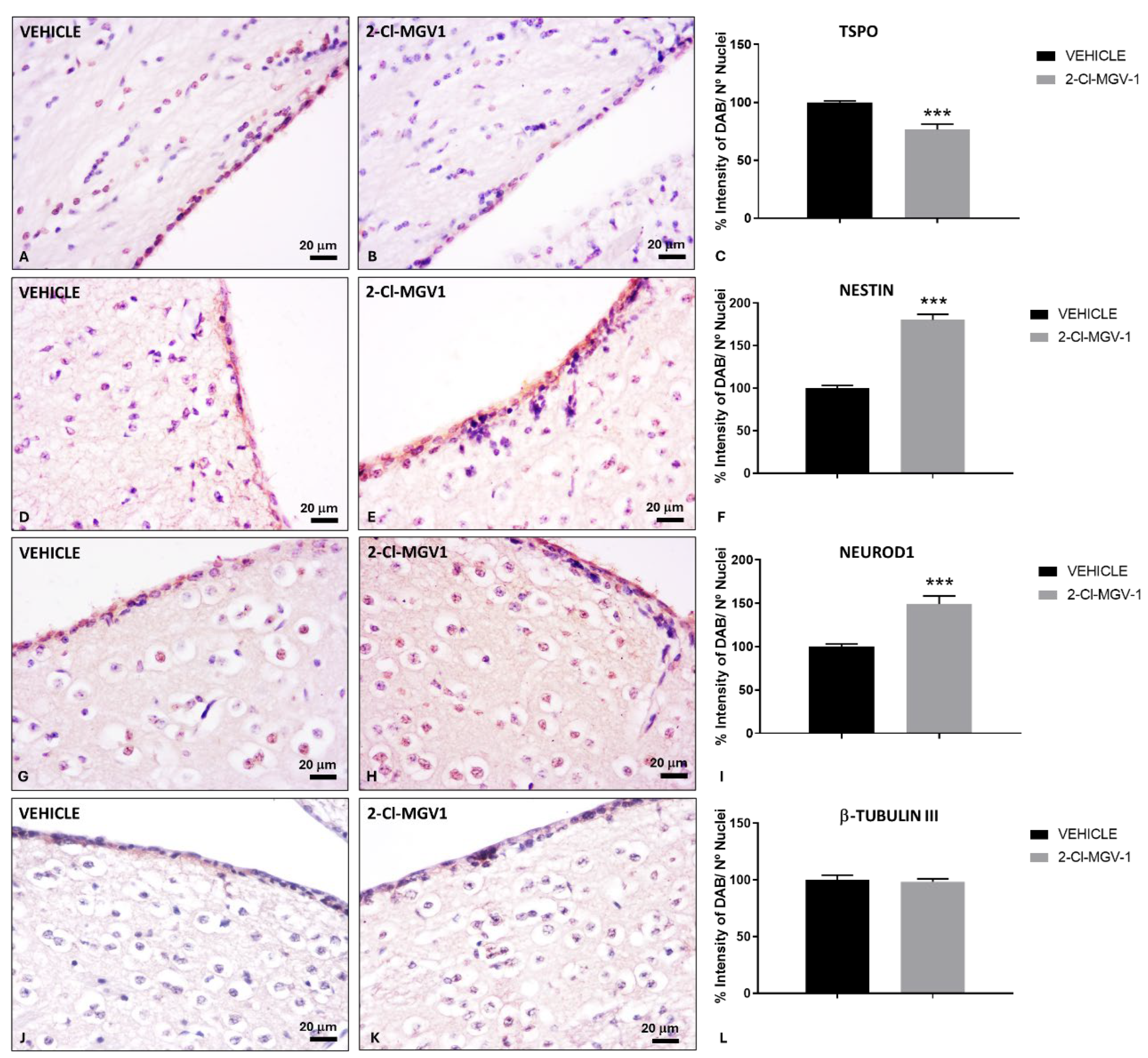
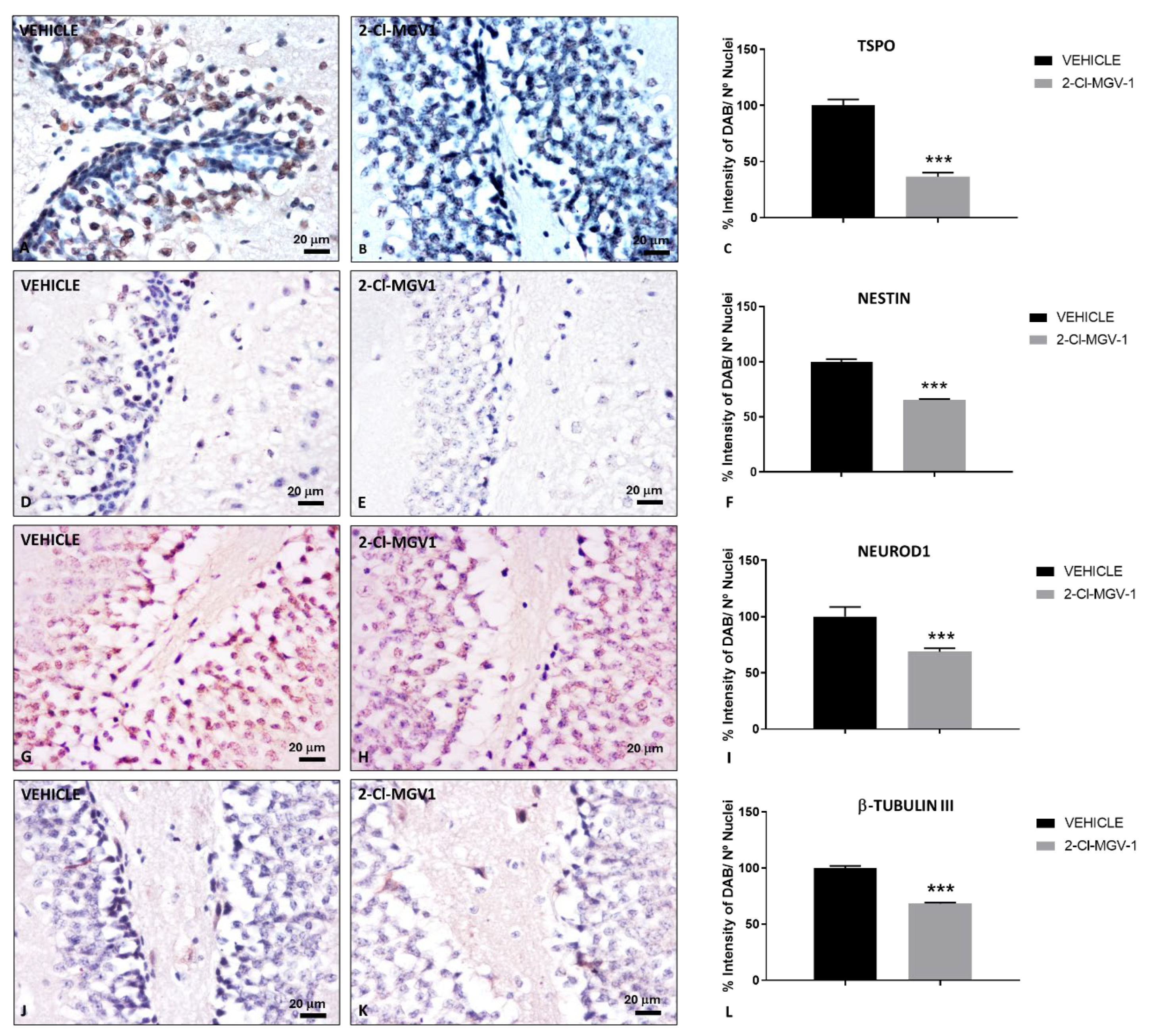
2.3. The TSPO Ligand 2-Cl-MGV-1 Enhances Neurogenesis in the SVZ Following TBI
2.4. The TSPO Ligand 2-Cl-MGV-1 Promotes Neuroblasts Migration from the SVZ to the Injured Brain Cortex Following TBI
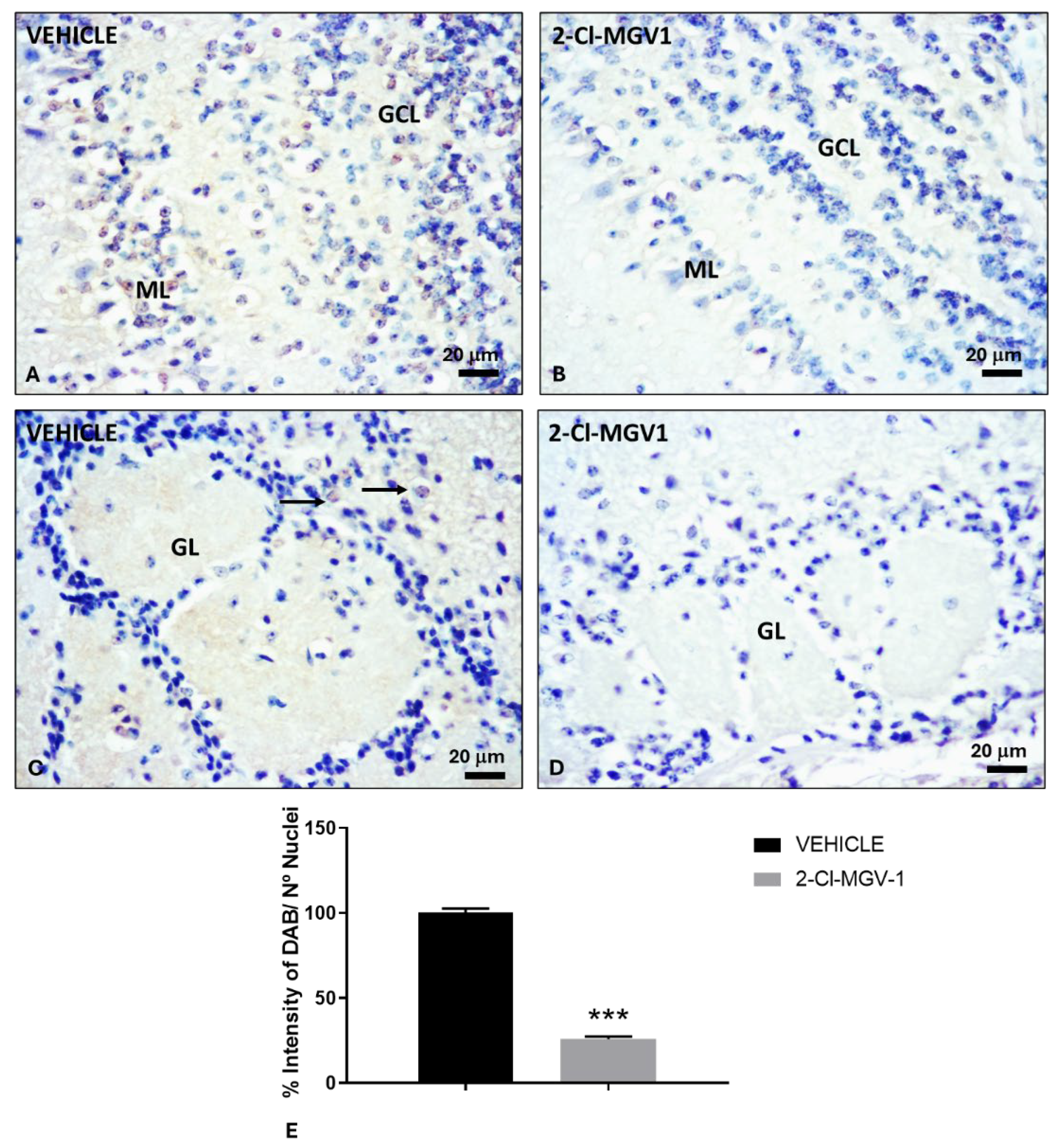
2.5. The TSPO Ligand 2-Cl-MGV-1 Reduces Neuroblasts Migration from the SVZ to the Olfatory Bulb Following TBI
2.6. The TSPO Ligand 2-Cl-MGV-1 Reduces Hippocampal Neurogenesis Following TBI
3. Discussion
4. Materials and Methods
4.1. Animals and TBI Model
4.2. Treatments
4.3. Histological Procedures
4.4. Immunohistochemistry
4.5. Images Analysis
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 2-Cl-MGV-1 | 2-(2-chlorophenyl) quinazolin-4-yl dimethylcarbamate |
| DMSO | Dimethyl sulfoxide |
| GCL | Granule cell layer |
| GL | Glomerular cell layer |
| Nestin | Neuroectodermal stem cell protein |
| NeuN | Neuronal nuclear antigen |
| NeuroD1 | Neurogenic differentiation factor 1 |
| SGZ | Subgranular zone |
| SVZ | Subventricular zone |
| TBI | Traumatic brain injury |
| TSPO | The 18-kDa translocator protein |
References
- Bao, Q.; Yuan, X.; Bian, X.; Wei, W.; Jin, P.; Jiang, W. Prognostic Significance of Translocator Protein in Brain Tissue Following Traumatic Brain Injury. Turkish neurosurgery 2023, 33, 736–744. [Google Scholar] [CrossRef] [PubMed]
- Mohan, M.C.; Anjana, A.S.; Hilmi Jaufer, T.A.; Deepti, A.; Krishnakumar, I.M.; Baby Chakrapani, P.S. Co-delivery of curcumin-resveratrol-carnosic acid complex promotes neurogenesis and cognitive recovery in a rodent model of repeated mild traumatic brain injury. Biomedicine & pharmacotherapy 2025, 183, 117818. [Google Scholar] [CrossRef]
- Ma, X.; Aravind, A.; Pfister, B.J.; Chandra, N.; Haorah, J. Animal Models of Traumatic Brain Injury and Assessment of Injury Severity. Molecular neurobiology 2019, 56, 5332–5345. [Google Scholar] [CrossRef] [PubMed]
- Khazaal, A.Q.; Ismaeel, H.M.; Cheah, P.S.; Nordin, N. Cellular Stem Cell Therapy for Treating Traumatic Brain Injury: Strategies for Enhancement of Therapeutic Efficacy. Molecular neurobiology, 2025. [Google Scholar] [CrossRef]
- Donat, C.K.; Scott, G.; Gentleman, S.M.; Sastre, M. Microglial Activation in Traumatic Brain Injury. Frontiers in aging neuroscience 2017, 9, 208. [Google Scholar] [CrossRef]
- Caballero, B.; Veenman, L.; Gavish, M. Role of mitochondrial translocator protein (18 kDa) on mitochondrial- related cell death processes. Recent patents on endocrine, metabolic & immune drug discovery 2013, 7, 86–101. [Google Scholar] [CrossRef]
- Caballero, B.; Veenman, L.; Bode, J.; Leschiner, S.; Gavish, M. Concentration-dependent bimodal effect of specific 18 kDa translocator protein (TSPO) ligands on cell death processes induced by ammonium chloride: potential implications for neuropathological effects due to hyperammonemia. CNS & neurological disorders drug targets 2014, 13, 574–592. [Google Scholar] [CrossRef]
- Veenman, L.; Vainshtein, A.; Gavish, M. TSPO as a target for treatments of diseases, including neuropathological disorders. Cell death & disease 2015, 6, e1911. [Google Scholar] [CrossRef]
- McNeela, A.M.; Bernick, C.; Hines, R.M.; Hines, D.J. TSPO regulation in reactive gliotic diseases. Journal of neuroscience research 2018, 96, 978–988. [Google Scholar] [CrossRef]
- Luo, L.F.; Weng, J.F.; Cen, M.; Dong, X.Q.; Yu, W.H.; Du, Q.; Yang, D.B.; Zheng, Y.K.; Hu, W.; Yu, L. , et al. Prognostic significance of serum translocator protein in patients with traumatic brain injury. Clinica chimica acta; international journal of clinical chemistry 2019, 488, 25–30. [Google Scholar] [CrossRef]
- Missault, S.; Anckaerts, C.; Blockx, I.; Deleye, S.; Van Dam, D.; Barriche, N.; De Pauw, G.; Aertgeerts, S.; Valkenburg, F.; De Deyn, P.P. , et al. Neuroimaging of Subacute Brain Inflammation and Microstructural Changes Predicts Long-Term Functional Outcome after Experimental Traumatic Brain Injury. Journal of neurotrauma 2019, 36, 768–788. [Google Scholar] [CrossRef]
- Bode, J.; Veenman, L.; Caballero, B.; Lakomek, M.; Kugler, W.; Gavish, M. The 18 kDa translocator protein influences angiogenesis, as well as aggressiveness, adhesion, migration, and proliferation of glioblastoma cells. Pharmacogenetics and genomics 2012, 22, 538–550. [Google Scholar] [CrossRef] [PubMed]
- Yasin, N.; Veenman, L.; Singh, S.; Azrad, M.; Bode, J.; Vainshtein, A.; Caballero, B.; Marek, I.; Gavish, M. Classical and Novel TSPO Ligands for the Mitochondrial TSPO Can Modulate Nuclear Gene Expression: Implications for Mitochondrial Retrograde Signaling. International journal of molecular sciences 2017, 18. [Google Scholar] [CrossRef]
- Dimitrova-Shumkovska, J.; Krstanoski, L.; Veenman, L. Diagnostic and Therapeutic Potential of TSPO Studies Regarding Neurodegenerative Diseases, Psychiatric Disorders, Alcohol Use Disorders, Traumatic Brain Injury, and Stroke: An Update. Cells 2020, 9. [Google Scholar] [CrossRef]
- Varga, B.; Marko, K.; Hadinger, N.; Jelitai, M.; Demeter, K.; Tihanyi, K.; Vas, A.; Madarasz, E. Translocator protein (TSPO 18kDa) is expressed by neural stem and neuronal precursor cells. Neuroscience letters 2009, 462, 257–262. [Google Scholar] [CrossRef]
- Palzur, E.; Sharon, A.; Shehadeh, M.; Soustiel, J.F. Investigation of the mechanisms of neuroprotection mediated by Ro5-4864 in brain injury. Neuroscience 2016, 329, 162–170. [Google Scholar] [CrossRef]
- Notter, T.; Schalbetter, S.M.; Clifton, N.E.; Mattei, D.; Richetto, J.; Thomas, K.; Meyer, U.; Hall, J. Neuronal activity increases translocator protein (TSPO) levels. Molecular psychiatry 2021, 26, 2025–2037. [Google Scholar] [CrossRef] [PubMed]
- Vainshtein, A.; Veenman, L.; Shterenberg, A.; Singh, S.; Masarwa, A.; Dutta, B.; Island, B.; Tsoglin, E.; Levin, E.; Leschiner, S. , et al. Quinazoline-based tricyclic compounds that regulate programmed cell death, induce neuronal differentiation, and are curative in animal models for excitotoxicity and hereditary brain disease. Cell death discovery 2015, 1, 15027. [Google Scholar] [CrossRef] [PubMed]
- Azrad, M.; Zeineh, N.; Weizman, A.; Veenman, L.; Gavish, M. The TSPO Ligands 2-Cl-MGV-1, MGV-1, and PK11195 Differentially Suppress the Inflammatory Response of BV-2 Microglial Cell to LPS. International journal of molecular sciences 2019, 20. [Google Scholar] [CrossRef]
- Shehadeh, M.; Palzur, E.; Apel, L.; Soustiel, J.F. Reduction of Traumatic Brain Damage by Tspo Ligand Etifoxine. International journal of molecular sciences 2019, 20. [Google Scholar] [CrossRef]
- Yasin N., V. L., Caballero B., Zeineh N., Gonzalez-Blanco L., Weizman A., Gavish G. TSPO ligand 2-Cl-MGV-1 mitigates trumatic brain INjury (TBI) in a mouse model. International Journal of Molecular Science 2025, 26. [Google Scholar] [CrossRef]
- Chen, Y.; Veenman, L.; Singh, S.; Ouyang, F.; Liang, J.; Huang, W.; Marek, I.; Zeng, J.; Gavish, M. 2-Cl-MGV-1 Ameliorates Apoptosis in the Thalamus and Hippocampus and Cognitive Deficits After Cortical Infarct in Rats. Stroke 2017, 48, 3366–3374. [Google Scholar] [CrossRef] [PubMed]
- Cachan-Vega, C.; Vega-Naredo, I.; Potes, Y.; Bermejo-Millo, J.C.; Rubio-Gonzalez, A.; Garcia-Gonzalez, C.; Antuna, E.; Bermudez, M.; Gutierrez-Rodriguez, J.; Boga, J.A. , et al. Chronic Treatment with Melatonin Improves Hippocampal Neurogenesis in the Aged Brain and Under Neurodegeneration. Molecules 2022, 27. [Google Scholar] [CrossRef]
- Gross, C.G. Neurogenesis in the adult brain: death of a dogma. Nature reviews. Neuroscience 2000, 1, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Krestinina, O.V.M., S. N.; Baburina, Y; Fadeev, R.S.; Azarashavili, T.S.; Akatov, V.S. The effects of isoquinoline carboxamide and melatonin on the differentation of N1E-115 mouse neuroblastoma cells (clone C-1300) and on the expression of the the TSPO translocation protein and 2´,3´-cyclonucleotide-3´-phosphodiesterase in these cells. Neurochem J 2017, 11. [Google Scholar] [CrossRef]
- Gonzalez-Blanco, L.; Bermejo-Millo, J.C.; Oliveira, G.; Potes, Y.; Antuna, E.; Menendez-Valle, I.; Vega-Naredo, I.; Coto-Montes, A.; Caballero, B. Neurogenic Potential of the 18-kDa Mitochondrial Translocator Protein (TSPO) in Pluripotent P19 Stem Cells. Cells 2021, 10. [Google Scholar] [CrossRef]
- Betlazar, C.; Harrison-Brown, M.; Middleton, R.J.; Banati, R.; Liu, G.J. Cellular Sources and Regional Variations in the Expression of the Neuroinflammatory Marker Translocator Protein (TSPO) in the Normal Brain. International journal of molecular sciences 2018, 19. [Google Scholar] [CrossRef]
- Lee, D.H.; Kang, S.K.; Lee, R.H.; Ryu, J.M.; Park, H.Y.; Choi, H.S.; Bae, Y.C.; Suh, K.T.; Kim, Y.K.; Jung, J.S. Effects of peripheral benzodiazepine receptor ligands on proliferation and differentiation of human mesenchymal stem cells. Journal of cellular physiology 2004, 198, 91–99. [Google Scholar] [CrossRef]
- Tanimoto, Y.; Yamasaki, T.; Nagoshi, N.; Nishiyama, Y.; Nori, S.; Nishimura, S.; Iida, T.; Ozaki, M.; Tsuji, O.; Ji, B. , et al. In vivo monitoring of remnant undifferentiated neural cells following human induced pluripotent stem cell-derived neural stem/progenitor cells transplantation. Stem cells translational medicine 2020, 9, 465–477. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Jiao, J. Molecular Biomarkers for Embryonic and Adult Neural Stem Cell and Neurogenesis. BioMed research international 2015, 2015, 727542. [Google Scholar] [CrossRef]
- Memberg, S.P.; Hall, A.K. Dividing neuron precursors express neuron-specific tubulin. Journal of neurobiology 1995, 27, 26–43. [Google Scholar] [CrossRef]
- Akter, M.; Kaneko, N.; Sawamoto, K. Neurogenesis and neuronal migration in the postnatal ventricular-subventricular zone: Similarities and dissimilarities between rodents and primates. Neuroscience research 2021, 167, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Navarro Quiroz, E.; Navarro Quiroz, R.; Ahmad, M.; Gomez Escorcia, L.; Villarreal, J.L.; Fernandez Ponce, C.; Aroca Martinez, G. Cell Signaling in Neuronal Stem Cells. Cells 2018, 7. [Google Scholar] [CrossRef]
- Defterali, C.; Moreno-Estelles, M.; Crespo, C.; Diaz-Guerra, E.; Diaz-Moreno, M.; Vergano-Vera, E.; Nieto-Estevez, V.; Hurtado-Chong, A.; Consiglio, A.; Mira, H. , et al. Neural stem cells in the adult olfactory bulb core generate mature neurons in vivo. Stem cells 2021, 39, 1253–1269. [Google Scholar] [CrossRef]
- Wang, Y.; Yue, X.; Kiesewetter, D.O.; Niu, G.; Teng, G.; Chen, X. PET imaging of neuroinflammation in a rat traumatic brain injury model with radiolabeled TSPO ligand DPA-714. European journal of nuclear medicine and molecular imaging 2014, 41, 1440–1449. [Google Scholar] [CrossRef] [PubMed]
- Israel, I.; Ohsiek, A.; Al-Momani, E.; Albert-Weissenberger, C.; Stetter, C.; Mencl, S.; Buck, A.K.; Kleinschnitz, C.; Samnick, S.; Siren, A.L. Combined [(18)F]DPA-714 micro-positron emission tomography and autoradiography imaging of microglia activation after closed head injury in mice. Journal of neuroinflammation 2016, 13, 140. [Google Scholar] [CrossRef]
- Monga, S.; Nagler, R.; Amara, R.; Weizman, A.; Gavish, M. Inhibitory Effects of the Two Novel TSPO Ligands 2-Cl-MGV-1 and MGV-1 on LPS-induced Microglial Activation. Cells 2019, 8. [Google Scholar] [CrossRef] [PubMed]
- Obeid, F.; Kahana, M.; Dahle, B.; Monga, S.; Zohar, Y.; Weizman, A.; Gavish, M. Novel TSPO Ligand 2-Cl-MGV-1 Can Counteract Lipopolysaccharide Induced Inflammatory Response in Murine RAW264.7 Macrophage Cell Line and Lung Models. Cells 2024, 13. [Google Scholar] [CrossRef]
- Monga, S.; Weizman, A.; Gavish, M. The Efficacy of the Novel TSPO Ligands 2-Cl-MGV-1 and 2,4-Di-Cl-MGV-1 Compared to the Classical TSPO Ligand PK 11195 to Counteract the Release of Chemokines from LPS-Stimulated BV-2 Microglial Cells. Biology 2020, 9. [Google Scholar] [CrossRef]
- Wang, Z.; Zheng, Y.; Zheng, M.; Zhong, J.; Ma, F.; Zhou, B.; Zhu, J. Neurogenic Niche Conversion Strategy Induces Migration and Functional Neuronal Differentiation of Neural Precursor Cells Following Brain Injury. Stem cells and development 2020, 29, 235–248. [Google Scholar] [CrossRef]
- Astakhova, O.; Ivanova, A.; Komoltsev, I.; Gulyaeva, N.; Enikolopov, G.; Lazutkin, A. Traumatic Brain Injury Promotes Neurogenesis and Oligodendrogenesis in Subcortical Brain Regions of Mice. Cells 2025, 14. [Google Scholar] [CrossRef]
- Chen, Y.; Veenman, L.; Liao, M.; Huang, W.; Yu, J.; Zeng, J. Enhanced angiogenesis in the thalamus induced by a novel TSPO ligand ameliorates cognitive deficits after focal cortical infarction. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism 2024, 44, 477–490. [Google Scholar] [CrossRef] [PubMed]
- Amanollahi, M.; Jameie, M.; Heidari, A.; Rezaei, N. The Dialogue Between Neuroinflammation and Adult Neurogenesis: Mechanisms Involved and Alterations in Neurological Diseases. Molecular neurobiology 2023, 60, 923–959. [Google Scholar] [CrossRef]
- Castro, E.S.J.H.; Pieropan, F.; Rivera, A.D.; Butt, A.M.; Costa, S.L. Agathisflavone Modulates Reactive Gliosis After Trauma and Increases the Neuroblast Population at the Subventricular Zone. Nutrients 2024, 16. [Google Scholar] [CrossRef]
- Badner, A.; Reinhardt, E.K.; Nguyen, T.V.; Midani, N.; Marshall, A.T.; Lepe, C.A.; Echeverria, K.; Lepe, J.J.; Torrecampo, V.; Bertan, S.H. , et al. Freshly Thawed Cryobanked Human Neural Stem Cells Engraft within Endogenous Neurogenic Niches and Restore Cognitive Function after Chronic Traumatic Brain Injury. Journal of neurotrauma 2021, 38, 2731–2746. [Google Scholar] [CrossRef] [PubMed]
- Badner, A.; Cummings, B.J. The endogenous progenitor response following traumatic brain injury: a target for cell therapy paradigms. Neural regeneration research 2022, 17, 2351–2354. [Google Scholar] [CrossRef] [PubMed]
- Hajinejad, M.; Ebrahimzadeh, M.H.; Ebrahimzadeh-Bideskan, A.; Rajabian, A.; Gorji, A.; Sahab Negah, S. Exosomes and Nano-SDF Scaffold as a Cell-Free-Based Treatment Strategy Improve Traumatic Brain Injury Mechanisms by Decreasing Oxidative Stress, Neuroinflammation, and Increasing Neurogenesis. Stem cell reviews and reports 2023, 19, 1001–1018. [Google Scholar] [CrossRef]
- Choi, B.Y.; Hong, D.K.; Kang, B.S.; Lee, S.H.; Choi, S.; Kim, H.J.; Lee, S.M.; Suh, S.W. Engineered Mesenchymal Stem Cells Over-Expressing BDNF Protect the Brain from Traumatic Brain Injury-Induced Neuronal Death, Neurological Deficits, and Cognitive Impairments. Pharmaceuticals 2023, 16. [Google Scholar] [CrossRef]
- Galgano, M.; Toshkezi, G.; Qiu, X.; Russell, T.; Chin, L.; Zhao, L.R. Traumatic Brain Injury: Current Treatment Strategies and Future Endeavors. Cell transplantation 2017, 26, 1118–1130. [Google Scholar] [CrossRef]
- Schepici, G.; Silvestro, S.; Bramanti, P.; Mazzon, E. Traumatic Brain Injury and Stem Cells: An Overview of Clinical Trials, the Current Treatments and Future Therapeutic Approaches. Medicina 2020, 56. [Google Scholar] [CrossRef]
- Deshpande, S.S.; Malik, S.C.; Conforti, P.; Lin, J.D.; Chu, Y.H.; Nath, S.; Greulich, F.; Dumbach, M.A.; Uhlenhaut, N.H.; Schachtrup, C. P75 neurotrophin receptor controls subventricular zone neural stem cell migration after stroke. Cell and tissue research 2022, 387, 415–431. [Google Scholar] [CrossRef]
- Schule, C.; Nothdurfter, C.; Rupprecht, R. The role of allopregnanolone in depression and anxiety. Progress in neurobiology 2014, 113, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Da Pozzo, E.; Giacomelli, C.; Costa, B.; Cavallini, C.; Taliani, S.; Barresi, E.; Da Settimo, F.; Martini, C. TSPO PIGA Ligands Promote Neurosteroidogenesis and Human Astrocyte Well-Being. International journal of molecular sciences 2016, 17. [Google Scholar] [CrossRef]
- Charalampopoulos, I.; Remboutsika, E.; Margioris, A.N.; Gravanis, A. Neurosteroids as modulators of neurogenesis and neuronal survival. Trends in endocrinology and metabolism: TEM 2008, 19, 300–307. [Google Scholar] [CrossRef]
- Hojo, Y.; Kawato, S. Neurosteroids in Adult Hippocampus of Male and Female Rodents: Biosynthesis and Actions of Sex Steroids. Frontiers in endocrinology 2018, 9, 183. [Google Scholar] [CrossRef] [PubMed]
- Sahab-Negah, S.; Hajali, V.; Moradi, H.R.; Gorji, A. The Impact of Estradiol on Neurogenesis and Cognitive Functions in Alzheimer's Disease. Cellular and molecular neurobiology 2020, 40, 283–299. [Google Scholar] [CrossRef] [PubMed]
- Tremolanti, C.; Angeloni, E.; Da Pozzo, E.; Germelli, L.; Giacomelli, C.; Scalzi, E.; Taliani, S.; Da Settimo, F.; Mensah-Nyagan, A.G.; Martini, C. , et al. Human oligodendrocyte-like cell differentiation is promoted by TSPO-mediated endogenous steroidogenesis. Biochimica et biophysica acta. Molecular basis of disease 2024, 1870, 167174. [Google Scholar] [CrossRef]
- Dash, P.K.; Mach, S.A.; Moore, A.N. Enhanced neurogenesis in the rodent hippocampus following traumatic brain injury. Journal of neuroscience research 2001, 63, 313–319. [Google Scholar] [CrossRef]
- Richardson, R.M.; Sun, D.; Bullock, M.R. Neurogenesis after traumatic brain injury. Neurosurgery clinics of North America 2007, 18, 169–181. [Google Scholar] [CrossRef]
- Ye, Y.; Zhao, Z.; Xu, H.; Zhang, X.; Su, X.; Yang, Y.; Yu, X.; He, X. Activation of Sphingosine 1-Phosphate Receptor 1 Enhances Hippocampus Neurogenesis in a Rat Model of Traumatic Brain Injury: An Involvement of MEK/Erk Signaling Pathway. Neural plasticity 2016, 2016, 8072156. [Google Scholar] [CrossRef]
- Weston, N.M.; Rolfe, A.T.; Freelin, A.H.; Reeves, T.M.; Sun, D. Traumatic brain injury modifies synaptic plasticity in newly-generated granule cells of the adult hippocampus. Experimental neurology 2021, 336, 113527. [Google Scholar] [CrossRef]
- Wang, X.; Gao, X.; Michalski, S.; Zhao, S.; Chen, J. Traumatic Brain Injury Severity Affects Neurogenesis in Adult Mouse Hippocampus. Journal of neurotrauma 2016, 33, 721–733. [Google Scholar] [CrossRef] [PubMed]
- Bielefeld, P.; Martirosyan, A.; Martin-Suarez, S.; Apresyan, A.; Meerhoff, G.F.; Pestana, F.; Poovathingal, S.; Reijner, N.; Koning, W.; Clement, R.A. , et al. Traumatic brain injury promotes neurogenesis at the cost of astrogliogenesis in the adult hippocampus of male mice. Nature communications 2024, 15, 5222. [Google Scholar] [CrossRef] [PubMed]
- Osier, N.D.; Carlson, S.W.; DeSana, A.; Dixon, C.E. Chronic Histopathological and Behavioral Outcomes of Experimental Traumatic Brain Injury in Adult Male Animals. Journal of neurotrauma 2015, 32, 1861–1882. [Google Scholar] [CrossRef]
- Simon-O'Brien, E.; Gauthier, D.; Riban, V.; Verleye, M. Etifoxine improves sensorimotor deficits and reduces glial activation, neuronal degeneration, and neuroinflammation in a rat model of traumatic brain injury. Journal of neuroinflammation 2016, 13, 203. [Google Scholar] [CrossRef]
- Jazvinscak Jembrek, M.; Radovanovic, V.; Vlainic, J.; Vukovic, L.; Hanzic, N. Neuroprotective effect of zolpidem against glutamate-induced toxicity is mediated via the PI3K/Akt pathway and inhibited by PK11195. Toxicology 2018, 406-407, 58–69. [Google Scholar] [CrossRef]
- Palzur, E.; Edelman, D.; Sakas, R.; Soustiel, J.F. Etifoxine Restores Mitochondrial Oxidative Phosphorylation and Improves Cognitive Recovery Following Traumatic Brain Injury. International journal of molecular sciences 2021, 22. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Chen, J. Moderate traumatic brain injury promotes neural precursor proliferation without increasing neurogenesis in the adult hippocampus. Experimental neurology 2013, 239, 38–48. [Google Scholar] [CrossRef]
- Khalin, I.; Jamari, N.L.; Razak, N.B.; Hasain, Z.B.; Nor, M.A.; Zainudin, M.H.; Omar, A.B.; Alyautdin, R. A mouse model of weight-drop closed head injury: emphasis on cognitive and neurological deficiency. Neural regeneration research 2016, 11, 630–635. [Google Scholar] [CrossRef]
- Kalish, B.T.; Whalen, M.J. Weight Drop Models in Traumatic Brain Injury. Methods in molecular biology 2016, 1462, 193–209. [Google Scholar] [CrossRef]
| Antibody | Antigen | Catalog Nº | Company |
|---|---|---|---|
| Anti-TSPO | The 18-kDa translocator protein | PA5-75544 | Invitrogen |
| Anti-Nestin | Neuroectodermal stem cell protein | N5413 | Sigma-Aldrich |
| Anti-NeuroD1 | Neurogenic differentiation factor 1 | #ABE991 | Millipore |
| Anti-TUBB3 | β-Tubulin III | T2200 | Sigma-Aldrich |
| Anti-NeuN | Neuronal nuclear antigen | AB4301175 | Sigma-Aldrich |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





