Submitted:
17 June 2025
Posted:
18 June 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
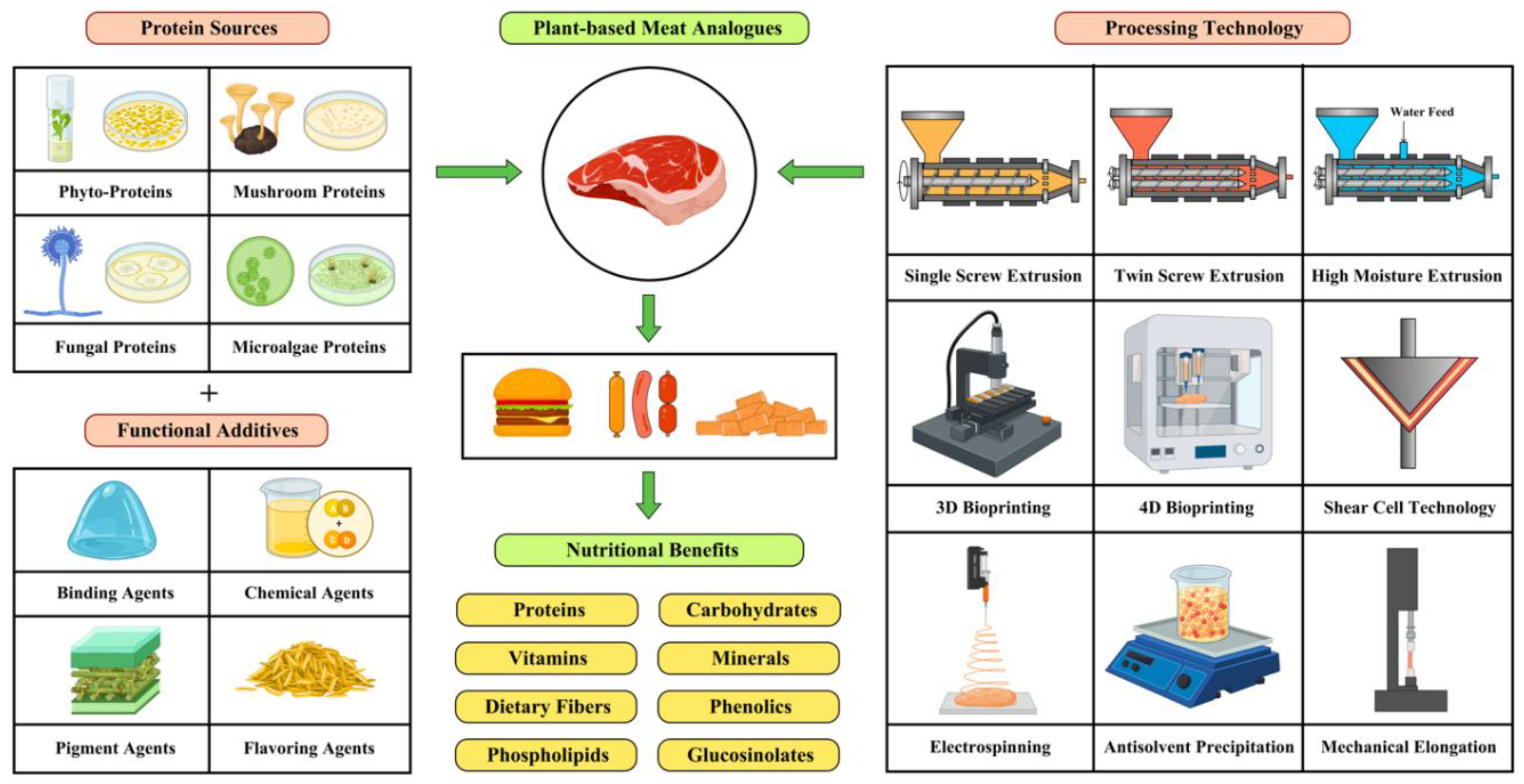
2. Plant Protein Sources
2.1. Proteins from Legumes
2.1.1. Soy Protein
2.1.2. Pea Protein
2.1.3. Mung Bean Protein
2.1.4. Faba Bean Protein
2.1.5. Lupin Bean Protein
2.1.6. Chickpea Protein
| Legume Proteins | Latin Name | Properties | Reference |
|---|---|---|---|
| Soy | Glycine max | Excellent water–holding, emulsification, strong gelling, good texturization. | [76] |
| Pea | Pisum sativum | High emulsifying and water–holding, moderate foaming and gelling stability. | [77] |
| Mung bean | Vigna radiata | Extrudability and texturization, gelling and foaming capacity under medium shear force. | [78] |
| Faba bean | Vicia faba | Good emulsification, foaming, and gelling, improves microstructural properties. | [79] |
| Lupin bean | Lupinus | Good emulsification, foaming, and water–binding, enhances firmness. | [80] |
| Chickpea | Cicer arietinum | Strong water and oil binding, moderate gelation, creates a soft texture. | [81] |
| Kidney bean | Phaseolus vulgaris | Moderate water–holding and gelation; improves texture and elasticity. | [82] |
| Lentil | Lens culinaris | Good water–binding, moderate gelation, emulsification, improves firmness and chewiness. | [83] |
2.2. Proteins from Oilseeds
2.2.1. Hemp Protein
2.2.2. Rapeseed Protein
2.2.3. Pumpkin Protein
| Oilseed Proteins | Latin Name | Properties | Reference |
|---|---|---|---|
| Peanut | Arachis hypogaea | Oil binding and foaming capacity comparable to SPI; higher viscosity and gel formation post–heating. | [107] |
| Hemp | Cannabis sativa | Superior functional attributes such as foaming, gel formation, and WHC. | [108] |
| Rapeseed | Brassica napus | Strong heat–set gel forms by cruciferin under alkaline conditions. | [109] |
| Pumpkin | Cucurbita maxima | High emulsifying capacity and stability, moderate foaming, low gelling, and high WHC. | [110] |
| Flaxseed | Linum usitatissimum | High nutritional value and good techno–functional properties i.e., solubility, foaming, emulsification, gelling, and WHC. | [111] |
| Chia | Salvia hispanica | Excellent water and oil binding properties; rich in plant–based omega–3 fatty acids. | [112] |
| Cottonseed | Gossypium hirsutum | Good emulsification and WHC; contains gossypol, which requires processing for safe consumption. | [113] |
| Sesame | Sesamum indicum | Excellent source of proteins and antioxidants; good emulsification and binding properties. | [114] |
| Safflower | Carthamus tinctorius | Rich in linoleic acid and good water retention aiding in texture formation. | [115] |
| Sunflower | Helianthus annuus | Stable emulsification and foaming with comparable gelling capacity. | [116] |
| Black Cumin | Nigella sativa | Flavor enhancement with its distinctive aroma. Good oil–holding capacity aids in juiciness and texture. | [117] |
2.3. Proteins from Cereal and Pseudocereal
2.3.1. Wheat Protein
2.3.2. Oat Protein
2.3.3. Rice Protein
| Cereal Proteins | Latin Name | Properties | Reference |
|---|---|---|---|
| Wheat | Triticum aestivum | High in gluten, providing elasticity and binding properties. | [141] |
| Oat | Avena sativa | Good solubility in water, high emulsifying, gelling, and WHC, moderate foaming capacity. | [142] |
| Rice | Oryza sativa | Good solubility in water, high emulsifying and stability, moderate foaming, and low gelling. | [143] |
| Corn (Zein) | Zea mays | Stabilizes oil–in–glycerol emulsion–gels, used as a fat analogue, and stabilizes foam and emulsions. | [144] |
| Barley | Hordeum vulgare | Contains β–glucans, contributing to water retention and gelation. Improves viscosity and mouthfeel. | [145] |
| Millet | Panicum miliaceum | Gluten–free and good water–binding capacity for improved texture. Rich in antioxidants and EAAs. | [146] |
| Sorghum | Sorghum bicolor | Polyphenols support texture and nutrition. Gluten–free good binding capacity for improved juiciness. | [147] |
| Pseudocereal Proteins | Latin Name | Properties | Reference |
|---|---|---|---|
| Quinoa | Chenopodium quinoa | Higher emulsifying capacity and stability than soy, wheat and pearl millet; low gelling capacity and good water holding capacity. | [148] |
| Amaranth | Amaranthus caudatus | Improves solubility, emulsification, foaming, gelling, and water–holding capabilities subject to pH, temperature, and enzymatic hydrolysis. | [149] |
| Buckwheat | Fagopyrum esculentum | Good water retention and strong emulsification capacity with gluten–free texture enhancement properties. Contributes to a slightly nutty flavor. | [150] |
2.4. Mushroom Species
2.4.1. Agaricus bisporus (Button Mushroom)
2.4.2. Pleurotus ostreatus (Oyster Mushroom)
2.4.3. Lentinus edodes (Shiitake Mushroom)
2.4.4. Coprinus comatus (Chicken Drumstick Mushroom)
| Mushroom Species | Latin Name | Properties | Reference |
|---|---|---|---|
| Button Mushroom | Agaricus bisporus | Enhances juiciness and nutritional profile due to its protein and fiber content, with antioxidant properties. | [177] |
| Oyster Mushroom | Pleurotus ostreatus | Increases protein and fiber content, reduces fat content, softer texture, antioxidant properties. | [178] |
| Shiitake Mushroom | Lentinus edodes | Increases moisture, fiber, nutrition and antioxidant activity, due to methionine and glutamic acid. | [179] |
| Chicken Mushroom | Coprinus comatus | Improves the textural profile and nutritional profile due to its high protein content. | [180] |
2.5. Algae Species
2.5.1. Auxenochlorella protothecoides
2.5.2. Chlorella vulgaris
2.5.3. Spirulina platensis
| Algae Species | Properties | Reference |
|---|---|---|
| Auxenochlorella protothecoides | High in lipids and carotenoids, potential for enhancing nutritional profile and color. | [200] |
| Chlorella vulgaris | High protein content, vitamins, minerals, and omega–3 fatty acids. Improves nutritional profile, shelf life, and sensory attributes. | [201] |
| Spirulina platensis | High protein content, vitamins, minerals, and rich in EAAs; contributes to improved texture, stability, and color. | [202] |
2.6. Fungi Species
2.6.1. Aspergillus oryzae (Koji Mold)
2.6.2. Neurospora intermedia
2.6.3. Fusarium venenatum
| Fungi Species | Properties | Reference |
|---|---|---|
| Aspergillus oryzae | Imparts a fibrous structure with high protein content and uses various substrates for cultivation. | [222] |
| Neurospora intermedia | Enhances chewability, gelation, and palatability when combined with soluble starch at optimal pH levels. | [223] |
| Fusarium venenatum | Mimics meat–like texture and enhance nutritional profile; widely used in commercial mycoprotein products like Quorn. | [224] |
3. Nutritional Amino Acid Profiling
3.1. Nutritional Profile of Plant Protein Sources
| Total Protein Content (g/100g) |
Chicken Breast [225] |
Soy [226] |
Pea [227] |
Mung bean [228] |
Faba bean [229] |
Lupin bean [230] |
Peanut [231] |
Oat [232] |
| Isoleucine (Ile) | 4.64 | 2.0 – 4.2 | 2.3 – 4.5 | 3.9 | 3.9 | 3.5 | 0.8 – 1.5 | 3.8 – 4.1 |
| Histidine (His) | 3.04 | 1.0 – 2.3 | 1.6 – 2.5 | 2.8 | 2.4 | 2.7 | 0.6 – 1.1 | 2.1 – 2.4 |
| Leucine (Leu) | 8.27 | 3.0 – 6.7 | 5.7 – 6.4 | 7.4 | 7.4 | 6.8 | 1.6 – 2.9 | 6.9 – 7.6 |
| Methionine (Met) | 2.84 | 0.5 – 1.1 | 0.3 – 1.1 | 1.3 | 0.8 | 2.8 | 0.3 – 0.5 | 2.2 – 3.3 |
| Lysine (Lys) | 7.55 | 2.7 – 5.3 | 4.7 – 5.7 | 6.2 | 7.0 | 4.5 | 0.9 – 1.5 | 3.5 – 4.1 |
| Phenylalanine (Phe) | 4.14 | 2.1 – 4.5 | 3.7 – 5.5 | 9.0 | 4.1 | 4.9 | 1.3 – 2.2 | 5.0 – 5.5 |
| Tryptophan (Trp) | 1.04 | 0.5 – 1.1 | 0.7 – 1 | 0.6 | 0.8 | 0.6 | 0.2 – 0.4 | 0.8 – 0.9 |
| Threonine (Thr) | 4.51 | 1.7 – 3.1 | 2.5 – 3.9 | 2.0 | 3.4 | 2.9 | 0.8 – 1.1 | 3.1 – 3.4 |
| Valine (Val) | 5.07 | 2.0 –4.0 | 2.7 – 5.0 | 4.6 | 4.3 | 3.2 | 1.0 – 1.8 | 5.2 – 5.8 |
| Alanine (Ala) | 5.33 | 1.9 – 3.5 | 3.2 – 4.3 | 3.6 | 4.1 | 2.9 | 0.9 – 1.7 | 4.4 – 4.9 |
| Arginine (Arg) | 6.44 | 3.1 – 6.6 | 6.6 | 6.4 | 9.4 | 10.0 | 3.0 – 5.0 | 6.1 – 7.1 |
| Aspartic acid (Asp)/Asparagine (Asn) | 9.06 | 10.2 – 12.0 | 8.9 – 11.5 | 8.5 | 10.7 | 9.7 | 3.0 – 4.9 | 7.6 – 8.7 |
| Cysteine (Cys) | 1.01 | 0.6 – 1.0 | 0.2 – 1.0 | 1.3 | 1.3 | 2.8 | 0.3 – 0.6 | 1.9 – 2.5 |
| Glutamic acid/Glutamine (Glu) | 13.52 | 7.8 –17.5 | 12.9 –13.2 | 12.5 | 16.5 | 22 | 5.2 – 8.3 | 20.9 – 27.3 |
| Glycine (Gly) | 4.0 | 1.8 – 3.6 | 2.8 – 4.1 | 3.2 | 4.3 | 3.7 | 1.5 | 4.6 – 5.3 |
| Proline (Pro) | 3.4 | 2.3 – 4.9 | 3.1 – 4.5 | 3.0 | 3.9 | 3.2 | 1.1 – 1.9 | 5.5 – 6.9 |
| Serine (Ser) | 3.99 | 2.3 – 4.5 | 3.6 – 5.3 | 3.8 | 4.6 | 4.3 | 1.2 – 1.7 | 3.8 – 5.6 |
| Tyrosine (Tyr) | 3.54 | 1.5 – 3.2 | 2.6 – 3.8 | 9.0 | 2.7 | 4.9 | 1.0 – 1.6 | 2.7 – 3.5 |
| Total Fat Content (g/100g) | Chicken Breast | Soy |
Pea |
Mung bean | Faba bean | Lupin bean |
Peanut |
Oat |
| Saturated fat (g) | 1.0 – 1.2 | 2.88 | 0.071 | 0.348 | 0.066 | 1.16 | 6.28 | 1.11 |
| Monounsaturated fat (g) | 0.8 – 1.2 | 4.4 | 0.035 | 0.161 | 0.079 | 3.94 | 24.4 | 1.98 |
| Polyunsaturated fat (g) | 0.8 | 11.3 | 0.187 | 0.384 | 0.164 | 2.44 | 15.6 | 2.3 |
| Essential fatty acids–Omega–3 (mg) | 32 – 107 | 1330 | 35 | 27 | 12 | 446 | 3.0 | 100 |
| EFA– Omega–6 (mg) | 559 | 9920 | 152 | 357 | 152 | 2000 | 15600 | 2200 |
| Vitamin Content | Chicken Breast | Soy |
Pea |
Mung bean | Faba bean | Lupin bean |
Peanut |
Oat |
| Vitamin A (RAE, UI) (μg, UI) | 6, 21 | 1, 22 | 38, 765 | 6, 114 | 1, 15 | – | – | – |
| Vitamin B1 (Thiamin) (mg) | 0.07 | 0.874 | 0.266 | 0.233 | 0.097 | 0.64 | 0.64 | 0.46 |
| Vitamin B2 (Riboflavin) (mg) | 0.114 | 0.87 | 0.132 | 0.621 | 0.089 | 0.22 | 0.135 | 0.155 |
| Vitamin B3 (Niacin) (mg) | 9.45 –13.7 | 1.62 | 2.09 | 2.25 | 0.711 | 2.19 | 12.1 | 1.125 |
| Vitamin B5 (Pantothenic acid) (mg) | 0.965 | 0.793 | 0.104 | 1.91 | 0.157 | 0.75 | 1.77 | – |
| Vitamin B6 (mg) | 0.6 –1.0 | 0.377 | 0.169 | 0.382 | 0.072 | 0.357 | 0.348 | 0.1 |
| Vitamin B9 (Folate) (μg) | 4.0 | 375 | 65 | 625 | 104 | 355 | 246 | 56 |
| Vitamin C (Total Ascorbic acid) (mg) | – | 6.0 | 40 | 4.8 | 0.3 | 4.8 | – | – |
| Vitamin D | 0.1 –2.5 | – | – | – | – | – | – | – |
| Vitamin E (alpha–toco–pherol) (mg) | 0.27 | 0.85 | 0.13 | 0.51 | 0.02 | – | 8.33 | 0.42 |
| Vitamin K (Phylloquinone) (μg) | 0.3 | 47 | 24.8 | 9.0 | 2.9 | – | – | 2.0 |
| Mineral Content | Chicken Breast | Soy |
Pea |
Mung bean | Faba bean | Lupin bean |
Peanut |
Oat |
| Calcium (Ca) (mg) | 6.0 –18.0 | 277 | 25 | 132 | 36 | 176 | 92 | 52 |
| Iron (Fe) (mg) | 1.04 –1.07 | 15.7 | 1.47 | 6.74 | 1.5 | 4.36 | 4.58 | 4.25 |
| Magnesium (Mg) (mg) | 29 | 280 | 33 | 189 | 43 | 198 | 168 | 138 |
| Phosphorous (P) (mg) | 228 | 704 | 108 | 367 | 125 | 440 | 376 | 410 |
| Potassium (K) (mg) | 343 –460 | 1880 | 244 | 1250 | 268 | 1010 | 705 | 362 |
| Sodium (Na) (mg) | 74 | 2.0 | 5.0 | 15 | 5.0 | 15 | 18 | 6.0 |
| Zinc (Zn) (mg) | 1.0 –1.6 | 4.89 | 1.24 | 2.68 | 1.01 | 4.75 | 3.27 | 3.64 |
| Copper (Cu) (mg) | 0.049 | 1.66 | 0.176 | 0.941 | 0.259 | 1.02 | 1.14 | 0.391 |
| Manganese (Mn) (mg) | 0.017 | 2.52 | 0.41 | 1.04 | 0.421 | 2.38 | 1.93 | – |
| Selenium (Se) (μg) | 28.4 | 17.8 | 1.8 | 8.2 | 2.6 | 8.2 | 7.3 | 28.9 |
| Nutritional Content | Chicken Breast | Soy |
Pea |
Mung bean | Faba bean | Lupin bean |
Peanut |
Oat |
| Ash (g/100g) | 1.06 | 4.87 | 0.87 | 3.32 | 0.81 | 3.28 | 2.33 | – |
| Carbohydrates (g/100g) | – | 30.2 | 14.4 | 62.6 | 19.6 | 40.4 | 16.1 | 67.7 |
| Total Dietary Fibers (g/100g) | – | 9.3 | 5.7 | 16.3 | 5.4 | 18.9 | 8.5 | 10.1 |
| Total Sugars (Glucose, Fructose, Lactose, Maltose, Galactose) (g/100g) | – | 7.33 | 5.67 | 6.6 | 1.82 | – | 4.72 | 0.99 |
| Calories (kcal) | 165 | 446 | 81 | 347 | 460 | 371 | 567 | 379 |
3.2. Nutritional Profile of Mushroom/Algae/Fungi Species
| Total Protein Content (g/100g) |
Chicken Breast [225] |
A. bisporus [233] |
P. ostreatus [234] |
L. edodes [168] |
A. oryzae . [209] |
A. protothecoides [235] |
P. limosum [236] |
F. venenatum [237] |
| Isoleucine (Ile) | 4.64 | 1.37 | 1.02 | 0.62 | 13.2 | 0.31 | NS | 1.51 |
| Histidine (His) | 3.04 | 0.17 | 0.27 | 0.84 | 0.75 | 0.09 – 0.96 | NS | 7.22 |
| Leucine (Leu) | 8.27 | 1.20 | 1.23 | 1.29 | 2.5 | NS | NS | 1.90 |
| Methionine (Met) | 2.84 | 0.22 | 0.26 | 0.42 | 0.59 | Low | NS | 4.21 |
| Lysine (Lys) | 7.55 | 1.09 | 1.18 | 1.91 | 1.83 | 0.184 – 0.224 | NS | 5.81 |
| Phenylalanine (Phe) | 4.14 | 0.19 | 0.25 | 0.83 | 4.7 | Low | NS | 3.01 |
| Tryptophan (Trp) | 1.04 | 0.3 | 0.4 | 0.28 | 0.15 | 0.36 | NS | NS |
| Threonine (Thr) | 4.51 | 0.36 | 0.38 | 1.01 | 0.3 | 0.23 | NS | 3.31 |
| Valine (Val) | 5.07 | 0.68 | 0.42 | 1.05 | 1.27 | NS | NS | 6.05 |
| Alanine (Ala) | 5.33 | 0.77 | 0.62 | 1.17 | 2.52 | NS | NS | 2.41 |
| Arginine (Arg) | 6.44 | 0.74 | 0.23 | 2.45 | 1.72 | NS | NS | 7.12 |
| Aspartic acid (Asp)/Asparagine (Asn) | 9.06 | 1.92 | 0.87 | 1.73 | 1.69 | NS | NS | NS |
| Cysteine (Cys) | 1.01 | 0.14 | 0.18 | 0.08 | 0.5 –1.0 | NS | NS | 2.11 |
| Glutamic acid/Glutamine (Glu) | 13.52 | 1.06 | 0.98 | 4.93 | 5.7 | 0.56 – 6.88 | NS | NS |
| Glycine (Gly) | 4.0 | 0.6 | 0.47 | 0.89 | 0.57 | NS | NS | 3.50 |
| Proline (Pro) | 3.4 | 0.69 | 0.68 | 0.82 | 2.5 | NS | NS | NS |
| Serine (Ser) | 3.99 | 0.62 | 0.26 | 1.04 | 6.19 | NS | NS | NS |
| Tyrosine (Tyr) | 3.54 | 0.28 | 0.65 | 0.54 | 0.72 | Low | NS | NS |
| Total Fat Content | Chicken Breast | A. bisporus | P. ostreatus | L. edodes | A. oryzae | A. protothecoides | P. limosum | F. venenatum |
| Total Fat Content (g) | 3.5 – 3.6 | 0.34 – 0.41 | 0.41 | 0.49 – 3.0 | 3 – 5 | 21 | 2.9 | 2.9 |
| Saturated fat (g) | 1.0 – 1.2 | 0.09 | 0.06 | ~0.23 | ~1.2 | NS | 0.7 | 0.7 |
| Monounsaturated fat (g) | 0.8 – 1.2 | 0.21 – 0.23 | 0.03 | ~0.3 | NS | NS | 0.5 | 0.5 |
| Polyunsaturated fat (g) | 0.8 | 0.07 – 0.1 | 0.12 | ~0.2– 0.24 | NS | High | 1.4 | 1.8 |
| Essential fatty acids–Omega–3 (mg) | 0.032– 0.107 | 0.1 | Low | Low | Low | High | NS | 6.9 |
| EFA– Omega–6 (mg) | 0.55 – 3.5 | 53 – 68 | 3.1 – 5.4 | 1.04 | 0.22 | 27.6 | 3.0 | 3.0 |
| Vitamin Content | Chicken Breast | A. bisporus | P. ostreatus | L. edodes | A. oryzae | A. protothecoides | P. limosum | F. venenatum |
| Vitamin A (RAE, UI) (mg, UI) | 0.006, 21 | 0 | 0.23 – 21, 2.93 | 0.01 | NS | NS | NS | 0 |
| Vitamin B1 (Thiamin) (mg) | 0.07 | 1.05 | NS | 0.05 | 0.2 | 0.1 | NS | 0.07 |
| Vitamin B2 (Riboflavin) (mg) | 0.114 | 0.42 | NS | 0.15 | 0.3 | 0.5 | NS | 0.114 |
| Vitamin B3 (Niacin) (mg) | 9.45 – 13.7 | 4.55 | NS | 0.99 | 3.6 | 2.0 | NS | ~13.7 |
| Vitamin B5 (Pantothenic acid) (mg) | 0.965 | 1.75 | NS | 0.5 | 1.5 | 0.5 | NS | 1.58 |
| Vitamin B6 (mg) | 0.6 – 1.0 | 0.082 | NS | 0.1 | 0.1 | 0.2 | NS | 0.921 |
| Vitamin B9 (Folate) (μg) | 4.0 | NS | NS | 21.51 | 500 | 100 | NS | NS |
| Vitamin C (Total Ascorbic acid) (mg) | – | NS | 16.46 | 2.1 | NS | 1.0 | NS | 0 |
| Vitamin D (μg) | 0.1 – 2.5 | 0.2 | 29 | NS | NS | NS | NS | 0.025 |
| Vitamin E (alpha–toco–pherol) (mg) | 0.27 | NS | 21.50 | NS | NS | 0.5 | NS | NS |
| Vitamin K (Phylloquinone) (μg) | 0.3 | NS | NS | NS | NS | NS | NS | NS |
| Mineral Content | Chicken Breast | A. bisporus | P. ostreatus | L. edodes | A. oryzae | A. protothecoides | P. limosum | F. venenatum |
| Calcium (Ca) (mg) | 6.0 –18 | 0.047 | 342 – 410 | 18 | 2.0 | 20 | NS | 5 |
| Iron (Fe) (mg) | 1.04 – 1.07 | 0.013 | 17 – 21 | 0.9 | 10 | 5.0 | NS | 0.45 |
| Magnesium (Mg) (mg) | 29 | 115 | 7.0 | 40.7 | NS | NS | NS | NS |
| Phosphorous (P) (mg) | 228 | 860 | 695 – 1060 | 778 | NS | NS | NS | NS |
| Potassium (K) (mg) | 343 – 460 | 4015 | 2080 – 2280 | 356 | 230 | 300 | NS | 255 |
| Sodium (Na) (mg) | 74 | 3.0 | 193 | NS | NS | NS | NS | NS |
| Zinc (Zn) (mg) | 1.0 – 1.6 | 0.013 | 12.96 | 1.0 | 1.5 | 1.0 | NS | 0.8 |
| Copper (Cu) (mg) | 0.049 | 52 – 350 | 91 – 116 | 14.8 | NS | NS | NS | NS |
| Manganese (Mn) (mg) | 0.017 | 4.8 | 16 – 23 | 2.0 | NS | NS | NS | NS |
| Selenium (Se) (μg) | 28.4 | NS | NS | 46.1 | NS | NS | NS | NS |
| Nutritional Content | Chicken Breast | A. bisporus | P. ostreatus | L. edodes | A. oryzae | A. protothecoides | P. limosum | F. venenatum |
| Ash (g/100g) | 1.06 | 1.35 | 8.22 | 6.0 | 4.0 | 4.0 | 4.0 | 2.0 |
| Carbohydrates (g/100g) | – | 3.3 | 43.42 | 64 | 50 | 20 | 50 | 12 |
| Total Dietary Fibers (g/100g) | – | 11.01 | 21 – 47 | 33.6 | 14 | NS | 30 | 6.0 |
| Total Sugars (Glucose, Fructose, Lactose, Maltose, Galactose) (g/100g) | – | 14.08 | 1.11 | 4.40 | ~2.0 | 3.5 | NS | 1.0 |
| Calories (kcal) | 165 | 22 | 33 | 34 | 300 | 300 | 300 | 93 |
4. Formulatory Composition
4.1. Binding Agents
4.1.1. Carboxymethyl Cellulose (CMC)
4.1.2. Methylcellulose (MC)
4.1.3. Xanthan Gum (X)
4.1.4. Carrageenan (CA)
4.1.5. Guar Gum (GuG)
4.1.6. Gellan Gum (GeG)
4.1.7. Other Hydrocolloid–Based Binders in PBMAs
| Hydrocolloids (Food additive code) | Latin Name | Properties | Reference |
|---|---|---|---|
| Carrageenan (E407) |
Chondrus crispus | Acts as a binder, stabilizer, and moisture retainer.
|
[264] |
| Xanthan gum (E415) |
Xanthomonas campestris | Exhibits pseudoplasticity. Maintains stability over a wide temperature (10–80°C) and pH (5–10) range. | [265] |
| Guar gum (E412) |
Cyamopsis tetragonoloba | Functions as a thickener, texture enhancer and fat substitute. Maintains stability in a wide pH range (1.0–10.5). | [266] |
| Konjac gum (E425) | Amorphophallus konjac | Exhibits texture control and forms thermo–reversible and irreversible gels. Stable at 85°C in the presence of mild alkali (pH 9–10). | [267] |
| Low acyl Gellan gum/ Gelzan (E418) | Sphingomonas elodea | Forms strong, brittle gels that are heat– and pH–stable, providing a fibrous structure. | [268] |
| Locust Bean Gum (E410) | Ceratonia siliqua | Functions as a thickener, emulsifier, and stabilizer. Has a neutral flavor and prebiotic properties that improve hydration. | [269] |
| Gum Arabic/Acacia (E414) | Senegalia senegal | Highly water-soluble, low viscosity, and optimal at pH 3.5. Structure–forming transitions shift to higher pH as protein content increases. | [270] |
| Flaxseed gum | Linum usitatissimum. | Improves mouthfeel, moisture retention, and uniform texture while preventing ingredient separation. | [271] |
| Starches | Latin Name | Properties | Reference |
|---|---|---|---|
| Corn starch | Zea mays | Enhances viscosity and elasticity. Exhibits WAC and gelatinization properties. Reduces off–flavors. | [272] |
| Modified starch | Amylum modificatum | Enhances viscosity, elasticity, resilience, chewiness, and ductility. Reduces off–flavors and off–colors. | [273] |
| Potato starch | Solanum tuberosum | Increases fibrous content through thermo–irreversible gelatinization and freeze-thawing processes. | [274] |
| Wheat starch | Triticum vulgare | Exhibits thermal stability, retrogradation properties, and swelling index. It develops fibrousness due to glutenins and gliadins ratio. | [275] |
| Maltodextrin | – | Functions as a thickener, binding agent, and mouthfeel enhancer. Provides a creamy, spreadable texture similar to hydrogenated fat. | [276] |
| Transglutaminase | – | Induces crosslinks between protein molecules, improving binding properties and cutting strength. Enhances the overall texture. | [277] |
| Cross–linking Gelling Agents | Latin Name | Properties | Reference |
|---|---|---|---|
| Calcium ions (Ca2+, lactate, acetate) + Sodium alginate/ alginin | – | Functions as an adhesive and forms a cold–set gel in the presence of divalent cations, improving texture and moisture retention. | [278] |
| Agar | Gracilaria verrucosa | Serves as a plant–based alternative to gelatin. Provides palatability and does not require high sugar concentrations to form a gel. | [279] |
| Pectin | Saccharomyces cerevisiae | High– or low–methyl–esterified pectin forms gels when combined with sugar and acid. | [280] |
4.2. Coloring Agents
| Coloring Agents | Latin Name | Shade | Pigments | Reference |
|---|---|---|---|---|
| Annatto extract/ Achiote | Bixa orellana | Yellow–Orange to Red | Carotenoids – Bixin and Norbixin | [286] |
| Caramel | Calamellus | Golden Brown | Heated sugars – Caramelization | [287] |
| Malt extract | Hordeum vulgare | Brown | Heated grains – Maillard reaction | [288] |
| Beet extract | Beta vulgaris | Red–Purple | Betalains – Betanin | [289] |
| Elderberry extract | Sambucus nigra | Purple–Red | Anthocyanins | [290] |
| Lycopene | Lycopersicon esculentum | Red | Carotenoids | [291] |
| Paprika | Capsicum annuum | Yellow–Orange to Red | Carotenoids – Capsanthin and Capsorubin | [292] |
| Turmeric |
Curcuma longa | Right Yellow | Curcuminoids | [293] |
| Spirulina extract | Arthrospira platensis | Blue–Green | Phycocyanins | [294] |
| Chlorophyllin | Chlorophylle | Green | Chlorophyll | [295] |
| Pomegranate concentrate | Punica granatum | Red–Purple | Anthocyanins and Ellagitannins | [296] |
4.3. Chemical Agents
| Cellulose | Chemical Formula | Properties | Reference |
|---|---|---|---|
| Carboxymethyl cellulose (CMC) | (C6H10O5)n | Odorless, white, or yellowish powder. Preserves structure and stops ingredient separation. | [304] |
| Methylcellulose (MC) (E461) | C20H38O11 | Known for its binding capacity and unique reversible thermal gelation. Functions as a stabilizer and emulsifier. | [241] |
| Hydroxypropyl methylcellulose (HPMC) (E464) | C56H108O30 | Provides binding, gelling, texture improvement, and stabilization. Functions as an emulsifier with thermal gelation properties. | [305] |
4.4. Flavoring Agents
| Powders and Pulps | Latin Name | Properties | Reference |
|---|---|---|---|
| Purple potato powder | Ipomoea batatas | Provides natural color, enhances texture, and is rich in antioxidants. | [311] |
| Konjac powder | Amorphophallus konjac | Functions as a gelling agent, imparts a chewy texture, and is low in calories. | [312] |
| Meat flavor powder |
– | Adds a savory, umami–rich flavor. | [313] |
| Paprika powder |
Capsicum annuum | Contributes color with a mild, slightly sweet flavor. | [314] |
| Cumin/Jeera powder |
Cuminum cyminum | Imparts an earthy, warm, and slightly nutty flavor. | [315] |
| Citric acid powder | Acidum citricum | Provides a tangy flavor and helps extend shelf life. | [316] |
| Ascorbic acid powder | Acidum ascorbicum | A source of vitamin C, prevents oxidation, and maintains freshness. | [317] |
| Mustard powder | Brassica juncea | Delivers a sharp, tangy, and slightly spicy flavor. | [318] |
| Onion powder |
Allium cepa | Adds a rich, savory base flavor. | [313] |
| Garlic powder | Allium sativum | Enhances depth and richness of flavor. | [314] |
| Panela/Jaggery powder | Saccharum officinarum | Provides a subtle sweetness that balances flavors. | [319] |
| Tomato powder | Solanum lycopersicum | Enhances natural color and imparts a rich, tangy flavor. | [320] |
| Pepper powder | Piper nigrum | Adds depth with a bold, spicy kick. | [321] |
| Flaxseed powder | Linum usitatissimum | High in omega–3 fatty acids and dietary fiber, contributing to texture and nutrition. | [322] |
| Ground jackfruit pulp | Artocarpus heterophyllus | Provides a fibrous texture and is a good source of dietary fiber. | [323] |
| Flavoring Agents | Properties | Reference |
|---|---|---|
| Monosodium glutamate/ Ajinomoto | A sodium salt of glutamic acid that enhances savory, umami flavors. | [324] |
| Yeast extracts powder | Provides umami flavor and helps mask bitter or earthy off–notes. | [301] |
| Soy leghemoglobin | A heme protein derived from genetically modified yeast; it enhances the meaty taste in plant–based products. | [325] |
| Beet and lemon juice | Adds natural red color and brightness to foods. | [310] |
| Cooked onion and carrot juice concentrates | Enhances savory depth, while natural carrot pigments improve color. | [326] |
| Salt | Enhances overall flavor by balancing and intensifying taste perception. | [327] |
| Sweeteners | Properties | Reference |
|---|---|---|
| Dextrose | 20% less sweet than sucrose, contributes to the Maillard reaction and caramelization when combined with cysteine. | [328] |
| Glucose | Actively participates in the Maillard reaction, enhancing browning and flavor development. | [329] |
| Sucrose | Serves as a bulking agent and preservative. Hydrolyzed into glucose and fructose during processing. | [330] |
| Fructose | Undergoes the Maillard reaction, though its effect is less pronounced compared to glucose and dextrose. | [331] |
| Sugar alcohols (erythritol, sorbitol) | Lower glycemic impact, contribute to a smooth, creamy texture, help retain moisture, and prevent drying. | [332] |
| Brown Sugar | Imparts a rich caramel flavor due to its molasses content. | [333] |
| Emulsifying Agents | Latin Name | Properties | Reference |
|---|---|---|---|
| Corn oil | Maydis oleum raffinatum | Rich in PUFAs, primarily linoleic acid (58–62%). Contains high levels of phytosterols (8,300–25,500 ppm) and tocopherols (1,130–1,830 ppm). | [334] |
| Soy oil | Soiae oleum raffinatum | Contains PUFAs like linoleic acid (48–58%) and isoflavones. | [335] |
| Peanut/ Groundnut oil |
Arachis hypogaea | High in monounsaturated fats, primarily oleic acid (45–72%). Mild flavor with a high smoke point. Used in oleogel production for fat stabilization. | [336] |
| Rapeseed oil | Brassica campestris | Rich in unsaturated fats, particularly oleic acid. Contributes to smooth texture and stability. | [337] |
| Canola oil | Brassica napus | Contains 7% saturated fat, monounsaturated fat, and ALA omega–3 fatty acids. It has a mild flavor, and a high smoke point. | [338] |
| Sunflower oil |
Helianthus annuus | High in PUFAs, mainly linoleic acid (55–75%). High smoke point and naturally rich in vitamin E. | [339] |
| Safflower oil | Carthamus tinctorius | Contains high levels of PUFAs, mainly linoleic acid. Enhances texture and moisture retention. | [340] |
| Palm oil |
Elaeis guineensis | Semi–solid at room temperature, contributing to moisture retention and succulence. | [341] |
| Red palm oil | Elaeis guineensis (or) Elaeis oleifera | Contains saturated and monounsaturated fats, with high palmitic acid and carotenoids. Enhances mouthfeel, juiciness, and richness in formulations. | [342] |
| Coconut oil | Cocos Nucifera | 92% saturated fat, making it a stable fat source. Used in 3D–printable fat analogues for meat substitutes when combined with glucomannan. | [343] |
| Orange oil | Citrus sinensis | Acts as a masking agent to reduce the bean odor of soy–based products. Exhibits strong antioxidant activity (DPPH radical scavenging activity). | [344] |
5. Existing Technology
5.1. Single Screw Extrusion
5.2. Twin Screw Extrusion
5.3. High Moisture Extrusion
5.4.3. D–Bioprinting
5.5.4. D–Bioprinting
5.6. Shear Cell Technology

5.7. Electrospinning
5.8. Antisolvent Precipitation
5.9. Mechanical Elongation
6. Conclusion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflict of Interest
References
- FAO (2018) The future of food and agriculture - Alternative pathways to 2050. 224.
- Singh U, Tiwari P, Kelkar S, et al. (2023) Edible mushrooms: A sustainable novel ingredient for meat analogs. eFood 4:e122. [CrossRef]
- Yuan X, Jiang W, Zhang D, et al. (2022) Textural, sensory and volatile compounds analyses in formulations of sausages analogue elaborated with edible mushrooms and soy protein isolate as meat substitute. Foods 11:52. [CrossRef]
- Gamarra-Castillo O, Echeverry-Montaña N, Marbello-Santrich A, et al. (2022) Meat Substitute Development from Fungal Protein (Aspergillus oryzae). Foods 11:2940. [CrossRef]
- Ritchie H (2019) Food production is responsible for one-quarter of the world’s greenhouse gas emissions. Our World in Data.
- Ederer P, Baltenweck I, Blignaut JN, et al. (2023) Affordability of meat for global consumers and the need to sustain investment capacity for livestock farmers. Anim Front 13:45. [CrossRef]
- 2015; IARC (2015) Red Meat and Processed Meat IARC Monographs on the Evaluation of Carcinogenic Risks to Humans Volume 114.
- Mazumder MAR, Sukchot S, Phonphimai P, et al. (2023) Mushroom–Legume-Based Minced Meat: Physico-Chemical and Sensory Properties. Foods 12:. [CrossRef]
- Zahari I, Ferawati F, Helstad A, et al. (2020) Development of High-Moisture Meat Analogues with Hemp and Soy Protein Using Extrusion Cooking. Foods 2020, Vol 9, Page 772 9:772. [CrossRef]
- Caporgno MP, Böcker L, Müssner C, et al. (2020) Extruded meat analogues based on yellow, heterotrophically cultivated Auxenochlorella protothecoides microalgae. Innovative Food Science & Emerging Technologies 59:102275. [CrossRef]
- Caputo V, Sun J, Staples AJ, Taylor H (2024) Market outlook for meat alternatives: Challenges, opportunities, and new developments. Trends Food Sci Technol 148:104474. [CrossRef]
- Małecki J, Muszy S, Sołowiej BG, et al. (2021) Proteins in Food Systems—Bionanomaterials, Conventional and Unconventional Sources, Functional Properties, and Development Opportunities. Polymers (Basel) 13:2506. [CrossRef]
- Taghian Dinani S, Broekema NL, Boom R, van der Goot AJ (2023) Investigation potential of hydrocolloids in meat analogue preparation. Food Hydrocoll 135:108199. [CrossRef]
- Mattice KD, Marangoni AG (2020) Comparing methods to produce fibrous material from zein. Food Research International 128:108804. [CrossRef]
- Ahmad MI, Farooq S, Ali U, et al. (2024) Molecular phenomena associated with the formation of fibrous structure of plant-based meat analogues. Trends Food Sci Technol 153:104743. [CrossRef]
- Gulkirpik E, Donnelly A, Nowakunda K, et al. (2023) Evaluation of a low-resource soy protein production method and its products. Front Nutr 10:1067621. [CrossRef]
- Choi HW, Hahn J, Kim HS, Choi YJ (2025) Microstructural and textural characteristics of blend gels and high-moisture meat analogs of soy protein isolate and faba bean protein concentrate. Food Chem 467:142184. [CrossRef]
- Jeon YH, Gu BJ, Ryu GH (2023) Investigating the Potential of Full-Fat Soy as an Alternative Ingredient in the Manufacture of Low- and High-Moisture Meat Analogs. Foods 2023, Vol 12, Page 1011 12:1011. [CrossRef]
- Zhang T, Dou W, Zhang X, et al. (2021) The development history and recent updates on soy protein-based meat alternatives. Trends Food Sci Technol 109:702–710. [CrossRef]
- Toomer OT, Oviedo-Rondón EO, Ali M, et al. (2024) Full-Fat Soybean Meals as an Alternative Poultry Feed Ingredient-Feed Processing Methods and Utilization-Review and Perspective. Animals (Basel) 14:. [CrossRef]
- Kumari S, Dambale AS, Samantara R, et al. (2025) Introduction, History, Geographical Distribution, Importance, and Uses of Soybean (Glycine max L.). Soybean Production Technology 1–17. [CrossRef]
- Lin W, Barbut S (2025) Effects of 0–12% soy proteins (four texturized and one isolate) on a lean hybrid meat system: cooking loss, texture, dynamic rheology, microstructure, and T2 NMR. Applied Food Research 5:100747. [CrossRef]
- Cho SY, Ryu GH (2021) Effects of Oyster Mushroom Addition on Physicochemical Properties of Full Fat Soy-based Meat Analog by Extrusion Process. Food Engineering Progress 25:85–94. [CrossRef]
- Hafizur Rahman Bhuiyan M, Yeasmen N, Ngadi M (2024) Restructuring plant-derived composites towards the production of meat-analog based coated fried food. Food Chem 443:138482. [CrossRef]
- Schreuders FKG, Dekkers BL, Bodnár I, et al. (2019) Comparing structuring potential of pea and soy protein with gluten for meat analogue preparation. J Food Eng 261:32–39. [CrossRef]
- Shanthakumar P, Klepacka J, Bains A, et al. (2022) The Current Situation of Pea Protein and Its Application in the Food Industry. Molecules 27:5354. [CrossRef]
- Rogers H, Dora M, Tsolakis N, Kumar M (2024) Plant-based Food Supply Chains: Recognising Market Opportunities and Industry Challenges of Pea Protein. Applied Food Research 4:100440. [CrossRef]
- Zhang L, Apea-Bah FB, Chen X, et al. (2023) The physicochemical and structural properties and in vitro digestibility of pea starch isolated from flour ground by milling and air classification. Food Chem 419:136086. [CrossRef]
- Zhang S, Huang W, Roopesh MS, Chen L (2022) Pre-treatment by combining atmospheric cold plasma and pH-shifting to prepare pea protein concentrate powders with improved gelling properties. Food Research International 154:111028. [CrossRef]
- Sha L, Koosis AO, Wang Q, et al. (2021) Interfacial dilatational and emulsifying properties of ultrasound-treated pea protein. Food Chem 350:129271. [CrossRef]
- Ge J, Sun C, Chang Y, et al. (2023) Understanding the differences in heat-induced gel properties of twelve legume proteins: A comparative study. Food Research International 163:112134. [CrossRef]
- Li Y, Qi X, Rong L, et al. (2024) Effect of gellan gum on the rheology, gelling, and structural properties of thermally induced pea protein isolate gel. Food Hydrocoll 147:109379. [CrossRef]
- Sajib M, Forghani B, Kumar Vate N, Abdollahi M (2023) Combined effects of isolation temperature and pH on functionality and beany flavor of pea protein isolates for meat analogue applications. Food Chem 412:135585. [CrossRef]
- Johansson M, Xanthakis E, Langton M, et al. (2021) Mixed legume systems of pea protein and unrefined lentil fraction: Textural properties and microstructure. LWT 144:111212. [CrossRef]
- Wang J, Kadyan S, Ukhanov V, et al. (2022) Recent advances in the health benefits of pea protein (Pisum sativum): bioactive peptides and the interaction with the gut microbiome. Curr Opin Food Sci 48:100944. [CrossRef]
- Sidiq M, Muzzaffar S, Masoodi FA, Irfan S (2024) Impact of ultrasonication on the physicochemical, structural, thermal and functional properties of Mung bean protein. Measurement: Food 16:100205. [CrossRef]
- Brishti FH, Chay SY, Muhammad K, et al. (2021) Texturized mung bean protein as a sustainable food source: Effects of extrusion on its physical, textural and protein quality. Innovative Food Science & Emerging Technologies 67:102591. [CrossRef]
- Han F, Moughan PJ, Li J, Pang S (2020) Digestible Indispensable Amino Acid Scores (DIAAS) of Six Cooked Chinese Pulses. Nutrients 12:3831. [CrossRef]
- Hammer L, Moretti D, Bétrix CA, et al. (2024) In vitro DIAAS of Swiss soybean cultivars using the INFOGEST model: Increase in protein quality from soybean to soymilk and tofu. Food Research International 178:113947. [CrossRef]
- Herreman L, Nommensen P, Pennings B, Laus MC (2020) Comprehensive overview of the quality of plant- And animal-sourced proteins based on the digestible indispensable amino acid score. Food Sci Nutr 8:5379–5391. [CrossRef]
- Siddiquy M, Ghamry M, Golshany H, et al. (2024) Structural and functional characterization of mung bean protein-peach gum conjugate through the Maillard reaction as a novel encapsulation agent. Prog Org Coat 188:108201. [CrossRef]
- Guo R, Sun B, Zhu Y, et al. (2024) Low-moisture extruded mung bean protein isolate and wheat gluten: Structure, techno-functional characteristics and establishment of rehydration kinetics of the products. LWT 210:116844. [CrossRef]
- De Angelis D, Opaluwa C, Pasqualone A, et al. (2023) Rheological properties of dry-fractionated mung bean protein and structural, textural, and rheological evaluation of meat analogues produced by high-moisture extrusion cooking. Curr Res Food Sci 7:100552. [CrossRef]
- Kartikeyan A, Vasudevan V, Peter AJ, et al. (2022) Effect of incubation period on the glycosylated protein content in germinated and ungerminated seeds of mung bean (Vigna radiata (L.) Wilczek). Int J Biol Macromol 217:633–651. [CrossRef]
- Hou D, Zhao Q, Yousaf L, et al. (2020) Consumption of mung bean (Vigna radiata L.) attenuates obesity, ameliorates lipid metabolic disorders and modifies the gut microbiota composition in mice fed a high-fat diet. J Funct Foods 64:103687. [CrossRef]
- Augustin MA, Cole MB (2022) Towards a sustainable food system by design using faba bean protein as an example. Trends Food Sci Technol 125:1–11. [CrossRef]
- Tuccillo F, Kantanen K, Wang Y, et al. (2022) The flavor of faba bean ingredients and extrudates: Chemical and sensory properties. Food Research International 162:112036. [CrossRef]
- Saldanha do Carmo C, Knutsen SH, Malizia G, et al. (2021) Meat analogues from a faba bean concentrate can be generated by high moisture extrusion. Future Foods 3:100014. [CrossRef]
- De Silva D, Liu Y, Smith MA, et al. (2024) Distribution of Vicine, Convicine and Levodopa in Faba Bean Plant Tissues Determined by UltraHigh Performance Liquid Chromatography-Electrospray Ionization Mass Spectrometry. Journal of Chromatography Open 5:100127. [CrossRef]
- Choi YM, Shin MJ, Lee S, et al. (2024) Anti-nutrient factors, nutritional components, and antioxidant activities of faba beans (Vicia faba L.) as affected by genotype, seed traits, and their interactions. Food Chem X 23:101780. [CrossRef]
- Liu C, Pei R, Heinonen M (2022) Faba bean protein: A promising plant-based emulsifier for improving physical and oxidative stabilities of oil-in-water emulsions. Food Chem 369:130879. [CrossRef]
- Fan Y, Annamalai PK, Bhandari B, Prakash S (2025) Characteristics of faba bean protein-based high-moisture meat analogues incorporating brewers’ spent grain through extrusion. Innovative Food Science & Emerging Technologies 100:103919. [CrossRef]
- Elshamy AF, Rosentrater KA, Ghnimi S, et al. (2025) High-moisture meat analogs produced from dry-fractionated Faba bean, yellow pea, and functional soy proteins: Effects of mixture design and extrusion parameters on texture properties. Innovative Food Science & Emerging Technologies 100:103927. [CrossRef]
- Kamani MH, Liu J, Fitzsimons SM, et al. (2024) Determining the influence of fava bean pre-processing on extractability and functional quality of protein isolates. Food Chem X 21:101200. [CrossRef]
- Kantanen K, Chamlagain B, Succi V, et al. (2024) Fermentation of faba bean flour as a pre-treatment in high-moisture extrusion for simultaneous fortification of vitamin B12 and reduction of raffinose oligosaccharides in meat analogues. Future Foods 10:100518. [CrossRef]
- Shrestha S, Hag L van t., Haritos VS, Dhital S (2021) Lupin proteins: Structure, isolation and application. Trends Food Sci Technol 116:928–939. [CrossRef]
- Kebede YS, Teferra TF (2023) Isoelectric point isolation and characterization of proteins from lupine cultivars as influenced by chemical and thermal treatments. Heliyon 9:e14027. [CrossRef]
- Palanisamy M, Töpfl S, Berger RG, Hertel C (2019) Physico-chemical and nutritional properties of meat analogues based on Spirulina/lupin protein mixtures. European Food Research and Technology 245:1889–1898. [CrossRef]
- Abreu B, Lima J, Rocha A (2023) Consumer Perception and Acceptability of Lupin-Derived Products: A Systematic Review. Foods 12:1241. [CrossRef]
- Mota J, Casimiro S, Fernandes J, et al. (2022) Lupin Protein Concentrate as a Novel Functional Food Additive That Can Reduce Colitis-Induced Inflammation and Oxidative Stress. Nutrients 14:2102. [CrossRef]
- Kamran F, Phillips M, Harman DG, Reddy N (2023) Antioxidant activities of lupin (Lupinus angustifolius) protein hydrolysates and their potential for nutraceutical and functional foods. Food Chemistry Advances 2:100297. [CrossRef]
- Yaver E, Bilgiçli N (2021) Ultrasound-treated lupin (Lupinus albus L.) flour: Protein- and fiber-rich ingredient to improve physical and textural quality of bread with a reduced glycemic index. LWT 148:. [CrossRef]
- Ramos-Diaz JM, Oksanen S, Kantanen K, et al. (2023) Characterization of texturized meat analogues containing native lupin flour and lupin protein concentrate/isolate. Heliyon 9:. [CrossRef]
- Ayalew DB, Abera BD, Adiss YL (2024) Effect of roasting temperature and soaking time on the nutritional, antinutrional and sensory properties of protein-based meat analog from lupine. Heliyon 10:e33122. [CrossRef]
- Kazemzadeh Pournaki S, Biswas A, Hall C (2024) Effects of storage conditions on chemistry and technological properties of different cultivars of Chickpea. J Agric Food Res 16:101066. [CrossRef]
- Sehar S, Rabail R, Munir S, et al. (2023) An insight into anticancer perspectives of chickpea bioactive compounds. Food Chemistry Advances 3:100453. [CrossRef]
- Madurapperumage A, Tang L, Thavarajah P, et al. (2021) Chickpea (Cicer arietinum L.) as a Source of Essential Fatty Acids – A Biofortification Approach. Front Plant Sci 12:734980. [CrossRef]
- Begum N, Khan QU, Liu LG, et al. (2023) Nutritional composition, health benefits and bio-active compounds of chickpea (Cicer arietinum L.). Front Nutr 10:1218468. [CrossRef]
- Xing Q, Dekker S, Kyriakopoulou K, et al. (2020) Enhanced nutritional value of chickpea protein concentrate by dry separation and solid state fermentation. Innovative Food Science & Emerging Technologies 59:102269. [CrossRef]
- Wetterauw K, Spitzer M, Nafingah R, et al. (2024) Dry fractionation of chickpea flour: Impact of de-oiling and flow aids. Powder Technol 446:120180. [CrossRef]
- Liu R, Flanagan BM, Ratanpaul V, Gidley MJ (2025) Valorising legume protein extraction side-streams: Isolation and characterisation of fibre-rich and starch-rich co-products from wet fractionation of five legumes. Food Hydrocoll 164:111191. [CrossRef]
- Yang JS, Dias FFG, Pham TTK, et al. (2024) A sequential fractionation approach to understanding the physicochemical and functional properties of aqueous and enzyme-assisted aqueous extracted black bean proteins. Food Hydrocoll 146:109250. [CrossRef]
- Ma Y, Zhang J, He J, et al. (2023) Effects of high-pressure homogenization on the physicochemical, foaming, and emulsifying properties of chickpea protein. Food Research International 170:112986. [CrossRef]
- Kong Y, Toh NP, Wu Y, Huang D (2023) Trypsin-treated chickpea protein hydrolysate enhances the cytoaffinity of microbeads for cultured meat application. Food Research International 173:113299. [CrossRef]
- Czapalay ES, Dobson S, Marangoni AG (2025) Legume Starch and Flour-Based Emulsion Gels as Adipose Tissue Mimetics in Plant-Based Meat Products. Future Foods 100578. [CrossRef]
- Lee EJ, Hong GP (2023) Effect of the double heating cycle on the thermal gelling properties of vicilin fractions from soy, mung bean, red bean and their mixture with soy glycinin. Food Hydrocoll 137:108370. [CrossRef]
- Kornet R, Yang J, Venema P, et al. (2022) Optimizing pea protein fractionation to yield protein fractions with a high foaming and emulsifying capacity. Food Hydrocoll 126:107456. [CrossRef]
- Seetapan N, Raksa P, Limparyoon N, et al. (2023) High moisture extrusion of meat analogues using mung bean (Vigna radiata L.) protein and flour blends: investigations on morphology, texture and rheology. Int J Food Sci Technol 58:1922–1930. [CrossRef]
- Boukid F, Castellari M (2022) How can processing technologies boost the application of faba bean (Vicia faba L.) proteins in food production? eFood 3:e18. [CrossRef]
- Arzami AN, de Carvalho DM, Vilaplana F, et al. (2022) Narrow-leafed lupin (Lupinus angustifolius L.): Characterization of emulsification and fibre properties. Future Foods 6:100192. [CrossRef]
- Patil ND, Bains A, Sridhar K, et al. (2024) Extraction, Modification, Biofunctionality, and Food Applications of Chickpea (Cicer arietinum) Protein: An Up-to-Date Review. Foods 13:1398. [CrossRef]
- Wani IA, Andrabi SN, Sogi DS, Hassan I (2020) Comparative study of physicochemical and functional properties of flours from kidney bean (Phaseolus vulgaris L.) and green gram (Vigna radiata L.) cultivars grown in Indian temperate climate. Legume Science 2:e11. [CrossRef]
- Carlin J; Der T;, Wanasundara JPD;, et al. (2023) Generating Multi-Functional Pulse Ingredients for Processed Meat Products—Scientific Evaluation of Infrared-Treated Lentils. Foods 12:1722. [CrossRef]
- Tănase Apetroaei V, Pricop EM, Istrati DI, Vizireanu C (2024) Hemp Seeds (Cannabis sativa L.) as a Valuable Source of Natural Ingredients for Functional Foods—A Review. Molecules 2024, Vol 29, Page 2097 29:2097. [CrossRef]
- Banskota AH, Jones A, Hui JPM, Stefanova R (2022) Triacylglycerols and Other Lipids Profiling of Hemp By-Products. Molecules 27:2339. [CrossRef]
- Chen H, Xu B, Wang Y, et al. (2023) Emerging natural hemp seed proteins and their functions for nutraceutical applications. Food Science and Human Wellness 12:929–941. [CrossRef]
- Santos-Sánchez G, Álvarez-López AI, Ponce-España E, et al. (2022) Hempseed (Cannabis sativa) protein hydrolysates: A valuable source of bioactive peptides with pleiotropic health-promoting effects. Trends Food Sci Technol 127:303–318. [CrossRef]
- Karabulut G, Kahraman O, Pandalaneni K, et al. (2023) A comprehensive review on hempseed protein: Production, functional and nutritional properties, novel modification methods, applications, and limitations. Int J Biol Macromol 253:127240. [CrossRef]
- Cabral EM, Zhu X, Garcia-Vaquero M, et al. (2023) Recovery of Protein from Industrial Hemp Waste (Cannabis sativa, L.) Using High-Pressure Processing and Ultrasound Technologies. Foods 12:2883. [CrossRef]
- Eckhardt L, Bu F, Franczyk A, et al. (2024) Hemp (Cannabis sativa L.) protein: Impact of extraction method and cultivar on structure, function, and nutritional quality. Curr Res Food Sci 8:100746. [CrossRef]
- Zahari I, Purhagen JK, Rayner M, et al. (2023) Extrusion of high-moisture meat analogues from hempseed protein concentrate and oat fibre residue. J Food Eng 354:111567. [CrossRef]
- Nasrollahzadeh F, Roman L, Swaraj VJS, et al. (2022) Hemp (Cannabis sativa L.) protein concentrates from wet and dry industrial fractionation: Molecular properties, nutritional composition, and anisotropic structuring. Food Hydrocoll 131:107755. [CrossRef]
- Tileuberdi N, Turgumbayeva A, Yeskaliyeva B, et al. (2022) Extraction, Isolation of Bioactive Compounds and Therapeutic Potential of Rapeseed (Brassica napus L.). Molecules 27:8824. [CrossRef]
- Peeters K, Tenorio AT (2022) Comparing Analytical Methods for Erucic Acid Determination in Rapeseed Protein Products. Foods 11:815. [CrossRef]
- Stolte N, Vettel J, Möllers C (2022) Genetic variation for seed storage protein composition in rapeseed (Brassica napus) and development of near-infrared reflectance spectroscopy calibration equations. Plant Breeding 141:408–417. [CrossRef]
- Duan X, Dong Y, Zhang M, et al. (2023) Identification and molecular interactions of novel ACE inhibitory peptides from rapeseed protein. Food Chem 422:136085. [CrossRef]
- Shen J, Liu Y, Wang X, et al. (2023) A Comprehensive Review of Health-Benefiting Components in Rapeseed Oil. Nutrients 15:999. [CrossRef]
- Jia W, Curubeto N, Rodríguez-Alonso E, et al. (2021) Rapeseed protein concentrate as a potential ingredient for meat analogues. Innovative Food Science & Emerging Technologies 72:102758. [CrossRef]
- Zhang Y, Shao F, Wan X, et al. (2024) Effects of rapeseed protein addition on soybean protein-based textured protein produced by low-moisture extrusion: Changes in physicochemical attributes, structural properties and barrel flow behaviors. Food Hydrocoll 149:109631. [CrossRef]
- Singh A, Kumar V (2024) Pumpkin seeds as nutraceutical and functional food ingredient for future: A review. Grain & Oil Science and Technology 7:12–29. [CrossRef]
- Aziz A, Noreen S, Khalid W, et al. (2023) Pumpkin and Pumpkin Byproducts: Phytochemical Constitutes, Food Application and Health Benefits. ACS Omega 8:23346. [CrossRef]
- Zerafatjou N, Amirzargar M, Biglarkhani M, et al. (2021) Pumpkin seed oil (Cucurbita pepo) versus tamsulosin for benign prostatic hyperplasia symptom relief: a single-blind randomized clinical trial. BMC Urol 21:. [CrossRef]
- Singh A, Kumar V (2022) Nutritional, phytochemical, and antimicrobial attributes of seeds and kernels of different pumpkin cultivars. Food Front 3:182–193. [CrossRef]
- Mehvish Habib, Sakshi Singh, Alok Sagar N, et al. (2025) Physicochemical and functional characterization of pumpkin seed protein isolate. Sustainable Food Technology. [CrossRef]
- Choi HW, Hahn J, Kim HS, Choi YJ (2025) Thermorheological properties and structural characteristics of soy and pumpkin seed protein blends for high-moisture meat analogs. Food Chem 464:141768. [CrossRef]
- Kong Y, Lin S, Chen S, et al. (2025) Pumpkin seed protein-based hydrogel as gelatin mimics and edible inks in 3D-Printed food. Food Hydrocoll 162:111014. [CrossRef]
- Kumar P, Sharma N, Ahmed MA, et al. (2022) Technological interventions in improving the functionality of proteins during processing of meat analogs. Front Nutr 9:1044024. [CrossRef]
- El-Sohaimy SA, Androsova NV, Toshev AD, El Enshasy HA (2022) Nutritional Quality, Chemical, and Functional Characteristics of Hemp (Cannabis sativa ssp. sativa) Protein Isolate. Plants 2022, Vol 11, Page 2825 11:2825. [CrossRef]
- Silventoinen P, Kortekangas A, Nordlund E, Sozer N (2022) Impact of Phytase Treatment and Calcium Addition on Gelation of a Protein-Enriched Rapeseed Fraction. Food Bioproc Tech 15:1422–1435. [CrossRef]
- Gadekar YP, Jairath G, Soni A, et al. (2025) Utilisation of pumpkin seed (Cucurbita maxima) as a meat matrix preservative: Influence on colour and lipid stabilities. Meat Sci 223:. [CrossRef]
- Kowalczewski L, Ró MB, Zá Nska ˙, et al. (2022) Flaxseed Bioactive Compounds: Chemical Composition, Functional Properties, Food Applications and Health Benefits-Related Gut Microbes. Foods 11:3307. [CrossRef]
- Motyka S, Skała E, Ekiert H, Szopa A (2023) Health-promoting approaches of the use of chia seeds. J Funct Foods 103:105480. [CrossRef]
- Huang X, Hu Y, Li Z, et al. (2025) Dephenolization Methods, Quality Characteristics, Applications, and Advancements of Dephenolized Cottonseed Protein: Review. Foods 2025, Vol 14, Page 628 14:628. [CrossRef]
- Wei P, Zhao F, Wang Z, et al. (2022) Sesame (Sesamum indicum L.): A Comprehensive Review of Nutritional Value, Phytochemical Composition, Health Benefits, Development of Food, and Industrial Applications. Nutrients 14:4079. [CrossRef]
- Song C, Lin Y, Hong P, et al. (2022) Low-Content Pre-Emulsified Safflower Seed Oil Enhances the Quality and Flavor of the Nemipterus Virgatus Surimi Gel. Gels 8:106. [CrossRef]
- Tantawy AA, Ali M, Kurbonova M, et al. (2025) Functional properties of sunflower protein concentrates extracted using different anti-greening agents- low-fat whipping cream preparation. LWT 218:117456. [CrossRef]
- Albakry Z, Karrar E, Mohamed Ahmed IA, et al. (2022) Nutritional Composition and Volatile Compounds of Black Cumin (Nigella sativa L.) Seed, Fatty Acid Composition and Tocopherols, Polyphenols, and Antioxidant Activity of Its Essential Oil. Horticulturae 8:575. [CrossRef]
- Wang J, Sun X, Xu X, et al. (2022) Wheat Flour-Based Edible Films: Effect of Gluten on the Rheological Properties, Structure, and Film Characteristics. Int J Mol Sci 23:11668. [CrossRef]
- Zhang X, Zhao Y, Zhang T, et al. (2023) Potential of hydrolyzed wheat protein in soy-based meat analogues: Rheological, textural and functional properties. Food Chem X 20:100921. [CrossRef]
- Wieser H, Koehler P, Scherf KA (2020) The Two Faces of Wheat. Front Nutr 7:517313. [CrossRef]
- Siddiqi RA, Singh TP, Rani M, et al. (2020) Diversity in Grain, Flour, Amino Acid Composition, Protein Profiling, and Proportion of Total Flour Proteins of Different Wheat Cultivars of North India. Front Nutr 7:141. [CrossRef]
- Zhang R, Yang Y, Liu Q, et al. (2023) Effect of Wheat Gluten and Peanut Protein Ratio on the Moisture Distribution and Textural Quality of High-Moisture Extruded Meat Analogs from an Extruder Response Perspective. Foods 2023, Vol 12, Page 1696 12:1696. [CrossRef]
- Sun Y, Dong M, Bai J, et al. (2024) Preparation and properties of high-soluble wheat gluten protein-based meat analogues. J Sci Food Agric 104:42–50. [CrossRef]
- Dai H, An H (2024) Study on the structural characteristics of wheat gluten enzymatic hydrolysates and their effect on the texturization degree of high-moisture plant-protein extrudates. J Cereal Sci 119:103974. [CrossRef]
- Flavourzyme® | Novozymes. https://www.novozymes.com/en/products/animal-protein/flavourzyme. Accessed 2 May 2025.
- Sun A, Chen L, Wu W, et al. (2023) The potential meat flavoring generated from Maillard reaction products of wheat gluten protein hydrolysates-xylose: Impacts of different thermal treatment temperatures on flavor. Food Research International 165:112512. [CrossRef]
- Wouters AGB, Nicolai T (2024) Self-assembly of oat proteins into various colloidal states as function of the NaCl concentration and pH. Food Hydrocoll 149:109603. [CrossRef]
- Rafique H, Dong R, Wang X, et al. (2022) Dietary-Nutraceutical Properties of Oat Protein and Peptides. Front Nutr 9:950400. [CrossRef]
- Kumar L, Sehrawat R, Kong Y (2021) Oat proteins: A perspective on functional properties. LWT 152:112307. [CrossRef]
- Zhou J, Li T, Peydayesh M, et al. (2022) Oat Plant Amyloids for Sustainable Functional Materials. Advanced Science 9:2104445. [CrossRef]
- Zhao J, Ni D, Bhandari B, et al. (2024) Impacts of oat flour fortification on the rheological, microstructural, digestibility, and sensory characteristics of low-fat, high-protein almond-based gels. Food Hydrocoll 151:109849. [CrossRef]
- Sargautis D, Kince T (2023) Effect of Enzymatic Pre-Treatment on Oat Flakes Protein Recovery and Properties. Foods 12:965. [CrossRef]
- Brückner-Gühmann M, Kratzsch A, Sozer N, Drusch S (2021) Oat protein as plant-derived gelling agent: Properties and potential of modification. Future Foods 4:100053. [CrossRef]
- Senarathna S, Mel R, Malalgoda M (2024) Utilization of cereal-based protein ingredients in food applications. J Cereal Sci 116:103867. [CrossRef]
- Wang D, Li T, Zhang X, et al. (2025) Modification of rice protein and its components: Enhanced fibrils formation and improved foaming properties. Food Hydrocoll 158:110575. [CrossRef]
- Rivero Meza SL, Cañizares L, Dannenberg B, et al. (2024) Sustainable rice bran protein: Composition, extraction, quality properties and applications. Trends Food Sci Technol 145:104355. [CrossRef]
- Li S, Jiang Z, Wang F, et al. (2020) Characterization of rice glutelin fibrils and their effect on in vitro rice starch digestibility. Food Hydrocoll 106:105918. [CrossRef]
- Lee JS, Choi I, Han J (2022) Construction of rice protein-based meat analogues by extruding process: Effect of substitution of soy protein with rice protein on dynamic energy, appearance, physicochemical, and textural properties of meat analogues. Food Research International 161:111840. [CrossRef]
- Lee JS, Oh H, Choi I, et al. (2022) Physico-chemical characteristics of rice protein-based novel textured vegetable proteins as meat analogues produced by low-moisture extrusion cooking technology. LWT 157:113056. [CrossRef]
- Charlie EA, Angrainy H, Kantrong H (2025) Exploring the use of rice bran and mung bean as soy substitutes in low-moisture extruded plant-based meat. Innovative Food Science & Emerging Technologies 100:103916. [CrossRef]
- Qu M, Jiang P, Zhu Y, et al. (2024) Effects of glutenin/gliadin ratio and calcium ion on the structure and gelatinity of wheat gluten protein under heat induction. Food Biosci 58:103704. [CrossRef]
- Boukid F (2021) Oat proteins as emerging ingredients for food formulation: where we stand? European Food Research and Technology 247:535–544. [CrossRef]
- Wu W, Li F, Wu X (2021) Effects of rice bran rancidity on oxidation, structural characteristics and interfacial properties of rice bran globulin. Food Hydrocoll 110:106123. [CrossRef]
- Li XL, Meng R, Xu BC, et al. (2022) Function emulsion gels prepared with carrageenan and zein/carboxymethyl dextrin stabilized emulsion as a new fat replacer in sausages. Food Chem 389:. [CrossRef]
- Dangi N, Yadav BS, Yadav RB (2020) Barley β-glucan concentrate and its acid hydrolysate for the modification of dough making and rheological properties of water chestnut flour. Int J Biol Macromol 164:253–264. [CrossRef]
- Abdollahzadeh A, Vazifedoost M, Didar Z, et al. (2024) Comparison of the effect of hydroxyl propyl methyl cellulose, pectin, and concentrated raisin juice on gluten-free bread based on rice and foxtail millet flour. Food Sci Nutr 12:439–449. [CrossRef]
- Bulgaru V, Netreba N, Ghendov-Mosanu A (2025) Pre-Treatment of Vegetable Raw Materials (Sorghum Oryzoidum) for Use in Meat Analog Manufacture. Applied Sciences 2025, Vol 15, Page 349 15:349. [CrossRef]
- Park J-H, Lee Y-J, Lim J-G, et al. (2021) Effect of Quinoa (Chenopodium quinoa Willd.) Starch and Seeds on the Physicochemical and Textural and Sensory Properties of Chicken Meatballs during Frozen Storage. Foods 2021, Vol 10, Page 1601 10:1601. [CrossRef]
- Zhu F (2023) Amaranth proteins and peptides: Biological properties and food uses. Food Research International 164:112405. [CrossRef]
- Sofi SA, Ahmed N, Farooq A, et al. (2022) Nutritional and bioactive characteristics of buckwheat, and its potential for developing gluten-free products: An updated overview. Food Sci Nutr 11:2256. [CrossRef]
- Bartkiene E, Zarovaite P, Starkute V, et al. (2023) Changes in Lacto-Fermented Agaricus bisporus (White and Brown Varieties) Mushroom Characteristics, including Biogenic Amine and Volatile Compound Formation. Foods 12:2441. [CrossRef]
- Keerthana K, Anukiruthika T, Moses JA, Anandharamakrishnan C (2020) Development of fiber-enriched 3D printed snacks from alternative foods: A study on button mushroom. J Food Eng 287:110116. [CrossRef]
- He M, Condict L, Richardson SJ, et al. (2024) Molecular characterization of interactions between lectin - a protein from common edible mushroom (Agaricus bisporus) - and dietary carbohydrates. Food Hydrocoll 146:109253. [CrossRef]
- Bell V, Silva CRPG, Guina J, Fernandes TH (2022) Mushrooms as future generation healthy foods. Front Nutr 9:1050099. [CrossRef]
- Kim H;, Jeon Y-E;, Kim S-M;, et al. (2023) Agaricus bisporus Extract Exerts an Anti-Obesity Effect in High-Fat Diet-Induced Obese C57BL/6N Mice by Inhibiting Pancreatic Lipase-Mediated Fat Absorption. Nutrients 15:4225. [CrossRef]
- Du X, Sissons J, Shanks M, Plotto A (2021) Aroma and flavor profile of raw and roasted Agaricus bisporus mushrooms using a panel trained with aroma chemicals. LWT 138:110596. [CrossRef]
- Fu Q, Yang J, Lv L, et al. (2023) Effects of replacing chicken breast meat with Agaricus bisporus mushrooms on the qualities of emulsion-type sausages. LWT 184:114983. [CrossRef]
- Törős G, El-Ramady H, Prokisch J (2022) Edible Mushroom of Pleurotus spp.:A Case Study of Oyster Mushroom (Pleurotus ostreatus L.). Environment, Biodiversity and Soil Security 6:51–59. [CrossRef]
- Ghafoor A, Niazi AR (2024) Pleurotus spp: an ultimate solution to the emerging calamities of the world. N Z J Bot. [CrossRef]
- Fulgoni VL, Agarwal S (2021) Nutritional impact of adding a serving of mushrooms on usual intakes and nutrient adequacy using National Health and Nutrition Examination Survey 2011–2016 data. Food Sci Nutr 9:1504. [CrossRef]
- Bouranis L, Aguchem RN, Chibuogwu CC, et al. (2021) Nutrient and Antinutrient Composition of Pleurotus ostreatus Grown on Different Substrates. Biology and Life Sciences Forum 2022, Vol 11, Page 69 11:69. [CrossRef]
- Mazumder MAR, Sujintonniti N, Chaum P, et al. (2023) Developments of Plant-Based Emulsion-Type Sausage by Using Grey Oyster Mushrooms and Chickpeas. Foods 2023, Vol 12, Page 1564 12:1564. [CrossRef]
- Vargas-Sánchez RD;, Rodríguez-Carpena M;, Fernández-López JG;, et al. (2022) Pleurotus Genus as a Potential Ingredient for Meat Products. Foods 11:779. [CrossRef]
- Demircan E, Aydar EF, Mertdinc (Mertdinç) Z, et al. (2023) 3D printable vegan plant-based meat analogue: Fortification with three different mushrooms, investigation of printability, and characterization. Food Research International 173:113259. [CrossRef]
- Zhang Z, Zang M, Chen J, et al. (2024) Effect of the mycelium of oyster mushrooms on the physical and flavor properties of a plant-based beef analogue. LWT 198:116029. [CrossRef]
- Hajdú P, Abdalla Z, El-Ramady H, Prokisch J (2022) Edible Mushroom of Lentinula spp.: A Case Study of Shiitake (Lentinula edodes L.) Cultivation. Environment, Biodiversity and Soil Security 6:41–49. [CrossRef]
- Kaya M, Cam M (2022) Eritadenine: Pressurized liquid extraction from Lentinula edodes and thermal degradation kinetics. Sustain Chem Pharm 29:100809. [CrossRef]
- Yu CX, Zhang YR, Ren YF, et al. (2023) Composition and contents of fatty acids and amino acids in the mycelia of Lentinula edodes. Food Sci Nutr 11:4038–4046. [CrossRef]
- Santhapur R, Jayakumar D, McClements DJ (2024) Development and Characterization of Hybrid Meat Analogs from Whey Protein-Mushroom Composite Hydrogels. Gels 2024, Vol 10, Page 446 10:446. [CrossRef]
- Choi H, Gwon HG, Kim D, et al. (2025) Structural characterization and rheological analysis of Lentinus edodes mycelium (mycoprotein)–hydrocolloid composites for food formulation. Future Foods 11:100568. [CrossRef]
- Dimopoulou M, Kolonas A, Mourtakos S, et al. (2022) Nutritional Composition and Biological Properties of Sixteen Edible Mushroom Species. Applied Sciences 2022, Vol 12, Page 8074 12:8074. [CrossRef]
- Stilinović N, Čapo I, Vukmirović S, et al. (2020) Chemical composition, nutritional profile and in vivo antioxidant properties of the cultivated mushroom Coprinus comatus. R Soc Open Sci 7:. [CrossRef]
- Ratnaningtyas NI, Hernayanti, Ekowati N, et al. (2022) Antioxidant Activities and Properties of Coprinus comatus Mushroom Both Mycelium and Fruiting Body Extracts In Streptozotocin-Induced Hyperglycemic Rats Model. Biosaintifika 14:9–21. [CrossRef]
- Zawadzka A, Kobus-Cisowska J, Szwajgier D, et al. (2022) Dual functional cholinesterase inhibitors and complexing of aluminum ions of five species of fungi family depended of drying conditions and extraction process - In vitro study. LWT 154:112712. [CrossRef]
- Yang H, Zheng Z, Zhou H, et al. (2022) Proteomics Reveals the Mechanism Underlying the Autolysis of Postharvest Coprinus comatus Fruiting Bodies. J Agric Food Chem 70:1346–1357. [CrossRef]
- Ren S, Zheng E, Zhao T, et al. (2022) Evaluation of physicochemical properties, equivalent umami concentration and antioxidant activity of Coprinus comatus prepared by different drying methods. LWT 162:113479. [CrossRef]
- Hollweg G, Trindade PCO, dos Santos BA, et al. (2024) Development of Plant-Based Burgers with Partial Replacement of Texturized Soy Protein by Agaricus bisporus: Effects on Physicochemical and Sensory Properties. Foods 13:. [CrossRef]
- Tokarczyk G, Felisiak K, Adamska I, et al. (2023) Effect of Oyster Mushroom Addition on Improving the Sensory Properties, Nutritional Value and Increasing the Antioxidant Potential of Carp Meat Burgers. Molecules 28:6975. [CrossRef]
- Rangel-Vargas E, Rodriguez JA, Domínguez R, et al. (2021) Edible Mushrooms as a Natural Source of Food Ingredient/Additive Replacer. Foods 10:2687. [CrossRef]
- Qu H, Dong Y, Liu C, et al. (2023) Effects of Coprinus comatus (chicken drumstick mushroom) on the quality characteristics of low nitrite-backfat Cantonese sausages. LWT 187:115339. [CrossRef]
- Liu K, Li J, Xing C, et al. (2023) Characterization of Auxenochlorella protothecoides acyltransferases and potential of their protein interactions to promote the enrichment of oleic acid. Biotechnology for Biofuels and Bioproducts 16:1–16. [CrossRef]
- Korozi E, Tsagou V, Kefalogianni I, et al. (2022) Continuous Culture of Auxenochlorella protothecoides on Biodiesel Derived Glycerol under Mixotrophic and Heterotrophic Conditions: Growth Parameters and Biochemical Composition. Microorganisms 10:541. [CrossRef]
- Mavrommatis A, Tsiplakou E, Zerva A, et al. (2023) Microalgae as a Sustainable Source of Antioxidants in Animal Nutrition, Health and Livestock Development. Antioxidants 2023, Vol 12, Page 1882 12:1882. [CrossRef]
- Maltsev Y, Maltseva K, Kulikovskiy M, Maltseva S (2021) Influence of Light Conditions on Microalgae Growth and Content of Lipids, Carotenoids, and Fatty Acid Composition. Biology 2021, Vol 10, Page 1060 10:1060. [CrossRef]
- Sägesser C, Kallfelz JM, Boulos S, et al. (2024) Structurability of microalgae, soy and pea protein for extruded high-moisture meat analogues. Food Hydrocoll 156:110290. [CrossRef]
- Mtaki K, Kyewalyanga MS, Mtolera MSP (2021) Supplementing wastewater with NPK fertilizer as a cheap source of nutrients in cultivating live food (Chlorella vulgaris). Ann Microbiol 71:1–13. [CrossRef]
- Abdur Razzak S, Bahar K, Islam KMO, et al. (2024) Microalgae cultivation in photobioreactors: sustainable solutions for a greener future. Green Chemical Engineering 5:418–439. [CrossRef]
- De Gol C, Snel S, Rodriguez Y, Beyrer M (2023) Gelling capacity of cell-disrupted Chlorella vulgaris and its texture effect in extruded meat substitutes. Food Structure 37:100332. [CrossRef]
- Farag MR, Alagawany M, Mahdy EAA, et al. (2023) Benefits of Chlorella vulgaris against Cadmium Chloride-Induced Hepatic and Renal Toxicities via Restoring the Cellular Redox Homeostasis and Modulating Nrf2 and NF-KB Pathways in Male Rats. Biomedicines 11:2414. [CrossRef]
- Expósito N, Carafa R, Kumar V, et al. (2021) Performance of chlorella vulgaris exposed to heavy metal mixtures: Linking measured endpoints and mechanisms. Int J Environ Res Public Health 18:1–19. [CrossRef]
- De Gol C, Snel S, Rodriguez Y, Beyrer M (2023) Gelling capacity of cell-disrupted Chlorella vulgaris and its texture effect in extruded meat substitutes. Food Structure 37:100332. [CrossRef]
- Bakhsh A, Park J, Baritugo KA, et al. (2023) A holistic approach toward development of plant-based meat alternatives through incorporation of novel microalgae-based ingredients. Front Nutr 10:1110613. [CrossRef]
- Gentscheva G, Nikolova K, Panayotova V, et al. (2023) Application of Arthrospira platensis for Medicinal Purposes and the Food Industry: A Review of the Literature. Life 13:845. [CrossRef]
- Balestri F, Podgórska-Kryszczuk I (2024) Spirulina—An Invaluable Source of Macro- and Micronutrients with Broad Biological Activity and Application Potential. Molecules 29:5387. [CrossRef]
- Mohammed IA, Ruengjitchatchawalya M, Paithoonrangsarid K (2023) Cultivation manipulating zeaxanthin-carotenoid production in Arthrospira (Spirulina) platensis under light and temperature stress. Algal Res 76:103315. [CrossRef]
- Singh U, Gandhi HA, Nikita, et al. (2023) Cyanometabolites: molecules with immense antiviral potential. Arch Microbiol 205:164. [CrossRef]
- Uzlaşır T, Selli S, Kelebek | Hasim (2024) Spirulina platensis and Phaeodactylum tricornutum as sustainable sources of bioactive compounds: Health implications and applications in the food industry. Future Postharvest and Food 1:34–46. [CrossRef]
- Guo J, Huang Y, Gu X, Meng Z (2025) Spirulina platensis protein-based emulsion gel as fat substitute in meat analogs: Evaluation performance across post-processing. Food Chem 463:141414. [CrossRef]
- Afdhaliah N, Ningrum A, Setiowati AD (2024) Red Palm Oil Gelled Emulsion Stabilized by Spirulina (Arthrospira platensis) Protein and Carrageenan as Fat Replacer in Beef Patty. Food Bioproc Tech 1–15. [CrossRef]
- Qu Y, Chen X, Ma B, et al. (2022) Extracellular Metabolites of Heterotrophic Auxenochlorella protothecoides: A New Source of Bio-Stimulants for Higher Plants. Mar Drugs 20:569. [CrossRef]
- Abdel-Moatamed BR, El-Fakhrany AEMA, Elneairy NAA, et al. (2024) The Impact of Chlorella vulgaris Fortification on the Nutritional Composition and Quality Characteristics of Beef Burgers. Foods 13:1945. [CrossRef]
- Sahil S, Bodh S, Verma P (2024) Spirulina platensis: A comprehensive review of its nutritional value, antioxidant activity and functional food potential. J Cell Biotechnol 1–14. [CrossRef]
- Yang H, Song C, Liu C, Wang P (2024) Synthetic Biology Tools for Engineering Aspergillus oryzae. Journal of Fungi 10:34. [CrossRef]
- Sun Z, Wu Y, Long S, et al. (2024) Aspergillus oryzae as a Cell Factory: Research and Applications in Industrial Production. Journal of Fungi 10:248. [CrossRef]
- Seidler Y, Rimbach G, Lüersen K, et al. (2024) The postbiotic potential of Aspergillus oryzae – a narrative review. Front Microbiol 15:1452725. [CrossRef]
- Yamashita H (2021) Koji starter and koji world in Japan. Journal of Fungi 7:. [CrossRef]
- Murai T, Annor GA (2025) Improving the Nutritional Profile of Intermediate Wheatgrass by Solid-State Fermentation with Aspergillus oryzae Strains. Foods 2025, Vol 14, Page 395 14:395. [CrossRef]
- Brandão M, Marques DJ, Sousa S, et al. (2025) Lactic Acid Bacteria and Yeast Fermentation to Improve the Nutritional Value of Ulva rigida. Marine Drugs 2025, Vol 23, Page 106 23:106. [CrossRef]
- Rousta N, Hellwig C, Wainaina S, et al. (2021) Filamentous Fungus Aspergillus oryzae for Food: From Submerged Cultivation to Fungal Burgers and Their Sensory Evaluation—A Pilot Study. Foods 2021, Vol 10, Page 2774 10:2774. [CrossRef]
- Maini Rekdal V, Villalobos-Escobedo JM, Rodriguez-Valeron N, et al. (2024) Neurospora intermedia from a traditional fermented food enables waste-to-food conversion. Nat Microbiol 9:2666. [CrossRef]
- Amara AA, El-Baky NA (2023) Fungi as a Source of Edible Proteins and Animal Feed. Journal of Fungi 2023, Vol 9, Page 73 9:73. [CrossRef]
- Rekdal VM, Villalobos-Escobedo JM, Rodriguez-Valeron N, et al. (2024) Multi-omics analysis of a traditional fermented food reveals a byproduct-associated subpopulation of Neurospora intermedia for waste-to-food upcycling. bioRxiv 3:2024.07.24.604980. [CrossRef]
- Toghiani J, Fallah N, Nasernejad B, et al. (2025) Production of protein-rich fungal biomass from pistachio dehulling waste using edible Neurospora intermedia. Sci Rep 15:5873. [CrossRef]
- Wang B, Lu H, Lou H, et al. (2024) Synthesis and characterization of Neurospora intermedia-based composite mycoprotein gel meat: Insight into the effect of pH and soluble starch on water-holding capacity and texture properties. Food Hydrocoll 155:110190. [CrossRef]
- Li K, Qiao K, Xiong J, et al. (2023) Nutritional Values and Bio-Functional Properties of Fungal Proteins: Applications in Foods as a Sustainable Source. Foods 12:4388. [CrossRef]
- Zhu J, Chen W, Chen Y, et al. (2025) CFD optimization of an air lift fermenter for Fusarium venenatum fermentation. Bioresour Technol Rep 29:102024. [CrossRef]
- Majumder R, Miatur S, Saha A, Hossain S (2024) Mycoprotein: production and nutritional aspects: a review. Sustainable Food Technology 2:81–91. [CrossRef]
- Tong S, Chen W, Hong R, et al. (2024) Efficient Mycoprotein Production with Low CO2 Emissions through Metabolic Engineering and Fermentation Optimization of Fusarium venenatum. J Agric Food Chem 72:604–612. [CrossRef]
- Akinsemolu AA, Onyeaka HN (2025) Mycoproteins as sustainable food sources: current applications and future prospects. Discover Applied Sciences 7:1–11. [CrossRef]
- D’Almeida AP, de Albuquerque TL (2025) Is It Possible to Produce Meat Without Animals? The Potential of Microorganisms as Protein Sources. Fermentation 2025, Vol 11, Page 24 11:24. [CrossRef]
- Li XL, Qi XN, Deng JC, et al. (2025) Characterization of Fusarium venenatum Mycoprotein-Based Harbin Red Sausages. Foods 14:556. [CrossRef]
- Devanthi PVP, Pratama F, Pramanda IT, et al. (2024) Exploring the Potential of Aspergillus oryzae for Sustainable Mycoprotein Production Using Okara and Soy Whey as Cost-Effective Substrates. Journal of Fungi 10:. [CrossRef]
- Elhalis H, See XY, Osen R, et al. (2023) The potentials and challenges of using fermentation to improve the sensory quality of plant-based meat analogs. Front Microbiol 14:. [CrossRef]
- Jeon H, Shin S, Winarto J, et al. (2024) Genetic approach to discover a valuable gene for enhanced nutritional value in the edible filamentous fungus Fusarium venenatum. Food Front 5:2556–2565. [CrossRef]
- Phumsombat P, Trisakwattana K, Ittithanaput N, et al. (2024) Synbiotic and protein-enriched low-fat Sao Hai rice ice cream. Quality Assurance and Safety of Crops and Foods 16:14–27. [CrossRef]
- Soybeans, mature seeds, raw - USDA FoodData Central Food Details. https://fdc.nal.usda.gov/food-details/174270/nutrients. Accessed 9 Mar 2025.
- Peas, green, raw - USDA FoodData Central Food Details. https://fdc.nal.usda.gov/food-details/170419/nutrients. Accessed 9 Mar 2025.
- Mung beans, mature seeds, raw - USDA FoodData Central Food Details. https://fdc.nal.usda.gov/food-details/174256/nutrients. Accessed 9 Mar 2025.
- Broadbeans (fava beans), mature seeds, cooked, boiled, without salt - USDA FoodData Central Food Details. https://fdc.nal.usda.gov/food-details/173753/nutrients. Accessed 9 Mar 2025.
- Lupins, mature seeds, raw - USDA FoodData Central Food Details. https://fdc.nal.usda.gov/food-details/172423/nutrients. Accessed 9 Mar 2025.
- Peanuts, all types, raw - USDA FoodData Central Food Details. https://fdc.nal.usda.gov/food-details/172430/nutrients. Accessed 9 Mar 2025.
- Oats, raw - USDA FoodData Central Food Details. https://fdc.nal.usda.gov/food-details/1101825/nutrients. Accessed 9 Mar 2025.
- Krishnamoorthi R, Srinivash M, Mahalingam PU, Malaikozhundan B (2022) Dietary nutrients in edible mushroom, Agaricus bisporus and their radical scavenging, antibacterial, and antifungal effects. Process Biochemistry 121:10–17. [CrossRef]
- Effiong ME, Umeokwochi CP, Afolabi IS, Chinedu SN (2023) Assessing the nutritional quality of Pleurotus ostreatus (oyster mushroom). Front Nutr 10:1279208. [CrossRef]
- Canelli G, Tarnutzer C, Carpine R, et al. (2020) Biochemical and Nutritional Evaluation of Chlorella and Auxenochlorella Biomasses Relevant for Food Application. Front Nutr 7:565996. [CrossRef]
- Zhang C, Wu X, Chen J, Zhou J (2024) Novel fungal alternative proteins from Penicillium limosum for enhancing structural and functional properties of plant-based meat analogues. Food Chem 444:138627. [CrossRef]
- Pobiega K, S˛ Ekul J, Pakulska A, et al. (2024) Fungal Proteins: Sources, Production and Purification Methods, Industrial Applications, and Future Perspectives. Applied Sciences 2024, Vol 14, Page 6259 14:6259. [CrossRef]
- Alshammari NA, Taylor MA, Stevenson R, et al. (2021) Effect of Intake of Food Hydrocolloids of Bacterial Origin on the Glycemic Response in Humans: Systematic Review and Narrative Synthesis. Nutrients 13:2407. [CrossRef]
- Vila-Clarà G, Vila-Martí A, Vergés-Canet L, Torres-Moreno M (2024) Exploring the Role and Functionality of Ingredients in Plant-Based Meat Analogue Burgers: A Comprehensive Review. Foods 13:1258. [CrossRef]
- Pirsa S, Hafezi K (2023) Hydrocolloids: Structure, preparation method, and application in food industry. Food Chem 399:133967. [CrossRef]
- Bakhsh A, Lee S-J, Lee E-Y, et al. (2021) A Novel Approach for Tuning the Physicochemical, Textural, and Sensory Characteristics of Plant-Based Meat Analogs with Different Levels of Methylcellulose Concentration. Foods 10:560. [CrossRef]
- Battacchi D, Verkerk R, Pellegrini N, et al. (2020) The state of the art of food ingredients’ naturalness evaluation: A review of proposed approaches and their relation with consumer trends. Trends Food Sci Technol 106:434–444. [CrossRef]
- Taghian Dinani S, Zhang Y, Vardhanabhuti B, Jan van der Goot A (2023) Enhancing textural properties in plant-based meat alternatives: The impact of hydrocolloids and salts on soy protein-based products. Curr Res Food Sci 7:. [CrossRef]
- Taghian Dinani S, Broekema NL, Boom R, van der Goot AJ (2023) Investigation potential of hydrocolloids in meat analogue preparation. Food Hydrocoll 135:108199. [CrossRef]
- Tan H, Nie S (2021) Functional hydrocolloids, gut microbiota and health: picking food additives for personalized nutrition. FEMS Microbiol Rev 45:. [CrossRef]
- Aga MB, Dar AH, Nayik GA, et al. (2021) Recent insights into carrageenan-based bio-nanocomposite polymers in food applications: A review. Int J Biol Macromol 192:197–209. [CrossRef]
- Liu F, Hou P, Zhang H, et al. (2021) Food-grade carrageenans and their implications in health and disease. Compr Rev Food Sci Food Saf 20:3918–3936. [CrossRef]
- Sha L, Xiong YL (2020) Plant protein-based alternatives of reconstructed meat: Science, technology, and challenges. Trends Food Sci Technol 102:51–61. [CrossRef]
- Cheng C, Chen S, Su J, et al. (2022) Recent advances in carrageenan-based films for food packaging applications. Front Nutr 9:. [CrossRef]
- Tahmouzi S, Meftahizadeh H, Eyshi S, et al. (2023) Application of guar (Cyamopsis tetragonoloba L.) gum in food technologies: A review of properties and mechanisms of action. Food Sci Nutr 11:4869–4897. [CrossRef]
- Yu J, Li D, Wang L, Wang Y (2023) Improving freeze-thaw stability and 3D printing performance of soy protein isolate emulsion gel inks by guar & xanthan gums. Food Hydrocoll 136:108293. [CrossRef]
- Salehi F (2020) Effect of coatings made by new hydrocolloids on the oil uptake during deep-fat frying: A review. J Food Process Preserv 44:. [CrossRef]
- Eghbaljoo H, Sani IK, Sani MA, et al. (2022) Advances in plant gum polysaccharides; Sources, techno-functional properties, and applications in the food industry - A review. Int J Biol Macromol 222:2327–2340. [CrossRef]
- Einhorn-Stoll U, Archut A, Eichhorn M, Kastner H (2021) Pectin - Plant protein systems and their application. Food Hydrocoll 118:106783. [CrossRef]
- Abka-khajouei R, Tounsi L, Shahabi N, et al. (2022) Structures, Properties and Applications of Alginates. Mar Drugs 20:364. [CrossRef]
- Gheorghita Puscaselu R, Lobiuc A, Dimian M, Covasa M (2020) Alginate: From Food Industry to Biomedical Applications and Management of Metabolic Disorders. Polymers (Basel) 12:2417. [CrossRef]
- Kyriakopoulou K, Keppler JK, van der Goot AJ (2021) Functionality of Ingredients and Additives in Plant-Based Meat Analogues. Foods 2021, Vol 10, Page 600 10:600. [CrossRef]
- Bisht B, Lohani UC, Kumar V, et al. (2022) Edible hydrocolloids as sustainable substitute for non-biodegradable materials. Crit Rev Food Sci Nutr 62:693–725. [CrossRef]
- Prasad N, Thombare N, Sharma SC, Kumar S (2022) Gum arabic – A versatile natural gum: A review on production, processing, properties and applications. Ind Crops Prod 187:115304. [CrossRef]
- Khezerlou A, Zolfaghari H, Banihashemi SA, et al. (2021) Plant gums as the functional compounds for edible films and coatings in the food industry: A review. Polym Adv Technol 32:2306–2326. [CrossRef]
- Inguglia ES, Song Z, Kerry JP, et al. (2023) Addressing Clean Label Trends in Commercial Meat Processing: Strategies, Challenges and Insights from Consumer Perspectives. Foods 12:2062. [CrossRef]
- Brassesco ME, Brandão TRS, Silva CLM, Pintado M (2021) Carob bean (Ceratonia siliqua L.): A new perspective for functional food. Trends Food Sci Technol 114:310–322. [CrossRef]
- Han JH, Keum DH, Hong SJ, et al. (2023) Comparative Evaluation of Polysaccharide Binders on the Quality Characteristics of Plant-Based Patties. Foods 2023, Vol 12, Page 3731 12:3731. [CrossRef]
- Udo T, Mummaleti G, Mohan A, et al. (2023) Current and emerging applications of carrageenan in the food industry. Food Research International 173:113369. [CrossRef]
- Chaturvedi S, Kulshrestha S, Bhardwaj K, Jangir R (2021) A Review on Properties and Applications of Xanthan Gum. Microbial Polymers: Applications and Ecological Perspectives 87–107. [CrossRef]
- Tahmouzi S, Meftahizadeh H, Eyshi S, et al. (2023) Application of guar (Cyamopsis tetragonoloba L.) gum in food technologies: A review of properties and mechanisms of action. Food Sci Nutr 11:4869–4897. [CrossRef]
- Gao Y, Luo C, Zhang J, et al. (2022) Konjac glucomannan improves the gel properties of low salt myofibrillar protein through modifying protein conformation. Food Chem 393:133400. [CrossRef]
- Dinani ST, Broekema NL, Boom RM, van der Goot AJ (2023) When and how should low acyl gellan gum be added to the protein blends to improve meat analogue texture? Journal of Food Measurement and Characterization 17:6609–6619. [CrossRef]
- Nasrallah K, Khaled S, El Khatib S, Krayem M (2024) Nutritional, biochemical and health properties of Locust beans and its applications in the food industry: a review. J Food Sci Technol 61:621–630. [CrossRef]
- Williams PA, Phillips GO (2021) Gum arabic. In: Handbook of Hydrocolloids. Elsevier, pp 627–652.
- Lorenc F, Jarošová M, Bedrníček J, et al. (2022) Structural Characterization and Functional Properties of Flaxseed Hydrocolloids and Their Application. Foods 11:2304. [CrossRef]
- Xia Y, Qian J, Zhao Y, et al. (2023) Effects of food components and processing parameters on plant-based meat texture formation and evaluation methods. J Texture Stud 54:394–409. [CrossRef]
- Dobson S, Laredo T, Marangoni AG (2022) Particle filled protein-starch composites as the basis for plant-based meat analogues. Curr Res Food Sci 5:892–903. [CrossRef]
- Bühler JM, Schlangen M, Möller AC, et al. (2022) Starch in Plant-Based Meat Replacers: A New Approach to Using Endogenous Starch from Cereals and Legumes. Starch - Stärke 74:. [CrossRef]
- Rashwan AK, Younis HA, Abdelshafy AM, et al. (2024) Plant starch extraction, modification, and green applications: a review. Environ Chem Lett 22:2483–2530. [CrossRef]
- Choi HW, Kim J-H, Ham SH, et al. (2025) Effect of heating time and drying method on the functional properties of soy protein isolate–maltodextrin conjugates for plant-based meringue cookies. Future Foods 11:100587. [CrossRef]
- Yu X, Li T, Yue M, et al. (2025) Impact of transglutaminase on structural and rheological properties of pea protein-cornmeal-wheat gluten blends for meat analogue production. J Food Eng 390:112412. [CrossRef]
- Taghian Dinani S, Zhang Y, Vardhanabhuti B, Jan van der Goot A (2023) Enhancing textural properties in plant-based meat alternatives: The impact of hydrocolloids and salts on soy protein-based products. Curr Res Food Sci 7:100571. [CrossRef]
- Öztürk-Kerimoğlu B (2021) A promising strategy for designing reduced-fat model meat emulsions by utilization of pea protein-agar agar gel complex. Food Structure 29:100205. [CrossRef]
- Zhang J, Liu P, Wu A, et al. (2024) Towards understanding pectin-protein interaction and the role of pectin in plant-based meat analogs constructing. LWT 202:116325. [CrossRef]
- Wu H, Sakai K, Zhang J, McClements DJ (2024) Plant-based meat analogs: color challenges and coloring agents. Food, Nutrition and Health 1:4. [CrossRef]
- Mariano E, Lee DY, Yun SH, et al. (2024) The Color-Developing Methods for Cultivated Meat and Meat Analogues: A Mini-Review. Food Sci Anim Resour 44:356–371. [CrossRef]
- Bakhsh A, Cho C, Baritugo KA, et al. (2023) Production and Analytical Aspects of Natural Pigments to Enhance Alternative Meat Product Color. Foods 12:1281. [CrossRef]
- Ryu KK, Kang YK, Jeong EW, et al. (2023) Applications of various natural pigments to a plant-based meat analog. LWT 174:114431. [CrossRef]
- Elhalis H, See XY, Osen R, et al. (2023) The potentials and challenges of using fermentation to improve the sensory quality of plant-based meat analogs. Front Microbiol 14:. [CrossRef]
- Nieto G, Martínez-Zamora L, Peñalver R, et al. (2023) Applications of Plant Bioactive Compounds as Replacers of Synthetic Additives in the Food Industry. Foods 13:47. [CrossRef]
- Shi X, Wang Z, Fang Z (2024) Effects of Incorporating Caramel, Carrot, and Tomato Powder on the Quality Characteristics of Soy Protein-Based Meat Patties. Foods 13:2224. [CrossRef]
- Bugoni M, Takiya CS, Grigoletto NathaliaTS, et al. (2022) Dry malt extract from barley partially replacing ground corn in diets of dairy cows: Nutrient digestibility, ruminal fermentation, and milk composition. J Dairy Sci 105:5714–5722. [CrossRef]
- Fernández-López J, Ponce-Martínez AJ, Rodríguez-Párraga J, et al. (2023) Beetroot juices as colorant in plant-based minced meat analogues: Color, betalain composition and antioxidant activity as affected by juice type. Food Biosci 56:103156. [CrossRef]
- Rocchetti G, Becchi PP, Lucini L, et al. (2022) Elderberry (Sambucus nigra L.) Encapsulated Extracts as Meat Extenders against Lipid and Protein Oxidation during the Shelf-Life of Beef Burgers. Antioxidants 11:2130. [CrossRef]
- Wen W, Chen X, Huang Z, et al. (2022) Dietary lycopene supplementation improves meat quality, antioxidant capacity and skeletal muscle fiber type transformation in finishing pigs. Animal Nutrition 8:256–264. [CrossRef]
- Ryu KK, Kang YK, Jeong EW, et al. (2023) Applications of various natural pigments to a plant-based meat analog. LWT 174:114431. [CrossRef]
- Augustyńska-Prejsnar A, Topczewska J, Ormian M, et al. (2022) The Effect of the Addition Turmeric on Selected Quality Characteristics of Duck Burgers Stored under Refrigeration. Applied Sciences 12:805. [CrossRef]
- Bakhsh A, Park J, Baritugo KA, et al. (2023) A holistic approach toward development of plant-based meat alternatives through incorporation of novel microalgae-based ingredients. Front Nutr 10:. [CrossRef]
- Martins T, Barros AN, Rosa E, Antunes L (2023) Enhancing Health Benefits through Chlorophylls and Chlorophyll-Rich Agro-Food: A Comprehensive Review. Molecules 28:5344. [CrossRef]
- El-Anany A, F.M. Ali R, Almujaydil MS, et al. (2024) Innovate plant-based burger patties using defatted sesame cake flour, chickpea flour, coffee silver skin and pomegranate juice as natural colorant: effects on nutritional and acceptability aspect. Nutr Food Sci 54:934–950. [CrossRef]
- Sun C, Ge J, He J, et al. (2021) Processing, Quality, Safety, and Acceptance of Meat Analogue Products. Engineering 7:674–678. [CrossRef]
- Guo Z, Teng F, Huang Z, et al. (2020) Effects of material characteristics on the structural characteristics and flavor substances retention of meat analogs. Food Hydrocoll 105:105752. [CrossRef]
- Zhao Y, Zhao X, Sun P, et al. (2023) Effects of adding other protein products on textural properties of soy protein concentrate-based meat analogs. J Texture Stud 54:410–419. [CrossRef]
- Chen Q, Chen Z, Zhang J, et al. (2023) Application of lipids and their potential replacers in plant-based meat analogs. Trends Food Sci Technol 138:645–654. [CrossRef]
- Jiang W, Yang X, Li L (2024) Flavor of extruded meat analogs: A review on composition, influencing factors, and analytical techniques. Curr Res Food Sci 8:100747. [CrossRef]
- Zhang C, Hua Y, Li X, et al. (2020) Key volatile off-flavor compounds in peas (Pisum sativum L.) and their relations with the endogenous precursors and enzymes using soybean (Glycine max) as a reference. Food Chem 333:127469. [CrossRef]
- Li R, Guo Y, Dong A, Yang X (2023) Protein-based emulsion gels as materials for delivery of bioactive substances: Formation, structures, applications and challenges. Food Hydrocoll 144:108921. [CrossRef]
- Cheng W, Zhu Y, Jiang G, et al. (2023) Sustainable cellulose and its derivatives for promising biomedical applications. Prog Mater Sci 138:101152. [CrossRef]
- Lu Z, Lee P-R, Yang H (2023) Using HPMC to improve sensory properties of vegan omelet analogue: Effect of HPMC on water retention, oil adsorption, and thermal gelation. Food Hydrocoll 144:108938. [CrossRef]
- Bao H, Wang Y, Huang Y, et al. (2025) The Beneficial Role of Polysaccharide Hydrocolloids in Meat Products: A Review. Gels 11:55. [CrossRef]
- Godschalk-Broers L, Sala G, Scholten E (2022) Meat Analogues: Relating Structure to Texture and Sensory Perception. Foods 11:2227. [CrossRef]
- Choton S, Gupta N, Bandral JD, et al. (2020) Extrusion technology and its application in food processing: A review. Pharma Innov 9:162–168. [CrossRef]
- Jang J, Lee D-W (2024) Advancements in plant based meat analogs enhancing sensory and nutritional attributes. NPJ Sci Food 8:50. [CrossRef]
- Fernández-López J, Ponce-Martínez AJ, Rodríguez-Párraga J, et al. (2023) Beetroot juices as colorant in plant-based minced meat analogues: Color, betalain composition and antioxidant activity as affected by juice type. Food Biosci 56:103156. [CrossRef]
- Jin S-K, Shin T-S, Yim D-G (2020) Effects of partial substitution of nitrites with purple-fleshed sweet potato powder on physicochemical characteristics of sausages. J Anim Sci Technol 62:702–712. [CrossRef]
- Huang L, Ren Y, Li H, et al. (2022) Create Fat Substitute From Soybean Protein Isolate/Konjac Glucomannan: The Impact of the Protein and Polysaccharide Concentrations Formulations. Front Nutr 9:. [CrossRef]
- (2020) The Flavor of Plant-Based Meat Analogues. Cereal Foods World 65:. [CrossRef]
- Kale P, Mishra A, Annapure US (2022) Development of vegan meat flavour: A review on sources and techniques. Future Foods 5:100149. [CrossRef]
- Liu X, Chen W, Sun M, et al. (2025) The Effect of Cumin on the Formation of β-Carboline Heterocyclic Amines in Smoked Meat and Simulated Systems. Foods 14:299. [CrossRef]
- Gu Z, Liu S, Duan Z, et al. (2021) Effect of citric acid on physicochemical properties and protein structure of low-salt restructured tilapia (<scp> Oreochromis mossambicus </scp>) meat products. J Sci Food Agric 101:1636–1645. [CrossRef]
- Wu H, Sakai K, Zhang J, McClements DJ (2024) Plant-based meat analogs: color challenges and coloring agents. Food, Nutrition and Health 1:4. [CrossRef]
- Grygier A (2023) Mustard Seeds as a Bioactive Component of Food. Food Reviews International 39:4088–4101. [CrossRef]
- Kaur R, Prasad K (2024) Development and characterization of chickpea based ready to use replacement beverage mix. Journal of Food Measurement and Characterization 18:3595–3618. [CrossRef]
- Lyu X, Ying D, Zhang P, Fang Z (2024) Effect of Whole Tomato Powder or Tomato Peel Powder Incorporation on the Color, Nutritional, and Textural Properties of Extruded High Moisture Meat Analogues. Food Bioproc Tech 17:231–244. [CrossRef]
- Kołodziejczak K, Onopiuk A, Szpicer A, Poltorak A (2021) Meat Analogues in the Perspective of Recent Scientific Research: A Review. Foods 11:105. [CrossRef]
- Baig MA, Ajayi FF, Hamdi M, et al. (2025) Recent Research Advances in Meat Analogues: A Comprehensive Review on Production, Protein Sources, Quality Attributes, Analytical Techniques Used, and Consumer Perception. Food Reviews International 41:236–267. [CrossRef]
- Achinna P, Malleboina P, Joshi AA, et al. (2024) Advancing plant-based meat analogs: Composite blend of pulse protein reinforcing structure with fibrous mushroom, jackfruit seed powder and carboxymethyl cellulose. Food Science and Technology International. [CrossRef]
- Kumar S, Kumar V, Sharma R, et al. (2021) Plant Proteins as Healthy, Sustainable and Integrative Meat Alternates. In: Veganism - a Fashion Trend or Food as a Medicine. IntechOpen.
- Reyes TF, Agrawal P, Chan T, et al. (2023) The Safety of Soy Leghemoglobin Protein Preparation Derived from Pichia pastoris Expressing a Soy Leghemoglobin Gene from Glycine max: In Vitro and In Vivo Studies. J Toxicol 2023:7398724. [CrossRef]
- Vega EN, Ciudad-Mulero M, Fernández-Ruiz V, et al. (2023) Natural Sources of Food Colorants as Potential Substitutes for Artificial Additives. Foods 12:4102. [CrossRef]
- Ryu J, Rosenfeld SE, McClements DJ (2024) Creation of plant-based meat analogs: Effects of calcium salt type on structure and texture of potato protein-alginate composite gels. Food Hydrocoll 156:110312. [CrossRef]
- Samborska K, Boostani S, Geranpour M, et al. (2021) Green biopolymers from by-products as wall materials for spray drying microencapsulation of phytochemicals. Trends Food Sci Technol 108:297–325. [CrossRef]
- Fu S, Ma Y, Wang Y, et al. (2023) Contents and Correlations of Nε-(carboxymethyl)lysine, Nε-(carboxyethyl)lysine, Acrylamide and Nutrients in Plant-Based Meat Analogs. Foods 12:1967. [CrossRef]
- Huang M, Mehany T, Xie W, et al. (2022) Use of food carbohydrates towards the innovation of plant-based meat analogs. Trends Food Sci Technol 129:155–163. [CrossRef]
- Peñaranda I, Garrido MD (2024) Viability of fructooligosaccharides as substitutes for methylcellulose reduction in plant-based burgers. Food Hydrocoll 154:110104. [CrossRef]
- Baydin T, Dille MJ, Aarstad OA, et al. (2023) The impact of sugar alcohols and sucrose on the physical properties, long-term storage stability, and processability of fish gelatin gels. J Food Eng 341:111334. [CrossRef]
- Liu J, Wan P, Xie C, Chen D-W (2021) Key aroma-active compounds in brown sugar and their influence on sweetness. Food Chem 345:128826. [CrossRef]
- Safaei P, Bayat G, Mohajer A (2024) Comparison of fish oil supplements and corn oil effects on serum lipid profile: a systematic review and meta-analysis of randomized controlled trials. Syst Rev 13:54. [CrossRef]
- Rizzo G, Baroni L (2018) Soy, Soy Foods and Their Role in Vegetarian Diets. Nutrients 10:43. [CrossRef]
- Arya SS, Salve AR, Chauhan S (2016) Peanuts as functional food: a review. J Food Sci Technol 53:31–41. [CrossRef]
- Shen J, Liu Y, Wang X, et al. (2023) A Comprehensive Review of Health-Benefiting Components in Rapeseed Oil. Nutrients 15:999. [CrossRef]
- Rahim MA, Ayub H, Sehrish A, et al. (2023) Essential Components from Plant Source Oils: A Review on Extraction, Detection, Identification, and Quantification. Molecules 28:6881. [CrossRef]
- Kołodziejczak K, Onopiuk A, Szpicer A, Poltorak A (2023) The Effect of Type of Vegetable Fat and Addition of Antioxidant Components on the Physicochemical Properties of a Pea-Based Meat Analogue. Foods 13:71. [CrossRef]
- N AA, Sontakke M (2023) Plant-based emulsifiers: Sources, extraction, properties and applications. Pharma Innov 12:08–16. [CrossRef]
- Ahmad Hairi AN, Taufik AM, Zainal Abidin SAS (2023) Product innovation: palm oil fat in plant-based meat. In: Innovation of Food Products in Halal Supply Chain Worldwide. Elsevier, pp 57–66.
- Loganathan R, Subramaniam KM, Radhakrishnan AK, et al. (2017) Health-promoting effects of red palm oil: evidence from animal and human studies. Nutr Rev 75:98–113. [CrossRef]
- Sanders C, Dobson S, Marangoni AG (2024) Effect of saturated and unsaturated fat on the physical properties of plant-based cheese. Curr Res Food Sci 9:100832. [CrossRef]
- Cho Y, Bae J, Lee J, Choi M-J (2023) Storage Stability of Meat Analogs Supplemented with Vegetable Oils. Foods 12:3586. [CrossRef]
- Vatansever S, Hall C (2024) Plant proteins for dry extruded products. In: Functionality of Plant Proteins. Elsevier, pp 339–372.
- Ribeiro G, Piñero M-Y, Parle F, et al. (2024) Optimizing Screw Speed and Barrel Temperature for Textural and Nutritional Improvement of Soy-Based High-Moisture Extrudates. Foods 13:1748. [CrossRef]
- He J, Evans NM, Liu H, Shao S (2020) A review of research on plant-based meat alternatives: Driving forces, history, manufacturing, and consumer attitudes. Compr Rev Food Sci Food Saf 19:2639–2656. [CrossRef]
- Mazumder MdAR, Panpipat W, Chaijan M, et al. (2023) Role of plant protein on the quality and structure of meat analogs: A new perspective for vegetarian foods. Future Foods 8:100280. [CrossRef]
- Arora S, Kataria P, Nautiyal M, et al. (2023) Comprehensive Review on the Role of Plant Protein As a Possible Meat Analogue: Framing the Future of Meat. ACS Omega 8:23305–23319. [CrossRef]
- Schmid E, Farahnaky A, Adhikari B, Torley PJ (2022) High moisture extrusion cooking of meat analogs: A review of mechanisms of protein texturization. Compr Rev Food Sci Food Saf 21:4573–4609. [CrossRef]
- Choi HW, Hahn J, Kim H-S, Choi YJ (2025) The influence of cooling die temperature gradients on the texture of high-moisture meat analogs. Food Chem 468:142403. [CrossRef]
- Maung TT, Gu BY, Kim MH, Ryu GH (2020) Fermentation of texturized vegetable proteins extruded at different moisture contents: effect on physicochemical, structural, and microbial properties. Food Sci Biotechnol 29:897–907. [CrossRef]
- Köllmann N, Schreuders FKG, Zhang L, van der Goot AJ (2023) On the importance of cooling in structuring processes for meat analogues. J Food Eng 350:111490. [CrossRef]
- Zhao L, Zhang M, Chitrakar B, Adhikari B (2021) Recent advances in functional 3D printing of foods: a review of functions of ingredients and internal structures. Crit Rev Food Sci Nutr 61:3489–3503. [CrossRef]
- Piyush, Kumar R, Kumar R (2020) 3D printing of food materials: A state of art review and future applications. Mater Today Proc 33:1463–1467. [CrossRef]
- Ko HJ, Wen Y, Choi JH, et al. (2021) Meat analog production through artificial muscle fiber insertion using coaxial nozzle-assisted three-dimensional food printing. Food Hydrocoll 120:106898. [CrossRef]
- Wen Y, Kim HW, Park HJ (2022) Effects of transglutaminase and cooking method on the physicochemical characteristics of 3D-printable meat analogs. Innovative Food Science & Emerging Technologies 81:103114. [CrossRef]
- Chao C, Park HJ, Kim HW (2024) Effect of l-cysteine on functional properties and fibrous structure formation of 3D-printed meat analogs from plant-based proteins. Food Chem 439:137972. [CrossRef]
- Akharume FU, Aluko RE, Adedeji AA (2021) Modification of plant proteins for improved functionality: A review. Compr Rev Food Sci Food Saf 20:198–224. [CrossRef]
- Chen J, Zhang M, Mujumdar AS, Phuhongsunge P (2022) 4D printing induced by microwave and ultrasound for mushroom mixtures: Efficient conversion of ergosterol into vitamin D2. Food Chem 387:132840. [CrossRef]
- Yang W, Tu A, Ma Y, et al. (2021) Chitosan and Whey Protein Bio-Inks for 3D and 4D Printing Applications with Particular Focus on Food Industry. Molecules 27:173. [CrossRef]
- Du Q, Tu M, Liu J, et al. (2023) Plant-based meat analogs and fat substitutes, structuring technology and protein digestion: A review. Food Research International 170:112959. [CrossRef]
- Cornet SH V., Snel SJE, Schreuders FKG, et al. (2022) Thermo-mechanical processing of plant proteins using shear cell and high-moisture extrusion cooking. Crit Rev Food Sci Nutr 62:3264–3280. [CrossRef]
- Wang Z, Tian Y, Lou F, Guo Z (2024) Effect of Pea Protein Isolate–Soybean Meal Ratio on Fiber Structure and Texture Properties of High-Moisture Meat Analogs. Foods 13:3818. [CrossRef]
- Wittek P, Ellwanger F, Karbstein HP, Emin MA (2021) Morphology Development and Flow Characteristics during High Moisture Extrusion of a Plant-Based Meat Analogue. Foods 2021, Vol 10, Page 1753 10:1753. [CrossRef]
- Chiang JH, Tay W, Ong DSM, et al. (2021) Physicochemical, textural and structural characteristics of wheat gluten-soy protein composited meat analogues prepared with the mechanical elongation method. Food Structure 28:100183. [CrossRef]
- Zahari I, Östbring K, Purhagen JK, Rayner M (2022) Plant-Based Meat Analogues from Alternative Protein: A Systematic Literature Review. Foods 11:2870. [CrossRef]
- Chen Y, Zhang M, Bhandari B, et al. (2021) 3D Printing of Steak-like Foods Based on Textured Soybean Protein. Foods 2021, Vol 10, Page 2011 10:2011. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





