Submitted:
17 June 2025
Posted:
18 June 2025
You are already at the latest version
Abstract
Keywords:
Introduction
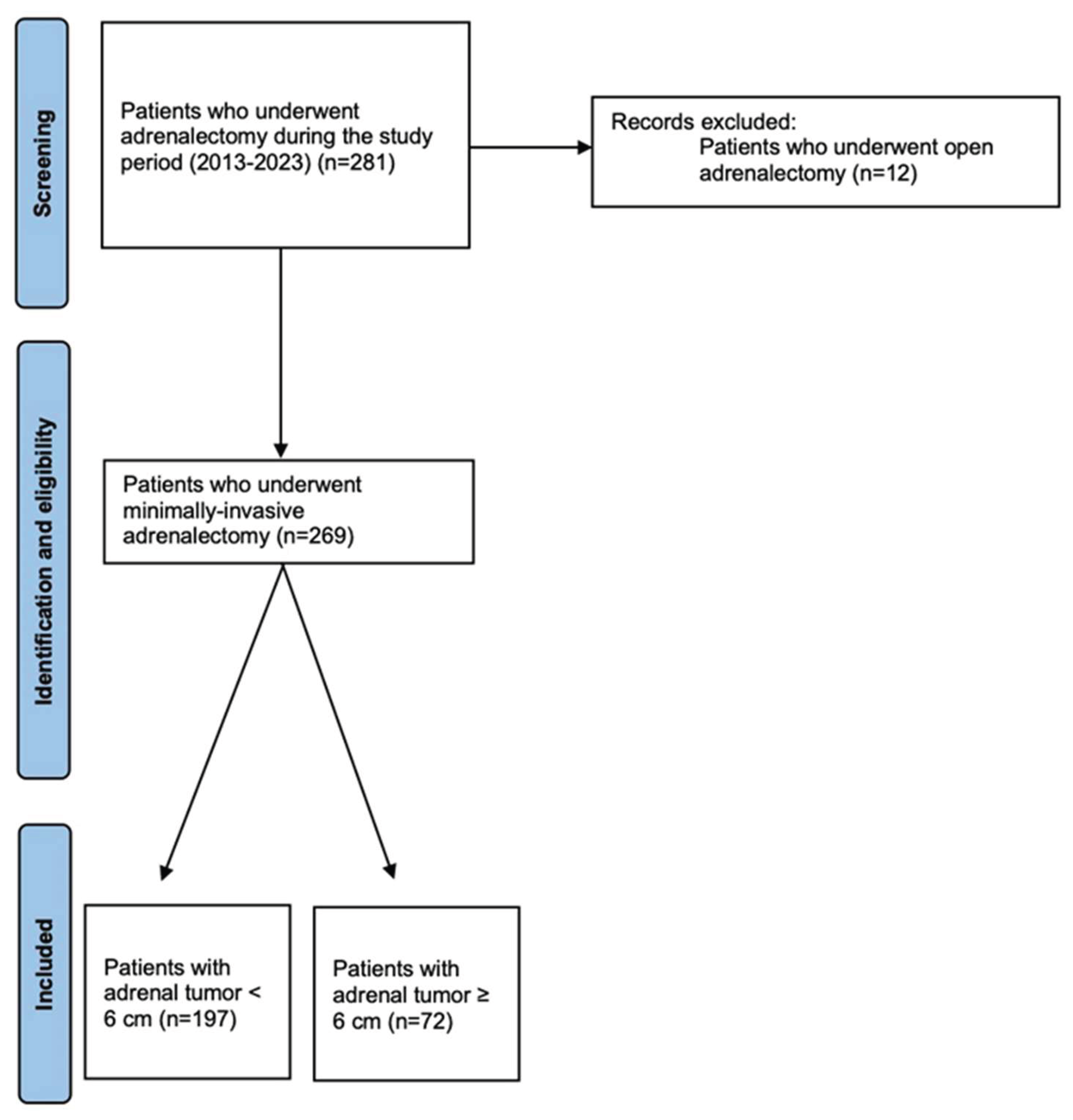
Material and Methods
Study Design and Population
Statistical Analysis
Results
Characteristics of the Study Population
Intraoperative and Postoperative Outcomes
Diagnostic Workup and Oncological Outcomes of ACC Patients
Multivariate Analyses
Discussion
Conclusion
Author Contributions
Funding
Institutional Review Board Statement
Informed consent
Data Availability Statement
Conflicts of Interest
References
- Gagner M, Lacroix A, Bolté E. Laparoscopic adrenalectomy in Cushing’s syndrome and pheochromocytoma. N Engl J Med. 1992 Oct 1;327(14):1033. [CrossRef]
- Higashihara E, Tanaka Y, Horie S, Aruga S, Nutahara K, Homma Y, et al. [A case report of laparoscopic adrenalectomy]. Nihon Hinyokika Gakkai Zasshi Jpn J Urol. 1992 Jul;83(7):1130–3.
- Heger P, Probst P, Hüttner FJ, Gooßen K, Proctor T, Müller-Stich BP, et al. Evaluation of Open and Minimally Invasive Adrenalectomy: A Systematic Review and Network Meta-analysis. World J Surg. 2017 Nov;41(11):2746–57. [CrossRef]
- Pogorzelski R, Toutounchi S, Krajewska E, Fiszer P, Kącka A, Piotrowski M, et al. The usefulness of laparoscopic adrenalectomy in the treatment of adrenal neoplasms - a single-centre experience. Endokrynol Pol. 2017;68(4):407–10. [CrossRef]
- Li J, Wang Y, Chang X, Han Z. Laparoscopic adrenalectomy (LA) vs open adrenalectomy (OA) for pheochromocytoma (PHEO): A systematic review and meta-analysis. Eur J Surg Oncol J Eur Soc Surg Oncol Br Assoc Surg Oncol. 2020 Jun;46(6):991–8. [CrossRef]
- Shen WT, Lim RC, Siperstein AE, Clark OH, Schecter WP, Hunt TK, et al. Laparoscopic vs open adrenalectomy for the treatment of primary hyperaldosteronism. Arch Surg Chic Ill 1960. 1999 Jun;134(6):628–31; discussion 631-632. [CrossRef]
- Hazzan D, Shiloni E, Golijanin D, Jurim O, Gross D, Reissman P. Laparoscopic vs open adrenalectomy for benign adrenal neoplasm. Surg Endosc. 2001 Nov;15(11):1356–8. [CrossRef]
- Materazzi G, Rossi L. Robot-assisted adrenalectomy: state of the art. Updat Surg. 2021 Jun;73(3):1131–46. [CrossRef]
- Guazzoni G, Montorsi F, Bocciardi A, Da Pozzo L, Rigatti P, Lanzi R, et al. Transperitoneal laparoscopic versus open adrenalectomy for benign hyperfunctioning adrenal tumors: a comparative study. J Urol. 1995 May;153(5):1597–600. [CrossRef]
- Zografos GN, Farfaras A, Vasiliadis G, Pappa T, Aggeli C, Vassilatou E, et al. Laparoscopic resection of large adrenal tumors. JSLS. 2010;14(3):364–8. [CrossRef]
- Dalvi AN, Thapar PM, Thapar VB, Rege SA, Deshpande AA. Laparoscopic adrenalectomy for large tumours: Single team experience. J Minimal Access Surg. 2012 Oct;8(4):125–8. [CrossRef]
- Hobart MG, Gill IS, Schweizer D, Sung GT, Bravo EL. Laparoscopic adrenalectomy for large-volume (> or = 5 cm) adrenal masses. J Endourol. 2000 Mar;14(2):149–54. [CrossRef]
- MacGillivray DC, Whalen GF, Malchoff CD, Oppenheim DS, Shichman SJ. Laparoscopic resection of large adrenal tumors. Ann Surg Oncol. 2002 Jun;9(5):480–5.
- Natkaniec M, Pędziwiatr M, Wierdak M, Major P, Migaczewski M, Matłok M, et al. Laparoscopic Transperitoneal Lateral Adrenalectomy for Large Adrenal Tumors. Urol Int. 2016;97(2):165–72. [CrossRef]
- Parente A, Verhoeff K, Wang Y, Wang N, Wang Z, Śledziński M, et al. Robotic and Laparoscopic Adrenalectomy for Pheochromocytoma: An International Multicenter Study. Eur Urol Focus. 2024 Sep 14;S2405-4569(24)00168-8. [CrossRef]
- Fassnacht M, Tsagarakis S, Terzolo M, Tabarin A, Sahdev A, Newell-Price J, et al. European Society of Endocrinology clinical practice guidelines on the management of adrenal incidentalomas, in collaboration with the European Network for the Study of Adrenal Tumors. Eur J Endocrinol. 2023 Jul 20;189(1):G1–42. [CrossRef]
- Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–83. [CrossRef]
- Daabiss, M. American Society of Anaesthesiologists physical status classification. Indian J Anaesth. 2011 Mar;55(2):111–5. [CrossRef]
- Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004 Aug;240(2):205–13.
- Slankamenac K, Graf R, Barkun J, Puhan MA, Clavien PA. The comprehensive complication index: a novel continuous scale to measure surgical morbidity. Ann Surg. 2013 Jul;258(1):1–7.
- Rashid R, Sohrabi C, Kerwan A, Franchi T, Mathew G, Nicola M, et al. The STROCSS 2024 guideline: strengthening the reporting of cohort, cross-sectional, and case-control studies in surgery. Int J Surg Lond Engl. 2024 Jun 1;110(6):3151–65. [CrossRef]
- Miller BS, Gauger PG, Hammer GD, Doherty GM. Resection of adrenocortical carcinoma is less complete and local recurrence occurs sooner and more often after laparoscopic adrenalectomy than after open adrenalectomy. Surgery. 2012 Dec;152(6):1150–7. [CrossRef]
- Wu K, Liu Z, Liang J, Tang Y, Zou Z, Zhou C, et al. Laparoscopic versus open adrenalectomy for localized (stage 1/2) adrenocortical carcinoma: Experience at a single, high-volumecenter. Surgery. 2018 Dec;164(6):1325–9.
- Donatini G, Caiazzo R, Do Cao C, Aubert S, Zerrweck C, El-Kathib Z, et al. Long-term survival after adrenalectomy for stage I/II adrenocortical carcinoma (ACC): a retrospective comparative cohort study of laparoscopic versus open approach. Ann Surg Oncol. 2014 Jan;21(1):284–91. [CrossRef]
- Balla A, Palmieri L, Meoli F, Corallino D, Ortenzi M, Ursi P, et al. Are Adrenal Lesions of 6 cm or More in Diameter a Contraindication to Laparoscopic Adrenalectomy? A Case-Control Study. World J Surg. 2020 Mar;44(3):810–8.
- Cicek MC, Gunseren KO, Senol K, Vuruskan H, Yavascaoglu I. Is 6 cm Diameter an Upper Limit for Adrenal Tumors to Perform Laparoscopic Adrenalectomy? J Laparoendosc Adv Surg Tech A. 2021 Mar;31(3):301–5.
- Agrusa A, Romano G, Frazzetta G, Chianetta D, Sorce V, Di Buono G, et al. Laparoscopic adrenalectomy for large adrenal masses: single team experience. Int J Surg Lond Engl. 2014;12 Suppl 1:S72-74. [CrossRef]
- Feo CV, Portinari M, Maestroni U, Del Rio P, Severi S, Viani L, et al. Applicability of laparoscopic approach to the resection of large adrenal tumours: a retrospective cohort study on 200 patients. Surg Endosc. 2016 Aug;30(8):3532–40. [CrossRef]
- Sharma R, Ganpule A, Veeramani M, Sabnis RB, Desai M. Laparoscopic management of adrenal lesions larger than 5 cm in diameter. Urol J. 2009;6(4):254–9.
- Prakobpon T, Santi-Ngamkun A, Usawachintachit M, Ratchanon S, Sowanthip D, Panumatrassamee K. Laparoscopic transperitoneal adrenalectomy in the large adrenal tumor from single center experience. BMC Surg. 2021 Feb 1;21(1):68. [CrossRef]
- Chen Y, Scholten A, Chomsky-Higgins K, Nwaogu I, Gosnell JE, Seib C, et al. Risk Factors Associated With Perioperative Complications and Prolonged Length of Stay After Laparoscopic Adrenalectomy. JAMA Surg. 2018 Nov 1;153(11):1036–41. [CrossRef]
- Tiberio GAM, Solaini L, Arru L, Merigo G, Baiocchi GL, Giulini SM. Factors influencing outcomes in laparoscopic adrenal surgery. Langenbecks Arch Surg. 2013 Jun;398(5):735–43. [CrossRef]
- Parnaby CN, Chong PS, Chisholm L, Farrow J, Connell JM, O’ Dwyer PJ. The role of laparoscopic adrenalectomy for adrenal tumours of 6 cm or greater. Surg Endosc. 2008 Mar;22(3):617–21. ttps://doi.org/10.1007/s00464-007-9709-7.
- NIH state-of-the-science statement on management of the clinically inapparent adrenal mass (‘incidentaloma’). NIH Consens State Sci Statements. 2002 Feb 4;19(2):1–25.
- Gan L, Meng C, Li K, Lei Peng null, Li J, Wu J, et al. Safety and effectiveness of minimally invasive adrenalectomy versus open adrenalectomy in patients with large adrenal tumors (≥5 cm): A meta-analysis and systematic review. Int J Surg Lond Engl. 2022 Aug;104:106779. [CrossRef]
- Cooper AB, Habra MA, Grubbs EG, Bednarski BK, Ying AK, Perrier ND, et al. Does laparoscopic adrenalectomy jeopardize oncologic outcomes for patients with adrenocortical carcinoma? Surg Endosc. 2013 Nov;27(11):4026–32. [CrossRef]
- Machado NO, Al Qadhi H, Al Wahaibi K, Rizvi SG. Laparoscopic Adrenalectomy for Large Adrenocortical Carcinoma. JSLS. 2015;19(3):e2015.00036. [CrossRef]
- Brix D, Allolio B, Fenske W, Agha A, Dralle H, Jurowich C, et al. Laparoscopic versus open adrenalectomy for adrenocortical carcinoma: surgical and oncologic outcome in 152 patients. Eur Urol. 2010 Oct;58(4):609–15. [CrossRef]
- Lombardi CP, Raffaelli M, De Crea C, Boniardi M, De Toma G, Marzano LA, et al. Open versus endoscopic adrenalectomy in the treatment of localized (stage I/II) adrenocortical carcinoma: results of a multiinstitutional Italian survey. Surgery. 2012 Dec;152(6):1158–64. [CrossRef]
| Parameter | Group A (< 6 cm) N=197 (73.2%) |
Group B (≥ 6 cm) N=72 (26.8%) |
p value |
|---|---|---|---|
| Age, mean ± SD (years) | 52 ± 13.6 | 55.5 ± 13.5 | 0.058 |
| BMI, mean ± SD (kg/m2) | 27.6 ± 6.8 | 27.9 ± 6.7 | 0.795 |
| Female, n. (%) Male, n. (%) |
75 (38.1) 123 (61.9) |
38 (54.2) 41(45.8) |
0.018 |
| Comorbidity, n. (%) | 180 (91.4) | 67 (93.1) | 0.655 |
| Charlson Comorbidity Index, mean ± SD | 1.9 ± 1.8 | 2.4 ± 2.0 | 0.049 |
| ASA score n. (%) 1 2 3 4 |
1 (0.5) 113 (56.3) 82 (42.1) 2 (1) |
5 (6.9) 26 (34.7) 48 (58.3) 0 (0) |
<0.001 |
| Prior abdominal surgery, n. (%) | 93 (47.2) | 28 (38.9) | 0.224 |
| Hormonal hypersecretion, n (%) Catecholamine, n (%) Aldosterone, n (%) Cortisol, n (%) Cortisol + Aldosterone, n (%) Catecholamine + Androgen, n (%) |
131 (66.5) 51 (25.9) 38 (19.3) 41 (20.8) 1 (0.5) 0 |
30 (41.7) 19 (26.4) 2 (2.8) 9 (12.5) 0 1 (1.4) |
<0.001 |
| Surgical approach Transperitoneal, n. (%) Retroperitoneal, n. (%) Robotic transperitoneal, n. (%) |
149 (75.6) 43 (21.8) 5 (2.5) |
69 (95.8) 1 (1.4) 2 (2.8) |
<0.001 |
| Tumor location: Right, n. (%) Left, n. (%) Bilateral, n. (%) |
95 (48.2) 95 (48.2) 7 (3.6) |
45 (62.5) 23 (31.9) 4 (5.6) |
0.055 |
| Genetic mutation, n. (%) 21-OHD, n. (%) MEN2A, n. (%) MEN2B, n. (%) NF1, n. (%) VHL, n. (%) Maffucci syndrome, n. (%) |
12 (6.1) 0 (0) 5 (2.5) 2 (1) 3 (1.5) 2 (1) 0 (0) |
7 (9.7) 1 (1.4) 4 (5.6) 0 (0) 1 (1.4) 0 (0) 1 (1.4) |
0.303 |
| Tumor size (major lesion), mean ± SD (cm) | 3.6 ± 1.3 | 7.6 ± 1.8 | <0.001 |
| Histology Cortical adenoma or cyst, n. (%) With AMH, n (%) Pheochromocytoma, n. (%) Adrenal malignancies, n. (%) ACC, n.(%) Angiosarcoma, n. (%) Adrenal metastasis, n. (%) Myelolipoma, n. (%) Others, n. (%) Lymphoma, n. (%) Ganglioneuroma, n. (%) Angiomyolipoma, n. (%) Hemangioma, n. (%) Lymphangioma, n. (%) Fibrous solitary tumor, n. (%) |
131 (66.5) 6 (4.6) 45 (22.8) 10 (5.1) 4 (2) 0 6 (3) 1 (0.5) 10 (5.1) 0 3 (1.5) 1 (0.5) 1 (0.5) 3 (1.5) 2 (1) |
28 (38.9) 2 (2.8) 17 (23.6) 9 (12.5) 2 (2.8) 1 (1.4) 6 (8.3) 14 (19.4) 4 (5.6) 2 (2.8) 2 (2.8) 0 0 0 0 |
<0.001 |
| Parameter | Group A (< 6 cm) N=197 (73.2%) |
Group B (≥ 6 cm) N=72 (28.8%) |
p value |
|---|---|---|---|
| Operative time, mean ± SD (min) | 97.8 ± 50.5 | 120 ± 56.2 | 0.002 |
| Associated surgeries, n. (%) | 23 (11.7) | 7 (9.7) | 0.652 |
| Conversion to open, n. (%) | 5 (2.5) | 6 (8.3) | 0.075 |
| Reason for conversion Technical difficulties, n. (%) Hemodynamic instability, n. (%) Others, n. (%) |
1 (0.5) 0 (0) 4 (2) |
4 (5.6) 2 (2.8) 0 (0) |
0.143 |
| Intra-operative complications No complications, n. (%) Hemorrhage, n. (%) Iatrogenic damage, n. (%) Others, n. (%) |
2 (1%) 195 (99.0) 0 (0) 1 (0.5) 1 (0.5) |
2 (2.8%) 70 (97.2) 1 (1.4) 0 (0) 1 (1.4) |
0.646 |
| Blood transfusions, n. (%) | 2 (1.0) | 3 (4.2) | 0.236 |
| Post-operative ICU, n. (%) | 106 (53.8) | 42 (58.3) | 0.508 |
| Length of ICU stay, mean ± SD (days) | 1 ± 0.2 | 1.1 ± 0.4 | 0.406 |
| Length of hospital stay, mean ± SD (days) | 3.1 ± 1.7 | 3.5 ± 2.3 | 0.112 |
| Postoperative complications, n. (%) | 19 (9.6) | 8 (11.1) | 0.723 |
| Clavien-Dindo classification Grade 1, n. (%) Grade 2, n. (%) Grade 3a, n. (%) Grade 4a, n. (%) |
6 (3) 10 (5.1) 1 (0.5) 2 (1) |
2 (2.8) 6 (8.3) 0 0 |
0.975 |
| Comprehensive Complications Index, mean ± SD | 2.0 ± 6.7 | 2.1 ± 6.1 | 0.857 |
| Readmission at 30 days, n. (%) | 4 (2) | 1 (1.4) | 0.730 |
| Adrenal-related mortality, n (%) | 0 | 0 | NA |
| Parameter | Coefficient | p-value | Odds Ratio | 95% CI | |
|---|---|---|---|---|---|
| Inferior | Superior | ||||
| Tumor size ≥ 6 cm | 0.149 | 0.768 | 1.161 | 0.432 | 3.117 |
| Age | -0.027 | 0.197 | 0.973 | 0.934 | 1.014 |
| Gender | 0.091 | 0.838 | 0.745 | 0.455 | 2.637 |
| ASA score | 0.525 | 0.240 | 1.691 | 0.704 | 4.063 |
| Charlson comorbidity index | 0.282 | 0.056 | 1.326 | 0.993 | 1.771 |
| Hormonal Hypersecretion |
0.378 | 0.426 | 1.459 | 0.575 | 3.701 |
| Tumor location (unilateral or bilateral) |
-0.223 | 0.851 | 0.800 | 0.078 | 8.162 |
| Surgical approach | -0.064 | 0.841 | 0.938 | 0.503 | 1.749 |
| Histology | 0.091 | 0.658 | 1.096 | 0.731 | 1.642 |
| Parameter | Coefficient | p-value | Odds Ratio | 95% CI | |
|---|---|---|---|---|---|
| Inferior | Superior | ||||
| Tumor size ≥ 6 cm | 1.758 | 0.031 | 5.800 | 1.177 | 28.595 |
| Age | 0.008 | 0.842 | 1.008 | 0.928 | 1.096 |
| Gender | 0.855 | 0.266 | 2.351 | 0.522 | 10.587 |
| ASA score | 1.662 | 0.057 | 5.271 | 0.950 | 29.253 |
| Charlson comorbidity index | 0.022 | 0.930 | 1.023 | 0.620 | 1.688 |
| Hormonal Hypersecretion |
-0.388 | 0.632 | 0.678 | 0.138 | 3.327 |
| Tumor location (unilateral or bilateral) |
0.853 | 0.526 | 2.346 | 0.168 | 32.808 |
| Surgical approach | 0.184 | 0.772 | 1.202 | 0.347 | 4.157 |
| Histology | 0.029 | 0.927 | 1.030 | 0.548 | 1.935 |
| Parameter | Coefficient | p-value | 95% CI | |
|---|---|---|---|---|
| Inferior | Superior | |||
| Tumor size ≥ 6 cm | 0.248 | 0.344 | -0.268 | 0.764 |
| Age | -0.011 | 0.332 | -0.034 | 0.011 |
| Gender | 0.203 | 0.378 | -0.251 | 0.657 |
| ASA score | 0.416 | 0.062 | -0.021 | 0.853 |
| Charlson comorbidity index | 0.211 | 0.015 | 0.041 | 0.381 |
| Hormonal Hypersecretion |
-0.276 | 0.239 | -0.737 | 0.185 |
| Tumor location (unilateral or bilateral) | 3.231 | <0.001 | 2.037 | 4.424 |
| Surgical approach | -0.193 | 0.207 | -0.494 | 0.108 |
| Histology | 0.051 | 0.630 | -0.157 | 0.259 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





