1. Introduction
Epilepsy is one of the most common chronic neurological disorders globally, affecting both humans and non-human species. The prevalence of epileptic seizures ranges from 1-3% in the human population [
1], and 0.5-5.7% in dogs [
2,
3]. Caring for an epileptic dog requires a substantial and ongoing commitment from the owners, particularly in terms of administering antiepileptic drugs (AEDs) and attending regular veterinary checks [
4,
5]. In addition to the medical burden, epilepsy also impacts the quality of life (QoL) of both affected dogs and their caregivers. Studies have shown that seizure frequency, rather than severity, is associated with lower perceived canine QoL [
6]. Dogs on third-line AEDs and those experiencing more severe adverse effects – such as increased sleeping and ataxia – have lower QoL scores [
6]. Beyond seizures, epilepsy may affect dogs’ neurobehavioral, emotional, and cognitive functioning as well [
7]. For caregivers, epilepsy management can be both emotionally and financially taxing, with a median monthly medication cost estimated between
$51 and
$75 [
8]. However, some evidence suggests that dog owners may not perceive the management burden as significantly diminishing QoL for themselves or their pets [
9].
While the causes and treatment of epilepsy are well-studied in humans e.g. [
1,
10], the disorder remains less well understood in non-human animals Epilepsy can be classified as idiopathic, structural, and reactive [
11]. Numerous parallels have been identified between human and canine epilepsy so far [
12]. Structural epilepsy arises from identifiable insults such as trauma, infections, or neurodegeneration. Idiopathic epilepsy, by contrast, is presumed to have a genetic basis and shows high heritability in certain dog breeds – such as Border Collies and Labrador Retrievers [
13]. Similarly, human epilepsy often involves a genetic component, though its polygenic nature complicates assessments of heritability [
14]. One notable example of genetic convergence is the identification of mutations in the LGI2 gene in both epileptic Lagotto Romagnolo dogs and children with epilepsy [
15]. Multidrug-resistant epilepsy also appears in both species [
16], and traumatic brain injury is a shared risk factor for post-traumatic epilepsy [
17,
18].
Electroencephalography (EEG) is a core diagnostic tool for epilepsy in humans, aiding in determining whether an episode is of epileptic origin, and enabling the classification of epilepsy syndromes. In veterinary medicine, however, EEG is underutilized. A recent survey found that fewer than 50% of veterinary neurologists perform EEG, and even among those who do, usage is infrequent [
19]. One of the most effective diagnostic contexts for human epilepsy is EEG recording during natural sleep e.g. [
20], but no such data exist for epileptic dogs, as clinical EEGs are typically performed under anaesthesia e.g. [
21], and even pioneering advances to use semi-invasive methods (needle electrode) in order to record natural EEG patterns in dogs (video-EEG), have focused on awake recordings [
22].
In addition to sleep’s diagnostic value, epilepsy is further associated with sleep-wake cycle disturbances in humans. Patients with focal epilepsy often exhibit reduced rapid eye movement (REM) sleep and decreased sleep efficiency, while those with generalized epilepsy show increased slow-wave sleep and similarly reduced sleep efficiency [
23]. Greater social jetlag and irregular sleep-wake patterns across weekdays and weekends have also been reported, along with a correlation between higher seizure frequency and poorer sleep quality [
24]. Patients with refractory temporal lobe epilepsy have been found to experience more awakenings after sleep onset compared to both frontal lobe epilepsy patients and healthy controls [
25]. Furthermore, epilepsy patients show non-REM sleep instability, characterized by increased cyclic alternating pattern rates and decreased A1 subtype percentages [
23]. Although AEDs may partially restore sleep architecture, they do not fully normalize sleep patterns in epilepsy patients [
23].
Recent advances in canine neurocognitive research have led to the development of fully non-invasive, welfare-compatible brain monitoring techniques for family dogs [
26]. Of particular interest is the canine polysomnography protocol [
27], which enables EEG recordings during natural sleep without the need for sedation or prior training. This approach has been successfully used in multiple studies exploring sleep physiology and behaviour in dogs [
28], demonstrating its practicality and compatibility with modern animal welfare guidelines.
The present study has two main aims. First, we investigate whether non-invasive sleep EEG, as implemented via canine polysomnography, can detect epileptiform activity in dogs with a clinical diagnosis of epilepsy. Second, we compare the sleep architecture of these epileptic dogs with that of age- and breed-matched healthy controls to identify potential abnormalities in sleep patterns associated with epilepsy.
2. Materials and Methods
Patients
Our subjects were N=11 adult pet dogs (from 2 to 11 years of age, mean: 6.27 years) all diagnosed with epilepsy (4 females, 7 males; all neutered;
Table 1). All participants were referred to by KHB’s veterinary practice, and were receiving antiepileptic medication at the time of the study. Diagnosed seizure types included focal-frontal lobe, generalised clonic, generalised tonic-clonic and mixed forms (e.g. generalised clonic + focal-frontal lobe), with varying intervals between the last seizure and the EEG recording. Magnetic resonance imaging (MRI) scans were available for N=7 of the dogs.
EEG Measurement
The EEG recordings were conducted using a fully non-invasive and previously validated protocol (Kis et al., 2014; Reicher et al., 2020), employing four active electrodes, including bilateral frontal placements (F7, F8) on the right and left zygomatic arch next to the eyes and another two over the anteroposterior midline of the skull (Fz, Cz). All four EEG electrodes were referenced to the G2 electrode, located at the posterior midline (external occipital protuberance). The ground electrode (G1) was placed on the left temporalis muscle. During electrode placement, all dogs were positively reinforced with social interaction (e.g., petting, praise) and/or food rewards. For visualisation purposes an additional EOG channel was computed as F7-F8 to aid eye movement identification.
EEG signals were collected, pre-filtered, amplified, and digitized at a sampling rate of 1024 Hz per channel using a SAM 25 R MicroMed Headbox (MicroMed Inc., Houston, TX, USA). The hardware passband was set to 0.5–256 Hz, with an anti-aliasing filter cutoff at 1 kHz, and 12-bit resolution across a voltage range of ±2 mV. Additionally, second-order software filters were applied (high-pass >0.016 Hz, low-pass <70 Hz) using the System Plus Evolution software (MicroMed Inc., Houston, TX, USA).
Visual Inspection
The recorded EEG traces were visually inspected by a practicing veterinarian (KHB) for any sign of epileptiform activity.
Control Subjects
In order to reveal any potential anomalies in the sleep structure of epileptic dogs, a group (N=11) of healthy dogs were also measured using the same non-invasive EEG recording protocol. The healthy control group was matched to the patients as much as possible regarding breed, age and gender (
Table 2).
Sleep Macrostructure Scoring
Sleep recordings were visually scored in 20 s epochs according to standardized criteria (Gergely et al., 2020) using a custom-developed software tool (Fercio © Ferenc Gombos, 2012). This method reliably distinguished between wakefulness, drowsiness, non-REM sleep and REM sleep. Given the variability in the duration of individual recordings the first 40 minutes (119 epochs) were analysed for all subjects to ensure standardisation. The coded data were then used to extract the following sleep macrostructure variables: sleep efficiency (% of the recording spent in drowsiness, non-REM and REM), sleep latency (time from recording onset to the first occurrence of non-REM sleep), wakings after sleep onset (total time awake after first drowsiness period; in minutes), drowsiness duration (minutes), non-REM duration (minutes), REM duration (minutes) and REM latency (time from first drowsiness to first REM sleep, in minutes).
Statistical Analysis
Epileptic and control dogs were compared on each sleep macrostructure variable using paired t-tests, as the data met the assumption of normality based on the Shapiro–Wilk test. All statistical tests were carried out with JASP software and SPSS was used for data visualisation.
3. Results
Descriptive Results
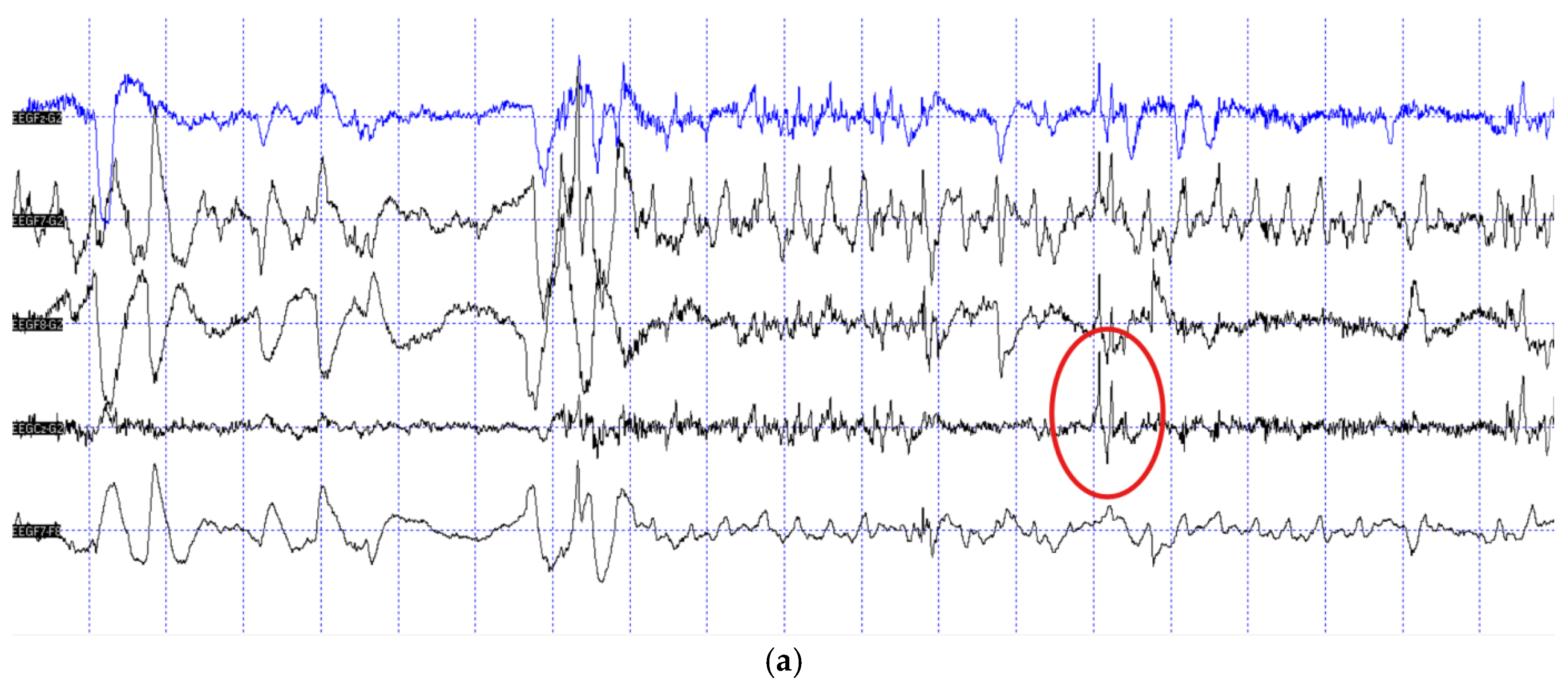
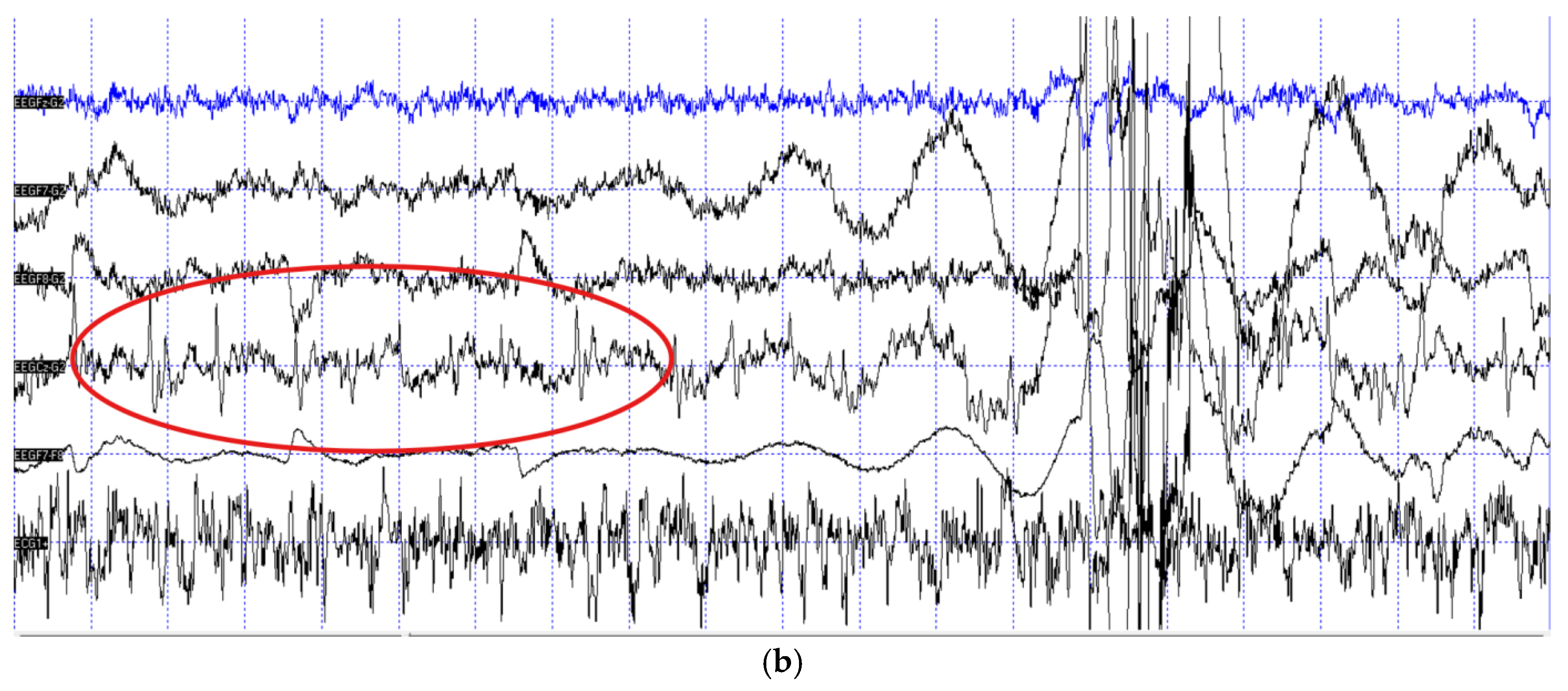
Out of the N=11 patient dogs, N=3 did not fall asleep during the measurement, thus their recordings could not be inspected for epileptiform activity due to muscle artefacts inherent of the awake muscle tone. From the remaining N=8 dogs N=2 provided epilepsy-positive EEG traces (
Figure 1), while the EEG recordings of N= 6 dogs were negative.
Sleep Macrostructure Differences
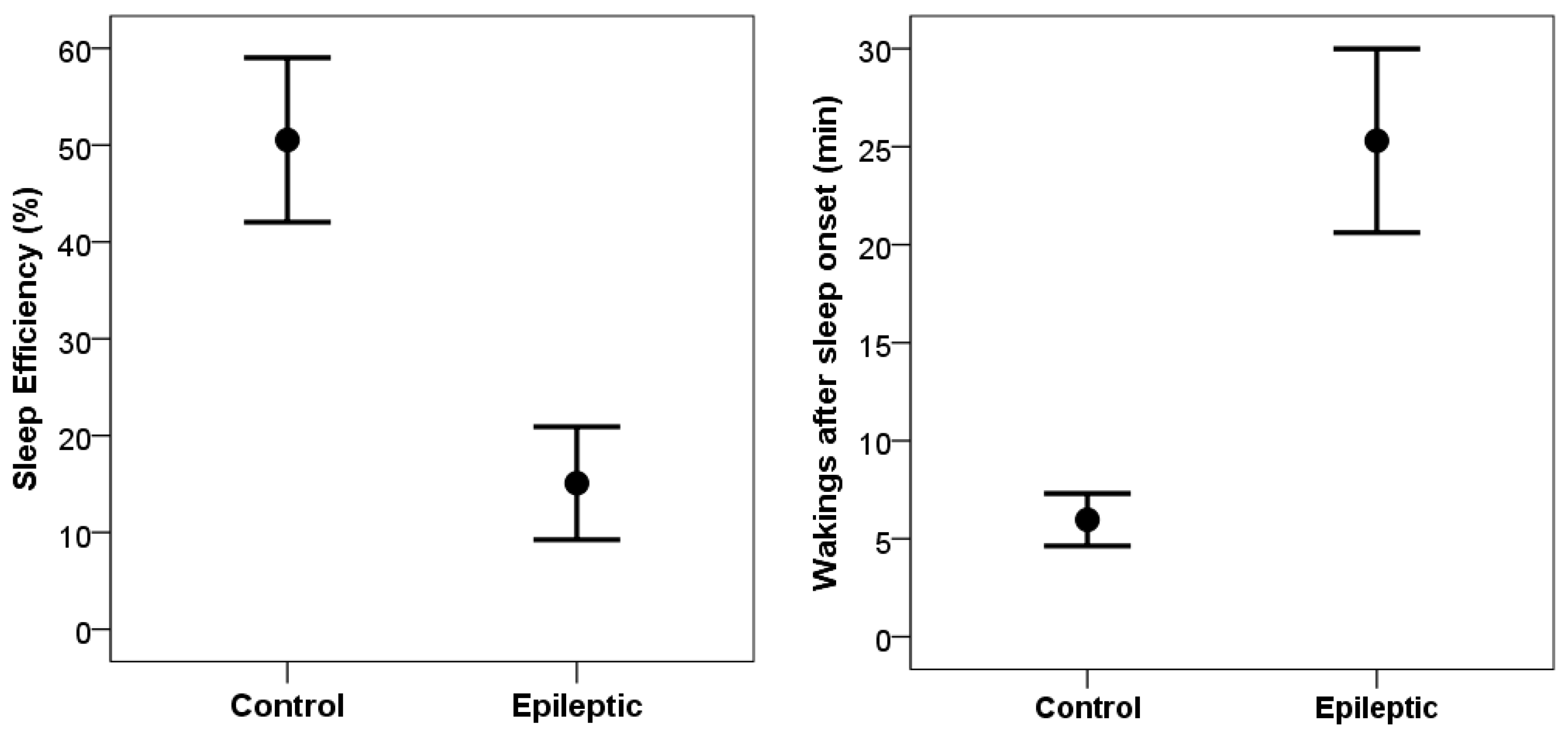
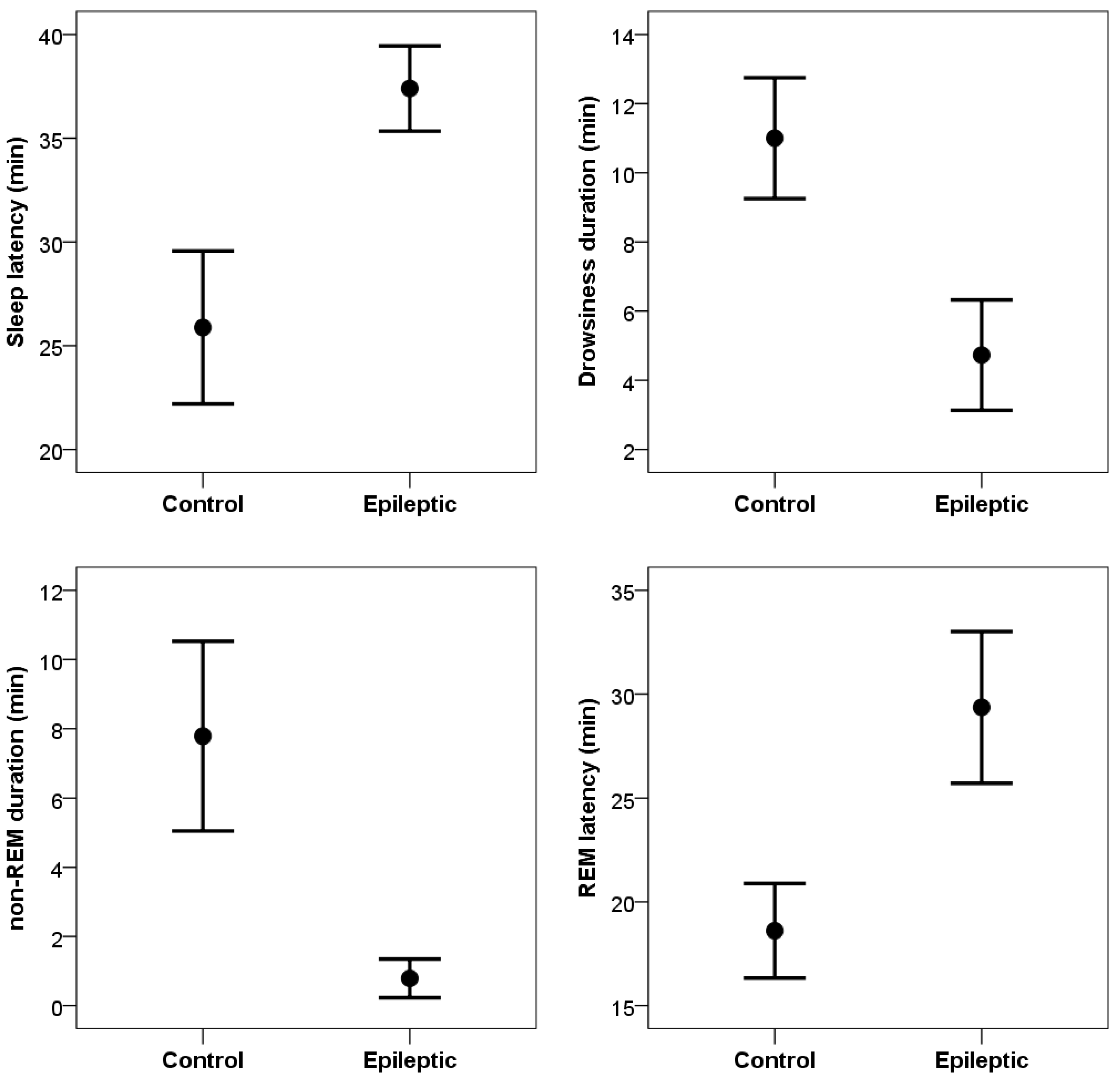
Compared to control subjects, dogs in the epileptic group (
Figure 2) had significantly lower sleep efficiency values (t
(10) = 3.79, p = 0.004; Cohen’s d = 1.14), significantly longer sleep latency (t
(10) = 2.45, p = 0.035; Cohen’s d = 0.74), and significantly more wakings after sleep onset (t
(10) = 4.26, p = 0.002; Cohen’s d = 1.29). Epileptic dogs also spent significantly less time in non-REM sleep (t
(10) = 3.86, p = 0.003; Cohen’s d =1.16) and also less time in drowsiness (t
(10) = 3.72, p = 0,004; Cohen’s d = 1.12). Time spent in REM sleep did not differ between epileptic and control groups (t
(10) = 1.55, p = 0,153; Cohen’s d = 0.47). Latency to reach REM sleep, however, was longer in the epileptic group (t
(10) = 2.84, p = 0.018; Cohen’s d = 0.86).
4. Discussion
The results of the current study demonstrate that epileptiform activity can be detected using EEG traces recorded with the fully non-invasive canine polysomnography protocol. However, some technical challenges were also encountered. A considerable proportion of dogs did not fall asleep during the single recording session, rendering their data unsuitable for analysis. This finding aligns with previous studies suggesting the need for an adaptation sleep session prior to conducting cognitive experiments [
29,
30]. Alongside existing guidelines indicating that sleep and drowsiness facilitate the activation of epileptiform discharges [
31], our results suggest that implementing longer and repeated recordings may be necessary for successful veterinary application of this protocol. A further limitation of the currently used setup is the lack of recording sites over the temporal lobe, which would be necessary to detect temporal lobe epilepsy.
It is also plausible that epileptiform activity is not consistently present in all epileptic dogs during the interval between seizures. Notably, in the current study, epileptiform discharges were observed only in two dogs whose most recent seizures occurred relatively recently (on the previous day and within two weeks), compared to those with negative EEGs where the last seizure occurred up to two months earlier. In human medicine, interictal discharges are detected in only ~50% of known epilepsy cases during the first routine EEG
[31], and earlier canine studies using needle electrodes reported abnormal EEG activity in 65% of epileptic dogs [
21]. Given these benchmarks, the current detection rate of 25% using a non-invasive protocol is promising. While EEG has long been a standard component of epilepsy diagnosis in human medicine, its veterinary application in dogs has typically relied on invasive methods involving sedation and/or using needle electrodes [
32]. This study highlights the potential of non-invasive EEG in the diagnosis of canine epilepsy, and will likely complement modern veterinary practices (such as video-EEG and actigraphy) already under testing [
33]. Nevertheless, it is important to consider that non-invasive EEG techniques are particularly sensitive to movement, electrode placement accuracy, and individual animal behaviour, all of which can influence data quality and interpretation [
34].
The second key finding of this study concerns the considerable alterations observed in the sleep architecture of epileptic dogs compared to healthy controls. These include decreased sleep efficiency, increased latency to both sleep onset and REM sleep, and reduced time spent in drowsiness and non-REM sleep. It is important to note that all epileptic dogs in the study were undergoing pharmacological treatment. Therefore, based solely on this dataset, it is not possible to disentangle the effects of epilepsy itself from those of the medications, or the possible interaction between the two. Previous studies using activity monitoring suggested that antiepileptic drugs are associated with lethargy and lower baseline activity levels in medicated dogs compared to untreated dogs with idiopathic epilepsy; however estimated sleep scored did not differ [
35]. Similarly, a study involving N=4 genetically epileptic Beagles found no difference in the percentage of time spent asleep or awake compared to healthy Beagles, although differences in sleep and REM latency were observed [
36] – the latter findings consistent with the present study. In humans, alterations in sleep architecture are known to depend on seizure type [
25]. Epileptic dogs in the current study exhibited heterogenic seizure types, however due to the limited sample size, we could not test if this has an interacting effect. It is also relevant to consider that antiseizure medications in humans have been shown to partially, but not fully, normalize sleep architecture in epileptic patients [
23]. Therefore, the observed sleep abnormalities in the current study are likely attributable at least in part to the underlying epileptic condition. Even greater differences might be expected between untreated epileptic dogs and healthy controls.
Beyond the welfare-compatible diagnostic potential of non-invasive EEG in dogs, the observed sleep structure alterations may have further implications for behaviour and cognition due to the deteriorating effect of poor sleep quality [
37]. Cognitive decline in epileptic dogs has been shown to be influenced by seizure frequency and the effects of medication [
38,
39]. Dogs experiencing more frequent seizures exhibit greater cognitive deterioration than those with less frequent episodes. Moreover, memory impairments and disorientation tend to worsen as the disease progresses [
38]. The risk of canine cognitive dysfunction is higher among epileptic dogs, particularly in those with a longer history of seizures or with seizure frequency exceeding one episode per week [
39]. At present, there is insufficient evidence to guide targeted treatment for the sleep and cognitive disturbances observed in epileptic dogs, and the causal relationship between these symptoms remains unclear. Some interventions have attempted to improve quality of life through physical exercise [
40]. While such interventions increased sleep scores as expected [
41], they were also associated with a higher monthly seizure frequency [
40], highlighting the complexity of epilepsy management.
In conclusion, while the diagnosis and treatment of canine epilepsy remain multifaceted and challenging, non-invasive methods such as canine polysomnography may offer valuable, welfare-conscious tools for improving both diagnosis and monitoring. These tools may ultimately contribute to a better understanding of the interplay between epilepsy, sleep, and cognition in dogs.
Author Contributions
Conceptualization, K.H.-B., A.P., and A.K.; methodology, A.K.; software, F.G.; formal analysis, K.H.-B., L.K., A.B. and A.K.; investigation, K.H.-B. and L.K.; data curation, all authors; writing—original draft preparation, A.K.; writing—review and editing, all atuthors; visualization, A.K.; supervision, K.H.-B., A.P.; project administration, all authors; funding acquisition, A.K., F.G. All authors have read and agreed to the published version of the manuscript.
Funding
AK was supported by the János Bolyai Research Scholarship and by the Hungarian Brain Research Program (HBRP) 3.0 NAP. FG was supported by the PPKE-BTK-KUT-23-1 project, with funding provided by the Faculty of Humanities and Social Sciences of Pázmány Péter Catholic University.
Institutional Review Board Statement
The present research was carried out following the Hungarian regulations on animal experimentation and the Guidelines for the Use of Animals in Research described by the Association for the Study of Animal Behavior (ASAB). Ethical approval was provided by the Hungarian “Animal Experiments Scientific and Ethical Committee” (PE/EA/55-4/2019).
Informed Consent Statement
All owners volunteered for their dog to participate in the study, and they gave written informed consent. Owners were made aware that they could interrupt the test at any time they felt that their dog was experiencing even minor stress, but this did not happen in any case.
Data Availability Statement
The raw data supporting the conclusions of this article is stored on the institutional NAS server and will be made available by the authors on request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Engel, J.; Timothy, A. Pedley, A.; Aicardi, J. Epilepsy: a comprehensive textbook, 2008. [Google Scholar]
- Podell, M.; Fenner, W.R.; Powers, J.D. Seizure classification in dogs from a nonreferral-based population. J. Am. Vet. Med. Assoc. 1995, 206, 1721–1728. [Google Scholar] [CrossRef] [PubMed]
- Heske, L.; Nødtvedt, A.; Jäderlund, K.H.; Berendt, M.; Egenvall, A. A cohort study of epilepsy among 665,000 insured dogs: Incidence, mortality and survival after diagnosis. Vet. J. 2014, 202, 471–476. [Google Scholar] [CrossRef] [PubMed]
- Lord, L.K.; Podell, M. Owner perception of the care of long-term phenobarbital-treated epileptic dogs. J. Small Anim. Pract. 1999, 40, 11–15. [Google Scholar] [CrossRef]
- Bhatti, S.F.; De Risio, L.; Muñana, K.; Penderis, J.; Stein, V.M.; Tipold, A.; Berendt, M.; Farquhar, R.G.; Fischer, A.; Long, S.; et al. International Veterinary Epilepsy Task Force consensus proposal: Medical treatment of canine epilepsy in Europe. BMC Vet Res 2015, 11, 176. [Google Scholar] [CrossRef]
- Wessmann, A.; Volk, H.A.; Packer, R.M.A.; Ortega, M.; Anderson, T.J. Quality-of-life aspects in idiopathic epilepsy in dogs. Vet. Rec. 2016, 179, 229. [Google Scholar] [CrossRef] [PubMed]
- Packer, R.M.A.; Volk, H.A. Epilepsy beyond seizures: A review of the impact of epilepsy and its comorbidities on health-related quality of life in dogs. Vet. Rec. 2015, 177, 306–315. [Google Scholar] [CrossRef]
- Nettifee, J.A.; Munana, K.R.; Griffith, E.H. Evaluation of the impacts of epilepsy in dogs on their caregivers. J. Am. Anim. Hosp. Assoc. 2017, 53, 143–149. [Google Scholar] [CrossRef]
- Masucci, M.; Di Stefano, V.; Donato, G.; Mangano, C.; De Majo, M. How owners of epileptic dogs living in italy evaluate their quality of life and that of their pet: A survey study. Vet. Sci. 2021, 8. [Google Scholar] [CrossRef]
- Goldberg, E.M.; Coulter, D.A. Mechanisms of epileptogenesis: A convergence on neural circuit dysfunction. Nat. Rev. Neurosci. 2013, 14, 337–349. [Google Scholar] [CrossRef]
- Shorvon, S.D. The etiologic classification of epilepsy. Epilepsia 2011, 52, 1052–1057. [Google Scholar] [CrossRef]
- Potschka, H.; Fischer, A.; Von Rüden, E.L.; Hülsmeyer, V.; Baumgärtner, W. Canine epilepsy as a translational model? Epilepsia 2013, 54, 571–579. [Google Scholar] [CrossRef] [PubMed]
- Chandler, K. Canine epilepsy: What can we learn from human seizure disorders? Vet. J. 2006, 172, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Löscher, W. Dogs as a Natural Animal Model of Epilepsy. Front. Vet. Sci. 2022, 9, 928009. [Google Scholar] [CrossRef]
- Jokinen, T.S.; Metsähonkala, L.; Bergamasco, L.; Viitmaa, R.; Syrjä, P.; Lohi, H.; Snellman, M.; Jeserevics, J.; Cizinauskas, S. Benign familial juvenile epilepsy in lagotto romagnolo dogs. J. Vet. Intern. Med. 2007, 21, 464–471. [Google Scholar] [CrossRef]
- Loscher, W.; Schwartz-Porsche, D.; Frey, H.H.; Schmidt, D. Evaluation of epileptic dogs as an animal model of human epilepsy. Arzneimittel-Forschung/Drug Res. 1985, 35, 82–87. [Google Scholar]
- Steinmetz, S.; Tipold, A.; Löscher, W. Epilepsy after head injury in dogs: A natural model of posttraumatic epilepsy. Epilepsia 2013, 54, 580–588. [Google Scholar] [CrossRef]
- Pitkänen, A.; Immonen, R.J.; Gröhn, O.H.J.; Kharatishvili, I. From traumatic brain injury to posttraumatic epilepsy: What animal models tell us about the process and treatment options. In Proceedings of the Epilepsia; 2009; Vol. 50; pp. 21–29. [Google Scholar]
- Luca, J.; McCarthy, S.; Parmentier, T.; Hazenfratz, M.; Linden, A. Zur; Gaitero, L.; James, F.M.K. Survey of electroencephalography usage and techniques for dogs. Front. Vet. Sci. 2023, 10. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Q.; Li, F.; Zhong, H. Early diagnosis, treatment and prognosis of epilepsy with continuous spikes and waves during slow sleep. Int. J. Clin. Exp. Med. 2015, 8, 4052–4058. [Google Scholar]
- Berendt, M.; Høgenhaven, H.; Flagstad, A.; Dam, M. Electroencephalography in dogs with epilepsy: Similarities between human and canine findings. Acta Neurol. Scand. 1999, 99, 276–283. [Google Scholar] [CrossRef]
- Folkard, E.; Niel, L.; Gaitero, L.; James, F.M.K. Tools and techniques for classifying behaviours in canine epilepsy. Front. Vet. Sci. 2023, 10. [Google Scholar] [CrossRef]
- Yeh, W.C.; Lin, H.J.; Li, Y.S.; Chien, C.F.; Wu, M.N.; Liou, L.M.; Hsieh, C.F.; Hsu, C.Y. Rapid eye movement sleep reduction in patients with epilepsy: A systematic review and meta-analysis. Seizure 2022, 96, 46–58. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.J.; Joo, E.Y.; Hong, S.B. Sleep-wake pattern, chronotype and seizures in patients with epilepsy. Epilepsy Res. 2016, 120, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Sudbrack-Oliveira, P.; Lima Najar, L.; Foldvary-Schaefer, N.; da Mota Gomes, M. Sleep architecture in adults with epilepsy: a systematic review. Sleep Med. 2019, 53, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Bunford, N.; Andics, A.; Kis, A.; Miklósi, Á.; Gácsi, M. Canis familiaris As a Model for Non-Invasive Comparative Neuroscience. Trends Neurosci. 2017. [Google Scholar] [CrossRef]
- Kis, A.; Szakadát, S.; Kovács, E.; Gácsi, M.; Simor, P.; Gombos, F.; Topál, J.; Miklósi, Á.; Bódizs, R. Development of a non-invasive polysomnography technique for dogs (Canis familiaris). Physiol. Behav. 2014, 130, 149–156. [Google Scholar] [CrossRef]
- Bódizs, R.; Kis, A.; Gácsi, M.; Topál, J. Sleep in the dog: comparative, behavioral and translational relevance. Curr. Opin. Behav. Sci. 2020, 33. [Google Scholar] [CrossRef]
- Kis, A.; Szakadát, S.; Gácsi, M.; Kovács, E.; Simor, P.; Török, C.; Gombos, F.; Bódizs, R.; Topál, J. The interrelated effect of sleep and learning in dogs (Canis familiaris); an EEG and behavioural study. Sci. Rep. 2017, 7, 41873. [Google Scholar] [CrossRef]
- Reicher, V.; Kis, A.; Simor, P.; Bódizs, R.; Gombos, F.; Gácsi, M. Repeated afternoon sleep recordings indicate first-night-effect-like adaptation process in family dogs. J. Sleep Res. 2020, 29. [Google Scholar] [CrossRef]
- De Risio, L.; Platt, S. Canine and feline epilepsy: Diagnosis and management; 2014; ISBN 9781780641096.
- Jeserevics, J.; Viitmaa, R.; Cizinauskas, S.; Sainio, K.; Jokinen, T.S.; Snellman, M.; Bellino, C.; Bergamasco, L. Electroencephalography Findings in Healthy and Finnish Spitz Dogs with Epilepsy: Visual and Background Quantitative Analysis. J. Vet. Intern. Med. 2007, 21, 1299–1306. [Google Scholar] [CrossRef]
- Folkard, E.; McKenna, C.; Monteith, G.; Niel, L.; Gaitero, L.; James, F.M.K. Feasibility of in-home electroencephalographic and actigraphy recordings in dogs. Front. Vet. Sci. 2023, 10. [Google Scholar] [CrossRef]
- Kulgod, A.; Linden, D. van der; França, L.G.S.; Jackson, M.; Zamansky, A. Non-invasive canine electroencephalography (EEG): a systematic review. bioRxiv, 5527. [Google Scholar]
- Barry, M.; Cameron, S.; Kent, S.; Barnes-Heller, H.; Grady, K. Daytime and nocturnal activity in treated dogs with idiopathic epilepsy compared to matched unaffected controls. J. Vet. Intern. Med. 2021, 35, 1826–1833. [Google Scholar] [CrossRef] [PubMed]
- Wauquier, A.; Van Den Broeck, W.A.E.; Edmonds, H.L. Sleep in epileptic beagles and antiepileptics. Funct. Neurol. 1986, 1, 53–61. [Google Scholar] [PubMed]
- Bolló, H.; Kovács, K.; Lefter, R.; Gombos, F.; Kubinyi, E.; Topál, J.; Kis, A. REM versus Non-REM sleep disturbance specifically affects inter-specific emotion processing in family dogs (Canis familiaris). Sci. Rep. 2020, 10. [Google Scholar] [CrossRef] [PubMed]
- Packer, R.M.A.; McGreevy, P.D.; Salvin, H.E.; Valenzuela, M.J.; Chaplin, C.M.; Volk, H.A. Cognitive dysfunction in naturally occurring canine idiopathic epilepsy. PLoS One 2018, 13. [Google Scholar] [CrossRef]
- Winter, J.; Packer, R.M.A.; Volk, H.A. Preliminary assessment of cognitive impairments in canine idiopathic epilepsy. Vet. Rec. 2018, 182. [Google Scholar] [CrossRef]
- Grady, K.; Cameron, S.; Kent, S.P.; Barnes Heller, H.; Barry, M.M. Effect of an intervention of exercise on sleep and seizure frequency in idiopathic epileptic dogs. J. Small Anim. Pract. 2023, 64, 59–68. [Google Scholar] [CrossRef]
- Bunford, N.; Reicher, V.; Kis, A.; Ákos, P.; Ferenc, G.; Bódizs, R.; Gácsi, M.; Pogány, Á.; Gombos, F.; Bódizs, R.; et al. Differences in pre-sleep activity and sleep location are associated with variability in daytime/nighttime sleep electrophysiology in the domestic dog. Sci. Rep. 2018, 8. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).