1. Introduction
The global COVID-19 vaccination campaign has been unprecedented in speed, scope, and ambition. Endorsed by health authorities worldwide as a necessary exit strategy from the COVID-19 crisis, the rollout was justified on the grounds of reducing viral transmission, severe illness, and death - as the Lancet Commission on lessons for the future from the COVID-19 pandemic put it: "Vaccines are the single most important medical technology to bring the COVID-19 pandemic under control" and their “early distribution in high-income countries offered the hope of a return to normalcy" (Sachs et al., 2022) (p. 21). However, as with any mass medical intervention, the assessment of benefits must be weighed against potential risks, especially when vulnerable populations, often excluded from initial clinical trials – such as individuals suffering from autoimmune disorders - are targeted for intervention precisely given their vulnerability (American College of Rheumatology, 2021).
In a recently completed scoping review of 109 peer-reviewed articles, we documented substantial and diverse reports of associations between COVID-19 vaccines and postvaccination autoimmune disorders, both among individuals with autoimmune disorders – in the forms of flares/relapses and new autoimmune disorders – as well as in individuals with no prior autoimmunity (Chaufan et al., 2025). Studies included well-documented clinical case reports, case series, and observational studies. Importantly, multiple studies provided mechanistic explanations for how COVID-19 vaccines could plausibly trigger autoimmune responses. These included established pathways such as molecular mimicry, bystander activation, epitope spreading, polyclonal activation, and adjuvant-induced immune stimulation - mechanisms historically observed in post-vaccination autoimmunity involving other vaccines (Cohen & Shoenfeld, 1996; Molina & Shoenfeld, 2005; Shoenfeld & Aron-Maor, 2000).
The study outlined in this protocol was prompted by the need to examine whether - and how - the literature on COVID-19 vaccines and autoimmunity has maintained consistency and transparency in its treatment of the risks and benefits of vaccination. While the biomedical literature has focused mostly on vaccine benefits – relying largely on modeling studies to assert that COVID-19 vaccination has saved “millions of lives” (Imperial College London 2022; Mellis 2022; Prof Peter Hotez MD PhD [@PeterHotez] 2025; Watson et al. 2022) - an emerging body of research actually points to COVID-19 vaccine harms – such as autoimmune complications, at least in some individuals (Guo et al., 2023; Jara et al., 2022; Lim et al., 2025; Mahroum & Shoenfeld, 2022; Watad et al., 2021).
The growing literature on autoimmune disorders postvaccination notwithstanding, consistent gaps remain. First, in the course of conducting our scoping review, we observed a disconnect between the presence of mechanistic plausibility and the conclusions drawn in many studies, with many authors documenting findings that supported a plausible vaccine-autoimmunity link yet concluding that no causal relationship could be established or implied. While such caution may be warranted given limitations of study design or data, it also raises questions about consistency in interpretive practice - precisely given study design and data limitations, a similar caution should apply to vaccine benefits, often claimed in the conclusion. Second, we found a tendency to apply different evidentiary thresholds to statements of vaccine benefits and vaccine harms, even within the same article. This epistemic asymmetry - wherein benefit claims are often asserted without corresponding rigor or explicit data, while harm claims are accompanied by hedging language and disclaimers - prompted us to explore this dynamic more systematically.
One other salient observation in our scoping review examining the relationship between COVID-19 vaccination and autoimmune disorders was that the Bradford Hill criteria – long recognized as foundational in evaluating causal relationships (Hill, 1965) – were notably underused or inconsistently applied. These criteria include key elements such as temporal sequence, strength and consistency of association, biological plausibility, and dose-response relationship. Their absence, combined with improperly supported benefits and safety claims and rhetorical minimization of risks, raises concerns about the standards being applied to interpret vaccine-related harms. This project aims to shed light on those interpretive standards, assess how the literature discusses the possibility of causality, and whether it does so with integrity.
This planned, two-phase follow-up study builds directly on our scoping review dataset and adopts a critical realist lens to explore both the biological and epistemic dimensions of the COVID-19 vaccine–autoimmunity literature. The first phase of the study will map the mechanisms of action described in the literature and assess whether these are consistent with the interpretive claims made by authors. The second phase will apply a structured coding tool to evaluate the epistemic integrity of each article, focusing on how consistently authors apply evidentiary standards to claims of benefits versus harms.
Although methodologically and conceptually aligned, each phase is self-contained. Together, however, both phases will trace a continuum - from biological plausibility to epistemological judgment - revealing how the interpretation of evidence about vaccine safety may be shaped not only by data, but by contrasting rhetorical, institutional, and epistemic norms.
2. Aims and Objectives
The two-phase planned study aims to evaluate how the biomedical literature addressing COVID-19 vaccines and autoimmune disorders frames questions of risk, benefit, and causality. Building on a comprehensive scoping review, it will seek to clarify how mechanistic plausibility and evidentiary reasoning are treated in the interpretation of findings. Phase 1 will assess whether the biological mechanisms reported by study authors are consistent with their interpretive conclusions. Phase 2 will apply an epistemic integrity framework to examine the standards of evidence applied to benefit and harm claims. By integrating mechanistic and epistemological analyses, the planned study will contribute a critical empirical and conceptual lens for assessing the evidentiary logic of COVID-19 vaccine safety research.
Objectives include:
3. Methods
Study Design
This protocol outlines a qualitative, two-phase, document-based study grounded in a critical realist philosophy of science. It builds directly on 109 studies previously selected and analyzed in a comprehensive scoping review using PRISMA-ScR guidelines and stored in full-text format (Chaufan et al., 2025). A qualitative content analysis approach will be applied to extract, categorize, and interpret data from these 109 full-text scientific articles and to appraise how these articles report, interpret, and contextualize associations between COVID-19 vaccination and autoimmune disorders. Qualitative content analysis is well suited for both phases of this project, as it enables systematic coding of textual data and facilitates the breakdown of published literature into manageable analytic units, while preserving contextual meaning (Hsieh & Shannon, 2005), thus aligning well with the study’s critical realist orientation by foregrounding the relationship between empirical observation and theoretical interpretation. It also allows for a clear mapping of how claims are framed relative to the data presented.
Each phase will employ a tailored methodological framework aligned with its respective objective. In Phase 1, this method will allow for the transparent classification of immunological mechanisms and the assessment of their interpretive treatment by study authors. In Phase 2, it will provide a basis for evaluating epistemic integrity using a reproducible typology that classifies articles based on internal consistency, evidentiary rigor, and framing asymmetries. This combination should support a robust and transparent examination of how scientific literature on COVID-19 vaccine safety represents uncertainty, causality, and risk.
This phase will extract mechanistic explanations provided by study authors and classify them into immunological categories such as molecular mimicry, bystander activation, cytokine dysregulation, and ASIA (autoimmune/inflammatory syndrome induced by adjuvants). It will also extract authors' conclusions regarding causality or lack thereof and evaluate the extent to which these are consistent with the biological mechanisms proposed. The goal is to examine whether the logic used in interpretation is coherent with the reported data. Attention will also be given to rhetorical strategies - hedging, disclaimers, or narrative distancing - in authors’ interpretive statements.
This phase will re-analyze the same set of articles using a structured typology of epistemic integrity. Quotations related to autoimmune harms, safety and efficacy claims, causal reasoning, and rhetorical framing will be extracted. Each article will be classified according to a four-level typology of epistemic integrity (high, moderate, low, or neutral), previously developed through inductive and deductive iteration during the scoping review. Coding will assess internal consistency, evidentiary rigour, and the symmetry of standards applied to benefit and harm claims.
A visual summary of the study design, including both phases and their respective goals, methods, and outputs, is provided in the flowchart below:
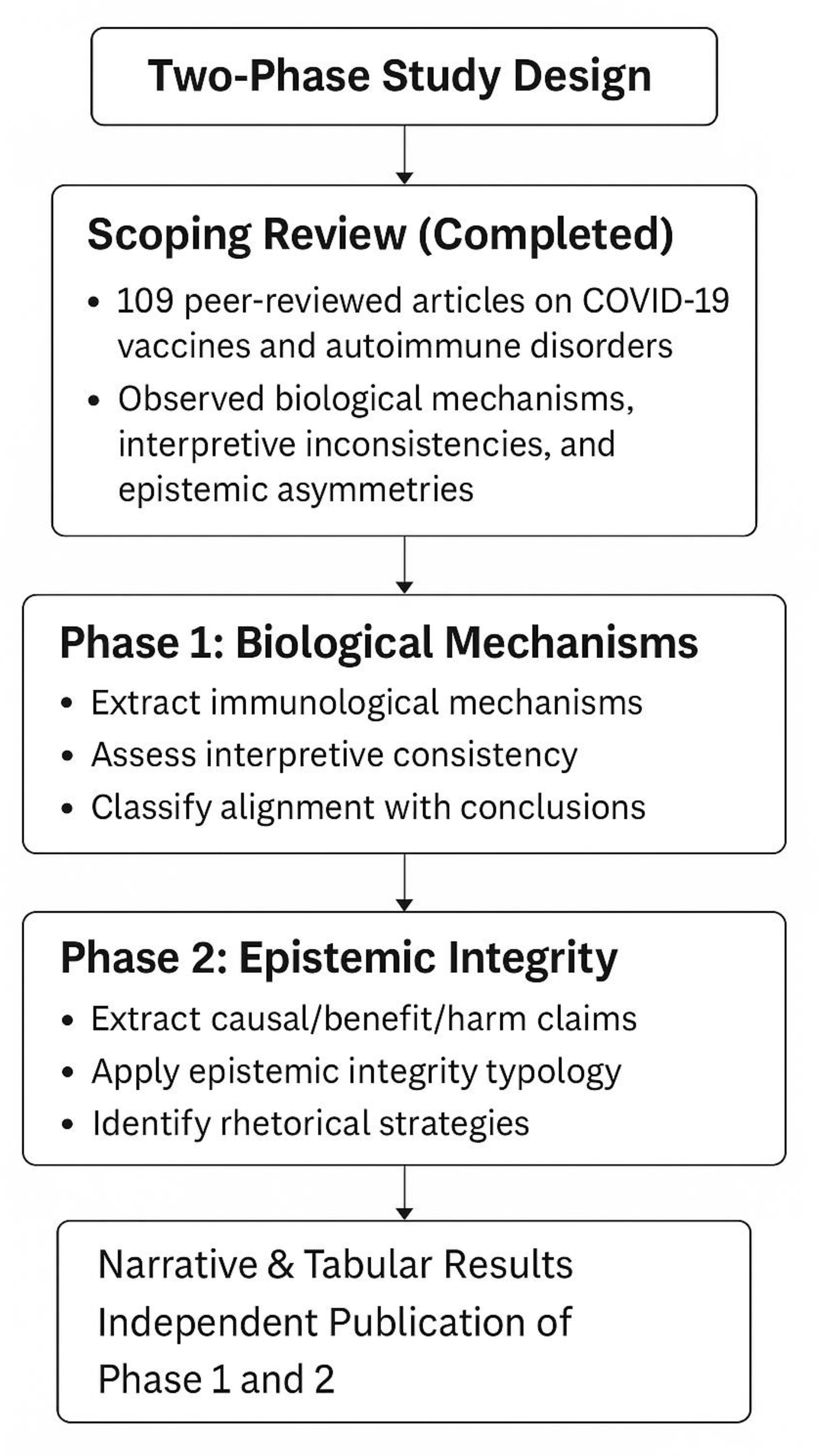
The data for this planned study have already been collected as part of a completed scoping review conducted in 2024–2025. The full dataset consists of 109 peer-reviewed articles, all of which are stored in full-text PDF format and have been archived for analysis. These texts represent the empirical foundation for the coding and classification phases described earlier.
Each phase will involve systematic coding of the 109 peer-reviewed articles selected in the published scoping review. Each article will be closely read by two independent coders, who will follow structured extraction templates tailored to each phase, with reflexive memos maintained to ensure transparency.
Articles will be coded for:
Mechanisms of action proposed to explain associations between COVID-19 vaccines and autoimmune disorders, classified into predefined immunological categories (e.g., molecular mimicry, bystander activation, cytokine dysregulation, epitope spreading, ASIA).
Descriptions of autoimmune outcomes (e.g., flares, new-onset conditions) and affected populations (e.g., pre-existing autoimmune disorder, no prior autoimmunity).
Interpretive conclusions related to causality (e.g., explicit denial, assertion, hedging, or neutrality).
Rhetorical framing strategies, such as minimization ("rare"), emphasis ("severe"), disclaimers, or qualification.
An Excel sheet will be used to document both mechanistic content and interpretive alignment. The analysis will examine whether mechanisms are used as support for causal reasoning or are rhetorically neutralized. Of note, it will not treat the absence of causal conclusions as evidence of bias. Rather, it will examine whether the level of caution applied to mechanistic findings is consistent with the level of certainty applied to claims of benefit within the same article. Quotations to support this analysis will be extracted verbatim and include page references.
This phase will apply a previously developed four-level typology of epistemic integrity (high, moderate, low, neutral) to evaluate how each article frames vaccine-related benefits and harms. Quotations will be extracted verbatim and recorded into an Excel sheet for:
Descriptions of autoimmune harms or adverse events
Statements about vaccine efficacy and safety
Causal framing and rhetorical qualifiers (e.g., “rare,” “anecdotal,” “safe,” “highly effective”)
The classification will be based on operational indicators across four domains: harms framing, efficacy claims, causal reasoning, and epistemic symmetry. Illustrative examples from the dataset will be included to show how the classification was applied.
Table 1 presents a typology summarizing the key indicators for each tier.
Finally, some statements may be relevant to both phases - for example, when conclusions reference mechanistic evidence while also asserting benefits or minimizing harms. In such cases, the same quotation may be analyzed in both phases but evaluated through distinct coding frameworks: Phase 1 will assess alignment between the author’s interpretation of their mechanistic evidence and their conclusion, while Phase 2 will assess the consistency of evidentiary standards applied across claims of benefit and harm. Coders will be trained to apply phase-specific criteria to ensure analytical separation.
Reliability Measures
Each article will be coded independently by two team members. Disagreements will be resolved through discussion. Inter-rater reliability will be assessed after the first 10 articles. If agreement falls below 80%, the coding guidelines will be refined to enhance consistency. This threshold aligns with established standards in qualitative research, where 80% agreement is commonly regarded as acceptable for ensuring coding reliability (Miles & Huberman, 1994).
In cases of persistent ambiguity or borderline judgments, the team will record consensus notes to document interpretive reasoning and ensure transparency. If necessary, the codebook will be iteratively revised to reflect these refinements and applied retroactively to previously coded material to ensure consistency across the dataset.
Data Analysis
For both phases of the study, data will be analyzed descriptively and thematically. Phase 1 will quantify the types of mechanisms reported, assesses their alignment with study conclusions, and identify interpretive patterns. Phase 2 will summarize epistemic integrity ratings and document recurring forms of rhetorical minimization, evidentiary asymmetry, and inconsistencies in causal reasoning. Findings will be presented in both narrative and tabular formats.
Across both phases, special attention will be given to the presence or absence of Bradford Hill criteria in authors' interpretations of causality. These criteria - such as temporality, biological plausibility, strength of association, and dose-response - serve as well-established heuristics in causal inference (Hill, 1965). In this planned study, criteria will be coded both individually and holistically. For example, temporality will be tracked as a discrete element in Phase 1, particularly in cases where acute autoimmune onset follows closely after vaccination but is dismissed without consideration. Meanwhile, the broader interpretive use (or neglect) of Bradford Hill–style reasoning will be qualitatively assessed in both phases to understand how authors engage with, or avoid, structured causal logic. To enhance interpretive transparency, clarify how judgments will be made regarding mechanistic consistency (Phase 1) and epistemic integrity (Phase 2), and highlight typical patterns of alignment or divergence between evidence and interpretation,
Table 2 includes representative examples to illustrate how these assessments will be operationalized.
Expected Outcomes
This two-phase planned study is expected to yield a range of scholarly and practical outputs across both mechanistic and epistemic domains. Each phase will generate distinct yet complementary contributions to the literature on vaccine safety, scientific communication, and methodological rigour.
A peer-reviewed article reporting on the classification of mechanistic pathways proposed in the 109 reviewed studies, alongside an analysis of whether and how these pathways align with authors’ conclusions.
A biological plausibility map that synthesizes common mechanisms of autoimmune reactivity, presented in tabular and narrative form.
A perspective article addressing debates on causality in clinical interpretation and drawing attention to rhetorical patterns and logical inconsistencies in the treatment of mechanistic evidence, suitable for a general science or public health audience
A peer-reviewed article detailing epistemic integrity classifications using the four-tiered typology across the same 109 articles.
A methodological paper outlining the development, operationalization, and application of the epistemic integrity framework.
A perspective article addressing epistemic norms in vaccine safety discourse, suitable for a general science or public health audience.
Collectively, the planned study is expected to generate systematic insights into how post-vaccination autoimmune harms are framed in the scientific literature - both in relation to biological mechanisms and epistemological reasoning. The results will contribute to a more rigorous and transparent evaluation of scientific communication, with implications for future vaccine safety research, clinical decision-making, and medical ethics.
Reflexivity and Researcher Positioning
Following Malterud (2001), reflexivity in this planned study is understood as methodological accountability - a recognition that all research is shaped by the perspectives, commitments, and situated knowledge of its investigators (Malterud, 2001). Reflexivity is not adopted here as personal disclosure or ideological positioning, but as a tool to enhance transparency and rigour across both phases of the study. The principal investigator (CC) brings to this project an interdisciplinary background in clinical medicine, medical sociology, bioethics, and critical policy studies. Her engagement with this topic stems from longstanding concerns about the narrowing of scientific discourse on COVID-19 vaccination and the exclusion of legitimate scrutiny, especially in the health and postsecondary education sectors (Chaufan, 2023; Chaufan & Hemsing, 2024). This perspective is grounded in the evidence compiled during the scoping review, which documented both a biological basis for autoimmune harms and inconsistent standards in their reporting. The overarching aim is not to affirm predetermined conclusions but to investigate how scientific claims are constructed and evaluated. The typology and coding frameworks used in each phase are designed to be reproducible, criteria-based, and sensitive to both high- and low-integrity reporting. Inter-rater calibration, paired coding, and consensus discussions are incorporated throughout, ensuring that classification decisions are dialogical and collectively reasoned. In this sense, the study will reflect dialogical reflexivity, where interpretations are shaped through collaborative scrutiny rather than individual bias (Barry et al., 1999). While this protocol is authored solely by the principal investigator, the study will be executed by a three female interdisciplinary team, including the second author of the completed scoping review and a junior investigator who provided research assistance in support of its publication.
Limitations
The study outlined in this protocol has several limitations. First, it will not assess the methodological quality of the reviewed studies or the clinical significance of their findings. This is because its purpose is not to validate or critique the biomedical content of each article, but to examine how that content is interpreted and framed - mechanistically and epistemologically. Second, some critics may argue that the planned study presupposes a skeptical or oppositional stance toward COVID-19 vaccination. However, in both phases, the analysis will be driven by structured frameworks - classification of mechanistic pathways in Phase 1, and a typology of epistemic integrity in Phase 2. These tools are designed to evaluate internal consistency and evidentiary reasoning, not to affirm or refute vaccine safety. Articles will be evaluated based on explicit criteria and assigned classifications reflective of their own content, including positive or high-integrity findings when appropriate. The risk of subjectivity, inherent to all interpretive research, will be mitigated through dual coding, inter-rater calibration, and consensus-based adjudication.
Third, critics may argue that the planned study’s reliance on a preselected sample - the 109 articles identified in a prior scoping review - limits the generalizability of its findings. However, the goal is not statistical generalization but analytical transferability. The aim is to develop and apply structured tools that can be used in other datasets and domains. While the sample reflects a specific subset of COVID-19 vaccine literature, the conceptual logic underpinning the coding frameworks is not bound to these articles or to autoimmunity specifically. Fourth, some may contend that the focus on autoimmune harms narrows the scope or limits the relevance of the project, especially compared to more widely discussed post-vaccination outcomes such as myocarditis. However, the selection of autoimmune disorders was both empirically and conceptually grounded. Autoimmune complications were reported early in the vaccine rollout yet received limited institutional or media attention. Moreover, as autoimmune conditions frequently involve diagnostic ambiguity and long-term progression, they offer a unique lens into how uncertainty and causality are interpreted in the scientific literature. The study builds on prior immunological research - such as the work of Shoenfeld and colleagues - while also applying a sociological lens to explore how emerging risks are rhetorically managed.
Finally, because the planned study spans two distinct domains—biological mechanisms and epistemic framing - some may view its goals as overly ambitious or heterogeneous. Yet it is precisely the combination of biological plausibility (Phase 1) and epistemic integrity (Phase 2) that will allow the project to trace how scientific claims are constructed, validated, or discounted. The frameworks used in each phase are distinct but aligned, and together they offer a novel contribution to the critical evaluation of scientific literature on vaccine safety. The value of the overall project lies in its capacity to expose interpretive dynamics that shape what is seen - and what is sidelined - in the production of medical knowledge.
Significance
The study outlined in this protocol should contribute to growing efforts to assess the epistemic and interpretive foundations of vaccine safety science. It will offer a two-phase model for evaluating how the peer-reviewed literature constructs, communicates, and potentially distorts scientific claims about COVID-19 vaccine safety - particularly in relation to autoimmune harms. By combining mechanistic analysis with an assessment of epistemic integrity, the planned study will trace how empirical signals are translated into interpretive claims, and how causal reasoning is comparatively applied to claims about benefits and claims about harms.
The planned study is also expected to yield findings of broader relevance to ongoing concerns about bias, transparency, and the politicization of science during declared public health emergencies. Inadequate epistemic practices - such as rhetorical minimization of harm, asymmetrical evidentiary standards, and uncritical repetition of safety claims - can undermine scientific progress, informed consent, and democratic public health governance. These concerns are magnified when access to raw clinical trial data remains restricted and when positive portrayals of pharmaceutical interventions are selectively emphasized (Gøtzsche, 2011). By focusing on epistemic consistency and interpretive transparency, this project will advance a structured framework for accountability in vaccine safety research. Further, its methodological tools may be transferable to other domains of biomedical literature where claims of harms and benefits remain contested, and where scientific narratives require closer scrutiny.
Funding
This protocol received no specific funding.
Ethical Considerations
As this study involves secondary analysis of publicly available documents, it does not involve human subjects and is exempt from research ethics board review.
Data Availability Statement
This study builds on 109 peer-reviewed articles previously identified and analyzed in a published scoping review (Chaufan et al. 2025; Preprints.org,
https://www.preprints.org/manuscript/202506.0831/v1). Full-text versions of all articles are publicly available through academic databases and have been catalogued by the research team. Data extraction will rely exclusively on these sources. No additional datasets were generated or analyzed for this protocol.
Acknowledgments
The author wishes to acknowledge earlier conversations with Dr. Laurie Manwell that stimulated this study and the research assistance of Annin Mohamed. Authorship in future publications will align with ICJME guidelines (ICMJE, 2019).
References
- American College of Rheumatology. (2021). ACR Releases Updated COVID-19 Vaccine Clinical Guidance. https://rheumatology.org/press-releases/acr-releases-updated-covid-19-vaccine-clinical-guidance-including-timing-3rd-doses-with-immunomodulatory-drugs.
- Barry, C. A., Britten, N., Barber, N., Bradley, C., & Stevenson, F. (1999). Using Reflexivity to Optimize Teamwork in Qualitative Research. Qualitative Health Research, 9(1), 26–44. [CrossRef]
- Chaufan, C. (2023). Is Covid-19 “vaccine uptake” in postsecondary education a “problem”? A critical policy inquiry. Health, 13634593231204169. [CrossRef]
- Chaufan, C., & Hemsing, N. (2024). Is resistance to Covid-19 vaccination a “problem”? A critical policy inquiry of vaccine mandates for healthcare workers. AIMS Public Health, 11(3), Article publichealth-11-03-035. [CrossRef]
- Chaufan, C., Manwell, L., Heredia, C., & McDonald, J. (2025). COVID-19 Vaccination and Autoimmune Disorders: A Scoping Review (2025060831). Preprints. [CrossRef]
- Cohen, A. D., & Shoenfeld, Y. (1996). Vaccine-induced autoimmunity. Journal of Autoimmunity, 9(6), 699–703. [CrossRef]
- Gøtzsche, P. C. (2011). Why we need easy access to all data from all clinical trials and how to accomplish it. Trials, 12(1), 249. [CrossRef]
- Guo, M., Liu, X., Chen, X., & Li, Q. (2023). Insights into new-onset autoimmune diseases after COVID-19 vaccination. Autoimmunity Reviews, 22(7), 103340. [CrossRef]
- Hill, S. A. B. (1965). The Environment and Disease: Association or Causation? 6.
- Hodges, B. D., Kuper, A., & Reeves, S. (2008). Discourse analysis. BMJ, 337, a879. [CrossRef]
- Hsieh, H.-F., & Shannon, S. E. (2005). Three approaches to qualitative content analysis. Qualitative Health Research, 15(9), 1277–1288. [CrossRef]
- ICMJE. (2019). Recommendations for the Conduct, Reporting, Editing, and Publication of Scholarly Work in Medical Journals. http://www.icmje.org/recommendations/.
- Imperial College London. (2022, June 24). Vaccinations may have prevented almost 20 million COVID-19 deaths worldwide | Imperial News | Imperial College London. Imperial News. https://www.imperial.ac.uk/news/237591/vaccinations-have-prevented-almost-20-million/.
- Jara, L. J., Vera-Lastra, O., Mahroum, N., Pineda, C., & Shoenfeld, Y. (2022). Autoimmune post-COVID vaccine syndromes: Does the spectrum of autoimmune/inflammatory syndrome expand? Clinical Rheumatology, 41(5), 1603–1609. [CrossRef]
- Lim, E., Kim, Y. H., Jeong, N.-Y., Kim, S.-H., Won, H., Bae, J.-S., Choi, N.-K., & Committee (CoVaSC), C.-19 V. S. (2025). The association between acute transverse myelitis and COVID-19 vaccination in Korea: Self-controlled case series study. European Journal of Neurology, 32(1), e70020. [CrossRef]
- Mahroum, N., & Shoenfeld, Y. (2022). COVID-19 vaccination can occasionally trigger autoimmune phenomena, probably via inducing age-associated B cells. International Journal of Rheumatic Diseases, 25(1), 5–6. [CrossRef]
- Malterud, K. (2001). Qualitative research: Standards, challenges, and guidelines. The Lancet, 358(9280), 483–488. [CrossRef]
- Mellis, C. (2022). Lives saved by COVID-19 vaccines. Journal of Paediatrics and Child Health, 10.1111/jpc.16213. [CrossRef]
- Miles, M. B., & Huberman, A. M. (1994). Qualitative Data Analysis: An Expanded Sourcebook (2nd edition). Sage Publications Inc.
- Molina, V., & Shoenfeld, Y. (2005). Infection, vaccines and other environmental triggers of autoimmunity. Autoimmunity, 38(3), 235–245. [CrossRef]
- Prof Peter Hotez MD PhD [@PeterHotez]. (2025, February 4). Here’s where the 3.2 million lives saved comes from https://t.co/A7Z1Vc2VL2 [Tweet]. Twitter. https://x.com/PeterHotez/status/1886573301977284968.
- Sachs, J. D., Karim, S. S. A., Aknin, L., Allen, J., Brosbøl, K., Colombo, F., Barron, G. C., Espinosa, M. F., Gaspar, V., Gaviria, A., Haines, A., Hotez, P. J., Koundouri, P., Bascuñán, F. L., Lee, J.-K., Pate, M. A., Ramos, G., Reddy, K. S., Serageldin, I., … Michie, S. (2022). The Lancet Commission on lessons for the future from the COVID-19 pandemic. The Lancet, 0(0). [CrossRef]
- Shoenfeld, Y., & Aron-Maor, A. (2000). Vaccination and Autoimmunity—‘vaccinosis’: A Dangerous Liaison? Journal of Autoimmunity, 14(1), 1–10. [CrossRef]
- Watad, A., De Marco, G., Mahajna, H., Druyan, A., Eltity, M., Hijazi, N., Haddad, A., Elias, M., Zisman, D., Naffaa, M. E., Brodavka, M., Cohen, Y., Abu-Much, A., Abu Elhija, M., Bridgewood, C., Langevitz, P., McLorinan, J., Bragazzi, N. L., Marzo-Ortega, H., … McGonagle, D. (2021). Immune-Mediated Disease Flares or New-Onset Disease in 27 Subjects Following mRNA/DNA SARS-CoV-2 Vaccination. Vaccines, 9(5), Article 5. [CrossRef]
- Watson, O. J., Barnsley, G., Toor, J., Hogan, A. B., Winskill, P., & Ghani, A. C. (2022). Global impact of the first year of COVID-19 vaccination: A mathematical modelling study. The Lancet Infectious Diseases, 22(9), 1293–1302. [CrossRef]
Table 1.
- Operational Criteria for Epistemic Integrity.
Table 1.
- Operational Criteria for Epistemic Integrity.
| Level |
Harms Reporting |
Safety/Efficacy Reporting |
Causal Reasoning |
Epistemic Symmetry |
| High Integrity |
Reports adverse events or harms without minimizing language. |
Benefit claims cautiously stated and directly supported by article data or external yet relevant data. |
Clear acknowledgment of uncertainty and transparency about causality limits; incorporates temporality, biological plausibility, or dose-response reasoning where applicable. |
Consistent evidentiary standards applied to both harms and benefits.
Avoids generalizing beyond scope of data |
| Moderate Integrity |
May acknowledge harms (e.g., flares) but qualifies them with minimization (e.g., 'rare'). |
Claims supported by data, yet external or not relevant to study population |
Causality mentioned inconsistently or without deeper engagement; may invoke plausible mechanisms but without clarity or follow-through. |
Partial symmetry: harms treated cautiously, benefits emphasized. |
| Low Integrity |
Downplays harms or uses dismissive terms (e.g., 'anecdotal'), or neutralizes them with unqualified benefit claims |
Unqualified claims or appeals to external authority with no supporting data. |
Causal attribution used only to dismiss harms; little to no engagement with plausible mechanisms or relevant temporal sequences.. |
Double standards: harms questioned, benefits accepted uncritically. |
| Epistemically Neutral |
Does not address harms or efficacy directly (e.g., purely serological or immunological studies).
May include studies with vaccine-adjacent data (e.g., serology) that are interpreted without framing efficacy or safety. |
No efficacy or policy claims included. |
May present data (e.g., serology, antibody response) without framing implications for vaccine efficacy or safety; no causal interpretation offered |
Not engaged: no interpretation or comparative framing. |
Table 2.
Illustrative Examples of Coding Assessments Across Study Phases.
Table 2.
Illustrative Examples of Coding Assessments Across Study Phases.
| Phase |
Case Summary |
Key Observation |
Interpretive Pattern |
Assessment |
| Phase 1: Mechanistic Plausibility |
Alroughani et al. (2022): MS flares and relapses post-vaccination documented in 26 individuals. |
Temporal proximity and clinical confirmation of relapses, but conclusion states no increased risk. |
Reaffirmation of safety despite data suggesting risk; no mechanistic rebuttal offered. |
Misalignment between findings and conclusions. |
| Phase 2: High Epistemic Integrity |
Singh et al. (2022): Case of post-vaccine seropositive rheumatoid arthritis. |
Temporal onset clearly described; mechanism discussed; no exaggeration of safety. |
Cautious, evidence-based interpretation without rhetorical minimization or unsupported claims. |
High epistemic integrity. |
| Phase 2: Low Epistemic Integrity |
Allen-Philbey et al. (2022): 193 MS patients, 87% had symptoms after first dose; two deaths reported. |
Adverse events minimized as “temporary” and “similar to general population” without data. |
Discrepancy between adverse event rate and safety claims; unrelated studies used to reaffirm safety. |
Low epistemic integrity. |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).






