Submitted:
11 June 2025
Posted:
12 June 2025
You are already at the latest version
Abstract
Keywords:
-
Highlights:
- It is possible to remove mixtures of dyes from different classes by more than >90% in a sequential reactor.
- Constant aeration is not necessary for effective removal of dyes when using immobilised mycelium.
- Strains within a species differ in their potential ability to remove pollutants, including the dominant mechanism.
1. Introduction
2. Materials and Methods
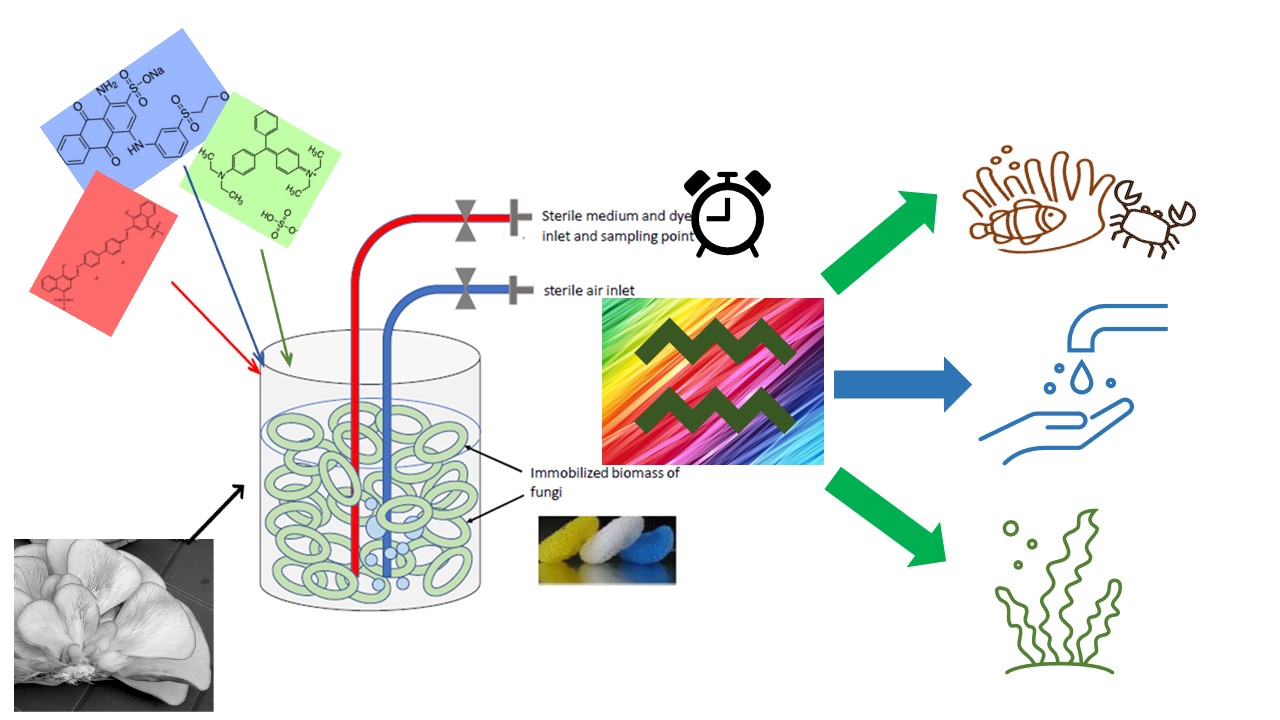
2.1. Bioreactors
2.2. Toxicity Assessment
3. Results and Discussion
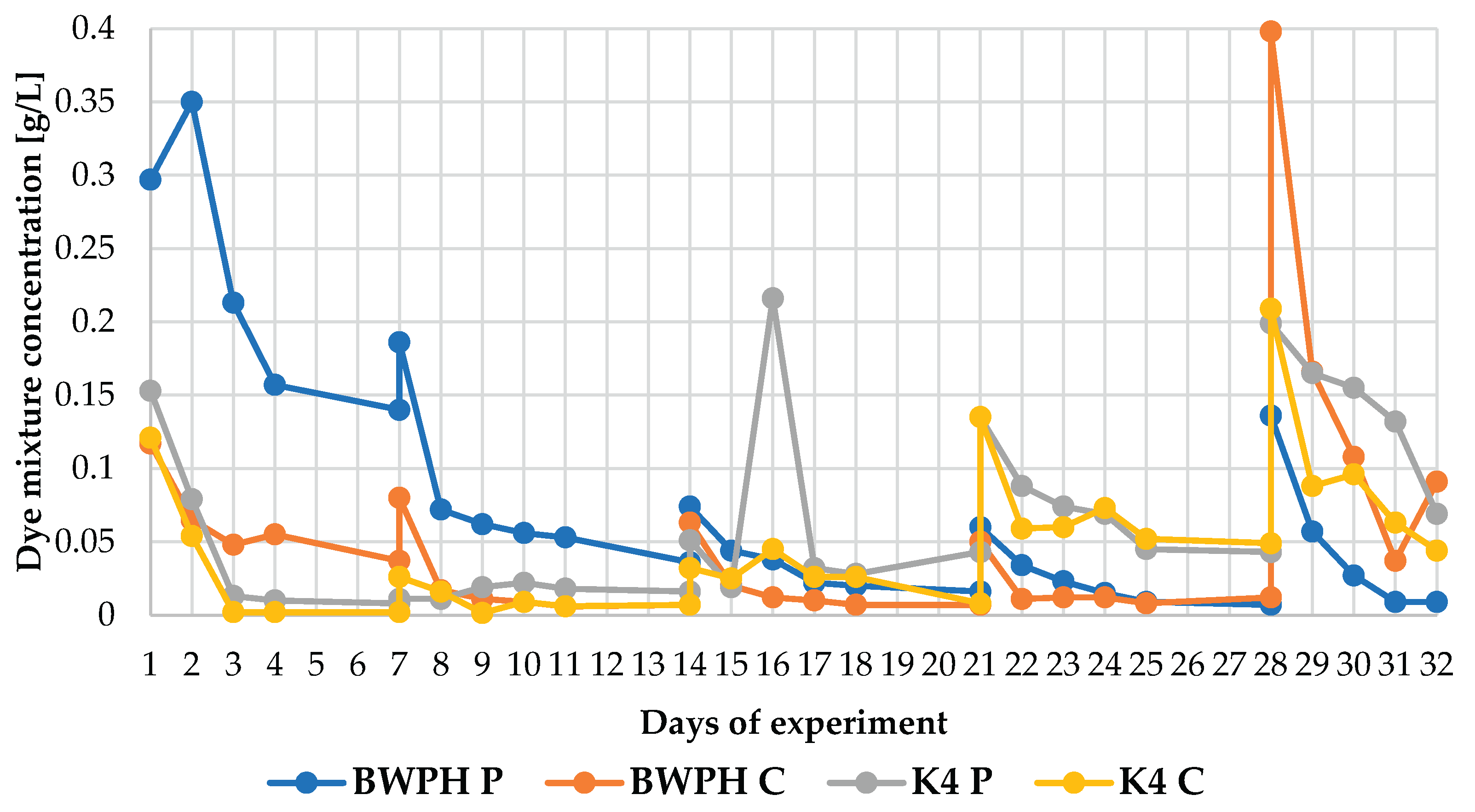
3.1. Removal of Dye Mixture in Bioreactor
3.2. Ecotoxicity of Postprocess Samples
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
Abbreviations
| BG | brilliant green dye |
| BWPH – | strain of Pleurotus ostreatus |
| BWPH P –, | bioreactor periodically aerated with strain BWPH |
| BWPH C – | bioreactor continuously aerated with strain BWPH |
| CR | Congo red dye |
| EC50 | half-maximal effective concentration |
| K4 – | strain of Pleurotus ostreatus |
| K4 P – | bioreactor periodically aerated with strain K4 |
| K4 C – | bioreactor continuously aerated with strain K4 |
| RBBR | remazol brilliant blue R dye |
| TUa | acute toxicity unit |
References
- Sekhar, C.P.; Kalidhasan, S.; Rajesh, V.; Rajesh, N. ; Bio-polymer adsorbent for the removal of malachite green from aqueous solution. Chemosphere 2009, 77, 842–847. [Google Scholar] [CrossRef] [PubMed]
- Solis, M.; Solis, A.; Perez, H.I.; Manjarrez, N.; Flores, M. ; Microbial decolouration of azo dyes: A review. Process. Bioch., 2012, 47, 1723–1748. [Google Scholar] [CrossRef]
- Mendes, M.; Cassoni, A.C.; Alves, S.; Pintado, M.E.; Castro, P.M.; Moreira, P. Screening for a more sustainable solution for decolorization of dyes and textile effluents using Candida and Yarrowia spp. J. Environ. Manag. 2022, 307, 114421. [Google Scholar] [CrossRef] [PubMed]
- Azmi, W.; Sani, K.R.; Banerjee, U.C. Biodegradation of triphenylmethane dyes. Enzyme. Microb. Technol. 1998, 22, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, P.; Malik, A. Fungal dye decolorization: recent advances and future potential. Environ. Int. 2009, 35, 127–141. [Google Scholar] [CrossRef] [PubMed]
- Knapp, J.S.; Newby, P.S.; Reece, L.P. Decolorization of wood-rotting basidomycete fungi. Enzyme. Microb. Technol. 1995, 17, 664–668. [Google Scholar] [CrossRef]
- Moreira, M.T.; Mielgo, I.; Feijoo, G.; Lema, J.M. Evaluation of different fungal strains in the decolorization of synthetic dyes. Biotechnol. Lett. 2000, 22, 1499–2000. [Google Scholar] [CrossRef]
- Radha, K.V.; Regupathi, A.; Arunagiri, A.; Murugesan, T. Decolorization studies of synthetic dyes using Phanerochaete chrysosporium and their kinetics. Process. Biochem. 2005, 40, 3337–3345. [Google Scholar] [CrossRef]
- Forootanfar, H.; Moezzi, A.; Aghaie-Khozani, M.; Mahmoudjanlou, Y.; Ameri, A.; Niknejad, F.; Faramarzi, M.A. Synthetic dye decolorization by three sources of fungal laccase. Iran. J. Environ. Health. Sci. Eng. 2012, 9, 27. [Google Scholar] [CrossRef] [PubMed]
- Radhika, R.; Jebapriya, G.R.; Gnanadoss, J.J. ; Decolorization of synthetic textile dyes using the edible mushroom fungi Pleurotus. Pakistan J Biological Sci. 2014, 17, 248–253. [Google Scholar] [CrossRef] [PubMed]
- Swamy, J.; Ramsay, J.A. . The evaluation of white rot fungi in the decoloration of textile dyes. Enzyme. Microb. Technol. 1999, 24, 130–137. [Google Scholar] [CrossRef]
- Przystaś, W.; Zabłocka-Godlewska, E.; Grabińska-Sota, E. Biological Removal of Azo and Triphenylmethane Dyes and Toxicity of Process By-Products. Water. Air. Soil. Pollut. 2012, 223, 1581–1592. [Google Scholar] [CrossRef] [PubMed]
- Przystaś, W.; Zablocka-Godlewska, E.; Grabińska-Sota, E. Effectiveness of dyes removal by mixed fungal cultures and toxicity of their metabolites. Water. Air. Soil. Pollut. 2013, 224, 1534. [Google Scholar] [CrossRef] [PubMed]
- Aksu, Z. Application of biosorption for the removal of organic pollutants: a review. Process. Biochem. 2005, 40, 997–1026. [Google Scholar] [CrossRef]
- Przystaś, W.; Zablocka-Godlewska, E.; Grabińska-Sota, E. Efficacy of fungal decolorization of a mixture of dyes belonging to different classes. Braz. J. Microbiol. 2015, 46, 415–424. [Google Scholar] [CrossRef] [PubMed]
- Kapdan, I.K.; Kargi, F. Biological decolorization of textile dyestuff containing wastewater by Coriolus versicolor In a rotating biological contractor. Enzyme. Microb. Technol. 2002, 30, 195–199. [Google Scholar] [CrossRef]
- Torres, J.M.O.; Cardenas, CH.V.; Moron, L.S.; Guzman, A.P.A.; dela Cruz, T.E.E. Dye decolorization activities of marine-derived fungi isolated from Manila Bay and Calatagan Bay, Philippines. Philippine. J. Sci. 2011, 140, 133–143. [Google Scholar]
- Kasinath, A.; Novotny, C.; Svobodova, K.; Patel, K.C.; Sasek, V. Decolorization of synthetic dyes by Irpex lacteus in liquid cultures and packed-bed bioreactor. Enzyme. Microb. Technol. 2003, 32, 167–173. [Google Scholar] [CrossRef]
- Mielgo, I.; Moreira, M.T.; Feijoo, G.; Lema, J.M. Biodegradation of a polymeric dye in a pulsed bed bioreactor by immobilized Phanerochaete chrysosporium. Water. Res. 2002, 36, 1896–1901. [Google Scholar] [CrossRef] [PubMed]
- Pakshirajan, K.; Kheria, S. Continuous treatment of coloured industry wastewater using immobilized Phanerochaete chrysosporium in a rotating biological contractor reactor. J. Environ. Manag. 2012, 101, 118–123. [Google Scholar] [CrossRef] [PubMed]
| Zootoxicity | Phytotoxicity | |||||
|---|---|---|---|---|---|---|
| Strain | Bioreactor type |
Cycle | EC50 | TUa (class) | EC50 | TUa (class) |
| BWPH | Periodically aerated (P) | 1 | 21.3 | 4.7 (III) | 3.7 | 27.0 (IV) |
| 2 | 18.4 | 5.4 (III) | 3.7 | 27.0 (IV) | ||
| 3 | 30.8 | 3.2 (III) | 1.7 | 58.8 (IV) | ||
| 4 | 22.8 | 4.4 (III) | 1.8 | 55.6 (IV) | ||
| 5 | 28.8 | 3.5 (III) | 1.8 | 55.6 (IV) | ||
| Continuously aerated (C) | 1 | 26.3 | 3.8 (III) | 3.7 | 27.0 (IV) | |
| 2 | 25.0 | 4.0 (III) | 2.3 | 43.5 (IV) | ||
| 3 | 28.8 | 3.5 (III) | 1.7 | 58.8 (IV) | ||
| 4 | 47.2 | 2.1 (III) | 2.7 | 37.0 (IV) | ||
| 5 | 31.6 | 3.2 (III) | 2.3 | 43.5 (IV) | ||
| K4 | Periodically aerated (P) | 1 | 1.2 | 83.3 (IV) | 3.6 | 27.8 (IV) |
| 2 | 1.4 | 71.4 (IV) | 2.3 | 43.5 (IV) | ||
| 3 | 2.3 | 43.5 (IV) | 7.3 | 13.7 (IV) | ||
| 4 | 2.6 | 38.5 (IV) | 2.3 | 43.5 (IV) | ||
| 5 | 2.6 | 38.5 (IV) | 2.3 | 43.5 (IV) | ||
| Continuously aerated (C) | 1 | 1.8 | 55.6 (IV) | 44.7 | 2.2 (III) | |
| 2 | 2.6 | 38.5 (IV) | 11.6 | 8.6 (III) | ||
| 3 | 3.4 | 29.4 (IV) | 3.7 | 27.0 (IV) | ||
| 4 | 3.2 | 31.3 (IV) | 4.3 | 23.3 (IV) | ||
| 5 | 3.2 | 31.3 (IV) | 4.3 | 23.3 (IV) | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





