Submitted:
11 June 2025
Posted:
11 June 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Methods
2.1. Ethical Consideration
2.2. Study Design
2.3. Laboratory and Imaging Investigation
2.4. Statistical Analysis
3. Results
4. Discussion
5. Study Limitation
6. Conclusion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- S. T. Hlebowicz M, Jakubowski P, “Streptococcus suis Meningitis: Epidemiology, Clinical Presentation and Treatment,” Vector Borne Zoonotic Dis, vol. 19, no. 8, pp. 557–562, 2019.
- A. Rayanakorn, B. H. Goh, L. H. Lee, T. M. Khan, and S. Saokaew, “Risk factors for Streptococcus suis infection: A systematic review and meta-analysis,” Sci. Rep., vol. 8, no. 1, pp. 1–9, 2018. [CrossRef]
- C. Neila-Ibáñez et al., “Risk factors associated with Streptococcus suis cases on pig farms in Spain,” Vet. Rec., vol. 193, no. 5, p. no, 2023. [CrossRef]
- A. van Samkar, M. C. Brouwer, C. Schultsz, A. van der Ende, and D. van de Beek, “Streptococcus suis Meningitis: A Systematic Review and Meta-analysis,” PLoS Negl. Trop. Dis., vol. 9, no. 10, pp. 1–10, 2015. [CrossRef]
- H. Tall et al., “Identification of Streptococcus suis Meningitis through Population-Based Surveillance, Togo, 2010–2014,” Emerg. Infect. Dis., vol. 22, no. 7, pp. 1262–1264, Jul. 2016. [CrossRef]
- K. Rieckmann, S.-M. Pendzialek, T. Vahlenkamp, and C. G. Baums, “A critical review speculating on the protective efficacies of autogenous Streptococcus suis bacterins as used in Europe,” Porc. Heal. Manag., vol. 6, no. 1, p. 12, Dec. 2020. [CrossRef]
- D. van de Beek, L. Spanjaard, and J. de Gans, “Streptococcus suis meningitis in the Netherlands,” J. Infect., vol. 57, no. 2, pp. 158–161, Aug. 2008. [CrossRef]
- J. Dutkiewicz et al., “Streptococcus suis: a re-emerging pathogen associated with occupational exposure to pigs or pork products. Part I – Epidemiology,” Ann. Agric. Environ. Med., vol. 24, no. 4, pp. 683–695, Dec. 2017. [CrossRef]
- P. Praphasiri et al., “Streptococcus suis Infection in Hospitalized Patients, Nakhon Phanom Province, Thailand,” Emerg. Infect. Dis., vol. 21, no. 2, pp. 345–348, Feb. 2015. [CrossRef]
- V. T. L. Huong et al., “Epidemiology, Clinical Manifestations, and Outcomes of Streptococcus suis Infection in Humans,” Emerg. Infect. Dis., vol. 20, no. 7, Jul. 2014. [CrossRef]
- E. MA et al., “Streptococcus suis infection in Hong Kong: an emerging infectious disease?,” Epidemiol. Infect., vol. 136, no. 12, pp. 1691–1697, Dec. 2008. [CrossRef]
- J. Arenas et al., “In vivo transcriptomes of Streptococcus suis reveal genes required for niche-specific adaptation and pathogenesis,” Virulence, vol. 10, no. 1, pp. 334–351, Jan. 2019. [CrossRef]
- G. Li et al., “Inhibition of suilysin activity and inflammation by myricetin attenuates Streptococcus suis virulence,” Life Sci., vol. 223, pp. 62–68, Apr. 2019. [CrossRef]
- Y. Zhu et al., “Comparative genetic analyses provide clues about capsule switching in Streptococcus suis 2 strains with different virulence levels and genetic backgrounds,” Microbiol. Res., vol. 250, p. 126814, Sep. 2021. [CrossRef]
- A. Kerdsin, M. Segura, N. Fittipaldi, and M. Gottschalk, “Sociocultural Factors Influencing Human Streptococcus suis Disease in Southeast Asia,” Foods, vol. 11, no. 9, p. 1190, Apr. 2022. [CrossRef]
- C. Zheng, M. Wei, M. Jia, and M. Cao, “Involvement of Various Enzymes in the Physiology and Pathogenesis of Streptococcus suis,” Vet. Sci., vol. 7, no. 4, p. 143, Sep. 2020. [CrossRef]
- J.-P. Auger, S. Payen, D. Roy, A. Dumesnil, M. Segura, and M. Gottschalk, “Interactions of Streptococcus suis serotype 9 with host cells and role of the capsular polysaccharide: Comparison with serotypes 2 and 14,” PLoS One, vol. 14, no. 10, p. e0223864, Oct. 2019. [CrossRef]
- A. M. Domado and J. Itable, “Streptococcus suis: Bacteremia presenting with fever, rashes, arthritis and neurologic deficits,” Androl. Gynecol. Curr. Res., vol. 05, no. 03, 2017. [CrossRef]
- K. Li et al., “Meningitis and sepsis caused by Streptococcus suis in an elderly woman: A CARE-compliant case report,” Medicine (Baltimore)., vol. 102, no. 43, p. e35780, Oct. 2023. [CrossRef]
- N. He, H. Li, and X. Zhang, “Human Streptococcus suis Infection,” in Radiology of Infectious Diseases: Volume 2, Dordrecht: Springer Netherlands, 2015, pp. 113–119. [CrossRef]
- D. T. N. Ho et al., “Risk Factors of Streptococcus suis Infection in Vietnam. A Case-Control Study,” PLoS One, vol. 6, no. 3, p. e17604, Mar. 2011. [CrossRef]
- N. M. Susilawathi et al., “Streptococcus suis –Associated Meningitis, Bali, Indonesia, 2014–2017,” Emerg. Infect. Dis., vol. 25, no. 12, pp. 2235–2242, Dec. 2019. [CrossRef]
- I. A. Aryasa et al., “<em>Streptococcus suis</em> meningitis related to processing and consuming raw pork during Balinese tradition, <em>Mebat</em>,” Med. J. Indones., vol. 29, no. 1, pp. 88–92, Mar. 2020. [CrossRef]
- N. M. A. Tarini et al., “Misidentification of S. suis as a Zoonotic Agent,” Open Access Maced. J. Med. Sci., vol. 7, no. 14, pp. 2309–2312, Jul. 2019. [CrossRef]
- M. Scarborough and G. E. Thwaites, “The diagnosis and management of acute bacterial meningitis in resource-poor settings,” Lancet Neurol., vol. 7, no. 7, pp. 637–648, Jul. 2008. [CrossRef]
- S. K. Lehman DC, Mahon CR, Textbook of diagnostic microbiology. 2016.
- Clinical and Laboratory Standards Institute, Performance standards for antimicrobial susceptibility testing; twenty-fifth informational supplement (M100-S27). Wayne (PA): The Institute, 2017.
- H. F. L. Wertheim et al., “Streptococcus suis, an Important Cause of Adult Bacterial Meningitis in Northern Vietnam,” PLoS One, vol. 4, no. 6, p. e5973, Jun. 2009. [CrossRef]
- T. Lakshmikanth et al., “Immune system adaptation during gender-affirming testosterone treatment,” Nature, vol. 633, no. 8028, pp. 155–164, Sep. 2024. [CrossRef]
- R. R. Sharma and A. Sharma, “Meningitis: Current Understanding and Management,” in The Microbiology of Central Nervous System Infections, Elsevier, 2018, pp. 3–27. [CrossRef]
- Y. Liu, Z. Kou, X. Wang, S. Chen, R. Li, and Q. Wang, “Case report: One human Streptococcus suis infection in Shandong Province, China,” Medicine (Baltimore)., vol. 102, no. 14, p. e33491, Apr. 2023. [CrossRef]
- S. Tanabe, M. Gottschalk, and D. Grenier, “Hemoglobin and Streptococcus suis cell wall act in synergy to potentiate the inflammatory response of monocyte-derived macrophages,” Innate Immun., vol. 14, no. 6, pp. 357–363, Dec. 2008. [CrossRef]
- M.-C. Jobin, M. Gottschalk, and D. Grenier, “Upregulation of prostaglandin E2 and matrix metalloproteinase 9 production by human macrophage-like cells: Synergistic effect of capsular material and cell wall from Streptococcus suis,” Microb. Pathog., vol. 40, no. 1, pp. 29–34, Jan. 2006. [CrossRef]
- A. Viallon, E. Bothelo-Nevers, and F. Zeni, “Clinical decision rules for acute bacterial meningitis: current insights,” Open Access Emerg. Med., p. 7, Apr. 2016. [CrossRef]
- A. Fongcom, S. Pruksakorn, P. Netsirisawan, R. Pongprasert, and P. Onsibud, “Streptococcus suis infection: a prospective study in northern Thailand.,” Southeast Asian J. Trop. Med. Public Health, vol. 40, no. 3, pp. 511–517, May 2009.
- I. M. Lucke et al., “Elevated leukocyte count in cerebrospinal fluid of patients with chronic inflammatory demyelinating polyneuropathy,” J. Peripher. Nerv. Syst., vol. 23, no. 1, pp. 49–54, Mar. 2018. [CrossRef]
- M. Zeinalizadeh, M. Shadkam, P. Afarinesh Khaki, A. Abdollahi, M. Douraghi, and M. Salehi, “Cerebrospinal Fluid Analysis in Patients with Post-neurosurgical Procedures: Meningitis vs. Non-meningitis,” Iran. J. Pathol., vol. 19, no. 3, pp. 342–347, Jul. 2024. [CrossRef]
- C. O’Leary et al., “Predictive performance of cerebrospinal fluid parameters for diagnosis of meningitis in infants: a cohort study,” Arch. Dis. Child., vol. 110, pp. 209–215, 2025. [CrossRef]
- R. B. Domingues, G. B. P. Fernandes, F. B. V. de M. Leite, and C. Senne, “Performance of lactate in discriminating bacterial meningitis from enteroviral meningitis,” Rev. Inst. Med. Trop. Sao Paulo, vol. 61, 2019. [CrossRef]
- L. Manning, M. Laman, T. Mare, I. Hwaiwhanje, P. Siba, and T. M. E. Davis, “Accuracy of cerebrospinal leucocyte count, protein and culture for the diagnosis of acute bacterial meningitis: a comparative study using <scp>B</scp> ayesian latent class analysis,” Trop. Med. Int. Heal., vol. 19, no. 12, pp. 1520–1524, Dec. 2014. [CrossRef]
- A. Kerdsin, “Human Streptococcus suis Infections in Thailand: Epidemiology, Clinical Features, Genotypes, and Susceptibility,” Trop. Med. Infect. Dis., vol. 7, p. 359, 2022. [CrossRef]
- M. C. Domínguez-Punaro, U. Koedel, T. Hoegen, C. Demel, M. Klein, and M. Gottschalk, “Severe cochlear inflammation and vestibular syndrome in an experimental model of Streptococcus suis infection in mice,” Eur. J. Clin. Microbiol. Infect. Dis., vol. 31, no. 9, pp. 2391–2400, Sep. 2012. [CrossRef]
- X. Xia, X. Wang, X. Wei, J. Jiang, and J. Hu, “Methods for the detection and characterization of Streptococcus suis: from conventional bacterial culture methods to immunosensors,” Antonie Van Leeuwenhoek, vol. 111, no. 12, pp. 2233–2247, Dec. 2018. [CrossRef]
- Z. Ágoston, G. Terhes, P. Hannauer, M. Gajdács, and E. Urbán, “Fatal case of bacteremia caused by Streptococcus suis in a splenectomized man and a review of the European literature,” Acta Microbiol. Immunol. Hung., vol. 67, no. 3, pp. 148–155, Oct. 2020. [CrossRef]
- L. Xu et al., “Interleukin-17A Contributed to the Damage of Blood-CNS Barriers During Streptococcus suis Meningitis,” Mol. Neurobiol., vol. 59, no. 4, pp. 2116–2128, Apr. 2022. [CrossRef]

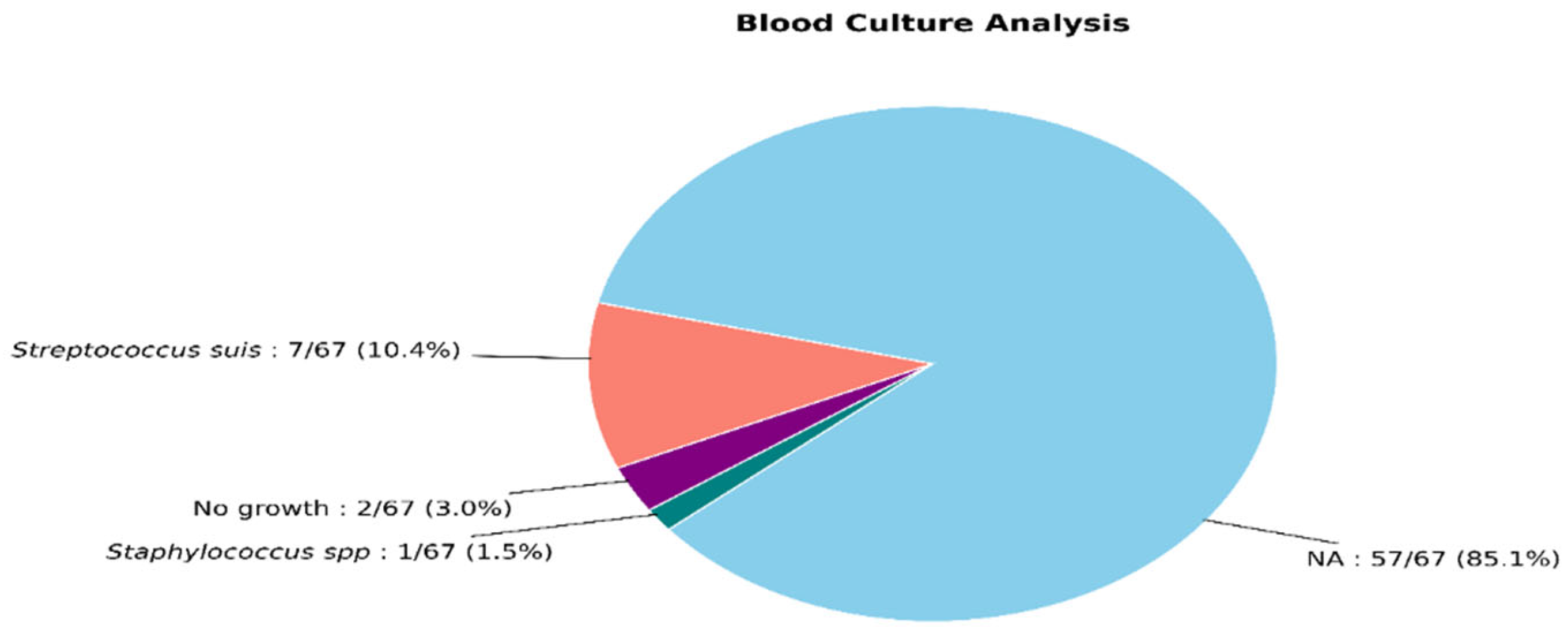
| Variables |
Total (N =67) |
Meningitis with sudden deafness (N=34) |
Meningitis without sudden deafness (N=33) |
p |
| Sex, N, (%) Male Female |
52 (77.7) 15 (22.4) |
27 (51.9) 7 (46.7) |
25 (48.1) 8 (53.3) |
0.474# |
| Age, (year), mean ±SD | 53.8 (14.9) | 53.7 (16.9) | 53.8 (12.9) | 0.965$ |
| Fever onset (day), mean ±SD | 2.8 (1.4) | 2.9 (1.6) | 2.6 (1.3) | 0.335$ |
| Length of stay (days), mean ±SD | 11.4 (3.8) | 11.4 (3.4) | 11.4 (4.3) | 0.044$ |
| Raw pork consumption, N (%) | 30 (44.8) | 17 (56.7) | 13 (43.3) | 0.151# |
| Sign and symptoms, N, (%) Headache Convulsion Ataxia Vomiting Hemiparesis Neck rigidity Mental state disturbance Septic arthritis |
66 (98.5) 8 (11.9) 4 (58.1) 63 (94) 6 (9) 67 (100) 43 (64.2) 9 (13.4) |
33 (50) 4 (50) 2 (50) 31 (49.2) 2 (33.3) 34 (50.7) 19 (44.2) 4 (44.4) |
33 (50) 4 (50) 2 (50) 32 (58.8) 4 (66.7) 33 (49.3) 24 (55.8) 5 (55.6) |
0.507* 0.628* 0.682* 0.318* 0.322* NA 0.151# 0.480* |
| ICU treatment | 3 ( 4.5) | 0 | 3 (100) | 0.114* |
| Outcome Survival |
64 (95.5) |
33 (51.6) |
31 (48.4) |
0.489* |
|
Total (N=67) |
Meningitis with sudden deafness (N=34) |
Meningitis without sudden deafness (N=33) |
p | |
| CBC at admission Hemoglobin (g/dL) Hematocrit (%) Leucocyte (x103/mL) Thrombocyte (x103/mL) |
13.3 (1.7)a 39.2 (5)a 13.6 (6.2)a 305 (125.3)a |
13.3 ± 1.9a 39.2 ± 5.7a 14.3 (7-38.2)b 302.9 ±130.1a |
13.3 ± 1.5a 39.2 ± 4.3a 10.9 (5.3-26.3)b 307.2 ± 122a |
NA NA 0.039# 0.892* |
| AST, IU/L | 47.6 (48)a | 37.5 (11-330)b | 33 (4-133)b | 0.261# |
| ALT, IU/L | 45.9 (37)a | 36 (19-224)b | 32 (13-85)b | 0.961 |
| BUN, mg/dL | 51 (9-211)a | 36.2 (22.9-188.2)b | 34.2 (16.1-65.2)b | 0.363# |
| SC, mg/dL | 41 (23.5)a | 0.7 (0.2-4.5)b | 0.7 (0.3-1.2)b | NA |
| Blood sugar, mg/dL | 138.2 (47.3)a | 125 (93-199)b | 123 (84-454)b | 0.337# |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





