Submitted:
10 June 2025
Posted:
10 June 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
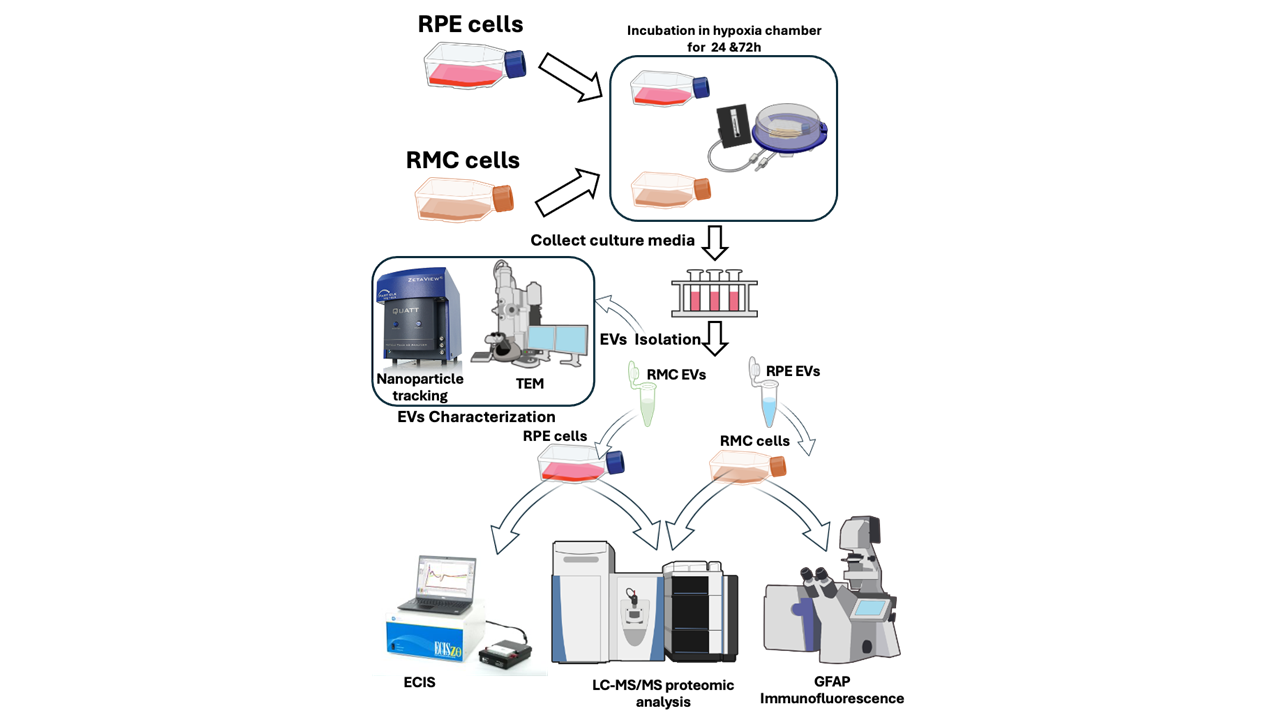
2. Materials and Methods
2.1. Retinal Pigmented (RPE) and Müller Cells (RMC) Culture
2.2. Isolation of Extracellular Vesicles (EVs)
2.3. EVs Characterizations
2.3.1. Nanoparticle Tracking of the Isolated Extracellular Vesicles
2.3.2. Transmission Electron Microscope (TEM)
2.4. Assessment of the Effect of Co-Culturing RMCs and RPE-EVs
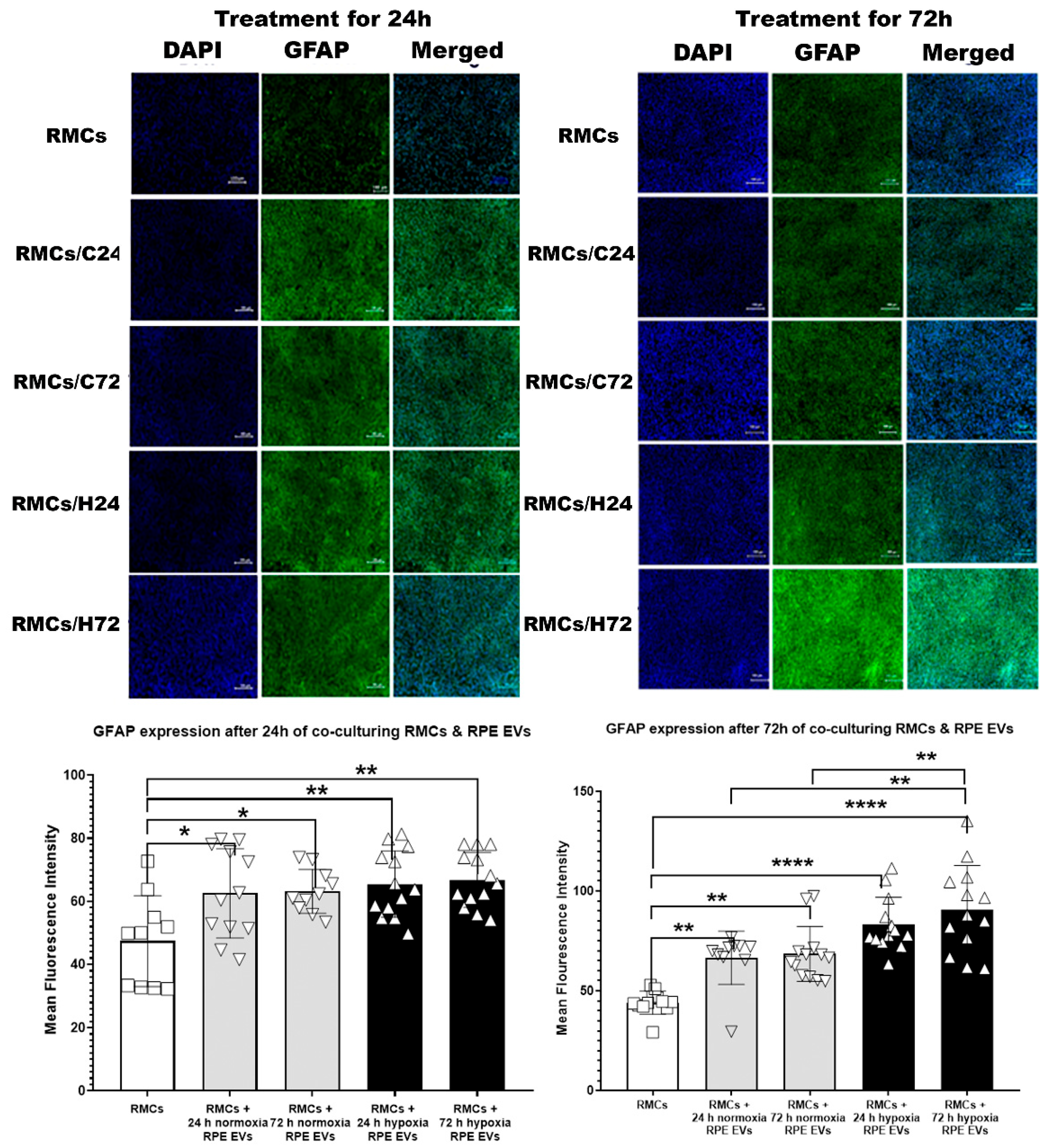
2.4.1. GFAP Immunofluorescence (IF) for RMCs
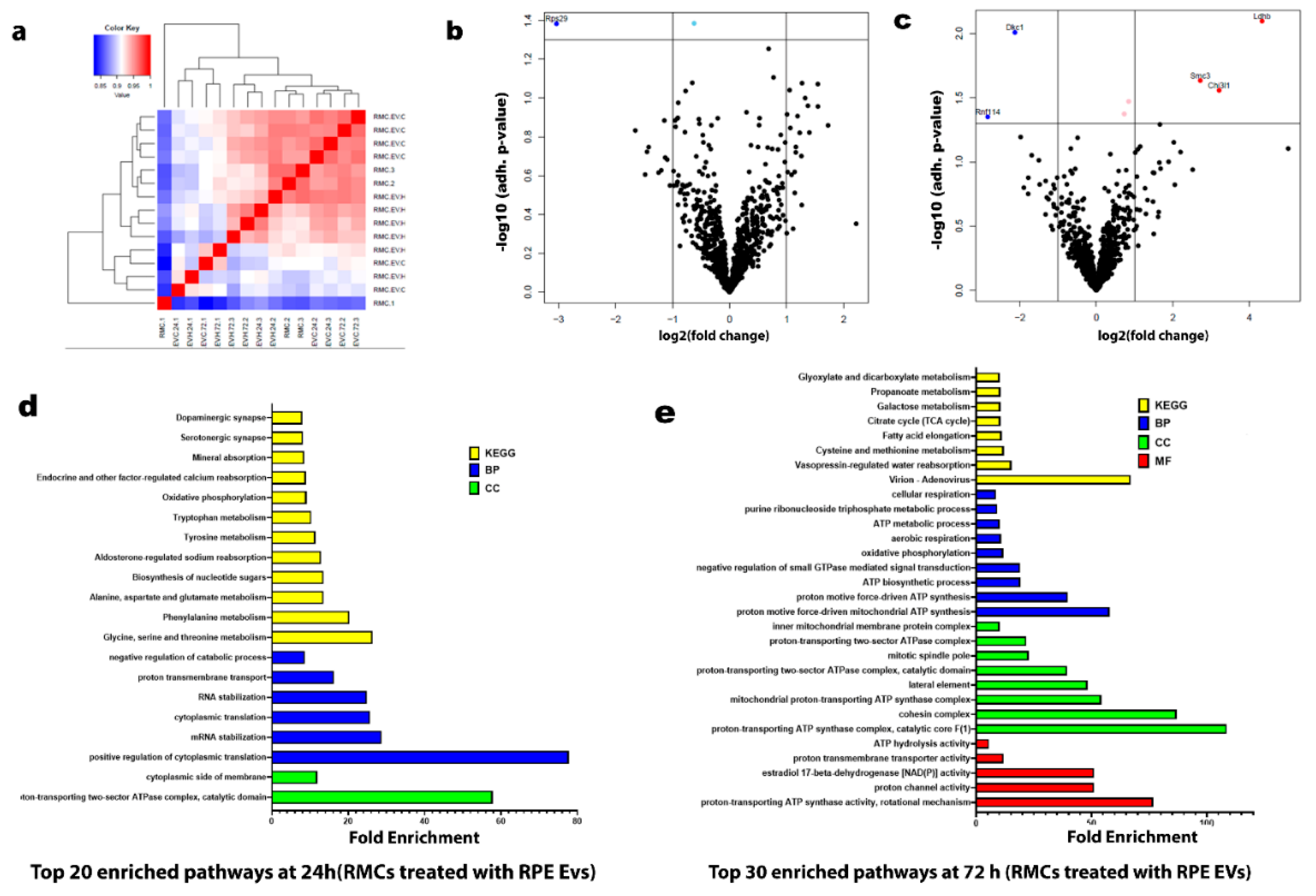
2.4.2. Proteomics for RMCs
2.5. Assessment of the effect of Co-Culturing RPE and RMCs-EVs.
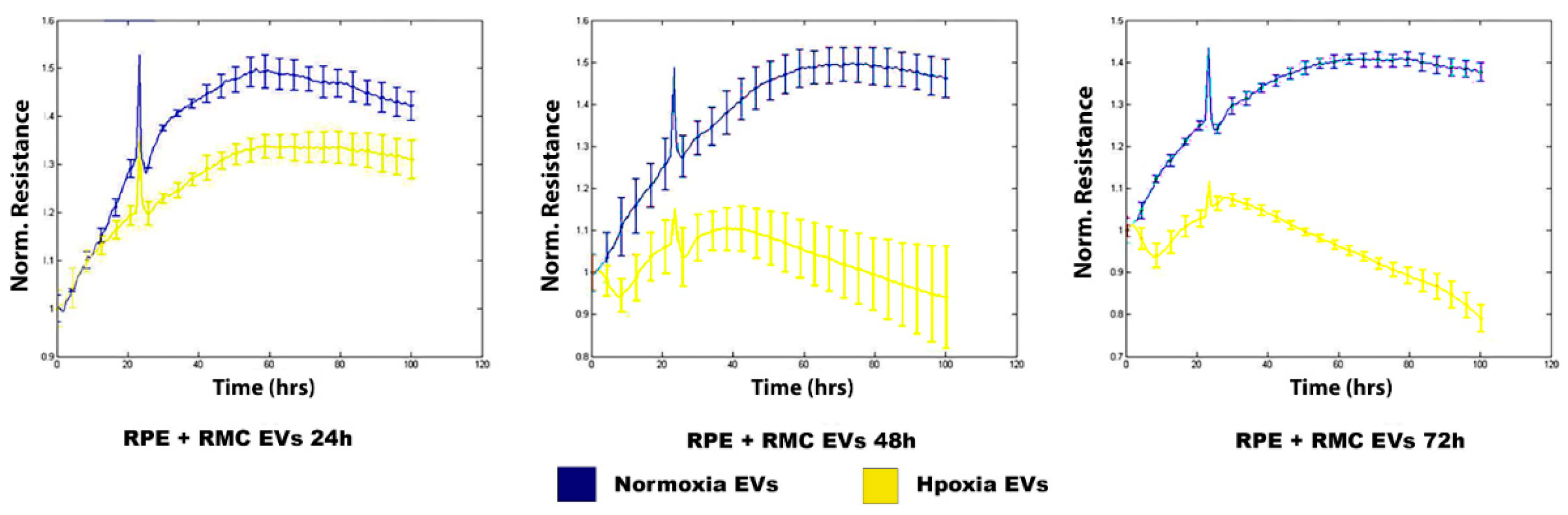
2.5.1. Electric Cell-Substrate Impedance Sensing Method (ECIS)
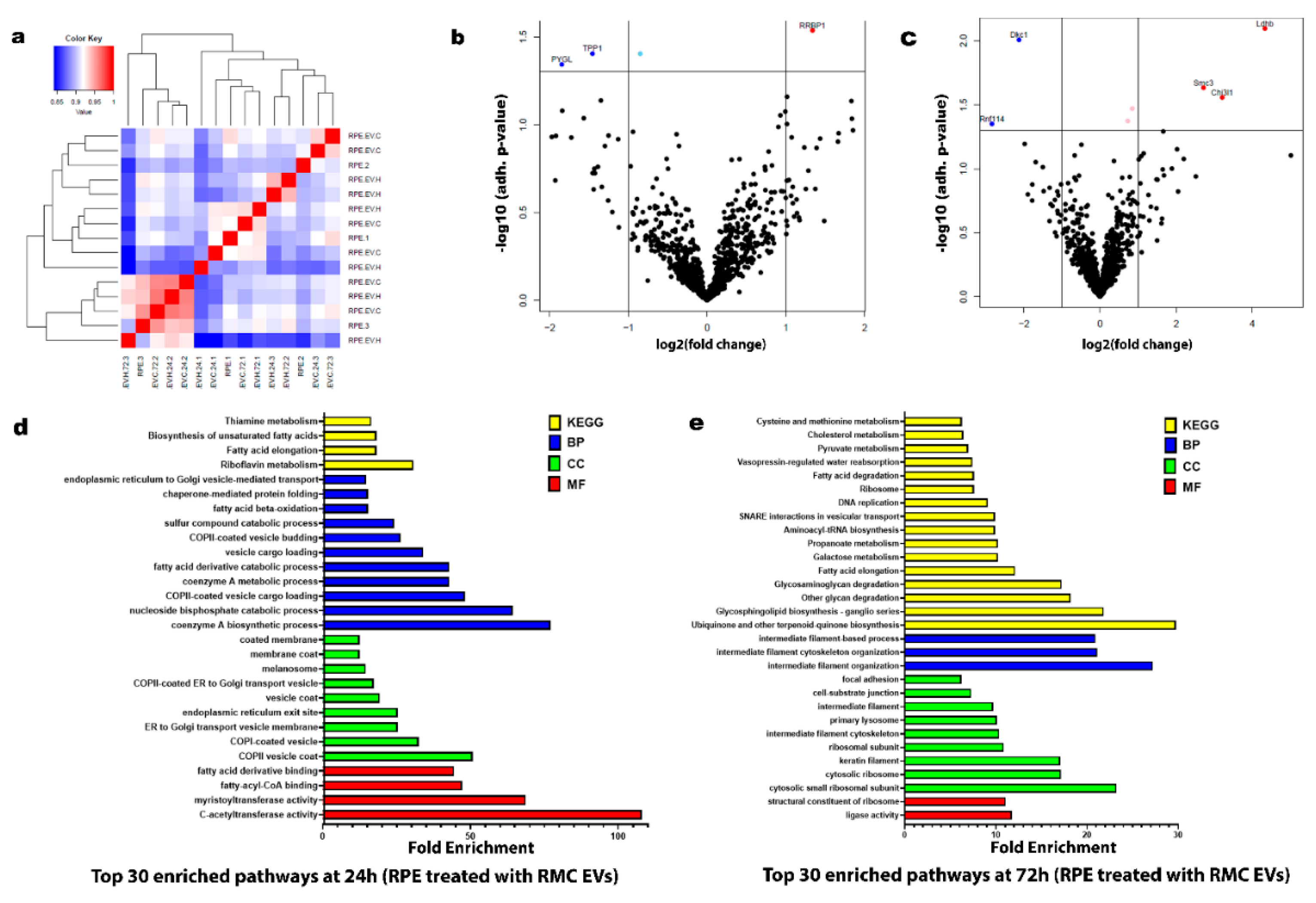
2.5.2. Proteomics for RPE
3. Results
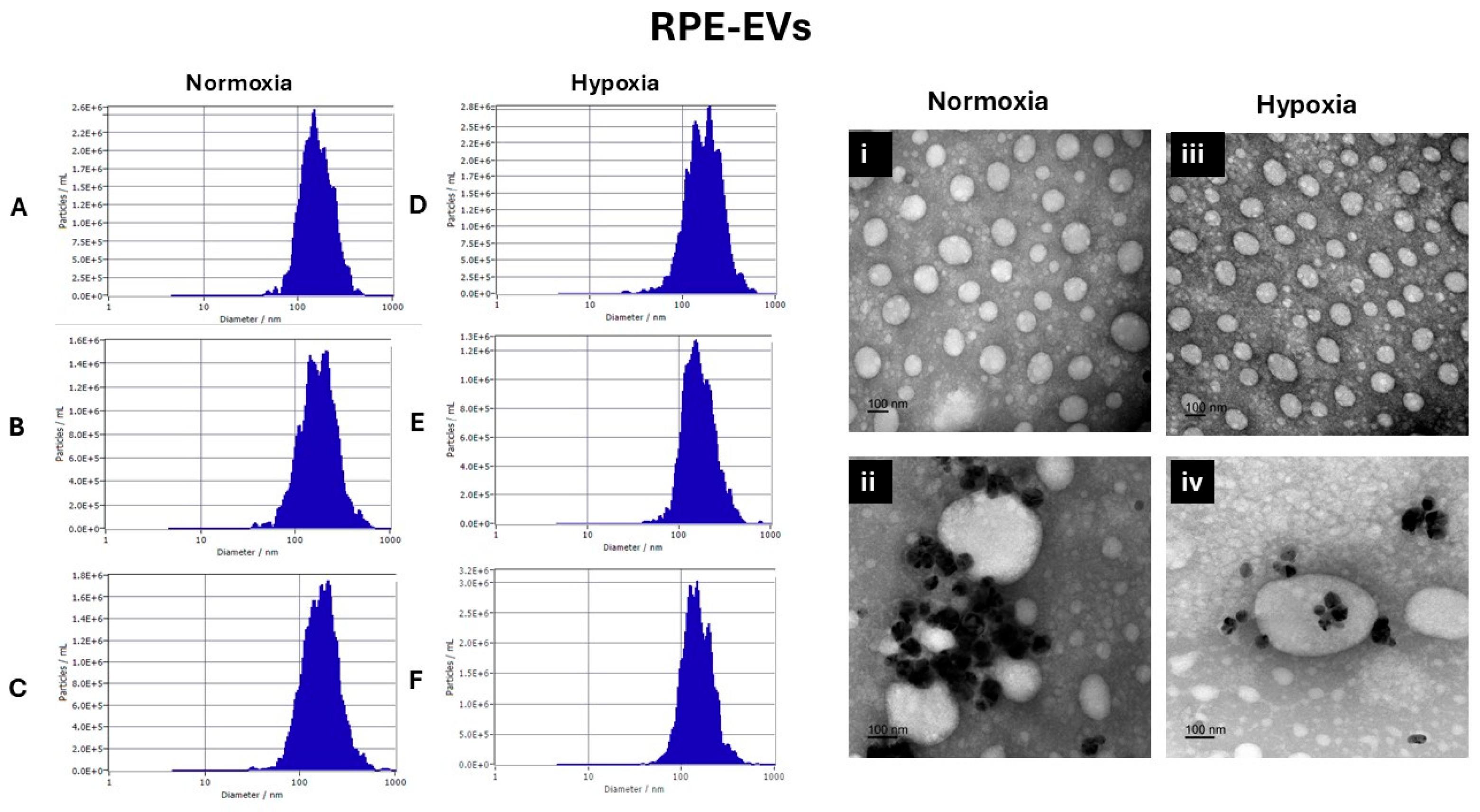
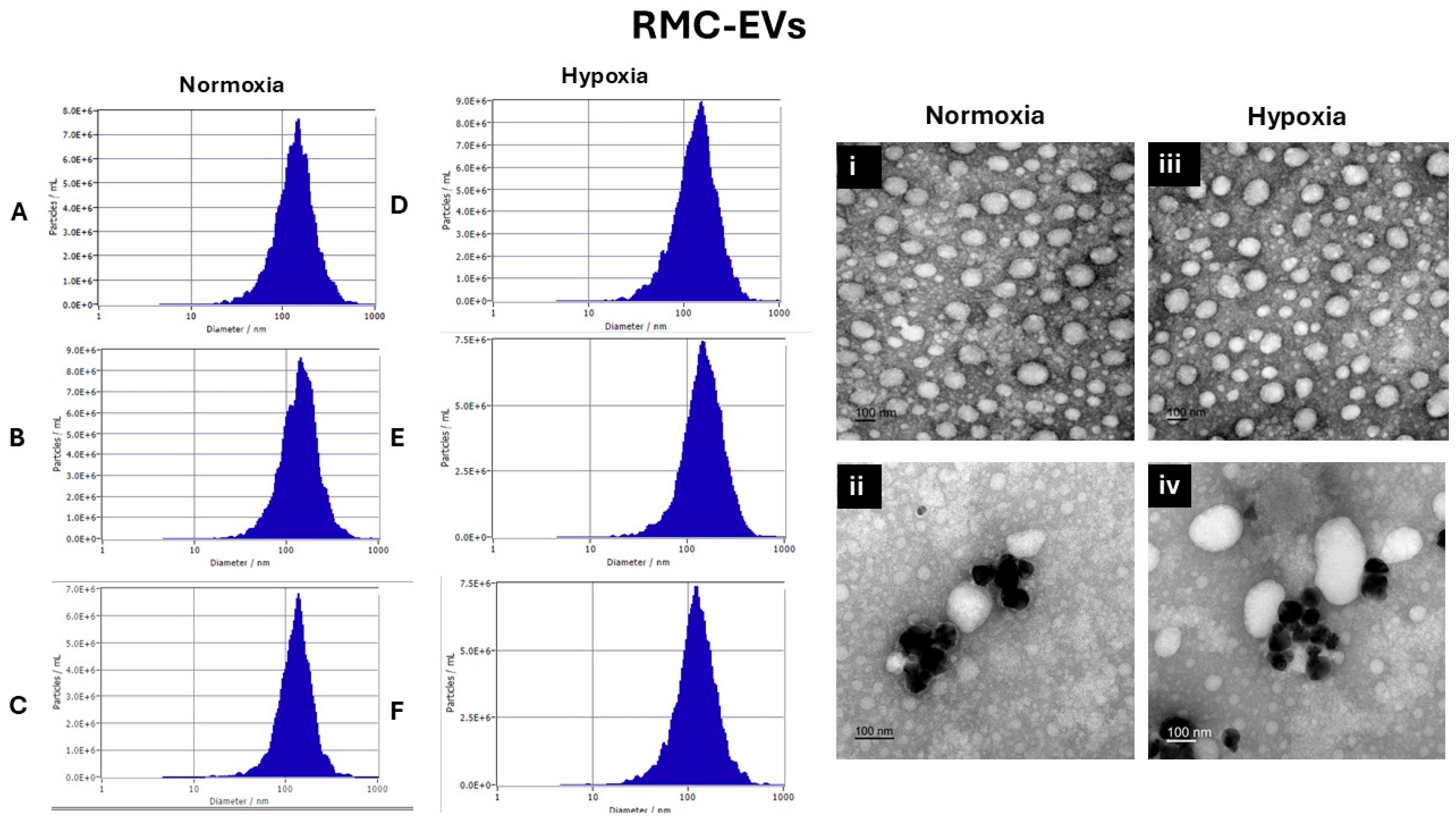
3.1. EVs Characterization
3.1.1. Nanoparticle Tracking Analysis by Zetaveiw Analysis
3.1.2. Transmission Electron Microscope
3.2. Co-Culture RMCs and RPE-EVs
3.2.1. Time-Dependent GFAP Upregulation in RMCs Treated with Hypoxic and Normoxic RPE EVs
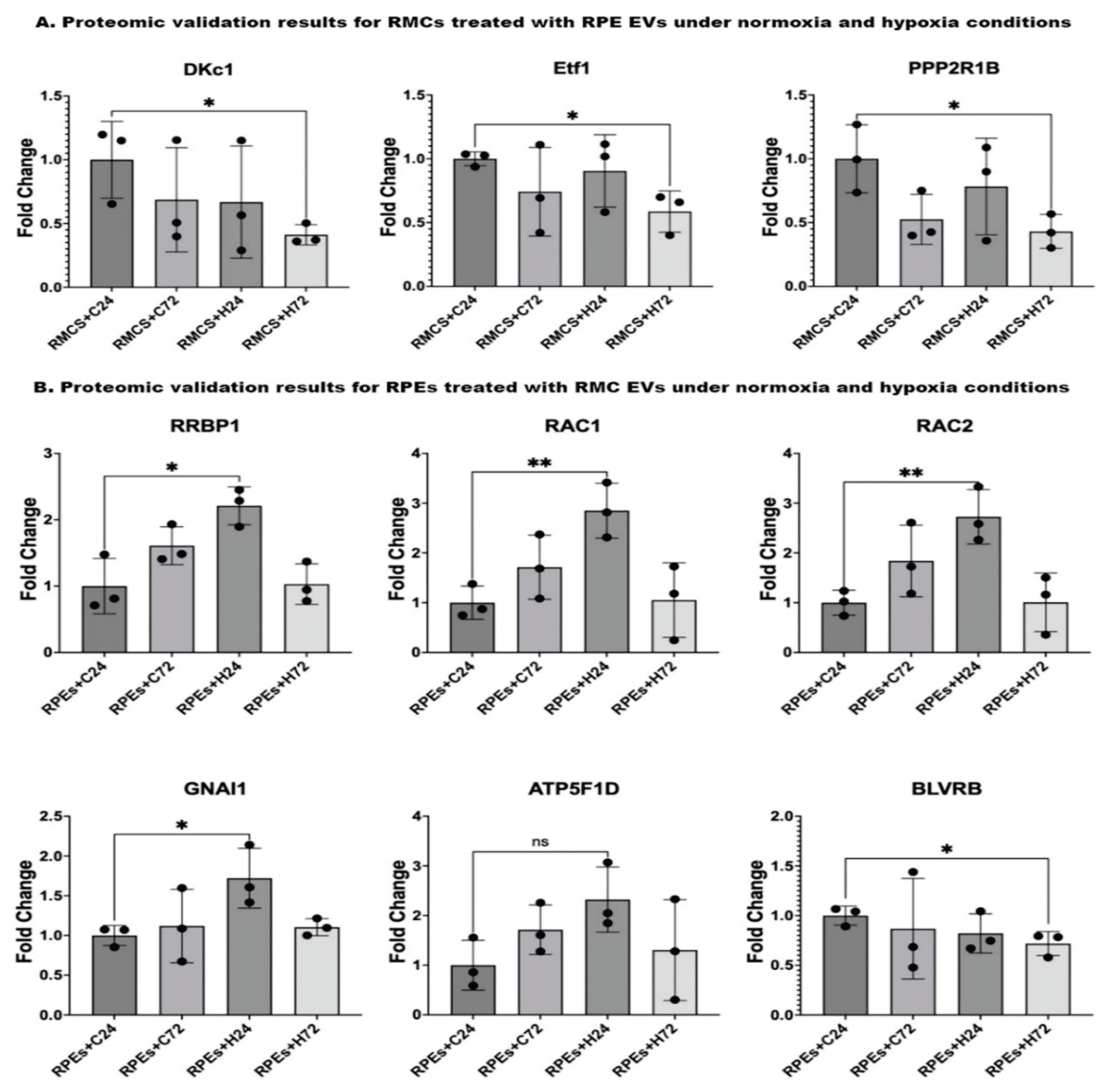
3.2.2. Proteomic Profiling Reveals Temporal Pathway Modulation in RMCs by Hypoxia-Induced RPE-EVs
3.3. Co-culture RPE and RMC-EVs
3.3.1. Hypoxic RMC-EVs Disrupt RPE Barrier Integrity in a Time-Dependent Manner
3.3.2. Hypoxic RMC-EVs Induce Distinct Temporal Proteomic Signatures in RPE Cells
3.4. Proteomic Validation Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DR | Diabetic retinopathy |
| AMD | Age-related macular retinopathy |
| GFAP | Glial fibrillary acidic protein |
| RPE | Retinal pigmented epithelium |
| EVs | Extracellular vesicles |
| oBRB | Outer blood-retinal barrier |
| PEDF | Pigment epithelial-derived factor |
| VEGF | Vascular epithelial growth factor |
| HIF-1 | Hypoxia-inducible factor 1 |
| ROS | Reactive oxygen species |
| RP | Retinitis pigmentosa |
| SD | Stargardt disease |
| FBS | Fetal bovine serum |
| PBS | Phosphate-buffered saline |
| TEM | Transmission electron microscope |
| ECIS | Electric cell-substrate impedance sensing method |
| TER | Transcellular electrical resistance |
| MF | Molecular function |
| CC | Cellular component |
| BP | Biological process |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| ER | Endoplasmic reticulum |
| PVR | Proliferative vitreoretinopathy |
| TCA | Tricarboxylic acid cycle |
| DKC1 | Dyskerin Pseudouridine Synthase 1 |
| snoRNPs | small nucleolar RNA ribonucleoproteins |
| ETF1 | Eukaryotic Translation Termination Factor 1 |
| PPP2R1B | Protein Ser/Thr phosphatases |
| TJ | Tight junctions |
| HRP | Horseradish peroxidase |
| OAT | Ornithine aminotransferase |
| UQ | Ubiquinone |
| CoQ | coenzyme Q |
| RRBP1 | Ribosome-binding protein 1 |
| URP | Unfolded protein response |
| RVO | Retinal vein occlusion |
| GNAI1 | Guanine Nucleotide-Binding Protein G(i) Subunit Alpha-1 |
| GPCRs | G-protein-coupled receptors |
| ATP5F1D | delta subunit of mitochondrial ATP synthase (Complex V) |
| OIR | Oxygen-induced retinopathy |
| BLVRB | Biliverdin Reductase B |
References
- Arjamaa, O. and M. Nikinmaa, Oxygen-dependent diseases in the retina: role of hypoxia-inducible factors. Experimental eye research, 2006. 83(3): p. 473–483. [CrossRef]
- Reichenbach, A. and A. Bringmann, Glia of the human retina. Glia, 2020. 68(4): p. 768–796. [CrossRef]
- Lupien, C., et al., Expression of glial fibrillary acidic protein in primary cultures of human Muller cells. Exp Eye Res, 2004. 79(3): p. 423–9. [CrossRef]
- Hippert, C., et al., RNAi-mediated suppression of vimentin or glial fibrillary acidic protein prevents the establishment of Muller glial cell hypertrophy in progressive retinal degeneration. Glia, 2021. 69(9): p. 2272–2290. [CrossRef]
- Yang, X.M., et al., Hypoxia-induced upregulation of pigment epithelium-derived factor by retinal glial (Muller) cells. J Neurosci Res, 2012. 90(1): p. 257–66.
- Huang, S., et al., Small extracellular vesicles of organoid-derived human retinal stem cells remodel Muller cell fate via miRNA: A novel remedy for retinal degeneration. J Control Release, 2024. 370: p. 405–420. [CrossRef]
- Goldman, D., Muller glial cell reprogramming and retina regeneration. Nat Rev Neurosci, 2014. 15(7): p. 431–42. [CrossRef]
- Xin, X., et al., Hypoxic retinal Muller cells promote vascular permeability by HIF-1-dependent up-regulation of angiopoietin-like 4. Proc Natl Acad Sci U S A, 2013. 110(36): p. E3425–34. [CrossRef]
- Rickman, C.B. and O. Strauss, The Function of the Retinal Pigment Epithelium. Adler's Physiology of the Eye E-Book, 2024: p. 339.
- Malavade, S., Overview of the ophthalmic system, in Nano-Biomaterials for ophthalmic drug delivery. 2016, Springer. p. 9–36.
- Fisher, C.R. and D.A. Ferrington, Perspective on AMD pathobiology: a bioenergetic crisis in the RPE. Investigative ophthalmology & visual science, 2018. 59(4): p. AMD41–AMD47. [CrossRef]
- Wang, S., et al., The retinal pigment epithelium: functions and roles in ocular diseases. Fundamental Research, 2023. [CrossRef]
- Wang, J., M.M. Barr, and A.M. Wehman, Extracellular vesicles. Genetics, 2024. 227(4): p. iyae088.
- Meldolesi, J., Exosomes and ectosomes in intercellular communication. Current Biology, 2018. 28(8): p. R435–R444. [CrossRef]
- Aheget, H., et al., Exosomes: their role in pathogenesis, diagnosis and treatment of diseases. Cancers, 2020. 13(1): p. 84.
- Habib, S., et al., Extracellular Vesicles and Diabetic Retinopathy: Nano-Sized Vesicles with Mega-Sized Hopes, in Diabetic Retinopathy - Advancement in Understanding the Pathophysiology and Management Strategies, M.I.I. Nawaz, Editor. 2024, IntechOpen: Rijeka. [CrossRef]
- Elmasry, K., et al., Possible Role of Endothelial-Derived Cellular and Exosomal-miRNAs in Lipid-Mediated Diabetic Retinopathy: Microarray Studies. Cells, 2024. 13(22). [CrossRef]
- Narayanan, R., C.C. Huang, and S. Ravindran, Hijacking the Cellular Mail: Exosome Mediated Differentiation of Mesenchymal Stem Cells. Stem Cells Int, 2016. 2016: p. 3808674. [CrossRef]
- Wong-Riley, M., Energy metabolism of the visual system. Eye and brain, 2010: p. 99–116. [CrossRef]
- Liu, H. and V. Prokosch, Energy metabolism in the inner retina in health and glaucoma. International journal of molecular sciences, 2021. 22(7): p. 3689. [CrossRef]
- Karti, O., I. Saatci, and A.O. Saatci, Vascular supply of the eye: clinical anatomy. Medical Hypothesis, Discovery and Innovation in Ophthalmology, 2025. 13(4): p. 176. [CrossRef]
- Kur, J., E.A. Newman, and T. Chan-Ling, Cellular and physiological mechanisms underlying blood flow regulation in the retina and choroid in health and disease. Progress in retinal and eye research, 2012. 31(5): p. 377–406. [CrossRef]
- Hurley, J.B., et al., Energy metabolism in the vertebrate retina. Vertebrate Photoreceptors: Functional Molecular Bases, 2014: p. 91–137.
- Gnanasambandam, B., et al., Addressing retinal hypoxia: pathophysiology, therapeutic innovations, and future prospects. Therapeutic Advances in Ophthalmology, 2024. 16: p. 25158414241280187. [CrossRef]
- Yang, S., J. Zhou, and D. Li, Functions and diseases of the retinal pigment epithelium. Frontiers in pharmacology, 2021. 12: p. 727870. [CrossRef]
- Reichenbach, A., et al., Müller cells in the healthy retina. 2010: Springer.
- Medina-Arellano, A.E., et al., Müller cells and retinal angiogenesis: critical regulators in health and disease. Frontiers in Cellular Neuroscience, 2024. 18: p. 1513686. [CrossRef]
- Martins, B., et al., Contribution of extracellular vesicles for the pathogenesis of retinal diseases: Shedding light on blood-retinal barrier dysfunction. Journal of Biomedical Science, 2024. 31(1): p. 48. [CrossRef]
- Wang, L. and X. Wei, Exosome-based crosstalk in glaucoma pathogenesis: a focus on oxidative stress and neuroinflammation. Frontiers in Immunology, 2023. 14: p. 1202704. [CrossRef]
- Ren, J., et al., Diabetic retinopathy: Involved cells, biomarkers, and treatments. Frontiers in Pharmacology, 2022. 13: p. 953691. [CrossRef]
- Martins, B., et al., Extracellular vesicles and MicroRNA: putative role in diagnosis and treatment of diabetic retinopathy. Antioxidants, 2020. 9(8): p. 705. [CrossRef]
- Atienzar-Aroca, S., et al., Oxidative stress in retinal pigment epithelium cells increases exosome secretion and promotes angiogenesis in endothelial cells. Journal of cellular and molecular medicine, 2016. 20(8): p. 1457–1466. [CrossRef]
- Coorey, N.J., et al., The role of glia in retinal vascular disease. Clinical and Experimental Optometry, 2012. 95(3): p. 266–281. [CrossRef]
- Choi, Y.K., Detrimental roles of hypoxia-inducible factor-1α in severe hypoxic brain diseases. International Journal of Molecular Sciences, 2024. 25(8): p. 4465. [CrossRef]
- Bringmann, A., et al., GABA and glutamate uptake and metabolism in retinal glial (Müller) cells. Frontiers in endocrinology, 2013. 4: p. 48. [CrossRef]
- Winkler, B.S., et al., Energy metabolism in human retinal Muller cells. Investigative ophthalmology & visual science, 2000. 41(10): p. 3183–3190.
- Garus, A. and C. Autexier, Dyskerin: an essential pseudouridine synthase with multifaceted roles in ribosome biogenesis, splicing, and telomere maintenance. Rna, 2021. 27(12): p. 1441–1458. [CrossRef]
- Liao, P., et al., Telomeres: dysfunction, maintenance, aging and cancer. Aging and Disease, 2023. 15(6): p. 2595.
- Rossiello, F., et al., Telomere dysfunction in ageing and age-related diseases. Nature cell biology, 2022. 24(2): p. 135–147. [CrossRef]
- Wouters, B.G., et al. Control of the hypoxic response through regulation of mRNA translation. in Seminars in cell & developmental biology. 2005. Elsevier. [CrossRef]
- Feng, Q., et al., The role of RNA modification in hepatocellular carcinoma. Frontiers in pharmacology, 2022. 13: p. 984453. [CrossRef]
- Lykke-Andersen, S. and T.H. Jensen, Nonsense-mediated mRNA decay: an intricate machinery that shapes transcriptomes. Nature reviews Molecular cell biology, 2015. 16(11): p. 665–677. [CrossRef]
- Zuo, Z., et al., The Role of Non-coding RNAs in Diabetic Retinopathy: Mechanistic Insights and Therapeutic Potential. Molecular Neurobiology, 2025: p. 1–32.
- Harel, S., et al., ETS1, ELK1, and ETV4 transcription factors regulate angiopoietin-1 signaling and the angiogenic response in endothelial cells. Frontiers in Physiology, 2021. 12: p. 683651. [CrossRef]
- Tang, J.X., et al., Mitochondrial OXPHOS biogenesis: co-regulation of protein synthesis, import, and assembly pathways. International Journal of Molecular Sciences, 2020. 21(11): p. 3820. [CrossRef]
- Zhang, S.X., et al., The unfolded protein response in retinal vascular diseases: implications and therapeutic potential beyond protein folding. Progress in Retinal and eye Research, 2015. 45: p. 111–131. [CrossRef]
- Swingle, M.R. and R.E. Honkanen, Inhibitors of serine/threonine protein phosphatases: biochemical and structural studies provide insight for further development. Current medicinal chemistry, 2019. 26(15): p. 2634–2660. [CrossRef]
- Van Hoof, C. and J. Goris, Phosphatases in apoptosis: to be or not to be, PP2A is in the heart of the question. Biochimica et Biophysica Acta (BBA)-Molecular Cell Research, 2003. 1640(2-3): p. 97–104.
- Antony, R., W.J. Lukiw, and N.G. Bazan, Neuroprotectin D1 induces dephosphorylation of Bcl-xL in a PP2A-dependent manner during oxidative stress and promotes retinal pigment epithelial cell survival. Journal of Biological Chemistry, 2010. 285(24): p. 18301–18308. [CrossRef]
- Barisic, S., et al., Identification of PP2A as a crucial regulator of the NF-κB feedback loop: its inhibition by UVB turns NF-κB into a pro-apoptotic factor. Cell Death & Differentiation, 2008. 15(11): p. 1681–1690. [CrossRef]
- Chen, Y., et al., Regulations of retinal inflammation: focusing on Müller glia. Frontiers in cell and developmental biology, 2022. 10: p. 898652. [CrossRef]
- O’Leary, F. and M. Campbell, The blood–retina barrier in health and disease. The FEBS Journal, 2023. 290(4): p. 878–891. [CrossRef]
- Kaur, C., W. Foulds, and E. Ling, Blood–retinal barrier in hypoxic ischaemic conditions: basic concepts, clinical features and management. Progress in retinal and eye research, 2008. 27(6): p. 622–647.
- Haydinger, C.D., T. Kittipassorn, and D.J. Peet, Power to see—Drivers of aerobic glycolysis in the mammalian retina: A review. Clinical & Experimental Ophthalmology, 2020. 48(8): p. 1057–1071. [CrossRef]
- Gabrielle, P.H., Lipid metabolism and retinal diseases. 2022, Wiley Online Library. [CrossRef]
- Wang, Y. and S. Hekimi, Understanding ubiquinone. Trends in cell biology, 2016. 26(5): p. 367–378. [CrossRef]
- Cui, X.A., H. Zhang, and A.F. Palazzo, p180 promotes the ribosome-independent localization of a subset of mRNA to the endoplasmic reticulum. PLoS biology, 2012. 10(5): p. e1001336. [CrossRef]
- Bartoszewska, S. and J.F. Collawn, Unfolded protein response (UPR) integrated signaling networks determine cell fate during hypoxia. Cellular & molecular biology letters, 2020. 25(1): p. 18. [CrossRef]
- McLaughlin, T., et al., Cellular stress signaling and the unfolded protein response in retinal degeneration: mechanisms and therapeutic implications. Molecular Neurodegeneration, 2022. 17(1): p. 25. [CrossRef]
- Parri, M. and P. Chiarugi, Rac and Rho GTPases in cancer cell motility control. Cell communication and signaling, 2010. 8: p. 1–14. [CrossRef]
- Zhang, P., et al., Rac1 activates HIF-1 in retinal pigment epithelium cells under hypoxia. Graefe's Archive for Clinical and Experimental Ophthalmology, 2009. 247: p. 633–639. [CrossRef]
- Sahajpal, N., A. Kowluru, and R.A. Kowluru, The regulatory role of Rac1, a small molecular weight GTPase, in the development of diabetic retinopathy. Journal of clinical medicine, 2019. 8(7): p. 965.
- Ramshekar, A., H. Wang, and M.E. Hartnett, Regulation of Rac1 activation in choroidal endothelial cells: insights into mechanisms in age-related macular degeneration. Cells, 2021. 10(9): p. 2414. [CrossRef]
- Kowluru, R.A., et al., TIAM1–RAC1 signalling axis-mediated activation of NADPH oxidase-2 initiates mitochondrial damage in the development of diabetic retinopathy. Diabetologia, 2014. 57: p. 1047–1056. [CrossRef]
- Wilkinson-Berka, J.L., et al., Reactive oxygen species, Nox and angiotensin II in angiogenesis: implications for retinopathy. Clinical science, 2013. 124(10): p. 597–615. [CrossRef]
- Lenin, R., S.M. Thomas, and R. Gangaraju, Endothelial activation and oxidative stress in neurovascular defects of the retina. Current pharmaceutical design, 2018. 24(40): p. 4742–4754. [CrossRef]
- Nomura-Komoike, K., F. Saitoh, and H. Fujieda, Phosphatidylserine recognition and Rac1 activation are required for Müller glia proliferation, gliosis and phagocytosis after retinal injury. Scientific reports, 2020. 10(1): p. 1488.
- Zhang, P., et al., Rac1 activates HIF-1 in retinal pigment epithelium cells under hypoxia. Graefes Arch Clin Exp Ophthalmol, 2009. 247(5): p. 633–9. [CrossRef]
- Li, Z.-W., et al., GNAI1 and GNAI3 reduce colitis-associated tumorigenesis in mice by blocking IL6 signaling and down-regulating expression of GNAI2. Gastroenterology, 2019. 156(8): p. 2297–2312. [CrossRef]
- Muir, A.M., et al., Variants in GNAI1 cause a syndrome associated with variable features including developmental delay, seizures, and hypotonia. Genetics in Medicine, 2021. 23(5): p. 881–887. [CrossRef]
- Bonkowski, E., E. Fathi, and H.C. Mefford, GNAI1-Related Neurodevelopmental Disorder. GeneReviews®[Internet], 2024.
- Wu, Y., et al., Exosomal miR-320d promotes angiogenesis and colorectal cancer metastasis via targeting GNAI1 to affect the JAK2/STAT3 signaling pathway. Cell Death & Disease, 2024. 15(12): p. 913.
- Oláhová, M., et al., Biallelic mutations in ATP5F1D, which encodes a subunit of ATP synthase, cause a metabolic disorder. The American Journal of Human Genetics, 2018. 102(3): p. 494–504. [CrossRef]
- Ferrington, D.A., C.R. Fisher, and R.A. Kowluru, Mitochondrial defects drive degenerative retinal diseases. Trends in molecular medicine, 2020. 26(1): p. 105–118. [CrossRef]
- Solaini, G., et al., Hypoxia and mitochondrial oxidative metabolism. Biochimica et Biophysica Acta (BBA)-Bioenergetics, 2010. 1797(6-7): p. 1171–1177.
- Chen, Y., et al., Metabolism dysregulation in retinal diseases and related therapies. Antioxidants, 2022. 11(5): p. 942. [CrossRef]
- O’Brien, L., et al., Biliverdin reductase isozymes in metabolism. Trends in Endocrinology & Metabolism, 2015. 26(4): p. 212–220. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




