Submitted:
09 June 2025
Posted:
09 June 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
3. Short-Term Intermediate Outcomes
3.1. Enhanced Toxin Clearance
3.2. Improved Hemodynamic Stability
3.3. Reduction of Intradialytic Cramps
3.4. Reduction in Inflammation and Oxidative Stress
3.5. Improved Anemia Management
3.6. Preservation of Residual Kidney Function
3.7. Reduction in Skin Hyperpigmentation
4. Middle-Term Intermediate Outcomes
4.1. ß2-Microglobulin Amyloidosis and Joint Symptoms Control
4.2. Improved Nutritional Status
4.3. Reduced Infection Risk
4.4. Cardiovascular Benefits
4.4. Peripheral Neuropathy Improvements
4.5. Cognitive and Quality of Life Benefits
5. Long-term outcomes
5.1. Randomized Controlled Trials
5.2. Meta-analyses: Expanding the Case for HVHDF
5.3. Reinforcement from Real World Evidence. Dose-response across observational studies
6. Conclusion
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- United States Renal Data System. 2024 USRDS Annual Data Report: Epidemiology of kidney disease in the United States; National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD, , 2024.
- Blankestijn, P.J.; Vernooij, R.W.M.; Hockham, C.; Strippoli, G.F.M.; Canaud, B.; Hegbrant, J.; Barth, C.; Covic, A.; Cromm, K.; Cucui, A.; et al. Effect of Hemodiafiltration or Hemodialysis on Mortality in Kidney Failure. N Engl J Med 2023, 389, 700-709. [CrossRef]
- Vernooij, R.W.M.; Hockham, C.; Strippoli, G.; Green, S.; Hegbrant, J.; Davenport, A.; Barth, C.; Canaud, B.; Woodward, M.; Blankestijn, P.J.; et al. Haemodiafiltration versus haemodialysis for kidney failure: an individual patient data meta-analysis of randomised controlled trials. Lancet (London, England) 2024, 404, 1742-1749. [CrossRef]
- Rose, M.; Fischer, F.H.; Liegl, G.; Strippoli, G.F.M.; Hockham, C.; Vernooij, R.W.M.; Barth, C.; Canaud, B.; Covic, A.; Cromm, K.; et al. The CONVINCE randomized trial found positive effects on quality of life for patients with chronic kidney disease treated with hemodiafiltration. Kidney Int 2024, 106, 961-971. [CrossRef]
- Strippoli, G.F.M.; Green, S.C. Actioning the findings of hard endpoint clinical trials as they emerge in the realm of chronic kidney disease care: a review and a call to action. Clin Kidney J 2024, 17, sfae035. [CrossRef]
- Peters, S.A.; Bots, M.L.; Canaud, B.; Davenport, A.; Grooteman, M.P.; Kircelli, F.; Locatelli, F.; Maduell, F.; Morena, M.; Nubé, M.J.; et al. Haemodiafiltration and mortality in end-stage kidney disease patients: a pooled individual participant data analysis from four randomized controlled trials. Nephrol Dial Transplant 2016, 31, 978-984. [CrossRef]
- Battaglia, Y.; Shroff, R.; Meijers, B.; Nistor, I.; Alfano, G.; Franssen, C.; Luyckx, V.; Liakopoulos, V.; Mantovani, A.; Baciga, F.; et al. Haemodiafiltration versus high-flux haemodialysis - a Consensus Statement from the EuDial Working Group of the ERA. Nephrol Dial Transplant 2025. [CrossRef]
- Canaud, B.; Blankestijn, P.J.; Grooteman, M.P.C.; Davenport, A. Why and how high volume hemodiafiltration may reduce cardiovascular mortality in stage 5 chronic kidney disease dialysis patients? A comprehensive literature review on mechanisms involved. Semin Dial 2022, 35, 117-128. [CrossRef]
- Lang, T.; Zawada, A.M.; Theis, L.; Braun, J.; Ottillinger, B.; Kopperschmidt, P.; Gagel, A.; Kotanko, P.; Stauss-Grabo, M.; Kennedy, J.P.; et al. Hemodiafiltration: Technical and Medical Insights. Bioengineering (Basel) 2023, 10. [CrossRef]
- Pedreros-Rosales, C.; Jara, A.; Lorca, E.; Mezzano, S.; Pecoits-Filho, R.; Herrera, P. Unveiling the Clinical Benefits of High-Volume Hemodiafiltration: Optimizing the Removal of Medium-Weight Uremic Toxins and Beyond. Toxins (Basel) 2023, 15. [CrossRef]
- Canaud, B.; Strippoli, G.; Davenport, A. High-Volume Hemodiafiltration Versus High-Flux Hemodialysis: A Narrative Review for the Clinician. J Clin Med 2025, 14. [CrossRef]
- Stuard, S.; Maddux, F.W. High-Volume Hemodiafiltration: Expanding the Evidence Beyond Randomized Trials—A Critical Perspective on the 2025 EuDial Consensus. Journal of Clinical Medicine 2025, 14, 3174.
- Vanholder, R.; De Smet, R.; Glorieux, G.; Argilés, A.; Baurmeister, U.; Brunet, P.; Clark, W.; Cohen, G.; De Deyn, P.P.; Deppisch, R.; et al. Review on uremic toxins: classification, concentration, and interindividual variability. Kidney Int 2003, 63, 1934-1943. [CrossRef]
- Neirynck, N.; Vanholder, R.; Schepers, E.; Eloot, S.; Pletinck, A.; Glorieux, G. An update on uremic toxins. International urology and nephrology 2013, 45, 139-150. [CrossRef]
- Rosner, M.H.; Reis, T.; Husain-Syed, F.; Vanholder, R.; Hutchison, C.; Stenvinkel, P.; Blankestijn, P.J.; Cozzolino, M.; Juillard, L.; Kashani, K.; et al. Classification of Uremic Toxins and Their Role in Kidney Failure. Clin J Am Soc Nephrol 2021, 16, 1918-1928. [CrossRef]
- Canaud, B.; Bosc, J.Y.; Leblanc, M.; Garred, L.J.; Vo, T.; Mion, C. Evaluation of high-flux hemodiafiltration efficiency using an on-line urea monitor. Am J Kidney Dis 1998, 31, 74-80. [CrossRef]
- Canaud, B.; Bragg-Gresham, J.L.; Marshall, M.R.; Desmeules, S.; Gillespie, B.W.; Depner, T.; Klassen, P.; Port, F.K. Mortality risk for patients receiving hemodiafiltration versus hemodialysis: European results from the DOPPS. Kidney Int 2006, 69, 2087-2093. [CrossRef]
- Pedrini, L.A.; De Cristofaro, V.; Comelli, M.; Casino, F.G.; Prencipe, M.; Baroni, A.; Campolo, G.; Manzoni, C.; Coli, L.; Ruggiero, P.; et al. Long-term effects of high-efficiency on-line haemodiafiltration on uraemic toxicity. A multicentre prospective randomized study. Nephrol Dial Transplant 2011, 26, 2617-2624. [CrossRef]
- Shinzato, T.; Kobayakawa, H.; Maeda, K. Comparison of various treatment modes in terms of beta 2-microglobulin removal: hemodialysis, hemofiltration, and push/pull HDF. Artif Organs 1989, 13, 66-70. [CrossRef]
- Vanholder, R.; Meert, N.; Schepers, E.; Glorieux, G. From uremic toxin retention to removal by convection: do we know enough? Contrib Nephrol 2008, 161, 125-131. [CrossRef]
- Santoro, A.; Ferramosca, E.; Mancini, E.; Monari, C.; Varasani, M.; Sereni, L.; Wratten, M. Reverse mid-dilution: new way to remove small and middle molecules as well as phosphate with high intrafilter convective clearance. Nephrol Dial Transplant 2007, 22, 2000-2005. [CrossRef]
- Canaud, B.; Gagel, A.; Peters, A.; Maierhofer, A.; Stuard, S. Does online high-volume hemodiafiltration offer greater efficiency and sustainability compared with high-flux hemodialysis? A detailed simulation analysis anchored in real-world data. Clin Kidney J 2024, 17, sfae147. [CrossRef]
- Canaud, B. The early years of on-line HDF: how did it all start? How did we get here? Contrib Nephrol 2011, 175, 93-109. [CrossRef]
- Lornoy, W.; De Meester, J.; Becaus, I.; Billiouw, J.M.; Van Malderen, P.A.; Van Pottelberge, M. Impact of convective flow on phosphorus removal in maintenance hemodialysis patients. J Ren Nutr 2006, 16, 47-53. [CrossRef]
- Zehnder, C.; Gutzwiller, J.P.; Renggli, K. Hemodiafiltration--a new treatment option for hyperphosphatemia in hemodialysis patients. Clin Nephrol 1999, 52, 152-159.
- Penne, E.L.; van der Weerd, N.C.; van den Dorpel, M.A.; Grooteman, M.P.; Levesque, R.; Nube, M.J.; Bots, M.L.; Blankestijn, P.J.; ter Wee, P.M.; Investigators, C. Short-term effects of online hemodiafiltration on phosphate control: a result from the randomized controlled Convective Transport Study (CONTRAST). Am J Kidney Dis 2010, 55, 77-87. [CrossRef]
- Davenport, A.; Gardner, C.; Delaney, M.; Pan Thames Renal Audit, G. The effect of dialysis modality on phosphate control : haemodialysis compared to haemodiafiltration. The Pan Thames Renal Audit. Nephrol Dial Transplant 2010, 25, 897-901. [CrossRef]
- Daugirdas, J.T. Comparison of measured vs kinetic-model predicted phosphate removal during hemodialysis and hemodiafiltration. Nephrol Dial Transplant 2022, 37, 2522-2527. [CrossRef]
- Movilli, E.; Camerini, C.; Gaggia, P.; Poiatti, P.; Pola, A.; Viola, B.F.; Zubani, R.; Jeannin, G.; Cancarini, G. Effect of post-dilutional on-line haemodiafiltration on serum calcium, phosphate and parathyroid hormone concentrations in uraemic patients. Nephrol Dial Transplant 2011, 26, 4032-4037. [CrossRef]
- Ok, E.; Asci, G.; Toz, H.; Ok, E.S.; Kircelli, F.; Yilmaz, M.; Hur, E.; Demirci, M.S.; Demirci, C.; Duman, S.; et al. Mortality and cardiovascular events in online haemodiafiltration (OL-HDF) compared with high-flux dialysis: results from the Turkish OL-HDF Study. Nephrol Dial Transplant 2013, 28, 192-202. [CrossRef]
- Maduell, F.; Moreso, F.; Pons, M.; Ramos, R.; Mora-Macia, J.; Carreras, J.; Soler, J.; Torres, F.; Campistol, J.M.; Martinez-Castelao, A.; et al. High-efficiency postdilution online hemodiafiltration reduces all-cause mortality in hemodialysis patients. J Am Soc Nephrol 2013, 24, 487-497. [CrossRef]
- Moon, S.J.; Lee, J.E.; Kim, J.K.; Yoon, S.Y.; Kang, S.W.; Choi, K.H.; Ha, S.K.; Park, H.C. The relationship between hemodialysis modality and insulin resistance in non-diabetic hemodialysis patients. Blood purification 2015, 39, 224-229. [CrossRef]
- Chen, H.; Han, X.; Cui, Y.; Ye, Y.; Purrunsing, Y.; Wang, N. Parathyroid Hormone Fragments: New Targets for the Diagnosis and Treatment of Chronic Kidney Disease-Mineral and Bone Disorder. Biomed Res Int 2018, 2018, 9619253. [CrossRef]
- Stein, G.; Franke, S.; Mahiout, A.; Schneider, S.; Sperschneider, H.; Borst, S.; Vienken, J. Influence of dialysis modalities on serum AGE levels in end-stage renal disease patients. Nephrol Dial Transplant 2001, 16, 999-1008. [CrossRef]
- Jørstad, S.; Smeby, L.C.; Balstad, T.; Widerøe, T.E. Generation and removal of anaphylatoxins during hemofiltration with five different membranes. Blood purification 1988, 6, 325-335. [CrossRef]
- Padrini, R.; Canova, C.; Conz, P.; Mancini, E.; Rizzioli, E.; Santoro, A. Convective and adsorptive removal of beta2-microglobulin during predilutional and postdilutional hemofiltration. Kidney Int 2005, 68, 2331-2337. [CrossRef]
- Lornoy, W.; Becaus, I.; Billiouw, J.M.; Sierens, L.; Van Malderen, P.; D'Haenens, P. On-line haemodiafiltration. Remarkable removal of beta2-microglobulin. Long-term clinical observations. Nephrol Dial Transplant 2000, 15 Suppl 1, 49-54. [CrossRef]
- Maduell, F.; del Pozo, C.; Garcia, H.; Sanchez, L.; Hdez-Jaras, J.; Albero, M.D.; Calvo, C.; Torregrosa, I.; Navarro, V. Change from conventional haemodiafiltration to on-line haemodiafiltration. Nephrol Dial Transplant 1999, 14, 1202-1207. [CrossRef]
- Roumelioti, M.E.; Trietley, G.; Nolin, T.D.; Ng, Y.H.; Xu, Z.; Alaini, A.; Figueroa, R.; Unruh, M.L.; Argyropoulos, C.P. Beta-2 microglobulin clearance in high-flux dialysis and convective dialysis modalities: a meta-analysis of published studies. Nephrol Dial Transplant 2018, 33, 542. [CrossRef]
- Locatelli, F.; Mastrangelo, F.; Redaelli, B.; Ronco, C.; Marcelli, D.; La Greca, G.; Orlandini, G. Effects of different membranes and dialysis technologies on patient treatment tolerance and nutritional parameters. The Italian Cooperative Dialysis Study Group. Kidney Int 1996, 50, 1293-1302. [CrossRef]
- Susantitaphong, P.; Tiranathanagul, K.; Katavetin, P.; Townamchai, N.; Praditpornsilpa, K.; Tungsanga, K.; Eiam-Ong, S. Efficacy of convective-controlled double high-flux hemodiafiltration versus on-line hemodiafiltration: 1-year prospective study. Blood purification 2010, 29, 35-43. [CrossRef]
- Ward, R.A.; Greene, T.; Hartmann, B.; Samtleben, W. Resistance to intercompartmental mass transfer limits beta2-microglobulin removal by post-dilution hemodiafiltration. Kidney Int 2006, 69, 1431-1437. [CrossRef]
- Guedes, M.; Vernooij, R.W.M.; Davenport, A.; Kuhlmann, M.K.; Aregger, F.; Pecoits-Filho, R. Clinical performance, intermediate and long-term outcomes of high-volume hemodiafiltration in patients with kidney failure. Semin Dial 2022, 35, 420-426. [CrossRef]
- Widjaja, A.; Kielstein, J.T.; Horn, R.; von zur Muhlen, A.; Kliem, V.; Brabant, G. Free serum leptin but not bound leptin concentrations are elevated in patients with end-stage renal disease. Nephrol Dial Transplant 2000, 15, 846-850. [CrossRef]
- Kim, S.; Oh, K.H.; Chin, H.J.; Na, K.Y.; Kim, Y.S.; Chae, D.W.; Ahn, C.; Han, J.S.; Kim, S.; Joo, K.W. Effective removal of leptin via hemodiafiltration with on-line endogenous reinfusion therapy. Clin Nephrol 2009, 72, 442-448. [CrossRef]
- Kuo, H.L.; Chou, C.Y.; Liu, Y.L.; Yang, Y.F.; Huang, C.C.; Lin, H.H. Reduction of pro-inflammatory cytokines through hemodiafiltration. Ren Fail 2008, 30, 796-800. [CrossRef]
- Morena, M.; Creput, C.; Bouzernidj, M.; Rodriguez, A.; Chalabi, L.; Seigneuric, B.; Lauret, C.; Bargnoux, A.S.; Dupuy, A.M.; Cristol, J.P. Randomised trial on clinical performances and biocompatibility of four high-flux hemodialyzers in two mode treatments: hemodialysis vs post dilution hemodiafiltration. Sci Rep 2019, 9, 18265. [CrossRef]
- Suzuki, S.; Moriyama, K.; Hara, Y.; Hinoue, T.; Kato, Y.; Hasegawa, D.; Kuriyama, N.; Nakamura, T.; Komatsu, S.; Yamashita, C.; et al. Comparison of myoglobin clearance in three types of blood purification modalities. Ther Apher Dial 2021, 25, 401-406. [CrossRef]
- Canaud, B.; Wizemann, V.; Pizzarelli, F.; Greenwood, R.; Schultze, G.; Weber, C.; Falkenhagen, D. Cellular interleukin-1 receptor antagonist production in patients receiving on-line haemodiafiltration therapy. Nephrol Dial Transplant 2001, 16, 2181-2187. [CrossRef]
- Krieter, D.H.; Falkenhain, S.; Chalabi, L.; Collins, G.; Lemke, H.D.; Canaud, B. Clinical cross-over comparison of mid-dilution hemodiafiltration using a novel dialyzer concept and post-dilution hemodiafiltration. Kidney Int 2005, 67, 349-356. [CrossRef]
- Bourguignon, C.; Chenine, L.; Bargnoux, A.S.; Leray-Moragues, H.; Canaud, B.; Cristol, J.P.; Morena, M. Hemodiafiltration improves free light chain removal and normalizes kappa/lambda ratio in hemodialysis patients. J Nephrol 2016, 29, 251-257. [CrossRef]
- Lukkanalikitkul, E.; Kidkaem, H.; Phonrat, M.; Prathompong, P.; Anutrakulchai, S. A randomized trial comparing medium cut-off membrane dialyzers with online hemodiafiltration for uremic toxins clearance in hemodialysis patients. Sci Rep 2025, 15, 5467. [CrossRef]
- Ward, R.A.; Schmidt, B.; Hullin, J.; Hillebrand, G.F.; Samtleben, W. A comparison of on-line hemodiafiltration and high-flux hemodialysis: a prospective clinical study. J Am Soc Nephrol 2000, 11, 2344-2350. [CrossRef]
- Ronco, C. Hemodiafiltration: Technical and Clinical Issues. Blood purification 2015, 40 Suppl 1, 2-11. [CrossRef]
- Meijers, B.K.; Van Kerckhoven, S.; Verbeke, K.; Dehaen, W.; Vanrenterghem, Y.; Hoylaerts, M.F.; Evenepoel, P. The uremic retention solute p-cresyl sulfate and markers of endothelial damage. Am J Kidney Dis 2009, 54, 891-901. [CrossRef]
- Sakurai, K.; Saito, T.; Hosoya, H.; Kurihara, Y.; Yamauchi, F. Therapeutic effect of high-efficiency online hemodiafiltration for recurrent restless legs syndrome in dialysis patients. J Artif Organs 2020, 23, 296-301. [CrossRef]
- den Hoedt, C.H.; Bots, M.L.; Grooteman, M.P.; van der Weerd, N.C.; Mazairac, A.H.; Penne, E.L.; Levesque, R.; ter Wee, P.M.; Nube, M.J.; Blankestijn, P.J.; et al. Online hemodiafiltration reduces systemic inflammation compared to low-flux hemodialysis. Kidney Int 2014, 86, 423-432. [CrossRef]
- Patrier, L.; Dupuy, A.M.; Granger Vallee, A.; Chalabi, L.; Morena, M.; Canaud, B.; Cristol, J.P. FGF-23 removal is improved by on-line high-efficiency hemodiafiltration compared to conventional high flux hemodialysis. J Nephrol 2013, 26, 342-349. [CrossRef]
- Nishizawa, Y.; Hosoda, Y.; Horimoto, A.; Omae, K.; Ito, K.; Higuchi, C.; Sakura, H.; Nitta, K.; Ogawa, T. Fibroblast growth factor 23 (FGF23) level is associated with ultrafiltration rate in patients on hemodialysis. Heart Vessels 2021, 36, 414-423. [CrossRef]
- Lima, J.D.; Guedes, M.; Rodrigues, S.D.; Florido, A.C.S.; Moreno-Amaral, A.N.; Barra, A.B.; Canziani, M.E.; Cuvello-Neto, A.; Poli-de-Figueiredo, C.E.; Pecoits-Filho, R.; et al. High-volume hemodiafiltration decreases the pre-dialysis concentrations of indoxyl sulfate and p-cresyl sulfate compared to hemodialysis: a post-hoc analysis from the HDFit randomized controlled trial. J Nephrol 2022, 35, 1449-1456. [CrossRef]
- Stuard, S.; Ridel, C.; Cioffi, M.; Trost-Rupnik, A.; Gurevich, K.; Bojic, M.; Karibayev, Y.; Mohebbi, N.; Marcinkowski, W.; Kupres, V. Hemodialysis Procedures for Stable Incident and Prevalent Patients Optimize Hemodynamic Stability, Dialysis Dose, Electrolytes, and Fluid Balance. Journal of Clinical Medicine 2024, 13, 3211.
- Bleyer, A.J.; Russell, G.B.; Satko, S.G. Sudden and cardiac death rates in hemodialysis patients. Kidney Int 1999, 55, 1553-1559. [CrossRef]
- Flythe, J.E.; Xue, H.; Lynch, K.E.; Curhan, G.C.; Brunelli, S.M. Association of mortality risk with various definitions of intradialytic hypotension. J Am Soc Nephrol 2015, 26, 724-734. [CrossRef]
- Burton, J.O.; Jefferies, H.J.; Selby, N.M.; McIntyre, C.W. Hemodialysis-induced repetitive myocardial injury results in global and segmental reduction in systolic cardiac function. Clin J Am Soc Nephrol 2009, 4, 1925-1931. [CrossRef]
- Locatelli, F.; Altieri, P.; Andrulli, S.; Bolasco, P.; Sau, G.; Pedrini, L.A.; Basile, C.; David, S.; Feriani, M.; Montagna, G.; et al. Hemofiltration and hemodiafiltration reduce intradialytic hypotension in ESRD. J Am Soc Nephrol 2010, 21, 1798-1807. [CrossRef]
- Donauer, J.; Schweiger, C.; Rumberger, B.; Krumme, B.; Bohler, J. Reduction of hypotensive side effects during online-haemodiafiltration and low temperature haemodialysis. Nephrol Dial Transplant 2003, 18, 1616-1622. [CrossRef]
- Sande, F.M.V.; Kooman, J.P.; Konings, C.J.; Leunissen, K.M.L. Thermal effects and blood pressure response during postdilution hemodiafiltration and hemodialysis: the effect of amount of replacement fluid and dialysate temperature. J Am Soc Nephrol 2001, 12, 1916-1920. [CrossRef]
- Morena, M.; Jaussent, A.; Chalabi, L.; Leray-Moragues, H.; Chenine, L.; Debure, A.; Thibaudin, D.; Azzouz, L.; Patrier, L.; Maurice, F.; et al. Treatment tolerance and patient-reported outcomes favor online hemodiafiltration compared to high-flux hemodialysis in the elderly. Kidney Int 2017, 91, 1495-1509. [CrossRef]
- Wang, A.Y.; Ninomiya, T.; Al-Kahwa, A.; Perkovic, V.; Gallagher, M.P.; Hawley, C.; Jardine, M.J. Effect of hemodiafiltration or hemofiltration compared with hemodialysis on mortality and cardiovascular disease in chronic kidney failure: a systematic review and meta-analysis of randomized trials. Am J Kidney Dis 2014, 63, 968-978. [CrossRef]
- Kawanishi, H. Is There Enough Evidence to Prove That Hemodiafiltration Is Superior? Blood purification 2018, 46, 3-6. [CrossRef]
- Sars, B.; van der Sande, F.M.; Kooman, J.P. Intradialytic Hypotension: Mechanisms and Outcome. Blood purification 2020, 49, 158-167. [CrossRef]
- Gross, M.; Gagel, A.; Maierhofer, A. The Donnan equilibrium is still valid in high-volume HDF. The International journal of artificial organs 2024, 47, 867-875. [CrossRef]
- Waniewski, J.; Pietribiasi, M.; Pstras, L. Calculation of the Gibbs-Donnan factors for multi-ion solutions with non-permeating charge on both sides of a permselective membrane. Sci Rep 2021, 11, 22150. [CrossRef]
- Rodriguez, A.; Morena, M.; Bargnoux, A.S.; Chenine, L.; Leray-Moragues, H.; Cristol, J.P.; Canaud, B. Quantitative assessment of sodium mass removal using ionic dialysance and sodium gradient as a proxy tool: Comparison of high-flux hemodialysis versus online hemodiafiltration. Artif Organs 2021, 45, E280-E292. [CrossRef]
- Chazot, C.; Deleuze, S.; Fadel, B.; Hebibi, H.; Jean, G.; Levannier, M.; Puyoo, O.; Attaf, D.; Stuard, S.; Canaud, B. Is high-volume post-dilution haemodiafiltration associated with risk of fluid volume imbalance? A national multicentre cross-sectional cohort study. Nephrology Dialysis Transplantation 2019, 34, 2089-2095. [CrossRef]
- La Milia, V.; Ravasi, C.; Carfagna, F.; Alberghini, E.; Baragetti, I.; Buzzi, L.; Ferrario, F.; Furiani, S.; Barbone, G.S.; Pontoriero, G. Sodium removal and plasma tonicity balance are not different in hemodialysis and hemodiafiltration using high-flux membranes. J Nephrol 2019, 32, 461-469. [CrossRef]
- Myburgh, J.A.; Mythen, M.G. Resuscitation fluids. N Engl J Med 2013, 369, 2462-2463. [CrossRef]
- Oberleithner, H. Vascular endothelium: a vulnerable transit zone for merciless sodium. Nephrol Dial Transplant 2014, 29, 240-246. [CrossRef]
- Daugirdas, J.T. Lower cardiovascular mortality with high-volume hemodiafiltration: a cool effect? Nephrol Dial Transplant 2016, 31, 853-856. [CrossRef]
- Maggiore, Q.; Pizzarelli, F.; Sisca, S.; Zoccali, C.; Parlongo, S.; Nicolo, F.; Creazzo, G. Blood temperature and vascular stability during hemodialysis and hemofiltration. Trans Am Soc Artif Intern Organs 1982, 28, 523-527.
- Agbas, A.; Canpolat, N.; Caliskan, S.; Yilmaz, A.; Ekmekci, H.; Mayes, M.; Aitkenhead, H.; Schaefer, F.; Sever, L.; Shroff, R. Hemodiafiltration is associated with reduced inflammation, oxidative stress and improved endothelial risk profile compared to high-flux hemodialysis in children. PLoS One 2018, 13, e0198320. [CrossRef]
- Filiopoulos, V.; Hadjiyannakos, D.; Metaxaki, P.; Sideris, V.; Takouli, L.; Anogiati, A.; Vlassopoulos, D. Inflammation and oxidative stress in patients on hemodiafiltration. Am J Nephrol 2008, 28, 949-957. [CrossRef]
- Marcelli, D.; Bayh, I.; Merello, J.I.; Ponce, P.; Heaton, A.; Kircelli, F.; Chazot, C.; Di Benedetto, A.; Marelli, C.; Ladanyi, E.; et al. Dynamics of the erythropoiesis stimulating agent resistance index in incident hemodiafiltration and high-flux hemodialysis patients. Kidney Int 2016, 90, 192-202. [CrossRef]
- Panichi, V.; Scatena, A.; Rosati, A.; Giusti, R.; Ferro, G.; Malagnino, E.; Capitanini, A.; Piluso, A.; Conti, P.; Bernabini, G.; et al. High-volume online haemodiafiltration improves erythropoiesis-stimulating agent (ESA) resistance in comparison with low-flux bicarbonate dialysis: results of the REDERT study. Nephrol Dial Transplant 2015, 30, 682-689. [CrossRef]
- Molina, P.; Vizcaino, B.; Molina, M.D.; Beltran, S.; Gonzalez-Moya, M.; Mora, A.; Castro-Alonso, C.; Kanter, J.; Avila, A.I.; Gorriz, J.L.; et al. The effect of high-volume online haemodiafiltration on nutritional status and body composition: the ProtEin Stores prEservaTion (PESET) study. Nephrol Dial Transplant 2018, 33, 1223-1235. [CrossRef]
- Aichi, M.; Kuragano, T.; Iwasaki, T.; Ookawa, S.; Masumoto, M.; Mizusaki, K.; Yahiro, M.; Kida, A.; Nanami, M. Hemodiafiltration Improves Low Levels of Health-Related Quality Of Life (Qol) and Nutritional Conditions of Hemodialysis Patients. ASAIO journal (American Society for Artificial Internal Organs : 1992) 2022, 68, 297-302. [CrossRef]
- Pecoits-Filho, R.; Larkin, J.; Poli-de-Figueiredo, C.E.; Cuvello-Neto, A.L.; Barra, A.B.L.; Goncalves, P.B.; Sheth, S.; Guedes, M.; Han, M.; Calice-Silva, V.; et al. Effect of hemodiafiltration on measured physical activity: primary results of the HDFIT randomized controlled trial. Nephrol Dial Transplant 2021, 36, 1057-1070. [CrossRef]
- Karkar, A.; Abdelrahman, M.; Locatelli, F. A Randomized Trial on Health-Related Patient Satisfaction Level with High-Efficiency Online Hemodiafiltration versus High-Flux Dialysis. Blood purification 2015, 40, 84-91. [CrossRef]
- Hazim, A.; Adarmouch, L.; Eloury, A.; Aasfara, J.; Asly, M.; Slassi, I. Hemodialysis-related headache: Still a challenge in 2020? Effect of conventional versus online hemodiafiltration from a study in Casablanca, Morocco. Artif Organs 2021, 45, 602-607. [CrossRef]
- Kantartzi, K.; Panagoutsos, S.; Mourvati, E.; Roumeliotis, A.; Leivaditis, K.; Devetzis, V.; Passadakis, P.; Vargemezis, V. Can dialysis modality influence quality of life in chronic hemodialysis patients? Low-flux hemodialysis versus high-flux hemodiafiltration: a cross-over study. Ren Fail 2013, 35, 216-221. [CrossRef]
- Vilar, E.; Fry, A.C.; Wellsted, D.; Tattersall, J.E.; Greenwood, R.N.; Farrington, K. Long-term outcomes in online hemodiafiltration and high-flux hemodialysis: a comparative analysis. Clin J Am Soc Nephrol 2009, 4, 1944-1953. [CrossRef]
- Zoccali, C.; Tripepi, G.; Carioni, P.; Fu, E.L.; Dekker, F.; Stel, V.; Jager, K.J.; Mallamaci, F.; Hymes, J.L.; Maddux, F.W.; et al. Antihypertensive Drug Treatment and the Risk for Intrahemodialysis Hypotension. Clin J Am Soc Nephrol 2024, 19, 1310-1318. [CrossRef]
- Rootjes, P.A.; Chaara, S.; de Roij van Zuijdewijn, C.L.M.; Nubé, M.J.; Wijngaarden, G.; Grooteman, M.P.C. High-Volume Hemodiafiltration and Cool Hemodialysis Have a Beneficial Effect on Intradialytic Hemodynamics: A Randomized Cross-Over Trial of Four Intermittent Dialysis Strategies. Kidney Int Rep 2022, 7, 1980-1990. [CrossRef]
- Neal, C.R.; Resnikoff, E.; Unger, A.M. Treatment of dialysis-related muscle cramps with hypertonic dextrose. Archives of internal medicine 1981, 141, 171-173.
- McGill, R.L.; Weiner, D.E. Dialysate Composition for Hemodialysis: Changes and Changing Risk. Semin Dial 2017, 30, 112-120. [CrossRef]
- Flythe, J.E.; Hilliard, T.; Castillo, G.; Ikeler, K.; Orazi, J.; Abdel-Rahman, E.; Pai, A.B.; Rivara, M.B.; St Peter, W.L.; Weisbord, S.D.; et al. Symptom Prioritization among Adults Receiving In-Center Hemodialysis: A Mixed Methods Study. Clin J Am Soc Nephrol 2018, 13, 735-745. [CrossRef]
- Punj, S.; Enaam, A.; Marquez, A.; Atkinson, A.J., Jr.; Batlle, D. A Survey on Dialysis-Related Muscle Cramping and a Hypothesis of Angiotensin II on Its Pathophysiology. Kidney Int Rep 2020, 5, 924-926. [CrossRef]
- Chillar, R.K.; Desforges, J.F. Muscular cramps during maintenance haemodialysis. Lancet (London, England) 1972, 2, 285. [CrossRef]
- Kolb, J.; Kitzler, T.M.; Tauber, T.; Morris, N.; Skrabal, F.; Kotanko, P. Proto-dialytic cardiac function relates to intra-dialytic morbid events. Nephrol Dial Transplant 2011, 26, 1645-1651. [CrossRef]
- Basile, C.; Lomonte, C. A neglected issue in dialysis practice: haemodialysate. Clin Kidney J 2015, 8, 393-399. [CrossRef]
- Beladi Mousavi, S.S.; Zeraati, A.; Moradi, S.; Mousavi, M.B. The effect of gabapentin on muscle cramps during hemodialysis: A double-blind clinical trial. Saudi J Kidney Dis Transpl 2015, 26, 1142-1148. [CrossRef]
- Noordzij, M.; Boeschoten, E.W.; Bos, W.J.; Dekker, F.W.; Bossuyt, P.M.; Krediet, R.T.; Korevaar, J.C.; Group, N.S. Disturbed mineral metabolism is associated with muscle and skin complaints in a prospective cohort of dialysis patients. Nephrol Dial Transplant 2007, 22, 2944-2949. [CrossRef]
- Campo, S.; Lacquaniti, A.; Trombetta, D.; Smeriglio, A.; Monardo, P. Immune System Dysfunction and Inflammation in Hemodialysis Patients: Two Sides of the Same Coin. J Clin Med 2022, 11. [CrossRef]
- Kokubo, K.; Kurihara, Y.; Kobayashi, K.; Tsukao, H.; Kobayashi, H. Evaluation of the Biocompatibility of Dialysis Membranes. Blood purification 2015, 40, 293-297. [CrossRef]
- Ağbaş, A.; Canpolat, N.; Çalışkan, S.; Yılmaz, A.; Ekmekçi, H.; Mayes, M.; Aitkenhead, H.; Schaefer, F.; Sever, L.; Shroff, R. Hemodiafiltration is associated with reduced inflammation, oxidative stress and improved endothelial risk profile compared to high-flux hemodialysis in children. PLoS One 2018, 13, e0198320. [CrossRef]
- Fischer, D.C.; Smith, C.; De Zan, F.; Bacchetta, J.; Bakkaloglu, S.A.; Agbas, A.; Anarat, A.; Aoun, B.; Askiti, V.; Azukaitis, K.; et al. Hemodiafiltration Is Associated With Reduced Inflammation and Increased Bone Formation Compared With Conventional Hemodialysis in Children: The HDF, Hearts and Heights (3H) Study. Kidney Int Rep 2021, 6, 2358-2370. [CrossRef]
- Santoro, A.; Mancini, E. Is hemodiafiltration the technical solution to chronic inflammation affecting hemodialysis patients? Kidney International 2014, 86, 235-237. [CrossRef]
- Cavallari, C.; Dellepiane, S.; Fonsato, V.; Medica, D.; Marengo, M.; Migliori, M.; Quercia, A.D.; Pitino, A.; Formica, M.; Panichi, V.; et al. Online Hemodiafiltration Inhibits Inflammation-Related Endothelial Dysfunction and Vascular Calcification of Uremic Patients Modulating miR-223 Expression in Plasma Extracellular Vesicles. J Immunol 2019, 202, 2372-2383. [CrossRef]
- Carracedo, J.; Merino, A.; Nogueras, S.; Carretero, D.; Berdud, I.; Ramírez, R.; Tetta, C.; Rodríguez, M.; Martín-Malo, A.; Aljama, P. On-line hemodiafiltration reduces the proinflammatory CD14+CD16+ monocyte-derived dendritic cells: A prospective, crossover study. J Am Soc Nephrol 2006, 17, 2315-2321. [CrossRef]
- Carracedo, J.; Merino, A.; Nogueras, S.; Carretero, D.; Berdud, I.; Ramirez, R.; Tetta, C.; Rodriguez, M.; Martin-Malo, A.; Aljama, P. On-line hemodiafiltration reduces the proinflammatory CD14+CD16+ monocyte-derived dendritic cells: A prospective, crossover study. J Am Soc Nephrol 2006, 17, 2315-2321. [CrossRef]
- Rama, I.; Llaudo, I.; Fontova, P.; Cerezo, G.; Soto, C.; Javierre, C.; Hueso, M.; Montero, N.; Martinez-Castelao, A.; Torras, J.; et al. Online Haemodiafiltration Improves Inflammatory State in Dialysis Patients: A Longitudinal Study. PLoS One 2016, 11, e0164969. [CrossRef]
- Panichi, V.; Tetta, C. The biological response to online hemodiafiltration. Contrib Nephrol 2007, 158, 194-200. [CrossRef]
- Canaud, B.; Chenine, L.; Henriet, D.; Leray, H. Online hemodiafiltration: a multipurpose therapy for improving quality of renal replacement therapy. Contrib Nephrol 2008, 161, 191-198. [CrossRef]
- Bowry, S.K.; Gatti, E. Impact of hemodialysis therapy on anemia of chronic kidney disease: the potential mechanisms. Blood purification 2011, 32, 210-219. [CrossRef]
- Bonforte, G.; Grillo, P.; Zerbi, S.; Surian, M. Improvement of anemia in hemodialysis patients treated by hemodiafiltration with high-volume on-line-prepared substitution fluid. Blood purification 2002, 20, 357-363. [CrossRef]
- Pedrini, L.A.; Comelli, M.; Ruggiero, P.; Feliciani, A.; Manfrini, V.; Cozzi, G.; Castellano, A.; Pezzotta, M.; Gatti, G.; Arazzi, M.; et al. Mixed hemodiafiltration reduces erythropoiesis stimulating agents requirement in dialysis patients: a prospective randomized study. J Nephrol 2020, 33, 1037-1048. [CrossRef]
- Lin, C.L.; Huang, C.C.; Yu, C.C.; Wu, C.H.; Chang, C.T.; Hsu, H.H.; Hsu, P.Y.; Yang, C.W. Improved iron utilization and reduced erythropoietin resistance by on-line hemodiafiltration. Blood purification 2002, 20, 349-356. [CrossRef]
- Macdougall, I.C. Role of uremic toxins in exacerbating anemia in renal failure. Kidney international. Supplement 2001, 78, S67-72. [CrossRef]
- Yamada, S.; Kataoka, H.; Kobayashi, H.; Ono, T.; Minakuchi, J.; Kawano, Y. Identification of an erythropoietic inhibitor from the dialysate collected in the hemodialysis with PMMA membrane (BK-F). Contrib Nephrol 1999, 125, 159-172. [CrossRef]
- Ayli, D.; Ayli, M.; Azak, A.; Yuksel, C.; Kosmaz, G.P.; Atilgan, G.; Dede, F.; Abayli, E.; Camlibel, M. The effect of high-flux hemodialysis on renal anemia. J Nephrol 2004, 17, 701-706.
- Aucella, F.; Scalzulli, R.P.; Vigilante, M.; Stallone, C. [The hemodiafiltration with endogenous reinfusion reduces the erythroid progenitor inhibition by uremic serum]. G Ital Nefrol 2004, 21 Suppl 30, S128-132.
- Allen, D.A.; Breen, C.; Yaqoob, M.M.; Macdougall, I.C. Inhibition of CFU-E colony formation in uremic patients with inflammatory disease: role of IFN-gamma and TNF-alpha. J Investig Med 1999, 47, 204-211.
- Panichi, V.; Rizza, G.M.; Paoletti, S.; Bigazzi, R.; Aloisi, M.; Barsotti, G.; Rindi, P.; Donati, G.; Antonelli, A.; Panicucci, E.; et al. Chronic inflammation and mortality in haemodialysis: effect of different renal replacement therapies. Results from the RISCAVID study. Nephrol Dial Transplant 2008, 23, 2337-2343. [CrossRef]
- Malyszko, J.; Malyszko, J.S.; Kozminski, P.; Mysliwiec, M. Type of renal replacement therapy and residual renal function may affect prohepcidin and hepcidin. Ren Fail 2009, 31, 876-883. [CrossRef]
- Bolasco, P.G.; Ghezzi, P.M.; Serra, A.; Corazza, L.; Murtas, S.; Mascia, M.; Cossu, M.; Ferrara, R.; Cogoni, G.; Cadinu, F.; et al. Hemodiafiltration with endogenous reinfusion with and without acetate-free dialysis solutions: effect on ESA requirement. Blood purification 2011, 31, 235-242. [CrossRef]
- Lutton, J.D.; Solangi, K.B.; Ibraham, N.G.; Goodman, A.I.; Levere, R.D. Inhibition of erythropoiesis in chronic renal failure: the role of parathyroid hormone. Am J Kidney Dis 1984, 3, 380-384. [CrossRef]
- Horl, W.H. The clinical consequences of secondary hyperparathyroidism: focus on clinical outcomes. Nephrol Dial Transplant 2004, 19 Suppl 5, V2-8. [CrossRef]
- Vilar, E.; Farrington, K. Emerging importance of residual renal function in end-stage renal failure. Semin Dial 2011, 24, 487-494. [CrossRef]
- Lin, C.L.; Huang, C.C.; Chang, C.T.; Wu, M.S.; Hung, C.C.; Chien, C.C.; Yang, C.W. Clinical improvement by increased frequency of on-line hemodialfiltration. Ren Fail 2001, 23, 193-206. [CrossRef]
- Moon, S.J.; Kim, D.K.; Chang, J.H.; Kim, C.H.; Kim, H.W.; Park, S.Y.; Han, S.H.; Lee, J.E.; Yoo, T.H.; Han, D.S.; et al. The impact of dialysis modality on skin hyperpigmentation in haemodialysis patients. Nephrol Dial Transplant 2009, 24, 2803-2809. [CrossRef]
- Shibata, M.; Nagai, K.; Usami, K.; Tawada, H.; Taniguchi, S. The quantitative evaluation of online haemodiafiltration effect on skin hyperpigmentation. Nephrol Dial Transplant 2011, 26, 988-992. [CrossRef]
- Locatelli, F.; Marcelli, D.; Conte, F.; Limido, A.; Malberti, F.; Spotti, D. Comparison of mortality in ESRD patients on convective and diffusive extracorporeal treatments. The Registro Lombardo Dialisi E Trapianto. Kidney Int 1999, 55, 286-293. [CrossRef]
- Cornelis, T.; van der Sande, F.M.; Eloot, S.; Cardinaels, E.; Bekers, O.; Damoiseaux, J.; Leunissen, K.M.; Kooman, J.P. Acute hemodynamic response and uremic toxin removal in conventional and extended hemodialysis and hemodiafiltration: a randomized crossover study. Am J Kidney Dis 2014, 64, 247-256. [CrossRef]
- Gal, R.; Korzets, A.; Schwartz, A.; Rath-Wolfson, L.; Gafter, U. Systemic distribution of beta 2-microglobulin-derived amyloidosis in patients who undergo long-term hemodialysis. Report of seven cases and review of the literature. Arch Pathol Lab Med 1994, 118, 718-721.
- Takayama, F.; Miyazaki, S.; Morita, T.; Hirasawa, Y.; Niwa, T. Dialysis-related amyloidosis of the heart in long-term hemodialysis patients. Kidney international. Supplement 2001, 78, S172-176. [CrossRef]
- Schiffl, H.; Lang, S.M.; Fischer, R. Ultrapure dialysis fluid slows loss of residual renal function in new dialysis patients. Nephrol Dial Transplant 2002, 17, 1814-1818. [CrossRef]
- Maeda, K.; Kobayakawa, H.; Fujita, Y.; Takai, I.; Morita, H.; Emoto, Y.; Miyazaki, T.; Shinzato, T. Effectiveness of push/pull hemodiafiltration using large-pore membrane for shoulder joint pain in long-term dialysis patients. Artif Organs 1990, 14, 321-327. [CrossRef]
- Paglialonga, F.; Monzani, A.; Prodam, F.; Smith, C.; De Zan, F.; Canpolat, N.; Agbas, A.; Bayazit, A.; Anarat, A.; Bakkaloglu, S.A.; et al. Nutritional and Anthropometric Indices in Children Receiving Haemodiafiltration vs Conventional Haemodialysis - The HDF, Heart and Height (3H) Study. J Ren Nutr 2023, 33, 17-28. [CrossRef]
- Mandolfo, S.; Borlandelli, S.; Imbasciati, E. Leptin and beta2-microglobulin kinetics with three different dialysis modalities. The International journal of artificial organs 2006, 29, 949-955. [CrossRef]
- Kalantar-Zadeh, K.; Kopple, J.D.; Humphreys, M.H.; Block, G. Comparing outcome predictability of markers of malnutrition-inflammation complex syndrome in haemodialysis patients. Nephrol Dial Transplant 2004, 19, 1507-1519. [CrossRef]
- Wolley, M.; Jardine, M.; Hutchison, C.A. Exploring the Clinical Relevance of Providing Increased Removal of Large Middle Molecules. Clin J Am Soc Nephrol 2018, 13, 805-814. [CrossRef]
- Haag-Weber, M.; Cohen, G.; Horl, W.H. Clinical significance of granulocyte-inhibiting proteins. Nephrol Dial Transplant 2000, 15 Suppl 1, 15-16. [CrossRef]
- Murtas, S.; Aquilani, R.; Iadarola, P.; Deiana, M.L.; Secci, R.; Cadeddu, M.; Bolasco, P. Differences and Effects of Metabolic Fate of Individual Amino Acid Loss in High-Efficiency Hemodialysis and Hemodiafiltration. J Ren Nutr 2020, 30, 440-451. [CrossRef]
- Bevier, A.; Novel-Catin, E.; Blond, E.; Pelletier, S.; Parant, F.; Koppe, L.; Fouque, D. Water-Soluble Vitamins and Trace Elements Losses during On-Line Hemodiafiltration. Nutrients 2022, 14. [CrossRef]
- Johansen, K.L.; Gilbertson, D.T.; Li, S.; Li, S.; Liu, J.; Roetker, N.S.; Ku, E.; Schulman, I.H.; Greer, R.C.; Chan, K.; et al. US Renal Data System 2023 Annual Data Report: Epidemiology of Kidney Disease in the United States. Am J Kidney Dis 2024, 83, A8-A13. [CrossRef]
- Silberzweig, J.I. Reducing Infections in Outpatient Hemodialysis: The Impact of Human Factors. Am J Kidney Dis 2024, 84, 4-5. [CrossRef]
- Kato, S.; Chmielewski, M.; Honda, H.; Pecoits-Filho, R.; Matsuo, S.; Yuzawa, Y.; Tranaeus, A.; Stenvinkel, P.; Lindholm, B. Aspects of immune dysfunction in end-stage renal disease. Clin J Am Soc Nephrol 2008, 3, 1526-1533. [CrossRef]
- Vanholder, R.; Ringoir, S. Infectious morbidity and defects of phagocytic function in end-stage renal disease: a review. J Am Soc Nephrol 1993, 3, 1541-1554. [CrossRef]
- Vaziri, N.D.; Pahl, M.V.; Crum, A.; Norris, K. Effect of uremia on structure and function of immune system. J Ren Nutr 2012, 22, 149-156. [CrossRef]
- den Hoedt, C.H.; Grooteman, M.P.; Bots, M.L.; Blankestijn, P.J.; van der Tweel, I.; van der Weerd, N.C.; Penne, E.L.; Mazairac, A.H.; Levesque, R.; ter Wee, P.M.; et al. The Effect of Online Hemodiafiltration on Infections: Results from the CONvective TRAnsport STudy. PLoS One 2015, 10, e0135908. [CrossRef]
- Allon, M.; Radeva, M.; Bailey, J.; Beddhu, S.; Butterly, D.; Coyne, D.W.; Depner, T.A.; Gassman, J.J.; Kaufman, A.M.; Kaysen, G.A.; et al. The spectrum of infection-related morbidity in hospitalized haemodialysis patients. Nephrol Dial Transplant 2005, 20, 1180-1186. [CrossRef]
- Kaplowitz, L.G.; Comstock, J.A.; Landwehr, D.M.; Dalton, H.P.; Mayhall, C.G. A prospective study of infections in hemodialysis patients: patient hygiene and other risk factors for infection. Infect Control Hosp Epidemiol 1988, 9, 534-541. [CrossRef]
- Akmal, M. Hemodialysis in diabetic patients. Am J Kidney Dis 2001, 38, S195-199. [CrossRef]
- Hoen, B.; Paul-Dauphin, A.; Hestin, D.; Kessler, M. EPIBACDIAL: a multicenter prospective study of risk factors for bacteremia in chronic hemodialysis patients. J Am Soc Nephrol 1998, 9, 869-876. [CrossRef]
- Schild, A.F.; Perez, E.; Gillaspie, E.; Seaver, C.; Livingstone, J.; Thibonnier, A. Arteriovenous fistulae vs. arteriovenous grafts: a retrospective review of 1,700 consecutive vascular access cases. J Vasc Access 2008, 9, 231-235.
- Canaud, B.; Popa, C.; Leray-Moragues, H.; Morena-Carrere, M.; Cristol, J.P. "Can Gut Instinct Guide the Detection of Intestinal Bacterial Translocation in Dialysis Patients?". Kidney Int Rep 2025, 10, 12-16. [CrossRef]
- Rootjes, P.A.; Grooteman, M.P.C.; Budding, A.E.; Bontkes, H.J.; Wijngaarden, G.; Nube, M.J.; de Roij van Zuijdewijn, C.L.M. Randomized Trial Demonstrating No Translocation of Intact Intestinal Bacteria During Hemodialysis or Hemodiafiltration. Kidney Int Rep 2025, 10, 109-119. [CrossRef]
- Nongnuch, A.; Ngampongpan, W.; Srichatrapimuk, S.; Wongsa, A.; Thongpraphai, S.; Boonarkart, C.; Sanmeema, N.; Chittaganpitch, M.; Auewarakul, P.; Tassaneetrithep, B.; et al. Immune response to influenza vaccination in ESRD patients undergoing hemodialysis vs. hemodiafiltration. PLoS One 2020, 15, e0227719. [CrossRef]
- Chuva, T.; Santos, T.; Goncalves, F.; Costa, L.; Alves, E.; Neves, I.; Paiva, A.; Carvalho, B.; Sousa, T.; Ramalheiro, A.; et al. Humoral immunity against Covid-19 six months after the Pfizer BNT162b2 vaccine in hemodialysis patients: data from five dialysis units. Is there a protective role for hemodiafiltration in the Covid-19 pandemic? J Nephrol 2022, 35, 1543-1545. [CrossRef]
- Lioulios, G.; Fylaktou, A.; Asouchidou, D.; Xochelli, A.; Nikolaidou, V.; Stai, S.; Christodoulou, M.; Giamalis, P.; Tsouchnikas, I.; Papagianni, A.; et al. Effect of Lymphocyte Phenotypic Alterations on the Humoral Response to Vaccination Against SARS-COV-2 in Dialysis Patients. Ann Lab Med 2023, 43, 451-460. [CrossRef]
- Hebibi, H.; Edeas, M.; Cornillac, L.; Beaudreuil, S.; Achiche, J.; Attaf, D.; Saibi, S.; Chazot, C.; Ouaaz, F.; Canaud, B. SARS-CoV-2 mRNA Vaccine Immunogenicity in Hemodialysis Patients: Promising Vaccine Protection That May Be Hindered by Fluid Overload. Kidney and Dialysis 2022, 2, 44-56.
- Carrera, F.; Jacobson, S.H.; Costa, J.; Marques, M.; Ferrer, F. Better Anti-Spike IgG Antibody Response to SARS-CoV-2 Vaccine in Patients on Haemodiafiltration than on Haemodialysis. Blood purification 2023, 52, 600-608. [CrossRef]
- Czifra, A.; Pall, A.; Kulcsar, J.; Barta, K.; Kertesz, A.; Paragh, G.; Lorincz, I.; Jenei, Z.; Agarwal, A.; Zarjou, A.; et al. Hemodialysis and hemodiafiltration differently modulate left ventricular diastolic function. BMC Nephrol 2013, 14, 76. [CrossRef]
- Mostovaya, I.M.; Blankestijn, P.J.; Bots, M.L.; Covic, A.; Davenport, A.; Grooteman, M.P.; Hegbrant, J.; Locatelli, F.; Vanholder, R.; Nube, M.J.; et al. Clinical evidence on hemodiafiltration: a systematic review and a meta-analysis. Semin Dial 2014, 27, 119-127. [CrossRef]
- Ohtake, T.; Oka, M.; Ishioka, K.; Honda, K.; Mochida, Y.; Maesato, K.; Moriya, H.; Hidaka, S.; Kobayashi, S. Cardiovascular protective effects of on-line hemodiafiltration: comparison with conventional hemodialysis. Ther Apher Dial 2012, 16, 181-188. [CrossRef]
- Charitaki, E.; Davenport, A. Does hemodiafiltration reduce vascular stiffness measured by aortic pulse wave velocity compared with high-flux hemodialysis? Hemodialysis international. International Symposium on Home Hemodialysis 2014, 18, 391-395. [CrossRef]
- Shroff, R.; Smith, C.; Ranchin, B.; Bayazit, A.K.; Stefanidis, C.J.; Askiti, V.; Azukaitis, K.; Canpolat, N.; Agbas, A.; Aitkenhead, H.; et al. Effects of Hemodiafiltration versus Conventional Hemodialysis in Children with ESKD: The HDF, Heart and Height Study. J Am Soc Nephrol 2019, 30, 678-691. [CrossRef]
- Chang, J.W.; Yang, W.S.; Seo, J.W.; Lee, J.S.; Lee, S.K.; Park, S.K. Continuous venovenous hemodiafiltration versus hemodialysis as renal replacement therapy in patients with acute renal failure in the intensive care unit. Scand J Urol Nephrol 2004, 38, 417-421. [CrossRef]
- Nistor, I.; Palmer, S.C.; Craig, J.C.; Saglimbene, V.; Vecchio, M.; Covic, A.; Strippoli, G.F. Convective versus diffusive dialysis therapies for chronic kidney failure: an updated systematic review of randomized controlled trials. Am J Kidney Dis 2014, 63, 954-967. [CrossRef]
- Lakshman, S.G.; Ravikumar, P.; Kar, G.; Das, D.; Bhattacharjee, K.; Bhattacharjee, P. A Comparative Study of Neurological Complications in Chronic Kidney Disease with Special Reference to its Stages and Haemodialysis Status. J Clin Diagn Res 2016, 10, OC01-OC04. [CrossRef]
- Arnold, R.; Issar, T.; Krishnan, A.V.; Pussell, B.A. Neurological complications in chronic kidney disease. JRSM Cardiovasc Dis 2016, 5, 2048004016677687. [CrossRef]
- Chillon, J.M.; Massy, Z.A.; Stengel, B. Neurological complications in chronic kidney disease patients. Nephrol Dial Transplant 2016, 31, 1606-1614. [CrossRef]
- Krishnan, A.V.; Kiernan, M.C. Neurological complications of chronic kidney disease. Nat Rev Neurol 2009, 5, 542-551. [CrossRef]
- Krishnan, A.V.; Phoon, R.K.; Pussell, B.A.; Charlesworth, J.A.; Kiernan, M.C. Sensory nerve excitability and neuropathy in end stage kidney disease. J Neurol Neurosurg Psychiatry 2006, 77, 548-551. [CrossRef]
- Krishnan, A.V.; Lin, C.S.; Kiernan, M.C. Nerve excitability properties in lower-limb motor axons: evidence for a length-dependent gradient. Muscle Nerve 2004, 29, 645-655. [CrossRef]
- Camargo, C.R.S.; Schoueri, J.H.M.; Alves, B.; Veiga, G.; Fonseca, F.L.A.; Bacci, M.R. Uremic neuropathy: an overview of the current literature. Rev Assoc Med Bras (1992) 2019, 65, 469-474. [CrossRef]
- Arnold, R.; Pussell, B.A.; Pianta, T.J.; Grinius, V.; Lin, C.S.; Kiernan, M.C.; Howells, J.; Jardine, M.J.; Krishnan, A.V. Effects of hemodiafiltration and high flux hemodialysis on nerve excitability in end-stage kidney disease. PLoS One 2013, 8, e59055. [CrossRef]
- Jiang, X.; Ji, F.; Chen, Z.W.; Huang, Q.L. Comparison of high-flux hemodialysis with hemodialysis filtration in treatment of uraemic pruritus: a randomized controlled trial. International urology and nephrology 2016, 48, 1533-1541. [CrossRef]
- Arzhan, S.; Roumelioti, M.E.; Unruh, M.L. Itch and Ache on Dialysis: New Approaches to Manage Uremic Pruritus and Restless Legs. Blood purification 2020, 49, 222-227. [CrossRef]
- Kang, A.; Arnold, R.; Gallagher, M.; Snelling, P.; Green, J.; Fernando, M.; Kiernan, M.C.; Hand, S.; Grimley, K.; Burman, J.; et al. Effect of Hemodiafiltration on the Progression of Neuropathy with Kidney Failure: A Randomized Controlled Trial. Clin J Am Soc Nephrol 2021, 16, 1365-1375. [CrossRef]
- Sehgal, A.R.; Grey, S.F.; DeOreo, P.B.; Whitehouse, P.J. Prevalence, recognition, and implications of mental impairment among hemodialysis patients. American Journal of Kidney Diseases 1997, 30, 41-49. [CrossRef]
- Kurella Tamura, M.; Yaffe, K. Dementia and cognitive impairment in ESRD: diagnostic and therapeutic strategies. Kidney Int 2011, 79, 14-22. [CrossRef]
- Murray, A.M. Cognitive Impairment in the Aging Dialysis and Chronic Kidney Disease Populations: An Occult Burden. Advances in Chronic Kidney Disease 2008, 15, 123-132. [CrossRef]
- Drew, D.A.; Weiner, D.E.; Tighiouart, H.; Duncan, S.; Gupta, A.; Scott, T.; Sarnak, M.J. Cognitive Decline and Its Risk Factors in Prevalent Hemodialysis Patients. Am J Kidney Dis 2017, 69, 780-787. [CrossRef]
- Van Campenhout, A.; Golledge, J. Osteoprotegerin, vascular calcification and atherosclerosis. Atherosclerosis 2009, 204, 321-329. [CrossRef]
- Fan, S.S.; Lin, L.F.; Chen, V.C.; Hsieh, C.W.; Hsiao, H.P.; McIntyre, R.S.; Iacobucci, M.; Coles, A.S.; Tsai, D.J.; Weng, J.C.; et al. Effects of Lower Past-Year Serum Sodium and Hyponatremia on Depression Symptoms and Cognitive Impairments in Patients With Hemodialysis. Ther Apher Dial 2020, 24, 169-177. [CrossRef]
- Olczyk, P.; Kusztal, M.; Golebiowski, T.; Letachowicz, K.; Krajewska, M. Cognitive Impairment in End Stage Renal Disease Patients Undergoing Hemodialysis: Markers and Risk Factors. Int J Environ Res Public Health 2022, 19. [CrossRef]
- Hsieh, H.L.; Yang, C.M. Role of redox signaling in neuroinflammation and neurodegenerative diseases. Biomed Res Int 2013, 2013, 484613. [CrossRef]
- Kurella Tamura, M.; Wadley, V.; Yaffe, K.; McClure, L.A.; Howard, G.; Go, R.; Allman, R.M.; Warnock, D.G.; McClellan, W. Kidney function and cognitive impairment in US adults: the Reasons for Geographic and Racial Differences in Stroke (REGARDS) Study. Am J Kidney Dis 2008, 52, 227-234. [CrossRef]
- Murray, A.M.; Tupper, D.E.; Knopman, D.S.; Gilbertson, D.T.; Pederson, S.L.; Li, S.; Smith, G.E.; Hochhalter, A.K.; Collins, A.J.; Kane, R.L. Cognitive impairment in hemodialysis patients is common. Neurology 2006, 67, 216-223. [CrossRef]
- Cervellati, C.; Romani, A.; Seripa, D.; Cremonini, E.; Bosi, C.; Magon, S.; Passaro, A.; Bergamini, C.M.; Pilotto, A.; Zuliani, G. Oxidative balance, homocysteine, and uric acid levels in older patients with Late Onset Alzheimer's Disease or Vascular Dementia. J Neurol Sci 2014, 337, 156-161. [CrossRef]
- MacEwen, C.; Sutherland, S.; Daly, J.; Pugh, C.; Tarassenko, L. Relationship between Hypotension and Cerebral Ischemia during Hemodialysis. J Am Soc Nephrol 2017, 28, 2511-2520. [CrossRef]
- Casserly, I.; Topol, E.J. Convergence of atherosclerosis and Alzheimer's disease: inflammation, cholesterol, and misfolded proteins. The Lancet 2004, 363, 1139-1146. [CrossRef]
- Breteler, M.M.; van Amerongen, N.M.; van Swieten, J.C.; Claus, J.J.; Grobbee, D.E.; van Gijn, J.; Hofman, A.; van Harskamp, F. Cognitive correlates of ventricular enlargement and cerebral white matter lesions on magnetic resonance imaging. The Rotterdam Study. Stroke 1994, 25, 1109-1115. [CrossRef]
- Chai, C.; Wang, H.; Chu, Z.; Li, J.; Qian, T.; Mark Haacke, E.; Xia, S.; Shen, W. Reduced regional cerebral venous oxygen saturation is a risk factor for the cognitive impairment in hemodialysis patients: a quantitative susceptibility mapping study. Brain Imaging Behav 2020, 14, 1339-1349. [CrossRef]
- Arieff, A.I. Aluminum and the Pathogenesis of Dialysis Encephalopathy. American Journal of Kidney Diseases 1985, 6, 317-321. [CrossRef]
- Han, M.; Guedes, M.; Larkin, J.; Raimann, J.G.; Lesqueves Barra, A.B.; Canziani, M.E.F.; Cuvello Neto, A.L.; Poli-de-Figueiredo, C.E.; Kotanko, P.; Pecoits-Filho, R. Effect of Hemodiafiltration on Self-Reported Sleep Duration: Results from a Randomized Controlled Trial. Blood purification 2020, 49, 168-177. [CrossRef]
- Kim, H.S.; Lee, S.; Kim, J.H. Real-world Evidence versus Randomized Controlled Trial: Clinical Research Based on Electronic Medical Records. J Korean Med Sci 2018, 33, e213. [CrossRef]
- Skarbinski, J.; Fischer, H.; Hong, V.; Liu, L.; Yau, V.M.; Incerti, D.; Qian, L.; Ackerson, B.K.; Amsden, L.B.; Shaw, S.F.; et al. Real-World Evidence to Supplement Randomized Clinical Trials: Tocilizumab for Severe COVID-19 Pneumonia vs. a Cohort Receiving Standard of Care. Clinical pharmacology and therapeutics 2023, 114, 1073-1081. [CrossRef]
- Sheldrick, R.C. Randomized Trials vs Real-world Evidence: How Can Both Inform Decision-making? Jama 2023, 329, 1352-1353. [CrossRef]
- Franklin, J.M.; Glynn, R.J.; Suissa, S.; Schneeweiss, S. Emulation Differences vs. Biases When Calibrating Real-World Evidence Findings Against Randomized Controlled Trials. Clinical pharmacology and therapeutics 2020, 107, 735-737. [CrossRef]
- Deleuran, M.; Vestergaard, C. Real-world evidence vs. randomized control trials. Br J Dermatol 2020, 182, 275-276. [CrossRef]
- Craig, J.C.; Irwig, L.M.; Stockler, M.R. Evidence-based medicine: useful tools for decision making. Med J Aust 2001, 174, 248-253. [CrossRef]
- Strippoli, G.F.; Craig, J.C.; Schena, F.P. The number, quality, and coverage of randomized controlled trials in nephrology. J Am Soc Nephrol 2004, 15, 411-419. [CrossRef]
- Grooteman, M.P.; van den Dorpel, M.A.; Bots, M.L.; Penne, E.L.; van der Weerd, N.C.; Mazairac, A.H.; den Hoedt, C.H.; van der Tweel, I.; Lévesque, R.; Nubé, M.J.; et al. Effect of online hemodiafiltration on all-cause mortality and cardiovascular outcomes. J Am Soc Nephrol 2012, 23, 1087-1096. [CrossRef]
- Ok, E.; Asci, G.; Toz, H.; Ok, E.S.; Kircelli, F.; Yilmaz, M.; Hur, E.; Demirci, M.S.; Demirci, C.; Duman, S.; et al. Mortality and cardiovascular events in online haemodiafiltration (OL-HDF) compared with high-flux dialysis: results from the Turkish OL-HDF Study. Nephrol Dial Transplant 2013, 28, 192-202. [CrossRef]
- Caskey, F.J.; Procter, S.; MacNeill, S.J.; Wade, J.; Taylor, J.; Rooshenas, L.; Liu, Y.; Annaw, A.; Alloway, K.; Davenport, A.; et al. The high-volume haemodiafiltration vs high-flux haemodialysis registry trial (H4RT): a multi-centre, unblinded, randomised, parallel-group, superiority study to compare the effectiveness and cost-effectiveness of high-volume haemodiafiltration and high-flux haemodialysis in people with kidney failure on maintenance dialysis using linkage to routine healthcare databases for outcomes. Trials 2022, 23, 532. [CrossRef]
- Susantitaphong, P.; Siribamrungwong, M.; Jaber, B.L. Convective therapies versus low-flux hemodialysis for chronic kidney failure: a meta-analysis of randomized controlled trials. Nephrol Dial Transplant 2013, 28, 2859-2874. [CrossRef]
- Neri, L.; Gurevich, K.; Zarya, Y.; Plavinskii, S.; Bellocchio, F.; Stuard, S.; Barbieri, C.; Canaud, B. Practice Patterns and Outcomes of Online Hemodiafiltration: A Real-World Evidence Study in a Russian Dialysis Network. Blood purification 2021, 50, 309-318. [CrossRef]
- Imamović, G.; Hrvačević, R.; Kapun, S.; Marcelli, D.; Bayh, I.; Grassmann, A.; Scatizzi, L.; Maslovarić, J.; Canaud, B. Survival of incident patients on high-volume online hemodiafiltration compared to low-volume online hemodiafiltration and high-flux hemodialysis. International urology and nephrology 2014, 46, 1191-1200. [CrossRef]
- Canaud, B.; Barbieri, C.; Marcelli, D.; Bellocchio, F.; Bowry, S.; Mari, F.; Amato, C.; Gatti, E. Optimal convection volume for improving patient outcomes in an international incident dialysis cohort treated with online hemodiafiltration. Kidney Int 2015, 88, 1108-1116. [CrossRef]
- Canaud, B.; Bayh, I.; Marcelli, D.; Ponce, P.; Merello, J.I.; Gurevich, K.; Ladanyi, E.; Ok, E.; Imamovic, G.; Grassmann, A.; et al. Improved survival of incident patients with high-volume haemodiafiltration: a propensity-matched cohort study with inverse probability of censoring weighting. Nephron 2015, 129, 179-188. [CrossRef]
- Maduell, F.; Varas, J.; Ramos, R.; Martin-Malo, A.; Pérez-Garcia, R.; Berdud, I.; Moreso, F.; Canaud, B.; Stuard, S.; Gauly, A.; et al. Hemodiafiltration Reduces All-Cause and Cardiovascular Mortality in Incident Hemodialysis Patients: A Propensity-Matched Cohort Study. Am J Nephrol 2017, 46, 288-297. [CrossRef]
- See, E.J.; Hedley, J.; Agar, J.W.M.; Hawley, C.M.; Johnson, D.W.; Kelly, P.J.; Lee, V.W.; Mac, K.; Polkinghorne, K.R.; Rabindranath, K.S.; et al. Patient survival on haemodiafiltration and haemodialysis: a cohort study using the Australia and New Zealand Dialysis and Transplant Registry. Nephrol Dial Transplant 2019, 34, 326-338. [CrossRef]
- Kikuchi, K.; Hamano, T.; Wada, A.; Nakai, S.; Masakane, I. Predilution online hemodiafiltration is associated with improved survival compared with hemodialysis. Kidney Int 2019, 95, 929-938. [CrossRef]
- Mercadal, L.; Franck, J.E.; Metzger, M.; Urena Torres, P.; de Cornelissen, F.; Edet, S.; Bechade, C.; Vigneau, C.; Drueke, T.; Jacquelinet, C.; et al. Hemodiafiltration Versus Hemodialysis and Survival in Patients With ESRD: The French Renal Epidemiology and Information Network (REIN) Registry. Am J Kidney Dis 2016, 68, 247-255. [CrossRef]
- Imamovic, G.; Hrvacevic, R.; Kapun, S.; Marcelli, D.; Bayh, I.; Grassmann, A.; Scatizzi, L.; Maslovaric, J.; Canaud, B. Survival of incident patients on high-volume online hemodiafiltration compared to low-volume online hemodiafiltration and high-flux hemodialysis. International urology and nephrology 2014, 46, 1191-1200. [CrossRef]
- Jirka, T.; Cesare, S.; Di Benedetto, A.; Perera Chang, M.; Ponce, P.; Richards, N.; Tetta, C.; Vaslaky, L. Mortality risk for patients receiving hemodiafiltration versus hemodialysis. Kidney Int 2006, 70, 1524; author reply 1524-1525. [CrossRef]
- Mercadal, L.; Franck, J.E.; Metzger, M.; Urena Torres, P.; de Cornelissen, F.; Edet, S.; Béchade, C.; Vigneau, C.; Drüeke, T.; Jacquelinet, C.; et al. Hemodiafiltration Versus Hemodialysis and Survival in Patients With ESRD: The French Renal Epidemiology and Information Network (REIN) Registry. Am J Kidney Dis 2016, 68, 247-255. [CrossRef]
- Valderrama, L.A.; Barrera, L.; Cantor, E.J.; Muñoz, J.; Arango, J.; Tobon, C.; Canaud, B. Mortality in High-Flux Hemodialysis vs. High-Volume Hemodiafiltration in Colombian Clinical Practice: A Propensity Score Matching Study. Kidney and Dialysis 2022, 2, 209-220.
- da Rocha, E.P.; Kojima, C.A.; Modelli de Andrade, L.G.; Costa, D.M.; Magalhaes, A.O.; Rocha, W.F.; de Vasconcelos Junior, L.N.; Rosa, M.G.; Wagner Martins, C.S. Comparing Survival Outcomes between Hemodialysis and Hemodiafiltration Using Real-World Data from Brazil. Journal of Clinical Medicine 2024, 13, 594.
- Zhang, Y.; Winter, A.; Ferreras, B.A.; Carioni, P.; Arkossy, O.; Anger, M.; Kossmann, R.; Usvyat, L.A.; Stuard, S.; Maddux, F.W. Real-world effectiveness of hemodialysis modalities: a retrospective cohort study. BMC Nephrology 2025, 26, 1-9. [CrossRef]
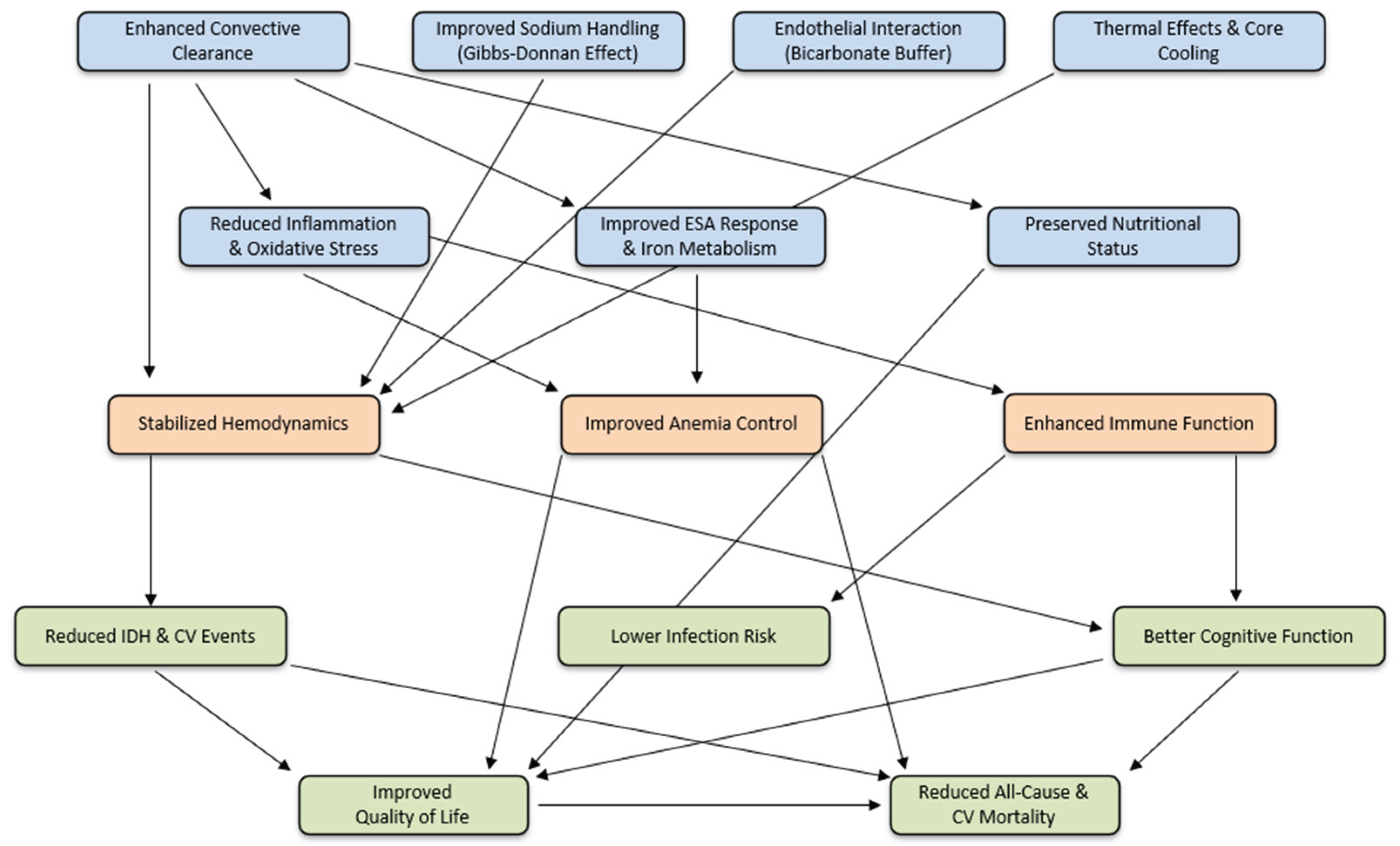
| Short-term Outcomes | Middle-Term Outcomes | Long-Term Outcomes |
|---|---|---|
| Enhanced toxin clearance | Reduced amyloidosis | Reduced all-cause mortality |
| Improved hemodynamic stability | Reduced joint pain | Reduced CV* mortality |
| Reduced inflammation | Improved nutritional status | |
| Reduced oxidative stress | Reduced infection risk | |
| Better anemia management | Cardiovascular benefits | |
| Residual kidney function protection | Peripheral Neuropathy Improvements: | |
| Reduced intradialytic cramps | Slow progression cognitive impairment | |
| Reduced skin hyperpigmentation | Improvement of QoL* |
| MMWs and PBTUs | MW (Da) | Clinical Relevance |
|---|---|---|
| Insulin [32] | 5,800 | Glucose metabolism |
| PTH Fragments [29,33] | 9,000 | CKD-MBD |
| AGE Products [23,34] | >10,000 | Oxidative vascular damage |
| Complement C3a/C5a [35] | 11,500 | Inflammation, immune response |
| Beta 2-Microglobulin [36,37,38,39,40,41,42,43] | 11,800 | Amyloidosis, inflammation |
| Leptin [44,45] | 16,000 | Appetite regulation |
| Tumor Necrosis Factor-alpha [46] | 17,000 | Systemic inflammation |
| Myoglobin [47,48] | 17,000 | Rhabdomyolysis marker |
| Interleukin-1 [49] | 17,000 | Inflammation, immune signaling |
| Retinol-Binding Protein [50] | 21,000 | Insulin resistance |
| Free Light Chains K/L [47,51,52] | 22,000 | Inflammation, dyscrasias |
| Beta-trace Protein [47] | 23,000 | GFR biomarker |
| Complement Factor D (Adipsin) [53] | 24,000 | Complement activation |
| Hepcidin [54,55] | 25,000 | Iron regulation |
| Alpha-1 Microglobulin [56] | 26,000 | Tubular injury, oxidative stress |
| Interleukin-6 [57] | 26,000 | Inflammation, CV risk |
| Fibroblast Growth Factor 23 [58,59] | 32,000 | CKD-MBD, vascular calcification |
| Alpha-1-Acid Glycoprotein [47] | 43,000 | Acute phase protein |
| Protein-bound p-Cresyl Sulfate [60] | 188 | Inflammation, atherosclerosis |
| Protein-bound Indoxyl Sulfate [60] | 213 | Vascular calcification, oxidative stress |
| Study | Country | Sample Size (HD/HDF) | Sub/Conv Vol (L/session) | Primary Outcome | Key Findings |
|---|---|---|---|---|---|
| ICS [65] | Italy | 70/40 | Sub 30-40 (pre) | ISH | ISH ↓ 50.9% with HDF |
| CONTRAST [205] | NL-CA | 356/358 | Sub 19.8 | All-cause mortality | No difference overall, but benefit with high-volume HDF |
| Turkish [206] | Turkey | 391/391 | 17.2/19.5 | All-cause mortality + CV event | No difference overall, better survival in high-efficiency HDF |
| ESHOL [31] | Spain | 450/456 | 21.8/23.9 | All-cause mortality | 30% lower all-cause mortality in HDF |
| FRENCHIE [68] | France | 191/190 | 20/21 | Intradialytic tolerance | Better tolerance; no difference in mortality |
| CONVINCE [2] | M | 677/683 | 23.0/25.5 | All-cause mortality | HDF ↓ all-cause mortality by 23% (HR 0.77) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





