1. Introduction
Floating aortic thrombi (FAT) represent a rare but serious medical condition [
1], especially on the ascending aorta and/or the aortic arch level, carrying a high risk of systemic embolization that can lead to severe complications such as stroke, mesenteric ischemia and other organs as well, and limb/arm ischemia[
2]. Despite their infrequency, the management of floating aortic thrombi poses a significant clinical challenge, with treatment options ranging from urgent surgical removal to conservative medical management with anticoagulation. The decision on whether to proceed with surgery or anticoagulation depends on several factors, including the thrombus's location, size, the presence of embolic events, patient comorbidities, and overall condition.
Floating aortic thrombi are thought to originate from a combination of endothelial injury, hypercoagulability, and abnormal flow dynamics. According to Virchow’s triad, any disruption in these components, as seen in atherosclerosis, malignancy or systemic inflammation, can predispose to thrombus formation even in high-flow vessels like the aorta. In fact, despite the high velocity and turbulent nature of aortic blood flow, thrombi can form and persist when local anatomical irregularities, like ulcers or plaques, provide anchoring points. Additionally, procoagulant states induced by neoplasms, autoimmune disorders or chronic infections may exacerbate thrombus development.
The rarity of FAT and the lack of prospective randomized trials have created uncertainty regarding its management. While some prefer early surgical removal, others suggest conservative treatment when the thrombus is stable and the operative risk is high. This controversy highlights the importance of individualized decision-making and the role of a multidisciplinary team.
In this review, we present two cases of patients with floating aortic thrombi. The first case involves a 59-year-old patient who underwent surgical resection of the thrombus due to the immediate threat of embolic events, including mesenteric ischemia and stroke. The second case describes an 88-year-old patient who was managed conservatively with anticoagulation therapy due to the stability of the thrombi and absence of acute ischemic symptoms.
2. PATIENT 1: Surgical Management of Floating Aortic Thrombus
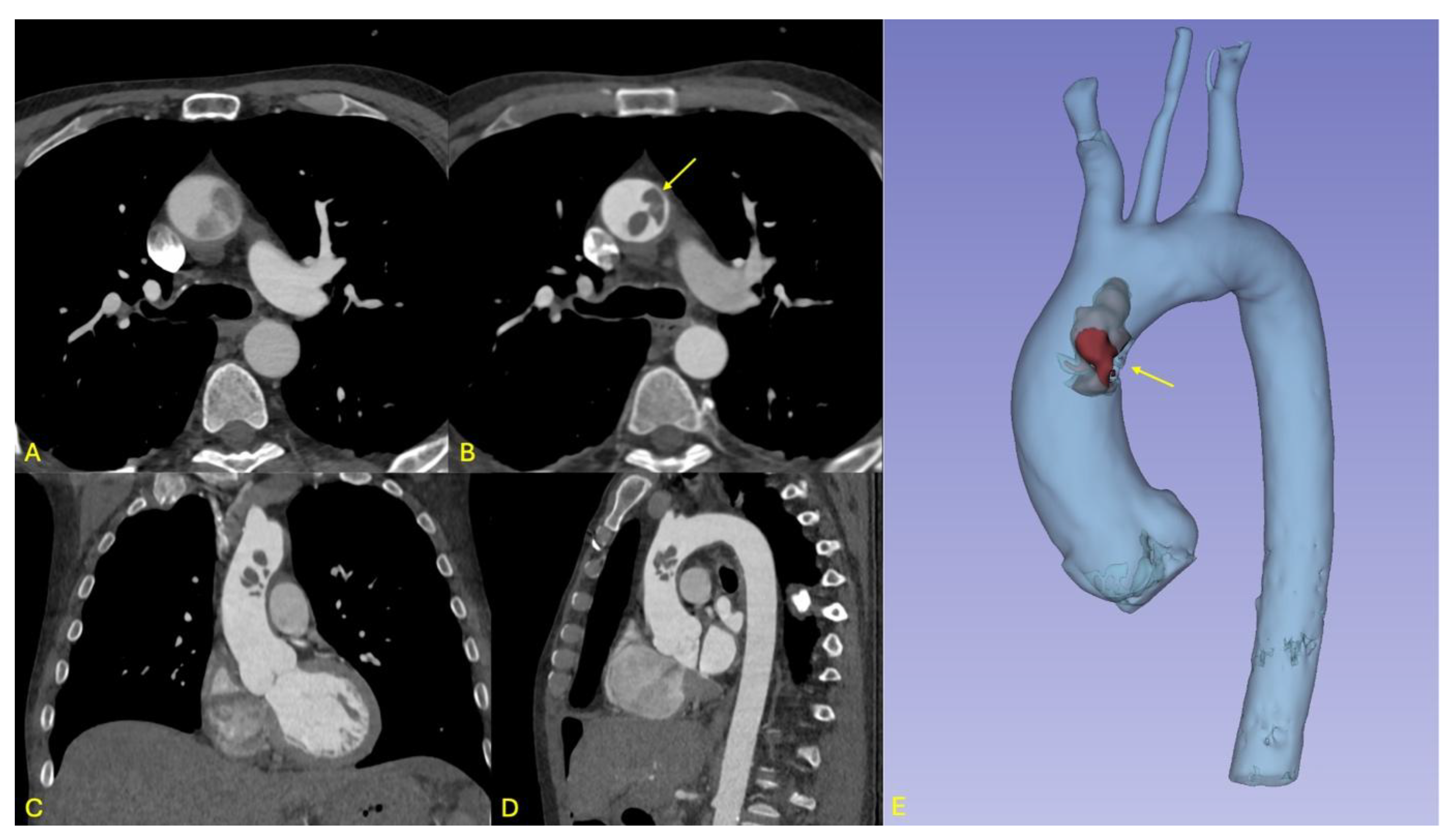
A 59-year-old male, known for active smoking, risky alcohol consumption, hypercholesterolemia, and hypertension, initially presented to the emergency department with abdominal pain persisting for two weeks and vomiting. Abdominal imaging revealed mesenteric ischemia and intestinal necrosis, necessitating an emergency laparotomy with resection of approximately two meters of ischemic jejunum. A few hours after the procedure, the patient experienced neurological symptoms, including speech difficulties and weakness in the right upper limb. A cranial CT revealed a subacute left Sylvian ischemic stroke. Full-body computed tomography (CT) further revealed a floating thrombus at the junction of the ascending aorta and aortic arch, representing a high-risk source of embolization to both cerebral and mesenteric vessels (see
Figure 1).
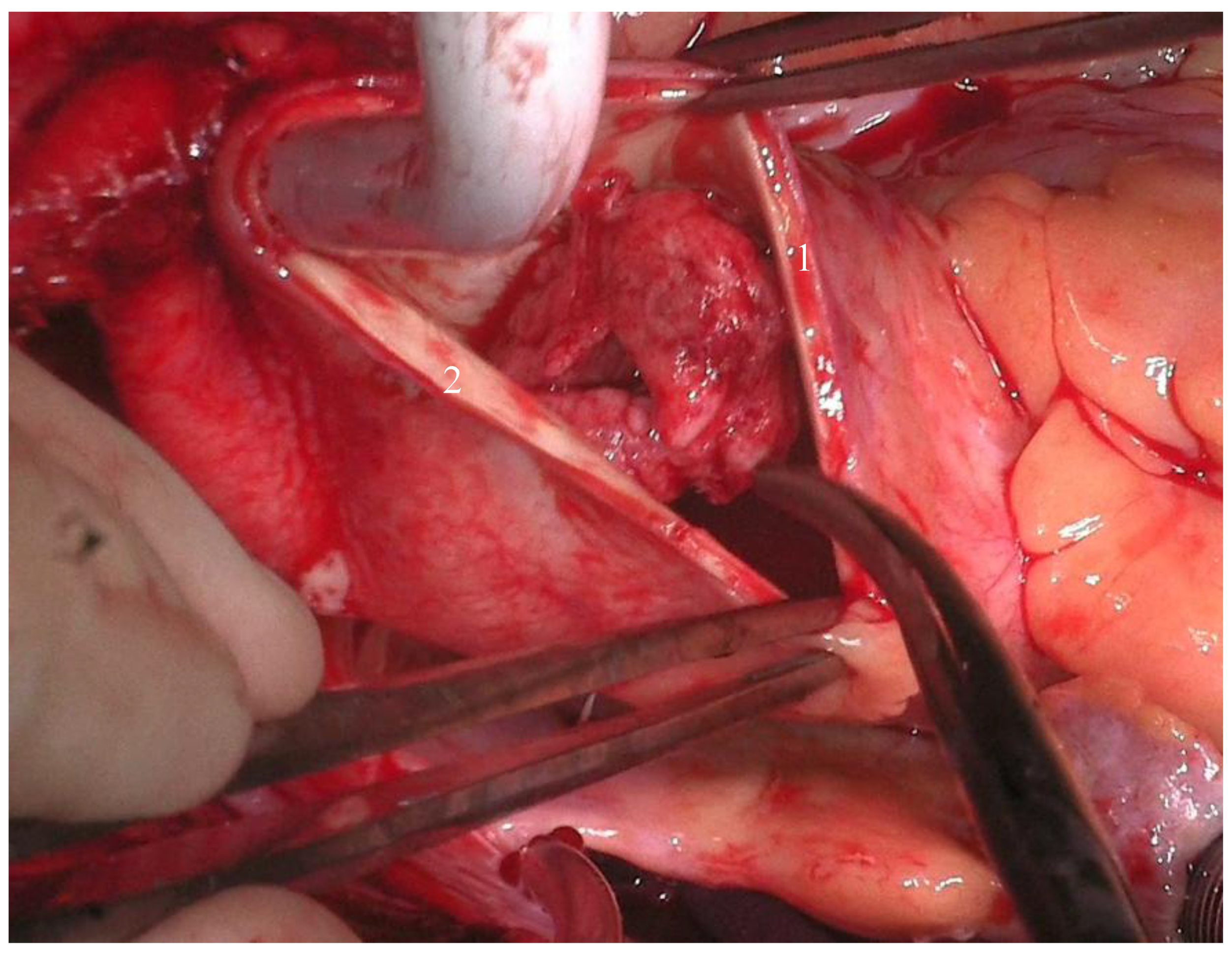
Given the thrombus's high embolic risk, a multidisciplinary discussion was held, and emergency surgery was deemed necessary. The patient underwent the surgery under general anesthesia in a supine position. After the usual disinfection and surgical draping, a complete surgical time-out was performed, and antibiotic prophylaxis was administered according to protocol. The procedure involved a median vertical sternotomy, followed by pericardiotomy and dissection of the ascending aorta, the brachiocephalic trunk with the right subclavian artery and right carotid artery, and dissection of the left carotid artery. General heparinization was carried out. Tangential clamping of the right subclavian artery was performed, followed by an anastomosis between the right subclavian artery and an 8 mm Dacron graft. An arterial cannula was placed in this graft, along with a double-staged venous cannula in the right atrium. Cardiopulmonary bypass was initiated, and cooling was carried out to 32°C. A left atrial suction cannula was inserted via the right superior pulmonary vein. The left carotid artery was closed, and clamping was applied to the brachiocephalic trunk. Selective cerebral circulation was maintained at 32°C. The ascending aorta was opened without applying an aortic cross clamp, to prevent the dissection and distal embolization of the voluminous floating thrombus. 100 ml of cardioplegic solution (Cardioplexol©) was injected into the left coronary ostium, followed by the right coronary ostium. The heart arrested in diastole. Two thrombi, each about 3x 4 cm size, were identified in distal part of the ascending aorta, with wall ulcerations. Further ulcerations were found at the beginning of the aortic arch (see
Figure 2). The walls were resected at this level, and a 28 mm Dacron graft was used for hemi-arch replacement, with an open anastomosis between the aortic arch and the Dacron graft. Clamps were placed on the prosthesis, the cerebral vessels were opened, and total perfusion was resumed. The patient was rewarmed to 36°C. The proximal anastomosis between the Dacron graft and the aortic root was done with a 5-0 Prolene suture. After de-airing the graft, the aorta was declamped, and the heart resumed a sinus rhythm at 36°C. CPB was weaned, and the patient was hemodynamically stable. A transesophageal echocardiogram showed good results. Decannulation, reversal of heparin with protamine, and hemostasis were achieved without complications. Two ventricular pacing wires were placed, and three Blake drains were positioned in the retrosternal, retro-cardiac, and right pleural spaces. The pericardium was closed with 2-0 Vicryl sutures, and the sternum was closed with 6-0 Ethibond sutures. Muscle and subcutaneous tissues were closed in layers, and sterile dressings were applied.
The patient’s postoperative course was uneventful, with rapid extubation and clinical improvement in the intensive care unit. Due to favorable hemodynamic progress, the patient was transferred to the neurology department for further management. Further investigations were conducted to explain the etiology of the floating aortic thrombus and raised the suspicion for hepatocellular carcinoma. As the patient was originally from Ireland and was only traveling in Switzerland, he wanted to return to his home country for the rest of his care. Once his condition was stable, he was safely transferred back to Ireland for further investigations.
This case underlines the diagnostic difficulty in identifying FAT in the context of non-specific gastrointestinal and neurological symptoms. The floating thrombus was discovered only after severe ischemic complications occurred, showing the potential benefit of earlier vascular imaging in patients with atypical or recurrent embolic events. Moreover, the short time frame between the abdominal and cerebral ischemia suggests a high embolic burden.
The surgical team faced a complex intraoperative challenge due to the volume and fragility of the thrombus. The choice to avoid cross-clamping before aortic opening was a critical safety measure to prevent distal embolization during manipulation. This technique ensured neuroprotection while allowing full thrombus resection. Such nuanced surgical strategies illustrate the necessity of advanced intraoperative planning and multidisciplinary discussion for management of FAT.
Postoperative suspicion of hepatocellular carcinoma raises the hypothesis of a malignancy-induced hypercoagulable state, which may have played a role in the thrombus formation. Although the diagnosis could not be confirmed due to the patient’s transfer abroad, this highlights the importance of investigating paraneoplastic causes in unexplained thrombotic events, especially in patients with no prior cardiovascular disease.
This case illustrates the importance of timely surgical intervention for patients with floating aortic thrombi, especially those at high risk for embolic complications and those who already have evidence of embolization. The surgical resection of the thrombus prevented further embolization, and the patient recovered well postoperatively.
Malignancies are known to increase the risk of thromboembolic events[
3], including the formation of FAT, primarily due to a hypercoagulable state. This hypercoagulability is often a result of tumor cells releasing procoagulant substances, which activate the coagulation cascade. Additionally, malignancies can induce the production of inflammatory cytokines[
4], leading to endothelial damage and further promoting thrombogenesis. These mechanisms contribute to the increased incidence of thromboembolic events in cancer patients, including the development of FAT.
Furthermore, the Trousseau sign of malignancy, also known as Trousseau's syndrome, describes the association between malignancy and migratory thrombophlebitis. This condition is characterized by recurrent, migratory thrombosis in superficial veins and is particularly associated with pancreatic, gastric, and lung cancers. The syndrome exemplifies how malignancies can create a systemic hypercoagulable state, leading to various thromboembolic complications, including FAT. This highlights the critical need for prompt diagnosis and management of thromboembolic events in cancer patients, as well as the importance of considering an underlying malignancy in patients presenting with unexplained thrombotic phenomena.
3. PATIENT 2: Conservative Management of Floating Aortic Thrombus
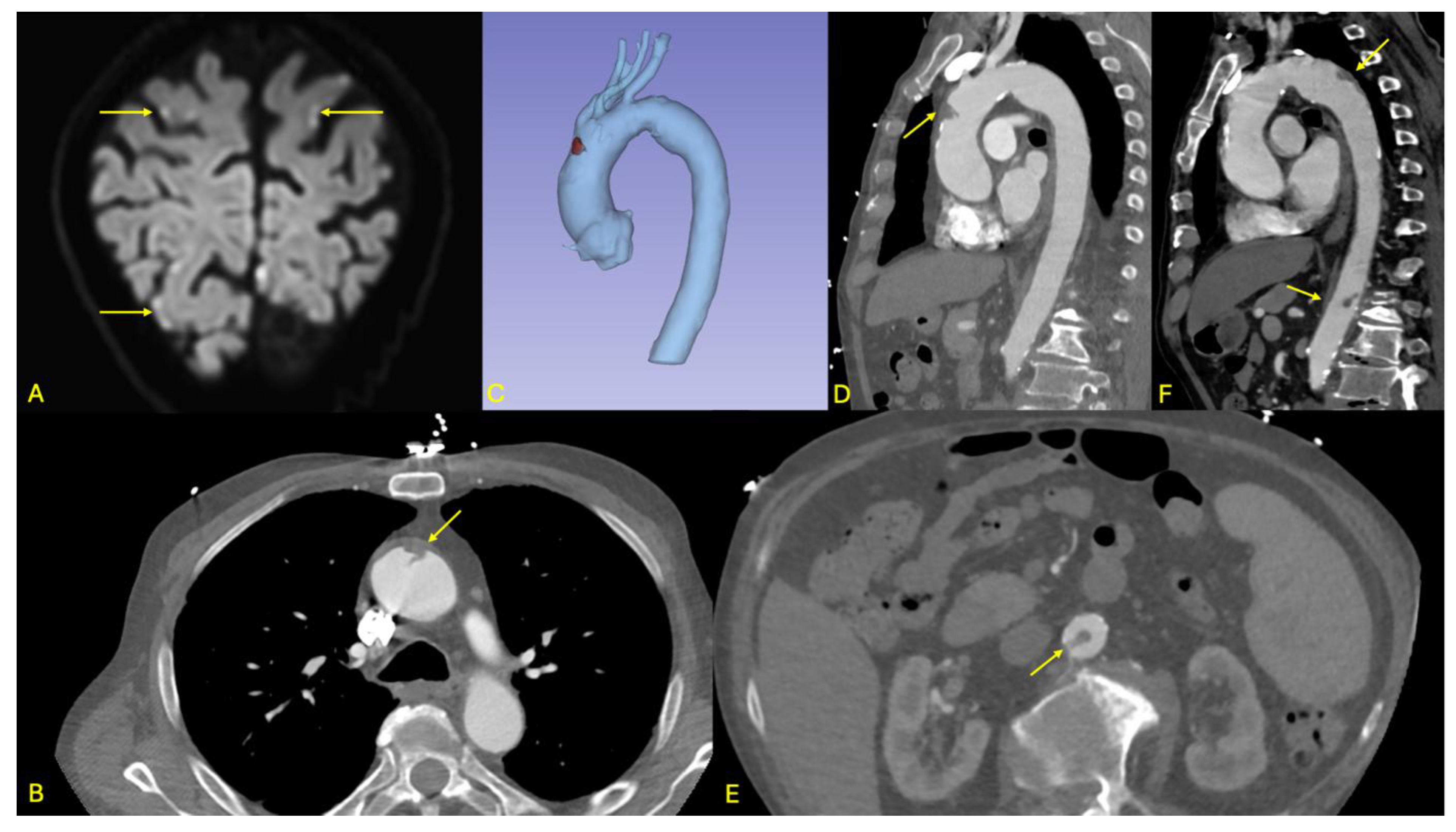
An 88-year-old male presented to the emergency department with bilateral paresthesia in both hands, raising suspicion of a stroke. The patient had a history of a right temporal ischemic stroke in 2021. On clinical examination, a right homonymous lateral hemiparesis with an NIHSS score of 2 was noted. Cerebral MRI showed acute supra- and infratentorial ischemic lesions, some with a hemorrhagic component, affecting multiple vascular territories, suggesting an embolic origin without a clear timing (see
Figure 3).
On the initial CT, a floating thrombus was observed in the aortic arch but required further assessment. This led to an emergency aortic angio-CT, which revealed stability of the floating thrombus in the distal ascending aorta (see
Figure 3). Additionally, the scan showed a small, ulcerated plaque in the abdominal aorta, just above the origin of the celiac trunk, as well as a second floating thrombus in the sub renal abdominal aorta. No ischemic damage to solid intra-abdominal organs was found, and the splanchnic arterial axes remained patent.
A multidisciplinary team reviewed the case, and it was decided that surgical intervention was not immediately necessary due to the stability of the thrombi, the absence of severe symptoms, the fragility of the patient and his age. The patient was started on low-molecular-weight heparin (LMWH), later transitioning to long-term anticoagulation therapy with vitamin K antagonists (VKA). The patient was monitored closely with regular INR checks to optimize anticoagulation levels.
The patient was followed up with regular clinical assessments and imaging. A repeat angio-CT at three months showed that the thrombi in both the aortic arch and the abdominal aorta remained stable, with no significant change in size or structure. Additionally, there was no evidence of embolization to other vascular territories, nor were new ischemic lesions identified. The multidisciplinary team concluded that continuing anticoagulation therapy was appropriate, and no surgical intervention was required at this stage.
In this case, the presence of bilateral ischemic cerebral lesions with hemorrhagic components complicated the therapeutic balance, as it limited the possibility of full-dose anticoagulation in the acute phase. The decision to initiate LMWH before switching to VKA was guided by a desire to optimize thrombus control while minimizing hemorrhagic risk. This reflects the complexity of managing embolic strokes in elderly, frail patients with multiples vascular risk factors.
The identification of two separate thrombi – one in the ascending aorta and the other one in the subrenal abdominal aorta – raises the question of systemic predisposition rather than localized pathology. While no definitive prothrombotic condition was identified during hospitalization, a full thrombophilia workup was deferred due to the patient’s age and stability. Finally, the positive clinical evolution without embolic recurrence after three months supports the viability of conservative management in selected patients. However, it also underscores the need for close radiologic follow-up and rigorous INR control to minimize risks. This shows that while anticoagulation may be sufficient, its success relies heavily on multidisciplinary coordination, patient adherence, and vigilant surveillance.
This case demonstrates the successful conservative management of a patient with a floating aortic thrombus using anticoagulation therapy. The close follow-up and absence of thrombus progression highlighted the importance of individualized treatment, particularly in elderly patients with stable thrombi and without acute ischemic symptoms.
4. Discussion
To conclude, floating aortic thrombus (FAT) is a rare condition, with an incidence of approximately 0.45% in the population. It is characterized by a thrombus that is not fully attached to the aortic wall, allowing it to move freely within the lumen. The descending aorta is the most affected site, followed by the aortic arch, while involvement of the ascending aorta is less frequent[
5], most probably related to the velocity of the blood flow.
The etiology of FAT is multifactorial. Common predisposing factors include atherosclerosis, hypercoagulable states, malignancies, as seen in our first patient, chronic inflammatory conditions, trauma, and prior surgical interventions. However, FAT can also occur in individuals without evident risk factors, making its pathogenesis in such cases less clear[
6].
Due to its potential to cause severe systemic embolization, including stroke and organ ischemia, FAT requires a prompt diagnosis and management. Treatment strategies vary based on the thrombus's characteristics and patient-specific factors, ranging from anticoagulation therapy to surgical intervention.
In our narrative review, the first patient, a 59-year-old male, presented with abdominal pain and vomiting, later developing neurological symptoms after emergency surgery for mesenteric ischemia. Imaging revealed a left Sylvian ischemic stroke and a large floating thrombus in the ascending aorta.
The second patient, an 88-year-old male with a history of stroke, presented with bilateral hand paresthesias. Imaging showed supra- and infratentorial ischemic lesions and stable floating thrombi in the ascending aorta and subrenal descending aorta, with no acute organ damage.
We presented two distinct approaches to manage floating aortic thrombi. One patient underwent urgent surgery due to the high risk of embolization, while the other was managed conservatively with anticoagulation because of the thrombus’s stability and the patient’s advanced age. Floating aortic thrombi pose a significant risk of systemic embolization, particularly to the brain, viscera, and limbs, making timely and individualized treatment critical. A review of the literature reveals that both surgical and conservative strategies can be effective, depending on the clinical context.
Most of the studies, such as those by Yang et al.[
7] and Choi et al.[
8], emphasize the critical role of surgery when the thrombus is large, mobile, or has already caused ischemic complications. In Yang et al.[
6] case, a large thrombus in the aortic arch was surgically removed due to its high embolic risk, mirroring our first patient's need for surgical intervention after developing mesenteric ischemia and stroke. Similarly, Choi et al.[
8] describe a patient with a floating thrombus extending into the left subclavian artery, which was also surgically removed due to the high risk of repeated embolization. Both studies support the use of surgery in cases where there is an imminent threat of further embolic events.
Noh et al.[
9] and Weiss et al.[
10] similarly reported cases where surgical removal of the thrombus was the preferred approach, especially when the thrombus was highly mobile and posed a risk for systemic embolism. Noh et al.[
9] patient had a floating thrombus in the aortic arch, leading to peripheral embolization, and surgery was deemed necessary to prevent further complications, just like in our first patient. Weiss et al.[
10], in a series of 10 patients, also demonstrated that surgical thrombectomy was an effective and safe treatment, with no recurrence of thrombus or embolic events post-surgery. These cases reflect our decision to proceed with surgery in the first patient, highlighting the importance of timely intervention in high-risk cases.
However, some studies advocate for conservative management in certain patient populations. Christou et al.[
11] presented a case where conservative anticoagulation was used successfully in a patient with a large floating thrombus in the aortic arch, opting against surgery due to the patient’s high perioperative risk. This aligns with our management of the second patient, where anticoagulation was chosen due to the stability of the thrombi and the patient’s advanced age. Parato et al.[
12] also reported successful long-term outcomes using anticoagulation in patients with floating thrombi, where surgery was avoided due to high operative risk or patient frailty. These cases demonstrate that, in select patients, conservative management can be a safe and effective alternative to surgery.
Moreover, Li et al.[
13] and Majdi et al[
14]. highlighted the importance of individualized treatment, emphasizing that while surgery is often necessary for large or symptomatic thrombi, anticoagulation may be sufficient in stable patients. In Li et al.'s case, a large thrombus extending from the ascending aorta to the aortic arch required surgical resection due to the high embolic risk. Similarly, Majdi et al. described two cases of floating aortic thrombi where surgery successfully prevented further embolization. These findings further prove that surgery remains the gold standard in cases where thrombi are large, mobile, or symptomatic.
Furthermore, risk stratification remains a major challenge in the management of FAT. Some authors have proposed preliminary decision-making criteria, like thrombus mobility, location, size and clinical presentation, to guide treatment choice. However, no widely accepted scoring system or standardized guideline currently exists. For example, thrombi located in the ascending aorta or arch are generally associated with a higher embolic risk compared to those in the descending aorta, which may justify more aggressive interventions. Likewise, the presence of symptomatic embolic events often shifts the balance toward surgery.
Long-term prognosis is also difficult to predict. In cases managed conservatively, recurrence rates remain uncertain, and the duration of anticoagulation is not standardized. Some suggest lifelong anticoagulation, especially in patients with persistent risk factors. Quality of life and functional status post-treatment have not been systematically studied, underlining the need for follow-up research focused of patient-centered outcomes.
Another important gap in current knowledge concerns the natural history of FAT. Since most diagnoses are incidental or made after embolic complications, the timeline from thrombus formation to clinical manifestation is unclear. This limits our ability to implement preventive strategies or early detection protocols. Future prospective registries may help define the incidence, evolution, and risk factors more precisely.
There is also an endovascular approach: in fact, the use of covered stents has emerged as an effective treatment option for floating thrombi in the descending aorta, particularly in cases where there is a high risk of embolization. Fueglistaler et al.[
15] reported the successful implantation of an endovascular stent graft for a symptomatic mobile thrombus in the thoracic aorta, highlighting its minimally invasive nature and effectiveness in preventing embolic complications. Similarly, Nguyen et al.[
16] reviewed management strategies for descending thoracic aortic thrombi and emphasized that endovascular stent grafts are a safe and feasible alternative to open surgery, particularly in patients for whom conservative management has failed or is unsuitable. Additionally, Rivera et al.[
17] demonstrated the high primary success rate and safety of endovascular techniques for treating floating thrombi in both the thoracic and abdominal aorta, further supporting their role as a first-line option in some cases. These studies collectively underscore the advantages of endovascular stent grafting as a viable and minimally invasive approach for managing floating aortic thrombi.
Overall, the literature reveals that the management of floating aortic thrombi must be tailored to each patient. Surgical intervention is generally favored when there is a high risk of embolization, especially in cases involving large, mobile thrombi[
18] or when embolic events have already occurred, as it was the case with our first patient. On the other hand, conservative management with anticoagulation can be a viable alternative for patients with stable thrombi, high surgical risk, or advanced age, as demonstrated in our second patient[
19]. These findings reflect the need for a multidisciplinary approach to ensure optimal outcomes in this challenging clinical condition[
20].
5. Conclusions
Floating aortic thrombi are rare but present a significant risk of systemic embolization, necessitating prompt and individualized management. The choice between surgical removal and anticoagulation must be tailored to each patient’s thrombus characteristics, comorbidities, and embolic risk. Our review underscores the importance of individualized care strategies.
However, the absence of standardized guidelines, validated risk scores, and long-term outcome data leaves many questions unanswered. Multicenter prospective studies and international registries are essential to establish evidence-based recommendations. Further prospective studies into novel imaging modalities, biomarkers of thrombus instability, and risk prediction tools will help refine our clinical approach. Until such data and registries are available, multidisciplinary discussion and shared decision-making remain the cornerstone of safe and effective FAT management.
Author Contributions
Conceptualization : CIKIRIKCIOGLU Mustafa, HUBER Christoph. Methodology : HUBER Christoph, DEMOULIN Estelle, CIKIRIKCIOGLU Mustafa. Investigation : DEMOULIN Estelle. Resources : WALDER Bernhard, SCHORER Raoul. Data curation : GLESSGEN Carl. Writing – original draft preparation : DEMOULIN Estelle. Writing – review and editing : CIKIRIKCIOGLU Mustafa, DEMOULIN Estelle, JOLOU Jalal. Supervision : CIKIRIKCIOGLU Mustafa, HUBER Christoph. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the review.
Data Availability Statement
Details were harvested from our local software with patient informations.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ghoweba M, Gnasigamany J, Chiluveri M, McClish J. A Ticking Time Bomb: A Case of Floating Distal Aortic Arch Intraluminal Thrombus. Cureus 2022, 14, e32212. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lupinetti FM, Warner J, Jones TK, Herndon SP. Comparison of human tissues and mechanical prostheses for aortic valve replacement in children. Circulation. 1997, 96, 321–325. [Google Scholar] [CrossRef] [PubMed]
- Mobile Menace: Floating Aortic Arch Thrombus. Schattner, Ami et al. The American Journal of Medicine 2016, 129, e23–e24. [Google Scholar]
- Varki, A. Trousseau's syndrome: multiple definitions and multiple mechanisms. Blood. 2007, 110, 1723–1729. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Avelino MC, de Miranda CLVM, de Sousa CSM, Bastos BB, de Sousa RSM. Free-floating thrombus in the aortic arch. Radiol Bras. 2017, 50, 406–407. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Oki, N. , Inoue, Y. & Kotani, S. Free-floating thrombus of the aorta: 3 case reports. Surg Case Rep 2021, 7, 141. [Google Scholar] [CrossRef] [PubMed]
- Yang, P. , Li, Y., Huang, Y. et al. A giant floating thrombus in the ascending aorta: a case report. BMC Surg 2020, 20, 321. [Google Scholar] [CrossRef] [PubMed]
- Choi JB, Choi SH, Kim NH, Jeong JW. Floating thrombus in the proximal aortic arch. Tex Heart Inst J. 2004, 31, 432–434. [Google Scholar] [PubMed] [PubMed Central]
- Noh TO, Seo PW. Floating thrombus in aortic arch. Korean J Thorac Cardiovasc Surg. 2013, 46, 464–466. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Weiss S, Bühlmann R, von Allmen RS, Makaloski V, Carrel TP, Schmidli J, Wyss TR. Management of floating thrombus in the aortic arch. J Thorac Cardiovasc Surg. 2016, 152, 810–817. [Google Scholar] [CrossRef] [PubMed]
- Christou N, Gourgiotis I, Dakis K, Liasidis C. Embolic strokes in a patient with a large floating thrombus in the ascending aorta. Hippokratia. 2021, 25, 172–174. [Google Scholar] [PubMed] [PubMed Central]
- Parato VM, Prifti E, Pezzuoli F, Labanti B, Baboci A. Huge ascending aortic aneurysm with an intraluminal thrombus in an embolic event-free patient. Interv Med Appl Sci. 2015, 7, 30–32. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li G, Chen Y, Wang H, Liu Y, Liu H, Sun H, Wang Z. Case report: Surgical strategies of a giant thrombus from the ascending aorta to the arch. Front Cardiovasc Med. 2023, 10, 1091303. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Majdi Gueldich, Mariantonietta Piscitelli, Haytham Derbel, Khaoula Boughanmi, Eric Bergoend, Nora Chanai, Thierry Folliguet, Antonio Fiore, Floating thrombus in the ascending aorta revealed by peripheral arterial embolism. Interactive CardioVascular and Thoracic Surgery 2020, 30, 762–764. [CrossRef]
- Endovascular stent graft for symptomatic mobile thrombus of the thoracic aorta. Fueglistaler, Philipp et al. Journal of Vascular Surgery 2005, 42, 781–783. [Google Scholar]
- Nguyen Q, Ma X, Vervoort D, Luc JGY. Management Strategies for Descending Thoracic Aortic Thrombus: A Review of the Literature. Innovations 2022, 17, 283–296. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Floating Thoracic and Abdominal Aortic Thrombus: Endovascular Solutions to Unexpected Findings. A Minimal Invasive Treatment With a High Primary Success. Rivera, Elena García et al. European Journal of Vascular and Endovascular Surgery 2019, 58, e733–e734. [Google Scholar]
- Zivkovic I, Milacic P, Mihajlovic V, Krasic S, Lesanovic J, Peric M, Zdravkovic D. Surgical treatment of ascending aorta floating thrombus in a patient with recent SARS-CoV-2 infection. Cardiovasc Diagn Ther. 2021, 11, 467–471. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mouselimis, D, Giesen, A, Donas, K. et al. Successful Conservative Treatment of Mobile Aortic Thrombus Causing Acute Limb Ischemia. J Am Coll Cardiol Case Rep. 2023, 11, 101770. [Google Scholar] [CrossRef]
- Grassl K, Hangler H, Gratl A, et al. A Free-Floating Aortic Thrombus: An Uncommon Approach to Handle a Rare Clinical Entity. Journal of Endovascular Therapy 2024, 15266028241256817. [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).