Submitted:
02 June 2025
Posted:
02 June 2025
You are already at the latest version
Abstract
Keywords:
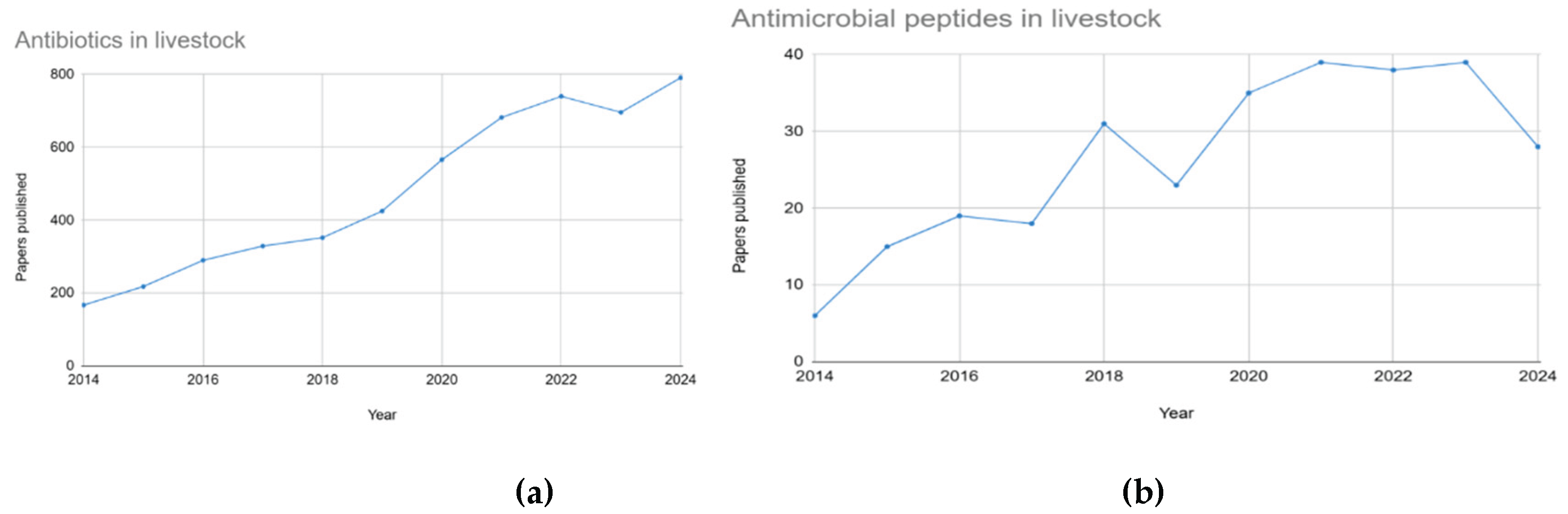
1. Introduction
2. AMPs against Bacteria, Fungi, and Parasites in Farm Animal Production
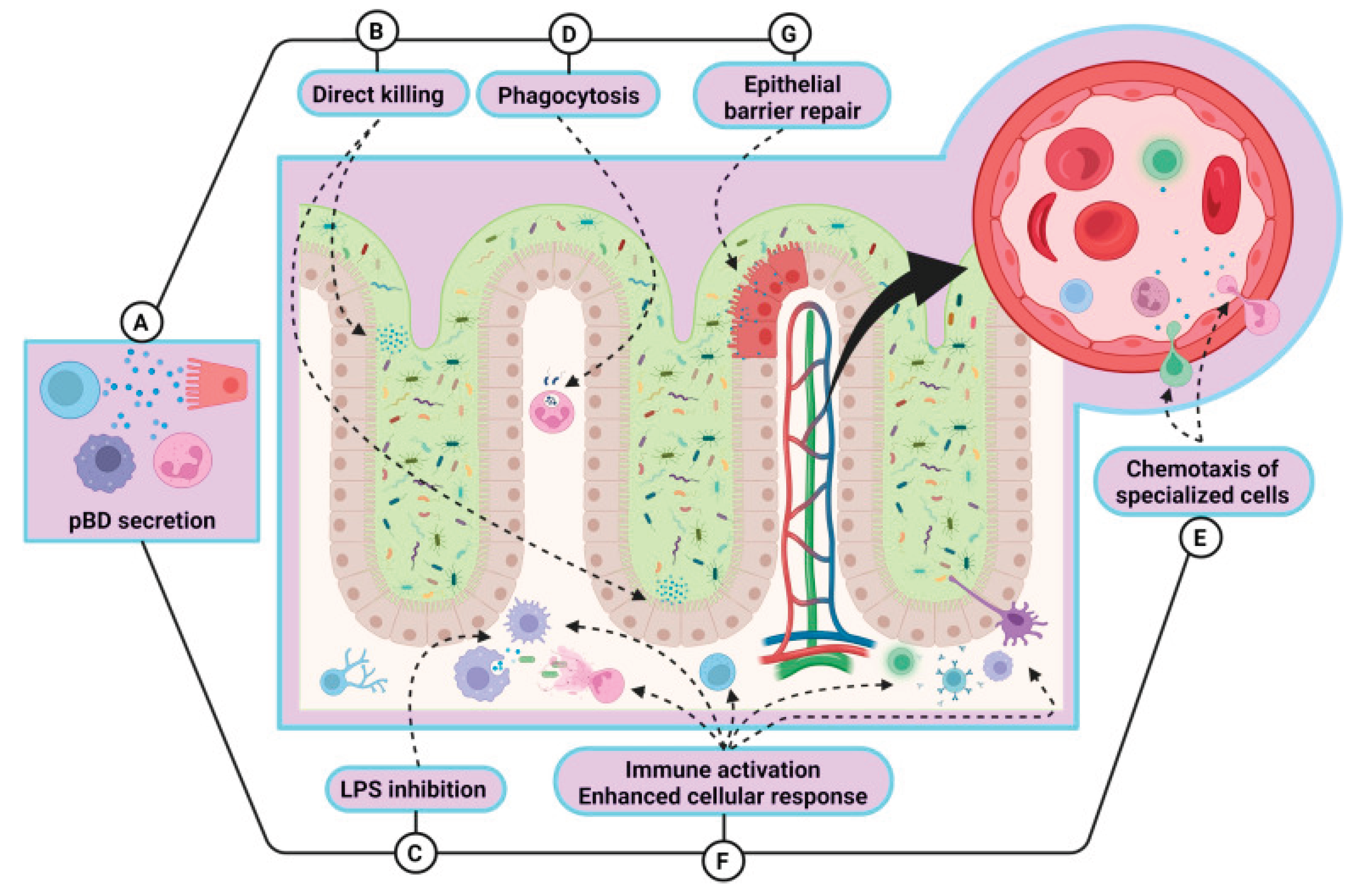
2.1. AMPs Against Bacterial Infections in Farm Animals
2.1.1. Application of AMPs in Poultry
2.1.2. Application of AMPs in Swine Production
2.1.3. Application of AMPs in Ruminants
2.2. AMPs against Fungal Infections in Farm Animals
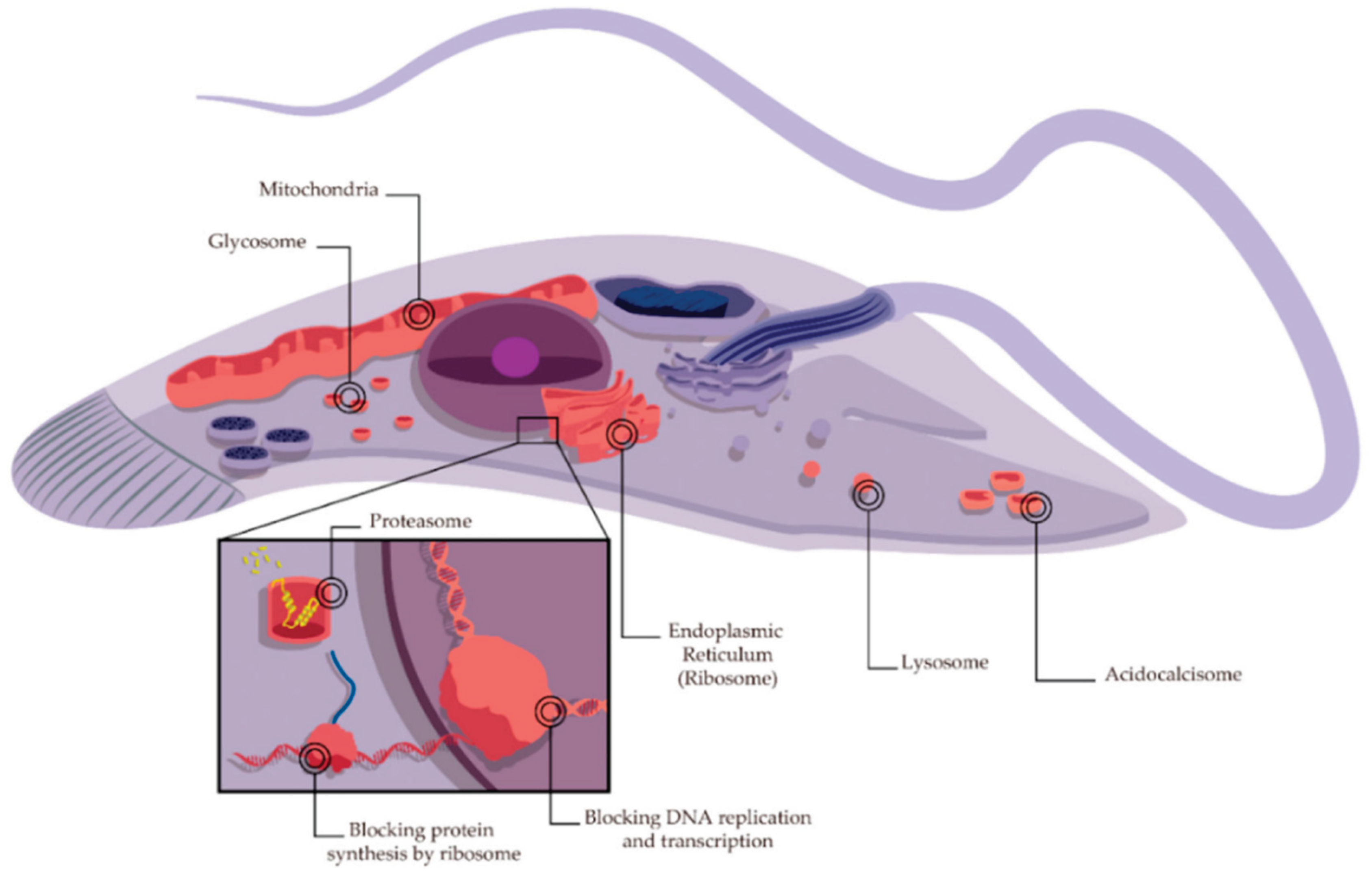
2.3. AMPs against Parasitic Infections in Farm Animals
2.4. Synthetic AMPs in Farm Animals
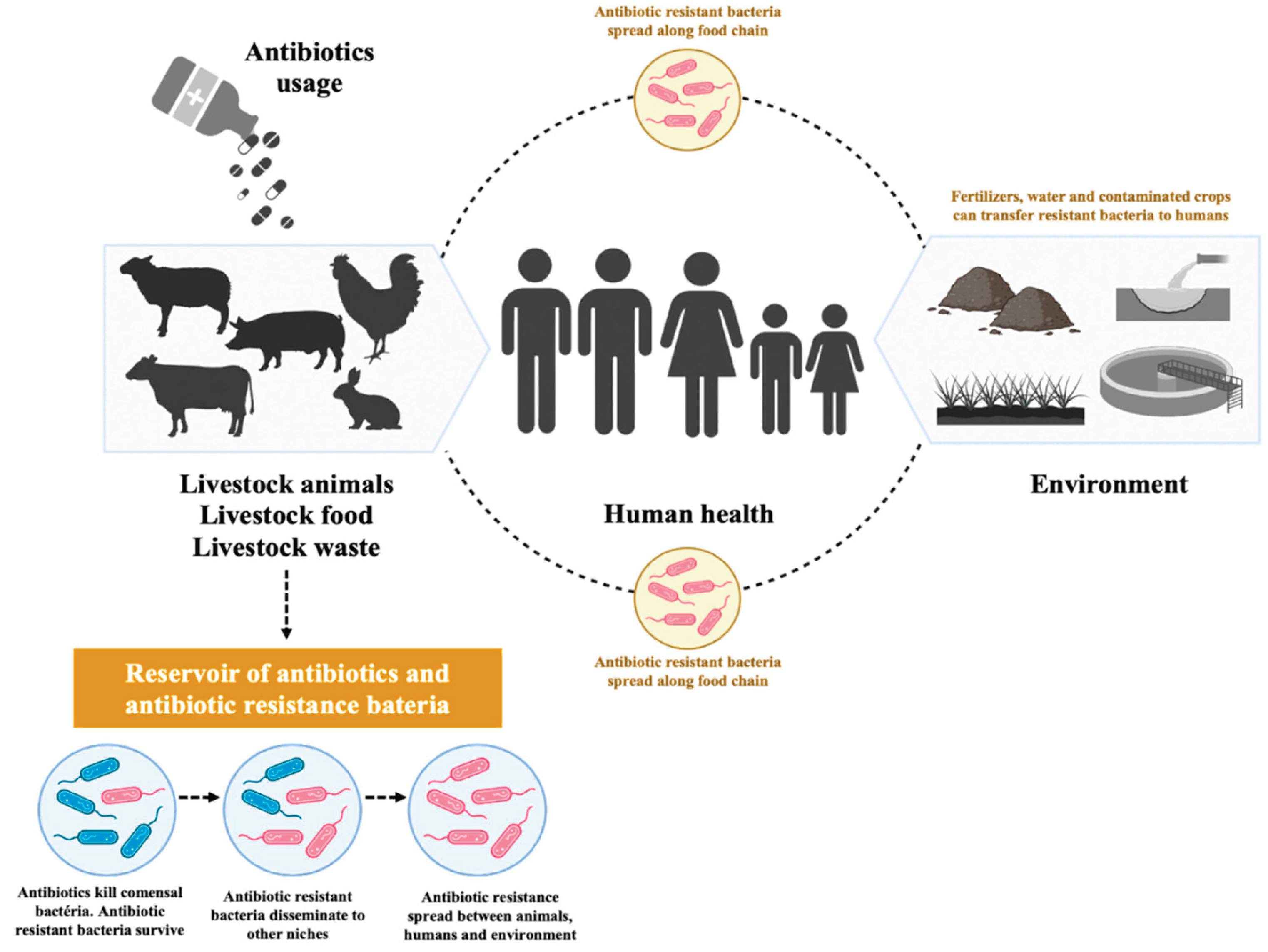
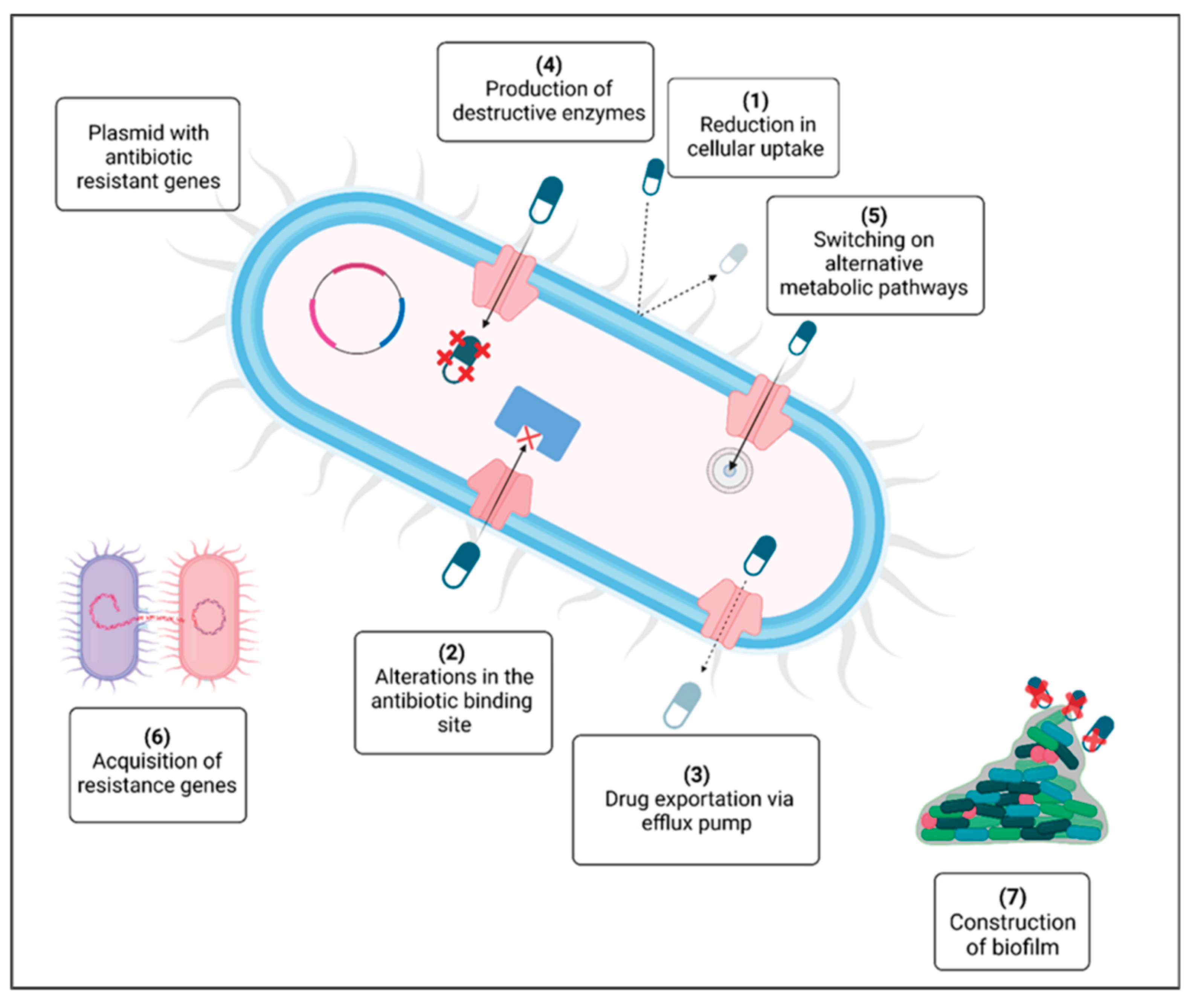
3. Antibiotics, Antifungals, and Antiparasitic Drugs in Farm Animal Production
3.1. Antibiotics in Farm Animal Production
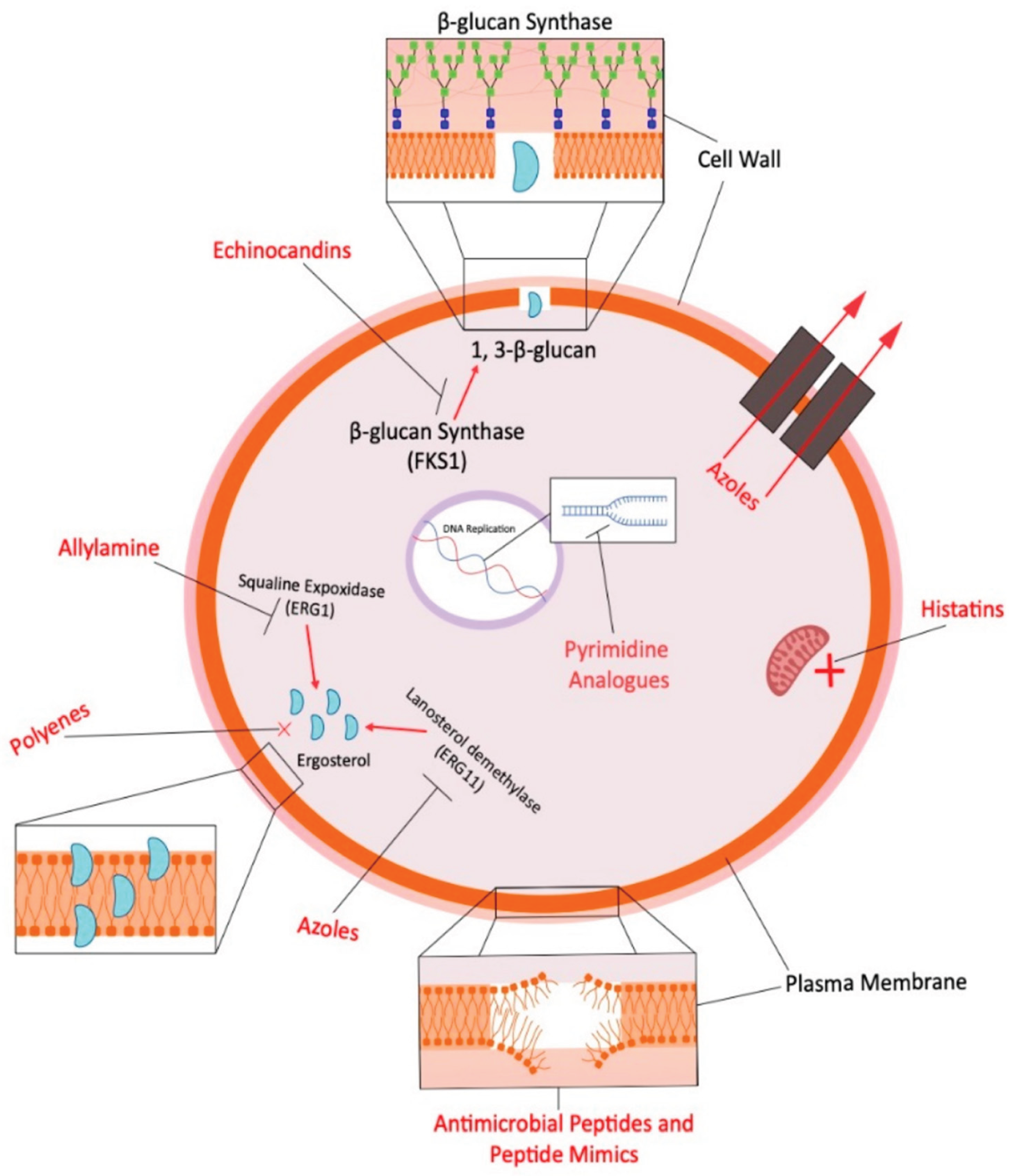
3.2. Antifungals in Farm Animal Production
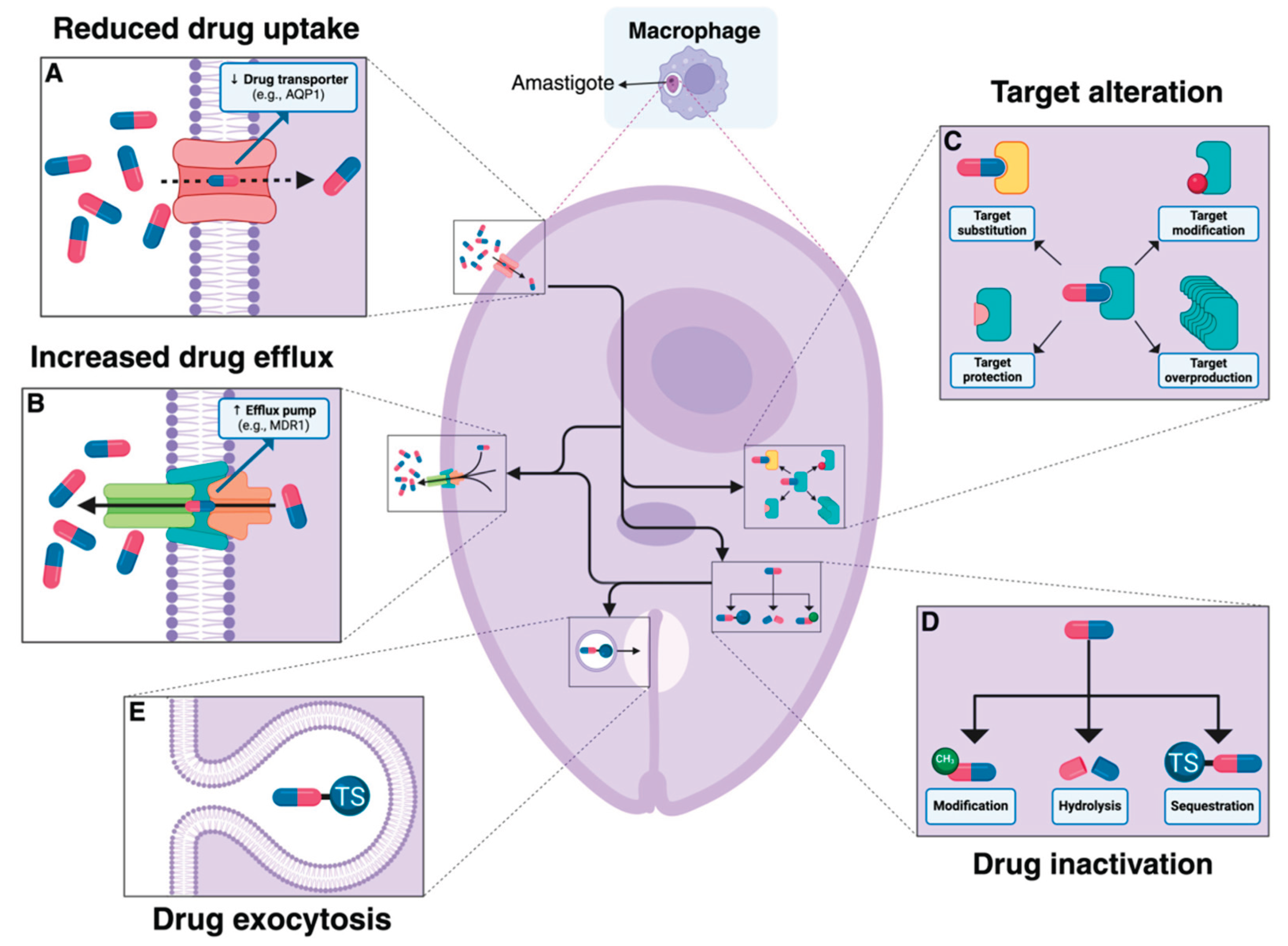
3.3. Antiparasitic Drugs in Farm Animal Production
4. Prospects of Antimicrobial Peptides Compared to Antibiotics in Treating Livestock Diseases
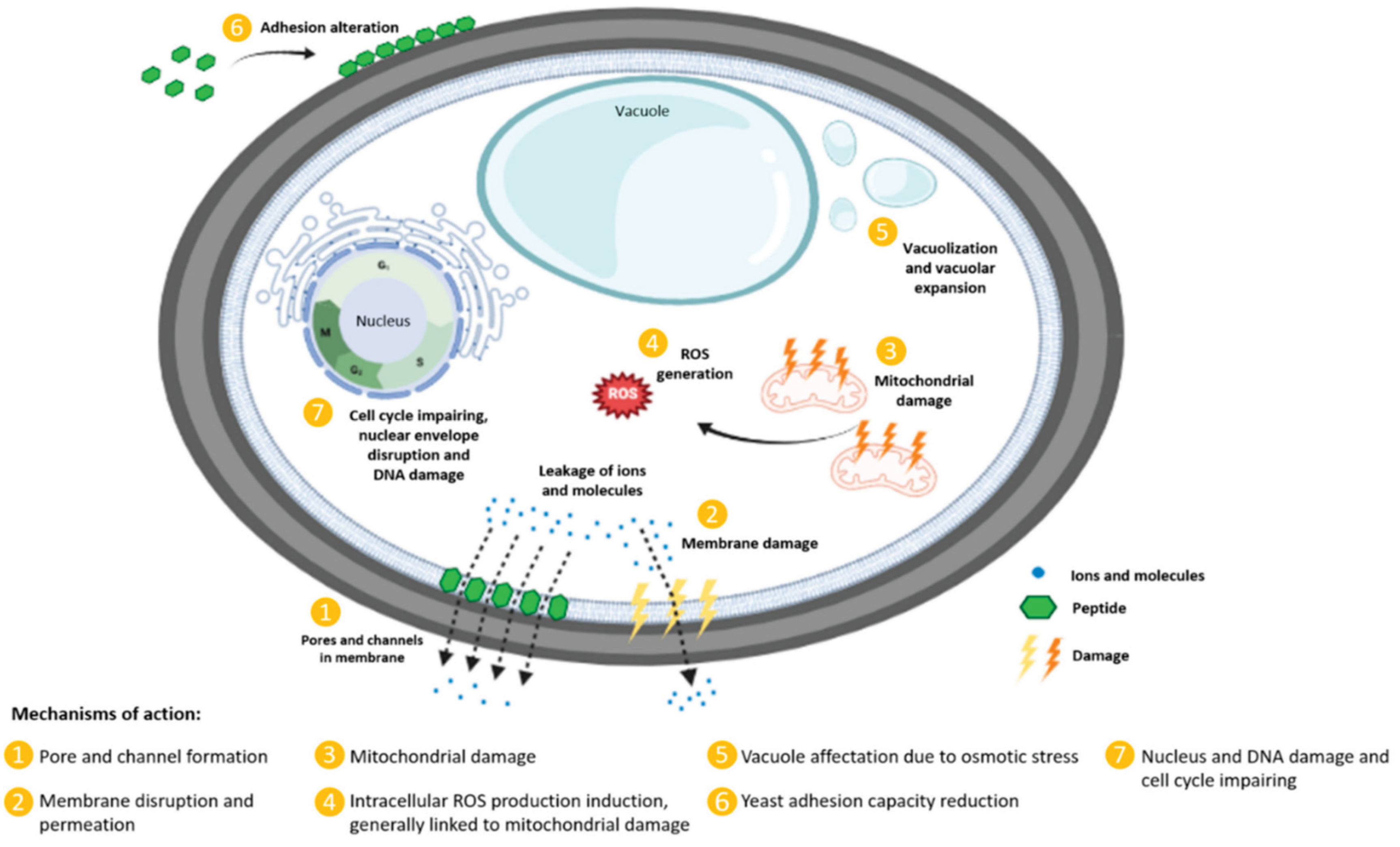
4.1. Antimicrobial Peptides as Alternatives to Conventional Antibiotics
4.2. Antifungal and Antiparasitic Properties of AMPs and Their Relevance
4.3. Addressing Challenges: Enhancing AMP Stability via Self-Assembly
5. Nano-Enabled Polymeric Systems for Pathogen Control in Animal Farming
6. Advantages and Future Perspectives
7. Conclusions
Author Contributions
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AMP | Antimicrobial Peptide |
| AFP | Antifungal Peptide |
| AMR | Antimicrobial Resistance |
| HDP | Host Defense Peptide |
| ROS | Reactive Oxygen Species |
References
- Ardakani, Z.; Aragrande, M.; Canali, M. Global Antimicrobial Use in Livestock Farming: An Estimate for Cattle, Chickens, and Pigs. Animal 2024, 18, 101060. [Google Scholar] [CrossRef] [PubMed]
- Sołek, P.; Stępniowska, A.; Koszła, O.; Jankowski, J.; Ognik, K. Antibiotics/Coccidiostat Exposure Induces Gut-Brain Axis Remodeling for Akt/mTOR Activation and BDNF-Mediated Neuroprotection in APEC-Infected Turkeys. Poultry Science 2025, 104, 104636. [Google Scholar] [CrossRef] [PubMed]
- Woo, W.-S.; Shim, S.H.; Kang, G.; Kim, K.-H.; Son, H.-J.; Sohn, M.-Y.; Lee, S.; Kim, J.; Seo, J.-S.; Kwon, M.-G.; et al. Assessment of Salinomycin’s Potential to Treat Microcotyle Sebastis in Korean Rockfish (Sebastes Schlegelii). Animals 2023, 13, 3233. [Google Scholar] [CrossRef] [PubMed]
- Dupuis, V.; Cerbu, C.; Witkowski, L.; Potarniche, A.-V.; Timar, M.C.; Żychska, M.; Sabliov, C.M. Nanodelivery of Essential Oils as Efficient Tools against Antimicrobial Resistance: A Review of the Type and Physical-Chemical Properties of the Delivery Systems and Applications. Drug Delivery 2022, 29, 1007–1024. [Google Scholar] [CrossRef]
- Veerapandian, R.; Abdul Azees, P.A.; Viswanathan, T.; Amaechi, B.T.; Vediyappan, G. Editorial: Developing Therapeutics for Antimicrobial Resistant Pathogens: Volume II. Front. Cell. Infect. Microbiol. 2025, 15, 1581237. [Google Scholar] [CrossRef]
- Allende, A.; Álvarez-Ordóñez, A.; Bortolaia, V.; Bover-Cid, S.; De Cesare, A.; Dohmen, W.; Guillier, L.; Herman, L.; Jacxsens, L.; et al.; EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards) Occurrence and Spread of Carbapenemase-producing Enterobacterales (CPE) in the Food Chain in the EU/EFTA. Part 1: 2025 Update. EFSA Journal 2025, 23. [Google Scholar] [CrossRef]
- O’Neill, J. Review on Antimicrobial Resistance. Antimicrobial Resistance: Tackling a Crisis for the Health and Wealth of Nations. 2014.
- Kamaruzzaman, N.F.; Tan, L.P.; Hamdan, R.H.; Choong, S.S.; Wong, W.K.; Gibson, A.J.; Chivu, A.; Pina, M.D.F. Antimicrobial Polymers: The Potential Replacement of Existing Antibiotics? IJMS 2019, 20, 2747. [Google Scholar] [CrossRef]
- Silva, A.; Silva, V.; Pereira, J.E.; Maltez, L.; Igrejas, G.; Valentão, P.; Falco, V.; Poeta, P. Antimicrobial Resistance and Clonal Lineages of Escherichia coli from Food-Producing Animals. Antibiotics 2023, 12, 1061. [Google Scholar] [CrossRef]
- Acosta, A.; Tirkaso, W.; Nicolli, F.; Van Boeckel, T.P.; Cinardi, G.; Song, J. The Future of Antibiotic Use in Livestock. Nat Commun 2025, 16, 2469. [Google Scholar] [CrossRef]
- Wu, D.; Fu, L.; Wen, W.; Dong, N. The Dual Antimicrobial and Immunomodulatory Roles of Host Defense Peptides and Their Applications in Animal Production. J Animal Sci Biotechnol 2022, 13, 141. [Google Scholar] [CrossRef]
- Finatto, A.N.; Meurens, F.; De Oliveira Costa, M. Piggybacking on Nature: Exploring the Multifaceted World of Porcine β-Defensins. Vet Res 2025, 56, 47. [Google Scholar] [CrossRef] [PubMed]
- Debbarma, S.; Narsale, S.A.; Acharya, A.; Singh, S.K.; Mocherla, B.P.; Debbarma, R.; Yirang, Y. Drawing Immune-Capacity of Fish-Derived Antimicrobial Peptides for Aquaculture Industry: A Comprehensive Review. Comparative Immunology Reports 2024, 6, 200150. [Google Scholar] [CrossRef]
- Mishra, S.K.; Akter, T.; Urmi, U.L.; Enninful, G.; Sara, M.; Shen, J.; Suresh, D.; Zheng, L.; Mekonen, E.S.; Rayamajhee, B.; et al. Harnessing Non-Antibiotic Strategies to Counter Multidrug-Resistant Clinical Pathogens with Special Reference to Antimicrobial Peptides and Their Coatings. Antibiotics 2025, 14, 57. [Google Scholar] [CrossRef]
- UniProt. Available online: https://www.uniprot.org/ (accessed on 16 May 2025).
- García-Beltrán, J.M.; Arizcun, M.; Chaves-Pozo, E. Antimicrobial Peptides from Photosynthetic Marine Organisms with Potential Application in Aquaculture. Marine Drugs 2023, 21, 290. [Google Scholar] [CrossRef]
- Yan, C.; Chen, M.; Xu, H.; Jin, J.; Liu, X.; Wang, Z.; Zhang, D. A Hypothetical Protein Fragment from Large Yellow Croaker (Larimichthys Crocea) Demonstrates Significant Activity Against Both Bacterial and Parasite. Fishes 2025, 10, 109. [Google Scholar] [CrossRef]
- Chen, M.; Lin, N.; Liu, X.; Tang, X.; Wang, Z.; Zhang, D. A Novel Antimicrobial Peptide Screened by a Bacillus Subtilis Expression System, Derived from Larimichthys Crocea Ferritin H, Exerting Bactericidal and Parasiticidal Activities. Front. Immunol. 2023, 14, 1168517. [Google Scholar] [CrossRef]
- Lei, Y.; Qiu, R.; Shen, Y.; Zhou, Y.; Cao, Z.; Sun, Y. Molecular Characterization and Antibacterial Immunity Functional Analysis of Liver-Expressed Antimicrobial Peptide 2 (LEAP-2) Gene in Golden Pompano (Trachinotus Ovatus). Fish & Shellfish Immunology 2020, 106, 833–843. [Google Scholar] [CrossRef]
- Ortega, L.; Carrera, C.; Muñoz-Flores, C.; Salazar, S.; Villegas, M.F.; Starck, M.F.; Valenzuela, A.; Agurto, N.; Montesino, R.; Astuya, A.; et al. New Insight into the Biological Activity of Salmo Salar NK-Lysin Antimicrobial Peptides. Front. Immunol. 2024, 15, 1191966. [Google Scholar] [CrossRef]
- Fernandes, J.M.O.; Saint, N.; Kemp, G.D.; Smith, V.J. Oncorhyncin III: A Potent Antimicrobial Peptide Derived from the Non-Histone Chromosomal Protein H6 of Rainbow Trout, Oncorhynchus Mykiss. Biochemical Journal 2003, 373, 621–628. [Google Scholar] [CrossRef]
- García-Vela, S.; Ben Said, L.; Soltani, S.; Guerbaa, R.; Fernández-Fernández, R.; Ben Yahia, H.; Ben Slama, K.; Torres, C.; Fliss, I. Targeting Enterococci with Antimicrobial Activity against Clostridium Perfringens from Poultry. Antibiotics 2023, 12, 231. [Google Scholar] [CrossRef]
- Sengkhui, S.; Klubthawee, N.; Aunpad, R. A Novel Designed Membrane-Active Peptide for the Control of Foodborne Salmonella Enterica Serovar Typhimurium. Sci Rep 2023, 13, 3507. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.T.T.; Allan, B.; Wheler, C.; Köster, W.; Gerdts, V.; Dar, A. Avian Antimicrobial Peptides: In Vitro and in Ovo Characterization and Protection from Early Chick Mortality Caused by Yolk Sac Infection. Sci Rep 2021, 11, 2132. [Google Scholar] [CrossRef] [PubMed]
- Satchanska, G.; Davidova, S.; Gergova, A. Diversity and Mechanisms of Action of Plant, Animal, and Human Antimicrobial Peptides. Antibiotics 2024, 13, 202. [Google Scholar] [CrossRef]
- Liu, Y.; Zai, X.; Weng, G.; Ma, X.; Deng, D. Brevibacillus Laterosporus: A Probiotic with Important Applications in Crop and Animal Production. Microorganisms 2024, 12, 564. [Google Scholar] [CrossRef]
- Hao, S.; Shi, W.; Chen, L.; Kong, T.; Wang, B.; Chen, S.; Guo, X. CATH-2-Derived Antimicrobial Peptide Inhibits Multidrug-Resistant Escherichia Coli Infection in Chickens. Front. Cell. Infect. Microbiol. 2024, 14, 1390934. [Google Scholar] [CrossRef]
- Bin Hafeez, A.; Jiang, X.; Bergen, P.J.; Zhu, Y. Antimicrobial Peptides: An Update on Classifications and Databases. IJMS 2021, 22, 11691. [Google Scholar] [CrossRef]
- Shen, S.; Ren, F.; He, J.; Wang, J.; Sun, Y.; Hu, J. Recombinant Antimicrobial Peptide OaBac5mini Alleviates Inflammation in Pullorum Disease Chicks by Modulating TLR4/MyD88/NF-κB Pathway. Animals 2023, 13, 1515. [Google Scholar] [CrossRef]
- Kathayat, D.; Closs, G.; Helmy, Y.A.; Lokesh, D.; Ranjit, S.; Rajashekara, G. Peptides Affecting the Outer Membrane Lipid Asymmetry System (MlaA-OmpC/F) Reduce Avian Pathogenic Escherichia Coli (APEC) Colonization in Chickens. Appl Environ Microbiol 2021, 87, e00567–21. [Google Scholar] [CrossRef]
- Wang, D.; Yu, P.; She, R.; Wang, K. Protective Effects of Rabbit Sacculus-Derived Antimicrobial Peptides on SPF Chicken against Infection with Very Virulent Infectious Bursal Disease Virus. Poultry Science 2024, 103, 103797. [Google Scholar] [CrossRef]
- Park, I.; Oh, S.; Nam, H.; Celi, P.; Lillehoj, H.S. Antimicrobial Activity of Sophorolipids against Eimeria Maxima and Clostridium Perfringens, and Their Effect on Growth Performance and Gut Health in Necrotic Enteritis. Poultry Science 2022, 101, 101731. [Google Scholar] [CrossRef]
- Chen, X.; Zhu, F.; Cao, Y.; Qiao, S. Novel Expression Vector for Secretion of Cecropin AD in Bacillus Subtilis with Enhanced Antimicrobial Activity. Antimicrob Agents Chemother 2009, 53, 3683–3689. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Zhang, F.; Huang, Z.; Liu, H.; Xie, C.; Zhang, J.; Thacker, P.A.; Qiao, S. Effects of the Antimicrobial Peptide Cecropin AD on Performance and Intestinal Health in Weaned Piglets Challenged with Escherichia Coli. Peptides 2012, 35, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.-N.; Pan, C.-Y.; Wu, H.-Y.; Chen, J.-Y. Antimicrobial Peptide Epinecidin-1 Promotes Complete Skin Regeneration of Methicillin-Resistant Staphylococcus Aureus-Infected Burn Wounds in a Swine Model. Oncotarget 2017, 8, 21067–21080. [Google Scholar] [CrossRef] [PubMed]
- Chee, P.Y.; Mang, M.; Lau, E.S.; Tan, L.T.-H.; He, Y.-W.; Lee, W.-L.; Pusparajah, P.; Chan, K.-G.; Lee, L.-H.; Goh, B.-H. Epinecidin-1, an Antimicrobial Peptide Derived From Grouper (Epinephelus Coioides): Pharmacological Activities and Applications. Front. Microbiol. 2019, 10, 2631. [Google Scholar] [CrossRef]
- Pränting, M.; Negrea, A.; Rhen, M.; Andersson, D.I. Mechanism and Fitness Costs of PR-39 Resistance in Salmonella Enterica Serovar Typhimurium LT2. Antimicrob Agents Chemother 2008, 52, 2734–2741. [Google Scholar] [CrossRef]
- Peng, Z.; Wang, A.; Xie, L.; Song, W.; Wang, J.; Yin, Z.; Zhou, D.; Li, F. Use of Recombinant Porcine β-Defensin 2 as a Medicated Feed Additive for Weaned Piglets. Sci Rep 2016, 6, 26790. [Google Scholar] [CrossRef]
- Wilson, H.L.; Buchanan, R.M.; Allan, B.; Tikoo, S.K. Milk-Derived Antimicrobial Peptides to Protect against Neonatal Diarrheal Disease: An Alternative to Antibiotics. Procedia in Vaccinology 2012, 6, 21–32. [Google Scholar] [CrossRef]
- Zhang, H.; Lv, J.; Ma, Z.; Ma, J.; Chen, J. Advances in Antimicrobial Peptides: Mechanisms, Design Innovations, and Biomedical Potential. Molecules 2025, 30, 1529. [Google Scholar] [CrossRef]
- Mihaylova-Garnizova, R.; Davidova, S.; Hodzhev, Y.; Satchanska, G. Antimicrobial Peptides Derived from Bacteria: Classification, Sources, and Mechanism of Action against Multidrug-Resistant Bacteria. IJMS 2024, 25, 10788. [Google Scholar] [CrossRef]
- Cornejo, S.; Barber, C.; Thoresen, M.; Lawrence, M.; Seo, K.S.; Woolums, A. Synthetic Antimicrobial Peptides Bac-5, BMAP-28, and Syn-1 Can Inhibit Bovine Respiratory Disease Pathogens in Vitro. Front. Vet. Sci. 2024, 11, 1430919. [Google Scholar] [CrossRef]
- Luo, C.; Yin, D.; Gao, X.; Li, Q.; Zhang, L. Goat Mammary Gland Expression of Cecropin B to Inhibit Bacterial Pathogens Causing Mastitis. Animal Biotechnology 2013, 24, 66–78. [Google Scholar] [CrossRef] [PubMed]
- Huan, Y.; Kong, Q.; Mou, H.; Yi, H. Antimicrobial Peptides: Classification, Design, Application and Research Progress in Multiple Fields. Front. Microbiol. 2020, 11, 582779. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Xue, Q.; Li, J.; Zhang, S. Antifungal Peptides from Living Organisms. Front. Microbiol. 2024, 15, 1511461. [Google Scholar] [CrossRef]
- Sargsyan, T.; Stepanyan, L.; Panosyan, H.; Hakobyan, H.; Israyelyan, M.; Tsaturyan, A.; Hovhannisyan, N.; Vicidomini, C.; Mkrtchyan, A.; Saghyan, A.; et al. Synthesis and Antifungal Activity of Fmoc-Protected 1,2,4-Triazolyl-α-Amino Acids and Their Dipeptides Against Aspergillus Species. Biomolecules 2025, 15, 61. [Google Scholar] [CrossRef]
- Buda De Cesare, G.; Cristy, S.A.; Garsin, D.A.; Lorenz, M.C. Antimicrobial Peptides: A New Frontier in Antifungal Therapy. mBio 2020, 11, e02123–20. [Google Scholar] [CrossRef]
- Gong, Y.; Li, H.; Wu, F.; Li, Y.; Zhang, S. Fungicidal Activity of AP10W, a Short Peptide Derived from AP-2 Complex Subunit Mu-A, In Vitro and In Vivo. Biomolecules 2022, 12, 965. [Google Scholar] [CrossRef]
- Perez-Rodriguez, A.; Eraso, E.; Quindós, G.; Mateo, E. Antimicrobial Peptides with Anti-Candida Activity. Int. J. Mol. Sci. 2022, 23, 9264. [Google Scholar] [CrossRef]
- Fodor, A.; Hess, C.; Ganas, P.; Boros, Z.; Kiss, J.; Makrai, L.; Dublecz, K.; Pál, L.; Fodor, L.; Sebestyén, A.; et al. Antimicrobial Peptides (AMP) in the Cell-Free Culture Media of Xenorhabdus Budapestensis and X. Szentirmaii Exert Anti-Protist Activity against Eukaryotic Vertebrate Pathogens Including Histomonas Meleagridis and Leishmania Donovani Species. Antibiotics 2023, 12, 1462. [Google Scholar] [CrossRef]
- Ünübol, N.; Çavuş, İ.; Polat, T.; Kurt, Ö.; Özbilgin, A.; Kocagöz, T. Antimicrobial Peptides and Their Anti-Leishmanial Efficacies on Leishmania Tropica Promastigotes In Vitro. Turkish Journal of Parasitology 2024, 135–141. [Google Scholar] [CrossRef]
- Aley, S.B.; Gillin, F.D. Killing of Giardia Lamblia by Cryptdins and Cationic Neutrophil Peptidest. 1994, 62.
- Wickramasuriya, S.S.; Park, I.; Lee, Y.; Kim, W.H.; Przybyszewski, C.; Gay, C.G.; Oosterwijk, J.G.V.; Lillehoj, H.S. Oral Delivery of Bacillus Subtilis Expressing Chicken NK-2 Peptide Protects Against Eimeria Acervulina Infection in Broiler Chickens. Front. Vet. Sci. 2021, 8, 684818. [Google Scholar] [CrossRef]
- Proaño-Bolaños, C.; Morán-Marcillo, G.; Espinosa De Los Monteros-Silva, N.; Bermúdez-Puga, S.; Salazar, M.A.; Blasco-Zúñiga, A.; Cuesta, S.; Molina, C.; Espinosa, F.; Meneses, L.; et al. Bioactivity of Synthetic Peptides from Ecuadorian Frog Skin Secretions against Leishmania Mexicana, Plasmodium Falciparum, and Trypanosoma Cruzi. Microbiol Spectr 2024, 12, e03339–23. [Google Scholar] [CrossRef] [PubMed]
- Pitale, D.M.; Kaur, G.; Baghel, M.; Kaur, K.J.; Shaha, C. Halictine-2 Antimicrobial Peptide Shows Promising Anti-Parasitic Activity against Leishmania Spp. Experimental Parasitology 2020, 218, 107987. [Google Scholar] [CrossRef] [PubMed]
- Sabiá Júnior, E.F.; Menezes, L.F.S.; De Araújo, I.F.S.; Schwartz, E.F. Natural Occurrence in Venomous Arthropods of Antimicrobial Peptides Active against Protozoan Parasites. Toxins 2019, 11, 563. [Google Scholar] [CrossRef]
- Rivas, L.; Rojas, V. Cyanobacterial Peptides as a Tour de Force in the Chemical Space of Antiparasitic Agents. Archives of Biochemistry and Biophysics 2019, 664, 24–39. [Google Scholar] [CrossRef]
- Kumar, R.; Ali, S.A.; Singh, S.K.; Bhushan, V.; Mathur, M.; Jamwal, S.; Mohanty, A.K.; Kaushik, J.K.; Kumar, S. Antimicrobial Peptides in Farm Animals: An Updated Review on Its Diversity, Function, Modes of Action and Therapeutic Prospects. Veterinary Sciences 2020, 7, 206. [Google Scholar] [CrossRef]
- Fasina, Y.O.; Obanla, T.; Dosu, G.; Muzquiz, S. Significance of Endogenous Antimicrobial Peptides on the Health of Food Animals. Front. Vet. Sci. 2021, 8, 585266. [Google Scholar] [CrossRef]
- Cheung, Q.C.K.; Turner, P.V.; Song, C.; Wu, D.; Cai, H.Y.; MacInnes, J.I.; Li, J. Enhanced Resistance to Bacterial Infection in Protegrin-1 Transgenic Mice. Antimicrob Agents Chemother 2008, 52, 1812–1819. [Google Scholar] [CrossRef]
- Takagi, S.; Hayashi, S.; Takahashi, K.; Isogai, H.; Bai, L.; Yoneyama, H.; Ando, T.; Ito, K.; Isogai, E. Antimicrobial Activity of a Bovine Myeloid Antimicrobial Peptide (BMAP-28) against Methicillin-susceptible and Methicillin-resistant Staphylococcus Aureus. Animal Science Journal 2012, 83, 482–486. [Google Scholar] [CrossRef]
- Guo, Y.; Xun, M.; Han, J. A Bovine Myeloid Antimicrobial Peptide (BMAP-28) and Its Analogs Kill Pan-Drug-Resistant Acinetobacter Baumannii by Interacting with Outer Membrane Protein A (OmpA). Medicine 2018, 97, e12832. [Google Scholar] [CrossRef]
- Hu, F.; Gao, X.; She, R.; Chen, J.; Mao, J.; Xiao, P.; Shi, R. Effects of Antimicrobial Peptides on Growth Performance and Small Intestinal Function in Broilers under Chronic Heat Stress. Poultry Science 2017, 96, 798–806. [Google Scholar] [CrossRef]
- Sun, Q.; Wang, K.; She, R.; Ma, W.; Peng, F.; Jin, H. Swine Intestine Antimicrobial Peptides Inhibit Infectious Bronchitis Virus Infectivity in Chick Embryos. Poultry Science 2010, 89, 464–469. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Dou, X.; Song, J.; Lyu, Y.; Zhu, X.; Xu, L.; Li, W.; Shan, A. Antimicrobial Peptides: Promising Alternatives in the Post Feeding Antibiotic Era. Medicinal Research Reviews 2019, 39, 831–859. [Google Scholar] [CrossRef] [PubMed]
- Petrov, P.D.; Davidova, S.; Satchanska, G. Polymer Micelles as Nanocarriers of Bioactive Peptides. Polymers 2025, 17, 1174. [Google Scholar] [CrossRef] [PubMed]
- Mughini-Gras, L.; Van Hoek, A.H.A.M.; Cuperus, T.; Dam-Deisz, C.; Van Overbeek, W.; Van Den Beld, M.; Wit, B.; Rapallini, M.; Wullings, B.; Franz, E.; et al. Prevalence, Risk Factors and Genetic Traits of Salmonella Infantis in Dutch Broiler Flocks. Veterinary Microbiology 2021, 258, 109120. [Google Scholar] [CrossRef]
- Wei, B.; Cha, S.-Y.; Zhang, J.-F.; Shang, K.; Park, H.-C.; Kang, J.; Lee, K.-J.; Kang, M.; Jang, H.-K. Antimicrobial Susceptibility and Association with Toxin Determinants in Clostridium Perfringens Isolates from Chickens. Microorganisms 2020, 8, 1825. [Google Scholar] [CrossRef]
- Mikulski, D.; Juśkiewicz, J.; Ognik, K.; Fotschki, B.; Tykałowski, B.; Jankowski, J. Gastrointestinal Response to the Early Administration of Antimicrobial Agents in Growing Turkeys Infected with Escherichia Coli. Poultry Science 2024, 103, 103720. [Google Scholar] [CrossRef]
- Orso, C.; Stefanello, T.B.; Franceschi, C.H.; Mann, M.B.; Varela, A.P.M.; Castro, I.M.S.; Frazzon, J.; Frazzon, A.P.G.; Andretta, I.; Ribeiro, A.M.L. Changes in the Ceca Microbiota of Broilers Vaccinated for Coccidiosis or Supplemented with Salinomycin. Poultry Science 2021, 100, 100969. [Google Scholar] [CrossRef]
- Cheng, X.; Zheng, H.; Wang, C.; Wang, X.; Fei, C.; Zhou, W.; Zhang, K. Effects of Salinomycin and Ethanamizuril on the Three Microbial Communities in Vivo and in Vitro. Front. Microbiol. 2022, 13, 941259. [Google Scholar] [CrossRef]
- Bampidis, V.; Azimonti, G.; Bastos, M. de L.; Christensen, H.; Durjava, M.; Dusemund, B.; Kouba, M.; López-Alonso, M.; Puente, S.L.; et al. Safety and Efficacy of a Feed Additive Consisting of Salinomycin Sodium (Sacox®) for Rabbits for Fattening (Huvepharma N.V.). EFSA Journal 2024, 22. [Google Scholar] [CrossRef]
- Nooreh, Z.; Taherpour, K.; Akbari Gharaei, M.; Shirzadi, H.; Ghasemi, H.A. Effects of a Dietary Direct-Fed Microbial and Ferulago Angulata Extract on Growth Performance, Intestinal Microflora, and Immune Function of Broiler Chickens Infected with Campylobacter Jejuni. Poultry Science 2021, 100, 100942. [Google Scholar] [CrossRef]
- Yuan, C.; Huang, X.; Zhai, R.; Ma, Y.; Xu, A.; Zhang, P.; Yang, Q. In Vitro Antiviral Activities of Salinomycin on Porcine Epidemic Diarrhea Virus. Viruses 2021, 13, 580. [Google Scholar] [CrossRef] [PubMed]
- Mysara, M.; Berkell, M.; Xavier, B. B.; De Backer, S.; Lammens, C.; Hautekiet, V.; Petkov, S.; Goossens, H.; Kumar-Singh, S.; Malhotra-Kumar, S. Assessing the Impact of Flavophospholipol and Virginiamycin Supplementation on the Broiler Microbiota: A Prospective Controlled Intervention Study. mSystems 2021, 6. [Google Scholar] [CrossRef] [PubMed]
- Dittoe, D.K.; Anderson, R.C.; Krueger, N.A.; Harvey, R.B.; Poole, T.L.; Crippen, T.L.; Callaway, T.R.; Ricke, S.C. Campylobacter Jejuni Response When Inoculated in Bovine In Vitro Fecal Microbial Consortia Incubations in the Presence of Metabolic Inhibitors. Pathogens 2023, 12, 1391. [Google Scholar] [CrossRef]
- Schwarz, C.; Mathieu, J.; Gomez, J.L.; Miller, M.R.; Tikhonova, M. ; Nagaraja, Tiruvoor. G.; Alvarez, P.J.J. Unexpected Finding of Fusobacterium Varium as the Dominant Fusobacterium Species in Cattle Rumen: Potential Implications for Liver Abscess Etiology and Interventions. Journal of Animal Science 2023, 101, skad130. [Google Scholar] [CrossRef]
- Warsi, O. M.; Upterworth, L. M.; Breidenstein, A.; Lustig, U.; Mikkelsen, K.; Nagy, T.; Dávid Szatmari; Hanne Ingmer; Andersson, D. I. Staphylococcus Aureus Mutants Resistant to the Feed-Additive Monensin Show Increased Virulence and Altered Purine Metabolism. mBio 2024, 15. [Google Scholar] [CrossRef]
- Vieira, A.M.; Soratto, T.A.T.; Cardinal, K.M.; Wagner, G.; Hauptli, L.; Lima, A.L.F.; Dahlke, F.; Peres Netto, D.; Moraes, P.D.O.; Ribeiro, A.M.L. Modulation of the Intestinal Microbiota of Broilers Supplemented with Monensin or Functional Oils in Response to Challenge by Eimeria Spp. PLoS ONE 2020, 15. [Google Scholar] [CrossRef]
- Pires, P.G.D.S.; Torres, P.; Teixeira Soratto, T.A.; Filho, V.B.; Hauptli, L.; Wagner, G.; Haese, D.; Pozzatti, C.D.; Moraes, P.D.O. Comparison of Functional-Oil Blend and Anticoccidial Antibiotics Effects on Performance and Microbiota of Broiler Chickens Challenged by Coccidiosis. PLoS ONE 2022, 17. [Google Scholar] [CrossRef]
- Kimura, J.; Kudo, H.; Fukuda, A.; Yamada, M.; Makita, K.; Oka, K.; Takahashi, M.; Tamura, Y.; Usui, M. Decreasing the Abundance of Tetracycline-Resistant Escherichia Coli in Pig Feces during Nursery Using Flavophospholipol as a Pig Feed Additive. Veterinary and Animal Science 2022, 15, 100236. [Google Scholar] [CrossRef]
- Lim, K.; Pennell, M.; Lewis, S.; El-Gazzar, M.; Gebreyes, W.A. Effects of Flavophospholipol on Conjugation and Plasmid Curing of Multidrug-Resistant Salmonella Enteritidis in Broiler Chickens. JAC-Antimicrobial Resistance 2021, 3. [Google Scholar] [CrossRef]
- Nair, S.; Farzan, A.; Weese, J.S.; Poljak, Z.; Friendship, R.M. Effect of Flavophospholipol on Fecal Microbiota in Weaned Pigs Challenged with Salmonella Typhimurium. Porc Health Manag 2020, 6, 14. [Google Scholar] [CrossRef]
- Shanmugasundaram, R.; Markazi, A.; Mortada, M.; Ng, T.T.; Applegate, T.J.; Bielke, L.R.; Syed, B.; Pender, C.M.; Curry, S.; Murugesan, G.R.; et al. Research Note: Effect of Synbiotic Supplementation on Caecal Clostridium Perfringens Load in Broiler Chickens with Different Necrotic Enteritis Challenge Models. Poultry Science 2020, 99, 2452–2458. [Google Scholar] [CrossRef] [PubMed]
- Xing, J.; Paithankar, S.; Liu, K.; Uhl, K.; Li, X.; Ko, M.; Kim, S.; Haskins, J.; Chen, B. Published Anti-SARS-CoV-2 in Vitro Hits Share Common Mechanisms of Action That Synergize with Antivirals. Briefings in Bioinformatics 2021, 22. [Google Scholar] [CrossRef] [PubMed]
- Woods, M.; McAlister, J.A.; Geddes-McAlister, J. A One Health Approach to Overcoming Fungal Disease and Antifungal Resistance. WIREs Mechanisms of Disease 2023, 15. [Google Scholar] [CrossRef]
- Russo, T.P.; Santaniello, A. Tackling Antibiotic and Antifungal Resistance in Domestic Animals, Synanthropic Species, and Wildlife: A Global Health Imperative. Antibiotics 2023, 12, 1632. [Google Scholar] [CrossRef]
- Hoenigl, M.; Arastehfar, A.; Arendrup, M.C.; Brüggemann, R.; Carvalho, A.; Chiller, T.; Chen, S.; Egger, M.; Feys, S.; Gangneux, J.-P.; et al. Novel Antifungals and Treatment Approaches to Tackle Resistance and Improve Outcomes of Invasive Fungal Disease. Clin Microbiol Rev 2024, 37, e00074–23. [Google Scholar] [CrossRef]
- Hossain, C.M.; Ryan, L.K.; Gera, M.; Choudhuri, S.; Lyle, N.; Ali, K.A.; Diamond, G. Antifungals and Drug Resistance. Encyclopedia 2022, 2, 1722–1737. [Google Scholar] [CrossRef]
- de Jong, J.E.; Heuvelink, A.E.; Dieste Pérez, L.; Holstege, M.M.C. Aspergillus Spp., Aspergillosis and Azole Usage in Animal Species in Europe: Results from a Multisectoral Survey and Review of Recent Literature. Medical Mycology 2025, 63. [Google Scholar] [CrossRef]
- Jiang, C.; Fang, W.; Chen, S.; Guo, X.; Gao, X.; Liu, P.; Hu, G.; Li, G.; Mai, W.; Liu, P. Genetic Framework Sequencing Analysis of Candida Tropicalis in Dairy Cow Mastitis and Study of Pathogenicity and Drug Resistance. BMC Microbiol 2024, 24, 428. [Google Scholar] [CrossRef]
- Van Dijk, M.A.M.; Buil, J.B.; Tehupeiory-Kooreman, M.; Broekhuizen, M.J.; Broens, E.M.; Wagenaar, J.A.; Verweij, P.E. Azole Resistance in Veterinary Clinical Aspergillus Fumigatus Isolates in the Netherlands. Mycopathologia 2024, 189, 50. [Google Scholar] [CrossRef]
- Sylvén, K.R.; Bergefur, A.-L.; Jacobson, M.; Wallgren, P.; Selling, L.E. Dermatophytosis Caused by Trichophyton Mentagrophytes Complex in Organic Pigs. Acta Vet Scand 2023, 65, 32. [Google Scholar] [CrossRef]
- Alghuthaymi, M.A.; Hassan, A.A.; Kalia, A.; Sayed El Ahl, R.M.H.; El Hamaky, A.A.M.; Oleksak, P.; Kuca, K.; Abd-Elsalam, K.A. Antifungal Nano-Therapy in Veterinary Medicine: Current Status and Future Prospects. JoF 2021, 7, 494. [Google Scholar] [CrossRef] [PubMed]
- Höglund, J.; Daş, G.; Tarbiat, B.; Geldhof, P.; Jansson, D.S.; Gauly, M. Ascaridia Galli - An Old Problem That Requires New Solutions. International Journal for Parasitology: Drugs and Drug Resistance 2023, 23, 1–9. [Google Scholar] [CrossRef] [PubMed]
- McIntyre, J.; Morrison, A.; Maitland, K.; Berger, D.; Price, D.R.G.; Dougan, S.; Grigoriadis, D.; Tracey, A.; Holroyd, N.; Bull, K.; et al. Chromosomal Genome Assembly Resolves Drug Resistance Loci in the Parasitic Nematode Teladorsagia Circumcincta. PLoS Pathog 2025, 21, e1012820. [Google Scholar] [CrossRef]
- Moncada-Diaz, M.J.; Rodríguez-Almonacid, C.C.; Quiceno-Giraldo, E.; Khuong, F.T.H.; Muskus, C.; Karamysheva, Z.N. Molecular Mechanisms of Drug Resistance in Leishmania spp. Pathogens 2024, 13, 835. Pathogens 2024, 13, 835. [Google Scholar] [CrossRef]
- Sun, H.; Su, X.; Fu, Y.; Hao, L.; Zhou, W.; Zhou, Z.; Huang, J.; Wang, Y.; Shi, T. Pathogenicity and Drug Resistance of the Eimeria Tenella Isolate from Yiwu, Zhejiang Province, Eastern China. Poultry Science 2023, 102, 102845. [Google Scholar] [CrossRef]
- Rogala-Hnatowska, M.; Gould, G.; Mehrotra, S.; Drażbo, A.; Konieczka, P.; Ramasami, P.; Kozłowski, K. Efficacy and Growth Performance between Two Different Ionophore Coccidiostats (Narasin and Salinomycin) in Broiler Chickens after Challenge with Eimeria spp. Animals 2024, 14, 2750. Animals 2024, 14, 2750. [Google Scholar] [CrossRef]
- Tsiouris, V.; Giannenas, I.; Bonos, E.; Papadopoulos, E.; Stylianaki, I.; Sidiropoulou, E.; Lazari, D.; Tzora, A.; Ganguly, B.; Georgopoulou, I. Efficacy of a Dietary Polyherbal Formula on the Performance and Gut Health in Broiler Chicks after Experimental Infection with Eimeria Spp. Pathogens 2021, 10, 524. [Google Scholar] [CrossRef]
- Qiu, Y.; Yin, Y.; Ruan, Z.; Gao, Y.; Bian, C.; Chen, J.; Wang, X.; Pan, X.; Yang, J.; Shi, Q.; et al. Comprehensive Transcriptional Changes in the Liver of Kanglang White Minnow (Anabarilius Grahami) in Response to the Infection of Parasite Ichthyophthirius Multifiliis. Animals 2020, 10, 681. [Google Scholar] [CrossRef]
- Denwood, M.J.; Kaplan, R.M.; McKendrick, I.J.; Thamsborg, S.M.; Nielsen, M.K.; Levecke, B. A Statistical Framework for Calculating Prospective Sample Sizes and Classifying Efficacy Results for Faecal Egg Count Reduction Tests in Ruminants, Horses and Swine. Veterinary Parasitology 2023, 314, 109867. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, W.; Du, H.; Dhakal, P.; Chen, X.; Wu, L.; Li, X.; Wang, R.; Zhang, L.; Zhang, S.; et al. Licochalcone a: A Promising Antiparasitic Drug against Giardiasis. International Journal for Parasitology: Drugs and Drug Resistance 2025, 27, 100573. [Google Scholar] [CrossRef]
- Kim, J.; Ahn, J. Emergence and Spread of Antibiotic-Resistant Foodborne Pathogens from Farm to Table. Food Sci Biotechnol 2022, 31, 1481–1499. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Yang, N.; Mao, R.; Hao, Y.; Li, Y.; Guo, Y.; Teng, D.; Huang, Y.; Wang, J. Self-Assembly Antimicrobial Peptide for Treatment of Multidrug-Resistant Bacterial Infection. J Nanobiotechnol 2024, 22, 668. [Google Scholar] [CrossRef] [PubMed]
- Javadmanesh, A.; Mohammadi, E.; Mousavi, Z.; Azghandi, M.; Tanhaiean, A. Antibacterial Effects Assessment on Some Livestock Pathogens, Thermal Stability and Proposing a Probable Reason for Different Levels of Activity of Thanatin. Sci Rep 2021, 11, 10890. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Lei, Y.; Wu, J.; Li, Z.; Zhang, X.; Jia, L.; Wang, Y.; Ma, Y.; Zhang, K.; Cheng, Q.; et al. Antimicrobial Peptides Act on the Rumen Microbiome and Metabolome Affecting the Performance of Castrated Bulls. J Animal Sci Biotechnol 2023, 14, 31. [Google Scholar] [CrossRef]
- Ji, F.; Yang, H.; Wang, Q.; Li, J.; Zhou, H.; Liu, S. Porcine Intestinal Antimicrobial Peptide as an In-Feed Antibiotic Alternative Improves Intestinal Digestion and Immunity by Shaping the Gut Microbiota in Weaned Piglets. Animal Nutrition 2023, 14, 43–55. [Google Scholar] [CrossRef]
- Xu, B.C.; Fu, J.; Zhu, L.Y.; Li, Z.; Wang, Y.Z.; Jin, M.L. Overall Assessment of Antimicrobial Peptides in Piglets: A Set of Meta-Analyses. Animal 2020, 14, 2463–2471. [Google Scholar] [CrossRef]
- Xu, B.; Fu, J.; Zhu, L.; Li, Z.; Jin, M.; Wang, Y. Overall Assessment of Antibiotic Substitutes for Pigs: A Set of Meta-Analyses. J Animal Sci Biotechnol 2021, 12, 3. [Google Scholar] [CrossRef]
- Patyra, E.; Kwiatek, K. Insect Meals and Insect Antimicrobial Peptides as an Alternative for Antibiotics and Growth Promoters in Livestock Production. Pathogens 2023, 12, 854. [Google Scholar] [CrossRef]
- Xia, J.; Ge, C.; Yao, H. Antimicrobial Peptides from Black Soldier Fly (Hermetia Illucens) as Potential Antimicrobial Factors Representing an Alternative to Antibiotics in Livestock Farming. Animals 2021, 11, 1937. [Google Scholar] [CrossRef]
- Van Eijk, M.; Boerefijn, S.; Cen, L.; Rosa, M.; Morren, M.J.H.; Van Der Ent, C.K.; Kraak, B.; Dijksterhuis, J.; Valdes, I.D.; Haagsman, H.P.; et al. Cathelicidin-Inspired Antimicrobial Peptides as Novel Antifungal Compounds. Medical Mycology 2020, 58, 1073–1084. [Google Scholar] [CrossRef]
- Youssef, F.S.; El-Banna, H.A.; Elzorba, H.Y.; Galal, A.M. Application of Some Nanoparticles in the Field of Veterinary Medicine. International Journal of Veterinary Science and Medicine 2019, 7, 78–93. [Google Scholar] [CrossRef] [PubMed]
- Maximiano, M.R.; Rios, T.B.; Campos, M.L.; Prado, G.S.; Dias, S.C.; Franco, O.L. Nanoparticles in Association with Antimicrobial Peptides (NanoAMPs) as a Promising Combination for Agriculture Development. Front. Mol. Biosci. 2022, 9, 890654. [Google Scholar] [CrossRef] [PubMed]
- Maleki Dizaj, S.; Salatin, S.; Khezri, K.; Lee, J.-Y.; Lotfipour, F. Targeting Multidrug Resistance With Antimicrobial Peptide-Decorated Nanoparticles and Polymers. Front. Microbiol. 2022, 13, 831655. [Google Scholar] [CrossRef]
- Meng, H.; Zhao, Y.; Cai, H.; You, D.; Wang, Y.; Wu, S.; Wang, Y.; Guo, W.; Qu, W. Hydrogels Containing Chitosan-Modified Gold Nanoparticles Show Significant Efficacy in Healing Diabetic Wounds Infected with Antibiotic-Resistant Bacteria. IJN 2024, Volume 19, 1539–1556. [Google Scholar] [CrossRef]
- Kuang, M.; Yu, H.; Qiao, S.; Huang, T.; Zhang, J.; Sun, M.; Shi, X.; Chen, H. A Novel Nano-Antimicrobial Polymer Engineered with Chitosan Nanoparticles and Bioactive Peptides as Promising Food Biopreservative Effective against Foodborne Pathogen E. Coli O157-Caused Epithelial Barrier Dysfunction and Inflammatory Responses. IJMS 2021, 22, 13580. [Google Scholar] [CrossRef]
- Carmona-Ribeiro, A.M.; Araújo, P.M. Antimicrobial Polymer−Based Assemblies: A Review. IJMS 2021, 22, 5424. [Google Scholar] [CrossRef]
- Sandhu, A.K.; Yang, Y.; Li, W.-W. In Vivo Antibacterial Efficacy of Antimicrobial Peptides Modified Metallic Implants─Systematic Review and Meta-Analysis. ACS Biomater. Sci. Eng. 2022, 8, 1749–1762. [Google Scholar] [CrossRef]
| AMP | Source Organism | Uniprot Entry Name | Sequence |
|---|---|---|---|
| PR-39 | Sus scrofa (Pig) | PR39_PIG | METQRASLCLGRWSLWLLLLGLVVPSASAQALSYREAVLRAVDRLNEQSSEANLYRLLELDQPPKADEDPGTPKPVSFTVKETVCPRPTRQPPELCDFKENGRVKQCVGTVTLNPSIHSLDISCNEIQSVRRRPRPPYLPRPRPPPFFPPRLPPRIPPGFPPRFPPRFPGKR |
| Beta-defensin 1 | Sus scrofa (Pig) | DEFB1_PIG | MRLHRLLLVFLLMVLLPVPGLLKNIGNSVSCLRNKGVCMPGKCAPKMKQIGTCGMPQVKCCKRK |
| Beta-defensin 10 | Bos taurus (Bovine) | DFB10_BOVIN | MRLHHLLLLLLLVVLSSGSGFTQGVRSYLSCWGNRGICLLNRCPGRMRQIGTCLAPRVKCCR |
| Cathelicidin-1 | Gallus gallus (Chicken) | CTHL1_CHICK | MLSCWVLLLALLGGACALPAPLGYSQALAQAVDSYNQRPEVQNAFRLLSADPEPGPNVQLSSLHNLNFTIMETRCQARSGAQLDSCEFKEDGLVKDCAAPVVLQGGRAVLDVTCVDSMADPVRVKRVWPLVIRTVIAGYNLYRAIKKK |
| Cathelicidin-2 | Gallus gallus (Chicken) | CTHL2_CHICK | MLSCWVLLLALLGGVCALPAPLSYPQALIQAVDSYNQRPEVQNAFRLLSADPEPGPGVDLSTLRALNFTIMETECTPSARLPVDDCDFKENGVIRDCSGPVSVLQDTPEINLRCRDASSDPVLVQRGRFGRFLRKIRRFRPKVTITIQGSARFG |
| Cathelicidin-4 | Bos taurus (Bovine) | CTHL4_BOVIN | MQTQRASLSLGRWSLWLLLLGLVVPSASAQALSYREAVLRAVDQLNELSSEANLYRLLELDPPPKDNEDLGTRKPVSFTVKETVCPRTIQQPAEQCDFKEKGRVKQCVGTVTLDPSNDQFDLNCNELQSVILPWKWPWWPWRRG |
| Cathelicidin-6 | Bos taurus (Bovine) | CTHL6_BOVIN | METQRASLSLGRWSLWLLLLGLALPSASAQALSYREAVLRAVDQFNERSSEANLYRLLELDPPPKEDDENPNIPKPVSFRVKETVCPRTSQQPAEQCDFKENGLVKQCVGTVTLDAVKGKINVTCEELQSVGRFKRFRKKFKKLFKKLSPVIPLLHLG |
| Gallinacin-1 | Gallus gallus (Chicken) | GLL1_CHICK | MRIVYLLLPFILLLAQGAAGSSQALGRKSDCFRKSGFCAFLKCPSLTLISGKCSRFYLCCKRIWG |
| Hepcidin | Larimichthys crocea (Large yellow croaker) | HEPC_LARCR | MKTFSVAVAVAVVLAFICLQESSAVPANEEQELEQQIYFADPEMPVESCKMPYYMRENRQGSPARCRFCCRCCPRMRGCGICCRF |
| LEAP 2 | Sus scrofa (Pig) | LEAP2_PIG | MWHLKLFAVLVICLLLAVQVHGSPIPELSSAKRRPRRMTPFWRAVSLRPIGASCRDDSECLTRLCRKRRCSLSVAQE |
| Oncorhyncin-1 | Salmo gairdneri (Rainbow trout) | ONC1_ONCMY | SKGKKANKDVELARG |
| Ostricacin-1 | Struthio camelus (Common ostrich) | OSTR1_STRCA | LFCRKGTCHFGGCPAHLVKVGSCFGFRACCKWPWDV |
| Piscidin-3 | Morone chrysops x Morone saxatilis (White bass x Striped bass) | PISC3_MORCS | FIHHIFRGIVHAGRSIGRFLTG |
| Protegrin-1 | Sus scrofa (Pig) | PG1_PIG | METQRASLCLGRWSLWLLLLALVVPSASAQALSYREAVLRAVDRLNEQSSEANLYRLLELDQPPKADEDPGTPKPVSFTVKETVCPRPTRQPPELCDFKENGRVKQCVGTVTLDQIKDPLDITCNEVQGVRGGRLCYCRRRFCVCVGRG |
| Peptide | Source Organism | Target Pathogen | Reference |
|---|---|---|---|
| Algal AMPs (various) | Marine photosynthetic organisms | Gram-positive & Gram-negative bacteria, parasites | [16] |
| Hepcidin | Fish | Bacteria, fungi, parasites | [13] |
| Lc149 | Large Yellow Croaker (Larimichthys crocea) | Escherichia coli, Vibrio harveyi, fish parasites | [17] |
| Lc1687 | C-terminal fragment of a Ferritin H in Larimichthys crocea | Gram-positive & Gram-negative bacteria | [18] |
| LEAP-2 | Golden pompano (fish) | Edwardsiella tarda and Streptococcus agalactiae | [19] |
| NK-lysin | Atlantic salmon (Salmo salar) | Piscirickettsia salmonis, Flavobacterium psychrophilum | [20] |
| Oncorhyncin III | Non-histone chromosomal protein H6 from Rainbow Trout | Gram-positive & Gram-negative bacteria | [21] |
| Piscidin | Fish | Bacteria, viruses, fungi, parasites | [13] |
| Peptide | Source Organism | Target Pathogen | Target Animal | Reference |
|---|---|---|---|---|
| A11 | Modified Acidocin J1132β (from L. acidophilus) | Salmonella Typhimurium | Poultry (Food chain) | [23] |
| ABD1 | Chicken | Gram-positive & Gram-negative bacteria | Chicken | [24,25] |
| Brevilaterins | Brevibacillus laterosporus | Bacteria, Fungi | Livestock (general, incl. aquaculture, poultry, and swine) | [26] |
| C2-2 | Modified chicken CATH-2 | Multidrug-resistant E. coli | Chicken | [27] |
| CATH-1(6–26) | Chicken | Gram-positive & Gram-negative bacteria | Chicken | [23] |
| Enterocin A and B | Enterococcus faecium from poultry | Clostridium perfringens | Poultry | [22] |
| Fowlicidins (cathelicidins from chicken) | Chicken | Gram-positive & Gram-negative bacteria | Chicken | [28] |
| OaBac5mini | E.coli recombinant system | Salmonella Pullorum | Chicken | [29] |
| Ostricacins | Ostrich | Bacteria | Ostrich | [25] |
| P1 (NPSRQERR) | Lactobacillus rhamnosus GG | Avian Pathogenic E. coli (APEC) | Chicken | [30] |
| Rabbit sacculus rotundus-derived AMP | Rabbit sacculus rotundus | vvIBDV (very virulent infectious bursal disease virus) | Chicken | [31] |
| Sophorolipids (SL1-SL4) | Candida bombicola and other yeasts | Eimeria maxima and Clostridium perfringens | Chicken | [32] |
| Peptide | Source Organism | Target Pathogen | Target Animal | Reference |
|---|---|---|---|---|
| Brevilaterins | Brevibacillus laterosporus | Bacteria, Fungi | Livestock (general, incl. aquaculture, poultry and swine) | [26] |
| Cecropin AD | Synthetic hybrid (insect cecropins A & D) | E. coli (enterotoxigenic strain) | Pig (weaned piglets) | [33,34] |
| Epinecidin-1 | Epinephelus coioides | MRSA | Pig | [35,36] |
| LEAP-2 | Golden pompano (fish); Pig liver | Salmonella Typhimurium | Aquaculture species; Pig | [12] |
| PR-39 | Pig (intestinal cathelicidin) | Salmonella Typhimurium | Pig (swine) | [37] |
| Porcine β-Defensins (pBDs) | Pig | Escherichia coli (ETEC – post-weaning diarrhea) | Pig (swine) | [38] |
| Protegrin-1 (PG-1) | Porcine leukocytes | Gram-positive & Gram-negative bacteria | Piglets | [25,39] |
| Peptide | Source Organism | Target Pathogen | Target Animal | Reference |
|---|---|---|---|---|
| BMAP-27 | Bovine myeloid antimicrobial peptide | E. coli | Calves (cattle) | [39] |
| Bac-7 | Bos taurus | E. coli, Salmonella typhimurium | Cattle | [40] |
| Bacteriocins | Gram-positive & Gram-negative bacteria | Gram-positive & Gram-negative bacteria | Eggs, poultry and dairy products | [23,41] |
| Cathelicidin 4 | Bos taurus | Gram-positive & Gram-negative bacteria | Cattle | [25,42] |
| Cecropin B | Insect (moth peptide, via transgenic expression) | Staphylococcus aureus (mastitis pathogen) | Goat (dairy goats) | [43] |
| Indolicidin | Bovine neutrophils; Bos taurus | Gram-positive & Gram-negative bacteria | Calves (cattle) | [25,39] |
| Lfcin B (Lactoferricin B) | Cattle milk (lactoferrin fragment) | Broad-spectrum bacteria | Cattle, dairy products | [44] |
| Peptide | Source Organism | Target Pathogen | Target Animal | Reference |
|---|---|---|---|---|
| Fmoc-dipeptide 7a | Synthetic (modeled for veterinary pathogens) | Aspergillus flavus, Aspergillus versicolor, Aspergillus candidus | Cattle | [46] |
| Defensins | Mammalian immune cells, Plants | Broad-spectrum (bacteria, fungi, viruses) | Multiple livestock species | [45,47] |
| Piscidin | Fish | Bacteria, viruses, fungi, parasites | Aquaculture species | [13,45] |
| Hepcidin | Fish | Bacteria, fungi, parasites | Aquaculture species | [13] |
| SMAP-29 | Sheep (Ovis aries) | C. albicans, C. neoformans, and R. rubra | Sheep | [45] |
| Cathelicidins | Sheep, Cattle, Pigs | Broad-spectrum: bacteria, fungi | Ruminants | [25,47] |
| Peptide | Source Organism | Target Pathogen | Target Animal | Reference |
|---|---|---|---|---|
| Fabclavine | Xenorhabdus szentirmaii | Histomonas meleagridis, Paenibacillus larvae, bacteria, parasites | Poultry, Honey bees | [50] |
| Indolicidin | Cattle (bovine neutrophil peptide) | Giardia lamblia | Cattle (calves) or general | [39,52] |
| Chicken NK-2 (cNK-2) | Chicken (NK-lysin peptide 2) | Eimeria acervulina (coccidian parasite) | Poultry (broiler chickens) | [53] |
| Sophorolipids (SL1-SL4) | Candida bombicola and other yeasts | Eimeria maxima and Clostridium perfringens | Chicken | [32] |
| Dermaseptin-SP2 | Agalychnis spurrelli (frog) | Plasmodium falciparum, Leishmania mexicana, T. cruzi | Livestock parasite models | [54] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





