1. Introduction
Delirium is a serious manifestation of acute encephalopathy[
1] that significantly hinders recovery in critically ill patients. It is highly prevalent in the ICU, with a meta-analysis of 16,595 patients reporting a prevalence of
31.8%[
2]. Depending on
age,
geographic region, and
ICU type, among other factors, delirium in the ICU, however, can range from
10% to 50% [
3,
4]
.
Defined in the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition[
5] as a “sudden deterioration in attention, awareness, and cognition”, one form of delirium is iatrogenic in nature, and caused by the intake of sedatives, opioids, and anticholinergic medications[
6]. Delirium can also emerge as a manifestation of endogenous, physiological conditions, such as ischemic or hemorrhagic stroke[
7], as well as disturbances originating outside of the CNS, such as sepsis[
8].
The diverse underlying causes of delirium reflect its distinct pathophysiological mechanisms. Recognizing this variability is crucial in evaluating new pharmacologic treatments for delirium; yet this concept has often been overlooked. Therefore, in the present study, we focused on ICU patients with delirium arising from physiological conditions rather than drug-induced causes, to better account for this distinction.
Traditionally, the butyrophenone antipsychotic haloperidol has been the first-line treatment for ICU delirium as most clinical trials to date assessed its efficacy[
9,
10,
11]. However, the authors of these studies concluded that there is no evidence indicating that haloperidol reduces ICU length of stay, and they expressed low certainty concerning its effectiveness in improving other outcomes, such as 28-day mortality or mortality at the longest reported follow-up.
Since the data on the efficacy of antipsychotics other than haloperidol in ICU patients with delirium
are more limited, in the present emulated clinical trial we first evaluated the efficacy of quetiapine (vs. control)
in shortening the length of ICU stay. In the second, head-to-head analysis, we compared the efficacy of quetiapine vs haloperidol in reducing the length of ICU stay. As there is now probative evidence that haloperidol does not reduce the length of ICU stay[
6],
we used it in the second analysis as a comparator to assess whether quetiapine has a differential effect on this outcome. We focused on the length of ICU stay because a large meta-analysis of forty-one studies showed that patients with delirium had ICU stays that were,
on average, 4.77 days longer than those without delirium[
12]
. Delirium not only increases the health risks associated with prolonged ICU stay, but also
elevates daily care costs to an average $600 per day, primarily due to factors like increased monitoring, and additional interventions[
13].
2. Materials and Methods
2.1. Study Design and Data Source
Implementing a large-sample retrospective analysis, we first assessed the length of ICU stay in hospitalized patients with delirium receiving quetiapine (relative to control patients) prior to ICU admission. Using the same procedure, we then assessed the length of ICU stay in patients receiving quetiapine vs haloperidol.
We obtained the data from the latest MIMIC-IV database (version 3.1) which contains electronic health records from over 65,000 patients admitted to the ICU at the Beth Israel Deaconess Medical Center (Boston, Massachusetts, USA) from 2008 to 2022. Deidentified data are available on PhysioNet upon signing Data Use Agreements and completing the required training. We merged data from different modules based on the patients’ id and hospital stay. The Beth Israel Deaconess Medical Center and Massachusetts Institute of Technology approved the release of the deidentified and publicly available data without a requirement for individual patient informed consent.
2.2. Patients, Exposure, Outcome and Covariates
We extracted the critically ill adults (age ≥ 18 years) with delirium who were admitted to the ICU. The following ICD codes were extracted the purpose of defining delirium in the present study: delirium due to known physiological condition, vascular dementia with delirium, subacute delirium, and senile dementia with delirium. We analyzed the first admission data in the event a patient was admitted multiple times. We excluded patients who received any other typical or atypical antipsychotic agent. Hence, in the first analysis, patients administered any or second-generation antipsychotic were excluded; in the second analysis, patients administered any antipsychotic other than quetiapine or haloperidol were excluded. Since causality requires temporal ordering, the administration of quetiapine/haloperidol had to occur during hospitalization but prior to the ICU admission. We coded control/quetiapine as 0/1 and haloperidol/quetiapine as 0/1 in the two analyses, respectively. The study outcome measure was the length of stay in the ICU. We collected the following factor (0=no; 1=yes) variables: antidepressant administration, benzodiazepine administration, cancer, chronic obstructive pulmonary disease, depression, diabetes, hypertension, myocardial infarction, opioid administration, sepsis, and stroke. Additionally, we collected age, and gender.
2.3. Statistical Analysis
We performed propensity score matching using the MatchIt[
14] package in R to address potential confounding and achieve covariate balance; first between the treatment (quetiapine) and control (no antipsychotic) groups; then between quetiapine and haloperidol groups. For both propensity matching procedures, we estimated propensity scores using a logistic regression model, with the treatment variable as the outcome and the following covariates: age, antidepressant administration, benzodiazepine administration, cancer, chronic obstructive pulmonary disease, depression, diabetes, gender, hypertension, myocardial infarction, opioid administration, sepsis, and stroke. To restrict matches within a reasonable distance, we used the nearest neighbor matching without replacement at a 1:1 ratio and a caliper of 0.2 standard deviations of the logit of the propensity score. We assessed model validity by calculating standardized mean differences (SMDs), with SMD < 0.1 indicative of a balanced distribution of confounding factors between groups.
Using the brms[
15] package in R and adjusting for all covariates used in propensity score matching, we conducted Bayesian generalized additive modeling (GAM) to assess the association between quetiapine exposure and length of ICU stay. We repeated the same model for the second GAM assessing the relationship between haloperidol
vs quetiapine and length of ICU stay. We centered and standardized age prior to fitting it in the GAM models, following which we included it as a smooth term to flexibly capture potential nonlinear effects. We specified informative priors to regularize estimates as normal (0,1) for linear coefficients and student_t (3,0,2) for the smoothness parameters of the spline term. We fitted the model with 4 Markov Chain Monte Carlo (MCMC) chains, each with 4000 iterations (1000 warmup). To improve sampler efficiency and convergence, we set the adapt_delta parameter to 0.995. We assessed convergence using the potential scale reduction factor R̂ and summarized posterior distributions using means and 95% credible intervals. We interpreted nonlinear effects of age based on the estimated smooth function and its credible intervals.
In the last step, we conducted a sensitivity analyses to assess the potential impact of unmeasured confounding on the estimated effect of treatment on length of stay. We implemented Bayesian nonlinear regression with an inverse Gaussian family and a log link. We incorporated an explicit bias term in a Bayesian nonlinear regression to account for potential unobserved confounding. We modeled the nonlinear formula as:
length of stay =exp (β0+ βquetiapine × quetiapine + bias)
where β0, βquetiapine, and bias were estimated intercept terms. To evaluate the robustness of our findings to unobserved confounding, we specified a prior on the bias parameter as ∼N (0,2.5). We repeated the sensitivity analysis twice - first following the first analysis (quetiapine vs control) and again following the second analysis (quetiapine versus haloperidol).
3. Results
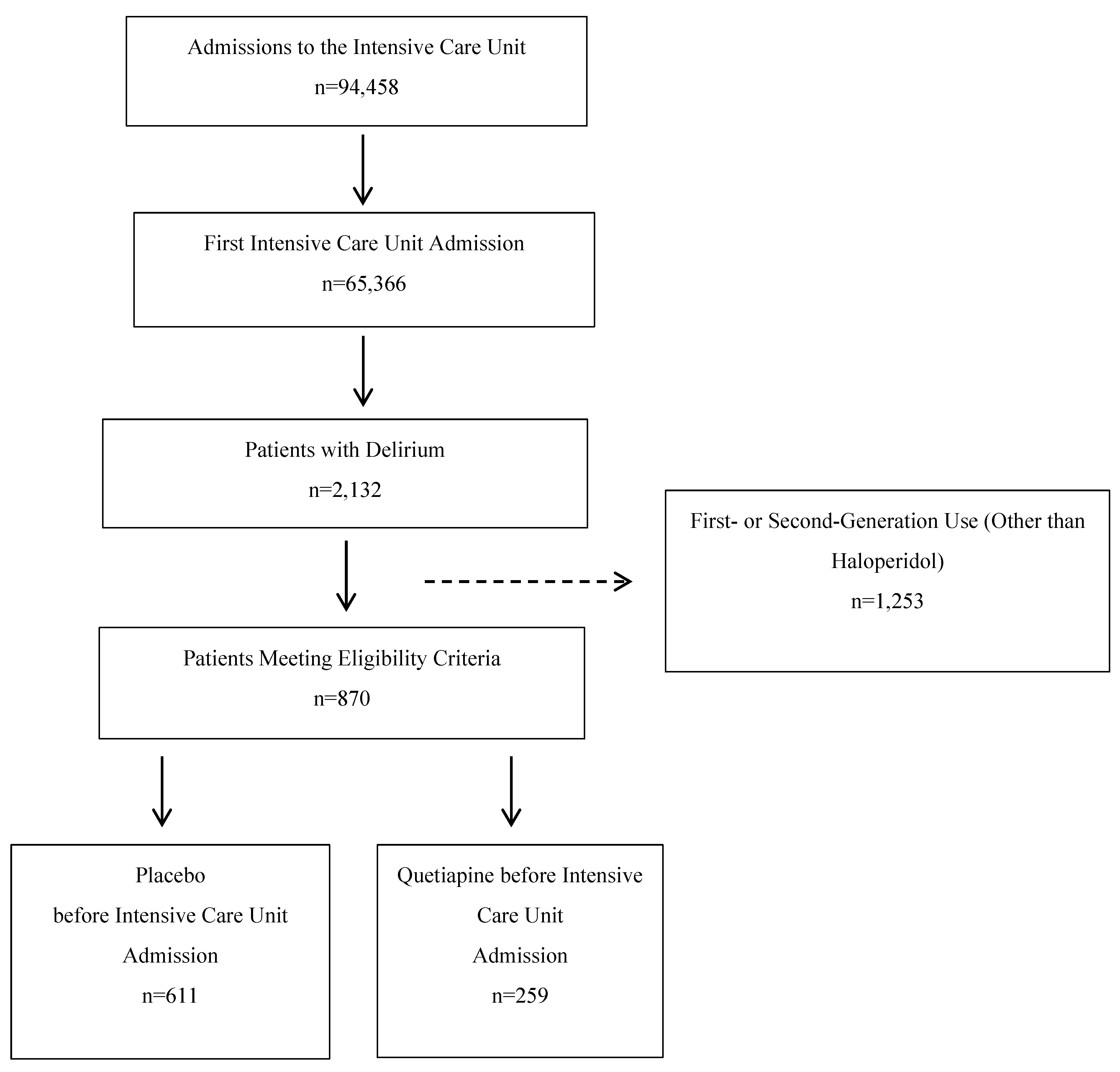
3.1. Participant Flow Diagrams
Of the 94,458 total ICU visits, 65,366 were first ICU visits, of which there were 2,132 patients with delirium. After the exclusion of patients who were administered antipsychotics other than quetiapine, 870 met the eligibility criteria. Of these, 259 received quetiapine (
Figure 1). The participant flow diagram for the second, quetiapine
vs haloperidol, analysis is identical other than the number patients meeting the inclusion criteria - 259 who received quetiapine and 168 who received haloperidol.
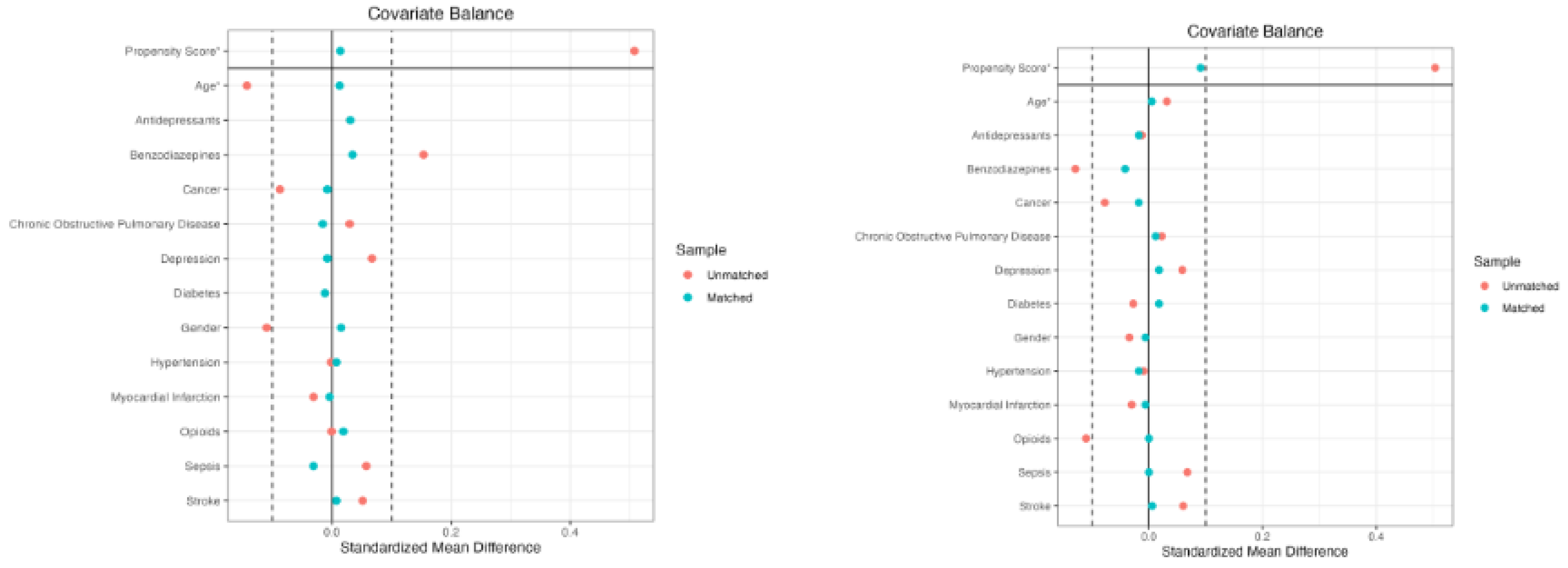
Propensity Score Matching – Quetiapine versus Control
After matching, the total of 259 individuals in the quetiapine group were matched to 259 individuals in the control group, resulting in a matched sample of 518 individuals. Prior to matching, the overall distance between the groups was 0.4888. Following the matching procedure, the overall distance was substantially reduced to 0.0135. All the covariates achieved adequate balance of absolute SMDs below 0.1 (see
Figure 2-left and
Table 1).
Propensity Score Matching – Quetiapine versus Haloperidol
The matching procedure of the 168 patients in the quetiapine group to the 168 patients in the haloperidol group resulted in a matched sample size of 336. Prior to matching, the overall distance between the groups was 0.4988, which was reduced to 0.0901. All the covariates achieved adequate balance of absolute SMDs below 0.1 (see
Figure 2-right and
Table 1).
Generalized Additive Modeling – Quetiapine versus Control
Quetiapine administration resulted in an increased length of stay (posterior mean = 0.36, 95% credible interval [CrI]: 0.14 to 0.59). Holding all variables constant, quetiapine increased the length of stay by 43%. That is, the baseline length of stay of 3.03 days was extended to 4.34 days (1.31 days) by quetiapine. Longer length of stay was also observed in patients with stroke (posterior mean = 0.35, 95% CrI: 0.08 to 0.65), sepsis (posterior mean = 0.32, 95% CrI: 0.06 to 0.60), and those receiving benzodiazepines (posterior mean = 0.42, 95% CrI: 0.20 to 0.65) or opioids (posterior mean = 0.24, 95% CrI: 0.01 to 0.46). There was no strong evidence of increased length of stay in patients with cancer, COPD, myocardial infarction, or other comorbidities, as the CrIs for these estimates included zero. The relationship between age and length of stay was non-linear, as indicated by the smoothing spline hyperparameter (posterior mean = 0.73, 95% CrI: 0.04 to 2.23). All chains converged successfully with
R̂ values of 1.00 for all parameters, indicating good mixing. Effective sample sizes (bulk effective sample size and tail effective sample size) were high across all parameters, suggesting reliable estimation of posterior distributions. See
Table 2 for details.
Table 1.
Standardized Mean Difference (SMD) According to Treatment Group after Propensity Score Matching.
Table 1.
Standardized Mean Difference (SMD) According to Treatment Group after Propensity Score Matching.
| Quetiapine vs Control Comparison |
Quetiapine vs Haloperidol Comparison |
| Variable |
Means |
SMD |
Means |
SMD |
| |
Quetiapine (n=259) |
Control (n=259) |
Quetiapine (n=168) |
Haloperidol
(n=168) |
| Propensity Score |
0.3375 |
0.3361 |
0.0135 |
0.5825 |
0.5723 |
0.0901 |
| Age |
71.7992 |
71.6178 |
0.0125 |
71.4167 |
71.3393 |
0.0053 |
| Antidepressants |
0.2317 |
0.2008 |
0.0732 |
0.2262 |
0.2440 |
-0.0423 |
| Benzodiazepines |
0.5676 |
0.5328 |
0.0701 |
0.5238 |
0.5655 |
-0.0840 |
| Cancer |
0.2201 |
0.2278 |
-0.0186 |
0.2798 |
0.2976 |
-0.0431 |
| Chronic Obstructive Pulmonary Disease |
0.1776 |
0.1931 |
-0.0404 |
0.1667 |
0.1548 |
0.0311 |
| Depression |
0.2432 |
0.2510 |
-0.0180 |
0.2024 |
0.1845 |
0.0416 |
| Diabetes |
0.3475 |
0.3591 |
-0.0243 |
0.3929 |
0.3750 |
0.0375 |
| Gender |
0.3707 |
0.3552 |
0.0320 |
0.3988 |
0.4048 |
-0.0123 |
| Hypertension |
0.4788 |
0.4710 |
0.0155 |
0.4702 |
0.4881 |
-0.0357 |
| Myocardial Infarction |
0.2317 |
0.2355 |
-0.0092 |
0.2560 |
0.2619 |
-0.0141 |
| Opioids |
0.6873 |
0.6680 |
0.0416 |
0.7976 |
0.7976 |
0.0000 |
| Sepsis |
0.2819 |
0.3127 |
-0.0687 |
0.2143 |
0.2143 |
0.0000 |
| Stroke |
0.2510 |
0.2432 |
0.0178 |
0.1964 |
0.1905 |
0.0137 |
Table 2.
Parametric and Smooth Coefficients from the General Additive Model.
Table 2.
Parametric and Smooth Coefficients from the General Additive Model.
| |
| Quetiapine vs Control |
Quetiapine vs Haloperidol |
| |
Estimate |
Estimated
Error |
Lower
95% CI |
Upper
95% CI |
R^ |
Bulk Effective Sample Size |
Tail Effective Sample Size |
Estimate |
Estimated
Error |
Lower
95% CI |
Upper
95% CI |
R^ |
Bulk Effective Sample Size |
Tail Effective Sample Size |
| (Intercept) |
1.1054 |
0.1517 |
0.8202 |
1.4135 |
1.0002 |
15050.8 |
9183.7 |
0.8707 |
0.3535 |
0.2448 |
1.6304 |
1.0004 |
9304.9 |
7770.6 |
| Age |
-0.2962 |
0.8889 |
-1.8913 |
1.5408 |
1.0001 |
6712.4 |
8406.9 |
-0.4389 |
0.9614 |
-2.2499 |
1.4932 |
1.0004 |
9733.1 |
8959.6 |
| Antidepressants |
-0.0262 |
0.1395 |
-0.2804 |
0.2655 |
1.0009 |
11642.8 |
7625.1 |
-0.1457 |
0.2733 |
-0.6314 |
0.4252 |
1.0000 |
9854.7 |
7752.8 |
| Benzodiazepines |
0.4224 |
0.1145 |
0.2012 |
0.6508 |
1.0002 |
13847.2 |
9062.8 |
0.4643 |
0.2204 |
0.0326 |
0.9068 |
1.0006 |
8155.2 |
7514.7 |
| Cancer |
0.0529 |
0.1370 |
-0.2045 |
0.3298 |
1.0004 |
13744.6 |
8865.3 |
-0.0630 |
0.2152 |
-0.4622 |
0.3814 |
1.0003 |
10877.2 |
8611.4 |
| Chronic Obstructive Pulmonary Disease |
0.0522 |
0.1465 |
-0.2170 |
0.3567 |
1.0000 |
14398.4 |
8627.2 |
0.1554 |
0.3290 |
-0.3888 |
0.9077 |
1.0007 |
10220.0 |
6952.2 |
| Depression |
-0.0450 |
0.1285 |
-0.2868 |
0.2122 |
1.0001 |
12973.0 |
9017.5 |
0.2824 |
0.3229 |
-0.2866 |
0.9845 |
1.0001 |
8208.7 |
7287.8 |
| Diabetes |
-0.0921 |
0.1246 |
-0.3332 |
0.1600 |
1.0001 |
11529.1 |
8523.1 |
0.2103 |
0.2291 |
-0.2175 |
0.6871 |
1.0003 |
10038.8 |
8217.5 |
| Gender |
-0.0257 |
0.1179 |
-0.2536 |
0.2125 |
1.0006 |
11947.3 |
8742.4 |
0.2107 |
0.2209 |
-0.2077 |
0.6638 |
1.0008 |
9340.2 |
8545.4 |
| Hypertension |
0.0732 |
0.1126 |
-0.1480 |
0.2969 |
1.0007 |
13561.5 |
8861.5 |
0.0373 |
0.2186 |
-0.3919 |
0.4767 |
1.0001 |
10571.8 |
8620.8 |
| Myocardial Infarction |
0.0293 |
0.1453 |
-0.2491 |
0.3259 |
1.0001 |
11403.8 |
9372.3 |
0.0575 |
0.2641 |
-0.4310 |
0.6068 |
1.0001 |
9124.3 |
7706.8 |
| Opioids |
0.2412 |
0.1148 |
0.0092 |
0.4630 |
1.0001 |
12971.3 |
8317.5 |
0.3800 |
0.2438 |
-0.1260 |
0.8311 |
1.0003 |
10288.7 |
7068.3 |
| Quetiapine |
0.3598 |
0.1135 |
0.1394 |
0.5881 |
1.0006 |
13568.9 |
8805.8 |
0.4766 |
0.2037 |
0.0857 |
0.8790 |
1.0001 |
10935.9 |
8509.2 |
| Sepsis |
0.3174 |
0.1352 |
0.0630 |
0.5982 |
1.0003 |
13171.7 |
8420.9 |
0.6023 |
0.2917 |
0.0891 |
1.2361 |
1.0008 |
9835.7 |
7022.8 |
| Stroke |
0.3502 |
0.1459 |
0.0781 |
0.6549 |
1.0004 |
10931.9 |
7207.3 |
0.4404 |
0.3018 |
-0.0984 |
1.0841 |
1.0006 |
8998.3 |
7678.7 |
Generalized Additive Modeling – Quetiapine versus Haloperidol
Quetiapine administration was associated with an increased length of stay (posterior mean = 0.48, 95% credible interval [CrI]: 0.09 to 0.88). Holding all variables constant, quetiapine increased the length of stay by 62%. That is, the baseline length of stay of 2.36 days was extended to 3.82 days (1.46 days) by quetiapine. Longer length of stay was also observed in patients with sepsis (posterior mean = 0.60, 95% CrI: 0.09 to 1.24) and those receiving benzodiazepines (posterior mean = 0.46, 95% CrI: 0.03 to 0.91). There was some indication of longer stays in patients with stroke (posterior mean = 0.44, 95% CrI: -0.10 to 1.08) and opioid use (posterior mean = 0.38, 95% CrI: -0.13 to 0.83), but the credible intervals included zero, indicating uncertainty. No strong evidence for increased length of stay was found in patients with cancer, COPD, myocardial infarction, or other comorbidities, as their CrIs included zero. The relationship between age and length of stay was modeled non-linearly with a smoothing spline hyperparameter estimate (posterior mean = 1.22, 95% CrI: 0.10 to 3.29), reflecting flexible age effects. All chains converged successfully with R̂ values of 1.00 for all parameters, indicating good mixing. Effective sample sizes (bulk and tail ESS) were high across parameters, suggesting reliable posterior estimation. See
Table 2 for details.
Sensitivity Analysis – Quetiapine versus Control
The posterior estimate for the bias parameter was close to zero (estimate = 0.012), with a wide 95% credible interval ranging from –0.95 to 1.00. Hence, any bias from unmeasured confounding appears to be minimal under the assumed moderate confounding scenario. The R̂ value was 1.00, indicating good convergence, and the bulk and tail effective sample sizes were 9,520 and 10,875, respectively, suggesting reliable posterior estimation. In summary, the findings suggest that the estimated effect of quetiapine is relatively robust to plausible levels of unknown confounding.
Sensitivity Analysis – Quetiapine versus Haloperidol
The posterior estimate for the bias parameter was close to zero (estimate = 0.005), with a wide 95% credible interval ranging from –0.97 to 0.98. This suggests little evidence of substantial bias due to unmeasured confounding. The R̂ value was 1.00, indicating good convergence, and the bulk and tail effective sample sizes were 11,177 and 12,283, respectively, supporting reliable posterior estimation.
4. Discussion
The present results indicate a significantly longer ICU stay in patients who received quetiapine relative to the control or patients who received haloperidol. These findings may guide more judicious selection of antipsychotic medications in the ICU; potentially reducing unnecessary risks and costs resulting from prolonged ICU stays.
The working group of the current delirium treatment guideline from Society of Critical Care Medicine [
6] concluded that there is insufficient evidence to
either support or refute the use of antipsychotic agents in ICU patients with delirium. This recommendation is based on the general lack of positive
results from rigorous haloperidol clinical trials
and the absence of well-powered studies involving other antipsychotic agents. For example, investigators of the double-blind, placebo-controlled, randomized Modifying the Incidence of Delirium (MIND) clinical trial [
11] administered haloperidol to
medical/surgical ICU patients with delirium across six tertiary care medical centers in the United States
. They reported no significant difference in ICU length of stay (
a secondary outcome) between the 179 patients who received placebo and the 192 who received haloperidol. Similarly, in the large Agents Intervening against Delirium in Intensive Care Unit (AID-ICU) trial, there was no statistically significant difference in
the length of hospital stay (a primary outcome) between 501 patients receiving haloperidol and
472 patients receiving placebo in this randomized, double-blind fashion conducted across 18 general intensive care units (ICUs) in Europe [
9]. Examining quetiapine, a retrospective study involving 47 ICU patients in Saudi Arabia reported no difference in the ICU length of stay among patients treated with quetiapine, haloperidol, risperidone, or olanzapine [
16]
– a finding similar to a prospective clinical trial conducted in Thailand involving 52 patients who received either quetiapine or haloperidol [
17]
. Conversely,
Zachary et[
18]
al reported a reduced ICU stay in 100 patients in Egypt randomized to haloperidol or placebo in a double-blind randomized fashion. Results of the study reported here differ from these three studies evaluating quetiapine vs haloperidol as we found that quetiapine extends the stay in the ICU relative to both haloperidol and control groups.
Although delirium involves dysfunction across multiple neurotransmitter systems, anticholinergic activity appears to play a particularly prominent role. This is suggested by the central cholinergic deficit hypothesis of delirium [
19], and supported by the finding of a greater likelihood for developing in-hospital delirium in patients with low pre-admission anticholinesterase levels [
20]. Further support comes from a study of 278 inpatients with delirium in whom exposure to medications with anticholinergic properties was independently associated with a subsequent increase in delirium symptom severity [
21]
. Notably, this association remained significant after adjusting for the initial severity of delirium, presence of comorbid conditions, as well as medications, suggesting that delirium is specific to the intake of medications with pronounced anticholinergic effects
.
In fact, the metabolite of quetiapine
norquetiapine exhibits appreciable affinity for
muscarinic receptors contributing to a
greater anticholinergic burden (e.g., sedation, dry mouth, and cognitive impairment) relative to
haloperidol,
risperidone, and
aripiprazole, which have
minimal to negligible muscarinic receptor affinity[
22,
23]. This, along with a strong antihistaminergic (H1-blocking) activity by quetiapine that causes sedation, may represent a mechanism of the quetiapine’s negative effect on the ICU discharge in patients with delirium reported in the present study.
It should be noted that the present findings apply to a subgroup of patients with delirium due to known physiological condition, vascular dementia, subacute delirium, and senile dementia with delirium. Delirium can also manifest from substance withdrawal, or acute effects of medications which may account for up to 39% of delirium cases[
24]. Hence, our study is limited in generalizability, which presents a limitation. Furthermore, though
selection bias can be minimized in emulated clinical trials such as the ones presented here, it cannot be
entirely eliminated. To deal with this, we have implemented
explicit inclusion/exclusion criteria that would be used in a hypothetical randomized controlled trial, thereby reducing arbitrary or biased selection. These study limitations should be evaluated against the strengths of the present study, such as a relatively large sample size, comparison of quetiapine to both control as well as haloperidol conditions, successful propensity score matching, and robustness of study findings as indicated by the results of our sensitivity analysis.
In conclusion, the present findings do not support administration of quetiapine to patients with delirium that is due to an underlying physiological condition. A relatively strong anticholinergic and antihistaminic profile of quetiapine may adversely affect the course of ICU stay, thereby exposing patients to risks and costs associated with spending more time in the ICU.
Author Contributions
Conceptualization, A.H.; methodology, A.H.; software, A.H.; validation, A.H.; formal analysis, A.H.; investigation, A.H.; resources, A.H. , J.D.; data curation, A.H.; writing—original draft preparation, A.H.; writing—review and editing, A.H.; visualization, A.H.; supervision, A.H.; project administration, A.H.; funding acquisition A.H., J.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study did not require Ethics Approval.
Informed Consent Statement
Deidentified data are available on PhysioNet upon signing Data Use Agreements and completing the required training. The Beth Israel Deaconess Medical Center and Massachusetts Institute of Technology approved the release of the deidentified and publicly available data without a requirement for individual patient informed consent.
Data Availability Statement
Restrictions apply to the availability of these data. Data were obtained from physionet.org and are available from physionet.org with the permission of physionet.org.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Slooter, A.J.C.; Otte, W.M.; Devlin, J.W.; Arora, R.C.; Bleck, T.P.; Claassen, J.; Duprey, M.S.; Ely, E.W.; Kaplan, P.W.; Latronico, N.; et al. Updated Nomenclature of Delirium and Acute Encephalopathy: Statement of Ten Societies. Intensive Care Med 2020, 46, 1020–1022. [Google Scholar] [CrossRef] [PubMed]
- Salluh, J.I.F.; Wang, H.; Schneider, E.B.; Nagaraja, N.; Yenokyan, G.; Damluji, A.; Serafim, R.B.; Stevens, R.D. Outcome of Delirium in Critically Ill Patients: Systematic Review and Meta-Analysis. BMJ 2015, 350, h2538. [Google Scholar] [CrossRef]
- Hshieh, T.T.; Inouye, S.K.; Oh, E.S. Delirium in the Elderly. Clin Geriatr Med 2020, 36, 183–199. [Google Scholar] [CrossRef] [PubMed]
- Stollings, J.L.; Kotfis, K.; Chanques, G.; Pun, B.T.; Pandharipande, P.P.; Ely, E.W. Delirium in Critical Illness: Clinical Manifestations, Outcomes, and Management. Intensive Care Med 2021, 47, 1089–1103. [Google Scholar] [CrossRef]
- American Psychiatric Association Diagnostic and Statistical Manual of Mental Disorders; Fifth Edition.; American Psychiatric Association, 2013; ISBN 978-0-89042-555-8.
- Lewis, K.; Balas, M.C.; Stollings, J.L.; McNett, M.; Girard, T.D.; Chanques, G.; Kho, M.E.; Pandharipande, P.P.; Weinhouse, G.L.; Brummel, N.E.; et al. A Focused Update to the Clinical Practice Guidelines for the Prevention and Management of Pain, Anxiety, Agitation/Sedation, Delirium, Immobility, and Sleep Disruption in Adult Patients in the ICU. Crit Care Med 2025, 53, e711–e727. [Google Scholar] [CrossRef] [PubMed]
- Rhee, J.Y.; Colman, M.A.; Mendu, M.; Shah, S.J.; Fox, M.D.; Rost, N.S.; Kimchi, E.Y. Associations Between Stroke Localization and Delirium: A Systematic Review and Meta-Analysis. J Stroke Cerebrovasc Dis 2022, 31, 106270. [Google Scholar] [CrossRef]
- Alıcı, Ş.; Öztürk Birge, A. The Frequency of Sepsis-Associated Delirium in Intensive Care Unit and Its Effect on Nurse Workload. J Clin Nurs 2025, 34, 1383–1397. [Google Scholar] [CrossRef]
- Andersen-Ranberg, N.C.; Poulsen, L.M.; Perner, A.; Wetterslev, J.; Estrup, S.; Hästbacka, J.; Morgan, M.; Citerio, G.; Caballero, J.; Lange, T.; et al. Haloperidol for the Treatment of Delirium in ICU Patients. N Engl J Med 2022, 387, 2425–2435. [Google Scholar] [CrossRef]
- Smit, L.; Slooter, A.J.C.; Devlin, J.W.; Trogrlic, Z.; Hunfeld, N.G.M.; Osse, R.J.; Ponssen, H.H.; Brouwers, A.J.B.W.; Schoonderbeek, J.F.; Simons, K.S.; et al. Efficacy of Haloperidol to Decrease the Burden of Delirium in Adult Critically Ill Patients: The EuRIDICE Randomized Clinical Trial. Crit Care 2023, 27, 413. [Google Scholar] [CrossRef]
- Girard, T.D.; Exline, M.C.; Carson, S.S.; Hough, C.L.; Rock, P.; Gong, M.N.; Douglas, I.S.; Malhotra, A.; Owens, R.L.; Feinstein, D.J.; et al. Haloperidol and Ziprasidone for Treatment of Delirium in Critical Illness. N Engl J Med 2018, 379, 2506–2516. [Google Scholar] [CrossRef]
- Dziegielewski, C.; Skead, C.; Canturk, T.; Webber, C.; Fernando, S.M.; Thompson, L.H.; Foster, M.; Ristovic, V.; Lawlor, P.G.; Chaudhuri, D.; et al. Delirium and Associated Length of Stay and Costs in Critically Ill Patients. Crit Care Res Pract 2021, 2021, 6612187. [Google Scholar] [CrossRef] [PubMed]
- Vasilevskis, E.E.; Chandrasekhar, R.; Holtze, C.H.; Graves, J.; Speroff, T.; Girard, T.D.; Patel, M.B.; Hughes, C.G.; Cao, A.; Pandharipande, P.P.; et al. The Cost of ICU Delirium and Coma in the Intensive Care Unit Patient. Med Care 2018, 56, 890–897. [Google Scholar] [CrossRef]
- Ho, D.E.; Imai, K.; King, G.; Stuart, E.A. MatchIt: Nonparametric Preprocessing for Parametric Causal Inference. J. Stat. Soft. 2011, 42. [Google Scholar] [CrossRef]
- Bürkner, P.-C. Brms: An R Package for Bayesian Multilevel Models Using Stan. J. Stat. Soft. 2017, 80. [Google Scholar] [CrossRef]
- Alghadeer, S.; Almesned, R.S.; Alshehri, E.A.; Alwhaibi, A. Evaluation of the Efficacy and Safety of Quetiapine in the Treatment of Delirium in Adult ICU Patients: A Retrospective Comparative Study. JCM 2024, 13, 802. [Google Scholar] [CrossRef] [PubMed]
- Maneeton, B.; Maneeton, N.; Srisurapanont, M.; Chittawatanarat, K. Quetiapine versus Haloperidol in the Treatment of Delirium: A Double-Blind, Randomized, Controlled Trial. Drug Des Devel Ther 2013, 7, 657–667. [Google Scholar] [CrossRef] [PubMed]
- Zakhary, T.; Ahmed, I.; Luttfi, I.; Montasser, M. Quetiapine Versus Haloperidol in the Management of Hyperactive Delirium: Randomized Controlled Trial. Neurocrit Care 2024, 41, 550–557. [Google Scholar] [CrossRef]
- Coffman, J.A.; Dilsaver, S.C. Cholinergic Mechanisms in Delirium. Am J Psychiatry 1988, 145, 382–383. [Google Scholar] [CrossRef]
- Bassi, T.; Rohrs, E.; Nicholas, M.; Reynolds, S. Meta-Analysis of Serological Biomarkers at Hospital Admission for the Likelihood of Developing Delirium during Hospitalization. Front Neurol 2023, 14, 1179243. [Google Scholar] [CrossRef]
- Han, L.; McCusker, J.; Cole, M.; Abrahamowicz, M.; Primeau, F.; Elie, M. Use of Medications with Anticholinergic Effect Predicts Clinical Severity of Delirium Symptoms in Older Medical Inpatients. Arch Intern Med 2001, 161, 1099–1105. [Google Scholar] [CrossRef]
- López-Muñoz, F.; Alamo, C. Active Metabolites as Antidepressant Drugs: The Role of Norquetiapine in the Mechanism of Action of Quetiapine in the Treatment of Mood Disorders. Front Psychiatry 2013, 4, 102. [Google Scholar] [CrossRef] [PubMed]
- Chew, M.L.; Mulsant, B.H.; Pollock, B.G.; Lehman, M.E.; Greenspan, A.; Kirshner, M.A.; Bies, R.R.; Kapur, S.; Gharabawi, G. A Model of Anticholinergic Activity of Atypical Antipsychotic Medications. Schizophr Res 2006, 88, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Rothberg, M.B.; Herzig, S.J.; Pekow, P.S.; Avrunin, J.; Lagu, T.; Lindenauer, P.K. Association between Sedating Medications and Delirium in Older Inpatients. J Am Geriatr Soc 2013, 61, 923–930. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).