Submitted:
29 May 2025
Posted:
29 May 2025
You are already at the latest version
Abstract

Keywords:
1. Introduction
2. Experimental Details
2.1. Materials
2.2. Preparation of the PyC layer
2.3. In-Situ Synthesis of Sc2SnC Coating
2.4. Characterization
3. Results
3.1. PyC Pre-Film Synthesis and Characterization
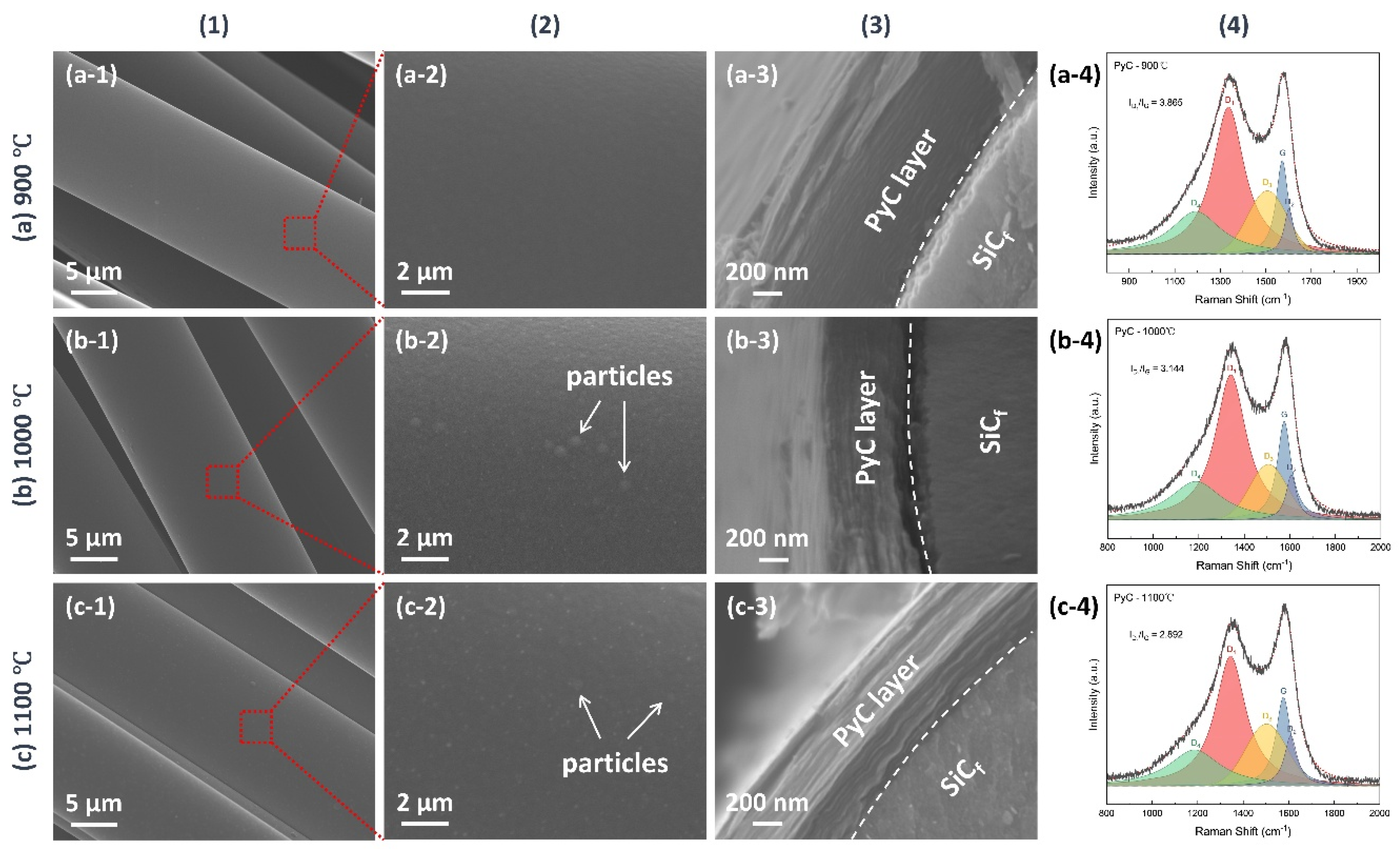
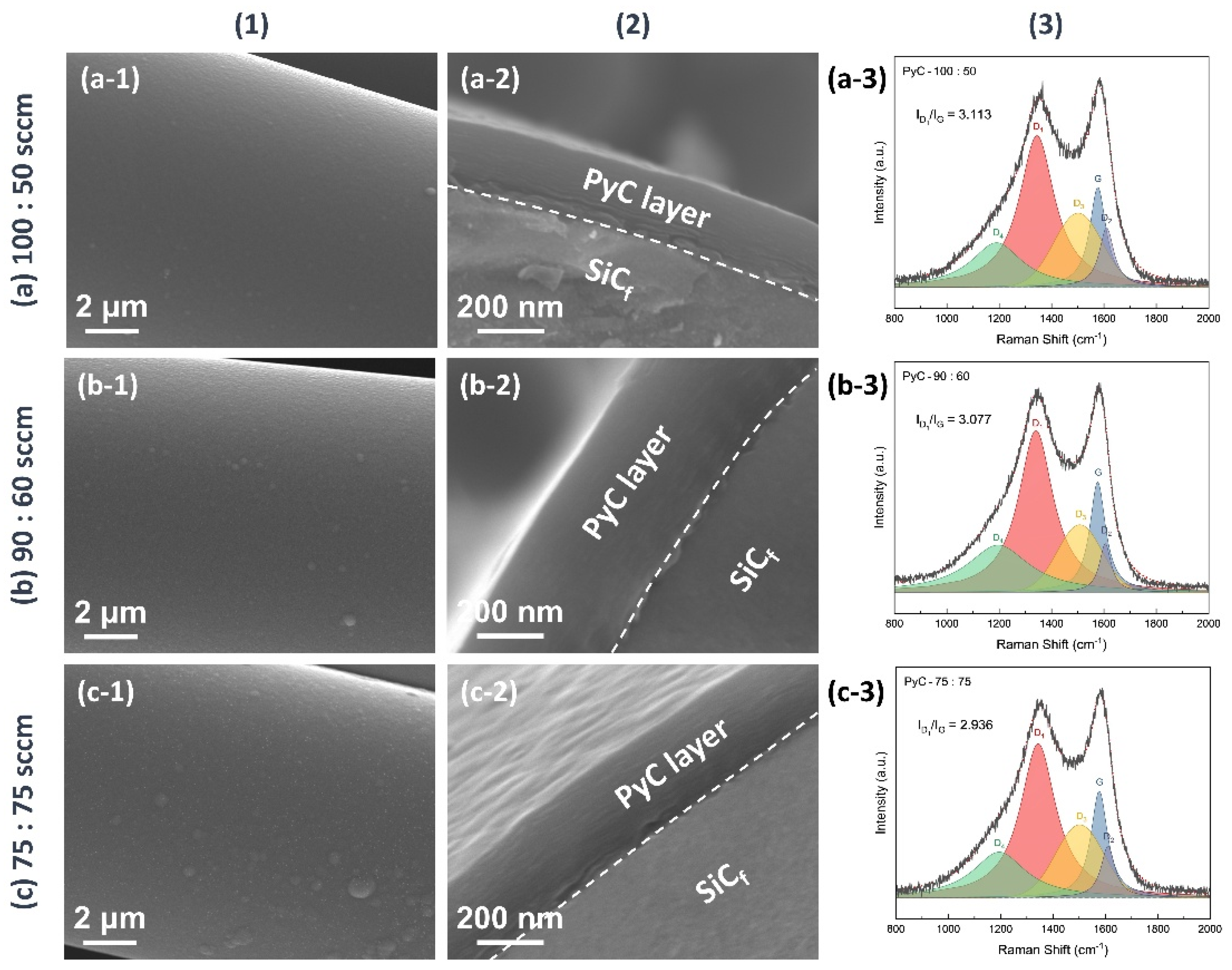
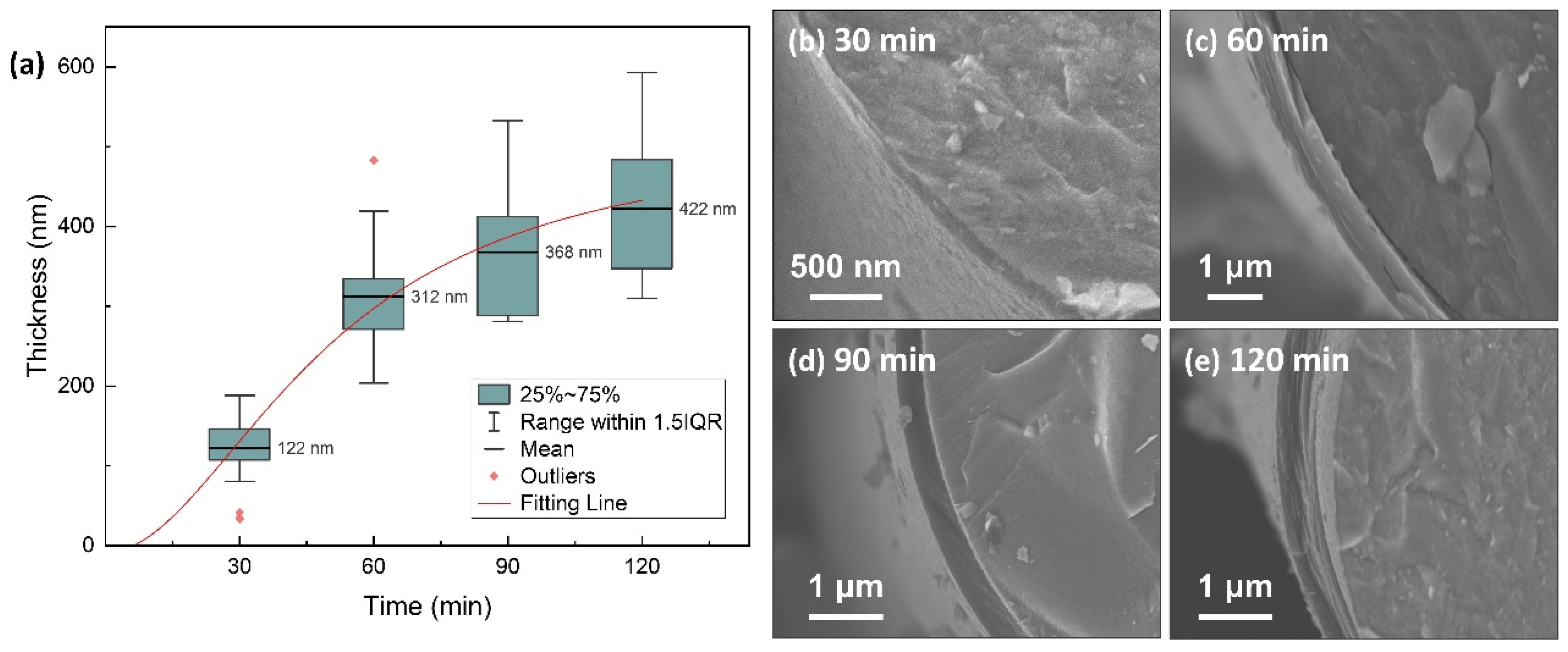
| Equation | |
| A1 | −8.957 ± 76.068 |
| A2 | 513.14 ± 77.330 |
| t0 | 50.17 ± 9.965 |
| p | 1.954 ± 0.656 |
| R-Square | 0.73704 |
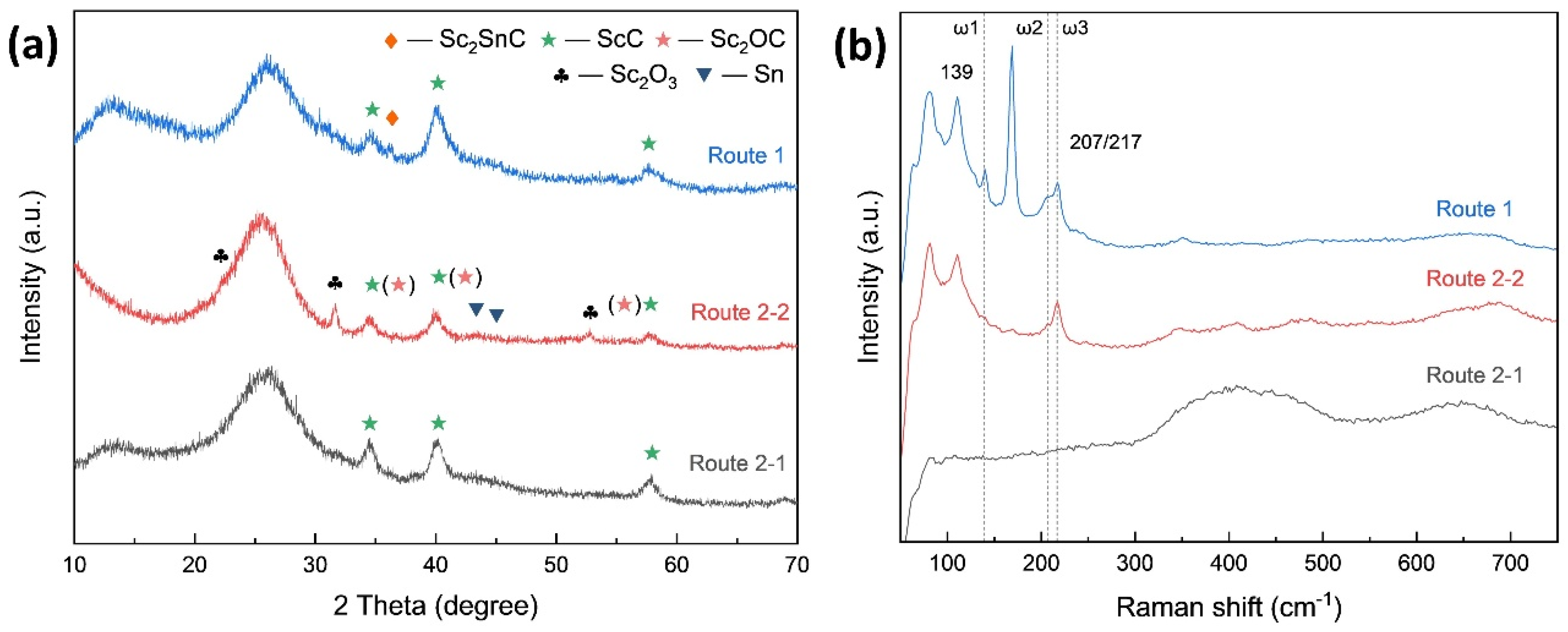
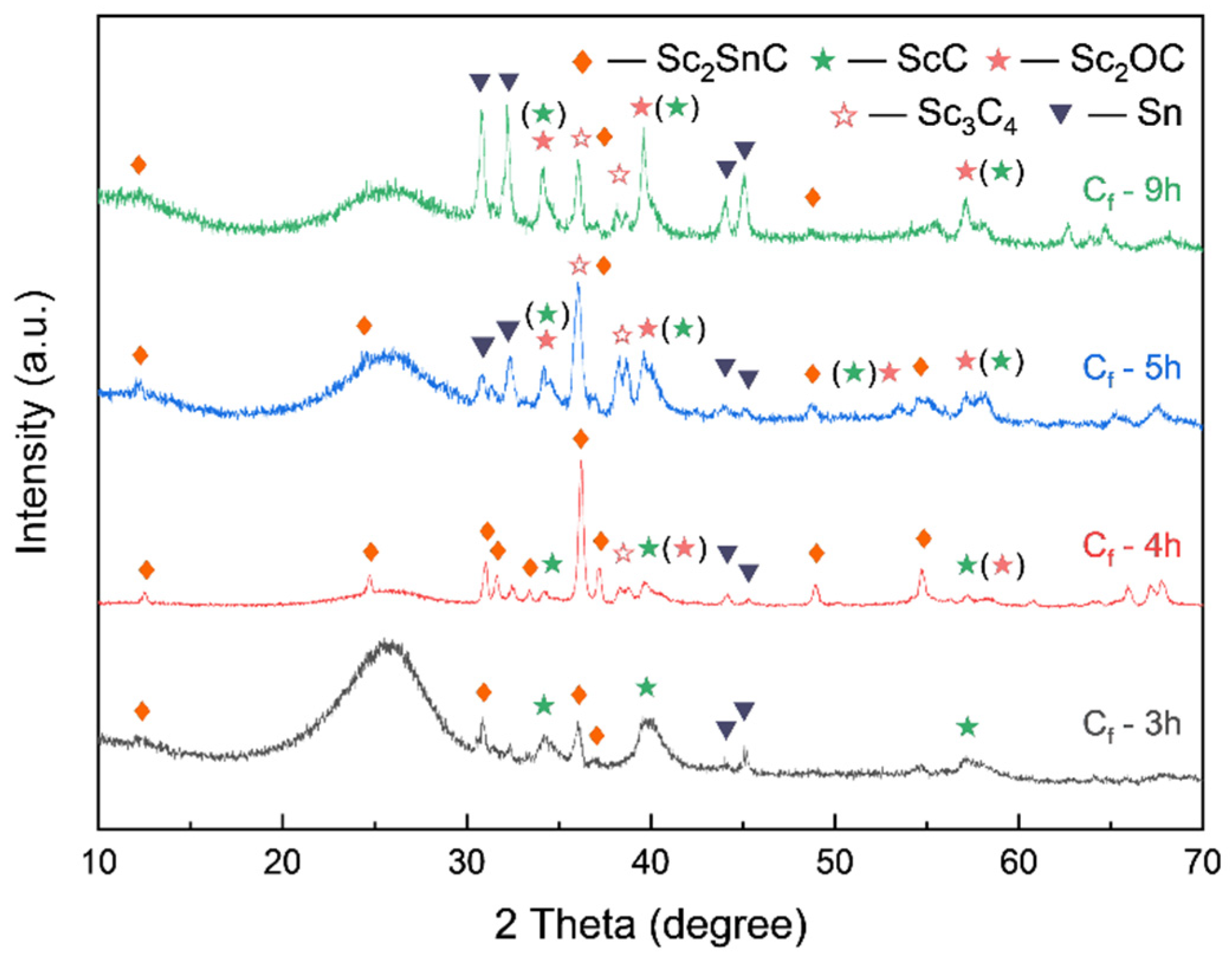
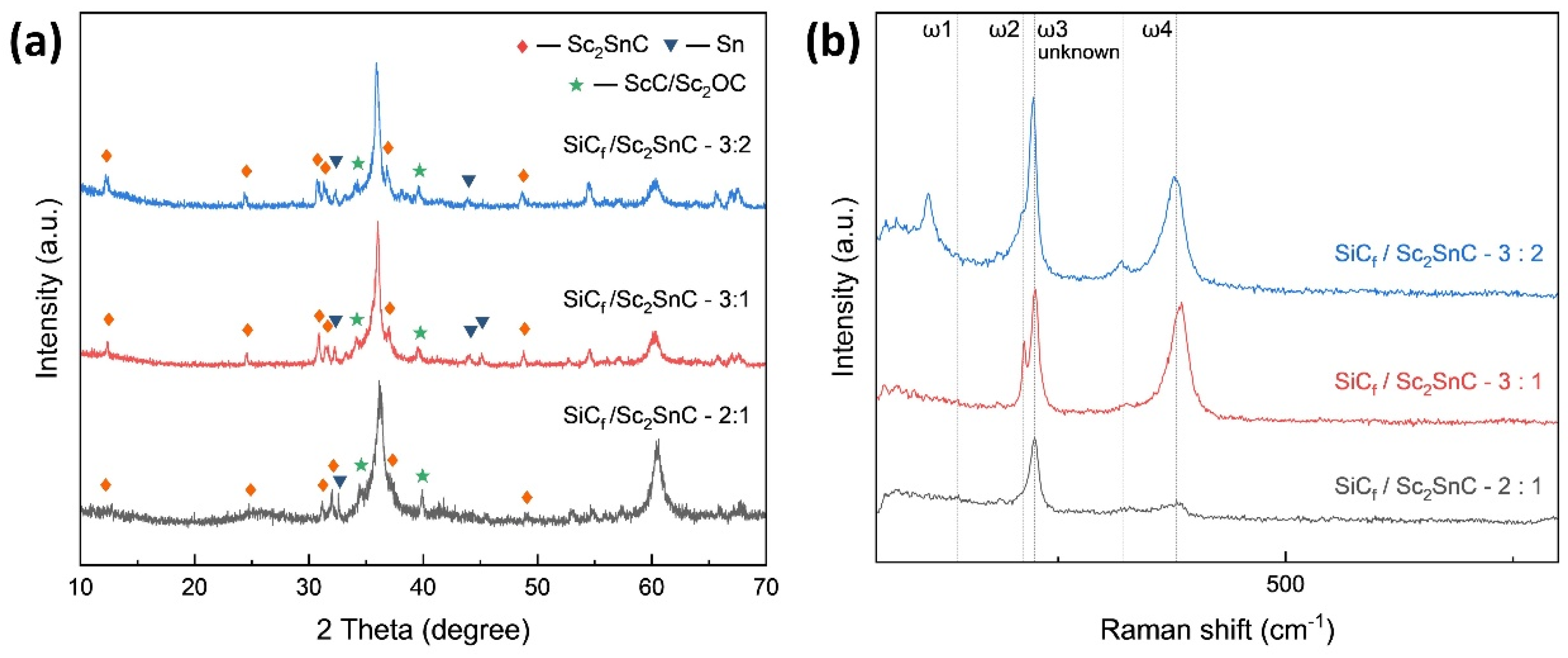
3.2. MAX Phase Coating
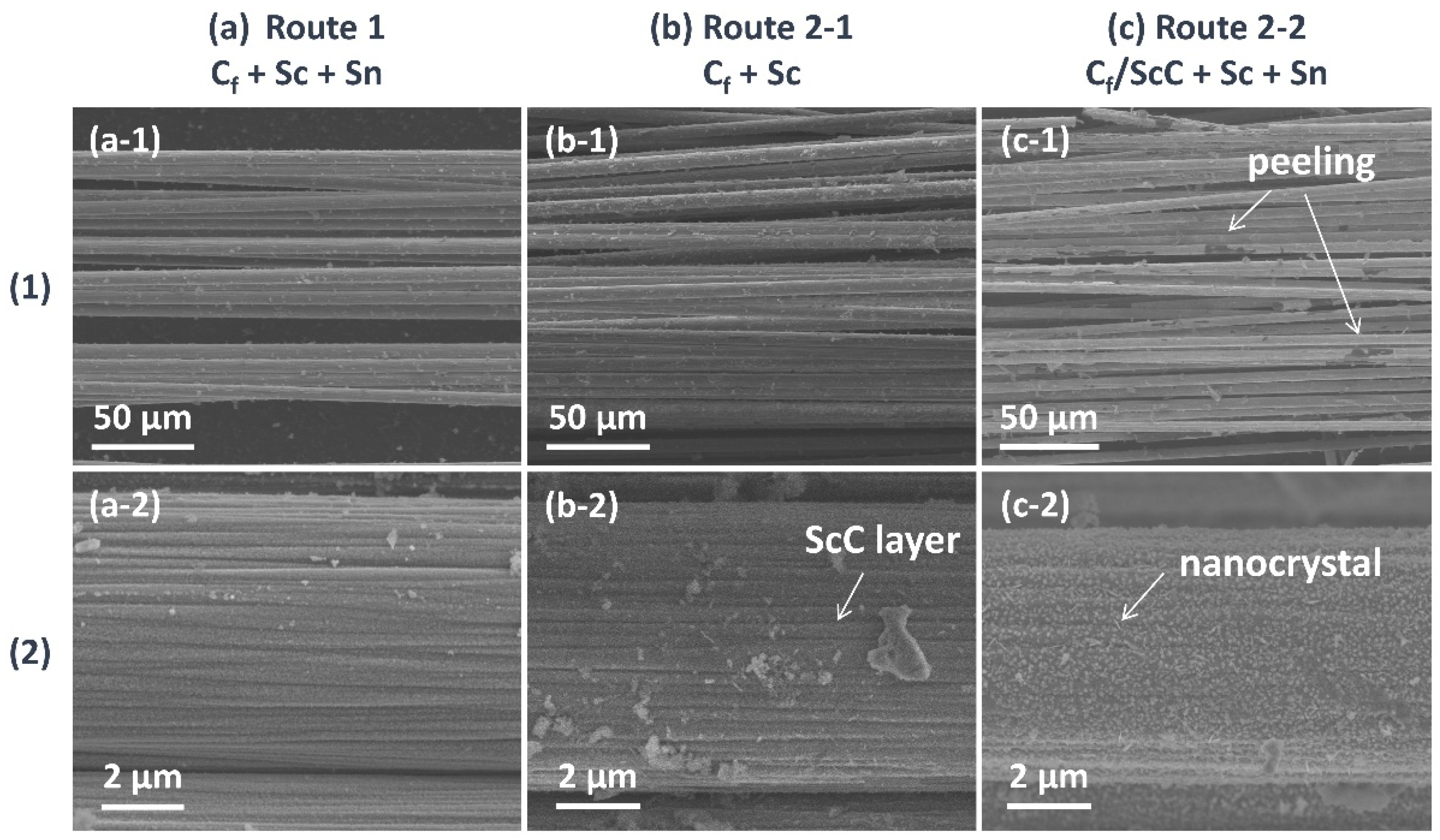
3.2.1. Carbon Fiber Coating (Cf/ScCx/Sc2SnC)
- Direct Molten Salt Route: Sc, Sn, and Cf were directly mixed to form Sc2SnC.
- Two-Step Molten Salt Route: Sc and Cf were first mixed to form an intermediate phase, ScC, followed by the introduction of Sn to synthesize Sc2SnC.
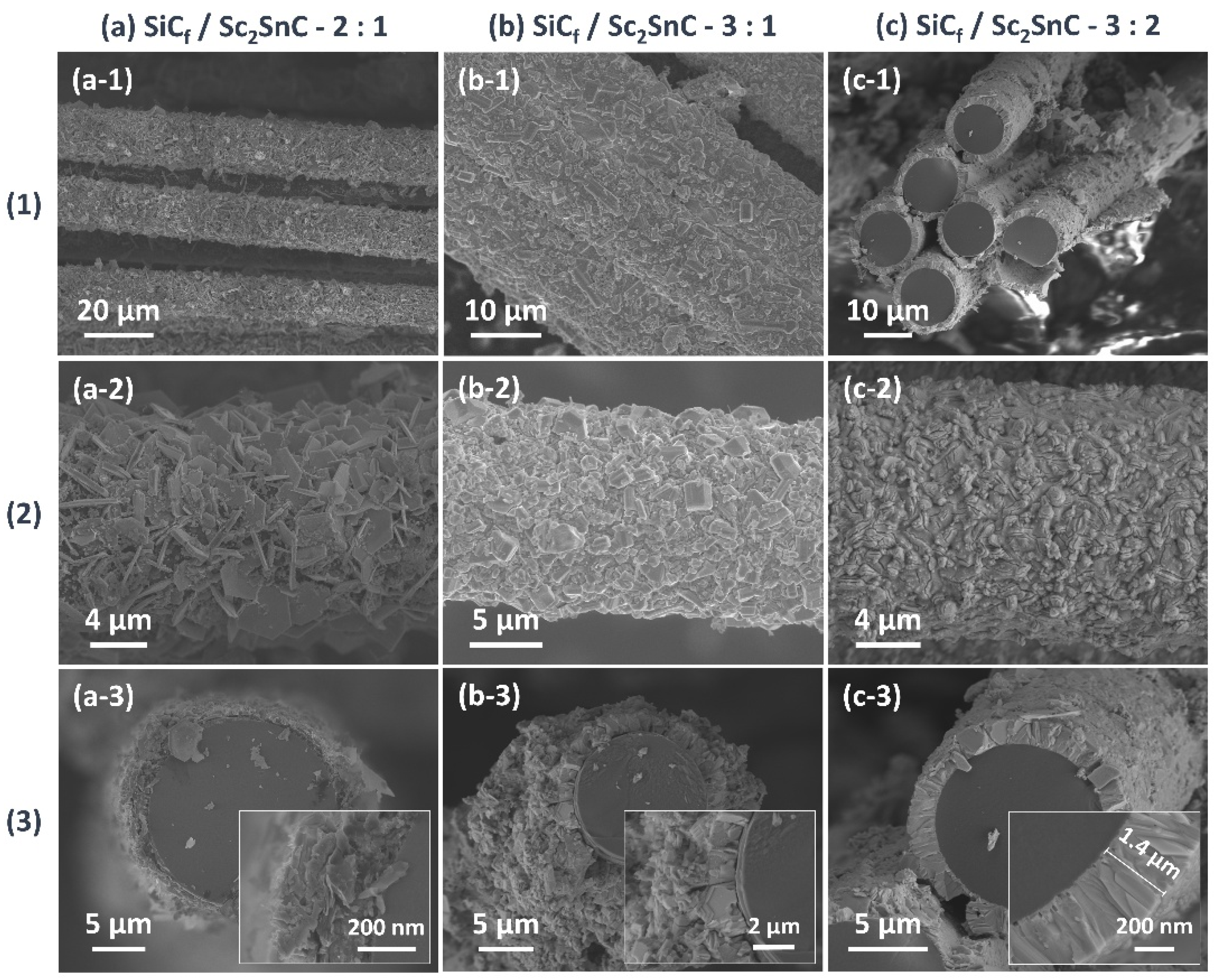
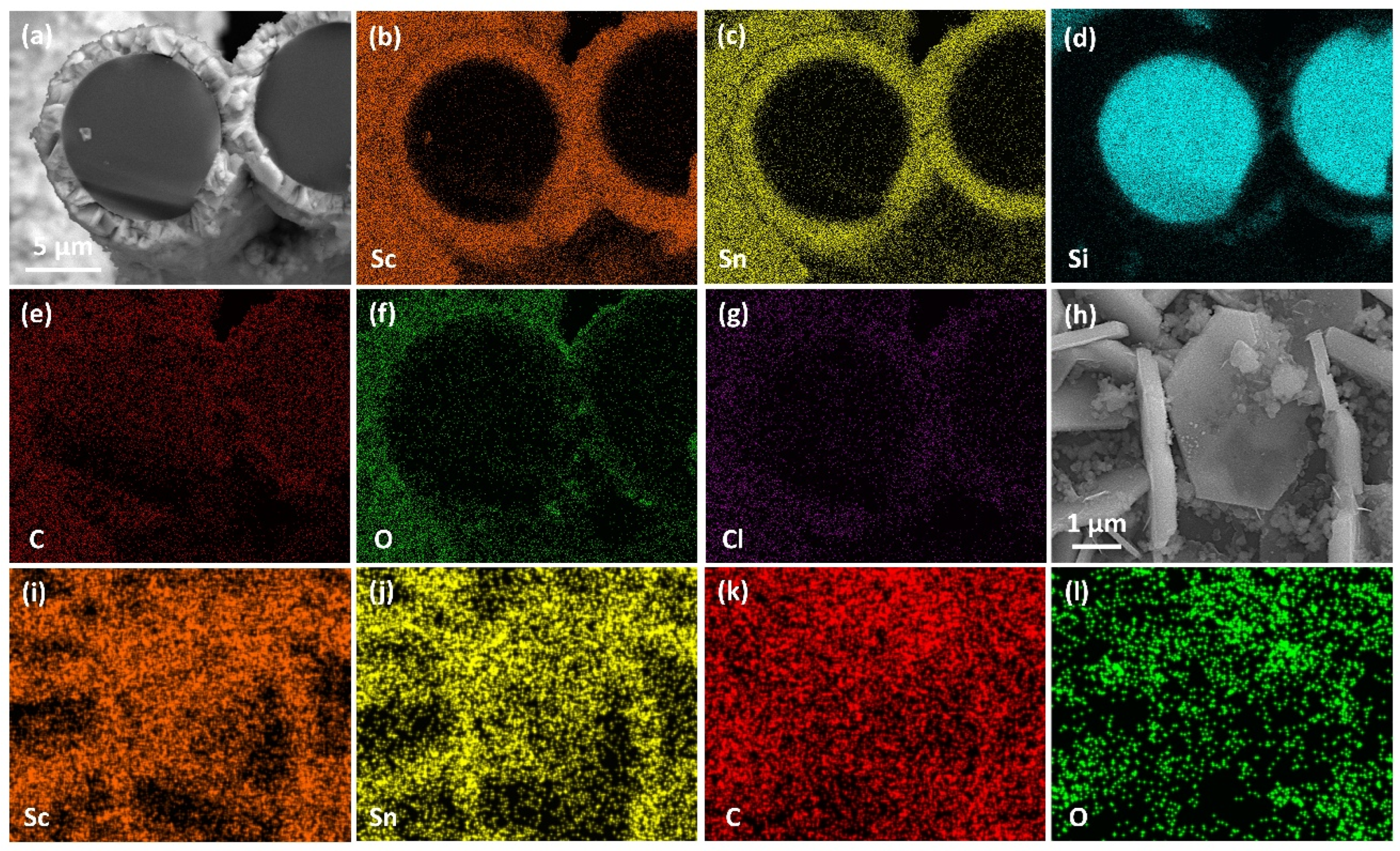
3.2.2. Silicon Carbide Fiber Coating (SiCf/ScCx/Sc2SnC)
4. Conclusions
- (1)
- A dense and uniformly coated PyC layer was successfully deposited on SiCf via CVD by precisely controlling the reaction temperature at 1000 °C and setting the CH4:C2H2 gas ratio to 90:60.
- (2)
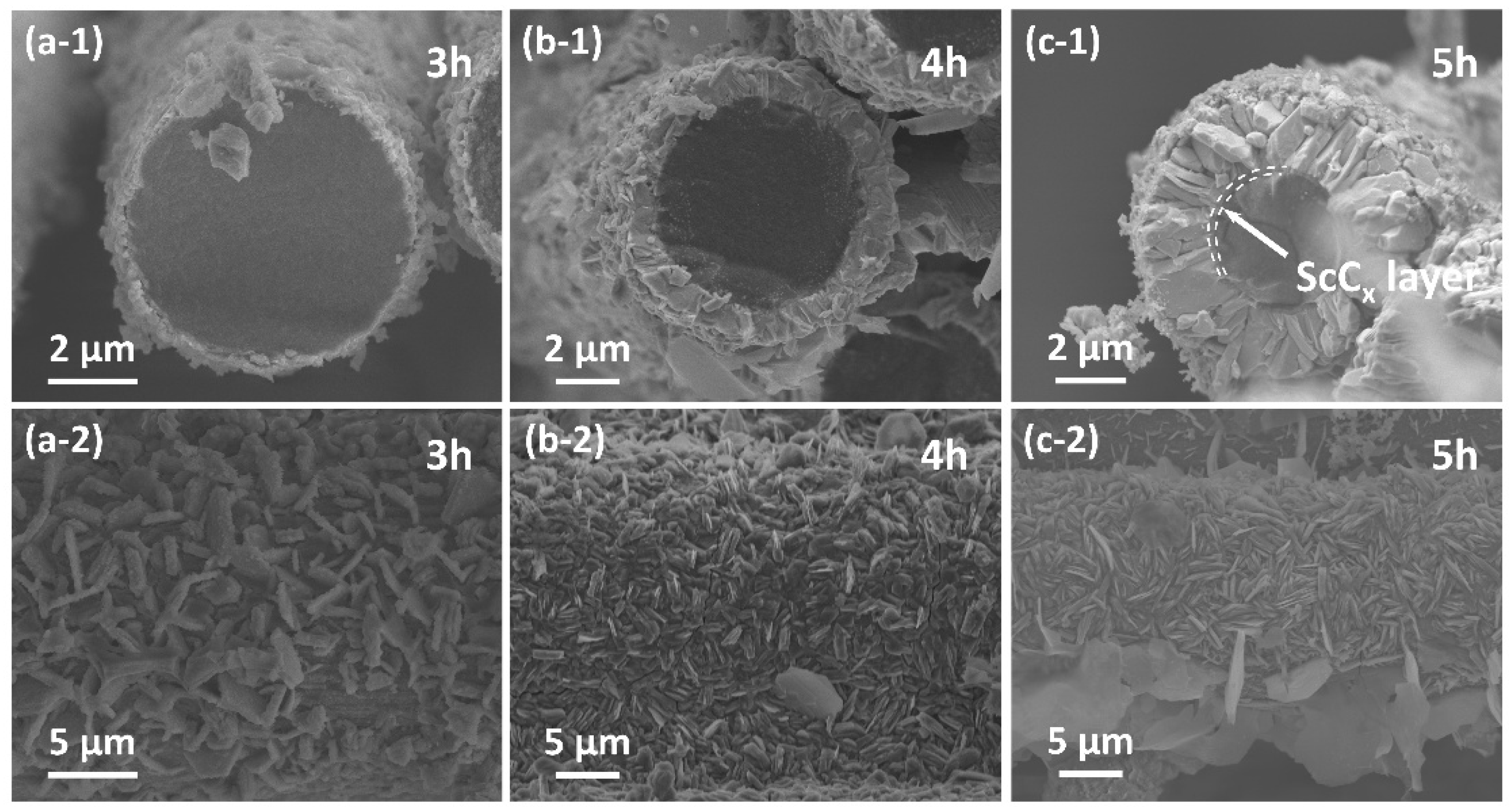
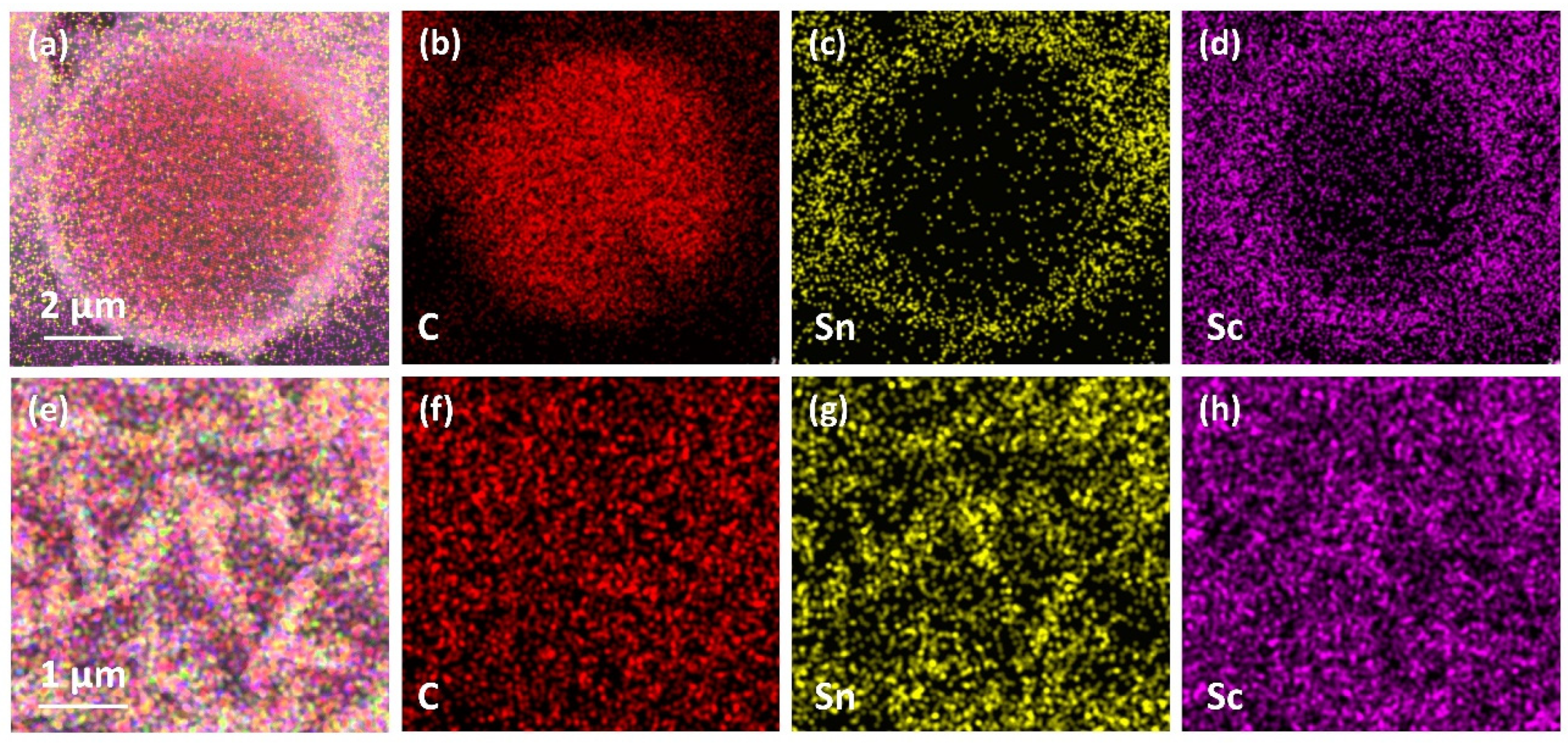
- ScCx/Sc2SnC composite coatings were synthesized on the surfaces of Cf and SiCf using a molten salt method. In the early stages of the reaction, isolated hexagonal Sc2SnC flakes nucleated and grew on the Cf surface. With extended reaction time, these nearly vertically oriented flakes gradually accumulated to form a continuous coating, with the thickness progressively increasing from 50 nm to 500 nm and ultimately to 2.9 μm.
- (3)
- During the reaction process, the formation of ScCx exhibited sluggish kinetics, making it a key intermediate that governed the overall reaction pathway. The high defect density and preferential orientation observed in ScCx contributed to the distinctive microstructure and growth direction of the resulting Sc2SnC phase. Raman spectroscopy confirmed the presence of both ScCx and Sc2SnC. However, due to its metastable nature, ScCx is prone to phase transformation into Sc2OC and Sc3C4, which may lead to cracking, pulverization, interfacial debonding, and eventual delamination of the coating—a challenge that remains difficult to mitigate.
Acknowledgments
References
- KATOH Y, OZAWA K, SHIH C, NOZAWA T, SHINAVSKI R J, HASEGAWA A, SNEAD L L. Continuous SiC fiber, CVI SiC matrix composites for nuclear applications: Properties and irradiation effects. Journal of Nuclear Materials 2014, 448, 448–476. [CrossRef]
- NASLAIN R, PAILLER R, LAMON J. Single- and Multi-Layered Interphases in SiC/SiC Composites Exposed to Severe Conditions: An Overview. Ceramics in Nuclear Applications. 2009, 1-18. [CrossRef]
- OUYANG Q, WANG Y F, XU J, LI Y S, PEI X L, MO G M, LI M A, LI P, ZHOU X B, GE F F, et al. Research Progress of SiC Fiber Reinforced SiC Composites for Nuclear Application. Journal of Inorganic Materials 2022, 37, 821–840. [CrossRef]
- DAHLQVIST M, BARSOUM M W, ROSEN J. MAX phases – Past, present, and future. Materials Today 2024, 72, 1–24. [CrossRef]
- DANG X, FAN X, YIN X, MA Y, MA X. Research Progress on Multi-functional Integration MAX Phases Modified Continuous Fiber-reinforced Ceramic Matrix Composites. Journal of Inorganic Materials 2020, 35, 29–34. [CrossRef]
- GONZALEZ-JULIAN J. Processing of MAX phases: From synthesis to applications. Journal of the American Ceramic Society 2021, 104, 659–690. [CrossRef]
- TALLMAN D J, HOFFMAN E N, CASPI E A N, GARCIA-DIAZ B L, KOHSE G, SINDELAR R L, BARSOUM M W. Effect of neutron irradiation on select MAX phases. Acta Materialia 2015, 85, 132–143. [CrossRef]
- MAGNUS C, COOPER D, JANTZEN C, LAMBERT H, ABRAM T, RAINFORTH M. Synthesis and high temperature corrosion behaviour of nearly monolithic Ti3AlC2 MAX phase in molten chloride salt. Corrosion Science 2021, 182, 109193. [CrossRef]
- ANG C, SILVA C, SHIH C, KOYANAGI T, KATOH Y, ZINKLE S J. Anisotropic swelling and microcracking of neutron irradiated Ti3AlC2–Ti5Al2C3 materials. Scripta Materialia 2016, 114, 74–78. [CrossRef]
- WANG C, YANG T, TRACY C L, LU C, ZHANG H, HU Y-J, WANG L, QI L, GU L, HUANG Q, et al. Disorder in Mn+1AXn phases at the atomic scale. Nature Communications 2019, 10, 622. [CrossRef]
- WANG J, SHU R, DONG Y, SHAO T, DENG Q H, ZHOU X B, HUANG F, DU S Y, WANG Z G, XUE J M, et al. Microstructure evolution of V2AlC coating on Zr substrate under He irradiation and their mechanical behavior. Scripta Materialia 2017, 137, 13–17. [CrossRef]
- TALLMAN D J, HE L, GARCIA-DIAZ B L, HOFFMAN E N, KOHSE G, SINDELAR R L, BARSOUM M W. Effect of neutron irradiation on defect evolution in Ti3SiC2 and Ti2AlC. Journal of Nuclear Materials 2016, 468, 194–206. [CrossRef]
- LI M, ZHOU X, YANG H, DU S, HUANG Q. The critical issues of SiC materials for future nuclear systems. Scripta Materialia 2018, 143, 149–153. [CrossRef]
- FILBERT-DEMUT I, BEI G, HöSCHEN T, RIESCH J, TRAVITZKY N, GREIL P. Influence of Ti3SiC2 Fiber Coating on Interface and Matrix Cracking in an SiC Fiber-Reinforced Polymer-Derived Ceramic. Advanced Engineering Materials 2015, 17, 1142–1148. [CrossRef]
- LI M, WANG K, WANG J, LONG D, LIANG Y, HE L, HUANG F, DU S, HUANG Q. Preparation of TiC/Ti2AlC coating on carbon fiber and investigation of the oxidation resistance properties. Journal of the American Ceramic Society 2018, 101, 5269–5280. [CrossRef]
- WANG K, LI M, LIANG Y, WANG J, HE L, DU S, HUANG Z, HUANG Q. Interface modification of carbon fibers with TiC/Ti2AlC coating and its effect on the tensile strength. Ceramics International 2019, 45, 4661–4666. [CrossRef]
- WANG J, WANG K, PEI X, LI M, YUAN Q, ZHU Y, YANG Y, ZHANG C, HE L, DU S, et al. Irradiation behavior of Cf/SiC composite with titanium carbide (TiC)-based interphase. Journal of Nuclear Materials 2019, 523, 10–15. [CrossRef]
- BARSOUM M W. The MN+1AXN phases: A new class of solids: Thermodynamically stable nanolaminates. Progress in Solid State Chemistry 2000, 28, 201–281. [CrossRef]
- LI Y B, QIN Y Q, CHEN K, CHEN L, ZHANG X, DING H M, LI M A, ZHANG Y M, DU S Y, CHAI Z F, et al. Molten Salt Synthesis of Nanolaminated Sc2SnC MAX Phase. Journal of Inorganic Materials 2021, 36, 773–778. [CrossRef]
- EKLUND P, BECKERS M, JANSSON U, HöGBERG H, HULTMAN L. The Mn+1AXn phases: Materials science and thin-film processing. Thin Solid Films 2010, 518, 1851–1878. [CrossRef]
- CHOWDHURY A, ALI M A, HOSSAIN M M, UDDIN M M, NAQIB S H, ISLAM A K M A. Predicted MAX Phase Sc2InC: Dynamical Stability, Vibrational and Optical Properties. Physica Status Solidi (B) 2018, 255, 1700235. [CrossRef]
- IVANOV L I, IVANOV V V, LAZORENKO V M, PLATOV Y M, TOVTIN V I, TOROPOVA L S. Radiation resistance and parameters of activation of aluminium-magnesium-scandium and aluminium-magnesium-vanadium alloys under neutron irradiation. Journal of Nuclear Materials 1992, 191–194, 1075–1079. [CrossRef]
- WANG C, TRACY C L, EWING R C. Radiation effects in Mn+1AXn phases. Applied Physics Reviews 2020, 7(4). [CrossRef]
- XIAO Y, MA C, XU H, LI G, LIU C, ZHENG R, LI L. Mechanical properties and microstructural evolution of Cansas-III SiC fibers after thermal exposure in different atmospheres. Ceramics International 2022, 48, 32804–32816. [CrossRef]
- MA Y, MENG X, CUI Y, KOU S, YANG S, GUO C, DENG J, FAN S. Effect of heat treatment on interface failure behavior in SiCf/PyC/SiC composites reinforced with Cansas-3 fibers. Ceramics International 2024, 50, 28102–28112. [CrossRef]
- WANG P, LIU F, WANG H, LI H, GOU Y. A review of third generation SiC fibers and SiCf/SiC composites. Journal of Materials Science & Technology 2019, 35, 2743–2750. [CrossRef]
- Narottam, P.B. Handbook of Ceramic Composites; Springer: New York, NY, USA, 2005. [Google Scholar] [CrossRef]
- BERNARD S, CORNU D, MIELE P, WEINMANN M, ALDINGER F. Polyborosilazane-Derived Ceramic Fibers in the Si-B-C-N Quaternary System for High-Temperature Applications [M]. Mechanical Properties and Performance of Engineering Ceramics and Composites: Ceramic Engineering and Science Proceedings. 2005, 35-42. [CrossRef]
- KANIYOOR A, RAMAPRABHU S. A Raman spectroscopic investigation of graphite oxide derived graphene. AIP Advances 2012, 2, 032183. [CrossRef]
- DING J, SHAO H, HU B, LIU D, SHEN L, SHEN Q. Effect of Heat Treatment on the Shear Strength of SiC/PyC/SiC Composites. Journal of Materials Engineering and Performance 2024, 33, 13803–13814. [CrossRef]
- YIFAN X, WEIJIE L, ZHONGWEI Z, XU P, YU L. Process Control of PyC Interphases Microstructure and Uniformity in Carbon Fiber Cloth. Journal of Inorganic Materials 2024, 39, 399–408. [CrossRef]
- ALEXANDER R, KAUSHAL A, RAO P T, PRAKASH J, DASGUPTA K. Identification and classification of disordered carbon materials in a composite matrix through machine learning approach integrated with Raman mapping. Diamond and Related Materials 2024, 142, 110741. [CrossRef]
- SADEZKY A, MUCKENHUBER H, GROTHE H, NIESSNER R, PöSCHL U. Raman microspectroscopy of soot and related carbonaceous materials: Spectral analysis and structural information. Carbon 2005, 43, 1731–1742. [CrossRef]
- DASH A, VAßEN R, GUILLON O, GONZALEZ-JULIAN J. Molten salt shielded synthesis of oxidation prone materials in air. Nature Materials 2019, 18, 465–470. [CrossRef]
- SUN Q, ZHU S, SHEN Z, LIU Y, WU C, KANG L, YANG Y. Molten-salt assisted synthesis of two-dimensional materials and energy storage application. Materials Today Chemistry 2023, 29, 101419. [CrossRef]
- LIU X, FECHLER N, ANTONIETTI M. Salt melt synthesis of ceramics, semiconductors and carbon nanostructures. Chemical Society Reviews 2013, 42, 8237–8265. [CrossRef]
- LI S, SONG J, CHE Y, JIAO S, HE J, YANG B. Advances in Molten Salt Synthesis of Non-oxide Materials. Energy & Environmental Materials 2023, 6, e12339. [CrossRef]
- PRESSER V, NAGUIB M, CHAPUT L, TOGO A, HUG G, BARSOUM M W. First-order Raman scattering of the MAX phases: Ti2AlN, Ti2AlC0.5N0.5, Ti2AlC, (Ti0.5V0.5)2AlC, V2AlC, Ti3AlC2, and Ti3GeC2. Journal of Raman Spectroscopy 2012, 43, 168–172. [CrossRef]
- SPANIER J E, GUPTA S, AMER M, BARSOUM M W. Vibrational behavior of the Mn+1AXn phases from first-order Raman scattering (M=Ti, V, Cr, A=Si, X=C, N). Physical Review B 2005, 71, 012103. [CrossRef]
- BENTZEL G W, NAGUIB M, LANE N J, VOGEL S C, PRESSER V, DUBOIS S, LU J, HULTMAN L, BARSOUM M W, CASPI E A N. High-Temperature Neutron Diffraction, Raman Spectroscopy, and First-Principles Calculations of Ti3SnC2 and Ti2SnC. Journal of the American Ceramic Society 2016, 99, 2233–2242. [CrossRef]
- YU J, CUI L, HE H, YAN S, HU Y, WU H. Raman spectra of RE2O3 (RE=Eu, Gd, Dy, Ho, Er, Tm, Yb, Lu, Sc and Y): laser-excited luminescence and trace impurity analysis. Journal of Rare Earths 2014, 32, 1–4. [CrossRef]
- KALEMOS A, MAVRIDIS A, HARRISON J F. Theoretical Investigation of Scandium Carbide, ScC. The Journal of Physical Chemistry A 2001, 105, 755–759. [CrossRef]
- KLEIN M V, HOLY J A, WILLIAMS W S. Raman scattering induced by carbon vacancies in TiCx. Physical Review B 1978, 17, 1546–1556. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





