1. Introduction
Adrenocortical carcinoma (ACC) is a rare and aggressive cancer of the adrenal cortex, often associated with hormonal hypersecretion and high mortality rates, particularly in its metastatic form [
1,
2]. Treatment for ACC primarily involves surgery in the early stages. Current pharmacological options are limited and are mostly used as adjuvant or palliative therapies [
1].
However, treatment remains a challenge, as ACC is typically highly resistant to chemotherapy and has a high recurrence rate following initial treatment. The 5-year survival rate is below 15% in patients with metastatic disease, depending on molecular, pathological, and clinical factors. This underscores the need to optimize existing treatments and explore new therapeutic strategies [
1,
3].
In general, the first-line treatment for patients with advanced or metastatic disease is mitotane, used either as monotherapy or in combination with chemotherapy [
4]. Mitotane is an adrenolytic drug. Its mechanism of action involves inhibition of the synthesis of glucocorticoids, mineralocorticoids, and other steroid hormones, as well as inducing destruction of adrenocortical tumor cells [
5].
Mitotane has low aqueous solubility and a high volume of distribution, with adipose tissue as its main site of distribution. This results in significant inter and intra-individual variability in bioavailability [
6]. It is administered orally, and its absorption improves with concurrent intake of high-fat foods. Its elimination half-life (t½) ranges from 18 to 159 days, with a median of 53 days [
7]. According to the drug label, the recommended initial dose is 2–3 g daily, with progressive increases to achieve therapeutic levels [
8].
Due to high inter and intra-individual pharmacokinetic variability, dosing must be individualized [
9]. For this reason, therapeutic drug monitoring (TDM) of plasma mitotane concentrations is a crucial tool to optimize therapeutic efficacy and minimize toxicity risks. TDM is recommended even in the drug’s official prescribing information.
Clinical studies have shown that maintaining minimum plasma concentrations (Cmin) within a specific therapeutic range (generally between 14–20 mg/L) significantly improves treatment response and reduces the likelihood of severe adverse effects such as neurotoxicity, hepatotoxicity, and endocrine disturbances [
11].
Plasma mitotane levels should be measured at least 12 hours after the last dose [
10].
This report presents the case of a male patient diagnosed with metastatic ACC who began treatment with mitotane in combination with chemotherapy. Cmin levels of mitotane were analyzed to ensure the efficacy and safety of treatment, and the influence of serum triglyceride and cholesterol levels on plasma concentrations of mitotane was evaluated.
2. Case Study
A 50-year-old male with a history of bilateral adrenalectomy performed five years earlier for oncocytic adrenocortical neoplasm was found on follow-up to have unresectable pulmonary and abdominal recurrence. The patient had a history of metabolic syndrome diagnosed at the age of 36, with good control of blood glucose and other cardiovascular risk factors, except for triglyceride (TAG) levels, which had fluctuated between 180 mg/dL and 350 mg/dL since diagnosis. He was receiving lipid-lowering therapy with rosuvastatin 20 mg/day and fenofibrate 145 mg/day, weighed 100 kg, was 180 cm tall, and had a body mass index (BMI) of 30.86 kg/m².
In January 2023, the patient began a monthly outpatient chemotherapy regimen with doxorubicin (40 mg/m² on day 1), etoposide (100 mg/m² on days 1, 2, and 3), and cisplatin (40 mg/m² on days 1 and 2), along with oral mitotane at 2 grams per day at home for ACC treatment. The first mitotane therapeutic drug monitoring (TDM) was conducted two weeks after treatment initiation and showed a concentration of 2.2 mg/L. As a result, the dose was increased to 4 grams per day, divided into three doses. Given the drug’s long elimination half-life (18–159 days, as indicated in the summary of product characteristics), follow-up TDM was scheduled every four weeks after dose adjustments. The patient’s baseline cholesterol level was 138 mg/dL and TAG level was 241 mg/dL. Minimum plasma concentrations (Cmin) were measured using high-performance liquid chromatography with ultraviolet detection.
During the initial months of treatment, the patient exhibited sharp fluctuations in mitotane levels [9.7–31 mg/L] in response to minor dose changes (maximum increase of 1g/day between tests), and even at the same dose. This led to the temporary suspension of mitotane on three occasions to avoid drug toxicity. The patient’s elimination half-life was estimated at 28 days. In November 2023, the final cycle of intravenous chemotherapy was administered, with mitotane monotherapy continued orally. A literature review was conducted to identify factors affecting mitotane pharmacokinetics. The patient reported no toxicity from mitotane.
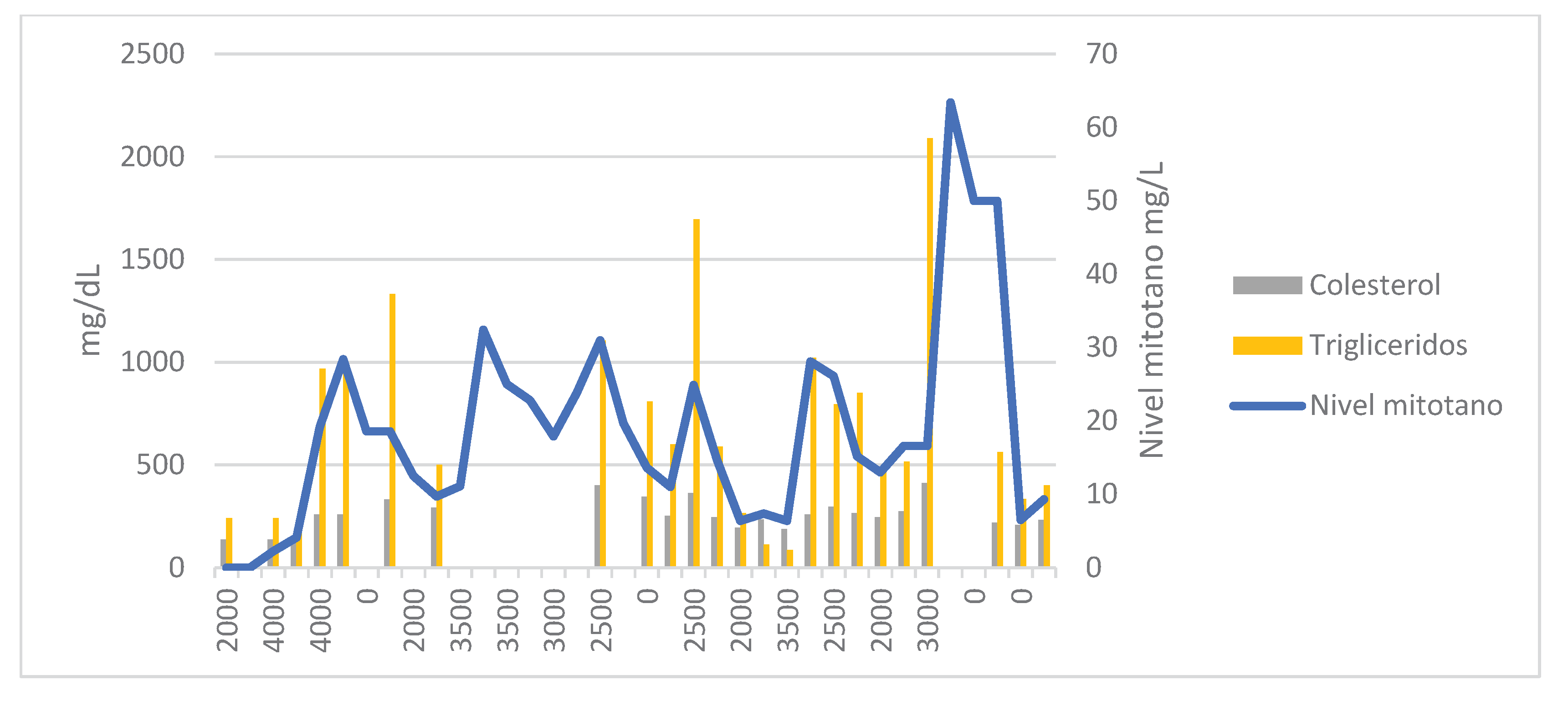
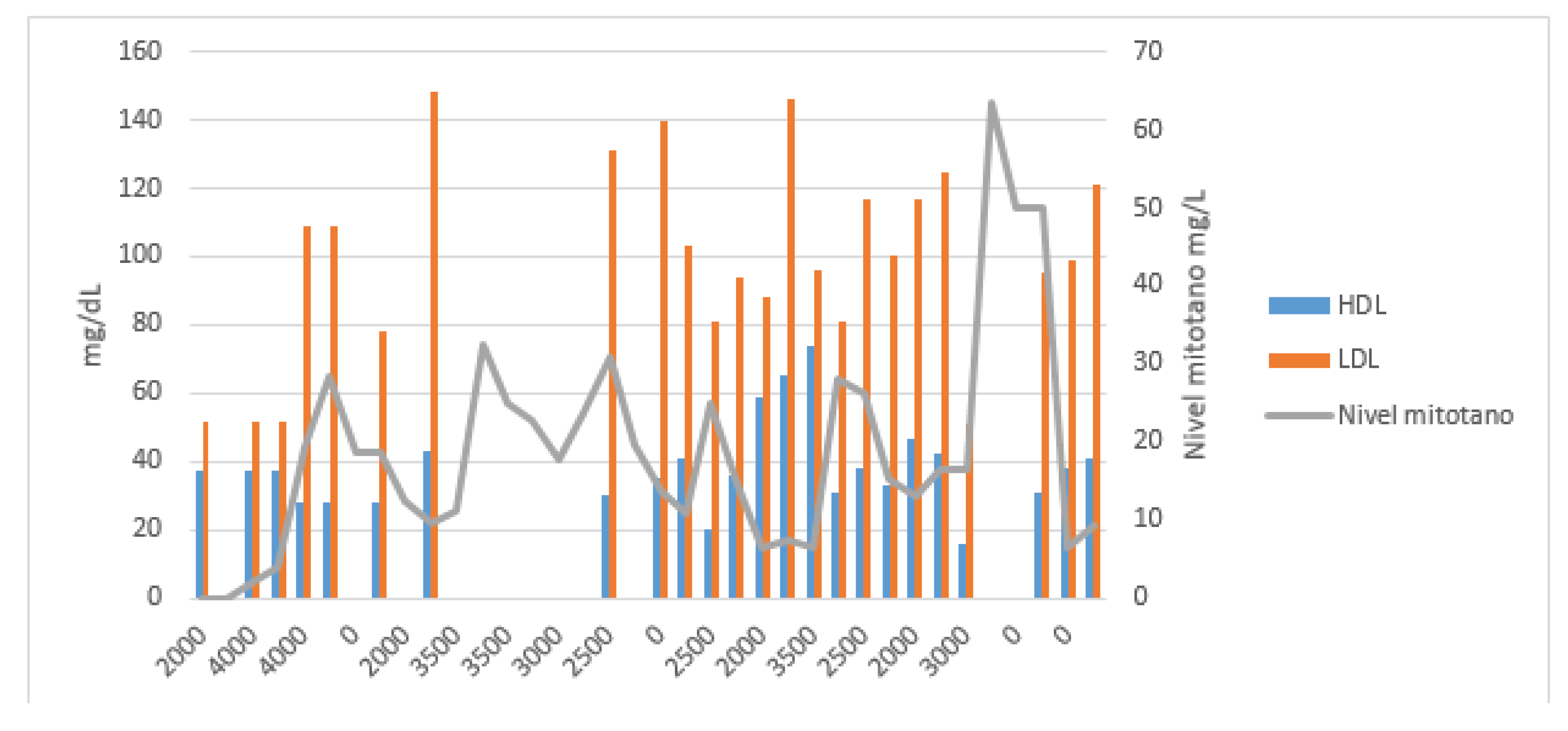
Due to a possible correlation between increased TAG and HDL levels and reduced mitotane clearance, a full lipid profile—including TAG, total cholesterol, HDL, and LDL—was ordered alongside each TDM (
Figure 1 and
Figure 2). Given mitotane’s lipophilic nature, the patient was advised to take the medication with half a glass of whole milk to minimize variability in absorption. His weight remained stable (98–102 kg), and his lipid-lowering regimen was changed to atorvastatin 80 mg/day + ezetimibe 10 mg/day + fenofibrate 250 mg/day. He was advised to limit daily caloric intake to 2000 kcal and follow a low-fat Mediterranean diet.
In the next two TDMs, increases in TAG levels were correlated with supratherapeutic mitotane concentrations. In March 2024, the patient was hospitalized in the neurosurgery department due to a cranio-cervical injury requiring microdiscectomy and anterior cervical fusion with plate placement. He remained hospitalized for 19 days, during which mitotane was continued at 2.5 g/day with a 1500 kcal/day hypocaloric diet. In the next two three-weekly tests, TAG levels were the lowest since the start of treatment [85–112 mg/dL], resulting in subtherapeutic mitotane levels (Cmin = 6.4 mg/L).
The mitotane dose was increased to 3.5 g/day, which led to a spike in TAG levels to 1022 mg/dL and a Cmin of 28.4 mg/L in the next test. Mitotane was again suspended for two weeks and restarted at 2.5 g/day. The patient was re-evaluated regarding his dietary habits. He was strongly advised to follow a fat-free diet and to take the medication with a slice of bread and olive oil. A new dose of 2 g/day was proposed, resulting in therapeutic levels of 15.2 mg/L.
In August 2024, disease progression was observed, and temozolomide (150 mg/m² for 5 days every 28 days orally) was added to the mitotane regimen due to a succinate dehydrogenase deficiency.
In October 2024, mitotane was increased to 3 g/day due to a Cmin of 13.0 mg/L (TAG = 462 mg/dL, cholesterol = 245 mg/dL). The patient reported nausea and vomiting following the initiation of temozolomide.
In January 2025, a Cmin of 63 mg/L was reported. The patient experienced grade 3 asthenia, confusion, disorientation, and grade 2 diarrhea. The last available TAG level was 2091 mg/dL, so omega-3 fatty acids were added to his treatment plan. Mitotane was again suspended for 6 weeks and then restarted at 2 g/day, which led to resolution of the neurological and gastrointestinal symptoms.
That same month, a follow-up CT scan showed disease progression. As a result, FOLFIRI was added to the treatment plan (irinotecan 180 mg/m² + folinic acid 200 mg/m² + 5-fluorouracil bolus 400 mg/m² + continuous infusion of 5-fluorouracil 2400 mg/m² over 46 hours every 14 days). The patient’s DPYD genotyping result was homozygous non-mutated (DPYD *1/*1). At this time, the patient had lost 4 kg in the past month due to clinical deterioration following drug intoxication, and maintained that weight the following month (BMI 29.63 kg/m²).
In the most recent available measurement, the patient had a Cmin of 9.3 mg/L, so the mitotane dose was increased to 2.5 g/day, with biweekly lipid profiles aligned with chemotherapy bloodwork and mitotane TDM every six weeks
3. Discussion
The high pharmacokinetic variability in mitotane Cmin levels significantly influences the potential success or failure of ACC (adrenocortical carcinoma) treatment, which currently has limited therapeutic options. Therefore, optimizing mitotane therapy through therapeutic drug monitoring (TDM) is a key tool in current clinical practice.
Published literature confirms that achieving Cmin levels between 14–20 mg/L is associated with increased treatment efficacy. Conversely, Cmin levels above 20 mg/L have been linked to the appearance of side effects such as dyslipidemia [
12].
Cazaubon et al. developed a one-compartment model for mitotane using data from patients diagnosed with ACC who had received this drug. A total of 38 patients and 503 samples were included. The study found that the variables influencing drug clearance were elevated triglyceride (TAG) and HDL levels, showing an inverse relationship with mitotane clearance [
13].
Based on this pharmacokinetic model, a pharmacogenetic study including genotypes of enzymes and transporters known to affect mitotane clearance was considered. However, due to the limited literature and the long turnaround time for these tests, it was decided to begin by requesting a lipid profile alongside each TDM, as these are routine laboratory tests.
In the presented case, high plasma TAG concentrations correlated with supratherapeutic mitotane levels, while HDL levels showed an inverse relationship. No such association was observed with LDL or total cholesterol levels. A relationship with BMI variations could not be established, as the patient maintained a stable weight throughout treatment, with only a slight 4 kg loss in the final month in a 100 kg individual.
It has been reported that mitotane treatment may increase cholesterol, triglycerides, LDL, and HDL levels. The mechanism is not well defined, but may involve mitotane-induced stimulation of HMG-CoA reductase [
14,
15]. As lipid-lowering therapy, rosuvastatin is preferred to avoid the inductive effect of mitotane on CYP3A4, which metabolizes many statins. However, in our case, switching from rosuvastatin to atorvastatin did not result in increased circulating cholesterol or LDL levels [
14].
Moreover, elevated TAG and cholesterol levels may lead to a mean overestimation of mitotane plasma levels by approximately 20% compared to normolipidemic patients. Therefore, optimized analytical techniques should be considered for dyslipidemic patients [
16]. This is particularly relevant, as in our case the patient only presented clinically significant adverse effects when Cmin exceeded 60 mg/L—no such effects were seen with Cmin between 20–35 mg/L, supporting the hypothesis of overestimation. Further clinical research is needed to determine if this has practical implications.
Close monitoring of the patient’s diet during hospitalization led to lipid profile normalization and subtherapeutic mitotane levels, highlighting the importance of ensuring adequate dietary adherence at home, and possibly implementing a food intake log.
4. Conclusions
In our case, we conclude that for patients undergoing treatment with mitotane, it is essential to routinely monitor factors that influence drug levels, such as lipid profiles, and to emphasize dietary measures in patients with dyslipidemia, along with a structured TDM protocol for the drug.
Furthermore, it is necessary to implement optimized analytical methods that prevent possible overestimation of mitotane levels in patients with hypertriglyceridemia, thereby avoiding the matrix effect and the risk of toxicity
References
- De Filpo G, Mannelli M, Canu L. Adrenocortical carcinoma: current treatment options. Curr Opin Oncol. 2020;33(1):16-22. [CrossRef]
- Del Rivero J, Else T, Hallanger-Johnson J et al. A review of mitotane in the management of adrenocortical cancer. Oncologist. 2024. [CrossRef]
- 3 Puglisi S, Calabrese A, Basile V et al. New perspectives for mitotane treatment of adrenocortical carcinoma. Best Pract Amp Res Clin Endocrinol Amp Metab. 2020;34(3):101415. [CrossRef]
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®): Neuroendocrine Tumors. Version 1.2025. Available at: https://www.nccn.org/professionals/physician_gls/pdf/neuroendocrine.pdf. Accessed April 1, 2025..
- Flauto F, De Martino MC, Vitiello C, Pivonello R, Colao A, Damiano V. A Review on Mitotane: A Target Therapy in Adrenocortical Carcinoma. Cancers. 2024;16(23):4061. [CrossRef]
- Haider MS, Ahmad T, Groll J, Scherf-Clavel O, Kroiss M, Luxenhofer R. The Challenging Pharmacokinetics of Mitotane: An Old Drug in Need of New Packaging. Eur J Drug Metab Pharmacokinet. 2021;46(5):575-593. [CrossRef]
- Moolenaar AJ, van Slooten H, van Seters AP, Smeenk D. Blood levels of o,p′-DDD following administration in various vehicles after a single dose and during long-term treatment. Chemother Pharmacol. 1981;7(1):51-54. [CrossRef]
- CIMA ::. FICHA TECNICA LYSODREN 500 mg COMPRIMIDOS. Accedido el 23 de mayo de 2025. https://cima.aemps.es/cima/dochtml/ft/04273001/FT_04273001.html.
- Yin A, Ettaieb MH, Swen JJ et al. Population Pharmacokinetic and Pharmacogenetic Analysis of Mitotane in Patients with Adrenocortical Carcinoma: Towards Individualized Dosing. Clin Pharmacokinet. 2020. [CrossRef]
- Paragliola RM, Torino F, Papi G, Locantore P, Pontecorvi A. Role of Mitotane in Adrenocortical Carcinoma – Review and State of the art. Eur Endocrinol. 2018;14(2):62. [CrossRef]
- Kerkhofs TM, Derijks LJ, Ettaieb MH et al. Short-term variation in plasma mitotane levels confirms the importance of trough level monitoring. Eur J Endocrinol. 2014;171(6):677-683. [CrossRef]
- Shawa H, Deniz F, Bazerbashi H et al. Mitotane-Induced Hyperlipidemia: A Retrospective Cohort Study. Int J Endocrinol. 2013;2013:1-7. [CrossRef]
- Cazaubon Y, Talineau Y, Feliu C et al. Population Pharmacokinetics Modelling and Simulation of Mitotane in Patients with Adrenocortical Carcinoma: An Individualized Dose Regimen to Target All Patients at Three Months? Pharmaceutics. 2019;11(11):566. [CrossRef]
- 14 Bianchini M, Puliani G, Chiefari A, Mormando M, Lauretta R, Appetecchia M. Metabolic and Endocrine Toxicities of Mitotane: A Systematic Review. Cancers. 2021;13(19):5001. [CrossRef]
- Gagnon N, Bernard S, Paquette M et al. Characterization of hyperlipidemia secondary to mitotane in adrenocortical carcinoma. Endocr Oncol. 2022;2(1):1-8. [CrossRef]
- Paci A, Hescot S, Seck A et al. Dyslipidemia causes overestimation of plasma mitotane measurements. Endocrinol Amp Metab Case Rep. 2016;2016. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).