Submitted:
22 May 2025
Posted:
23 May 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
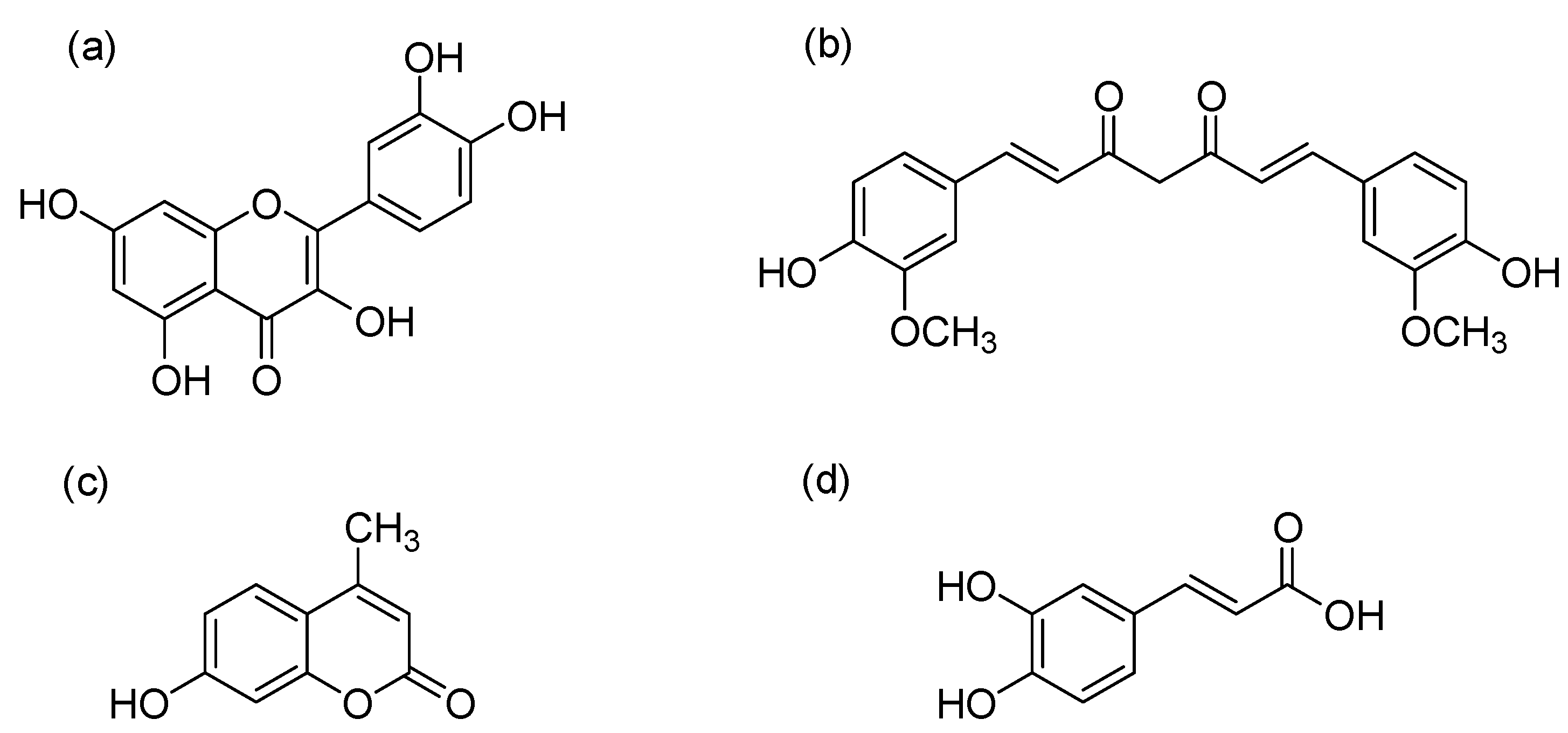
2.1. Raw Materials and Chemicals
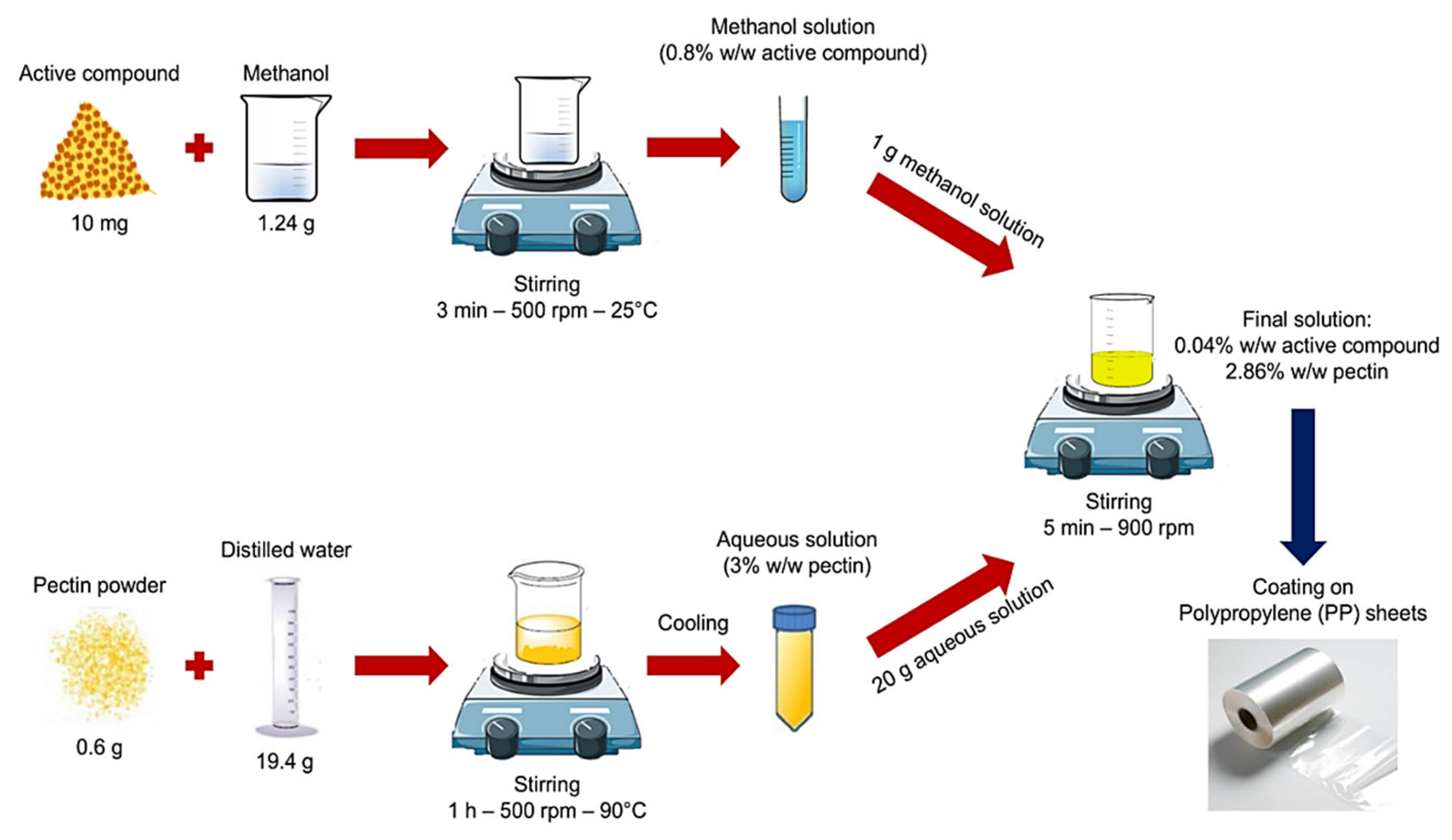
2.2. Preparation of Coating Solutions and Application to Plastic Films
2.3. Characterization of Films
2.4. Statistical Analysis
3. Results & Discussion
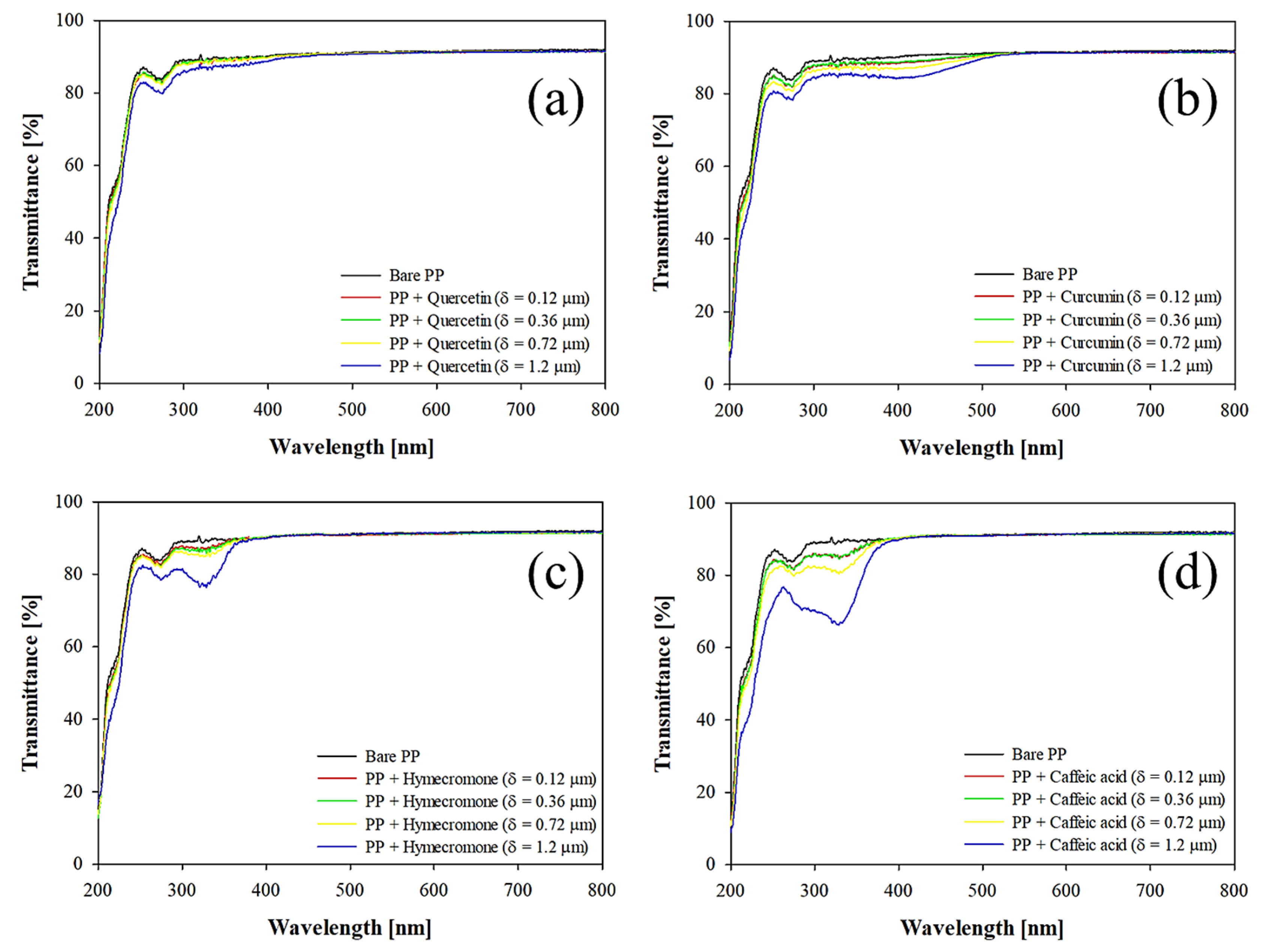
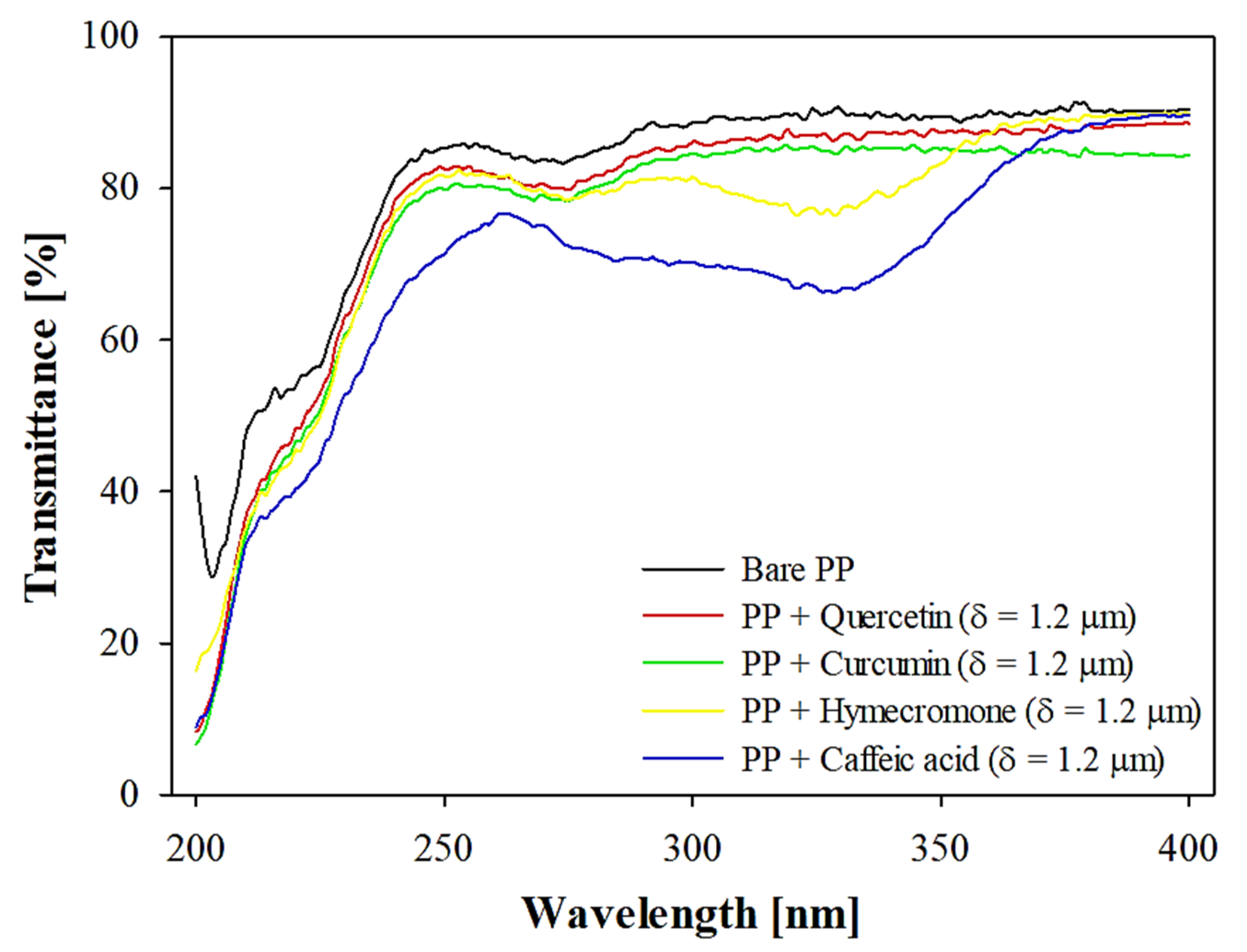
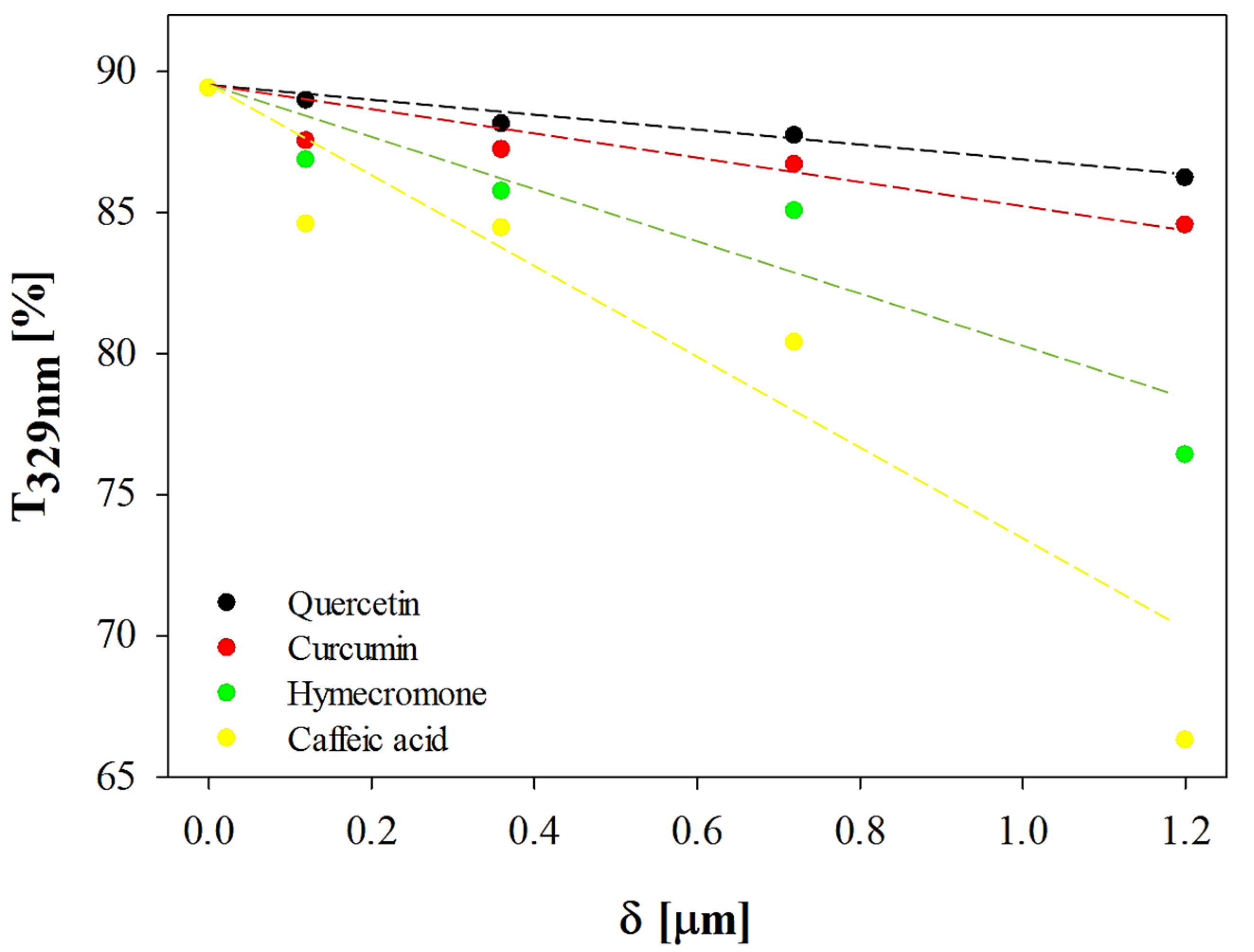
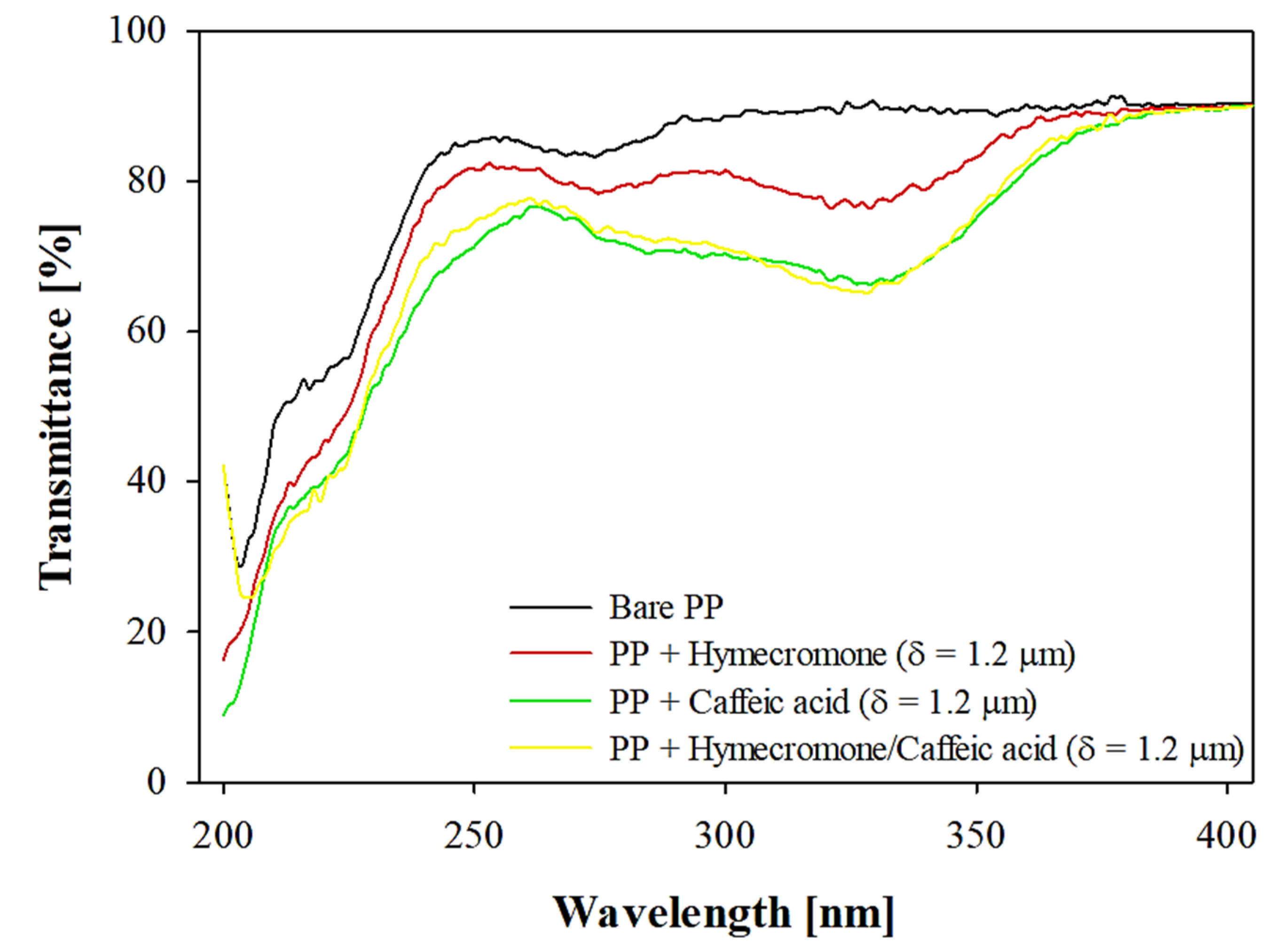
3.1. Effect of Coating Thickness on the Optical Properties of the Plastic Substrate
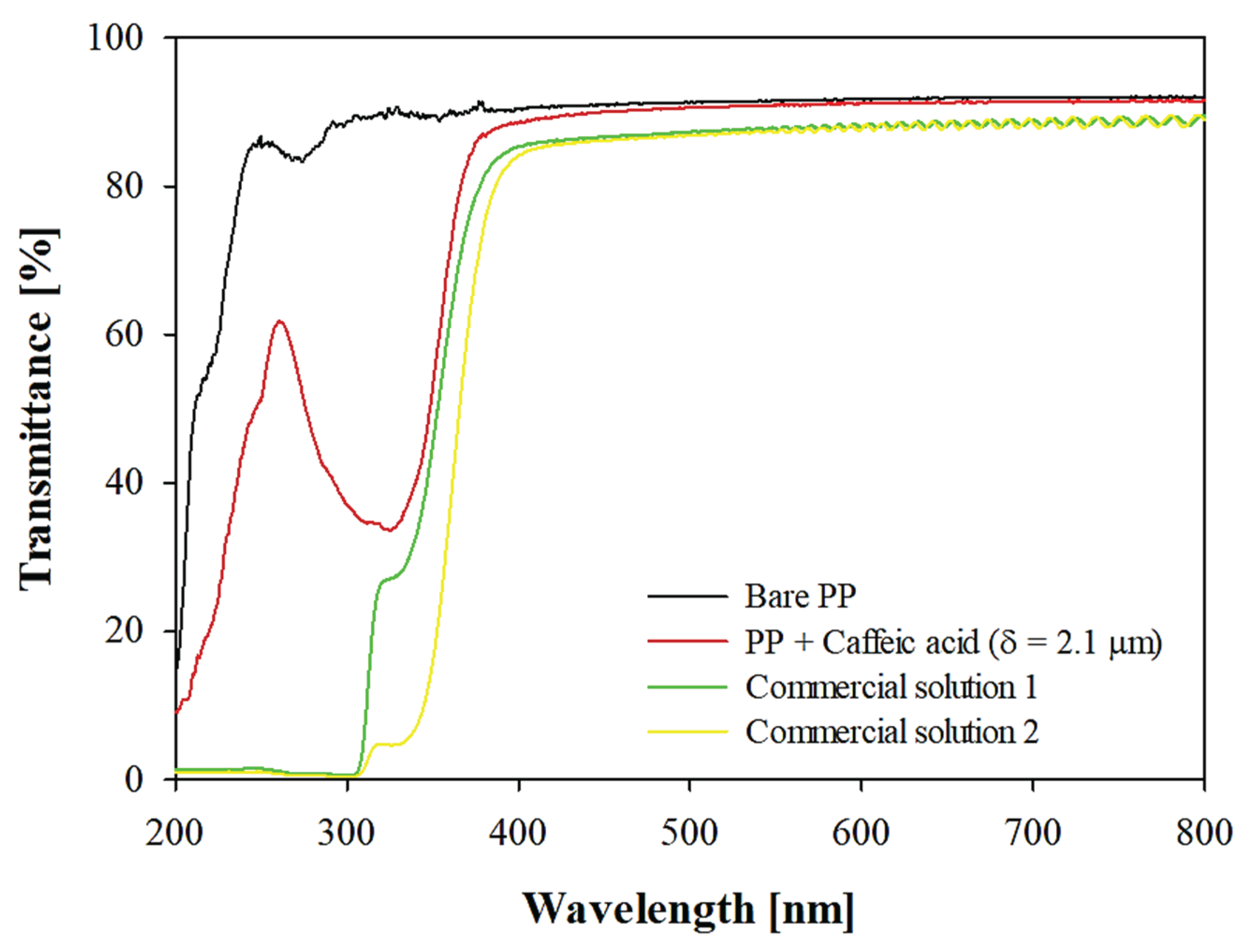
3.2. Pectin-Coated OPP Films vs. Commercial Solutions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Versino, F.; Ortega, F.; Monroy, Y.; Rivero, S.; López, O.V.; García, M.A. Sustainable and bio-based food packaging: A review on past and current design innovations. Foods 2023, 12, 1057. [Google Scholar] [CrossRef] [PubMed]
- Sani, M.A.; Khezerlou, A.; Tavassoli, M.; Abedini, A.H.; McClements, D. J. Development of sustainable UV-screening food packaging materials: A review of recent advances. Trends Food Sci. Technol. 2024, 145, 104366. [Google Scholar] [CrossRef]
- Ezati, P.; Khan, A.; Priyadarshi, R.; Bhattacharya, T.; Tammina, S.K.; Rhim, J.W. Biopolymer-based UV protection functional films for food packaging. Food Hydrocoll. 2023, 142, 108771. [Google Scholar] [CrossRef]
- Chandran, G.U.; Kumar, A.A.; Menon, S.K.; Sambhudevan, S.; Shankar, B. The potential role of flavonoids in cellulose-based biopolymeric food packaging materials for UV radiation protection. Cellulose 2024, 31, 4733 – 4773.
- Galgano, F.; Caruso, M.C.; Ventura, N.M.; Magno, C.; Favati, F. Effects of anti-UV film and protective atmosphere on fresh-cut iceberg lettuce preservation. Acta Aliment. 2017, 46, 35 – 42.
- Gore, A.H.; Prajapat, A.L. Biopolymer nanocomposites for sustainable UV protective packaging. Front. Mater. 2022, 9, 855727. [Google Scholar] [CrossRef]
- Zayat, M.; Garcia-Parejo, P.; Levy, D. Preventing UV-light damage of light sensitive materials using a highly protective UV-absorbing coating. Chem. Soc. Rev. 2007, 36, 1270–1281. [Google Scholar] [CrossRef]
- Loste, J.; Lopez-Cuesta, J.M.; Billon, L.; Garay, H.; Save, M. Transparent polymer nanocomposites: An overview on their synthesis and advanced properties. Progr. Polym. Sci. 2019, 89, 133–158. [Google Scholar] [CrossRef]
- Guzman-Puyol, S.; Hierrezuelo, J.; Benítez, J.J.; Tedeschi, G.; Porras-Vázquez, J.M.; Heredia, A., Athanassiou, A.; Romero, D.; Heredia-Guerrero, J.A. Transparent, UV-blocking, and high barrier cellulose-based bioplastics with naringin as active food packaging materials. Int. J. Biol. Macromol. 2022, 209, 1985-1994.
- Störmer, A.; Bott, J.; Kemmer, D.; Franz, R. Critical review of the migration potential of nanoparticles in food contact plastics. Trends Food Sci. Technol. 2017, 63, 39–50. [Google Scholar] [CrossRef]
- Roy, S.; Ramakrishnan, R.; Goksen, G.; Singh, S.; Lopusiewicz, L. Recent progress on UV-light barrier food packaging films–a systematic review. IFSET 2023, 103550. [Google Scholar] [CrossRef]
- Zhu, F. Polysaccharide based films and coatings for food packaging: Effect of added polyphenols. Food Chem. 2021, 359, 129871. [Google Scholar] [CrossRef]
- Kaczmarek-Szczepańska, B.; Grabska-Zielińska, S.; Michalska-Sionkowska, M. The Application of Phenolic Acids in The Obtainment of Packaging Materials Based on Polymers—A Review. Foods 2023, 12, 1343. [Google Scholar] [CrossRef]
- Canales, D.; Montoille, L.; Rivas, L.M.; Ortiz, J.A.; Yañez-S, M.; Rabagliati, F.M.; Ulloa, M.T.; Alvarez, E.; Zapata, P.A. Fungicides Films of Low-Density Polyethylene (LDPE)/Inclusion Complexes (Carvacrol and Cinnamaldehyde) Against Botrytis Cinerea. Coatings 2019, 9, 795. [Google Scholar] [CrossRef]
- Quiles-Carrillo, L.; Montava-Jordà, S.; Boronat, T.; Sammon, C.; Balart, R.; Torres-Giner, S. On the use of gallic acid as a potential natural antioxidant and ultraviolet light stabilizer in cast-extruded bio-based high-density polyethylene films. Polymers 2019, 12, 31. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Jaiswal, A.K.; Duffy, B.; Jaiswal, S. Ferulic acid incorporated active films based on poly (lactide)/poly (butylene adipate-co-terephthalate) blend for food packaging. Food Packag. Shelf Life 2020, 24, 100491. [Google Scholar] [CrossRef]
- Xiao, Z.P.; Peng, Z.Y.; Peng, M.J.; Yan, W.B.; Ouyang, Y.Z.; Zhu, H. L. Flavonoids health benefits and their molecular mechanism. Mini Rev. Med. Chem. 2011, 11, 169–177. [Google Scholar] [CrossRef]
- Deshmukh, R.K. , Gaikwad, K. K. Natural antimicrobial and antioxidant compounds for active food packaging applications. Biomass Convers. Biorefin. 2024, 14, 4419–4440. [Google Scholar] [CrossRef]
- Carullo, D.; Vergani, L.; Franzoni, G.; Mapelli, F.; Ferrante, A.; Borin, S.; Farris, S. Pectin-Based Films for Applications in the Horticultural Sector: a Preliminary Characterization. Chem. Eng. Trans. 2024, 110, 283–288. [Google Scholar]
- Farris, S.; Mora, L.; Capretti, G.; Piergiovanni, L. Charge Density Quantification of Polyelectrolyte Polysaccharides by Conductometric Titration: An Analytical Chemistry Experiment. J. Chem. Educ. 2012, 89, 121–124. [Google Scholar] [CrossRef]
- Hossain, M.T.; Shahid, M.A.; Mahmud, N.; Habib, A.; Rana, M.M.; Khan, S.A.; Hossain, M.D. Research and application of polypropylene: a review. Discov. Nano 2024, 19, 1–21. [Google Scholar] [CrossRef]
- Ma, R.; Tang, P.; Feng, Y.; Li, D. UV absorber co-intercalated layered double hydroxides as efficient hybrid UV-shielding materials for polypropylene. Dalton Trans. 2019, 48, 2750–2759. [Google Scholar] [CrossRef]
- Carullo, D.; Rovera, C.; Bellesia, T.; Büyüktaş, D.; Ghaani, M.; Santo, N.; Romano, D.; Farris, S. Acid-derived bacterial cellulose nanocrystals as organic filler for the generation of high-oxygen barrier bio-nanocomposite coatings. Sustain. Food Technol. 2023, 1, 941–950. [Google Scholar] [CrossRef]
- Ghaani, M.; Soltanzadeh, M.; Carullo, D.; Farris, S. Development of a Biopolymer-Based Anti-Fog Coating with Sealing Properties for Applications in the Food Packaging Sector. Polymers 2024, 16, 1745. [Google Scholar] [CrossRef] [PubMed]
- Aleixandre-Tudo, J.L.; Du Toit, W. The Role of UV-Visible Spectroscopy for Phenolic Compounds Quantification in Winemaking. In Frontiers and New Trends in the Science of Fermented Food and Beverages; Solis-Oviedo, R.L., De La Cruz Pech-Canul, A., Eds.; IntechOpen, London, 2019, Chapter 3.
- Farris, S.; Introzzi, L.; Piergiovanni, L. Evaluation of a bio-coating as a solution to improve barrier, friction and optical properties of plastic films. Packag. Tech. Sci. 2009, 22, 69–83. [Google Scholar] [CrossRef]
- Regulation (EU) 2025/40 of the European Parliament and of the Council on packaging and packaging waste (https://eur-lex.europa.eu/eli/reg/2025/40/oj/eng). Last Accessed: 10th April 2025. 20 April.
- Cazón, P.; Vázquez, M.; Velazquez, G. Cellulose-glycerol-polyvinyl alcohol composite films for food packaging: Evaluation of water adsorption, mechanical properties, light-barrier properties and transparency. Carbohydr. Polym. 2018, 195, 432–443. [Google Scholar] [CrossRef]
- Cozzolino, C.A.; Castelli, G.; Trabattoni, S.; Farris, S. Influence of colloidal silica nanoparticles on pullulan-coated BOPP film. Food Packag. Shelf Life 2016, 8, 50–55. [Google Scholar] [CrossRef]
- Carullo, D.; Casson, A.; Rovera, C.; Ghaani, M.; Bellesia, T.; Guidetti, R.; Farris, S. Testing a coated PE-based mono-material for food packaging applications: an in-depth performance comparison with conventional multi-layer configurations. Food Packag. Shelf Life 2023, 39, 101143. [Google Scholar] [CrossRef]
- Lee, J.W.; Son, S.M.; Hong, S.I. Characterization of protein-coated polypropylene films as a novel composite structure for active food packaging application. J. Food Eng. 2008, 86, 484–493. [Google Scholar] [CrossRef]
| Bar n° | Wire diameter [mm] | Wet coating thickness [μm] | Dry coating thickness [μm] |
|---|---|---|---|
| 1 | 0.05 | 4 | 0.12 |
| 2 | 0.15 | 12 | 0.36 |
| 3 | 0.30 | 24 | 0.72 |
| 4 | 0.51 | 40 | 1.2 |
| Active Compound |
DR [% μm-1] |
R2 | δT329nm=50% [μm] |
CB ratio [%] |
|---|---|---|---|---|
| Quercetin | 2.63 | 0.98 | 14.98 | 27.24 |
| Curcumin | 4.17 | 0.90 | 9.45 | 19.11 |
| Hymecromone | 9.67 | 0.90 | 4.07 | 9.23 |
| Caffeic acid | 18.71 | 0.92 | 2.10 | 4.98 |
| Material | Haze [%] |
|---|---|
| Bare PP | 3.90 ± 0.12 ab |
| PP + Quercetin | 4.26 ± 0.15 b |
| PP + Curcumin | 3.78 ± 0.25 a |
| PP + Hymecromone | 3.57 ± 0.06 a |
| PP + Caffeic acid | 5.40 ± 0.08 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





