1. Introduction
The first antibody approved by the FDA was the anti-CD3 antibody muromonab, which was produced by a hybridoma cell line. Since that time significant efforts have been made to reduce the portion of non-human protein sequences (humanization of mabs) to minimze antibody responses in patients (Strohl & Strohl 2012). Nowadays, antibodies are produced recombinantly which allows the production of molecules with fully human sequences. Therapeutic antibodies belong to the fastest growing class of new drugs and are used in the clinic for diverse therapeutic applications, such as autoimmune and cardiovascular diseases, cancer treatment, and many more (Crescioli et al., 2025). Antibodies are composed of two protein chains that are required for antigen-binding: The heavy chain (HC) and the light chains (LC). In mammals, antibodies can be classified in five distinct classes, IgA, IgD, IgE, IgG and IgM, depending on the type of heavy chain used. In the case of IgM and IgA, a third protein-chain, the joining (J)-chain, is incorporated into the architecture of the antibody. The J-chain is structural integrated within the C-terminal extensions of the IgM and IgA heavy chains and is required for the PIGR-mediated secretion of IgM and IgA (Li et al., 2020; Giannone et al.,2024). Both, the heavy and the lights chain are composed of a variable domain and a constant domain. The antigen-binding site, or paratope, is formed by both variable domains. In humans, there are two different light chains encoded by two different gene loci: The k-light chain (gene locus 2p11.2) and the l-light chain (gene locus 22q11.2) (McBride et al., 1982). Both light chain types consist of a variable domain and a constant domain, which determines the light chain type. Both chains show structural and functional homologies that suggest a common evolutionary origin (Titani et al., 1962; Solomon and Weiss 1995).
Antibody-producing cells originate from white blood cells known as B-lymphocytes or B-cells. Upon stimulation with a corresponding antigen, these B-cells differentiate into antibody producing cells, called plasma cells. The first antibody type produced by the immune system is IgM, which can be changed to another class during the immune response through a process known as class switching. During this process, the genes for the constant domains of the heavy chains are rearranged in a recombinational manner (Stavnezer et al., 2009). However, B-cells only express one type of light chain, either the k-light chain or the l-light chain, but not both. The exact mechanism for the selection of the light chain type is not known, but it is assumed, that the l genes are used only after unsuccessful k-light chain recombination (Durdik et al., 1984, van der Burg et al., 2001). Interestingly, the ratio of k/l-light chains used in mature antibodies differs between different species. For example, in dogs, cats, horses and cattle, the l-light chain is used in 90% or more (Arun et al, 1996) whereas in mice, the ratio of k/l-light chain is ~95% / 5% (Haughton et al., 1978; Woloschak and Krco, 1987). In humans, the κ-light chain is the predominant form and the k/l ratio has been reported to be ~ 60% / 40% (Molé et al., 1994; Popov et al., 1999). The reason for this discrepancy between different species is still unknown.
Interestingly, most of the clinically validated antibodies are those containing a k-light chain. Referring to the data provided by the antibody society, there are 228 antibody products approved or under regulatory review. Of these, 179 antibodies contain only the k-light chain, 26 antibodies contain only the l-light chain, 5 antibodies have a l– as well as a k-light chain, 2 products are mixtures of two antibodies, wherein one antibody contains the k-light chain and the other the l-light chain and one antibody has a light chain containing the variable domain from the l-light chain and the constant domain from the k-light chain (The Antibody Society, 2025). It is frequently stated that usage of the l-light chain has disadvantages during antibody developability, e.g. a higher propensity for aggregation (Lehmann et al., 2015). Further reasons why l-light chain containing antibodies are underrepresented in the clinic may be, that many antibodies have been generated in mice, which have a 99% / 5% ratio of k to l-light chains, therefore incorporation of the λ-light chain might not have been evaluated as an alternative during further antibody development. Additionally, most in vitro antibody engineering strategies are based on antibodies using the k-light chain. In nature, the usage of the l-light chain is probably not determined by chance. It has been reported, that more complex antigens, such as influenza, led to an antibody response with a higher λ to k-light chain ratio compared to an antibody response against the diphtheria toxin (Smith et al., 2016). Moreover, in a recent report it was described that the yields of IgM antibodies can be increased tremendously by incorporation of a λ-light chain instead of a κ-light chain (Gong et al., 2022). To better understand the impact of the light chain type on productivity, we compared the production of adalimumab and trastuzumab as IgG and IgM with either the κ or the λ-light chain in HEK293 cells.
During the early research phase, most antibodies are produced in HEK293 cells, since HEK293 cells can be easily transfected transiently leading to the production of sufficient amounts of protein to be used in initial screening assays (Tan et. al., 2021). Usually, there is a continuous cell culture ongoing, which is renewed in a regularly manner, e.g. after 30-40 passages. We wondered whether such frequent renewal of a cell line is actually required during the early research phase. To our knowledge, a comparable analysis of expression yields for antibodies comparing a young cell line (< 15 passages) with an old cell line (> 250 passages) has not been published. We have continued culturing HEK293 cells for > 250 passages and used this cell line in direct comparison to a young cell line for the production of two clinically validated antibodies: Adalimumab and trastuzumab
During development the cell line is usually switched to CHO cells, where stable cell lines can be generated that can produce >10 g/ L in large stirred bioreactors (Kim et al., 2012). As reported previously, CHO cells preferably incorporate the k-light chain over the λ-light chain when both genes are supplied. This is in contrast to HEK293 cells, for which no preference of a particular light chain type was reported (Zhou et al, 2023). To get a deeper understanding of the preference of HEK293 or CHO cells for either the k or the l-light chain, we selected two clinically tested IgG antibodies with either a k (adalimumab [anti-TNFα] and trastuzumab [anti-Erb2]) or l (anti-CD38- and anti-PD-L1-antibody) -light chain and produced them in both cell lines offering both light chain genes for expression.
2. Materials and Methods
2.1. Antibody Constructs, Protein Expression and Purification
Sequences of the variable domains for the used antibodies adalimumab and trastuzumab were obtained from the DrugBank (
https://go.drugbank.com/; trastuzumab: DB00072; adalimumab: DB0051). The Fc-sequences and sequences for the constant domains of the light chains were obtained from the UniProt database (
https://www.uniprot.org/; IgG1: P01857-1; IgM: P01871-1; J-chain: P01591, k-light chain: P01834, l-light chain: P0DOY2). Corresponding DNAs were synthesized (Thermo Fisher Scientific, Waltham, MA, USA) and cloned into an expression vector under a CMW promoter and a leader sequence directing the proteins into the culture supernatant. A corresponding expression construct was made for the J-chain comprising amino acids 23-159. Plasmids were used in equimolar ratios for all transfections.
HEK293 cells (FreeStyle HEK293-F, Thermo Fisher Scientific) were cultured in 600 mL TubeSpin bioreactors (TPP Techno Plastic Products AG, Trasadingen, Switzerland) with 300 mL FreeStyle F17 medium (Thermo Fisher Scientific) supplemented with 6 mM glutamine and 0.02% Kolliphor P180 (Sigma-Aldrich) at 150 rpm, 37°C and 8% CO2. For maintenance of the main cell cultures, cells were passaged 3 times per week. For cell cultures used for transfection, cell density was ~1.5 × 106 at the time of transfection as measured with an automated cell counter (Vi-CELL BLU, Beckman Coulter). DNA was mixed with linear polyethylenimine (PEI, Polysciences, Warrington, PA) in a ratio of 1 : 3 in FreeStyle F17-medium (Thermo Fisher Scientific). After incubation of 20 min at room temperature transfection mixtures were added to the cell cultures and cultivation was continued for 6 days. Cells were harvested by centrifugation (2000 g, 20°C, 30 min) and supernatants were 0.22 µm filtered (RapidFlow Filter Units, 0,2 µM PES Membrane, Thermo Fisher Scientific). An internal CHO-K1 derived cell line was used for protein expression. Cells were cultured in 600 mL TubeSpin bioreactors with 250 mL FreeStyle F17 medium and supplements and conditions as used for HEK293 cells. For maintenance of the main cell cultures, cells were passaged 3 times per week. For cell cultures used for transfection cell density was ~4 × 106 after centrifugation and media was exchanged to new FreeStyle F17 medium with supplements. DNA was added with linear polyethylenimine in a ~ ratio of 4 : 6 (260 µg DNA, 440 µg sheared salmon sperm DNA [Thermo Fisher Scientific] / 1000 µg PEI). After 2 hours, 15 % CHO CD EfficientFeed B (Thermo Fisher Scientific) was added. After transfection, cells were cultivated for 10 days. Cells were harvested as described above.
IgG proteins were purified using a protein A MabSelect Sure column (Cytiva) followed by a desalting step and size exclusion chromatography (Superdex 200 20/60 pg column, Cytiva) as described before (Becker et al., 2019). Running buffer for the size exclusion chromatography was 10 mM Histidine, 150 mM NaCl, pH 6.0 (PAN-Biotech, Aidenbach, Germany). All experiments were done in duplicates or triplicates.
2.2. MS-Analysis
Composition of purified antibody preparations were analysed by LC-MS. Protein samples were diluted to 0.25 mg/ml with water (LC-MS grade, Fisher Scientific). For glycosylation, 1.0 µl PNGase F (New England Biolabs, #P0705L) was added to 25 µl sample and samples were incubated at 37°C overnight. LC-MS-analysis was done with an 6545XT advance Bio QTOF/LC MS-system (Agilent Technologies, Santa Clara, CA). For the reversed phase HPLC a PLRP-S column was used (Agilent Technologies, #PL1912-1502). 2 µl of sample were injected and eluted with increasing acetonitrile concentration. Eluents used were buffer A containing LC-MS grade water and 0.1 formic acid and buffer B containing 90 % acetonitrile, 10 % LC water and 0.1 formic acid. Obtained mass data were analysed with the MassHunter software (Agilent Technologies, V B9044.)
2.3. Capillary Gelelectrophoresis (cGE)
For capillary gel electrophoresis, protein samples were diluted to a concentration of 0.5 mg/mL and analyzed on a LabChip GXII Touch (Perkin Elmer). Samples were analysed under reducing conditions (50 mM DTT) following the manufacturer´s instructions using the Protein-Clear HR reagent kit (#CLS960014) and a Protein Clear HR LabChip Touch (#CLS148695, Revvity Inc., Waltham, Mass, USA).
2.4. SDS-PAGE Analysis
Protein samples were mixed with either 4 × LDS sample buffer (Thermo Fisher Scietific) for non-reducing SDS-PAGE or with 4 × LDS sample buffer with 100 mM dithiothreitol for reducing SDS-PAGE. For SDS-PAGEs under reducing conditions, samples were incubated for 5 min at 99°C before loading on the gels. 4-12 % BisTris or 12 % BisTris gels were used with MES or MOPS as running buffer (Life Technologies/ Thermo Fisher Scientific). SDS-PAGE gels were stained with Coomassie-blue (Instant Blue, Expedeon, Cambridgeshire, UK).
3. Results
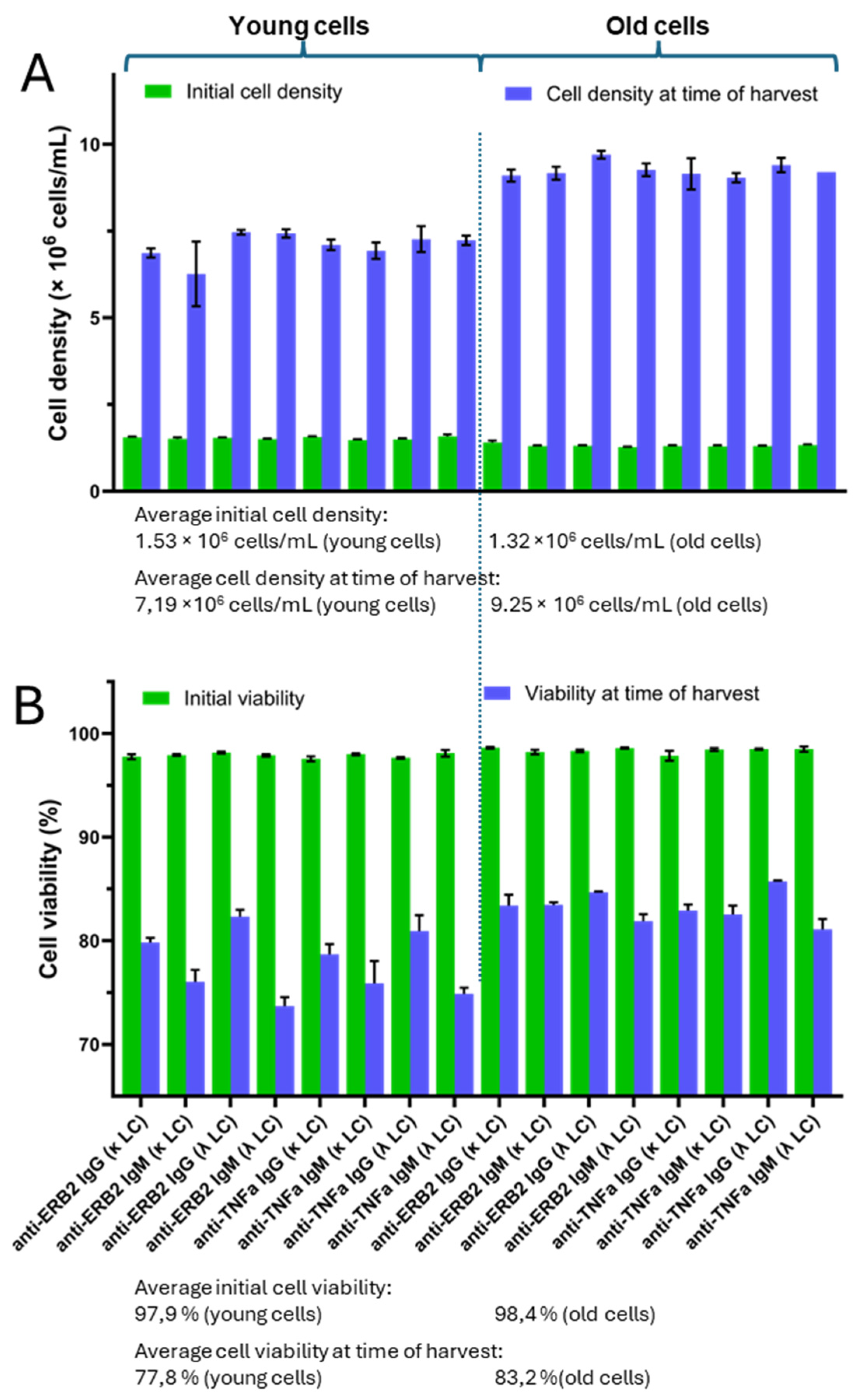
To address the preference of the light chain type for either IgG or IgM, we produced both antibody-types as variants of the anti-TNFα-antibody adalimumab and anti-Erb2 antibody trastuzumab with either k or l-light chain. All protein expression experiments were done using transiently transfected HEK293 cells. Concomitantly we compared the expression of these in antibodies in “young cells” (<15 passages) with the expression in “old cells” (>250 passages). Young cells were transfected after 10 passages in culture after cells were withdrawn from the cell bank stored in liquid nitrogen. The “old cells” had been cultured in suspension for at least 250 passages. All cell cultures were transfected under the same conditions having cell densities of ~1,3 - 1.5 × 10
6 cells/mL and a cell viability >95 % (
Figure 1A). It should be noted that all cell cultures were cultivated at the same time. Before cell harvest, cell density and viability were measured again. For the young cells, cell densities ranged between 6.8 – 7.5 × 10
6 cells / mL. In contrast, the old cells had cell densities in the range of 9.1 – 9.7 × 10
6 cells /mL (
Figure 1A). The cell viability of the young cells at the time of harvest was between ~75 - 81 % whereas cell viability of the old cells was in the range between 81-86 % (
Figure 1B). We observed that the viability of the young cells at the time of harvest producing IgG antibodies was slightly higher compared to the IgM producing cultures (
Figure 1 B). Interestingly, this seems not to be the case for the old cells. In addition, the production of κ- or λ-light chain containing antibody variants showed no significant differences in respect to cell density and viability at time of harvest.
After cultivation, cells were harvested and the culture supernatants were analysed by SDS-PAGE (
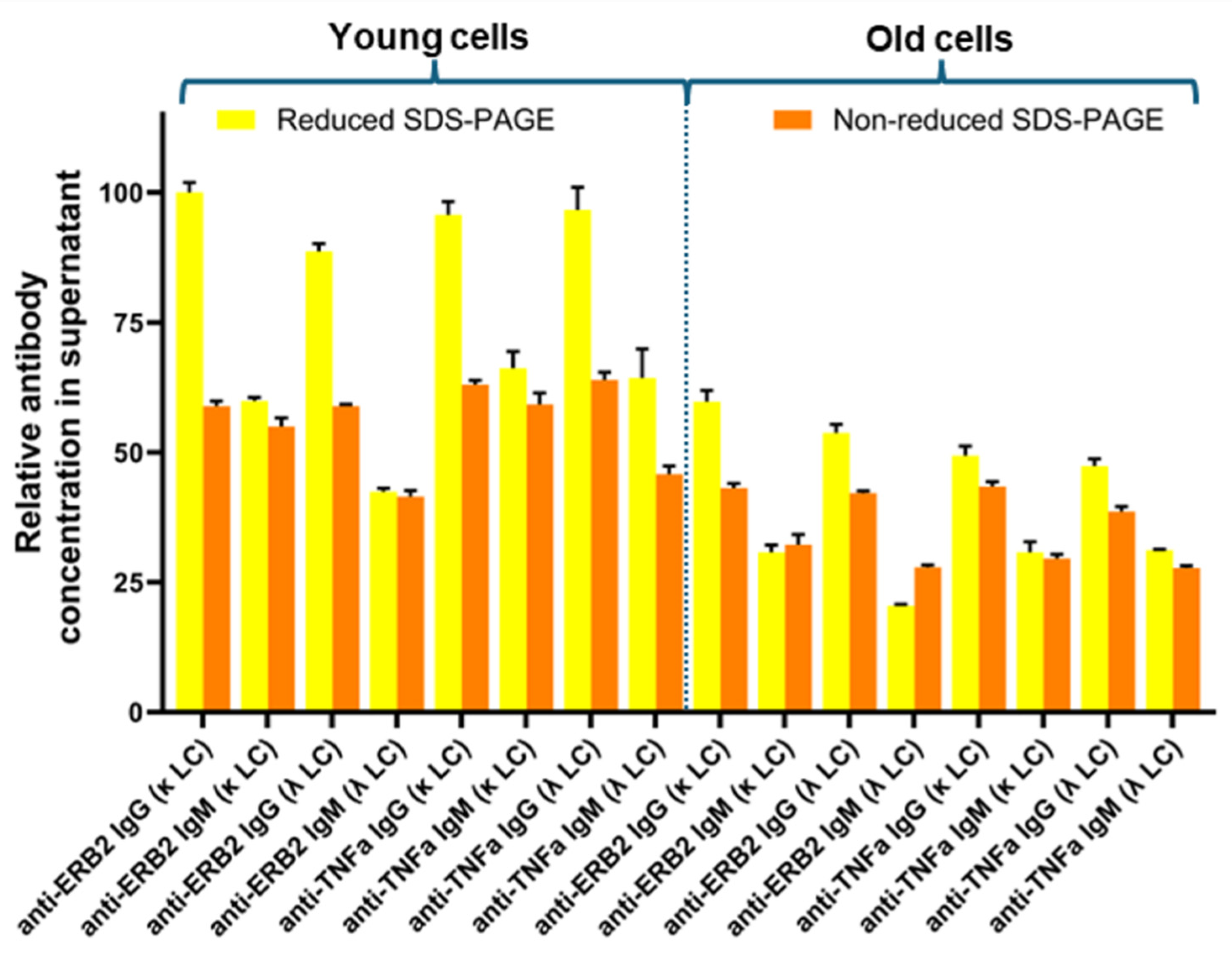
Figure S1). The expressed antibodies were clearly visible as distinct protein bands. In all cultures, excessive production of light chains is also clearly visible. As estimated from the SDS-PAGE, the amount of free light chains is higher for the k-light chain compared to the l-light chain (
Figure S1). To compare the expression levels of the different antibody variants, the staining intensity of the bands in both the reduced and non-reduced SDS-PAGE gels was measured and the value measured for anti-Erb2-IgG (k) was arbitrarily set as 100 %. For the reduced-SDS-PAGES the staining intensities of both the heavy and light chain were considered (
Figure S2,
Table S1). The relative expression data are shown in
Figure 2. Based on the reduced SDS-PAGEs the expression yields are higher for the IgG variants compared to the IgM-variants. This observation is more evident for the young cells compared to the old cells. The reason for this is probably that in the calculation for the reduced antibodies the amount of free light chain is also considered. For the IgG-variants, the usage of either the k or the l-light chain does not have a big impact on expression yield. For the IgM variants, yields seem to be slightly lower when the l-light chain is used. However, this effect is even smaller for the anti-TNFα-IgM variants compared to the anti-Eer2-IgM variant. In the case of the old cells, there is no difference in expression levels between anti-TNFα-IgM(k) and anti-TNFα-IgM(l). In general, the expression levels are generally lower for the older cells compared to the fresh cells.
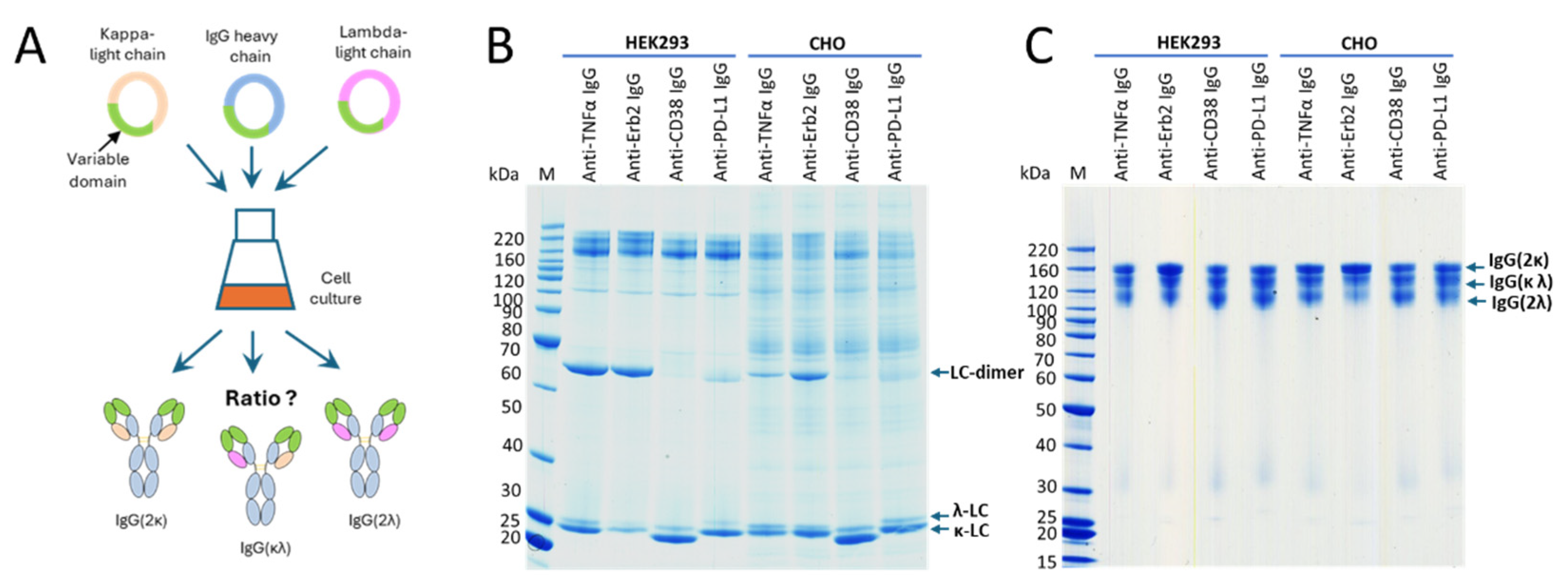
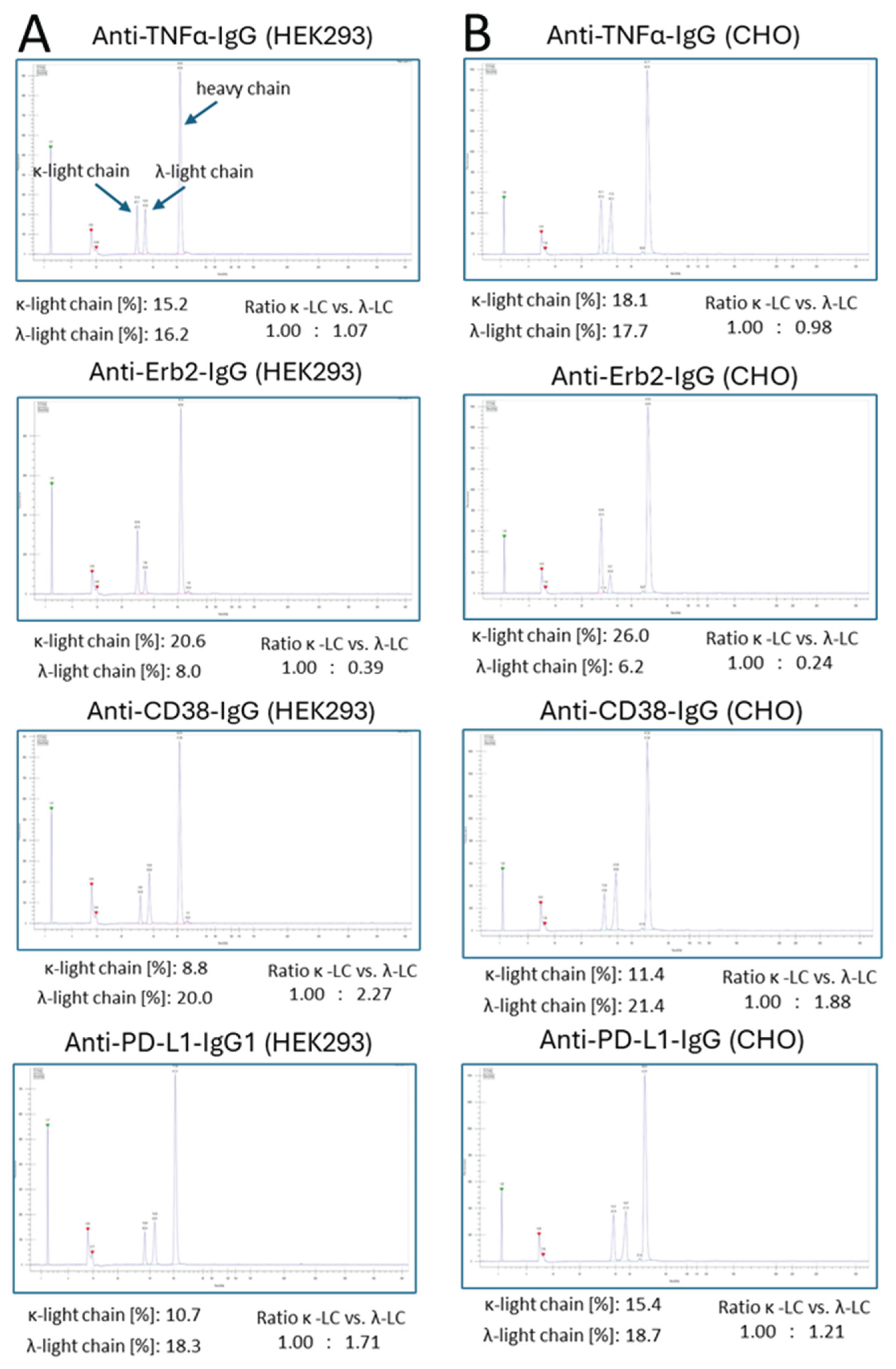
It has been reported earlier that CHO cells have a strong preference for incorporation of the k-light chain over the l-light chain. For HEK293 cells, no preference for either light chain isotype was reported (Zhou et al., 2023). To further address the question whether a certain light chain isoform is preferred over the other one, we expressed four clinically validated antibodies as IgG offering both, the k- and the l-light chain gene for expression simultaneously. The antibodies with an original k-light chain are an anti-TNFα- and an anti-Erb2-IgG, the antibodies with an original l-light chain are an anti-CD38- and an anti-PD-L1-IgG. HEK293 and CHO cells were transiently transfected using the same plasmids with 3 plasmids in a 1:1:1 ratio encoding for the heavy chain, a k- and a l-light chain (
Figure 3A). At the time of harvest, samples from the cell culture supernatants were analysed by SDS-PAGE (
Figure 3B). As shown, both light chains were highly expressed and free light chains were secreted. As estimated from the staining intensities of the bands in the SDS-PAGE, secretion levels are higher for the free k-light chain compared to the free l-light chain. Interestingly, the amount of light chain dimer formation is different between the different antibodies. High amounts of light chain dimers are clearly visible for adalimumab and trastuzumab (originally with k-light chain), but only few amounts for the anti-CD38- and anti-PD-L1-IgG (originally with l-light chain). The results obtained for excess light chain expression from HEK293 and CHO cells are comparable (
Figure 3B). These observations are in accordance with the results obtained in the previous experiment. SDS-PAGE analysis was also done with the purified proteins under reducing and non-reducing conditions using two different SDS-PAGE running conditions. Using MES as running buffer, separation of the different-species could not be observed (
Figure S3). In contrast, when using a MOPS-based running buffer, a clear separation of the different antibody species could be observed (
Figure 3C). The separation is obviously due to the different isoelectric points of the antibodies having 2 κ-, 2 λ- or 1κ- and 1λ-light chain. Under reducing conditions, distinct bands are clearly visible for the heavy and the light chain. However, no separation of the k- and the l-light chains was observed in the SDS-PAGEs of the purified IgG antibodies under reducing conditions (
Figure S3B, C) despite this is clearly visible in the SDS-PAGE gels of the culture supernatants under non-reducing conditions (
Figure 3B). To estimate the ratio between the different antibody-species, the staining intensity of the corresponding bands from the SDS-PAGE were measured (
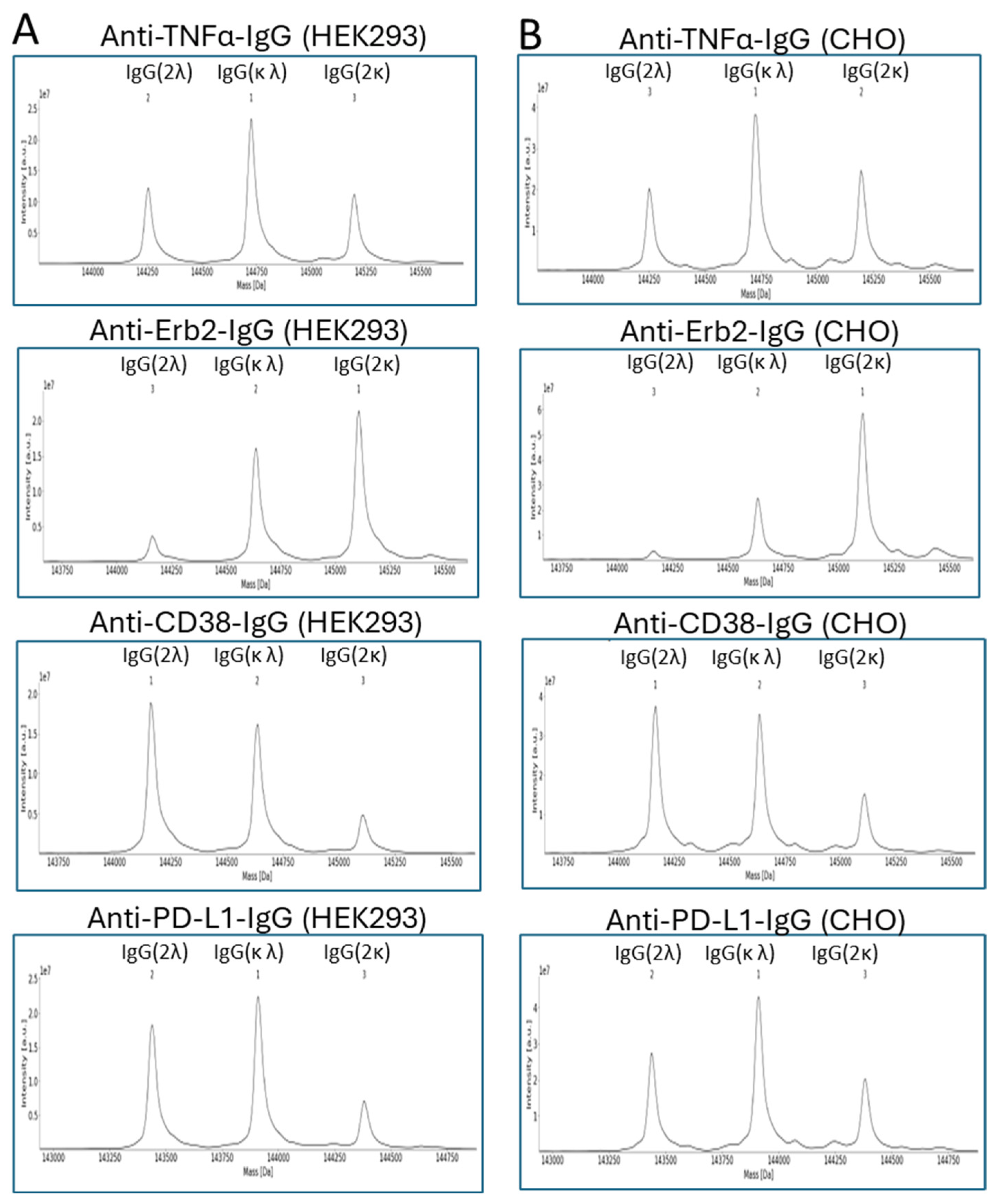
Figure S4). Purified proteins were also analysed by mass-spectrometry (
Figure 4). No differences in light chain incorporation between antibodies expressed in HEK293 or CHO cells could be observed. The obtained masses were in good accordance with the calculated masses (
Table S2). The results from the volumetric analysis of the stained SDS-PAGE and signal intensities obtained by MS are given in
Table 1. Despite signal intensities obtained by MS do not necessarily allow a quantification of the analysed species, the obtained data from the two different methods are in good accordance. All antibodies were also analysed under reducing condition by cGE, where a good separation of the k- and l-light chain was achieved. Based on the obtained signal intensities, the ratio of incorporated k- vs- l-light chain into an IgG was calculated (
Figure 5). Data from cGE are in accordance with data from MS and SDS-PAGE.
Taken together, in the case of anti-TNFα-IgG, HEK293 cells obviously do not discriminate between k- and l-light chain. Both light chains are used in equal amounts. A slight preference for usage of the k-light chain is observed for CHO cells compared to HEK293 cells. In contrast to anti-TNFα-IgG, for the anti-Erb2-IgG the incorporation of a k-light chain is clearly preferred by both HEK293 and CHO cells. Again, CHO cells had a slightly higher incorporation rate for the k-light chain compared to HEK293 cells. For the anti-CD38-IgG, the incorporation of the l-light chain is clearly preferred in both cell types. Again, CHO cells incorporated more k-light chain compared to HEK293 cells. Similar results as for the anti-CD38-IgG were obtained for the anti-PD-L1-IgG, albeit for the latter a slightly higher percentage of k-light chain was incorporated. There is a trend perceptible within the data. Overall, CHO cells actually seem to prefer the k-light chain over the l-light chain compared to HEK293 cells, but this extent is rather small. However, it is evident that that selection and incorporation of a particular light chain isotype does obviously not depend on the host cell type, but rather on the original antibody isoform.
4. Discussion
In the first part of our study, we compared the expression of two validated monoclonal antibodies, adalimumab and trastuzmab, in different antibody–isoforms, IgM and IgG, and analysed whether the usage of either the k- or l-light chain had a beneficial effect on expression yields. These experiments were done using transient transfection of HEK293 cell line having different passages (<15 young cells, > 250 old cells). All expression experiments were done at the same time under the same conditions to allow for best comparison between the experiments. All cell cultures had comparable initial cell density and viability (
Figure 1A). We observed that at the time of harvest, cell densities and viabilities of cultures with old cells were higher than cell cultures with young cells. Within the young cells, viabilities were higher for cells expressing IgG compared to IgM. This bias could not be observed for the old cells. The reason for the faster cell growth of the older cells might be, that during the “aging” period cells have been adapted to the cultivation conditions. The older cells have evolved into a fast-growing culture rather than a good production cell line as evident from the reduced expression levels compared to the young cells (
Figure 2). The underlying changes for these observations are unknown as we did no genomic or transcriptional profiling of the cell lines. However, when aiming at high expression levels it is expedient to use fresh cells. It is well known that cells can adapt to their environment by altering their genomic and transcriptomic profiles. A well characterized example is the evolution of the parental HEK293 cell line and their derivatives (Malm et al., 2020). In contrast to the HEK293 cell lines analysed by Malm et al, which have evolved over several years or decades, the changes observed with our cells are evident after 250 passages (3 passages / week, ~1.6 years) without any further selection pressure.
Next, we evaluated the expression levels of two IgM variants and two IgG antibodies with the k- as well as the l-light chain. As it has been reported earlier, the usage of the l-light chain for IgM expression instead of the k-light chain should have a huge beneficial effect on expression yields. Gong et al. evaluated the expression of four antibodies as IgG, IgA and IgM with either the k-or l-light chain. Their observation was that IgM variants with a k-light chain showed only very little expression. Upon usage of the l-light chain instead of the k-light chain expression levels for one IgM variant improved with a factor of 12 (VRC01). For the other IgM variants increase of expression levels of ~ 7000 fold (PGT121), ~11.600 fold (33C6) and even up to nearly ~20.000 fold (Fm-6) was described (Gong et al. 2022). Despite having only a limited data set, we could not observe a meaningful change of expression levels for the IgM antibodies when using the l-light chain instead of the k-light chain.
During the early research phase, monoclonal antibodies are usually produced in transiently transfected HEK293 cells. This cell line is derived from human embryonic kidney cells and used because of its ease of transformation and high expression titers (Tan et. al., 2021). For development and production of monoclonal antibodies the expression host is usually switched to CHO cells, which are derived from the ovary of the chinese hamster
Cricetulus griseus. CHO cells can easily be adapted for growth in large stirred bioreactors required for large scale production of biotherapeutics with high expression titers of <10g/L (Kim et al., 2012). Most of the clinically validated IgG have the k-light chain as antibodies with a l-light chain may have a tendency for aggregation (Lehmann et al., 2015). Hence, the light chain type chosen for production is usually the k-light chain. Further support for using the k-light chain for CHO-cell expression was a recent report by Zhou et al, wherein the preferred incorporation of the k-light chain over the l light chain was described for CHO cells, but not for HEK293 cells (Zhou et al., 2023). Upon co-transfection of CHO and HEK293 cells with a heavy chain and the k-and l-light chain, a 99:1 k : l-light chain usage was reported for CHO cells and a ~1 : 1 ratio for HEK293 cells (Zhou et al. 2023). To further understand the impact of the k-or the l-light chain, we choose two validated IgG antibodies with a k-light chain and two IgGs with a l-light chain and transiently transfected either CHO or HEK293 cells with three plasmids coding for the heavy chain, the k- and the l light chain in a 1:1:1 ration. (
Figure 3). The purified IgGs were analysed by SDS-PAGE, cGE and MS. We observed only slight differences in the usage of either light chain isotype between HEK293 and CHO cells. Interestingly, we noticed that the originally used light chain isotype is the preferred one in both cell lines. This effect is largely dependent on the particular antibody used. In the case for the anti-TNFα antibody adalimumab, the light chain isoform does not have an influence on incorporation in the IgG. In contrast, for the anti-Erb2 antibody trastuzumab, the usage of the k-light chain is clearly preferred over the l-light chain in both cell lines to similar extents. This phenomenon was also observed for the two antibodies having naturally a l-light chain. The trend for using the l-light chain is more pronounced for the anti-CD38 antibody compared to the anti-PD1 antibody. Overall, there seems to be a slightly higher usage of the k-light chain in CHO cells compared to HEK293 cells; but the most important factor for selecting the best light-chain isotype seems to be the usage of the original light chain isotype that was selected by the antibody producing B-cell. This is an in important factor to be considered when switching the light chain type from a l to the k-isotype. Possibly, additional engineering efforts might be required.
In summary, we addressed several topics that frequently arise during the early research phase for antibodies. HEK293 cells quickly adapt to culture conditions; in our case older cells were growing faster but produced proteins in lower yields. We did not observe a positive effect upon usage of the l light chain for enhanced expression for IgM antibodies. Furthermore, only a very slight preference of CHO cells for the k- over the l-light chain was observed. In conclusion, the light chain isotype that was originally selected by the antibody producing B-cell is a critical factor to be considered for therapeutic antibody generation.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org.
Conflicts of Interest statement
A.V., D.L., P.G., I.F., W.D.L., C.B., S.W., E.R. and T.L. are employees of Sanofi-Aventis Deutschland GmbH and may hold company shares and/or stock options. A.V. was during the time of work a registered student at Provadis School of International Management and Technology, Frankfurt am Main and working at Sanofi-Aventis Deutschland GmbH. However, the authors declare no additional conflict of interest. The authors are grateful to Franziska Arlt, Ellen Kessler and Stephanie Otto for great technical assistance (all employees of Sanofi-Aventis Deutschland GmbH).
References
- Arun SS, Breuer W, Hermanns W. Immunohistochemical examination of light-chain expression (lambda/kappa ratio) in canine, feline, equine, bovine and porcine plasma cells. Zentralbl Veterinarmed A. 1996;43(9):573-576. [CrossRef]
- Becker W, Scherer A, Faust C, et al. A fully automated three-step protein purification procedure for up to five samples using the NGC chromatography system. Protein Expr Purif. 2019;153:1-6. [CrossRef]
- Crescioli S, Kaplon H, Wang L, Visweswaraiah J, Kapoor V, Reichert JM. Antibodies to watch in 2025. MAbs. 2025;17(1):2443538. [CrossRef]
- Durdik J, Moore MW, Selsing E. Novel kappa light-chain gene rearrangements in mouse lambda light chain-producing B lymphocytes. Nature. 1984;307(5953):749-752. [CrossRef]
- Giannone C, Mess X, He R, et al. How J-chain ensures the assembly of immunoglobulin IgM pentamers. EMBO J. Published online , 2024. 4 December. [CrossRef]
- Gong S, Gautam S, Coneglio JD, Scinto HB, Ruprecht RM. Antibody Light Chains: Key to Increased Monoclonal Antibody Yields in Expi293 Cells?. Antibodies (Basel). 2022;11(2):37. Published 2022 May 18. [CrossRef]
- Haughton G, Lanier LL, Babcock GF. The murine kappa light chain shift. Nature. 1978;275(5676):154-157. [CrossRef]
- Keyt BA, Baliga R, Sinclair AM, Carroll SF, Peterson MS. Structure, Function, and Therapeutic Use of IgM Antibodies. Antibodies (Basel). 2020;9(4):53. Published 2020 Oct 13. [CrossRef]
- Kim JY, Kim YG, Lee GM. CHO cells in biotechnology for production of recombinant proteins: current state and further potential. Appl Microbiol Biotechnol. 2012;93(3):917-930. [CrossRef]
- Lehmann A, Wixted JH, Shapovalov MV, Roder H, Dunbrack RL Jr, Robinson MK. Stability engineering of anti-EGFR scFv antibodies by rational design of a lambda-to-kappa swap of the VL framework using a structure-guided approach. MAbs. 2015;7(6):1058-1071. [CrossRef]
- Li Y, Wang G, Li N, et al. Structural insights into immunoglobulin M. Science. 2020;367(6481):1014-1017. [CrossRef]
- Malm M, Saghaleyni R, Lundqvist M, et al. Evolution from adherent to suspension: systems biology of HEK293 cell line development [published correction appears in Sci Rep. 2021 Mar 2;11(1):5407. doi: 10.1038/s41598-021-85105-9.]. Sci Rep. 2020;10(1):18996. Published 2020 Nov 4. [CrossRef]
- McBride OW, Hieter PA, Hollis GF, Swan D, Otey MC, Leder P. Chromosomal location of human kappa and lambda immunoglobulin light chain constant region genes. J Exp Med. 1982;155(5):1480-1490. [CrossRef]
- Molé CM, Béne MC, Montagne PM, Seilles E, Faure GC. Light chains of immunoglobulins in human secretions. Clin Chim Acta. 1994;224(2):191-197. [CrossRef]
- Popov AV, Zou X, Xian J, Nicholson IC, Brüggemann M. A human immunoglobulin lambda locus is similarly well expressed in mice and humans. J Exp Med. 1999;189(10):1611-1620. [CrossRef]
- Schroeder HW Jr, Cavacini L. Structure and function of immunoglobulins. J Allergy Clin Immunol. 2010;125(2 Suppl 2):S41-S52. [CrossRef]
- Smith K, Shah H, Muther JJ, Duke AL, Haley K, James JA. Antigen nature and complexity influence human antibody light chain usage and specificity. Vaccine. 2016;34(25):2813-2820. [CrossRef]
- Solomon A and Weiss DT. Structural and functional properties of human lambda-light-chain variable-region subgroups. Clin Diagn Lab Immunol. 1995;2(4):387-394. 1995. [CrossRef]
- Stavnezer J, Guikema JE, Schrader CE. Mechanism and regulation of class switch recombination. Annu Rev Immunol. 2008;26:261-292. [CrossRef]
- Strohl WR & Strohl LM (2012) Therapeutic Antibody Engineering, Woodhead Publishing,111-595. [CrossRef]
- Tan E, Chin CSH, Lim ZFS, Ng SK. HEK293 Cell Line as a Platform to Produce Recombinant Proteins and Viral Vectors. Front Bioeng Biotechnol. 2021;9:796991. Published 2021 Dec 13. [CrossRef]
- The Antibody Society. Therapeutic monoclonal antibodies approved or in regulatory review. (access April 2025); www.antibodysociety.org/antibody-therapeutics-product-data.
- Titani K, Wikler M, Putnam FW. Evolution of immunoglobulins: structural homology of kappa and lambda Bence Jones proteins. Science. 1967;155(3764):828-835. [CrossRef]
- van der Burg M, Tümkaya T, Boerma M, de Bruin-Versteeg S, Langerak AW, van Dongen JJ. Ordered recombination of immunoglobulin light chain genes occurs at the IGK locus but seems less strict at the IGL locus. Blood. 2001;97(4):1001-1008. [CrossRef]
- Woloschak GE, Krco CJ. Regulation of kappa/lambda immunoglobulin light chain expression in normal murine lymphocytes. Mol Immunol. 1987;24(7):751-757. [CrossRef]
- Zhou J, Yan GG, Cluckey D, et al. Exploring Parametric and Mechanistic Differences between Expi293FTM and ExpiCHO-STM Cells for Transient Antibody Production Optimization. Antibodies (Basel). 2023;12(3):53. Published 2023 Aug 10. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).