1. Introduction
Weeds pose a major threat to agricultural productivity in cultivated areas. They compete with crops for essential resources such as water, nutrients, and sunlight, leading to decreased yields and overall agricultural output [
1]. Traditional manual weeding methods, while effective, are highly labor-intensive and becoming increasingly unsustainable due to rural labor shortage and declining birth rate [
1,
2]. As a result, chemical herbicides have become the primary weed control method in modern agriculture, offering both high efficacy and cost efficiency [
3]. This shift is particularly crucial for rice, a staple crop for over half of the global population, which faces escalating yield losses from weed infestation, especially as cultivation transitions from transplanting to direct seeding [
4,
5]. Herbicide application, combined with herbicide-resistant rice varieties, offers an effective weed management solution with minimal labor demands, making it indispensable for large-scale rice production [
6].
The identification of herbicide-resistance genes is crucial for developing herbicide-resistant crops. By isolating these genes, which often are derived from resistant weeds, bacteria, or other plant species, researchers can employ genetic engineering to introduce herbicide tolerance into crops, ultimately producing elite cultivars [
7,
8]. Through the introduction and upregulation of genes responsible for herbicide detoxification, scientists can enhance the herbicide resistance of crops without negatively affecting their agronomic performance or yield [
8,
9].
To date, numerous synthetic herbicides with diverse selectivity and modes of action have been commercialized and widely adopted [
10,
11]. Developing crop cultivars resistant to various herbicides and implementing rotations of crops with different herbicide resistances are essential strategies to mitigate the risk of evolved weed resistance, which often results from the overreliance on single herbicide-resistant crops [
2,
12]. Diquat, a bipyridyl herbicide with structural similarity to paraquat, exerts its phytotoxic effects by accepting electrons from chloroplast photosystem I (PSI) under light conditions. The subsequent transfer of these electrons to molecular oxygen generates reactive oxygen species, inducing severe oxidative stress and leading to rapid plant tissue necrosis [
13,
14]. Compared to paraquat, diquat maintains comparable herbicidal efficacy but exhibits lower mammalian toxicity. In recent years, diquat has been widely used to replace paraquat in many countries [
15,
16,
17]. Additionally, diquat can be absorbed by soil particles and undergoes degradation, resulting in reduced environmental persistence and enhanced ecological safety [
13].
In our previous study, we identified a bipyridyl herbicide-resistance gene named
DTX6 in
Arabidopsis [
18]. This gene encodes a MATE (multidrug and toxic compound extrusion) family transporter. A glycine-to-glutamic acid substitution at position 311 (G311E) confers enhanced paraquat resistance in
Arabidopsis [
18,
19]. DTX6 comprises 12 transmembrane domains with dual localization to both vacuolar and plasma membranes. Though its in vivo substrate remains undetermined, it mediates bipyridyl herbicide detoxification through vacuolar sequestration and exocytotic extrusion [
18]. While these findings established the molecular basis of resistance in dicots, whether this mutation confers cross-species resistance or induces pleiotropic effects remains unknown. In this study, we demonstrate that the G311E-mutated DTX6 (designated DTX6m) confers robust resistance to bipyridyl herbicides in rice. Furthermore, DTX6m induces mild dwarfism and accelerates flowering time without compromising grain yield, while significantly increasing seed amino acid content. These results highlight its agricultural potential for developing herbicide-resistant crops through genetic breeding without sacrificing agronomic performance, and also provide mechanistic insights for investigating its physiological substrates.
2. Materials and Methods
2.1. Rice Variety and Growth Conditions
All rice materials used in this study are of Oryza sativa L. cv. Zhonghua 11 (ZH11) background. For green house assay, they were grown under a 12-h-light (30°C)/12-h-dark (22°C) photoperiod with 50% humidity unless otherwise specified. For field assay, rice seeds were germinated and grown in green house for three weeks, and then transplanted to the paddy field within the rice transgene experimental base located in Fudan university campus on June and harvested on October.
2.2. Generation of Transgenic Lines
The open reading frames of
DTX6-
G311E were cloned into the pCAMBIA1301-N-eYFP vector by KpnI/ BamHIsites. The construct was transformed into rice using the
Agrobacterium tumefaciens-mediated transformation method [
20], with the assistance provided by EDGENE Biotechnology Co., Ltd (Wuhan, China). The primers used in clone construction for overexpression are listed in Supplemental
Table S1.
2.3. RNA Extraction and RT-qPCR
Total RNA was extracted using the TRIzol reagent (TaKaRa, Japan) and reverse-transcribed with PrimeScript RT reagent (TaKaRa, Japan). qPCR was performed with the SYBR Premix Ex Taq kit (TaKaRa, Japan) using the CFX96 real-time PCR detection system (Bio-Rad, USA). Rice
UBQ5 gene was used as an internal control to normalize different samples. RT-qPCR primer sequences are given in Supplemental
Table S1.
2.4. Herbicide Spraying Assay of Soil-Grown Seedlings
For paraquat resistance assay in greenhouse, the homozygous overexpression lines and ZH11 wild type were grown in soil for one month, and then sprayed with 0.5 g/L mM paraquat. 10 days after spraying, the survival rates and wilted leaves of different lines were documented and plants were photographed. For diquat resistance assay, plants were grown for 25 days and then sprayed with 0, 0.5, 1, 2, and 5 g/L diquat. 3 days post-application, the phenotypes were recorded and the survival rate was calculated. For the field test, the overexpression lines and ZH11 wild type were grown side-by-side for two months till the reproductive stage, then 2 g/L diquat was sprayed and pictures were taken after 10 days.
2.5. Agronomic Trait Analysis of Transgenic Rice Plants
Rice plants were grown in the paddy field within the rice transgene experimental base located in Fudan university campus from June to October. At least 12 plants of each line were randomly chosen in the field for further agronomic trait analysis, including plant height, flowering time, effective panicle number per plant, grain number per panicle, panicle length, one thousand-grain weight and grain size. One thousand-grain weight was measured using the harvested seeds that were air-dried for two weeks. Grain size including the length and width were analyzed by the automatic seed-examining high-speed document camera (Shenzhen Liangtian, China) and the thickness were measured manually by a vernier caliper.
2.6. Nutritional Component Determination of Rice Seeds
About 2 g dehulled rice seeds were ground into fine powder using a grinding mill and then used for the following nutritional component determination.
2.6.1. Free Fatty Acids Determination
Free fatty acids (FFAs) were extracted and quantified according to the established protocol with modifications [
21,
22]. Briefly, 0.1 g of rice powder was homogenized in 1 mL of chloroform-heptane-methanol (200:150:7, v/v/v) containing silicic acid (0.05 g/mL) to eliminate phospholipids. The mixture was agitated for 3 h at room temperature, followed by centrifugation at 8,000 × g, 4°C for 10 min. The supernatant was collected and reacted with freshly prepared Cu-TEA solution [0.05 M Cu(NO
3)
2, 0.1 M triethanolamine, NaCl-saturated (~33 g/100 mL), pH 8.1] to form FFA-Cu complexes. The FFA content was then determined using a commercial Free Fatty Acid Determination Kit (Suzhou Keming Co. Ltd., China).
2.6.2. Total Sugar Determination
Total sugar content was determined using the 3,5-dinitrosalicylic acid (DNS) method [
23]. Briefly, 0.1 g of rice powder was homogenized in 1.5 mL of ddH
2O, followed by the addition of 1 mL of 6 N HCl. The mixture was incubated in a 95°C water bath for 30 min to hydrolyze polysaccharides. After cooling to room temperature, the sample was neutralized with ~1 mL of 6 N NaOH, diluted to a final volume of 10 mL with ddH
2O, and centrifuged at 8000 × g for 10 min at 25°C. The supernatant was then analyzed for sugar content using a Total Sugar Determination Kit (Suzhou Keming Co., Ltd., China) according to the manufacturer’s instructions.
2.6.3. Soluble Sugar Determination
Soluble sugar content was determined using the anthrone colorimetric method [
24] with a Plant Soluble Sugar Determination Kit (Suzhou Keming Co. Ltd., China). The soluble sugar was extracted from 0.1 g of rice powder with 1 mL of distilled water at 95°C for 10 min. After cooling, samples were centrifuged at 8000 × g for 10 min at 25°C. The supernatant was collected, diluted to 10 mL with ddH
2O, and 40 µL aliquots were used for soluble sugar quantification according to the manufacturer’s protocol.
2.6.4. Starch Content Determination
Starch were extracted and determined as described previously [
25] with some modifications: 0.1g rice powder was extracted with 1 mL of 80% ethanol at 80°C for 30 min. After centrifugation at 3,000 × g for 5 min, the supernatant was discarded and the pellet was air-dried. The air-dried residue was resuspended in 0.5 mL ddH
2O, heated in a boiling water bath for 15 min, and cooled. For starch extraction, 0.35 mL of 9.2 M perchloric acid was added and the mixture was incubated for 15 min at room temperature. The solution was then diluted with 0.85 mL ddH₂O and centrifuged at 3,000 × g for 10 min. The resulting supernatant was analyzed for starch content using Plant Starch Content Determination Kit (Suzhou Keming Co. Ltd., China) according to the manufacturer's instructions.
2.6.5. Protein Level Determination
0.02 g of rice powder was subjected to sequential extractions to obtain the four major rice storage proteins (albumin, globulin, prolamin, and glutelin) according to the method described previously [
26]. The contents of different protein ingredients were determined using the Modified Bradford Protein Assay Kit (Sangon Biotech, China).
2.6.6. Free Amino Acid Level Determination
0.1 g rice powder was extracted with 1 mL of distilled water in a boiling water bath for 15 min. After cooled to room temperature, samples were centrifuged at 10,000× g for 10 min. The supernatant was used for total amino acid determination by ninhydrin method [
27].
2.7. Phylogenetic Analysis
The amino acid sequences of Arabidopsis and rice MATE family proteins were extracted from the TAIR (
www.arabidopsis.org) and RAP-DB (
https://rapdb.dna.affrc.go.jp/) websites. 56
Arabidopsis MATE family proteins and 46 rice MATE proteins (for genes with multiple transcripts, only the first transcript was chosen) were analyzed for their phylogenetic relationship using
selaginella_moellendorffii as the alien species. Multiple sequence alignment was performed by Clustal X using default parameters. The resulting alignment was output in Phylip format and uploaded to the online PhyML (
http://www.atgc-montpellier.fr/) website to construct the phylogenetic tree using the maximal likelihood method by standard bootstrap analysis with 1000 replicates [
28]. The resulting phylogenetic tree was visualized using the iTOL tool (
https://itol.embl.de).
3. Results
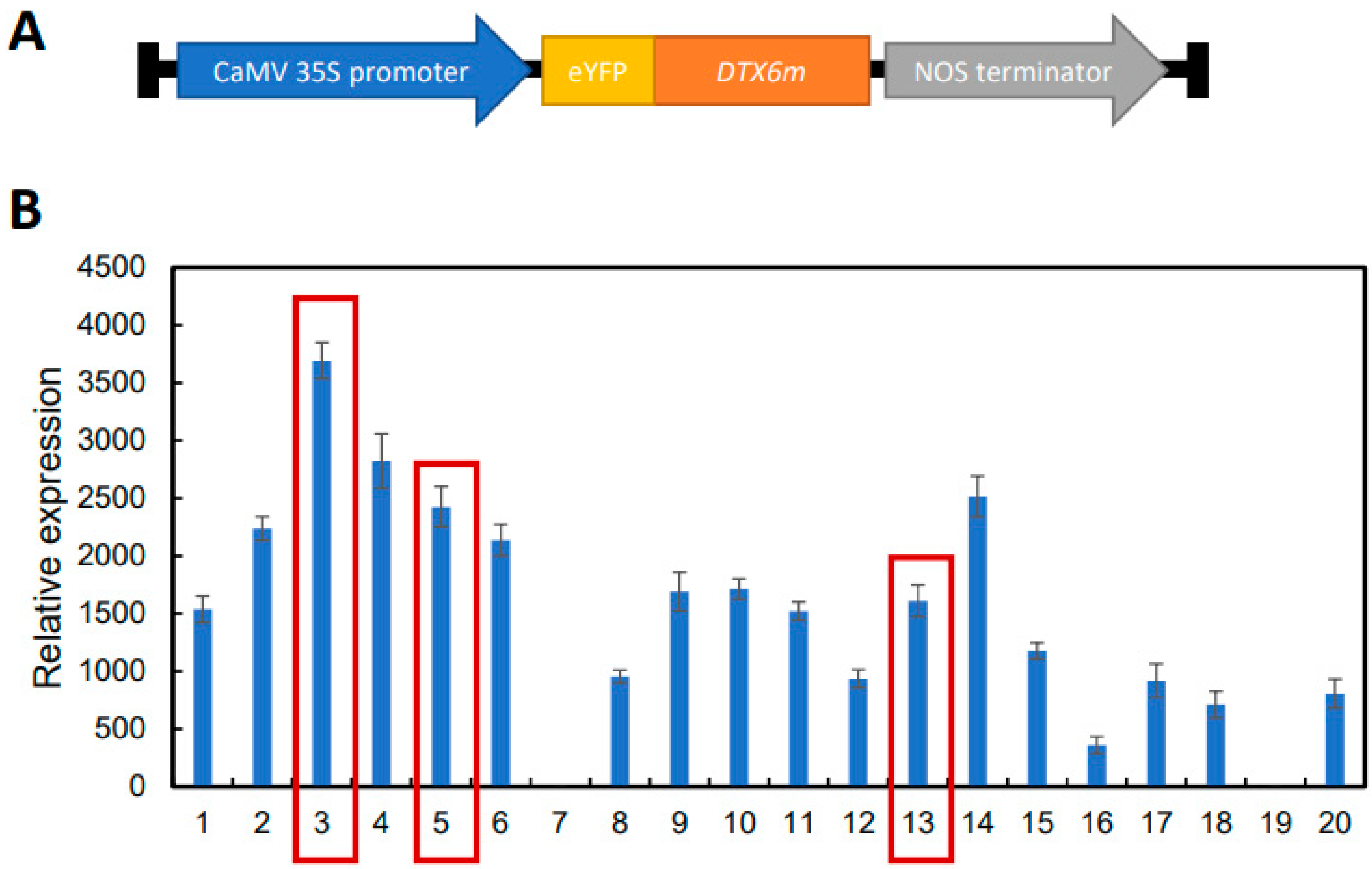
3.1. Generation of DTX6m-Expressing Rice Lines
The G311E-mutated
DTX6 gene allele,
DTX6m, was cloned into the pCAMBIA-N-eYFP vector under the promoter of CaMV 35S and transformed into ZH11 (
Figure 1A). Screening of T
0 transgenic plants identified 20 independent lines harboring the
DTX6m transgene, which were designated OE1 to OE20. Quantitative RT-PCR analysis revealed substantial variation in
DTX6m expression level across these lines (
Figure 1B), with the majority exhibiting significant transgene expression. To assess the correlation between expression levels and herbicide resistance, three representative lines: OE-3 (highest level), OE-5 (intermediate level), and OE-13 (relatively low level), were chosen for phenotypic characterization.
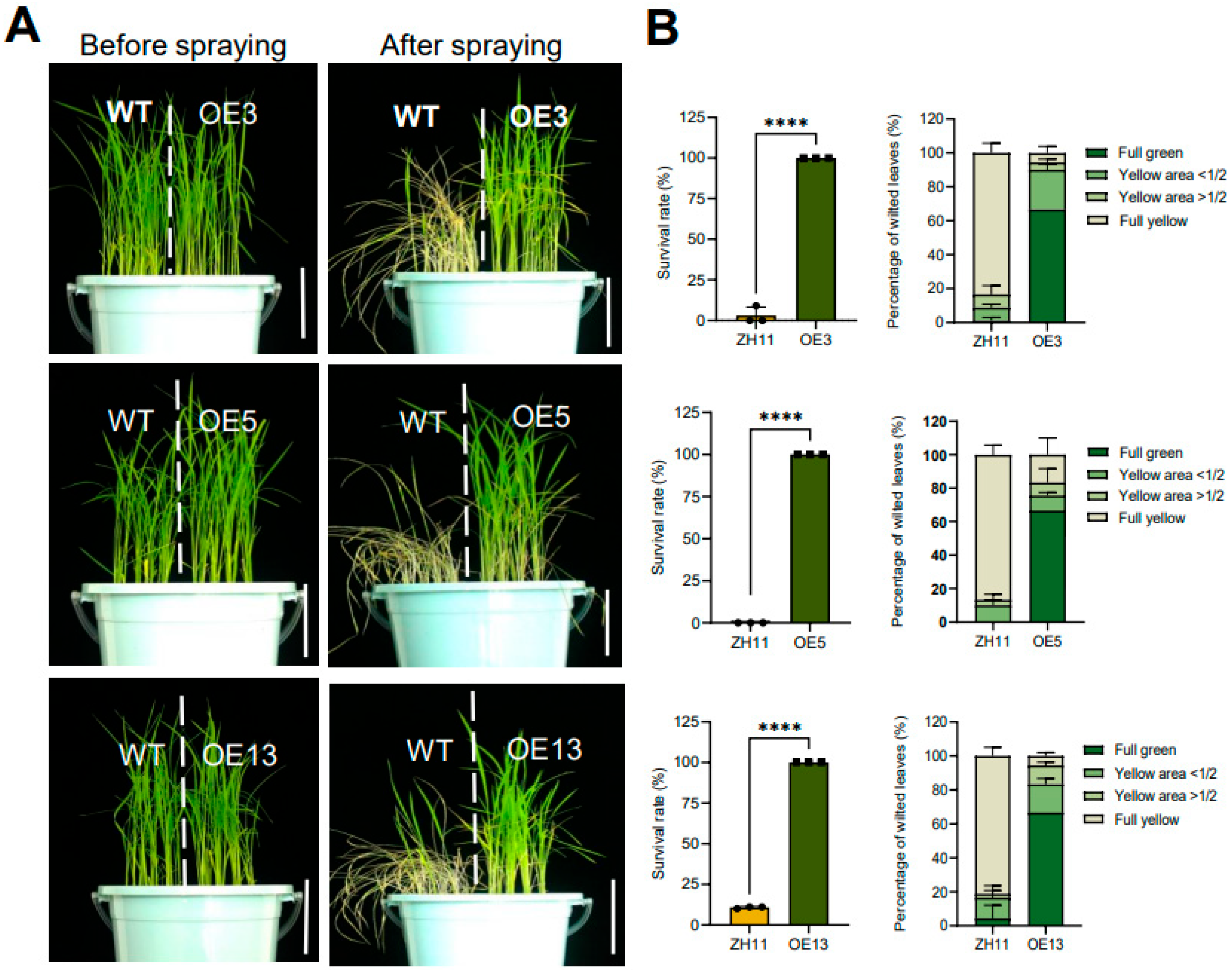
3.2. DTX6m-Overexpression Lines Exhibit Strong Bipyridyl Herbicide Resistance
To evaluate resistance to bipyridyl herbicides, we utilized the T3 generation of the representative lines whose homozygosity were confirmed by the absence of segregation in hygromycin resistance. To preliminarily observe the herbicide resistance on culture media, OE3, 5, 13 and the ZH11 control were grown on 1/2 MS media supplemented with 0.3 µM paraquat for 8 days. As shown in
Figure S1, all overexpression lines had strikingly higher seedling heights and longer primary roots compared to the wild type control, which were severely stunted by the herbicide in the media. To compare their resistance to foliar-applicated herbicide, seedlings grown in soil for 25 days were foliar-sprayed with 0.5 g/L paraquat. Ten days after application, leaves of the ZH11 wild type exhibited severe necrosis, whereas all three overexpression lines maintained green foliage with minimal phytotoxicity symptoms (
Figure 2A). Quantitative analysis confirmed significantly higher survival rates and more retention of functional green leaves in transgenic lines relative to the wild type (
Figure 2B). These findings conclusively demonstrate that constitutive expression of
DTX6m confers substantial paraquat tolerance in rice.
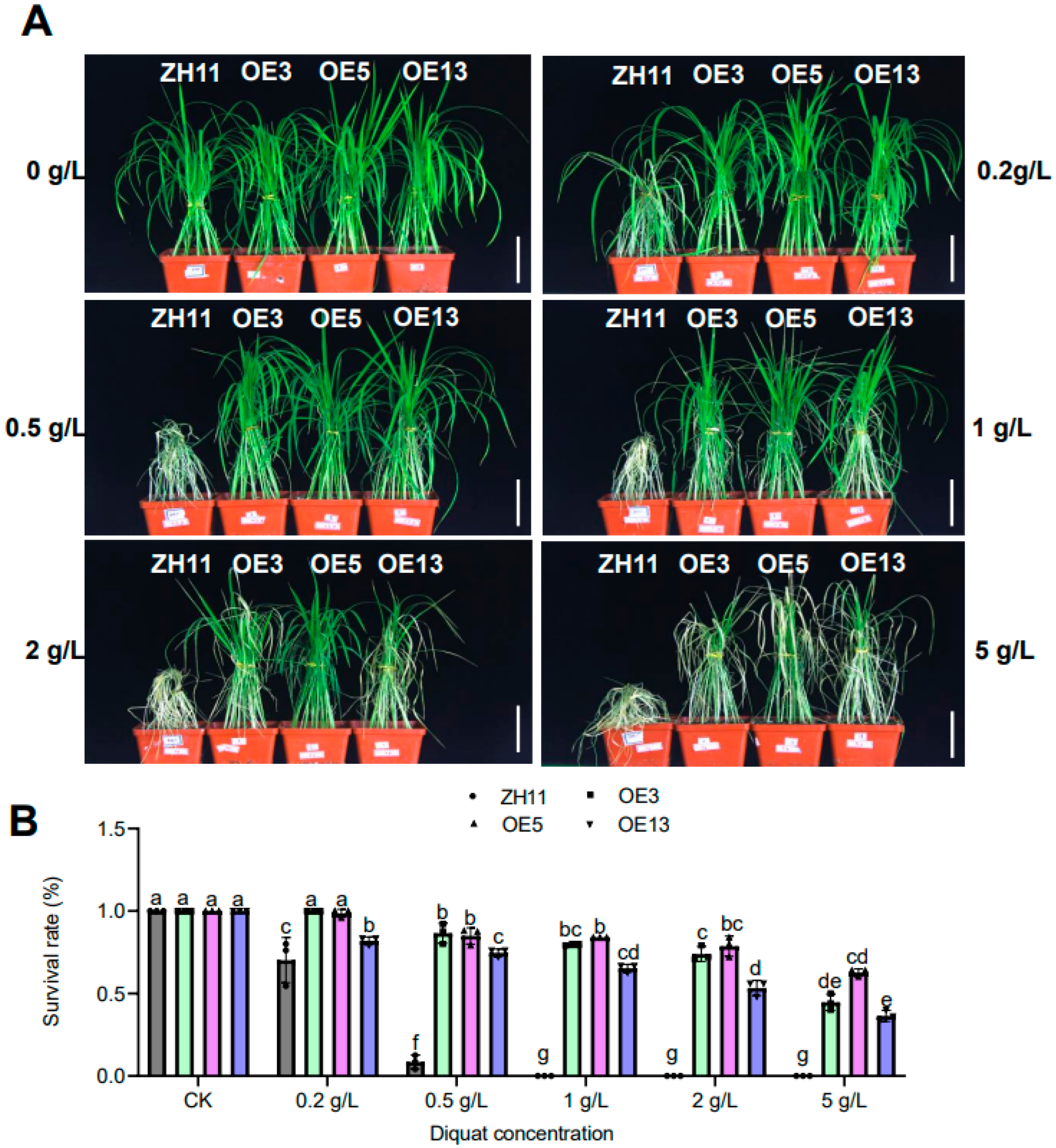
Given the prevalent agricultural usage of diquat as an alternative bipyridyl herbicide to paraquat in many countries, we evaluated diquat resistance in rice plants through multi-stage physiological assessments. Initial screening at the seedling stage employed 25-day-old soil-grown plants subjected to foliar application of diquat at escalating concentrations (0, 0.5, 1, 2, and 5 g/L). Post-treatment evaluation after three days revealed a concentration-dependent response: ZH11 plants exhibited severe chlorosis at all tested concentrations, whereas transgenic lines maintained significant chlorophyll integrity even at 5 g/L (5×the commercial field application rate) (
Figure 3A). Quantitative analysis demonstrated differential tolerance among transgenic lines, with OE3 and OE5 showing significantly stronger resistance compared to OE13 (
Figure 3B), correlating with the higher expression level of
DTX6m in OE3 and OE5 (
Figure 1). Field validation experiments involved diquat application (2 g/L) at the reproductive stage (2 months post-transplant in the paddy field). 10 days after treatment, ZH11 plants displayed complete systemic necrosis and mortality, while all transgenic lines survived without chlorosis (
Figure 4). This translational evidence confirms that
DTX6m overexpression confers robust cross-environmental tolerance to diquat.
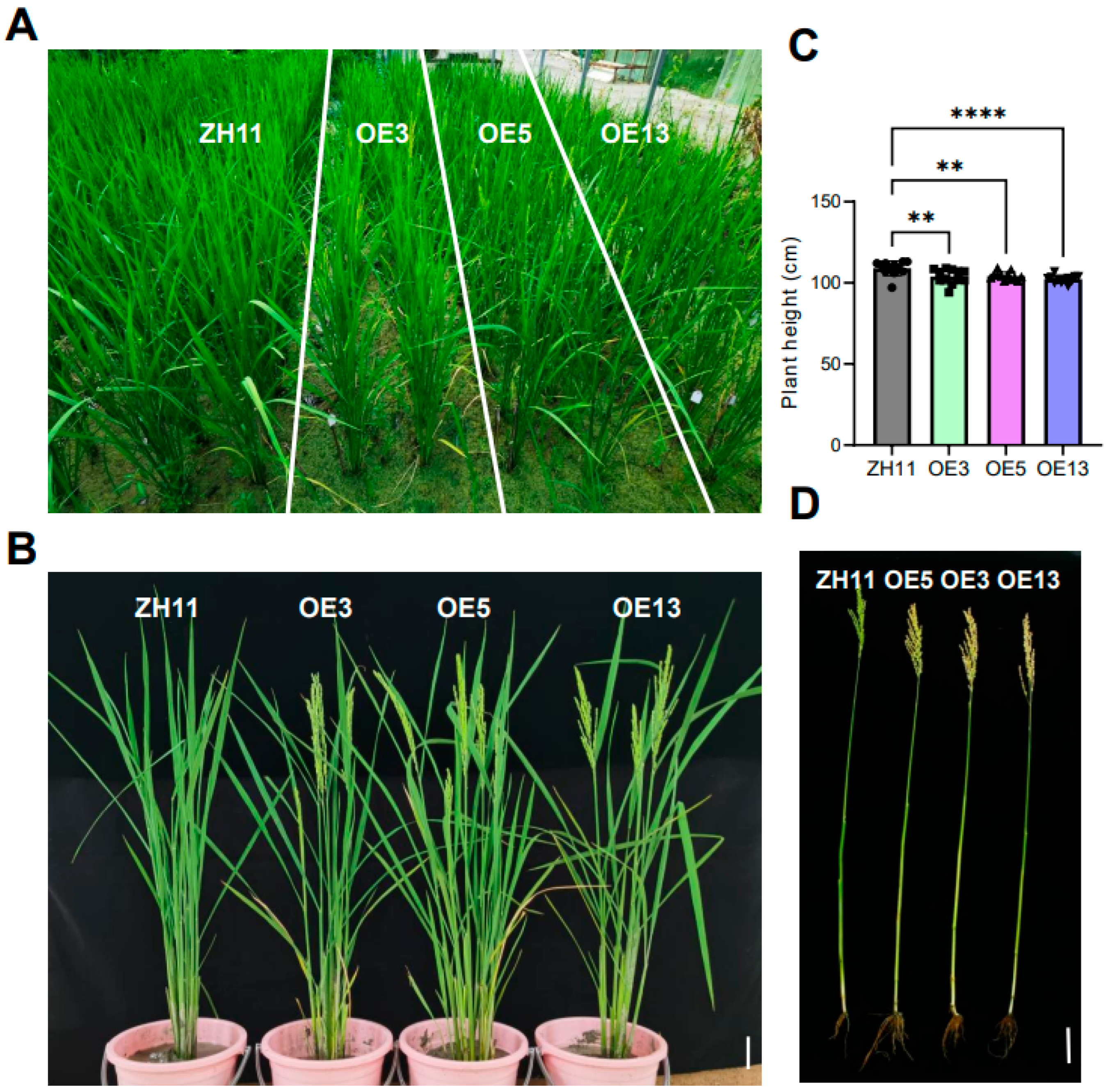
3.3. DTX6m-Overexpression Lines Had Early Flowering and Semi-Dwarf Growth Phenotypes in the Field
To systematically characterize the possible pleiotropic effects of
DTX6m- overexpression in rice, we conducted side-by-side field trials of transgenic lines and ZH11 wild-type controls. While vegetative growth showed no significant differences between the groups, pronounced phenotypic variations emerged during maturation. The
DTX6m-overexpressing lines displayed reduced plant height and an approximately 3-4 days earlier flowering time, accompanied by earlier leaf senescence (
Figure 5A–C). Analysis of primary tillers revealed that transgenic lines produced significantly shorter tillers with precocious maturation relative to ZH11 (
Figure 5D). These findings demonstrate that
DTX6m also functions as a developmental regulator in rice, in addition to the herbicide-detoxifying capacity.
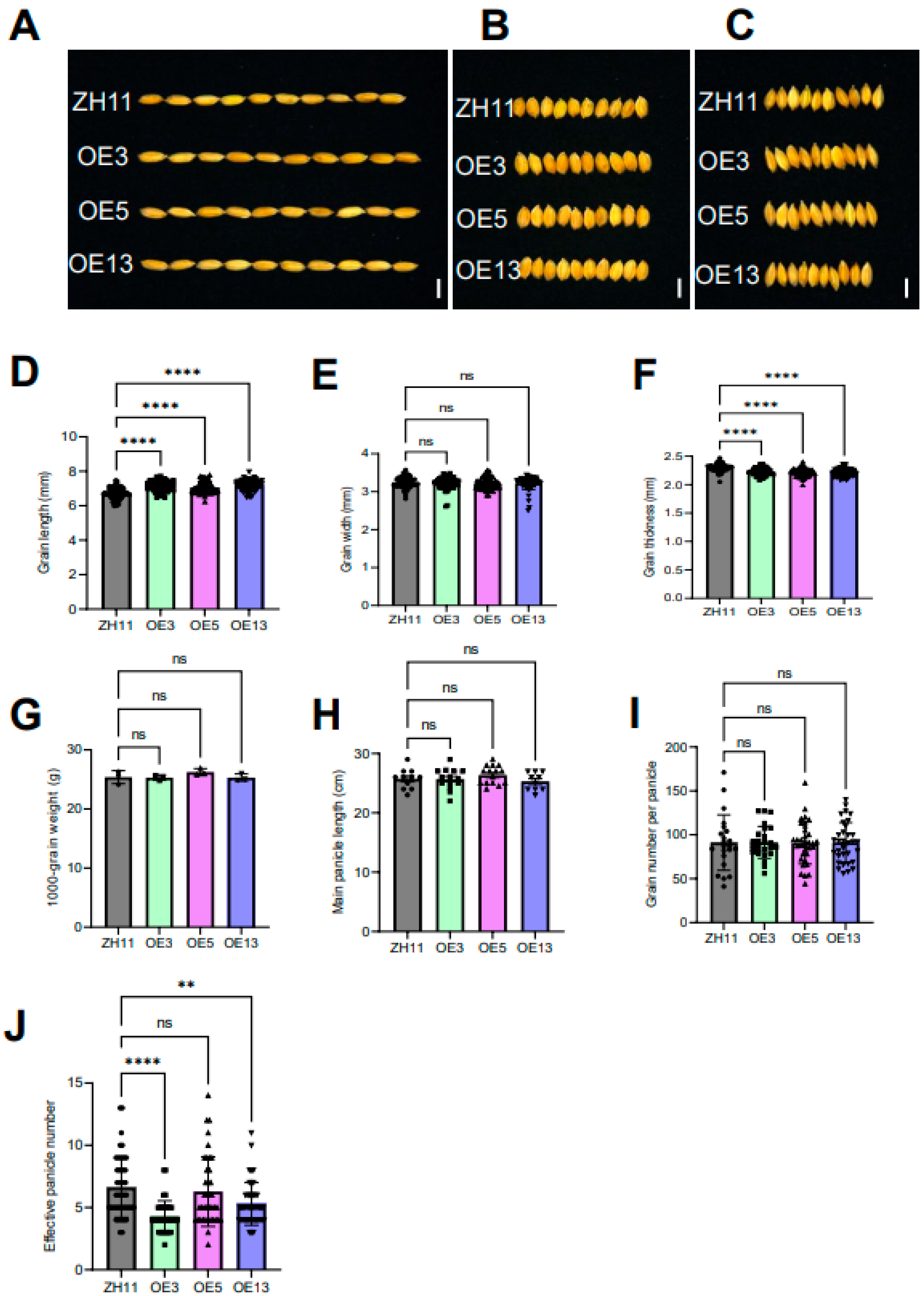
3.4. Analysis of the Field Agronomic Traits of DTX6m-Transgenic Lines
Yield traits are essential considerations in the application of genetically modified crops. We therefore evaluated the impact of
DTX6m overexpression on agronomic traits associated with grain yield, including grain size, 1000-grain weight, number of effective panicles per plant, panicle length, and grain number per panicle. As shown in
Figure 6A–F, OE3, OE5, and OE13 lines displayed three distinct morphological characteristics: (1) increased grain length, (2) comparable grain width, and (3) a slight reduction in grain thickness compared to ZH11. Statistical analysis revealed no significant differences in 1000-grain weight between
DTX6m-OE lines and ZH11 (
Figure 6G). Evaluation of panicle architecture showed consistent patterns in both main panicle length and grain number per panicle across all lines (
Figure 6H,I). However, phenotypic divergence emerged in effective panicle numbers, with OE3 and OE13 showing significant reductions relative to ZH11, while OE5 maintained wild-type levels (
Figure 6J). This differential performance suggests that field-effective panicle formation exhibits environmental sensitivity, with the OE5 line demonstrating better field adaptability, likely attributable to the preserved tillering capacity compared to other transgenic lines.
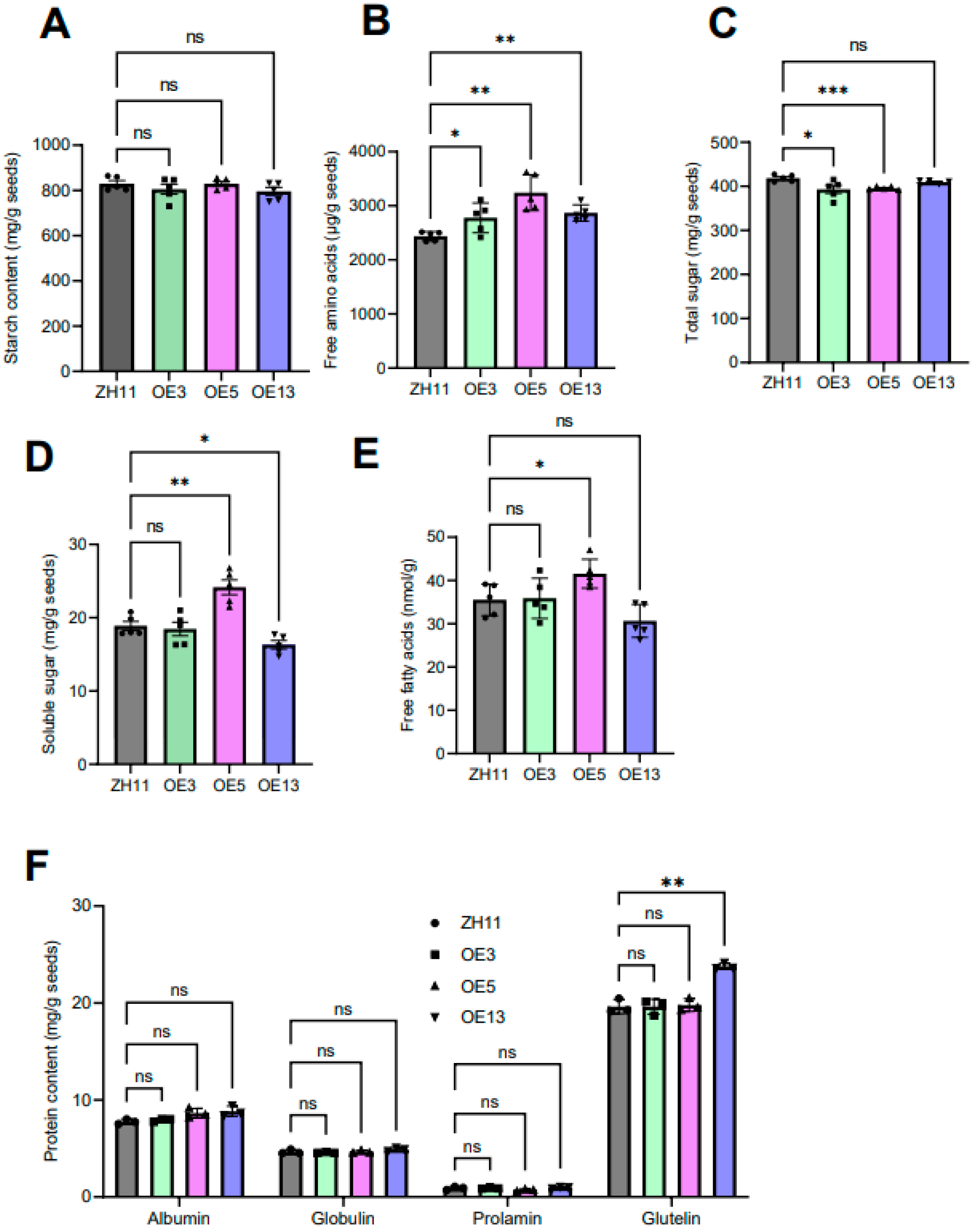
3.5. The Seed Storage Reserve Accumulation Is Not Consistently Affected in the Transgenic Lines Except the Free Amino Acid Level
Rice seeds, as a staple food, serve as a source of carbohydrates, proteins, and lipids for human consumption. To determine whether the overexpression of
DTX6m altered the nutritional quality of rice seeds, we analyzed the levels of starch, total sugar, soluble sugars, free lipidic acids, free amino acids and proteins in transgenic rice lines. The results revealed that the transformation of
DTX6m did not change the starch content (
Figure 7A), but did elevate the free amino acid level in all three transgenic lines (
Figure 7B). As regards the total sugar, soluble sugar and free lipidic acid levels, there were no consistent changes in three transgenic lines (
Figure 7C–E), suggesting that their content might be affected by factors other than
DTX6m. The protein content such as albumin, globulin, prolamin and glutelin levels remained unchanged in the transgenic lines (
Figure 7F). Among the three
DTX6m-overexpression lines, OE5 performed exceptionally well (
Figure 7A–E), exhibiting significantly higher levels of free fatty acids, free amino acids and soluble sugar compared to ZH11.
4. Discussion
4.1. Evolutionary Analysis of Arabidopsis and rice MATE Family Genes
MATE family proteins, belonging to a large superfamily of evolutionarily conserved membrane transporters, are widely distributed across diverse organisms ranging from bacteria to plants and animals [
29]. In plants, these proteins exhibit distinct subcellular localizations, including the plasma membrane [
30,
31], tonoplast [
32,
33,
34,
35], Golgi apparatus [
36], and late endosome/prevacuolar compartment [
37].They mediate a wide spectrum of physiological processes such as heavy metal and herbicide detoxification [
18,
19,
30]; aluminum tolerance [
38,
39]; iron homeostasis regulation [
31,
36,
40]; vacuolar sequestration of secondary metabolites [
32,
33,
35,
41]; hormone delivery [
42,
43,
44,
45]; leaf senescence and turgor modulation [
34,
37]; and pathogen resistance [
46,
47,
48]. Notably, the substrate specificity of MATE family members shows remarkable diversity, necessitating individual characterization for each transporter.
The MATE transporter family comprises 56 members in
Arabidopsis thaliana [
49] and 46 members in rice (
Oryza sativa) [
50]. Phylogenetic analysis of MATE proteins from both species revealed that the rice homologs LOC_Os01g31980, LOC_Os01g49120 and LOC_Os05g48040 show closest evolutionary proximity to
Arabidopsis DTX6 (
Figure S2). Although clustered within the same phylogenetic subgroup, these proteins occupy distinct branches with relatively low sequence identities to DTX6 (55.8%, 53.9% and 50.7%, respectively), highlighting substantial divergence of MATE transporters between monocots and dicots. To functionally explore their relationship, we employed single-base editing technique [
51] to introduce a G311E substitution (equivalent to DTX6's critical glycine residue) in LOC_Os01g31980 (
Figure S3). Unexpectedly, the edited rice plants exhibited no altered resistance phenotypes to paraquat or diquat herbicides, suggesting that LOC_Os01g31980 may not serve as the functional ortholog of DTX6 in rice.
4.2. DTX6m Largely Increased the Bipyridyl Herbicides Resistance of Rice
Weed infestation poses a significant threat to global rice production, with yield losses exceeding 40% in severe cases [
8]. In large-scale rice cultivation, the integration of herbicide-resistant rice cultivars with compatible herbicides has emerged as an effective weed control strategy. Recent advances in understanding plant-herbicide interactions have driven the development of herbicide-tolerant rice through three principal strategies including traditional mutagenesis and phenotypic screening, precise editing of endogenous resistance genes and transgenic introduction of exogenous resistance determinants [
2,
37]. In previous studies, we found that DTX6 mediates paraquat and diquat detoxification through exocytosis and vacuolar sequestration mechanisms. Notably, when glycine at position 311 in
Arabidopsis DTX6 is substituted with acidic amino acid (G311E or G311D), the protein exhibits enhanced detoxification capacity [
18]. In this study, we investigated the potential application of the
DTX6m gene and its side effects in monocot crop rice. Three independent
DTX6m-overexpression lines (OE3, OE5 and OE13) demonstrated remarkable bipyridyl herbicide resistance compared to the ZH11 wild-type. Greenhouse trials with rice seedlings showed that while ZH11 plants completely wilted in 3 days after 1 g/L diquat treatment, all three transgenic lines maintained >80% survival rates. Even at 5 g/L diquat (5× concentration), a significant number of the transgenic plants survived, with OE5 showing the strongest resistance (over 60%). Field trials conducted on two-month-old plants revealed complete mortality of ZH11 within 10 days after 2 g/L diquat application, whereas all transgenic lines survived, demonstrating stable, long-term diquat resistance that appears more effective in mature plants. These results suggest that DTX6m functions similarly in rice and
Arabidopsis, likely through the same mechanisms.
4.3. The Transgenic Rice Has Slightly Early Flowering and Dwarf phenotype Without Compromised Yield and Nutritional Value
The potential pleiotropic effects of DTX6m transgenesis on plant growth, development, yield, and nutrient assimilation were systematically investigated. In field trials, all three transgenic lines exhibited two phenotypes with significant agricultural value: slightly early flowering and semi-dwarfism. Early flowering shortens the growth cycle, optimizing land use efficiency. Meanwhile, the semi-dwarf trait enhances lodging resistance, which is particularly advantageous in typhoon-prone regions.
Before implementing
DTX6m in genetic manipulation, it is crucial to determine its impact on crop yield and grain quality. Therefore, we evaluated key rice yield components, including the number of panicles per plant, the number of grains per panicle, and 1000-grain weight. The results showed that the OE3 and OE13 transgenic lines produced fewer productive panicles than the wild-type ZH11, while the OE5 line did not exhibit such a reduction. In all three transgenic lines, the number of grains per panicle and 1000-grain weight remained unchanged. However, compared to ZH11, all
DTX6m-overexpression lines had altered seed morphology, with increased grain length, unchanged grain width, and reduced grain thickness. Additionally, no significant differences were detected in starch or total protein content between the transgenic and wild-type plants. Overall, among the transgenic lines, OE5 exhibited the most superior performance, demonstrating robust resistance to bipyridyl herbicides while maintaining rice yield and nutritional quality. These findings highlight the critical need for screening large transgenic populations to identify optimally performing lines for agricultural implementation. The significantly elevated accumulation of free amino acids in all three independent
DTX6m-OE lines (
Figure 7B) implies a potential role for DTX6 in amino acid homeostasis. However, the precise function of DTX6 in
Arabidopsis still remains elusive at present. Future research will focus on identifying the endogenous substrates of DTX6 to clarify its physiological role.
5. Conclusions
Our results reveal that the DTX6m gene effectively confers resistance to bipyridyl herbicides in rice without compromising yield potential. Moreover, its overexpression leads to early flowering, semi-dwarfism, and increased free amino acid levels in rice grains. The phenotypes induced by DTX6m in rice suggest generalizability to other monocot cereal crops. Collectively, our findings in rice and previous results in Arabidopsis indicate that DTX6m holds broad utility for improving herbicide resistance in both monocot and dicot crops.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org.
Author Contributions
Conceptualization, Xiaochun Ge; Data curation, Gaoan Chen, Jiaying Han and Ziyan Sun; Funding acquisition, Xiaochun Ge; Investigation, Gaoan Chen, Jiaying Han, Ziyan Sun, Mingming Zhao and Zihan Zhang; Methodology, Shuo An, Muyu Shi and Jinxiao Yang; Resources, Jinxiao Yang; Supervision, Xiaochun Ge; Validation, Gaoan Chen; Writing – original draft, Jiaying Han and Xiaochun Ge; Writing – review & editing, Xiaochun Ge.
Funding
This work was supported by the Natural Science Foundation of Shanghai Municipality (No. 23ZR1403400) and National Natural Science Foundation of China (No. 31970343).
Data Availability
Data will be made available on request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Kaur, S.; Kaur, R.; Chauhan, B. S. , Understanding crop-weed-fertilizer-water interactions and their implications for weed management in agricultural systems. Crop Prot 2018, 103, 65–72. [Google Scholar] [CrossRef]
- Kuang, Y. J.; Yu, H. Y.; Qi, F. Y.; Zhou, X. P.; Li, X. J.; Zhou, H. B. , Developing herbicide-resistant crops through genome editing technologies: A review. Crop Prot 2024, 183, 106745. [Google Scholar] [CrossRef]
- Hussain, A.; Ding, X.; Alariqi, M.; Manghwar, H.; Hui, F. J.; Li, Y. P.; Cheng, J. Q.; Wu, C. L.; Cao, J. L.; Jin, S. X. Herbicide Resistance: Another Hot Agronomic Trait for Plant Genome Editing. Plants-Basel 2021, 10, 621. [Google Scholar] [CrossRef]
- Farooq, M.; Siddique, K. H. M.; Rehman, H.; Aziz, T.; Lee, D. J.; Wahid, A. , Rice direct seeding: Experiences, challenges and opportunities. Soil Till Res 2011, (2), 87–98. [Google Scholar] [CrossRef]
- Mahajan, G.; Chauhan, B. S. , Weed control in dry direct-seeded rice using tank mixtures of herbicides in South Asia. Crop Prot 2015, 72, 90–96. [Google Scholar] [CrossRef]
- Kumar, V.; Bellinder, R. R.; Gupta, R. K.; Malik, R. K.; Brainard, D. C. , Role of herbicide-resistant rice in promoting resource conservation technologies in rice-wheat cropping systems of India: A review. Crop Prot 2008, 27, 290–301. [Google Scholar] [CrossRef]
- Beckie, H. J.; Busi, R.; Bagavathiannan, M. V.; Martin, S. L. , Herbicide resistance gene flow in weeds: Under-estimated and under-appreciated. Agr Ecosyst Environ 2019, 283, 106566. [Google Scholar] [CrossRef]
- Jin, M.; Chen, L.; Deng, X. W.; Tang, X. Y. , Development of herbicide resistance genes and their application in rice. Crop J 2022, 10, 26–35. [Google Scholar] [CrossRef]
- Shah, D. M.; Horsch, R. B.; Klee, H. J.; Kishore, G. M.; Winter, J. A.; Tumer, N. E.; Hironaka, C. M.; Sanders, P. R.; Gasser, C. S.; Aykent, S.; Siegel, N. R.; Rogers, S. G.; Fraley, R. T. , Engineering Herbicide Tolerance in Transgenic Plants. Science 1986, 233, 478–481. [Google Scholar] [CrossRef]
- Das, T. K.; Behera, B.; Nath, C. P.; Ghosh, S.; Sen, S.; Raj, R.; Ghosh, S.; Sharma, A. R.; Yaduraju, N. T.; Nalia, A.; Dutta, A.; Kumar, N.; Singh, R.; Pathak, H.; Singh, R. G.; Hazra, K. K.; Ghosh, P. K.; Layek, J.; Patra, A.; Paramanik, B. , Herbicides use in crop production: An analysis of cost-benefit, non-target toxicities and environmental risks. Crop Prot 2024, 181, 106691. [Google Scholar] [CrossRef]
- Gianessi, L. P. , The increasing importance of herbicides in worldwide crop production. Pest Manag Sci 2013, 69, 1099–1105. [Google Scholar] [CrossRef] [PubMed]
- Vencill, W. K.; Nichols, R. L.; Webster, T. M.; Soteres, J. K.; Mallory-Smith, C.; Burgos, N. R.; Johnson, W. G.; McClelland, M. R. , Herbicide Resistance: Toward an Understanding of Resistance Development and the Impact of Herbicide-Resistant Crops. Weed Sci 2012, 60, 2–30. [Google Scholar] [CrossRef]
- Farrington, J. A.; Ebert, M.; Land, E. J.; Fletcher, K. , Bipyridylium Quaternary-Salts and Related Compounds.5. Pulse-Radiolysis Studies of Reaction of Paraquat Radical with Oxygen - Implications for Mode of Action of Bipyridyl Herbicides. Biochim Biophys Acta 1973, 314, 372–381. [Google Scholar] [CrossRef]
- Fussell, K. C.; Udasin, R. G.; Gray, J. P.; Mishin, V.; Smith, P. J. S.; Heck, D. E.; Laskin, J. D. , Redox cycling and increased oxygen utilization contribute to diquat-induced oxidative stress and cytotoxicity in Chinese hamster ovary cells overexpressing NADPH-cytochrome P450 reductase. Free Radical Bio Med 2011, 50, 874–882. [Google Scholar] [CrossRef]
- Guo, H. H.; Li, L.; Gao, L. , Paraquat and Diquat: Recent Updates on Their Pretreatment and Analysis Methods since 2010 in Biological Samples. Molecules 2023, 28, 684. [Google Scholar] [CrossRef]
- Kelemen, A.; Garda, T.; Kónya, Z.; Erdödi, F.; Ujlaky-Nagy, L.; Juhász, G. P.; Freytag, C.; M-Hamvas, M.; Máthé, C. , Treatments with Diquat Reveal the Relationship between Protein Phosphatases (PP2A) and Oxidative Stress during Mitosis in Arabidopsis thaliana Root Meristems. Plants-Basel 2024, 13, 1896. [Google Scholar] [CrossRef]
- Schulteis, B. M.; Moisinho, I. S.; Butler-Jones, A.; Besançon, T. E.; Brunharo, C.; Sosnoskie, L. M. , Response of Horseweed from New York Vineyards and Orchards to Paraquat and Diquat. Hortscience 2025, 60, 554–562. [Google Scholar] [CrossRef]
- Lv, Z.; Zhao, M. M.; Wang, W. J.; Wang, Q.; Huang, M. Q.; Li, C. Q.; Lian, Q. C.; Xia, J. Q.; Qi, J.; Xiang, C. B.; Tang, H. R.; Ge, X. C. , Changing Gly311 to an acidic amino acid in the MATE family protein DTX6 enhances resistance to the dihydropyridine herbicides. Mol Plant 2021, 14, 2115–2125. [Google Scholar] [CrossRef]
- Xia, J. Q.; Nazish, T.; Javaid, A.; Ali, M.; Liu, Q. Q.; Wang, L.; Zhang, Z. Y.; Zhang, Z. S.; Huang, Y. J.; Wu, J.; Yang, Z. S.; Sun, L. F.; Chen, Y. X.; Xiang, C. B. , A gain-of-function mutation of the MATE family transporter DTX6 confers paraquat resistance in Arabidopsis. Mol Plant 2021, 14, 2126–2133. [Google Scholar] [CrossRef]
- Hiei, Y.; Komari, T. , -mediated transformation of rice using immature embryos or calli induced from mature seed. Nat Protoc 2008, 3, 824–834. [Google Scholar] [CrossRef]
- Laurell, S.; Tibbling, G. , Colorimetric Micro-Determination of Free Fatty Acids in Plasma. Clin Chim Acta 1967, 16, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Hron, W. T.; Menahan, L. A. , A Sensitive Method for the Determination of Free Fatty-Acids in Plasma. J Lipid Res 1981, 22, 377–381. [Google Scholar] [CrossRef]
- Miller, G. L. , Use of Dinitrosalicylic Acid Reagent for Determination of Reducing Sugar. Anal Chem 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Yemm, E. W.; Willis, A. J. , The Estimation of Carbohydrates in Plant Extracts by Anthrone. Biochem J 1954, 57, 508–514. [Google Scholar] [CrossRef] [PubMed]
- Hansen, J.; Moller, I. , Percolation of Starch and Soluble Carbohydrates from Plant-Tissue for Quantitative-Determination with Anthrone. Anal Biochem 1975, 68, 87–94. [Google Scholar] [CrossRef]
- Yu, J.; Li, S. M.; Xu, Q. Y.; Zheng, X.; Shao, Y. F.; Zhu, D. W. , Extraction and determination of rice protein components. Quality and Safety of Agro-Products 2023, 2, 10–14. [Google Scholar]
- Doi, E.; Shibata, D.; Matoba, T. , Modified Colorimetric Ninhydrin Methods for Peptidase Assay. Anal Biochem 1981, 118, 173–184. [Google Scholar] [CrossRef]
- Guindon, S.; Dufayard, J. F.; Lefort, V.; Anisimova, M.; Hordijk, W.; Gascuel, O. , New Algorithms and Methods to Estimate Maximum-Likelihood Phylogenies: Assessing the Performance of PhyML 3.0. Syst Biol 2010, 59, 307–321. [Google Scholar] [CrossRef] [PubMed]
- Omote, H.; Hiasa, M.; Matsumoto, T.; Otsuka, M.; Moriyama, Y. , The MATE proteins as fundamental transporters of metabolic and xenobiotic organic cations. Trends Pharmacol Sci 2006, 27, 587–593. [Google Scholar] [CrossRef]
- Li, L. G.; He, Z. Y.; Pandey, G. K.; Tsuchiya, T.; Luan, S. , Functional cloning and characterization of a plant efflux carrier for multidrug and heavy metal detoxification. J Biol Chem 2002, 277, 5360–5368. [Google Scholar] [CrossRef]
- Rogers, E. E.; Guerinot, M. L. , FRD3, a member of the multidrug and toxin efflux family, controls iron deficiency responses in Arabidopsis. Plant Cell 2002, 14, 1787–1799. [Google Scholar] [CrossRef] [PubMed]
- Debeaujon, I.; Peeters, A. J. M.; Léon-Kloosterziel, K. M.; Koornneef, M. , The TRANSPARENT TESTA12 gene of Arabidopsis encodes a multidrug secondary transporter-like protein required for flavonoid sequestration in vacuoles of the seed coat endothelium. Plant Cell 2001, 13, 853–871. [Google Scholar] [CrossRef] [PubMed]
- Marinova, K.; Pourcel, L.; Weder, B.; Schwarz, M.; Barron, D.; Routaboul, J. M.; Debeaujon, I.; Klein, M. , The MATE transporter TT12 acts as a vacuolar flavonoid/H+-antiporter active in proanthocyanidin-accumulating cells of the seed coat. Plant Cell 2007, 19, 2023–2038. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H. W.; Zhao, F. G.; Tang, R. J.; Yu, Y. X.; Song, J. L.; Wang, Y.; Li, L. G.; Luan, S. , Two tonoplast MATE proteins function as turgor-regulating chloride channels in Arabidopsis. P Natl Acad Sci USA 2017, 114, E2036–E2045. [Google Scholar] [CrossRef]
- Zhao, J.; Huhman, D.; Shadle, G.; He, X. Z.; Sumner, L. W.; Tang, Y. H.; Dixon, R. A. , MATE2 Mediates Vacuolar Sequestration of Flavonoid Glycosides and Glycoside Malonates in Medicago truncatula. Plant Cell 2011, 23, 1536–1555. [Google Scholar] [CrossRef]
- Seo, P. J.; Park, J.; Park, M. J.; Kim, Y. S.; Kim, S. G.; Jung, J. H.; Park, C. M. , A Golgi-localized MATE transporter mediates iron homoeostasis under osmotic stress in Arabidopsis. Biochem J 2012, 442, 551–561. [Google Scholar] [CrossRef]
- Jia, M.; Liu, X. Y.; Xue, H.; Wu, Y.; Shi, L.; Wang, R.; Chen, Y.; Xu, N.; Zhao, J.; Shao, J. X.; Qi, Y. F.; An, L. J.; Sheen, J.; Yu, F. , Noncanonical ATG8-ABS3 interaction controls senescence in plants. Nat Plants 2019, 5, 212–224. [Google Scholar] [CrossRef]
- Hoekenga, O. A.; Maron, L. G.; Piñeros, M. A.; Cançado, G. M. A.; Shaff, J.; Kobayashi, Y.; Ryan, P. R.; Dong, B.; Delhaize, E.; Sasaki, T.; Matsumoto, H.; Yamamoto, Y.; Koyama, H.; Kochian, L. V. , AtALMT1, which encodes a malate transporter, is identified as one of several genes critical for aluminum tolerance in Arabidopsis. P Natl Acad Sci USA 2006, 103, 9738–9743. [Google Scholar] [CrossRef]
- Zhou, G. F.; Delhaize, E.; Zhou, M. X.; Ryan, P. R. The barley MATE gene, HvAACT1, increases citrate efflux and Al3+ tolerance when expressed in wheat and barley. Ann Bot-London 2013, 112, 603–612. [Google Scholar] [CrossRef]
- Durrett, T. P.; Gassmann, W.; Rogers, E. E. , The FRD3-mediated efflux of citrate into the root vasculature is necessary for efficient iron translocation. Plant Physiol 2007, 144, 197–205. [Google Scholar] [CrossRef]
- Morita, M.; Shitan, N.; Sawada, K.; Van Montagu, M. C. E.; Inzé, D.; Rischer, H.; Goossens, A.; Oksman-Caldentey, K. M.; Moriyama, Y.; Yazaki, K. , Vacuolar transport of nicotine is mediated by a multidrug and toxic compound extrusion (MATE) transporter in Nicotiana tabacum. P Natl Acad Sci USA 2009, 106, 2447–2452. [Google Scholar] [CrossRef]
- Zhang, H. W.; Zhu, H. F.; Pan, Y. J.; Yu, Y. X.; Luan, S.; Li, L. G. A DTX/MATE-Type Transporter Facilitates Abscisic Acid Efflux and Modulates ABA Sensitivity and Drought Tolerance in Arabidopsis. Mol Plant 2014, 7, 1522–1532. [Google Scholar] [CrossRef]
- Qin, P.; Zhang, G. H.; Hu, B. H.; Wu, J.; Chen, W. L.; Ren, Z. J.; Liu, Y. L.; Xie, J.; Yuan, H.; Tu, B.; Ma, B. T.; Wang, Y. P.; Ye, L. M.; Li, L. G.; Xiang, C. B.; Li, S. G. , Leaf-derived ABA regulates rice seed development via a transporter-mediated and temperature-sensitive mechanism. Sci Adv 2021, 7, eabc8873. [Google Scholar] [CrossRef]
- Nawrath, C.; Heck, S.; Parinthawong, N.; Métraux, J. P. , EDS5, an essential component of salicylic acid-dependent signaling for disease resistance in Arabidopsis, is a member of the MATE transporter family. Plant Cell 2002, 14, 275–286. [Google Scholar] [CrossRef] [PubMed]
- Serrano, M.; Wang, B. J.; Aryal, B.; Garcion, C.; Abou-Mansour, E.; Heck, S.; Geisler, M.; Mauch, F.; Nawrath, C.; Métraux, J. P. , Export of Salicylic Acid from the Chloroplast Requires the Multidrug and Toxin Extrusion-Like Transporter EDS5. Plant Physiol 2013, 162, 1815–1821. [Google Scholar] [CrossRef] [PubMed]
- Sun, X. L.; Gilroy, E. M.; Chini, A.; Nurmberg, P. L.; Hein, I.; Lacomme, C.; Birch, P. R. J.; Hussain, A.; Yun, B. W.; Loake, G. J. , encodes a MATE-transporter that negatively regulates plant disease resistance. New Phytol 2011, 192, 471–482. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, M.; Sharma, D.; Singh, M.; Tripathi, R. D.; Trivedi, P. K. Expression of OsMATE1 and OsMATE2 alters development, stress responses and pathogen susceptibility in Arabidopsis. Sci Rep-Uk 2014, 4, 3964. [Google Scholar] [CrossRef] [PubMed]
- Dobritzsch, M.; Lübken, T.; Eschen-Lippold, L.; Gorzolka, K.; Blum, E.; Matern, A.; Marillonnet, S.; Böttcher, C.; Dräger, B.; Rosahl, S. , MATE Transporter-Dependent Export of Hydroxycinnamic Acid Amides. Plant Cell 2016, 28, 583–596. [Google Scholar] [CrossRef]
- Wang, L.; Bei, X.; Gao, J.; Li, Y.; Yan, Y.; Hu, Y. , The similar and different evolutionary trends of MATE family occurred between rice and Arabidopsis thaliana. BMC Plant Biol 2016, 16, 207. [Google Scholar] [CrossRef]
- Du, Z. X.; Su, Q. T.; Wu, Z.; Huang, Z.; Bao, J. Z.; Li, J. B.; Tu, H.; Zeng, C. H.; Fu, J. R.; He, H. H. , Genome-wide characterization of MATE gene family and expression profiles in response to abiotic stresses in rice (Oryza sativa). Bmc Ecol Evol 2021, 21, 1–14. [Google Scholar] [CrossRef]
- Zhang, C. W.; Wang, Y.; Wang, F. P.; Zhao, S.; Song, J. L.; Feng, F.; Zhao, J. R.; Yang, J. X. , Expanding base editing scope to near-PAMless with engineered CRISPR/Cas9 variants in plants. Mol Plant 2021, 14, 191–194. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).