1. Introduction
Kawasaki disease (KD) is an autoimmune vasculitis syndrome. The most serious complication is coronary artery lesion (CAL). Without effective treatment, the risk of cardiovascular damage can be as high as 15-20% [
1]. It has become the most common acquired heart disease in pediatrics. With the widespread application of IVIG, the incidence of CAL has decreased year by year. However, there are still IVIG-R KD, which is also a high-risk factor for coronary artery aneurysms (CAA) [
2]. Clinicians urgently hope to find an indicator that can predict IVIG-R KD, CAL and MOD in the acute phase. Growth stimulation expressed gene 2 protein (ST
2) belongs to the interleukin-1 receptor family and includes two forms: soluble ST2 (sST2) and transmembrane form sST2 (sST2L). Currently, it is believed that sST2 is a myocardial protein produced by myocardial cells under the action of biomechanical forces. These two forms of ST2 proteins are directly related to the progression of heart disease. Previous research on sST2 mainly focused on heart failure. In recent years, it has been found that sST2 is associated with the progression of various diseases such as heart disease, tumors, and inflammation such as sepsis [
3].
The acute phase of KD is characterized by necrotizing vasculitis, mainly infiltrated by neutrophils and macrophages, with elevated inflammatory markers. Studies had shown that sST2 is elevated in the acute phase of Kawasaki disease [
4]. Exploring the relationship between the elevation of sST2 in the acute phase of Kawasaki disease and coronary artery damage, IVIG-R KD, and multi-organ involvement can help in the timely treatment and prevention of serious complications of KD. It is an acute febrile exanthematous disease with systemic small and medium-sized arterial inflammatory lesions as the main pathological changes. The most serious complication is CAA. IVIG-R KD can involve multiple organs throughout the body. Systemic small and medium-sized arteritis is the main pathological change of acute febrile exanthematous disease.
2. Methods
Patients: A total of 287 children with KD who were diagnosed and treated in the Department of Pediatric Cardiology, Shengjing Hospital Affiliated to China Medical University from November 2021 to December 2022. And 4 cases with ST2 > 200 ng/mL were followed up until April 2025.
2.1. Inclusion Criteria
Children with KD who met the diagnostic criteria of KD in the 2017 American Heart Association (AHA) [
5] and the sixth revised diagnostic criteria of KD in Japan [
6], and who received treatment and were followed up. Diagnosis of CAL: according to the sixth revised criteria in Japan. The determination of Z value:Japan Kobayashi(
http://raise.umin.jp/zsp/CALculator)
2.2. Exclusion Criteria
①The duration of fever exceeded 10 days at the time of admission; ②sST2 was not detected (sST2 can only be detected on working days); ③Those who received gamma globulin or corticosteroid treatment within 1 month.
2.3. Groups
Based laboratery detection results, and changes in electrocardiogram (ECG), ECHO, 287 KD patients were divided into ① myocardial damage (MD): yes= Group A (17 cases) vs no = Group B (270 cases); ②CAL: yes= Group C (48 cases) vs no = Group D (239 cases); ③≥ 3 organs involved (MOD): yes = Group E (58 cases) vs < 3 organs involved = Group F (229 cases); IVIG-R KD: yes = Group G (24 cases) vs no = Group H (263 cases). Data collection: sST2: the concentration of sST2 in serum was quantitatively detected on a 96-well plate using a double-antibody sandwich ELISA method. 2 ml of venous blood was collected from the children. The kit was from Shanghai Ruidi Biotechnology Co., Ltd., and the detection was performed using the Freedom Evolyzer-2100 fully automatic enzyme immunoassay integrated machine from TECAN, Switzerland. At the same time, the biochemical indicators such as complete blood count, C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), interleukin 6 (IL-6), troponin I, high-sensitivity troponin T, N-terminal pro-B-type natriuretic peptide (NT-pro BNP), serum albumin (ALB), D-dimer, etc., as well as imaging data such as ECG, ECHO, electroencephalogram (EEG), and thoracic CT of 287 children were collected.
2.4. Statistical Analysis
The data were analyzed using SPSS 27.0 statistical software. Measurement data conforming to the normal distribution were expressed as (x ± s). For data with normal distribution and equal variance, an independent samples t-test was used. For data with normal distribution but unequal variance, a t,-test was used. Skewed distribution data were expressed as median (interquartile range), and the Mann-Whitney U test was used. Enumeration data were expressed as the number of cases and percentage, and the chi-square test was used for comparison. Binary logistic regression analysis was used for the analysis of influencing factors. The receiver operating characteristic curve (ROC) was used to evaluate the predictive value of sST2 for KD patients. p<0.05 indicates statistically significant difference. Spearman correlation analysis was used to analyze the correlation between each indicator and sST2.
3. Results
1.The age and gender distribution of children with KD
Among the 287 children with KD, 213 (72.20%) were under 3 years old, and the male-to-female ratio was 1.58:1. The proportion of males and the age were both lower in the KD with CAL group (P<0.05).
Table 1.
General information.
Table 1.
General information.
| |
groups |
age (year) |
male (%) |
P年龄
|
P性别
|
| MD |
A (17) |
2.0 (0.6~3.0) |
9(52.94) |
0.061 |
0.464 |
| B (270) |
2.5 (1.5~4.0) |
167(61.85) |
| CAL |
C (48) |
1.9 (0.8~2.7) |
40(83.33) |
0.003 |
<0.001 |
| D (239) |
2.5 (1.5~4.0) |
136(56.90) |
| MOD |
E (58) |
2.7 (1.0~4.0) |
36(62.07) |
0.849 |
0.896 |
| F (229) |
2.4 (1.4~4.0) |
140(61.14) |
| IVIG-R KD |
G (24) |
2.8 (1.8~5.0) |
17(70.83) |
0.109 |
0.318 |
| H (263) |
2.4 (1.4~4.0) |
159(60.46) |
2.Comparison of sST2 levels among different groups
The sST2 levels in group A, C, E and G were significantly higher than those in group B, D, F, and H (P<0.05).
Table 2.
Comparison of sST2 levels among different groups.
Table 2.
Comparison of sST2 levels among different groups.
| |
groups |
sST2 (ng/mL) |
Z |
P |
| MD |
A (17) |
55.53(41.97~120.58) |
-3.150 |
0.002 |
| B (270) |
38.28(27.25~57.60) |
| CAL |
C (48) |
42.82(32.24~71.78) |
-2.086 |
0.037 |
| D (239) |
38.35(27.14~57.46) |
| MOD |
E (58) |
59.58(37.47~96.14) |
-5.380 |
<0.001 |
| F (229) |
37.49(26.33~51.83) |
| IVIG-R KD |
G (24) |
65.67(43.96~183.66) |
-4.214 |
<0.001 |
| H (263) |
37.73(27.29~55.62) |
3.Comparison of other indicators among different groups
The level of CRP、NT-pro BNP and D-dimer in group A、C、E, and G were respectively higher than those in groups B, D, F, and H (P<0.05).
Table 3.
Comparison of other indicators among different groups.
Table 3.
Comparison of other indicators among different groups.
| |
PWBC
|
PHB
|
PPLT
|
PCRP
|
PIL-6
|
PESR
|
PBNP
|
PD-dimer
|
PALB
|
| A vs B |
0.001 |
0.134 |
0.046 |
0.018 |
0.002 |
0.348 |
<0.001 |
0.003 |
0.072 |
| C vs D |
0.37 |
0.001 |
0.011 |
0.039 |
0.076 |
0.933 |
0.037 |
0.005 |
0.032 |
| E vs F |
0.002 |
<0.001 |
0.002 |
<0.001 |
<0.001 |
0.772 |
<0.001 |
<0.001 |
<0.001 |
| G vs H |
0.032 |
<0.001 |
0.05 |
<0.001 |
0.001 |
0.288 |
0.009 |
0.002 |
<0.001 |
4.Correlation analysis between sST2 and other indicators
The correlation coefficient r was calculated using Spearman correlation analysis. A correlation was considered weak when 0.3 ≤ |r| < 0.5. sST2 had a weak positive correlation with WBC, CRP, IL-6, NT-pro BNP, and D-dimer, and a weak negative correlation with HB and ALB. There was no correlation between sST2 and ESR or PLT.
Table 4.
Correlation analysis between sST2 and other indexs.
Table 4.
Correlation analysis between sST2 and other indexs.
| |
Indexs |
r |
Sig. |
95% confidence interval (CI) |
| lower limit |
upper limit |
| sST2 |
WBC |
0.301 |
<0.001 |
0.188 |
0.405 |
| HB |
-0.333 |
<0.001 |
-0.434 |
-0.222 |
| PLT |
0.196 |
<0.001 |
0.079 |
0.308 |
| CRP |
0.412 |
<0.001 |
0.308 |
0.506 |
| IL-6 |
0.456 |
<0.001 |
0.352 |
0.548 |
| ESR |
0.105 |
0.08 |
-0.016 |
0.223 |
| NT-pro BNP |
0.419 |
<0.001 |
0.315 |
0.514 |
| D-dimer |
0.367 |
<0.001 |
0.258 |
0.467 |
| ALB |
-0.403 |
<0.001 |
-0.499 |
-0.299 |
3.1. KD Combined with MD
According to the differences between Group A and Group B in
Table 3, sST2, WBC, PLT, CRP, IL-6, D-dimer, and NT-proBNP were included as independent variables in the univariate binary Logistic regression analysis. The increases in sST2, WBC, and CRP were promoting factors for KD complicated with MD(
P<0.05).
Table 5.
Univariate logistic regression analysis of KD combined with MD.
Table 5.
Univariate logistic regression analysis of KD combined with MD.
| Influence factor |
B |
SE |
Wald |
P |
OR |
95%CI |
| lower limit |
upper limit |
| sST2 |
0.011 |
0.004 |
7.043 |
0.008 |
1.011 |
1.003 |
1.020 |
| WBC |
0.099 |
0.035 |
7.788 |
0.005 |
1.104 |
1.030 |
1.183 |
| CRP |
0.012 |
0.004 |
10.034 |
0.002 |
1.012 |
1.004 |
1.019 |
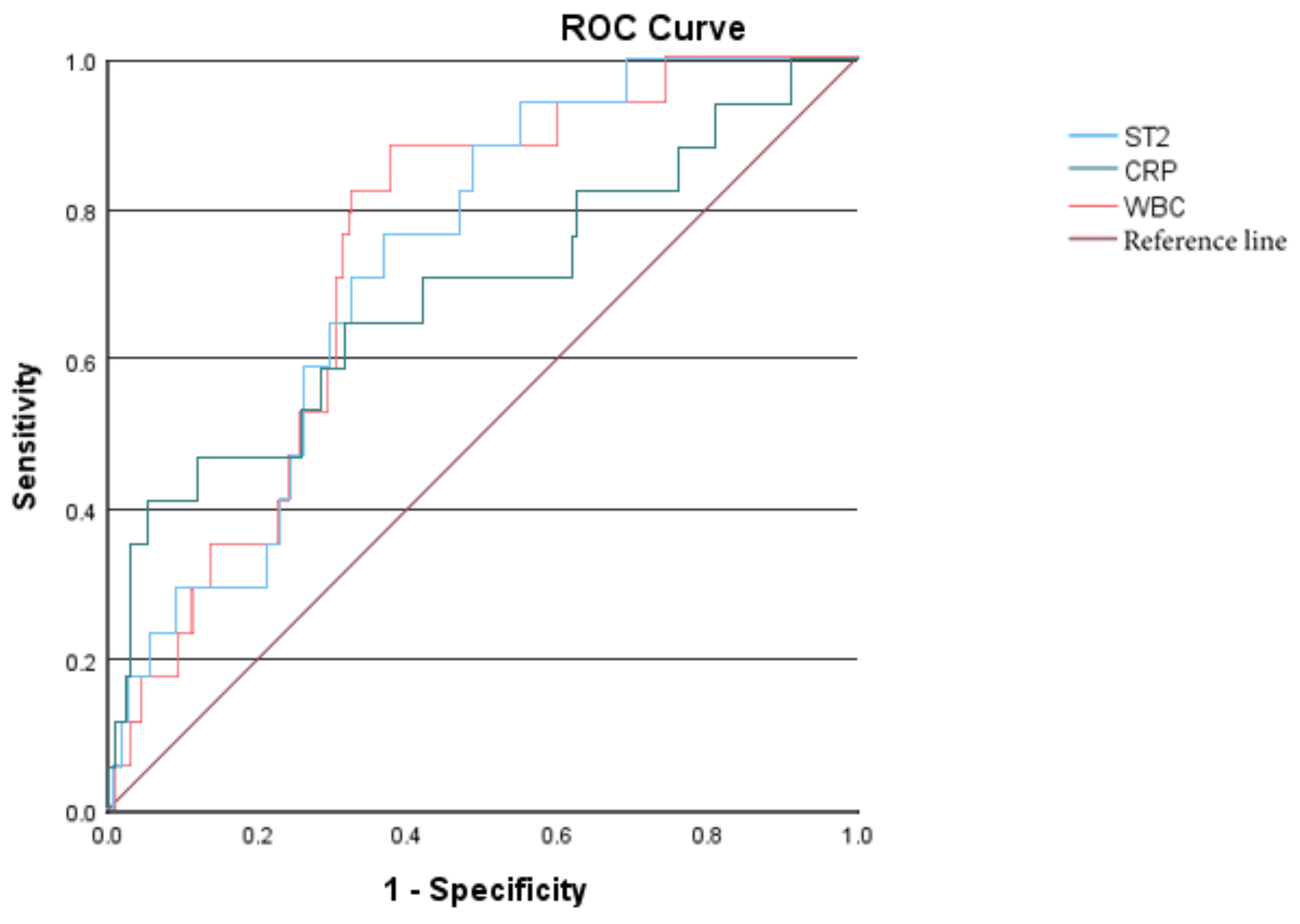
Further perform the receiver operating characteristic (ROC) curve analysis. The areas under the curve (AUC) of sST2, WBC, CRP are 0.728, 0.738, and 0.686 respectively; The optimal cut-off value of sST2 for predicting MD is 44.247 ng/ml.
Figure 1.
ROC curves of sST2, WBC, and CRP predicting KD combined with MD.
Figure 1.
ROC curves of sST2, WBC, and CRP predicting KD combined with MD.
3.2. KD Combined with MOD
According to the differences between Group E and Group F in
Table 3, sST2, WBC, HB, PLT, CRP, IL-6, NT-pro BNP, D-dimer, and ALB were included as independent variables in the univariate binary Logistic regression analysis. The results showed that the models constructed with sST2, WBC, HB, PLT, IL-6, and D-dimer were successful and had a good goodness of fit. These above independent variables were further included in the multivariate binary Logistic regression analysis, which showed that the increases in sST2 and IL-6 and the decrease in HB were independent risk factors for multiple organ involvement (
P<0.05).
Table 6.
Logistic regression analysis of KD combined with MOD.
Table 6.
Logistic regression analysis of KD combined with MOD.
| factor |
influence factor |
B |
SE |
Wald |
P |
OR |
95%CI |
| lower limit |
upper limit |
| single |
sST2 |
0.025 |
0.005 |
24.92 |
<0.001 |
1.025 |
1.015 |
1.035 |
| WBC |
0.078 |
0.026 |
8.91 |
0.003 |
1.081 |
1.027 |
1.137 |
| HB |
-0.085 |
0.016 |
28.99 |
<0.001 |
0.918 |
0.890 |
0.947 |
| PLT |
0.002 |
0.001 |
7.97 |
0.005 |
1.002 |
1.001 |
1.004 |
| IL-6 |
0.005 |
0.001 |
22.39 |
<0.001 |
1.005 |
1.003 |
1.008 |
| D-dimer |
0.001 |
0.000 |
16.44 |
<0.001 |
1.001 |
1.001 |
1.002 |
| multi |
sST2 |
0.013 |
0.005 |
6.01 |
0.014 |
1.013 |
1.003 |
1.024 |
| HB |
-0.067 |
0.021 |
10.65 |
0.001 |
0.935 |
0.898 |
0.974 |
| IL-6 |
0.003 |
0.001 |
5.79 |
0.016 |
1.003 |
1.001 |
1.006 |
| WBC |
-0.021 |
0.040 |
0.28 |
0.600 |
0.979 |
0.905 |
1.059 |
| PLT |
0.001 |
0.001 |
0.51 |
0.477 |
1.001 |
0.998 |
1.003 |
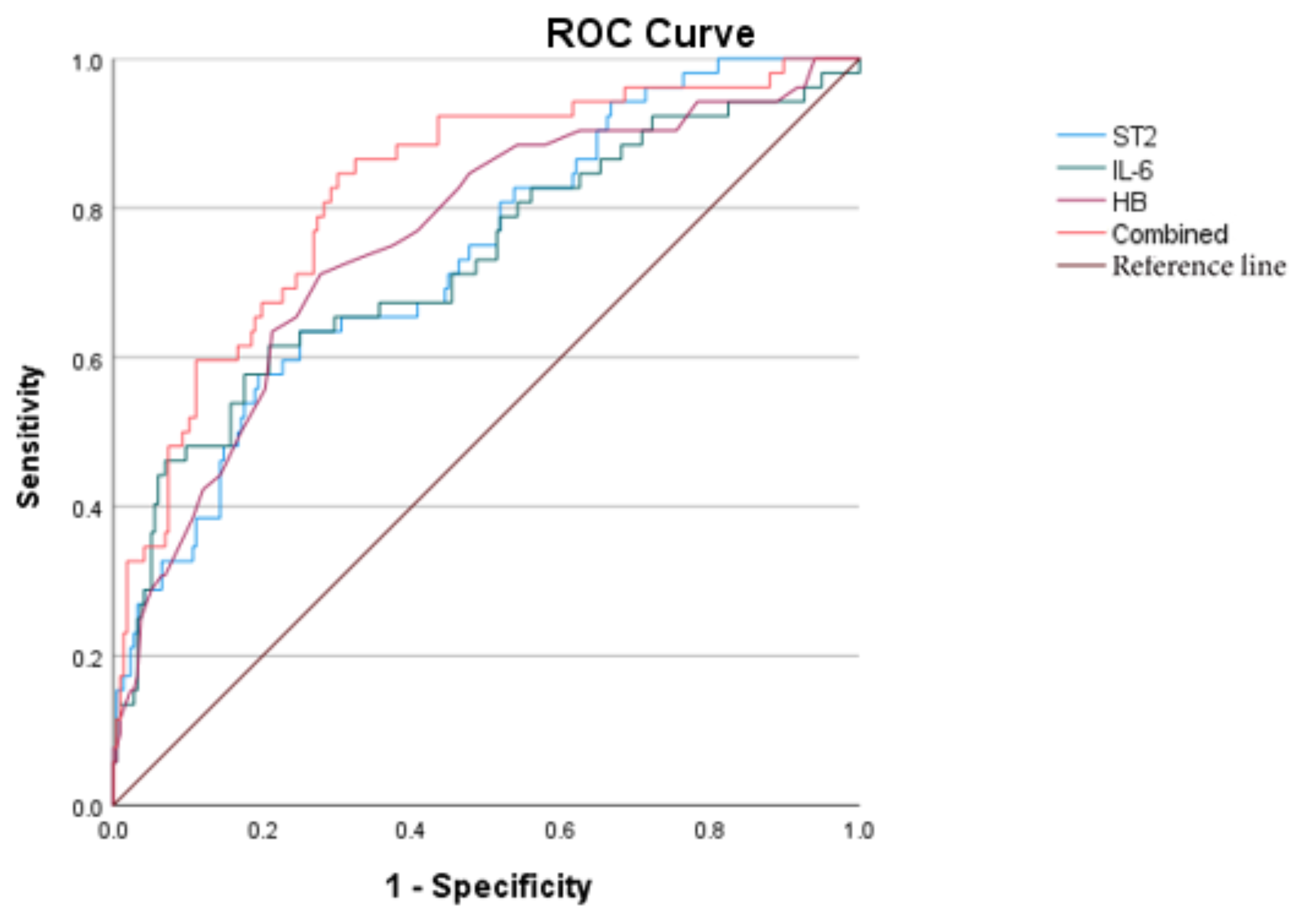
Further perform the receiver operating characteristic (ROC) curve analysis. The AUC of sST2, IL-6,HB are 0.735、0.728、0.756 respectively; The combined AUC of the three is 0.823. The optimal cut-off value of sST2 for predicting MOD is 51.264ng/ml.
Figure 2.
ROC curves of sST2, IL-6, HB, and the combined diagnosis for KD with MOD.
Figure 2.
ROC curves of sST2, IL-6, HB, and the combined diagnosis for KD with MOD.
3.3. IVIG-R KD
Binary logistic regression analysis showed that sST2, HB, CRP, IL-6, ALB and IVIG-R KD models were successfully constructed with good fit; sST2 and HB were independent risk factors for IVIG-R KD (P<0.05).
Table 7.
Logistic regression analysis of IVIG-R KD.
Table 7.
Logistic regression analysis of IVIG-R KD.
| factor |
influence factor |
B |
SE |
Wald |
P |
OR |
95%CI |
| lower limit |
upper limit |
| single |
sST2 |
0.026 |
0.005 |
24.142 |
<0.001 |
1.025 |
1.016 |
1.037 |
| HB |
-0.107 |
0.022 |
23.786 |
<0.001 |
0.899 |
0.861 |
0.938 |
| CRP |
0.017 |
0.003 |
24.584 |
<0.001 |
1.017 |
1.010 |
1.024 |
| IL-6 |
0.003 |
0.001 |
6.239 |
0.013 |
1.003 |
1.001 |
1.005 |
| ALB |
-0.243 |
0.069 |
12.369 |
<0.001 |
0.785 |
0.685 |
0.898 |
| multi |
sST2 |
0.017 |
0.006 |
7.987 |
0.005 |
1.017 |
1.005 |
1.029 |
| HB |
-0.062 |
0.027 |
5.354 |
0.021 |
0.940 |
0.892 |
0.991 |
| CRP |
0.006 |
0.005 |
1.143 |
0.285 |
1.006 |
0.995 |
1.016 |
| IL-6 |
0.000 |
0.001 |
0.416 |
0.519 |
1.000 |
0.999 |
1.002 |
| ALB |
0.059 |
0.086 |
0.477 |
0.490 |
1.061 |
0.897 |
1.256 |
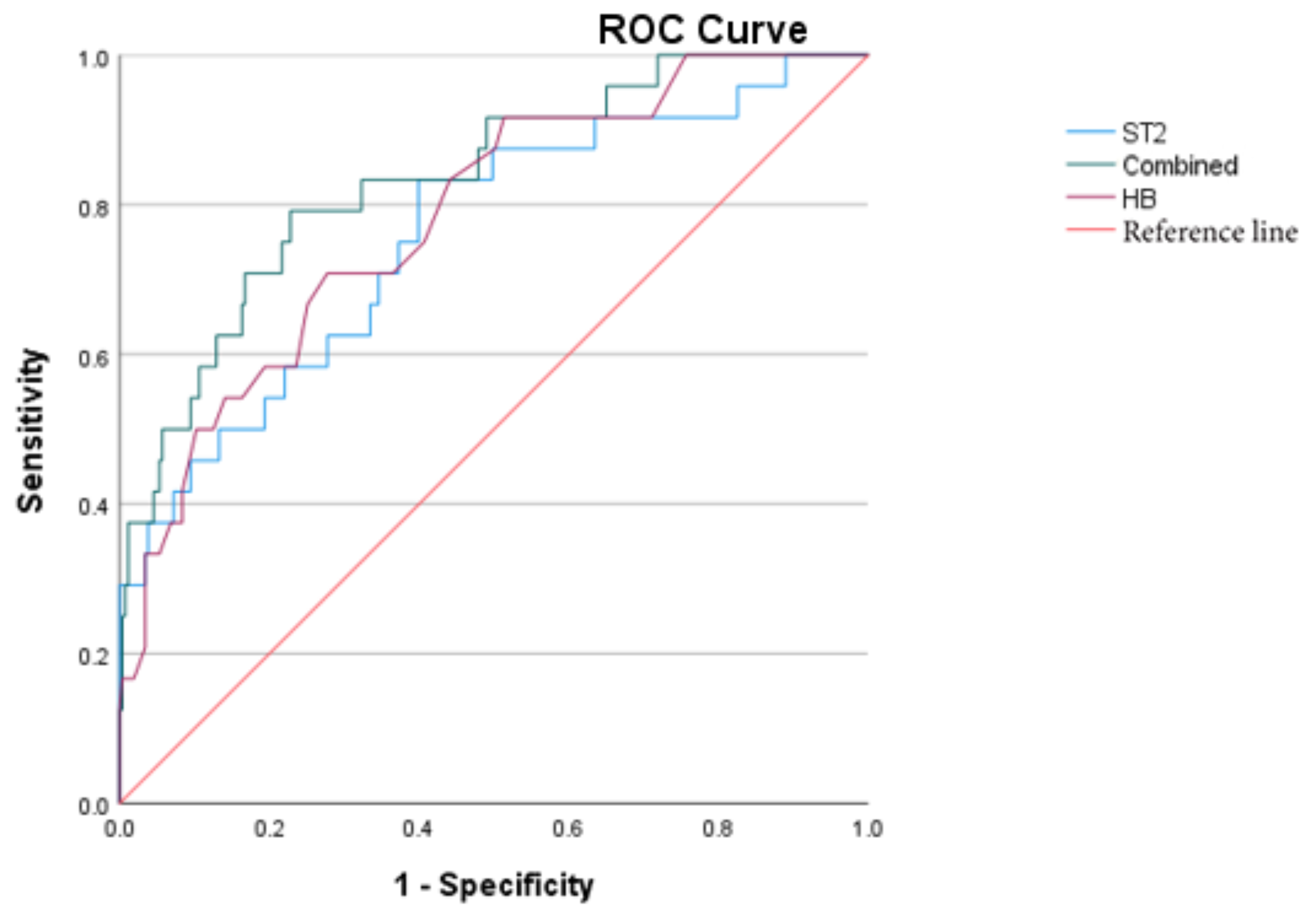
Further perform the receiver operating characteristic (ROC) curve analysis. The AUC of sST2, HB are 0.760、0.783 respectively; The combined AUC of them is 0.835. sST2 increases earlier than HB decreases. The optimal cut-off value of sST2 for predicting IVIG-R KD is 43.412ng/ml.
Figure 3.
ROC curves of sST2, HB, and the combined prediction of IVIG-R KD.
Figure 3.
ROC curves of sST2, HB, and the combined prediction of IVIG-R KD.
3.4. Clinical Data of Four Cases with ST2>200ng/ml
Table 8.
Clinical data of four cases with ST2>200.
Table 8.
Clinical data of four cases with ST2>200.
| Case |
Gender |
Age |
ST2
(ng/ml) |
Fever |
Treatment |
MOD |
1#
19kg |
F |
3.5y |
>200 |
Admission 7d
Regressive10d |
IVIG 4g/kg
Dex5mg* 2d
Methyl methicone:
2mg/kg*7d
1.5mg/kg*7d
1mg/kg*1d
Prednisone Po 14d
ALB IV 40g |
Cardiogenic shock,
Acute heart failure,
Hypoproteinemia(27.1g/L),
Hypokalemia, hyponatremia,
Pneumonia,
Aseptic encephalitis (EEG 2-3Hz),
Localized peritonitis,
Thrombocytopenia |
2#
13.5 kg
102 cm |
M |
3y |
>200 |
Admission 9d
Regressive20d |
IVIG 2g/kg
Methyl methicone:
20mg/kg*3d
2mg/kg*3d
1mg/kg*1d
Prednisone Po 7d
ALB IV 10g |
CAA: LM4.7mm,Z=6.07, 3m recvered
Liver damage (ALT95U/L)
Hypoproteinemia(24g/L)
Leukemoid reaction
Aseptic encephalitis (EEG 5-7Hz)
Pneumonia,
|
3#
9.3kg
82cm |
M |
23m |
285.4 |
Admission 5d
Regressive27d |
IVIG 4g/kg
Methyl methicone
20mg/kg*3d
10mg/kg*3d
2mg/kg*4d
1mg/kg*10d
Prednisone Po 10d
TNF inhibitor 5mg/kg
ALBI V 70g |
CAA: LM5.6mm(Z=11.1)
RCA6.5mm(Z=12)
Liver damage (ALT 434U/L)
Hypoproteinemia (24.2g/L)
Aseptic encephalitis
(CSF:WBC66, Pro 0.56)
Pleural effusion
Moderate anemia(HGB=76g/L) |
4#
29kg
130cm |
F |
9y |
287.2 |
Admission 6d
Regressive22d |
IVIG 3g/kg
Methyl methicone:
2mg/kg*6d
1mg/kg*7d
0.7md/kg*3d
Prednison Po 10d
ALBIV 60g
CTX 2mg/kg IV |
CAA: LAD 6.9mm(Z= 7.63) persist
RCA7.7mm(Z=10.63) persist
Hypoproteinemia(20.6g/L)
Aseptic encephalitis (EEG 4-7Hz)
Knee joint effusion
Granulocytopenia
Hyponatremia,
Moderate anemia(HGB=86g/L) |
4. Discussion
ST2 is a member of the interleukin-1 receptor family. IL-33 is a specific ligand for ST2,as a member of the interleukin-1 family. Research on the correlation between the IL-33/ST2 pathway and diseases is currently one of the hotspots in basic and clinical medicine. The relationship between ST2 and the heart was first reported by Japanese scholar Shin ichi Tominaga in 1989. In 2002, Richard Lee’s team at Brigham Women’s Hospital first elucidated the relationship between ST2 and the heart. The specific ligand IL-33 for ST2 was discovered in 2005[
7,
8]. There are two forms of human ST2: soluble ST2 (sST2) and transmembrane form ST2 (ST2L). IL-33 binds to the receptor sST2L to form the ST2/IL-33 signaling pathway. Literature has shown that when the physiological state of sST2 concentration is low, it can inhibit myocardial cell hypertrophy and cardiac fibrosis, thereby exerting a cardio protective effect [
8]. The protective effect of sST2 binding to IL-33 disappears. sST2 is a novel biomarker for myocardial injury, myocardial fibrosis, and cardiac remodeling. In this study, the concentration of sST2 in patients with concomitant CAA was significantly higher than that in patients with normal coronary arteries, consistent with literature reports [
4]. Compared with NT-Pro BNP, the concentration of sST2 is not affected by renal function [
9]. Continued fever or remittent fever in Acute phase of KD patients may cause dehydration, leading to pre renal insufficiency. Therefore, in predicting CAL in KD patients during the acute phase, sST2 has an advantage over NT-Pro BNP. In this study, all patients with CAL had elevated levels of sST2, but this was also confirmed by 3 cases without concomitant NT Pro BNP elevation. Some scholars believe that sST2 in KD patients is triggered by mid coronary artery necrosis, which in turn explains the significant increase in sST2 in children with CAA. Patients without CAA but with MOD or IVIG-R KD have mild elevation of sST2, while KD patients without complications have normal sST2 levels. A total of 4 patients had sST2>200ng/mL, all of which developed IVIG-R KD and MOD: case 1# with 8 organs damag, cardiogenic shock was corrected within 8 days and heart failure recovered within 10 days, and aseptic meningitis recovered within 3 months. Case 2# with 6 organs damage, leukemia reaction recovered within 3 weeks, midle CAA recovered within 2 yesrs. Case 3# ferver continued over 3 weeks and with 6 organs, the giant CAA rcovered within 2 years. Case 4# ferver continued for 22 days and with 7 organs damage, the huge CAA will last until April 2025. The mechanism may be that the IL-33/sST2 signaling pathway is involved in the pathophysiological processes of various inflammatory diseases and is related to inflammation and immune tolerance [
11].
There is a study comparing the serum sST2 levels of sepsis patients with or without shock, and it was found that the serum sST2 levels of sepsis shock patients were higher than those of the normal sepsis group. This suggests that the more severe the condition, the higher the serum sST2 levels of patients [
12]. In patients with ordinary sepsis and severe sepsis, those who ultimately end up dead have higher levels of sST2 compared to survivors, indicating that serum sST2 levels can reflect the patient’s outcome. The higher the level of sST2 in patients, the worse the prognosis may be. In this study, elevated sST2 may indicate IVIG-R KD, CAL, MOD, and MD. Through multiple factor binary logistic regression analysis, it was found that elevated sST2, IL-6, and decreased HB were independent risk factors for multi organ involvement. Through ROC curve analysis, it was found that HB reduction had better predictive value for MOD among the three, followed by sST2. Further comparison showed that sST2 elevation appeared earlier than HB reduction during the course of the disease, which could early indicate MOD. The combination of the three was the best.
In the multivariate binary logistic regression analysis of IVIG-R KD, it was found that sST2 and HB were independent risk factors for IVIG-R KD, and sST2 had better predictive value. In addition, this study noted that compared to the group without CAL, patients in the CAL group are younger and predominantly male. The difference was statistical significance, which consistent with literature reports [
13]. Patients with MD have higher sST2 levels than those without MD, and the difference was statistical significance. Univariate binary logistic regression analysis showed that elevated sST2 was a promoting factor for myocardial and CAL, but further multivariate logistic regression analysis showed no significant effect. This result may be related to the short research time and small sample size. Further research is needed to expand the sample size.
It is reported that more children have developed MIS-C after being infected with COVID-19 [
14]. The main manifestations of this disease are similar to KD, including fever, rash, conjunctivitis, limb edema, and mucosal changes. In addition, this disease often presents with severe respiratory symptoms, low blood pressure, and involvement of multiple organs (such as the heart, gastrointestinal tract, kidneys, blood, skin, and nervous system) [
15], and can be fatal in severe cases. Elevated sST2 may indicate severe cases of inflammatory response and cause severe myocardial damage and other multi-organ involvement. The systemic inflammatory syndrome caused by COVID-19 infection involves multiple organs, causing megaphagocytic system activation such as acute myocarditis, cardiogenic shock, aseptic encephalitis, progressive anemia and thrombocytopenia. How much impact will KD combined with persistent CAA have on the future of the patient? This is the most concerning issue for pediatricians and parents. Study showed that ST2 levels in such patients often increase. Their autopsy showed significant myocardial fibrosis, which may be related to chronic inflammation of the coronary arteries and myocardial ischemia. Long term monitoring of ST2 has predictive significance for such patients.
In summary, a significant increase in sST2 during the acute phase of KD may indicate severe inflammatory reactions, such as causing IVIG-R KD, CAL, MOD, and MD. We believe that detecting sST2 during the acute phase of KD can serve as a means of detecting severe cases, especially early atypical cases. Early intervention with immune agents and active management of systemic complications can reduce the occurrence of CAA and severe complications.
What is particularly exciting is that studies have shown that the IL-33/sST2 axis may be involved in the occurrence and development of KD vasculitis; The IL-33/sST2 axis may be a target for KD therapy [
17]. For patients with KD combined with persistent CAA, the significance of long-term follow-up of their sST2 needs to be further expanded in sample size and further studied.
Authors Contributions
Zhanghua YANG: Data collection, data analysis, and Chinese editing of manuscript; Yunming XU: Co-first author: the diagnosis, treatment and follow-up of some patients, analysis of some data, image production, and English editing of manuscript; Yanqiu CHU: The diagnosis, treatment and follow-up of some patients; Jinghao LI: The diagnosis, treatment and follow-up of some patients; Hong WANG: corresponding author. The design of the project, ethical application, diagnosis, treatment and follow-up of the vast majority of patients, editing of the preface, conclusions and discussions of the paper, as well as English revision.
Funding
No project funding surported.
Institutional Review Board Statement
This study had agreed by Shengjing hospital affiliated to China medical university ethics committee, ethical number:2023PS152J
Acknowledgments
Thank you, professor Xianyi YU, for the diagnosis and follow-up of some patients, professor Rui CHEN and Ce WANG, for the follow-up of some patients.
Conflicts of Interest
There is no conflict of interest among all the authors.
References
- Fuller, M.G. Kawasaki Disease in Infancy. Adv. Emerg. Nurs. J. 2019, 41, 222–228. [CrossRef]
- Fabi, M.; Andreozzi, L.; Frabboni, I.; Dormi, A.; Corinaldesi, E.; Lami, F.; Cicero, C.; Tchana, B.; Francavilla, R.; Sprocati, M.; et al. Non-coronary cardiac events, younger age, and IVIG unresponsiveness increase the risk for coronary aneurysms in Italian children with Kawasaki disease. Clin. Rheumatol. 2020, 40, 1507–1514. [CrossRef]
- Kakkar, R.; Lee, R.T. The IL-33/ST2 pathway: therapeutic target and novel biomarker. Nat. Rev. Drug Discov. 2008, 7, 827–840. [CrossRef]
- Okada, S.; Yasudo, H.; Ohnishi, Y.; Matsuguma, C.; Fukano, R.; Motonaga, T.; Waniishi, T.; Hasegawa, S. Interleukin-33/ST2 Axis as Potential Biomarker and Therapeutic Target in Kawasaki Disease. Inflammation 2022, 46, 480–490. [CrossRef]
- McCrindle, B.W.; Rowley, A.H.; Newburger, J.W.; Burns, J.C.; Bolger, A.F.; Gewitz, M.; Baker, A.L.; Jackson, M.A.; Takahashi, M.; Shah, P.B.; et al. Diagnosis, Treatment, and Long-Term Management of Kawasaki Disease: A Scientific Statement for Health Professionals from the American Heart Association. Circulation 2017, 135, e927–e999. [CrossRef]
- Kobayashi, T.; Ayusawa, M.; Suzuki, H.; Abe, J.; Ito, S.; Kato, T.; Kamada, M.; Shiono, J.; Suda, K.; Tsuchiya, K.; et al. Revision of diagnostic guidelines for Kawasaki disease (6th revised edition). Pediatr. Int. 2020, 62, 1135–1138. [CrossRef]
- Schmitz, J.; Owyang, A.; Oldham, E.; Song, Y.; Murphy, E.; McClanahan, T.K.; Zurawski, G.; Moshrefi, M.; Qin, J.; Li, X.; et al. IL-33, an Interleukin-1-like Cytokine that Signals via the IL-1 Receptor-Related Protein ST2 and Induces T Helper Type 2-Associated Cytokines. Immunity 2005, 23, 479–490. [CrossRef]
- Kotsiou, O.S.; Gourgoulianis, K.I.; Zarogiannis, S.G. IL-33/ST2 Axis in Organ Fibrosis. Front. Immunol. 2018, 9, 2432. [CrossRef]
- van Vark, L.C.; Lesman-Leegte, I.; Baart, S.J.; Postmus, D.; Pinto, Y.M.; Orsel, J.G.; Westenbrink, B.D.; Rocca, H.P.B.-L.; van Miltenburg, A.J.; Boersma, E.; et al. Prognostic Value of Serial ST2 Measurements in Patients With Acute Heart Failure. Circ. 2017, 70, 2378–2388. [CrossRef]
- Okada, S.; Sakai, A.; Ohnishi, Y.; Yasudo, H.; Motonaga, T.; Fukano, R.; Waniishi, T.; Sugiyama, M.; Hasegawa, S. Necrotic Change of Tunica Media Plays a Key Role in the Development of Coronary Artery Lesions in Kawasaki Disease. Circ. J. 2024, 88, 1709–1714. [CrossRef]
- Homsak, E.; Gruson, D. Soluble ST2: A complex and diverse role in several diseases. Clin. Chim. Acta 2020, 507, 75–87. [CrossRef]
- Pastille, E.; Wasmer, M.-H.; Adamczyk, A.; Vu, V.P.; Mager, L.F.; Phuong, N.N.T.; Palmieri, V.; Simillion, C.; Hansen, W.; Kasper, S.; et al. The IL-33/ST2 pathway shapes the regulatory T cell phenotype to promote intestinal cancer. Mucosal Immunol. 2019, 12, 990–1003. [CrossRef]
- Wang, Y.; Lin, K.; Zhang, L.; Lin, Y.; Yu, H.; Xu, Y.; Fu, L.; Pi, L.; Li, J.; Mai, H.; et al. The rs7404339 AA Genotype in CDH5 Contributes to Increased Risks of Kawasaki Disease and Coronary Artery Lesions in a Southern Chinese Child Population. Front. Cardiovasc. Med. 2022, 9, 760982. [CrossRef]
- Algarni, A.S.; Alamri, N.M.; Khayat, N.Z.; Alabdali, R.A.; Alsubhi, R.S.; Alghamdi, S.H. Clinical practice guidelines in multisystem inflammatory syndrome (MIS-C) related to COVID-19: a critical review and recommendations. World J. Pediatr. 2022, 18, 83–90. [CrossRef]
- Kabeerdoss, J.; Pilania, R.K.; Karkhele, R.; Kumar, T.S.; Danda, D.; Singh, S. Severe COVID-19, multisystem inflammatory syndrome in children, and Kawasaki disease: immunological mechanisms, clinical manifestations and management. Rheumatol. Int. 2020, 41, 19–32. [CrossRef]
- Hoshino, S.; Jain, S.; Shimizu, C.; Roberts, S.; He, F.; Daniels, L.B.; Kahn, A.M.; Tremoulet, A.H.; Gordon, J.B.; Burns, J.C. Biomarkers of inflammation and fibrosis in young adults with history of Kawasaki disease. IJC Hear. Vasc. 2021, 36, 100863. [CrossRef]
- Okada S, Yasudo H, Ohnishi Y, et al. Interleukin-33/sST2 Axis as Potential Biomarker and Therapeutic Target in Kawasaki Disease. Inflammation. 2022 Oct 8. Epub ahead of print. PMID: 36208354. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).