Introduction
Obesity has emerged as a pressing public health issue, closely linked to metabolic conditions such as type 2 diabetes, hypertension, and cardiovascular diseases (Mohajan, 2023). Sedentary lifestyles and unhealthy dietary habits significantly contribute to obesity by disrupting lipid metabolism, resulting in elevated fasting triglyceride levels, insulin resistance, and inflammation (Ujianti et al., 2022). These factors highlight the urgent need for natural interventions that can enhance lipid metabolism and mitigate obesity-related health risks.
Sea cucumber extract is a promising candidate for managing triglyceride levels and addressing the complications associated with obesity. This marine organism boasts a rich composition of bioactive compounds, including saponins, chondroitin sulfates, glycosaminoglycans, and fatty acids, which exhibit anti-inflammatory, antioxidant, antidiabetic, and other health-promoting properties. Research reveals its ability to modulate lipid metabolism through mechanisms such as inhibiting pancreatic lipase activity, promoting fat oxidation, and reducing fat synthesis (Shi et al., 2016). Additionally, studies note its role in reducing body weight and fat mass, improving insulin sensitivity, and regulating glucose metabolism in animal models (Lu et al., 2022). Nevertheless, balanced nutrition and physical activity remain critical components of obesity prevention and management (Fitriani et al., 2024).
Despite its potential, the mechanisms of action of sea cucumber extract remain incompletely understood. Recent studies (Liu et al., 2023) have shed light on pathways targeted by its bioactive compounds, particularly those involved in lipid metabolism and fat formation. However, further research, particularly involving human subjects, is necessary to fully elucidate these mechanisms and identify key active ingredients. A comprehensive methodology combining computational modeling, in vitro experimentation, and in vivo studies offers a robust approach to understanding its impacts on fasting triglycerides and obesity.
In light of the global obesity epidemic, the exploration of sea cucumber extract as a traditional medicinal ingredient provides a promising alternative for obesity treatment. By integrating biological research with computational predictions, in vitro and in vivo study, this research highlights its pharmacological potential and paves the way for innovative strategies to combat obesity and associated metabolic disorders, thereby advancing public health.
Materials and Methods
Holothuria atra Sampling
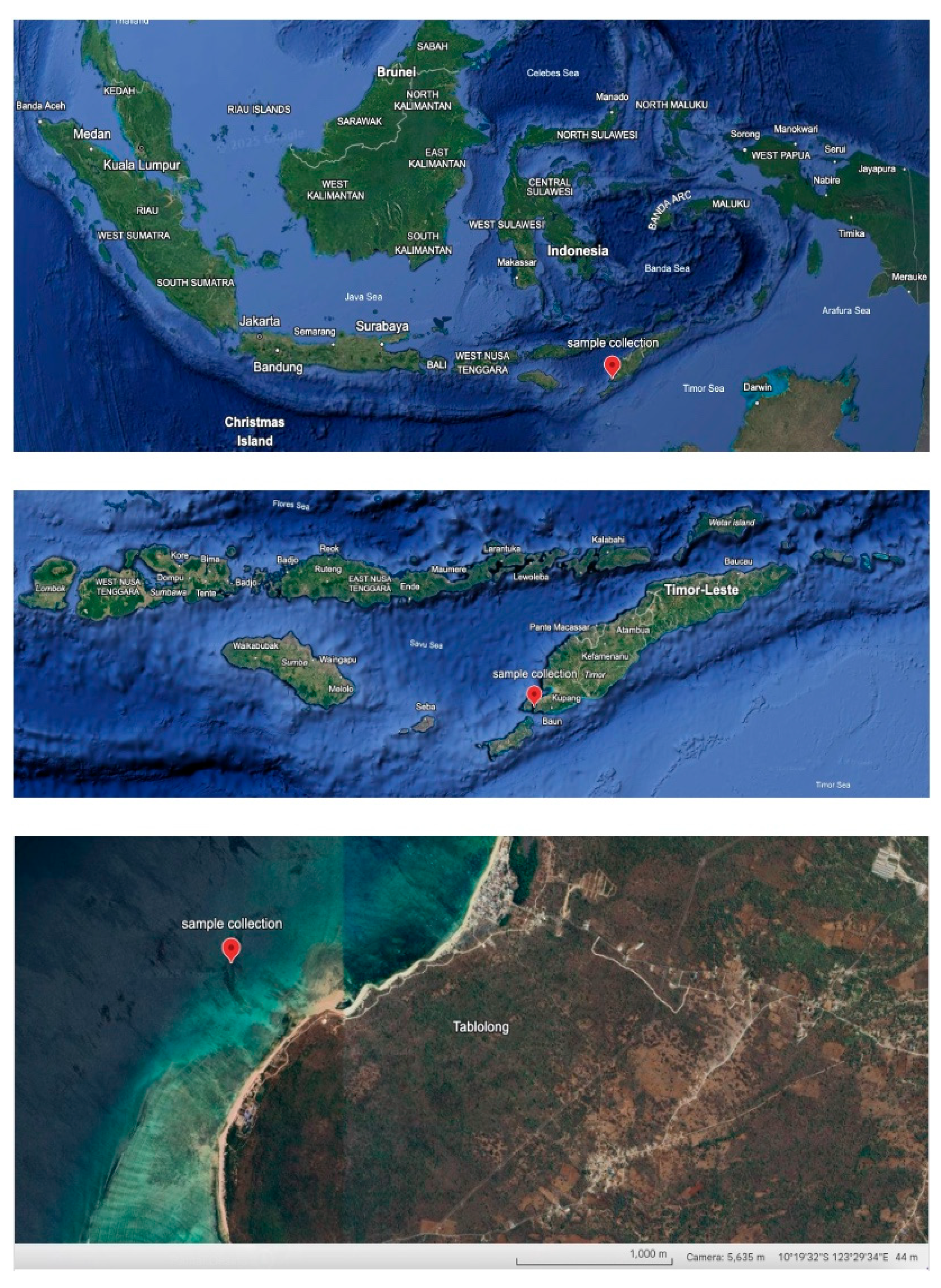
Holothuria atra (Jäger, 1957) was identified from a specimen collected by daytime diving in Tablolong Beach Kupang 10°19'07"S 123°27'46", East Nusa Tenggara, Indonesia (Mei 17–30, 2022) as
Figure 1. Morphological characteristics (elongated, firm body; reddish-black coloration; numerous dorsal papillae; densely packed ventral tube feet) matched published descriptions and aligned with known features of
H. atra. (Tehranifard, A., Rahimibashar, M.R., 2012) Taxonomic classification: Animalia; Echinodermata; Holothuroidea; Aspidochirotida; Holothuriidae;
Holothuria;
H. atra. This species is common in Indonesian coastal habitats.( Setyastuti, A., et al., 2019) DNA barcoding (COI gene) analysis from Tablolong Beach, Kupang, confirmed the identification (99–100% similarity to
H. atra in NCBI GenBank (Kautsari et al., 2024
In Silico Methods
Screening of Potentially Active Compounds and Toxicity Analysis of Sea Cucumber
The qualitative analysis of bioactive compounds in sea cucumber (Holothuroidea atra) extract identified several key compounds, including flavonoids, triterpenoids, tannins, saponins, and quinones. These were evaluated using a microscopy-microchemical method, with results confirming their presence. According to Lipinski's Rule of Five, flavonoids, tannins, and quinones show potential as drug-like molecules, while saponins and triterpenoids have either exceeded molecular weight thresholds or shown borderline drug-likeness . These compounds were further analyzed for their molecular properties, such as hydrogen bond donors and acceptors, molecular weight, and LogP values, to assess their physicochemical characteristics .
Quantitative Structure-Activity Relationship (QSAR) Analysis
The analysis of bioactive compounds in H. atra for their anti-obesity potential was conducted using the WAY2DRUG PASS prediction system. This tool evaluates compounds by comparing their structural features to those of compounds with known properties through Structure-Activity Relationship analysis. The system's predictions become more reliable when there are stronger structural similarities between the analyzed compounds. This approach is founded on the principle that compounds with similar structures are likely to exhibit similar properties. The system produces a Pa value (Probability to be Active) that indicates how likely a compound is to demonstrate specific effects, with higher Pa values suggesting greater confidence in the prediction's accuracy.
Prediction of Protein Targets and Network Analysis
Potential of seacucumber extract targets were identified through the SuperPred database analysis, with only high-confidence results exceeding 80% in both accuracy and probability metrics being considered. The target identification process involved analyzing the previously determined SMILES notation. The DisGeNET database was consulted to identify genes and proteins linked to cervical cancer, establishing disease relevance. Further investigation of teripang's Echinoside B protein targets utilized STRING (Search Tool for the Retrieval of Interacting Genes/Proteins) version 12.0. The STRING analysis was configured with specific settings: selecting Homo sapiens as the studied organism, utilizing the complete STRING network format, and implementing a medium confidence threshold of 0.4. The network data, exported in TSV format from STRING, was then processed using Cytoscape version 10.0 to create and examine protein interaction networks.
In Vitro Methods
Mesenchim Cell Culture and Treatment
The study received approval from the Health Research Ethics Committee of the Faculty of Medicine, University Muhammadiyah Prof.Dr.Hamka, under the number KEPKK/FK/071/17/2022. MSC from Human umbilical cord (hUC) were cultured in culture well plate 12 using Amem medium supplemented with 10% Platelet Rich Plasma (PRP). The cells were incubated at a temperature of 37 °C with 5% CO2. The culture medium was replaced every 2-3 days to ensure optimal cell growth.
Before any interventions were performed, a standardized protocol was followed for subculturing the cells. This involved rinsing the cells with phosphate-buffered saline (PBS), followed by a 2-minute incubation with 1 mL of 0.05% trypsin-EDTA. The cells were then transferred to a 15 mL tube containing 10 mL of Amem medium supplemented with 10% PRP and centrifuged at a speed of 1200 rpm for 10 minutes at a temperature of 20°C.(Pawitan et al., 2015) The supernatant was discarded, and the cell pellet was resuspended in 1 mL of medium. Cell viability was assessed using Tryphan Blue assay. Subsequently, the cells were seeded in a culture well plate 12 at a density of 5000 cells/cm2 per well and incubated at 37 °C with 5% CO2. The adipogenesis medium induction was performed and replaced every 2-3 days. The induction of adipogenesis was done concurrently with the application of sea cucumber extract at concentrations of 9 ûgr/ml and 19 ûgr/ml
Biochemical Parameters of NFκB and FAS
The cells were lysed in Radio-Immunoprecipitation Assay (RIPA) buffer (Sigma-Aldrich, MO, USA), then centrifuged at 10,000 rpm at 2–8 °C for 10 minutes. The total protein concentration was determined with a Bicinchoninic acid (BCA) protein assay kit. medium was prepared according to the protocol and collected directly from the cell well culture. It was then centrifuged at 2500 rpm at 2–8 °C for 5 minutes and subsequently stored at −80 °C for the insulin parameter check. NFκB in the cell lysate and medium were measured using an ELISA kit (Fine Test, Wuhan, China), following the manufacturer’s protocol.
In Vivo Methods
Animals and Categorization
Ten-week-old healthy male Sprague Dawley rats were obtained from Institut Pertanian Bogor (IPB). The animals underwent a one-week acclimatization period in the Central Animal Lab at the Department of Chemistry, University of Indonesia, where environmental conditions were maintained at 24°C with 50% humidity. After acclimatization, the rats were randomly divided into five experimental groups of 5 rats each: control diet group, high-fat diet group, high-fat diet plus extract group, high-fat diet with exercise group, and high-fat diet with both extract and exercise group. The rats were housed individually throughout the study period, with environmental conditions maintained between 18-26°C and 50% humidity. They were fed twice daily and had free access to water, which was replaced every two days. The researchers monitored each rat's weight, size, and waist measurements monthly. Blood tests measuring serum lipids, glucose, and insulin levels were performed at the study's conclusion. The research protocol was approved by the ethics committee of Universitas Muhammadiyah Prof.Dr.HAMKA under permit number KEPKK/FK/058/05/2024.
Exercise Protocol
Swimming training was conducted in a barrel filled with water at 33–35°C to a depth of 40–50 cm, allowing the rats to swim freely. The first session lasted 15 minutes, and the duration increased by 5 minutes daily until 30 minutes. Rats in the HFD+Exercise and HFD+Exercise+extract groups swam for 30 minutes daily, 5 days a week, for 6 weeks, while those in the other groups remained sedentary in cages (Lu et al., 2016)
Measurement of Fasting Glucose, Fasting Triglyceride, and Triglyceride Index
Each rat’s body weight, body size, and abdominal circumference were measured before and after treatment. Abdominal circumference (AC) was assessed on the largest zone of the rat abdomen using a plastic non-extensible measuring tape, and body size was defined as the length from nose to anus. Lee’s obesity index was calculated using the formula: Lee’s index = cube root of body weight (g)/body size (cm). After the rats were sacrificed, aorta tissues were sampled for homogenate tissue and Hematoxylin-Eosin (HE) staining. After a 12-hour fast, blood samples were collected from rat tails, and levels of fasting blood glucose (FBG), triglycerides (TG), high-density lipoprotein (HDL), and low-density lipoprotein (LDL) were measured every 8 weeks using a Hitachi 7600 automatic biochemical analyzer (Hitachi; Tokyo, Japan). The Triglyceride-Glucose (TyG) Index by comparing between triglyceride and glucose levels. The Triglyceride-Glucose (TyG) Index is indeed used as a surrogate marker for insulin resistance. Some studies have proposed that TyG Index < 8.3: Low likelihood of insulin resistance TyG Index 8.3 - 8.9: Moderate likelihood of insulin resistance TyG Index > 8.9: High likelihood of insulin resistance.
Statistical Analysis
For in vivo study Statistical results are presented as means ± standard deviations, with experimental groups containing 5-7 animals each. The specific number of animals used in each experiment is indicated in the corresponding figure legends. We performed one-way ANOVA to assess differences between groups, followed by Tukey's post-hoc test for multiple comparisons. Results were considered statistically significant when P<0.05. All statistical analyses were conducted using SPSS version 20.0 software.
For in vitro study To confirm that the data were normally distributed, the Shapiro–Wilk test was performed. For group comparisons of NFκB with one-way Analysis of Variance (ANOVA) with Tukey’s post hoc test was performed. Additionally, the Pearson correlation analysis was used to analyze the correlation between medium and cell lysate insulin levels. All experiments were conducted in duplicate. SPSS version 25.0 (IBM, Chicago, IL, USA) was used to perform statistical analysis. Data were expressed as mean ± standard deviation.
Results
Phytochemical Study and Toxicity Analysis
Phytochemical analysis was performed to evaluate the bioactive compounds in the extract. Using the microscopy-microchemical method, the results showed in
Table 1 that flavonoid, triterpenoid, tannins, and saponins were present.
Table 1 showed that extracts contain bioactive compounds such as flavonoids, triterpenoids, tannins, saponins, and quinones, with drug-like properties analyzed based on Lipinski’s Rule of Five. Flavonoids, tannins, and quinones comply with the rule, showing potential as drug-like molecules, while saponins exceed the acceptable thresholds for molecular weight and hydrogen bonding, indicating poor drug-likeness. Triterpenoids exhibit borderline drug-likeness due to slightly exceeding the molecular weight criterion.
Quantitative Structure-Activity Relationship (QSAR) Analysis
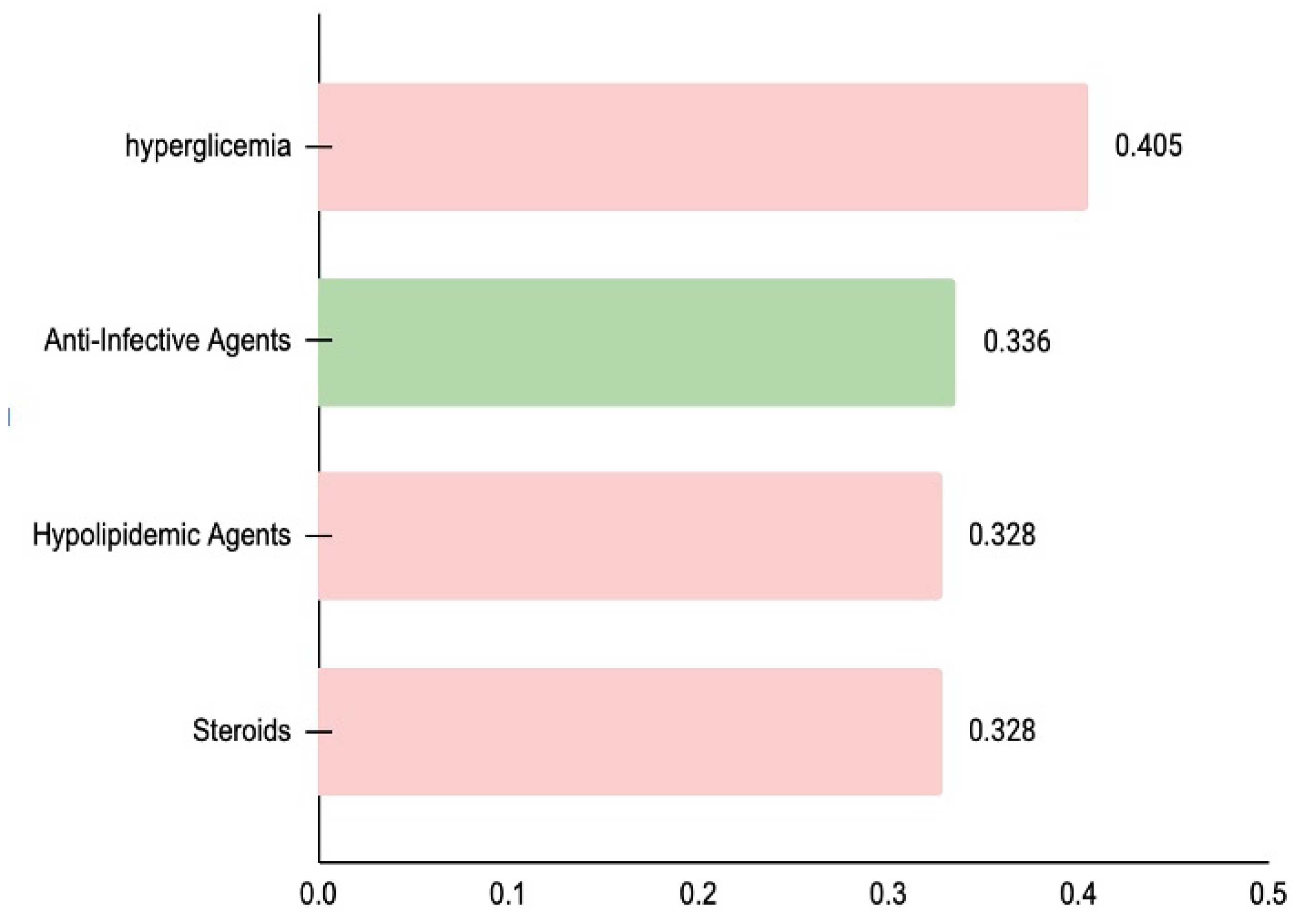
Based on SAR analysis using Way2Drug Pass Online, bioactive compounds in sea cucumbers were found to have good potential for various applications related to metabolic disease prevention. Compounds in sea cucumbers also show structural similarity to some drugs, such as diabetes drugs (PA Score 0.405), and drugs for dyslipidemia (PA Score 0.328), which can also act as obesity drugs, as shown in
Figure 2.
Network Analysis
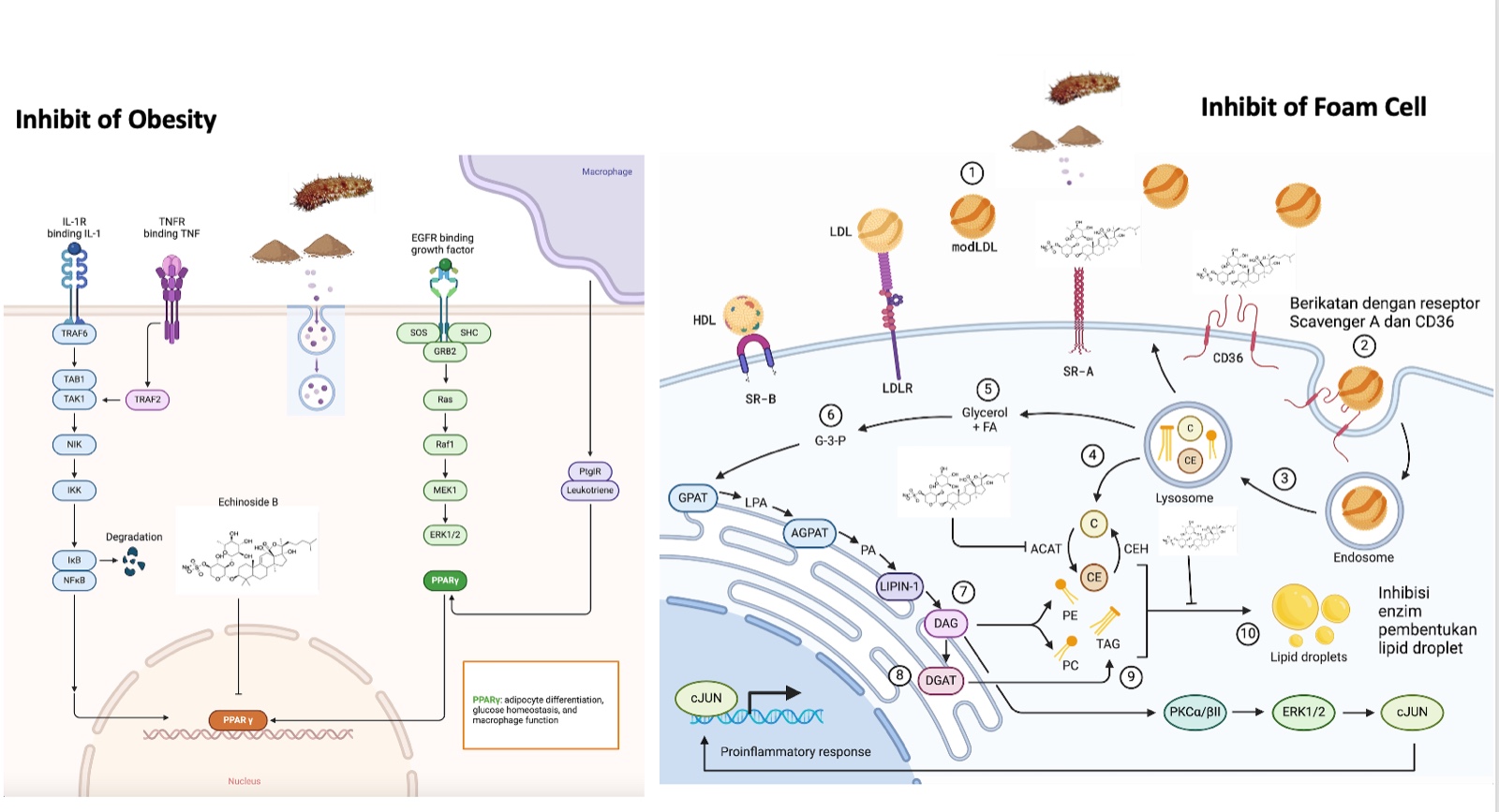
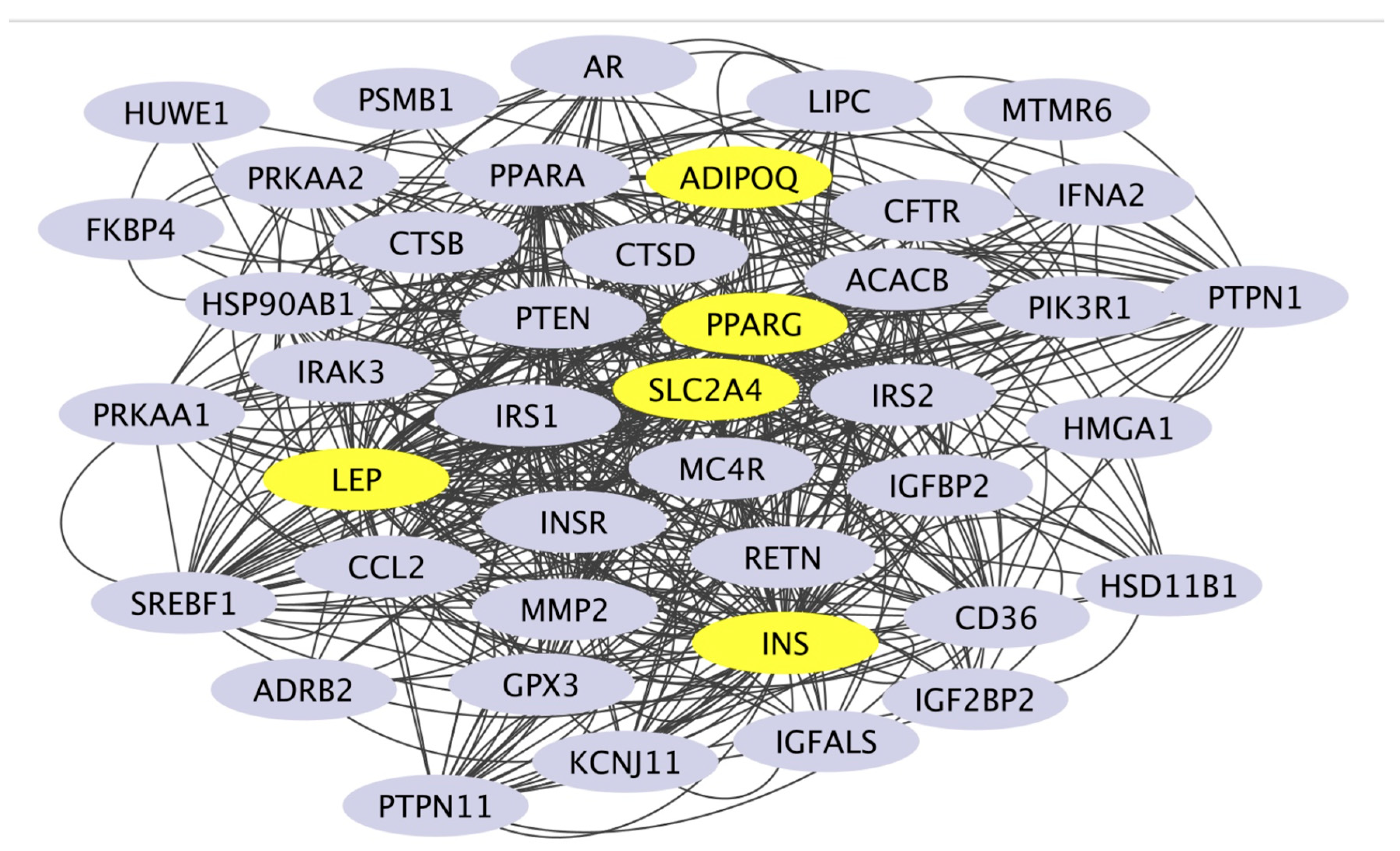
PPAR-γ is considered a potential target because it has the highest values in terms of betweenness centrality, closeness centrality, degree, and cervical cancer overall score compared to other targets (
Table 2,
Figure 3). Based on these analyses, it is predicted that
H.atra, specifically through the PPAR-γ pathway, may be effective in targeting obesity, dyslipidemia, and insulin resistance.
In Vitro Result
In Vitro Differentiation
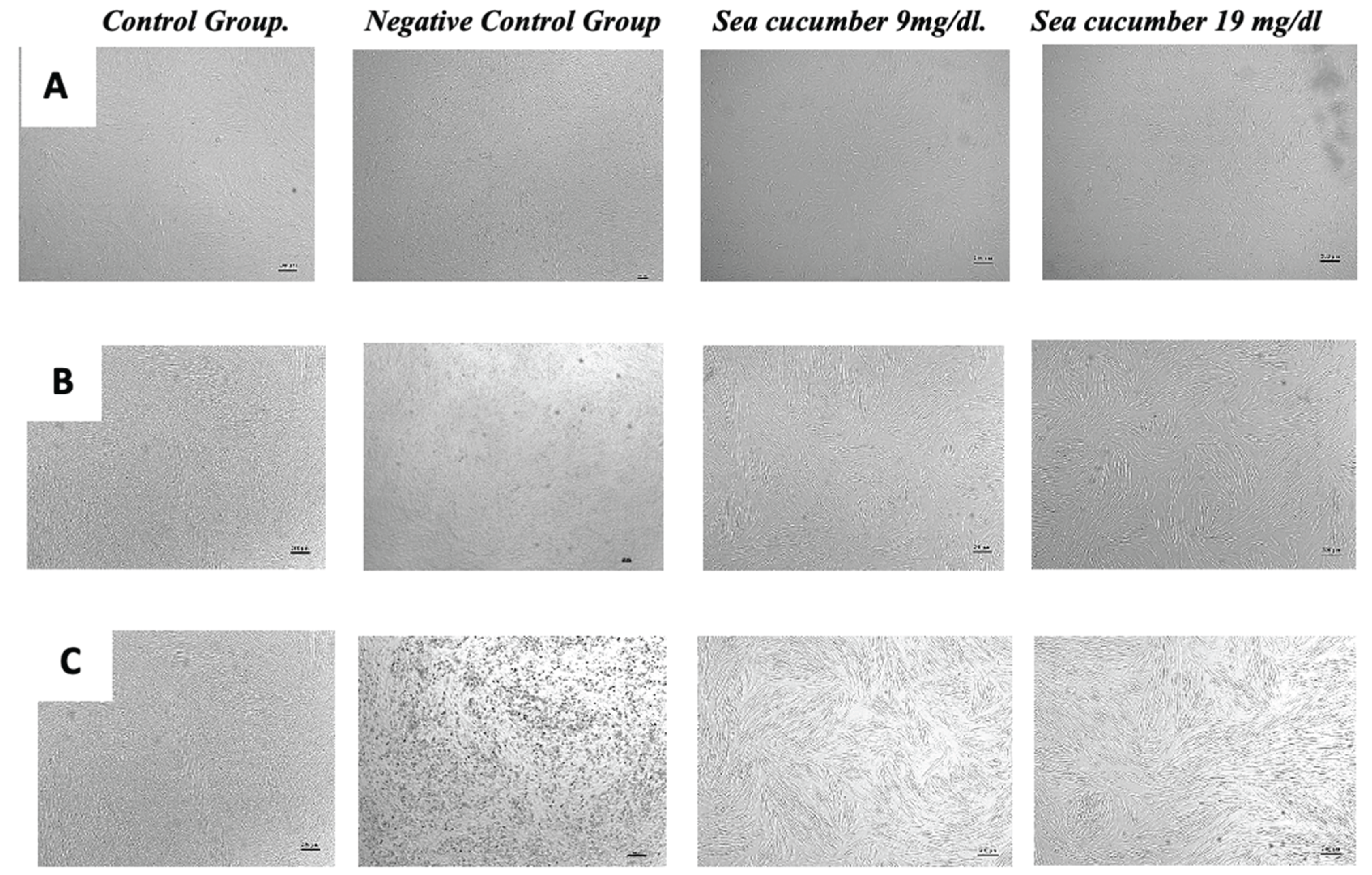
At the outset, the HU-MSCs show a customary fibroblast-like morphology, featuring small cell bodies with elongated cell processes across all treatment groups. Upon reaching the 5th passage, during the commencement of the treatment, control group cells demonstrated differentiation towards mature adipocytes. This resulted in cells adopting a spherical shape, their cytoplasm riddled with large fat droplets in preparation for forming a single lipid droplet. Control group cells without differentiation retained the fibroblast-like morphology typical of MSCs at the beginning of the treatment. In contrast, the sea cumber extract treatment group elucidated a fibroblast-like structure but showed the presence of a cytoplasm filled with minimal fat vacuoles. The treatment group given a 9 ug/ml extract dose displayed larger vacuoles than the group treated with a concentration of 19 ug/ml, showed in
Figure 4.
NFκB - FAS Level During Adipogenesis
During our study, we confirmed the existence of proteins such as NFκB and FAS within the cell culture. As displayed in
Table 3, we observed the minimum protein concentration in the treatment cells related to NFκB and FAS. When the NFκB levels were evaluated, there was a significantly higher concentration in the differentiation control group and lowest concentration is tratment group with concentration 9 mg/dl. Interestingly, a similar trend was noted in the FAS levels. The highest concentration was found in the differentiation control group and the lowest concentration is treatment group with concentration 9 mg/dl.
The effect of seacucumber extract on NFκB and FAS parameters. Data are presented as mean ± SD values. The statistical differences were revealed by one-way ANOVA and Tukey's post hoc test. The mean differences were significant compared with the positive control group (**P < 0.01, ***P < 0.001)
In Vivo Results
In Vivo Biochemical Results
Based on the data in the table, the administration of extracts and exercise, either separately or together, can improve the disturbed biochemical parameters caused by a high-fat diet. This includes reducing triglycerides, glucose, triglyceride index, and weight gain. The combination of extract administration and exercise in the HFD + Extract + Exercise group showed the most optimal results in improving these parameters as showed in
Table 4.
Discussion
The bioactive compound flavonoid and triterpenoid, derived from sea cucumbers, has shown significant potential as a natural treatment for obesity and related metabolic complications. This is supported by in silico, in vitro, and in vivo studies. It targets obesity-related pathways, offering a holistic approach to addressing obesity and its related metabolic dysfunctions (Jin et al., 2018). These findings are further supported by several studies (Kalinin et al., 2020; Kang et al., 2015; Shi et al., 2016).
In silico studies provide foundational insights into how sea cucumber compounds interact with genes associated with obesity. Investigations by Hoang and Amir et al. revealed that Echinoside B modulates metabolic and inflammatory pathways. It increases adiponectin (ADIPOQ) levels, enhances insulin sensitivity, and suppresses pro-inflammatory cytokines like TNF-α (Jayaraman et al., 2021). Moreover, the compound activates PPAR-γ, a critical regulator of lipid metabolism (Zhang et al., 2024). It also restores the activity of SLC2A4/GLUT4, improves glucose uptake, and reduces insulin resistance (Pérez-García et al., 2022). These mechanisms collectively enhance insulin receptor function and support effective glucose metabolism, preventing metabolic disorders (Shi et al., 2016; 2019). Further in vitro research has validated these molecular mechanisms (Liu et al., 2023). Studies have shown that administering sea cucumber extracts reduces NFκB levels, directly inhibiting fat cell production or adipogenesis (Al Madhoun et al., 2023). AAmir et al. demonstrated that NFκB regulates PPAR-γ expression, which is essential for adipocyte differentiation, highlighting the extract’s ability to control fat accumulation (Aamir et al., 2020). Additionally, the extracts inhibit lipid storage regulators like fatty acid synthase (FAS) and the NFκB/IKK signaling pathway (Jayaraman et al., 2021). These results align with in vitro findings by Parida et al., which indicated a reduction in lipid accumulation markers, including TNF-α and NFκB (Parida et al., 2019). Overall, the anti-adipogenic and anti-inflammatory effects of sea cucumber extracts, as demonstrated in vitro, show promise for managing obesity.
In vivo studies further build on these findings, highlighting the physiological effects of consuming sea cucumber extracts in animal models of obesity. In mice fed a high-fat diet (HFD), those treated with sea cucumber extracts experienced significant weight loss compared to the control group (Li et al., 2021; Chen et al., 2018). These effects were attributed to Echinoside B’s ability to suppress fat production by inhibiting PPAR-γ and SREBF1 and lowering leptin levels, which regulate appetite (Lu et al., 2022; Wen et al., 2022). Additionally, Echinoside B promoted fatty acid breakdown through PPAR-α activation, reduced triglyceride synthesis by inhibiting enzymes like DGAT and FAS, and improved blood lipid profiles by increasing LDL receptor (LDLR) expression (Zhang & Hao, 2020). Together, these mechanisms not only reduced obesity but also addressed key markers of insulin resistance, improving glucose metabolism and systemic insulin sensitivity (Han et al., 2019). Physical activity also plays an essential role in improving metabolic health by increasing adiponectin, PPAR-γ, and GLUT4 levels, which enhance insulin sensitivity and promote glucose and lipid metabolism (Polito et al., 2020). Exercise complements the effects of sea cucumber extracts by reducing leptin levels, supporting appetite control, and improving blood lipid profiles through increased PPAR-α activation (Lu et al., 2016). When combined with sea cucumber extracts, physical activity optimizes insulin signaling pathways, enhances fat breakdown, and reduces inflammatory markers. This dual approach offers protection against oxidative stress, leading to better weight management, glucose uptake, and overall metabolic regulation (Pérez-García et al., 2022).
The combined findings from in vitro and in vivo studies emphasize the potential clinical application of Echinoside B. For instance, experiments with C57/BL6 mice fed a high-fat diet and supplemented with sea cucumber saponins and phospholipids enriched with eicosapentaenoic acid (EPA-PL) by Hen et al., showed improved glucose tolerance and insulin sensitivity (Han et al., 2019). Similarly, liposomal formulations of sea cucumber saponins proved highly effective in reducing obesity and cholesterol levels in mouse models (Chen et al., 2018). These results align with prior research and highlight the compound’s ability to regulate glucose and lipid metabolism (Jin et al., 2018).
In conclusion, evidence from in silico, in vitro, and in vivo studies provides solid insights into the anti-obesity potential of sea cucumber-derived compounds, particularly flavonoid and triterpenoid. Flavonoids and triterpenoids are the two most potent bioactive compounds in inhibiting adipogenesis via the PPARγ pathway. While flavonoids exhibit additional effects, such as regulating the AMPK pathway and demonstrating antioxidant properties, triterpenoids are considered more specific and directly target PPARγ by disrupting its ligand-binding activity. Therefore, when considering direct influence on PPARγ, triterpenoids are likely to have the greatest impact. By modulating adipogenesis, fat production, and glucose metabolism while reducing systemic inflammation, sea cucumber extracts emerge as a promising natural therapy for obesity and related metabolic disorders (Hosseini et al., 2022). When combined with lifestyle changes like physical activity, the therapeutic effects of this compound are enhanced, offering a comprehensive strategy for managing obesity and improving metabolic health (Li et al., 2021). Future efforts should focus on enhancing its bioavailability, isolating and characterizing specific bioactive components, exploring underlying molecular pathways, and conducting clinical trials to confirm its therapeutic efficacy. These findings highlight the clinical potential of sea cucumber extract as a natural alternative for managing obesity.
Conclusion
This multidisciplinary study provides insights into the therapeutic potential of sea cucumber extract for managing obesity and related metabolic disorders. The findings suggest that the combination of sea cucumber extract and exercise can effectively regulate lipid metabolism, improve insulin sensitivity, and support weight management. Further research is needed to fully elucidate the underlying mechanisms and explore the clinical applications of sea cucumber-based treatments for obesity and related health conditions.
Abbreviation
AC - Abdominal Circumference; ALogP - Atom-based LogP; ANOVA - Analysis of Variance; CMNPD - Comprehensive Marine Natural Products Database; DGAT - Diacylglycerol Acyltransferase; FAS - Fatty Acid Synthase; FBG - Fasting Blood Glucose; HFD - High-Fat Diet; HUC-MSCs - Human umbilical cord mesenchymal stem cells; INSR - Insulin Receptor; IRS1 - Insulin Receptor Substrate 1; LDL - Low-Density Lipoprotein; LDLR - Low-Density Lipoprotein Receptor; LEP - Leptin; NFκB - Nuclear Factor Kappa B; Pa - Probability to be Active; PASS - Prediction of Activity Spectra for Substances; PPAR - Peroxisome Proliferator-Activated Receptor; PTEN - Phosphatase and Tensin Homolog; QSAR - Quantitative Structure-Activity Relationship; SAR - Structure-Activity Relationship; SCS - Sea Cucumber Saponins; SREBF1 - Sterol Regulatory Element-Binding Protein 1; STRING DB - Search Tool for the Retrieval of Interacting Genes/Proteins Database; TG - Triglycerides; TyG Index- Triglyceride-Glucose Index; WAY2DRUG PASS - Prediction of Activity Spectra for Substances
Acknowledgments
The authors would like to thank Ministry of Education, Culture, Research, and Technology Indonesia for supporting this study with number 7308/E2/DT.01.00/2023.
References
- Al Madhoun, A.; Kochumon, S.; Haddad, D.; Thomas, R.; Nizam, R.; Miranda, L.; Sindhu, S.; Bitar, M.S.; Ahmad, R.; Al-Mulla, F. Adipose Tissue Caveolin-1 Upregulation in Obesity Involves TNF-α/NF-κB Mediated Signaling. Cells 2023, 12, 1019. [Google Scholar] [CrossRef] [PubMed]
- Aamir, K.; Khan, H.U.; Sethi, G.; Hossain, M.A.; Arya, A. Wnt signaling mediates TLR pathway and promote unrestrained adipogenesis and metaflammation: Therapeutic targets for obesity and type 2 diabetes. Pharmacol. Res. 2020, 152, 104602. [Google Scholar] [CrossRef]
- Chen, C.; Han, X.; Dong, P.; Li, Z.; Yanagita, T.; Xue, C.; Zhang, T.; Wang, Y. Sea cucumber saponin liposomes ameliorate obesity-induced inflammation and insulin resistance in high-fat-diet-fed mice. Food Funct. 2018, 9, 861–870. [Google Scholar] [CrossRef]
- Fitriana, R.; Ujianti, I.; Sasongko, T.H.; Yashiro, T. Examining Oxygen Saturation and Peak Volume Correlation in Respiratory Disorders: A Study at PCM/PCA Ciledug. Sanus Med J. 2024, 5, 37–41. [Google Scholar] [CrossRef]
- Han, X.-Q.; Zhang, L.-Y.; Ding, L.; Shi, H.-H.; Xue, C.-H.; Zhang, T.-T.; Wang, Y.-M. Synergistic effect of sea cucumber saponins and EPA-enriched phospholipids on insulin resistance in high-fat diet-induced obese mice. Food Funct. 2019, 10, 3955–3964. [Google Scholar] [CrossRef]
- Hawas, U.W.; El-Kassem, L.T.A.; Shaher, F.M.; Ghandourah, M.; Al-Farawati, R. Sulfated Triterpene Glycosides from the Saudi Red Sea Cucumber Holothuria atra with Antioxidant and Cytotoxic Activities. Thalass. Int. J. Mar. Sci. 2021, 37, 817–824. [Google Scholar] [CrossRef]
- Hoang, T.H.; Liang, Q.; Luo, X.; Tang, Y.; Qin, J.G.; Zhang, W. Bioactives From Marine Animals: Potential Benefits for Human Reproductive Health. Front. Mar. Sci. 2022, 9. [Google Scholar] [CrossRef]
- Hosseini, S.F.; Rezaei, M.; McClements, D.J. Bioactive functional ingredients from aquatic origin: a review of recent progress in marine-derived nutraceuticals. Crit. Rev. Food Sci. Nutr. 2020, 62, 1242–1269. [Google Scholar] [CrossRef]
- Jayaraman, S.; Devarajan, N.; Rajagopal, P.; Babu, S.; Ganesan, S.K.; Veeraraghavan, V.P.; Palanisamy, C.P.; Cui, B.; Periyasamy, V.; Chandrasekar, K. β-Sitosterol Circumvents Obesity Induced Inflammation and Insulin Resistance by down-Regulating IKKβ/NF-κB and JNK Signaling Pathway in Adipocytes of Type 2 Diabetic Rats. Molecules 2021, 26, 2101. [Google Scholar] [CrossRef]
- Jin, Q.; Yu, H.; Li, P. The Evaluation and Utilization of Marine-derived Bioactive Compounds with Anti-Obesity Effect. Curr. Med. Chem. 2018, 25, 861–878. [Google Scholar] [CrossRef]
- Kang, Y.; Hengbo, S.; Jun, L.; Jun, L.; Wangsheng, Z.; Huibin, T.; Huaiping, S. PPARG Modulated Lipid Accumulation in Dairy GMEC via Regulation of ADRP Gene. J. Cell. Biochem. 2014, 116, 192–201. [Google Scholar] [CrossRef]
- Kautsari, N.; Djonu, A.; Valen, F.S. DNA barcoding and phylogenetic of lollyfish, Holothuria atra (Jaeger, 1833)(Actinopoda: Holothuriidae), from Tablolong Beach, Kupang, East Nusa Tenggara, Indonesia. Aquac. Aquar. Conserv. Legis. 2024, 17, 1–12. [Google Scholar]
- Kalinin, V.; Anisimov, M.; Prokofieva, N.; Avilov, S.; Afiyatullov, S.; Stonik, V.A. Biological activities and biological role of triterpene glycosides from holothuroids (Echinodermata). In Echinoderm Studies 5 (1996); CRC Press: Boca Raton, FL, USA, 2020; pp. 139–181. [Google Scholar]
- Li, R.; Meng, J.; Shi, H.; Wang, C.; Li, Z.; Xue, C.; Wang, Y.; Zhang, T. Dietary Supplementation with Sea Cucumber Saponins and Exercise Can Significantly Suppress Adipose Accumulation in Mice Fed with High-Fat Diet. J. Ocean Univ. China 2021, 20, 629–640. [Google Scholar] [CrossRef]
- Liu, L.; Chen, Y.; Chen, B.; Xu, M.; Liu, S.; Su, Y.; Qiao, K.; Liu, Z. Advances in Research on Marine-Derived Lipid-Lowering Active Substances and Their Molecular Mechanisms. Nutrients 2023, 15, 5118. [Google Scholar] [CrossRef]
- Lu, Y.; Li, H.; Shen, S.-W.; Shen, Z.-H.; Xu, M.; Yang, C.-J.; Li, F.; Feng, Y.-B.; Yun, J.-T.; Wang, L.; et al. Swimming exercise increases serum irisin level and reduces body fat mass in high-fat-diet fed Wistar rats. Lipids Heal. Dis. 2016, 15, 1–8. [Google Scholar] [CrossRef]
- Lu, Z.; Sun, N.; Dong, L.; Gao, Y.; Lin, S. Production of Bioactive Peptides from Sea Cucumber and Its Potential Health Benefits: A Comprehensive Review. J. Agric. Food Chem. 2022, 70, 7607–7625. [Google Scholar] [CrossRef]
- Parida, S.; Siddharth, S.; Sharma, D. Adiponectin, Obesity, and Cancer: Clash of the Bigwigs in Health and Disease. Int. J. Mol. Sci. 2019, 20, 2519. [Google Scholar] [CrossRef]
- Pérez-García, A.; Torrecilla-Parra, M.; Frutos, M.F.-D.; Martín-Martín, Y.; Pardo-Marqués, V.; Ramírez, C.M. Posttranscriptional Regulation of Insulin Resistance: Implications for Metabolic Diseases. Biomolecules 2022, 12, 208. [Google Scholar] [CrossRef]
- Polito, R.; Monda, V.; Nigro, E.; Messina, A.; Di Maio, G.; Giuliano, M.T.; Orrù, S.; Imperlini, E.; Calcagno, G.; Mosca, L.; et al. The Important Role of Adiponectin and Orexin-A, Two Key Proteins Improving Healthy Status: Focus on Physical Activity. Front. Physiol. 2020, 11, 356. [Google Scholar] [CrossRef]
- Setyastuti, A. , Wirawati, I., Permadi, S., & Vimono, I. B. (2019). Teripang indonesia: jenis, sebaran dan status nilai ekonomi. PT. Media Sains-Jakarta, 75.
- Shi, S.; Feng, W.; Hu, S.; Liang, S.; An, N.; Mao, Y. Bioactive compounds of sea cucumbers and their therapeutic effects. Chin. J. Oceanol. Limnol. 2015, 34, 549–558. [Google Scholar] [CrossRef]
- Shi, J.; Fan, J.; Su, Q.; Yang, Z. Cytokines and Abnormal Glucose and Lipid Metabolism. Front. Endocrinol. 2019, 10, 703. [Google Scholar] [CrossRef] [PubMed]
- Tehranifard, A.; Rahimibashar, M.R. Description a Sea Cucumber Spesies Holothuria atra Jaeger, 1833 from Kiss Island Iran (Echinodermata: Holothuridea). J Basic Appl Sci Res 2012, 2, 12660–12664. [Google Scholar]
- Ujianti, I.; Lakhsmi, B.S.; Nurusshofa, Z.; Sukarya, W.S. Evaluation of the potential of Stichopus Herrmanni extract in inhibiting cervical cancer cell proliferation. Phytomedicine Plus 2024, 4. [Google Scholar] [CrossRef]
- Ujianti, I.; Lakshmi, B.S.; Nurusshofa, Z.; Sukarya, W.; Indriyanti, L. Network Pharmacology Analysis Reveals Bioactive Compounds and Potential Targets of Sea cucumber for Cervical Cancer Therapy. F1000Research 2023, 12, 1358. [Google Scholar] [CrossRef]
- Ujianti, I.; Sianipar, I.R.; Prijanti, A.R.; Santoso, D.I.S. CONSUMPTION OF Hibiscus sabdariffa DRIED CALYX ETHANOL EXTRACT IMPROVED REDOX IMBALANCE AND GLUCOSE PLASMA IN VITAMIN B12 RESTRICTION DIET IN RATS. Malays. Appl. Biol. 2022, 51, 33–40. [Google Scholar] [CrossRef]
- Ujianti, I.; Sianipar, I.R.; Prijanti, A.R.; Hasan, I.; Arozal, W.; Jusuf, A.A.; Wibowo, H.; Prihartono, J.; Amani, P.; Santoso, D.I.S. Effect of Roselle Flower Extract (Hibiscus sabdariffa Linn.) on Reducing Steatosis and Steatohepatitis in Vitamin B12 Deficiency Rat Model. Medicina 2023, 59, 1044. [Google Scholar] [CrossRef]
- Wen, L.; Li, R.; Zhao, Y.-C.; Yang, J.-Y.; Li, X.-Y.; Xue, C.-H.; Zhang, T.-T.; Wang, Y.-M. A Comparative Study of the Anti-Obesity Effects of Dietary Sea Cucumber Saponins and Energy Restriction in Response to Weight Loss and Weight Regain in Mice. Mar. Drugs 2022, 20, 629. [Google Scholar] [CrossRef]
- Zhang, X.; Hao, Y. Beneficial Effects of Echinacoside on Diabetic Cardiomyopathy in Diabetic Db/Db Mice. Drug Des. Dev. Ther. 2020, ume 14, 5575–5587. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, X.-Y.; Shi, S.-R.; Ma, C.-N.; Lin, Y.-P.; Song, W.-G.; Guo, S.-D. Natural products in atherosclerosis therapy by targeting PPARs: a review focusing on lipid metabolism and inflammation. Front. Cardiovasc. Med. 2024, 11, 1372055. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).