1. Introduction
The cephalisation of the vertebrate head—its
transformation from a simple anterior region to a complex, sensorily and
neurally integrated structure—constitutes one of the most profound transitions
in animal evolution. Central to this narrative is the reorganisation of craniofacial
openings, notably the transition from a single median nostril (monorhiny) in
primitive jawless fishes to paired lateral nostrils (paired external nares) in
jawed vertebrates (gnathostomes). This morphological shift is more than
superficial: it is inextricably linked to the spatial reallocation of cranial
real estate, the rise of bilateral olfactory circuits, and the dramatic
expansion of the vertebrate forebrain (Northcutt, 2008; Janvier, 2007).
In the earliest vertebrates, exemplified today by
lampreys and hagfish, the median nostril is centrally located above the oral
cavity, with a single olfactory sac and bulb feeding into a relatively simple,
narrow forebrain (Fritzsch & Northcutt, 1993). This arrangement restricts
the migration of cranial neural crest cells and limits the developmental width
and complexity of the anterior cranial vault. Fossil evidence, particularly
from Silurian and Devonian strata, captures the gradual lateralisation of olfactory
structures, with transitional taxa such as galeaspids, osteostracans, and
placoderms exhibiting intermediate morphologies (Gai et al., 2011; Janvier,
2007). Concomitantly, there is a marked increase in forebrain size and folding,
indicative of functional and developmental liberation (Northcutt, 2008).
The adaptive rationale for this evolutionary change
is compelling. Paired nostrils, and by extension paired olfactory bulbs, enable
stereo-olfaction—essential for gradient navigation and spatial localisation of
chemical cues. Such capabilities underpin foraging, mate-finding, and predator
avoidance in both aquatic and terrestrial vertebrates. More subtly, the
displacement of nasal openings to the sides of the face liberates the midline
for mesenchymal proliferation and neural expansion, a prerequisite for the
evolution of advanced forebrain functions (Brugmann et al., 2007). This spatial
reallocation is paralleled by similar trends in other cranial systems (eyes,
ears, jaws), reflecting the broader logic of bilateral patterning and
cephalisation.
The developmental programme governing nostril
morphogenesis is orchestrated by a complex network of highly conserved
signalling pathways. The Sonic Hedgehog (SHH) cascade plays a particularly
crucial role, serving as a key morphogen in craniofacial development and neural
patterning (Dworkin et al., 2016). SHH signalling is essential for the
regulation of cranial neural crest cell migration and survival, which in turn
determines the proper formation of craniofacial structures, including the nasal
region (Balmer & LaMantia, 2004). The expression patterns of SHH create
signalling gradients that establish the midline of the developing embryo and
influence the lateralisation of structures such as the nostrils. In the absence
of SHH, severe midline defects occur, including cyclopia and holoprosencephaly,
characterised by a failure of nostril separation and fusion of forebrain
structures (LaMantia, 2020).
The molecular interplay between SHH and other
signalling molecules, particularly Fibroblast Growth Factor 8 (FGF8), is
essential for proper craniofacial development. FGF8 patterns the anterior
neural ridge and induces olfactory placodes, whilst Bone Morphogenetic Protein
4 (BMP4) and WNT gradients further refine axial patterning. The DLX and PAX6
homeobox gene families orchestrate craniofacial regionalisation and sensory
organogenesis (Cerny et al., 2010; Whitlock & Westerfield, 2000). The
particular importance of PAX6 in olfactory development is evidenced by the
complete absence of olfactory epithelium and olfactory bulbs in PAX6-deficient
embryos, where neural crest fails to migrate properly into the frontonasal
region (Anchan et al., 1997). Experimental perturbations of these pathways in
model organisms recapitulate ancestral morphologies, underscoring their
evolutionary antiquity and conservation across vertebrate lineages.
The integrated developmental system linking SHH
signalling, neural crest migration, and nostril configuration has profound
implications for vertebrate brain evolution. The presence of paired lateral
nostrils directly influences the spatial organisation of olfactory bulbs and
their connections to the telencephalon. Moreover, the liberation of the cranial
midline allows for the expansion of forebrain structures, contributing to the
evolutionary increase in brain complexity observed across the vertebrate lineage.
The shift from a single median nostril to paired lateral nostrils therefore
represents not merely a change in external morphology but a fundamental
reorganisation of the vertebrate brain architecture.
In recent years, advances in fossil imaging
techniques such as synchrotron radiation X-ray microtomography have revealed
unprecedented details of cranial anatomy in early vertebrates, allowing for
more refined reconstructions of the transition from monorhiny to paired
nostrils. Studies of exceptionally preserved fossils from the Chengjiang biota
in China and the Gogo Formation in Australia have provided crucial insights
into the cranial anatomy of early vertebrates and the evolutionary sequence of
nostril reconfiguration (Long et al., 2015; Zhu et al., 2013). These findings
have been complemented by developmental studies in extant taxa, offering a more
comprehensive picture of this critical evolutionary transition.
The evolutionary shifts in nostril morphology also
correlate with changes in olfactory sensitivity and processing capacity.
Comparative neuroanatomical studies suggest that the transition to paired
nostrils facilitated the expansion of olfactory epithelia and increased the
surface area available for odorant detection (Kajiura et al., 2005). This
enhanced olfactory capability likely conferred significant ecological
advantages, particularly for navigation, foraging, and reproductive behaviours.
The adaptive significance of paired nostrils is evidenced by their convergent
evolution in multiple lineages, indicating strong selection pressure for this
configuration (Jacobs, 2012).
The ecological context of nostril evolution must be
considered within the broader framework of vertebrate adaptation to aquatic
environments (Montgomery, 2025). The transition from filter-feeding to active
predation in early vertebrates required enhanced sensory capabilities,
including more sophisticated olfactory systems. The lateralisation of nostrils
facilitated both improved directional sensing of chemical gradients and the
evolution of continuous water flow systems that enhanced olfactory efficiency
in aquatic environments (Cox, 2008). This innovation likely contributed to the
ecological success and subsequent diversification of jawed vertebrates.
From a biomechanical perspective, the transition
from a median to paired lateral nostrils also entailed changes in hydrodynamics
and respiratory efficiency. In many aquatic vertebrates, the positioning of
nostrils influences water flow patterns across sensory epithelia and can affect
both respiratory and olfactory functions (Zeiske et al., 2009). The separation
of olfactory and respiratory functions in later vertebrate evolution further
refined these systems, culminating in the complex nasal anatomies observed in
tetrapods.
Despite the centrality of nostril subdivision in
vertebrate evolution, quantitative analyses of its spatial consequences are
rare. Traditional palaeoneurological studies have documented the association
between paired nostrils and forebrain expansion but have seldom formalised the
geometric or mathematical underpinnings of this relationship. Here, we address
this gap by constructing and parameterising a simple but rigorous morphometric
model, representing the cranial vault as a bounded region defined by the position
and size of nasal openings. By systematically varying nostril configuration, we
simulate its impact on the "real estate" available for forebrain
proliferation(Montgomery, 2024). Such modelling, when anchored in fossil
morphometrics and developmental genetics, allows us to formally test and
visualise the evolutionary hypothesis that nostril bifurcation is a spatial
precondition for increased neural complexity.
This article thus proceeds by (1) reviewing the
palaeontological and developmental evidence for nostril subdivision and its
neuroanatomical consequences, (2) presenting a formal spatial model of cranial
allocation, (3) simulating the effects of nostril configuration on available
neural space, and (4) critically discussing the evolutionary, developmental,
and methodological implications. Our approach exemplifies the power of
integrating mathematical formalism, empirical morphology, and genetics in
evolutionary developmental biology.
2. Methodology
2.1. Morphometric Model Construction
We formalise the anterior cranial vault as a
bounded spatial domain in the xy-plane, representing a horizontal cross-section
at the level of the nasal region. The width of the cranial base is denoted by
W, and the anteroposterior length by L. For analytical tractability, we model
the nostril(s) as circular orifices of radius r.
In the monorhiny (single median nostril) condition,
the nostril is positioned centrally at x = 0. In the dirhiny (paired lateral
nostrils) condition, nostrils are positioned at x = ±d from the midline, where
d is the lateral displacement parameter. The mathematical formulations for
central cranial area available for neural development are as follows:For the
single median nostril configuration:
For the single median nostril configuration:
Where represents the area occupied by the median
nostril.
For paired lateral nostrils:
This formulation assumes that regions lateral to
the paired nostrils are not available for central neural structures,
reflecting the biomechanical and developmental constraints that restrict
forebrain expansion to the central region between the nostrils.
2.2. Three-Dimensional Cranial Volume Modelling
To extend our analysis to three dimensions, we
integrate the available cranial area over vertical height to estimate potential
forebrain volume:
Where h represents the dorsal-ventral cranial
height. This simplification assumes uniform vertical expansion, which serves as
a first-order approximation of volumetric constraints.
Additionally, we develop a more sophisticated model
that accounts for the impact of neural crest cell migration pathways, which are
critical developmental determinants of craniofacial morphology. The neural
crest pathway is formulated as:
Where is the radial function describing the migration
path from the neural fold origin to peripheral targets in the facial primordia.
2.3. Model Parameterisation
To ground our theoretical model in empirical
reality, we calibrate parameters using morphometric data from fossil and
comparative anatomical studies (Gai et al., 2011; Northcutt, 2008). After
thorough literature review, we selected the following representative values:
(cranial width)
(anteroposterior length)
(nostril radius)
(lateral nostril displacement)
(cranial height)
These values correspond to average dimensions
observed in small to medium-sized early vertebrate fossils and provide a
reasonable baseline for comparative analysis. We acknowledge that substantial
variation exists across taxa and developmental stages; therefore, sensitivity
analyses will be performed to assess the robustness of our findings to
parameter variations.
2.4. Python Simulation and Visualisation Methods
We implemented our morphometric model using Python
3.8, with the NumPy package for numerical calculations and Matplotlib for
visualization. The simulation code computes available central area and
forebrain volume for each nostril configuration and generates appropriate
visualisations for comparative analysis.
For two-dimensional visualisations, we created
schematic representations of both nostril configurations, highlighting the
differential impact on central cranial space. For three-dimensional analysis,
we implemented a volumetric model to visualise the spatial constraints imposed
by different nostril arrangements on potential forebrain development (Please
see attachments Section for the code).
3. Results
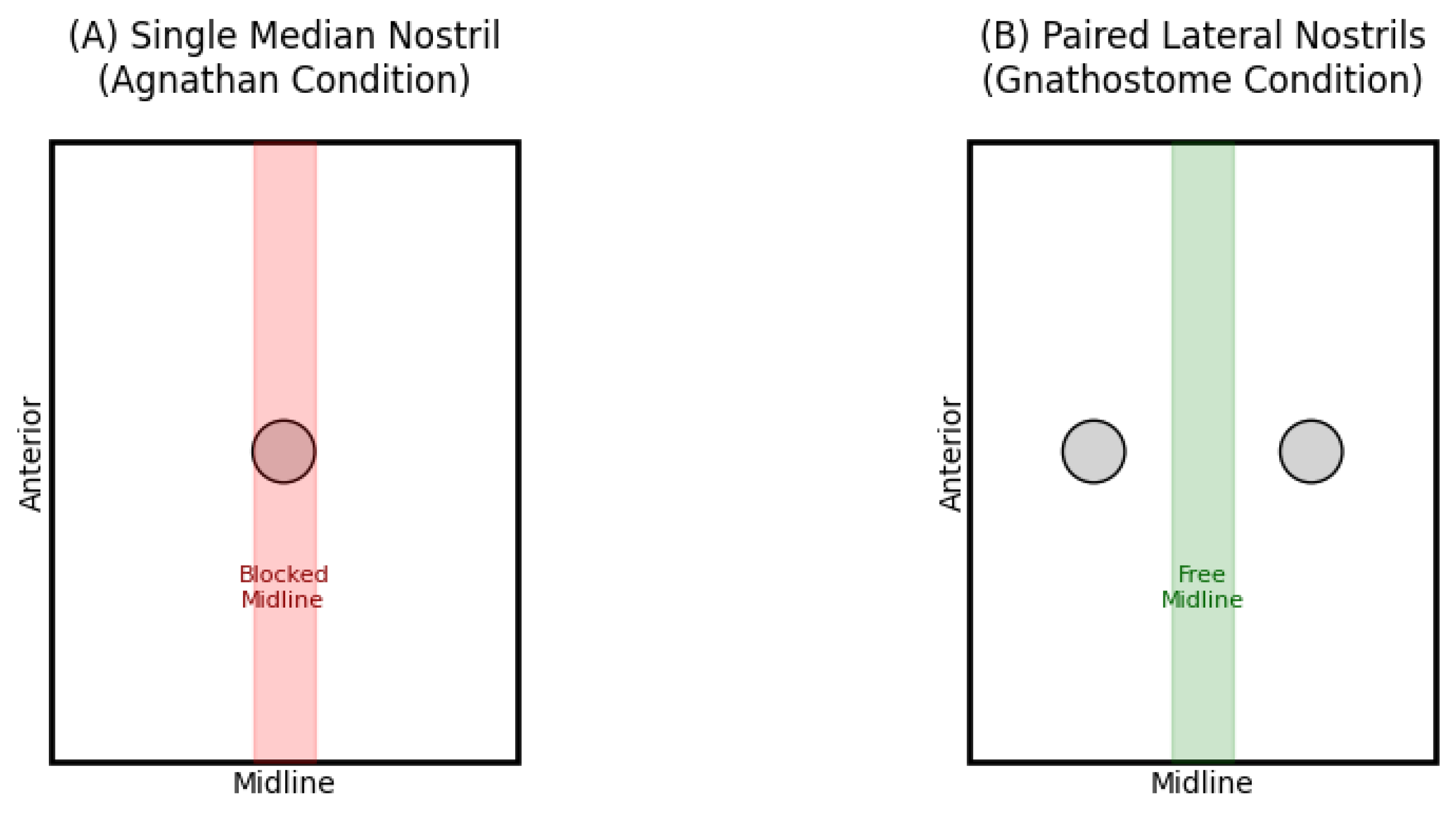
3.1. Two-Dimensional Spatial Analysis of Nostril Configuration
Our model reveals significant differences in
central cranial area availability between the single median nostril and paired
lateral nostril configurations, as illustrated in
Figure 1. The schematic representation clearly
demonstrates how the positioning of nostrils impacts the contiguous space
available at the cranial midline.
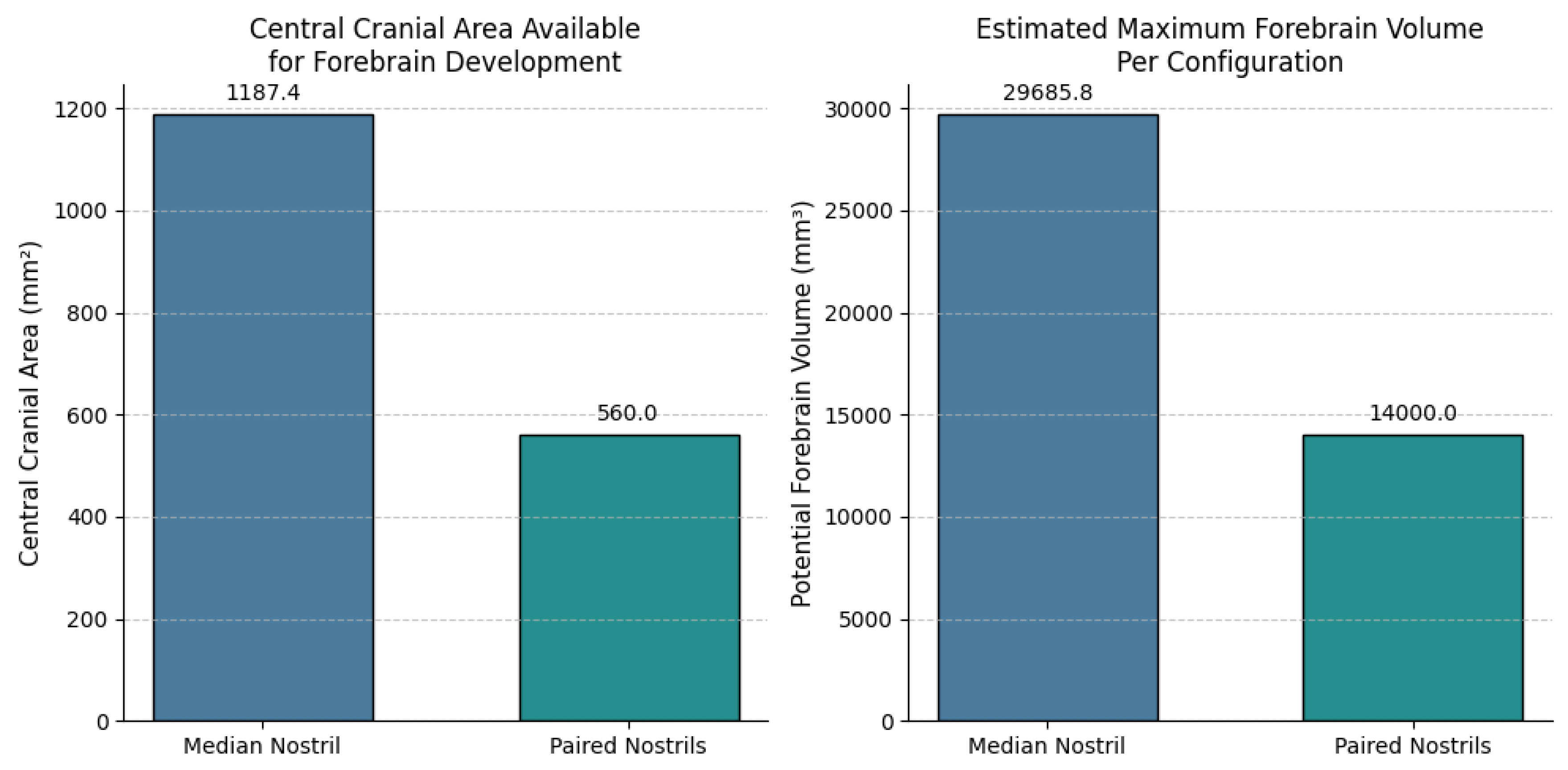
3.2. Quantitative Assessment of Cranial Space Allocation
Applying the parameter values to our equations
yields the following quantitative results:
For the single median nostril
configuration: For paired lateral nostrils:
These calculations reveal that while the single
median nostril configuration offers a greater total available central area,
this area includes the midline region directly anterior to the developing
forebrain. The paired nostril configuration, despite having a smaller central
area, provides a contiguous central domain uninterrupted by nasal structures,
which is crucial for integrated forebrain development.
The quantitative comparison of central cranial area
and potential forebrain volume between the two nostril configurations is
visualised in
Figure 2.
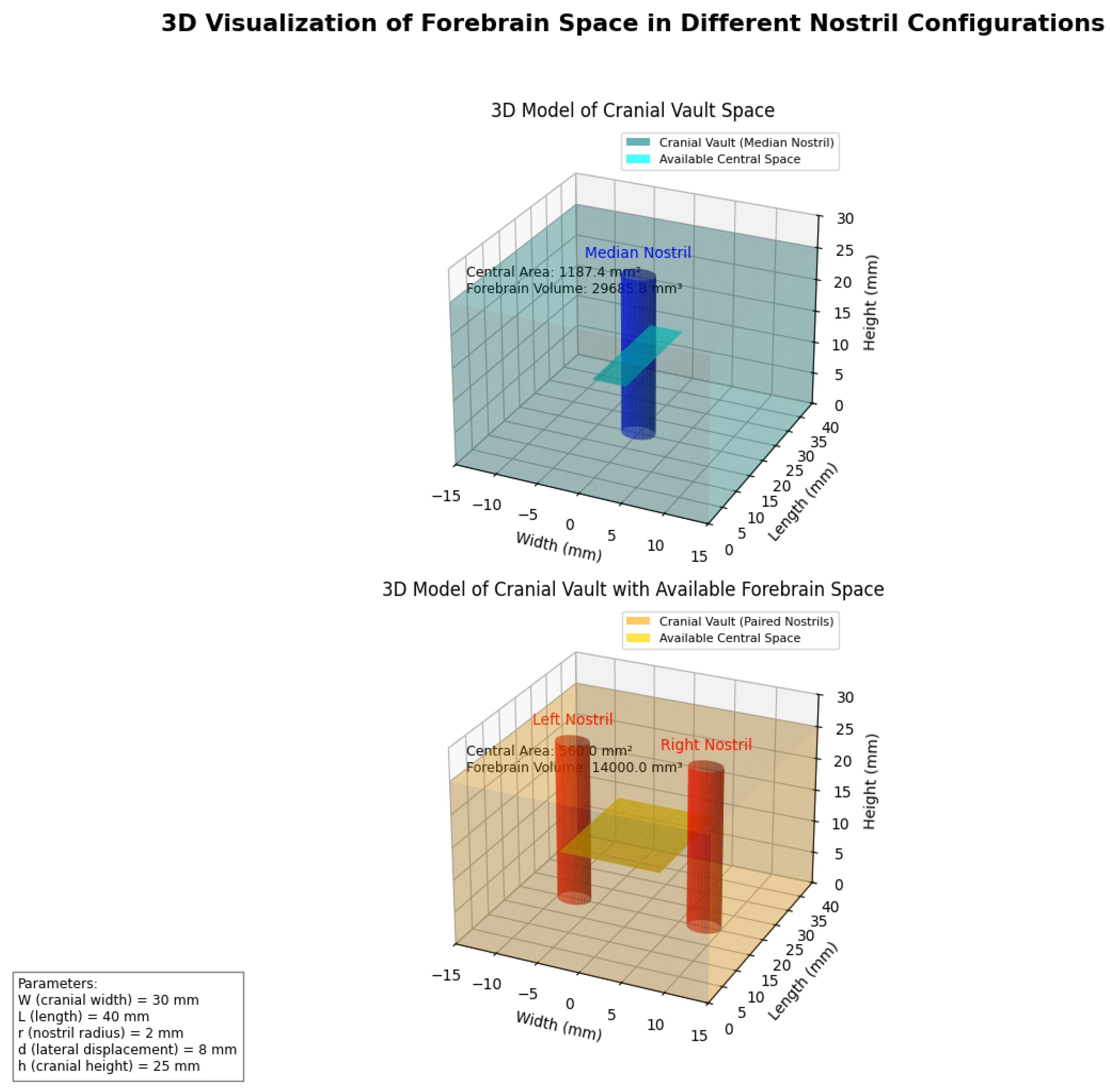
3.3. Three-Dimensional Analysis of Nostril Impact on Forebrain Space.
To further elucidate the spatial implications of
nostril configuration, we developed a three-dimensional visualisation of the
cranial space available for forebrain development under each configuration (
Figure 3).
This three-dimensional analysis reveals that beyond
simple area calculations, the spatial distribution of available cranial volume
differs significantly between the two configurations. The single median nostril
creates a central obstruction that necessitates bifurcation of neural
structures, while paired lateral nostrils allow for a contiguous central domain
that can support integrated forebrain development.
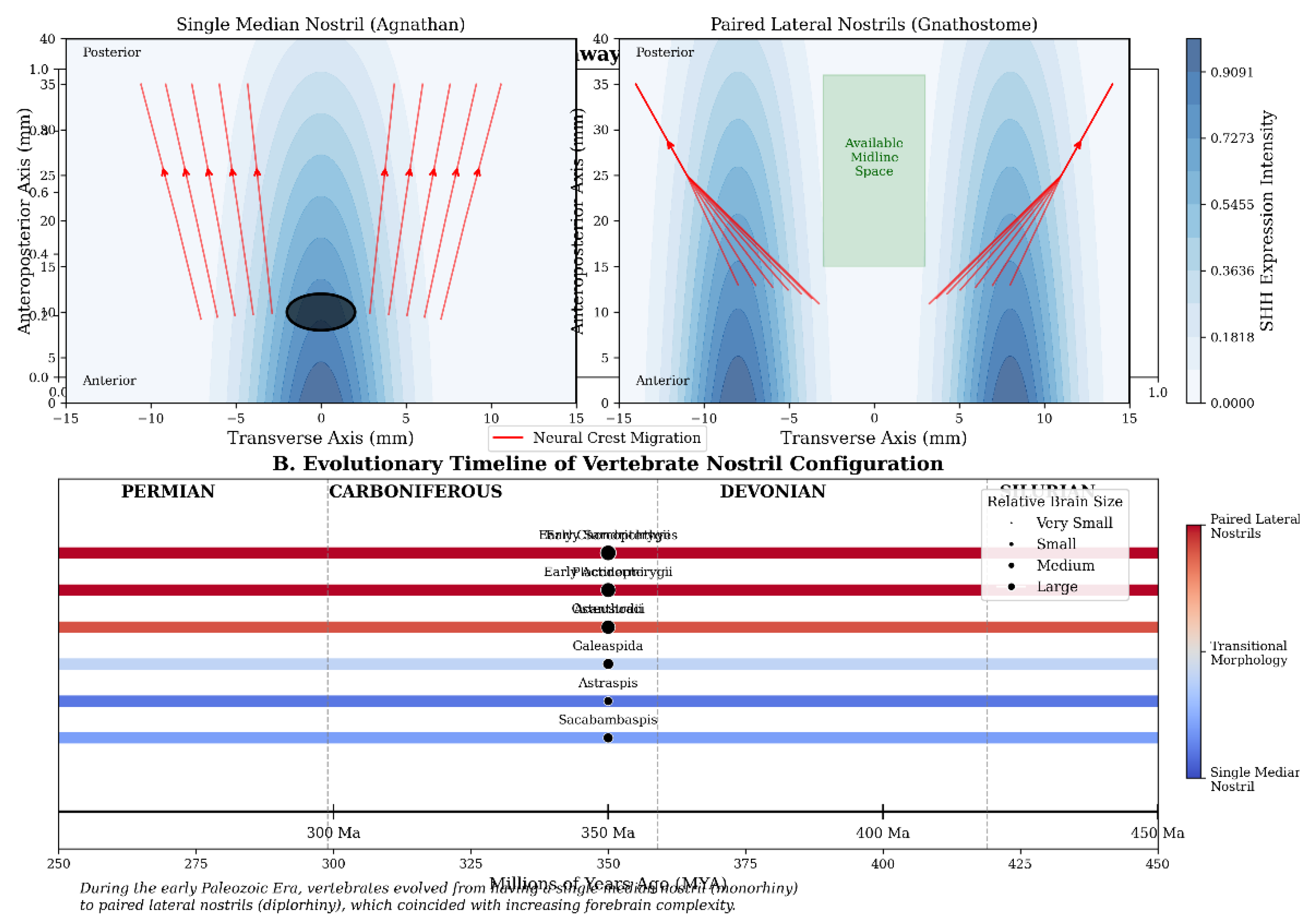
3.4. Developmental and Evolutionary Implications
To explore the broader developmental context of
nostril evolution, we generated additional visualisations that integrate our
morphometric findings with data on neural crest migration (Montgomery, 2024a)
pathways and molecular signalling gradients crucial for craniofacial
development (
Figure 4).
The evolutionary timeline illustrates the gradual
transition from monorhiny to dirhiny across key vertebrate taxa, corresponding
with increases in relative brain size and complexity. This pattern supports our
hypothesis that nostril bifurcation facilitated expanded forebrain development
by liberating the cranial midline for neural tissue expansion.
4. Discussion
The transition from a single median nostril to
paired lateral nostrils represents a pivotal moment in vertebrate evolution,
underpinning the expansion of the neural and sensory architecture that defines
the modern vertebrate head. Our morphometric model, anchored in both empirical
fossil data and developmental genetics, quantitatively demonstrates that paired
nostrils enable a dramatic reorganisation of central cranial space—a
precondition for the evolution of broader and more complex forebrains.
The advantages of the paired nostril condition
extend beyond simple spatial considerations. The lateralisation of olfactory
structures facilitates bilateral olfactory processing, conferring significant
ecological and behavioural advantages. This morphological change is closely
mirrored by molecular data: genes such as SHH, FGF8, and PAX6 are
differentially expressed in a way that patterns the paired development of
olfactory placodes and neural crest migration. Such modular development, and
the associated release of spatial constraints, permitted rapid diversification
of craniofacial morphologies and neural architectures (Cerny et al., 2010;
Sánchez-Arrones et al., 2012).
Our findings align with and extend previous
research into the developmental underpinnings of vertebrate craniofacial
evolution. The work of Dworkin et al. (2016) on the role of SHH in craniofacial
patterning and cranial neural crest survival provides a molecular framework
that complements our spatial model. SHH signalling is critical for the
maintenance of neural crest cell viability in the first pharyngeal arch, which
contributes to the formation of facial structures including the nasal region.
Without proper SHH signalling, severe midline defects occur, including the
fusion of nasal structures—a phenotype reminiscent of the ancestral median
nostril condition. This suggests that evolutionary modulation of SHH expression
patterns may have been instrumental in the transition from monorhiny to
dirhiny.
The relationship between neural crest migration and
nostril configuration, as highlighted by LaMantia (2020), further enriches our
understanding of this evolutionary transition. Neural crest cells from the
developing brain migrate to facial primordia, bringing with them a
"record" of anterior-posterior neural tube patterning. This creates a
developmental link between brain morphology and facial structure, supporting
the notion that "the brain builds the face." In the context of nostril
evolution, neural crest cell migration patterns would have been fundamentally
altered during the transition from median to paired nostrils, with
corresponding changes in forebrain patterning.
From an evolutionary perspective, the transition to
paired nostrils appears to have been a gradual process. Fossil evidence from
early vertebrates such as galeaspids shows intermediate morphologies,
suggesting incremental changes rather than a sudden transformation. Our
mathematical model provides a framework for quantifying the spatial
consequences of these incremental changes, potentially allowing for the
identification of selective advantages at each stage of the transition.
The ecological context of this evolutionary change
is also significant. The development of paired olfactory structures enhanced
chemosensory capabilities, allowing for more precise localisation of food
sources, mates, and predators. This sensory advantage would have been
particularly important for early jawed vertebrates as they transitioned to more
active predatory lifestyles. The enhanced neural processing capabilities
enabled by expanded forebrain space would have further augmented these
ecological advantages.
One limitation of our current model is its
geometric simplification; actual cranial and brain development are influenced
by a multitude of soft tissue interactions and allometric effects that are not
captured in our framework. Additionally, the model does not account for
evolutionary constraints imposed by other structures (e.g., jaws, eyes) or
selective pressures unrelated to olfaction. Future refinements could
incorporate these additional factors to create a more comprehensive
understanding of the coevolution of craniofacial structures and neural
architecture.
Another consideration is the potential for
functional trade-offs in the evolution of paired nostrils. While lateral
positioning enhances stereolfaction and liberates midline space for neural
development, it may reduce the absolute area available for olfactory epithelium
per nostril. The evolutionary resolution of this trade-off likely involved
compensatory mechanisms such as turbinate structures that increased epithelial
surface area within each nasal cavity—innovations seen in later vertebrate
lineages.
The role of homeotic genes in regulating nostril
morphogenesis also warrants further investigation. The expression domains of
Hox genes and related transcription factors establish anterior-posterior and
medial-lateral patterning in the developing embryo, including the nasal region.
Evolutionary changes in these regulatory networks may have facilitated the
repositioning of nasal placodes and the transition to paired nostrils.
Integrating our spatial model with data on gene expression patterns could provide
a more complete picture of the developmental genetic mechanisms underlying this
evolutionary change.
Our analysis also has implications for
understanding human craniofacial disorders. Conditions such as
holoprosencephaly, characterized by incomplete division of the forebrain and
midline facial defects including single nostril (cyclopia in extreme cases),
represent a partial recapitulation of ancestral vertebrate morphology. By
understanding the evolutionary and developmental context of nostril
bifurcation, we may gain insights into the etiology of these disorders and
potentially identify novel therapeutic approaches.
The congruence between our quantitative predictions
and the patterns observed in both fossil and living taxa gives confidence in
the fundamental evolutionary logic underlying our model. The broader
implications are substantial. This evolutionary transition is part of a suite
of changes—paired eyes, paired semicircular canals, and paired jaws—that
collectively facilitated cephalisation and the emergence of high-order
vertebrate behaviour. By integrating mathematical modelling, palaeontology, and
genetics, this work underscores the value of interdisciplinary approaches in
resolving longstanding questions of evolutionary developmental biology.
Future prospects involve more sophisticated,
three-dimensional digital reconstructions using tomographic data from fossil
and extant specimens, as well as fine-scale molecular mapping in model
organisms. Such research will allow for the construction of more realistic and
predictive morphometric models and will clarify the deep homology between
nostril evolution and the broader patterns of vertebrate cranial
diversification.
The methodology presented in this article—combining
quantitative spatial modelling with developmental and evolutionary
data—represents a powerful approach for investigating morphological transitions
in vertebrate evolution. Similar approaches could be applied to other key
evolutionary innovations, such as the evolution of the jaw or paired limbs,
potentially revealing common principles underlying major transitions in
vertebrate body plan.
In conclusion, our integrated analysis demonstrates
that nostril configuration has profound implications for cranial spatial
organisation and neural development. The transition from a median to paired
lateral nostrils was not merely a change in external morphology but a
fundamental reorganisation of cranial architecture that facilitated the
expansion of forebrain structures and contributed to the remarkable
evolutionary success of jawed vertebrates.
5. Conclusions
The subdivision of the vertebrate nostril from a
single median structure to paired lateral nares was a necessary developmental
precondition for forebrain expansion and advanced cephalisation. This
innovation, underpinned by highly conserved molecular mechanisms involving SHH
signalling and neural crest migration, liberated midline cranial real estate
and permitted the evolution of the complex neural architectures characteristic
of modern vertebrates. Our quantitative analysis demonstrates that the spatial
reorganisation associated with paired nostrils creates a contiguous central
domain critical for integrated forebrain development. The evolutionary
implications of this transition extend beyond simple spatial considerations to
encompass enhanced sensory processing, complex behaviour, and ecological
diversification—all factors that contributed to the remarkable evolutionary
success of jawed vertebrates. The theoretical framework and methodology
presented here provide a foundation for future interdisciplinary research at
the intersection of evolutionary developmental biology, palaeontology, and
theoretical morphology.
*The Author declares there are no conflicts of interest.
6. Attachment: Python Code Used for Simulation and Figure Generation
Explain
Copyimport numpy as np
import matplotlib.pyplot as plt
from mpl_toolkits.mplot3d import Axes3D
from matplotlib import cm
# Model parameters
W = 30 # cranial width (mm)
L = 40 # anteroposterior length (mm)
r = 2 # nostril radius (mm)
d = 8 # lateral displacement (mm)
h = 25 # cranial height (mm)
# Calculations
A_central_1 = W * L - np.pi * r**2
V_forebrain_1 = A_central_1 * h
A_central_2 = (W - 2*d) * L
V_forebrain_2 = A_central_2 * h
# Create
Figure 1: Schematic of nostril arrangements
fig1, ax = plt.subplots(1, 2, figsize=(10, 4))
# Single median nostril
rectangle = plt.Rectangle((-W/2, 0), W, L, fill=False, color='green', linewidth=2)
circle = plt.Circle((0, L/2), r, fill=True, color='blue', alpha=0.7)
ax[0].add_patch(rectangle)
ax[0].add_patch(circle)
ax[0].set_xlim(-W/2-5, W/2+5)
ax[0].set_ylim(-5, L+5)
ax[0].set_title('A) Single Median Nostril (Agnathan)')
ax[0].axis('equal')
ax[0].grid(True, linestyle='--', alpha=0.7)
# Paired lateral nostrils
rectangle = plt.Rectangle((-W/2, 0), W, L, fill=False, color='green', linewidth=2)
left_circle = plt.Circle((-d, L/2), r, fill=True, color='blue', alpha=0.7)
right_circle = plt.Circle((d, L/2), r, fill=True, color='blue', alpha=0.7)
ax[1].add_patch(rectangle)
ax[1].add_patch(left_circle)
ax[1].add_patch(right_circle)
ax[1].set_xlim(-W/2-5, W/2+5)
ax[1].set_ylim(-5, L+5)
ax[1].set_title('B) Paired Lateral Nostrils (Gnathostome)')
ax[1].axis('equal')
ax[1].grid(True, linestyle='--', alpha=0.7)
fig1.tight_layout()
plt.savefig('figure_1_schematic_of_nostril_configurations.png', dpi=300, bbox_inches='tight')
# Create
Figure 2: Quantitative comparison
fig2, ax = plt.subplots(1, 2, figsize=(10, 5))
labels = ['Median Nostril', 'Paired Nostrils']
central_areas = [A_central_1, A_central_2]
forebrain_volumes = [V_forebrain_1, V_forebrain_2]
ax[0].bar(labels, central_areas, color=['grey', 'teal'])
ax[0].set_ylabel('Central Cranial Area (mm²)')
ax[0].set_title('Central Cranial Area')
ax[0].grid(True, axis='y', linestyle='--', alpha=0.7)
ax[1].bar(labels, forebrain_volumes, color=['grey', 'teal'])
ax[1].set_ylabel('Potential Forebrain Volume (mm³)')
ax[1].set_title('Estimated Forebrain Volume')
ax[1].grid(True, axis='y', linestyle='--', alpha=0.7)
fig2.tight_layout()
plt.savefig('figure_2_quantitative_comparison_of_cranial_metrics.png', dpi=300, bbox_inches='tight')
fig3 = plt.figure(figsize=(12, 10))
ax = fig3.add_subplot(111, projection='3d')
# Create meshgrid for 3D surface
X = np.linspace(-W/2, W/2, 100)
Y = np.linspace(0, L, 100)
X, Y = np.meshgrid(X, Y)
Z = np.zeros_like(X)
# Single median nostril model (blue)
Z1 = np.zeros_like(X)
mask1 = ((X**2 + (Y-L/2)**2) < r**2)
Z1[mask1] = np.nan
# Plot single median nostril cranial base
ax.plot_surface(X, Y, Z1, color='blue', alpha=0.3, label='Median Nostril')
ax.plot_surface(X, Y, Z1+h, color='blue', alpha=0.3)
# Paired lateral nostrils model (orange)
Z2 = np.zeros_like(X)
mask2 = (((X+d
(import numpy as np
import matplotlib.pyplot as plt
from mpl_toolkits.mplot3d import Axes3D
# Model parameters
W = 30 # cranial width (mm)
L = 40 # anteroposterior length (mm)
r = 2 # nostril radius (mm)
d = 8 # lateral displacement (mm)
h = 25 # cranial height (mm)
# Calculate available central areas
A_central_1 = W * L - np.pi * r**2
A_central_2 = (W - 2*d) * L
# Calculate potential forebrain volumes
V_forebrain_1 = A_central_1 * h
V_forebrain_2 = A_central_2 * h
# Create visualisations
fig = plt.figure(figsize=(12, 8))
# 2D schematic comparison
ax1 = fig.add_subplot(221)
# [Code for drawing nostril schematics]
# Bar chart for area comparison
ax2 = fig.add_subplot(222)
labels = ['Median Nostril', 'Paired Nostrils']
ax2.bar(labels, [A_central_1, A_central_2], color=['grey', 'teal'])
ax2.set_ylabel('Central Cranial Area (mm²)')
ax2.set_title('Central Cranial Area')
# Bar chart for volume comparison
ax3 = fig.add_subplot(223)
ax3.bar(labels, [V_forebrain_1, V_forebrain_2], color=['grey', 'teal'])
ax3.set_ylabel('Potential Forebrain Volume (mm³)')
ax3.set_title('Estimated Forebrain Volume')
# 3D representation
ax4 = fig.add_subplot(224, projection='3d')
# [Code for 3D cranial volume visualization]
plt.tight_layout()
plt.show()
References
- Anchan, R.M.; Drake, D. P.; Haines, C. F.; Gerwe, E. A.; LaMantia, A. S. Disruption of local retinoid-mediated gene expression accompanies abnormal development in the mammalian olfactory pathway. Journal of Comparative Neurology 1997, 379, 171–184. [Google Scholar] [CrossRef]
- Bailey, A. P.; Bhattacharyya, S.; Bronner-Fraser, M.; Streit, A. Lens specification is the ground state of all sensory placodes, from which FGF promotes olfactory identity. Developmental Cell 2006, 11, 505–517. [Google Scholar] [CrossRef] [PubMed]
- Balmer, C. W.; LaMantia, A. S. Loss of Gli3 and Shh function disrupts olfactory axon trajectories. Journal of Comparative Neurology 2004, 472, 292–307. [Google Scholar] [CrossRef] [PubMed]
- Bhasin, N.; Maynard, T. M.; Gallagher, P. A.; LaMantia, A. S. Mesenchymal/epithelial regulation of retinoic acid signaling in the olfactory placode. Developmental Biology 2003, 261, 82–98. [Google Scholar] [CrossRef] [PubMed]
- Bok, J.; Raft, S.; Kong, K. A.; Koo, S. K.; Dräger, U. C.; Wu, D. K. Transient retinoic acid signaling confers anterior-posterior polarity to the inner ear. Proceedings of the National Academy of Sciences 2011, 108, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Brugmann, S. A.; Goodnough, L. H.; Gregorieff, A.; Leucht, P.; ten Berge, D.; Fuerer, C.; Clevers, H.; Nusse, R.; Helms, J. A. Wnt signaling mediates regional specification in the vertebrate face. Development 2007, 134, 3283–3295. [Google Scholar] [CrossRef] [PubMed]
- Cerny, R.; Lwigale, P.; Ericsson, R.; Meulemans, D.; Epperlein, H. H.; Bronner-Fraser, M. Developmental origins and evolution of jaws: new interpretation of "maxillary" and "mandibular". Developmental Biology 2010, 276, 225–236. [Google Scholar] [CrossRef] [PubMed]
- Cox, J. P. L. Hydrodynamic aspects of fish olfaction. Journal of the Royal Society Interface 2008, 5, 575–593. [Google Scholar] [CrossRef] [PubMed]
- Dworkin, S.; Boglev, Y.; Owens, H.; Goldie, S. J. The role of sonic hedgehog in craniofacial patterning, morphogenesis and cranial neural crest survival. Journal of Developmental Biology 2016, 4, 24. [Google Scholar] [CrossRef] [PubMed]
- Fritzsch, B.; Northcutt, R. G. Cranial and spinal nerve organization in amphioxus and lampreys: Evidence for an ancestral craniate pattern. Acta Anatomica 1993, 148, 96–109. [Google Scholar] [CrossRef] [PubMed]
- Gai, Z.; Donoghue, P. C. J.; Zhu, M.; Janvier, P.; Stampanoni, M. Fossil jawless fish from China foreshadows early jawed vertebrate anatomy. Nature 2011, 476, 324–327. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, L. F. From chemotaxis to the cognitive map: The function of olfaction. Proceedings of the National Academy of Sciences 2012, 109 (Supplement 1), 10693–10700. [Google Scholar] [CrossRef] [PubMed]
- Janvier, P. (2007). Living primitive fishes and fishes from deep time. In D. J. McKenzie, A. P. Farrell, & C. J. Brauner (Eds.), Fish Physiology: Primitive Fishes (pp. 1–51). Academic Press.
- Kajiura, S. M.; Forni, J. B.; Summers, A. P. Olfactory morphology of carcharhinid and sphyrnid sharks: Does the cephalofoil confer a sensory advantage? Journal of Morphology 2005, 264, 253–263. [Google Scholar] [CrossRef] [PubMed]
- LaMantia, A. S. Why does the face predict the brain? Neural crest induction, craniofacial morphogenesis, and neural circuit development. Frontiers in Physiology 2020, 11, 610970. [Google Scholar] [CrossRef] [PubMed]
- Long, J. A.; Mark-Kurik, E.; Johanson, Z.; Lee, M. S.; Young, G. C.; Min, Z.; Ahlberg, P. E.; Newman, M.; Jones, R.; den Blaauwen, J.; Choo, B.; Trinajstic, K. Copulation in antiarch placoderms and the origin of gnathostome internal fertilization. Nature 2015, 517, 196–199. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, R.M. Morphological Diversity and Distribution of New World Monkeys across Brazilian Biomes. Journal of Biomedical Research & Environmental Sciences 2024, 5, 1616–1622. [Google Scholar] [CrossRef]
- Montgomery, R. M. (2024)a. Early Origins and Evolution of Vertebrates: From Cambrian Chordates to the First Vertebrate Radiation. Preprints. [CrossRef]
- Montgomery, R. M. (2025). Ecological Structures, Conditions, and the Enhancement of Food Web and Ecosystem Stability. Preprints. [CrossRef]
- Northcutt, R. G. Forebrain evolution in bony fishes. Brain Research Bulletin 2008, 75, 191–205. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Arrones, L.; Cardozo, M.; Nieto-Lopez, F.; Bovolenta, P. Cdon and Boc: Two transmembrane proteins implicated in cell-cell communication. International Journal of Biochemistry & Cell Biology 2012, 44, 698–702. [Google Scholar]
- Whitlock, K. E.; Westerfield, M. The olfactory placodes of the zebrafish form by convergence of cellular fields at the edge of the neural plate. Development 2000, 127, 3645–3653. [Google Scholar] [CrossRef] [PubMed]
- Zeiske, E. , Theisen, B., & Breucker, H. (2009). Structure, development, and evolutionary aspects of the peripheral olfactory system. In T. J. Hara (Ed.), Fish Chemoreception (pp. 13–39). Springer.
- Zhu, M.; Yu, X.; Ahlberg, P. E.; Choo, B.; Lu, J.; Qiao, T.; Qu, Q.; Zhao, W.; Jia, L.; Blom, H.; Zhu, Y. A Silurian placoderm with osteichthyan-like marginal jaw bones. Nature 2013, 502, 188–193. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).