Submitted:
15 May 2025
Posted:
16 May 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Extraction
2.2. Phytochemical Profile of Extracts
2.3. Materials Used in the Formulations
2.4. Determination of Partition Coefficient LogP and Skin Permeability Potential
2.5. Physicochemical and Rheological Characterizationation Methods
| Formulation | Extraction method | Extract & Concentration (w/w) | Gel components | In vivo Unique Name |
Description |
|---|---|---|---|---|---|
| OG | ETOH99.5 | OB 5% |
Sunflower oil 80% Glyceryl dibehenate 15% |
OG_OB_ETOH99.5 | Oleogel with basil extract in absolute ethanol, based on glyceryl dibehenate and sunflower oil |
| OG | ETOH99.5 | BS 5% |
Sunflower oil 80% Glyceryl dibehenate 15% |
OG_BS_ETOH99.5 | Oleogel with frankincense extract in absolute ethanol, based on glyceryl dibehenate and sunflower oil |
| OG | ETOH99.5 | SNF 5% |
Olive oil 80% Glyceryl dibehenate 15% |
OG_SNF_ETOH99.5 | Oleogel with elderflower extract in absolute ethanol, based on glyceryl dibehenate and olive oil |
| OG | ETOH99.5 | SNB 5% |
Sunflower oil 80% Glyceryl dibehenate 15% |
OG_SNB_ETOH99.5 | Oleogel with elder bark extract in absolute ethanol, based on glyceryl dibehenate and sunflower oil |
| OG | ETOH99.5 | GV 5% |
Olive oil 80% Glyceryl dibehenate 15% |
OG_GV_ETOH99.5 | Oleogel with Galium verum extract in absolute ethanol, based on glyceryl dibehenate and olive oil |
| OG | ETOH70 | OB 5% |
Isopropyl myristate 80% Glyceryl dibehenate 15% |
OG_OB_ETOH70 | Oleogel with basil extract in 70% ethanol, based on glyceryl dibehenate and isopropyl myristate |
| OG | ETOH70 | BS 5% |
Sunflower oil 80% Glyceryl dibehenate 15% |
OG_BS_ETOH70 | Oleogel with frankincense extract in 70% ethanol, based on glyceryl dibehenate and sunflower oil |
| OG | ETOH70 | SNF 5% |
Diethylene glycol monoethyl ether 80% Glyceryl dibehenate 15% |
OG_SNF_ETOH70 | Oleogel with elderflower extract in 70% ethanol, based on glyceryl dibehenate and diethylene glycol monoethyl ether |
| OG | ETOH99.5 | BS_OB_SNF_GV 1.25:1.25:1.25:1.25% |
Sunflower oil 20% Olive oil 20% Isopropyl myristate 20% Diethylene glycol monoethyl ether 20% Glyceryl dibehenate 15% |
OG_BS_OB_SNF_GV_ETOH99.5 | Oleogel with 4 plant extracts in absolute ethanol |
| OG | ETOH70 | BS_OB_SNF_GV 1.25:1.25:1.25:1.25% |
Sunflower oil 20% Olive oil 20% Isopropyl myristate 20% Diethylene glycol monoethyl ether 20% Glyceryl dibehenate 15% |
OG_BS_OB_SNF_GV_ETOH70 | Oleogel with 4 plant extracts in 70% ethanol |
| HG | ETOH99.5 | OB 5% |
Purified water 60% Poloxamer 407 25% Glycerol 10% |
HG_OB_ETOH99.5 | Hydrogel with basil extract in absolute ethanol |
| HG | ETOH99.5 | BS 5% |
Purified water 60% Poloxamer 407 25% Glycerol 10% |
HG_BS_ETOH99.5 | Hydrogel with frankincense extract in absolute ethanol |
| HG | ETOH99.5 | SNF 5% |
Purified water 60% Poloxamer 407 25% Glycerol 10% |
HG_SNF_ETOH99.5 | Hydrogel with elderflower extract in absolute ethanol |
| HG | ETOH99.5 | GV 5% |
Purified water 60% Poloxamer 407 25% Glycerol 10% |
HG_GV_ETOH99.5 | Hydrogel with Galium verum extract in absolute ethanol |
| HG | ETOH70 | OB 5% |
Purified water 60% Poloxamer 407 25% Glycerol 10% |
HG_OB_ETOH70 | Hydrogel with basil extract in 70% ethanol |
| HG | ETOH70 | BS 5% |
Purified water 60% Poloxamer 407 25% Glycerol 10% |
HG_BS_ETOH70 | Hydrogel with frankincense extract in 70% ethanol |
| HG | ETOH70 | SNF 5% |
Purified water 60% Poloxamer 407 25% Glycerol 10% |
HG_SNF_ETOH70 | Hydrogel with elderflower extract in 70% ethanol |
| HG | ETOH99.5 | SNB 5% |
Purified water 60% Poloxamer 407 25% Glycerol 10% |
HG_SNB_ETOH99.5 |
Hydrogel with elder bark extract extract in absolute ethanol |
| HG | ETOH70 | BS_OB_SNF_GV 1.25:1.25:1.25:1.25% |
Purified water 60% Poloxamer 407 25% Glycerol 10% |
HG_BS_OB_SNF_GV_ETOH70 | Hydrogel with 4 plant extracts in 70% ethanol |
| HG | ETOH99.5 | BS_OB_SNF_GV 1.25:1.25:1.25:1.25% |
Purified water 60% Poloxamer 407 25% Glycerol 10% |
HG_BS_OB_SNF_GV_ETOH99.5 | Hydrogel with 4 plant extracts in absolute ethanol |
| Pre-burn | N/A | Baseline | N/A | Pre-burn_baseline | Control for the healthy skin |
| Untreated_ Burn | N/A | Untreated_Burn | N/A | Untreated_burn_control | Control for the untreated burn |
| OG | N/A | OG_base |
Sunflower oil 21.5% Olive oil 21.5% Isopropyl myristate 21.5% Diethylene glycol monoethyl ether 21.5% Glyceryl dibehenate 15% |
OG_base |
Control oleogel (without bioactive component) |
| HG | N/A | HG_base |
Purified water 65% Poloxamer 407 25% Glycerol 10% |
HG_base | Control hydrogel (without bioactive component), based on poloxamer 407 and glycerol, purified water |
2.6. Statistical Analysis
2.7. Methodology for Physicochemical and Rheological Characterization
2.7.1. Determination of Macroscopic Properties and pH
2.7.2. Rheological Measurements
2.7.3. pH Analysis: Formulations and Burn Wounds
2.8. Animal Study and Clinical Parameters
2.8.1. In Vivo Study and Experimental Protocol
2.8.2. Anesthesia, Preoperative Preparation and Pain Management
2.8.3. Burn Induction and Wound Standardization
2.8.4. Treatment Application and Dressing Protocol
2.8.5. Statistical Analysis
3. Results
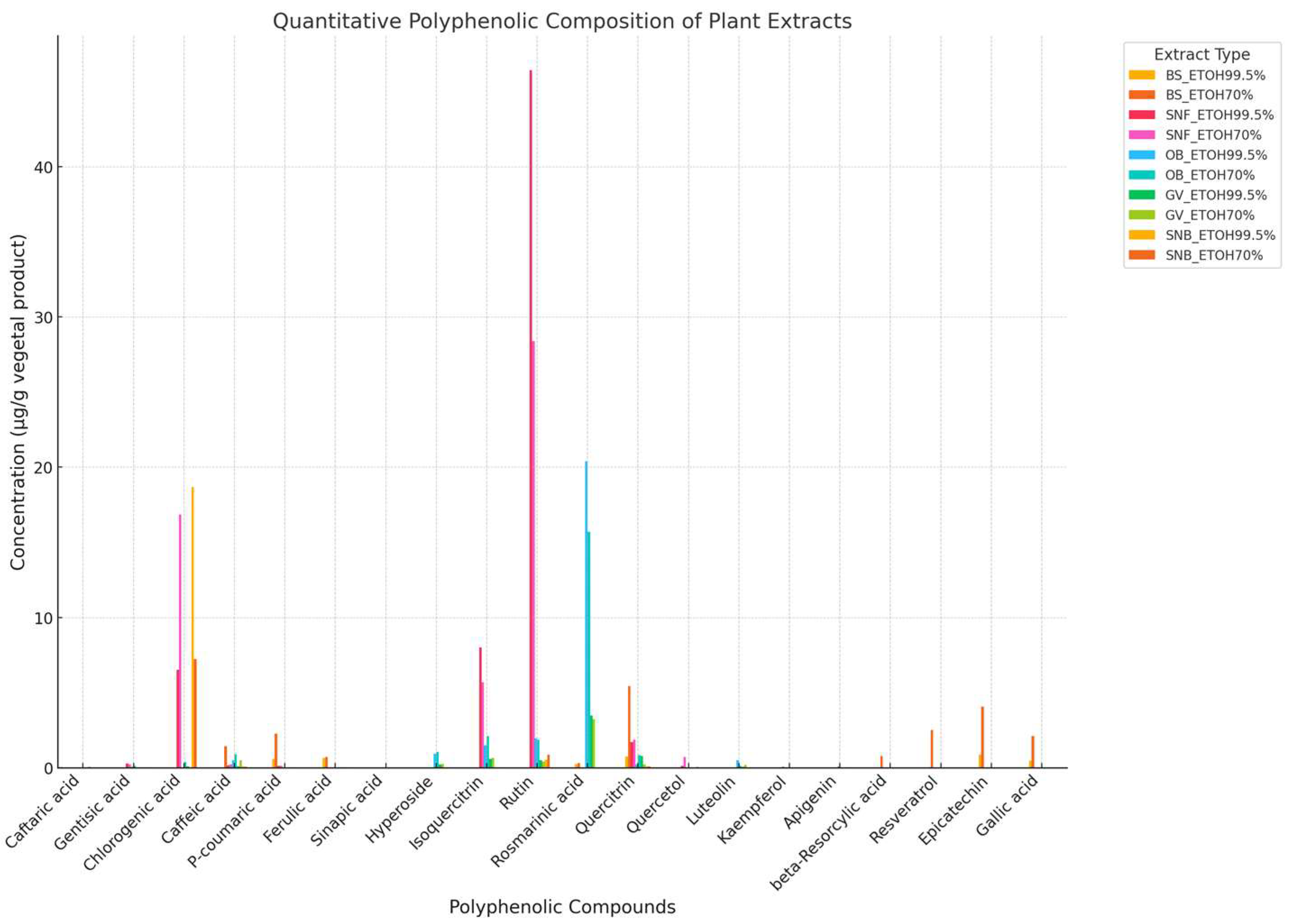
3.1. Quantitative Phytochemical Profile of Extracts
| No. | Compound Name | Molecular Weight (g/mol) | Permeability Potential [28] | Log Po/w |
|---|---|---|---|---|
| 1 | Caftaric acid | 311 | High | -0.29 |
| 2 | Gentisic acid | 153 | High | 0.74 |
| 3 | Chlorogenic acid | 353 | High | -0.43 |
| 4 | Caffeic acid | 179 | High | 0.93 |
| 5 | P-coumaric acid | 163 | High | 1.26 |
| 6 | Ferulic acid | 193 | High | 1.36 |
| 7 | Sinapic acid | 223 | High | 1.31 |
| 8 | Hyperoside | 463 | High | -0.38 |
| 9 | Isoquercitrin | 463 | High | -0.48 |
| 10 | Rutin | 609 | Low | -1.51 |
| 11 | Rosmarinic acid | 359 | High | 5.15 |
| 12 | Quercitrin | 447 | High | -0.05 |
| 13 | Quercetol | 301 | High | 1.23 |
| 14 | Luteolin | 285 | High | 1.73 |
| 15 | Kaempferol | 285 | High | 1.58 |
| 16 | Apigenin | 269 | High | 2.11 |
| 17 | Gaelic acid | 169 | High | 0.21 |
| 18 | Epicatechin | 289 | High | 0.83 |
| 19 | Beta-Resorcylic | 153 | High | 0.77 |
| 20 | Rosveratrol | 227 | High | 2.48 |
3.2. Physicochemical and Rheological Properties
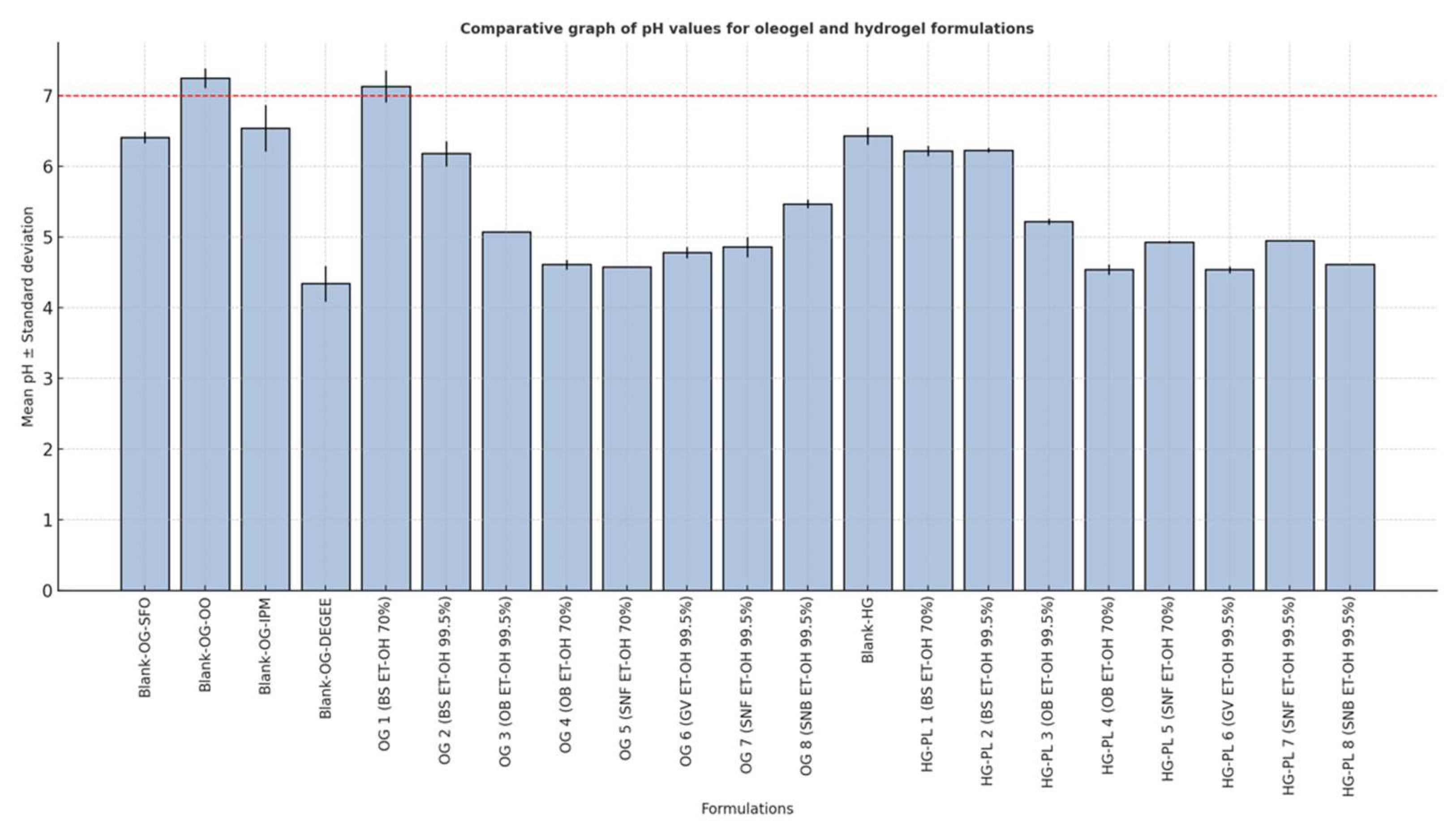
3.2.1. Determination of Macroscopic Properties and pH
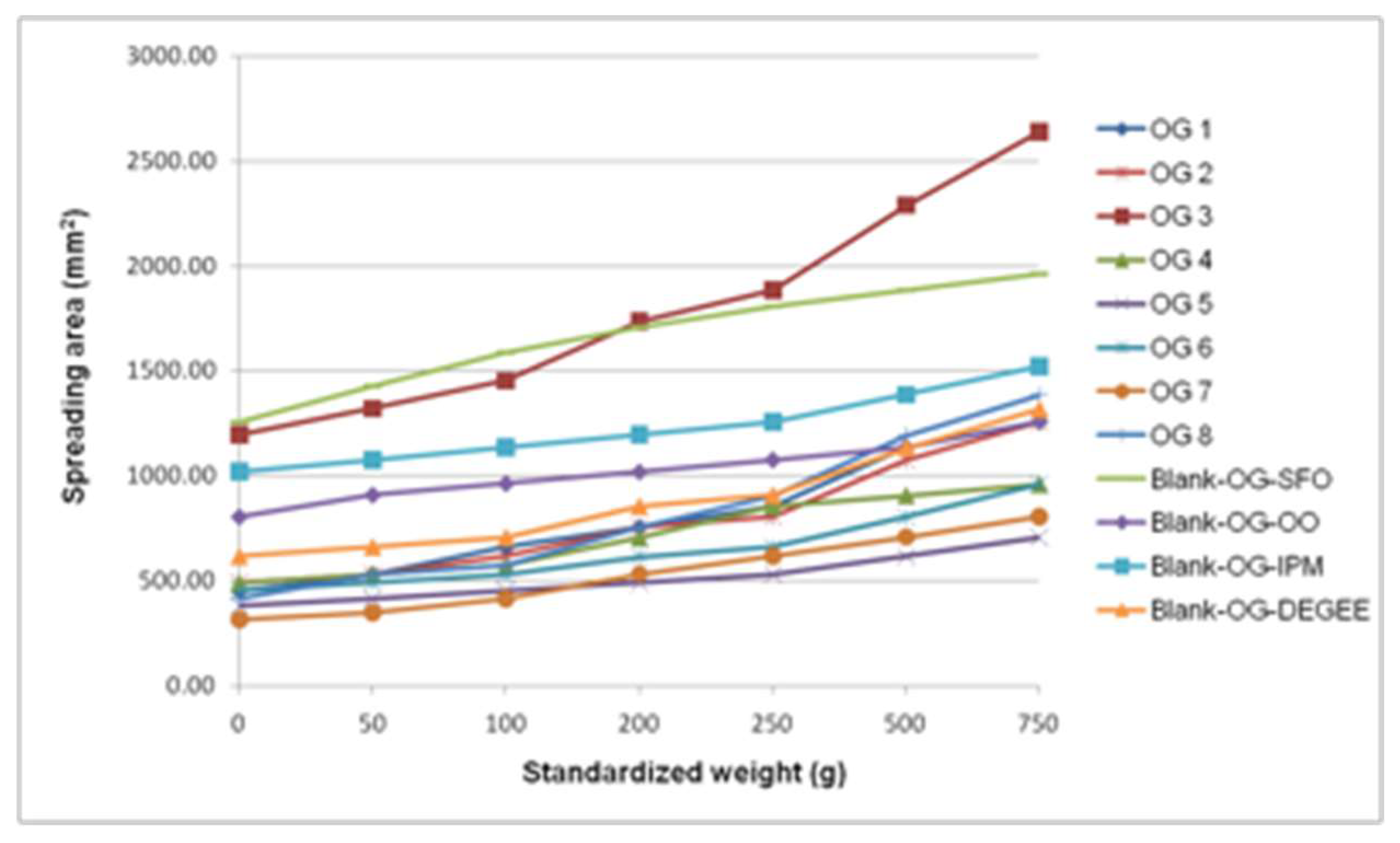
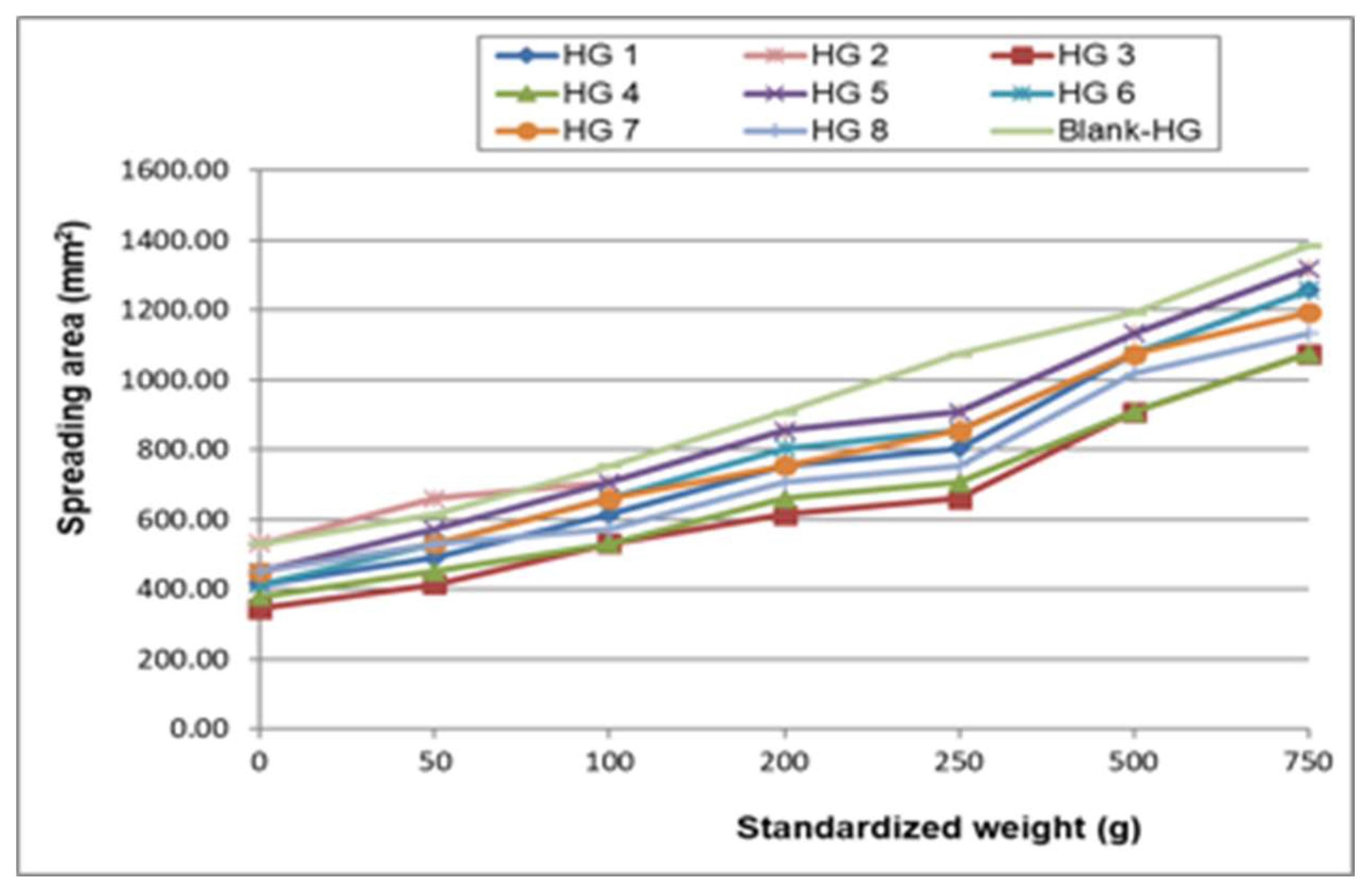
3.2.2. Standardized Macroscopic Evaluation of Topical Formulations

3.3. Physicochemical Characterization
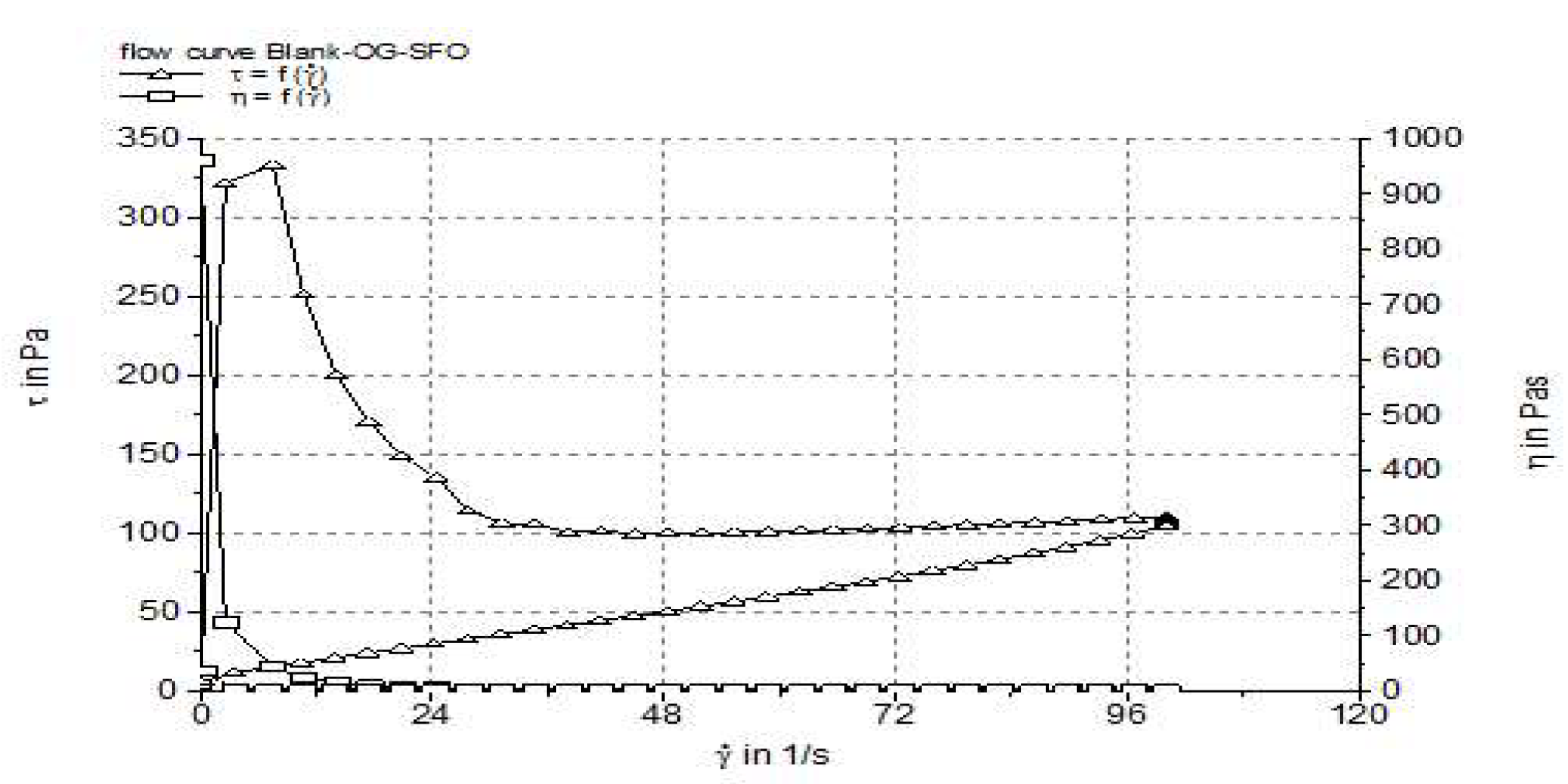
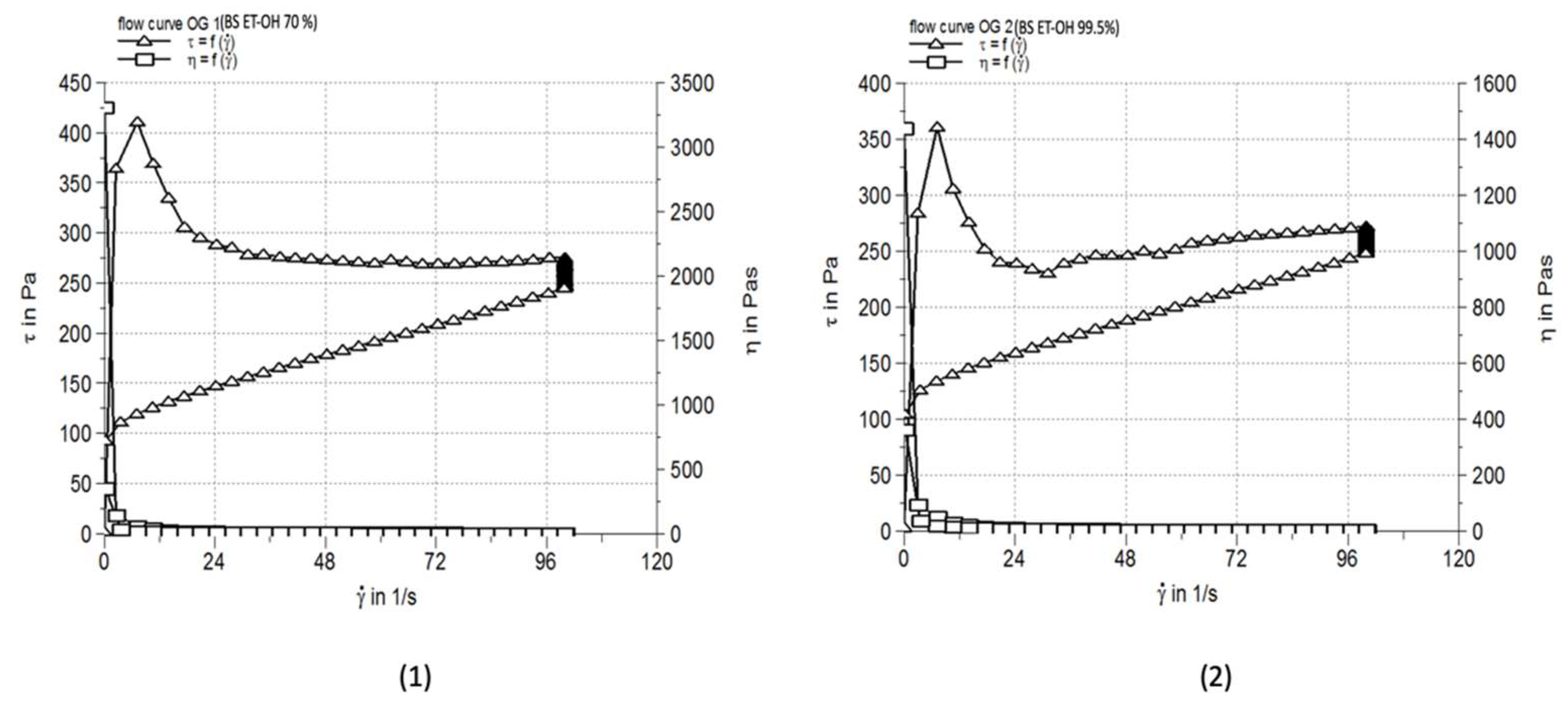
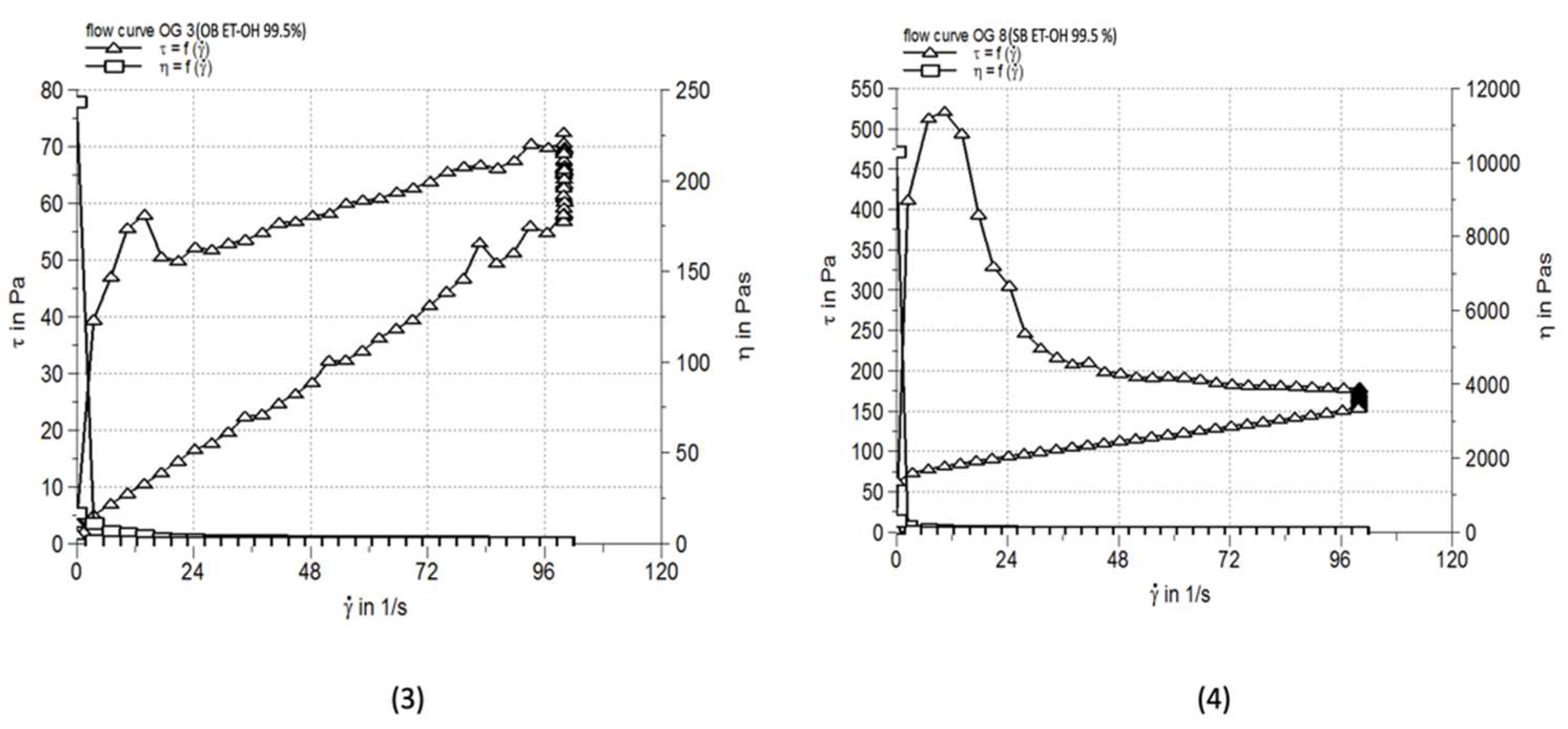
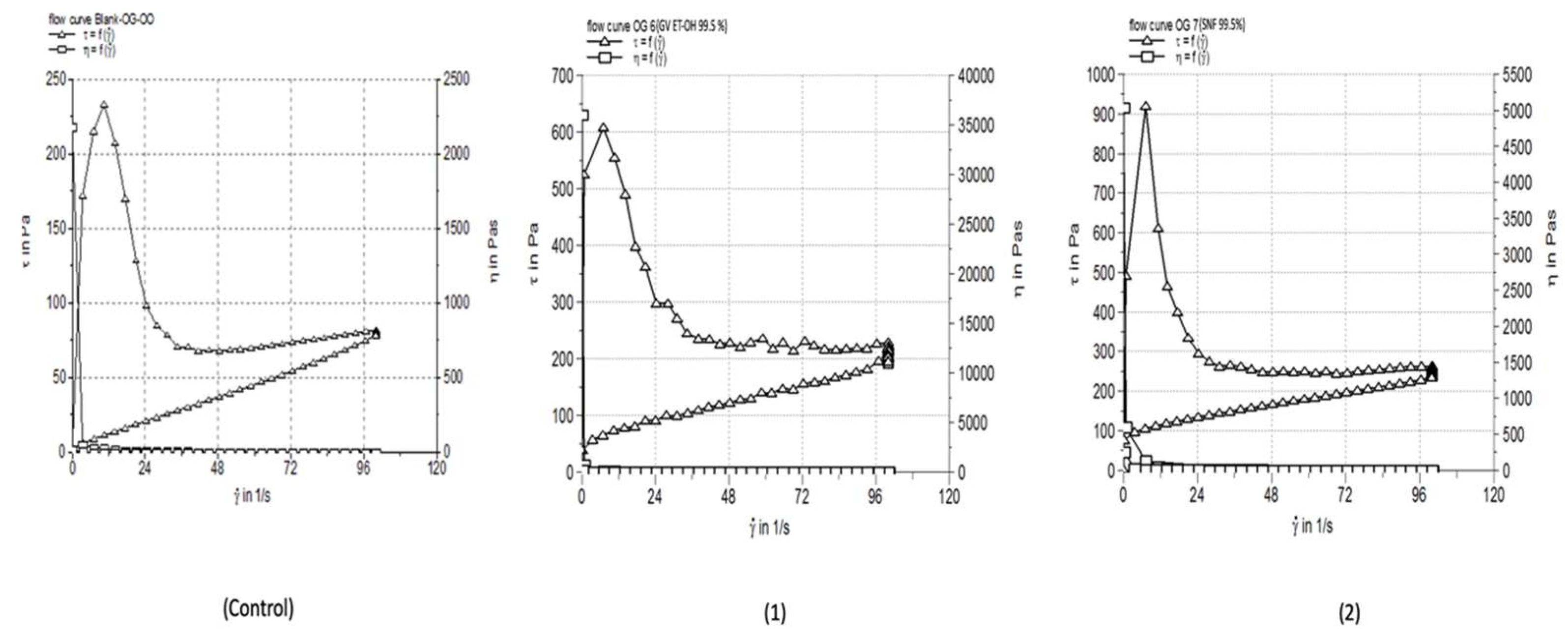
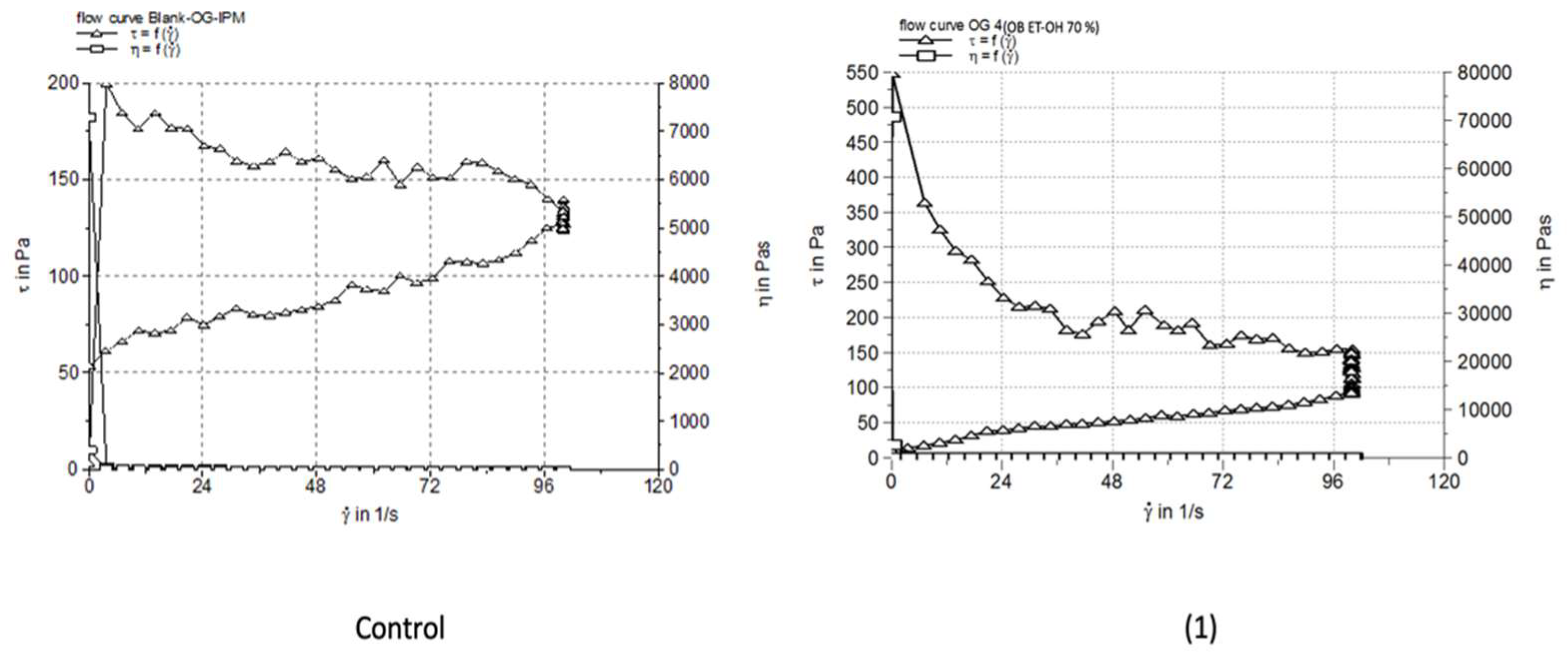
3.4. Rheological Characterization
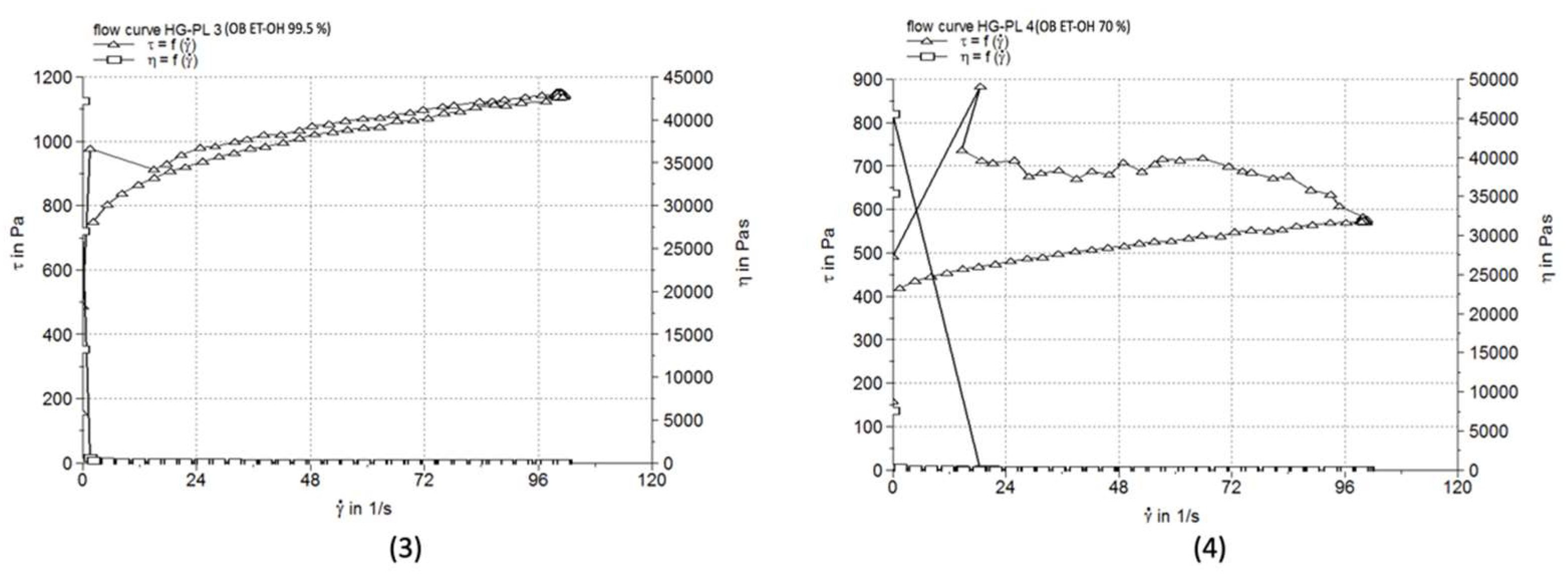
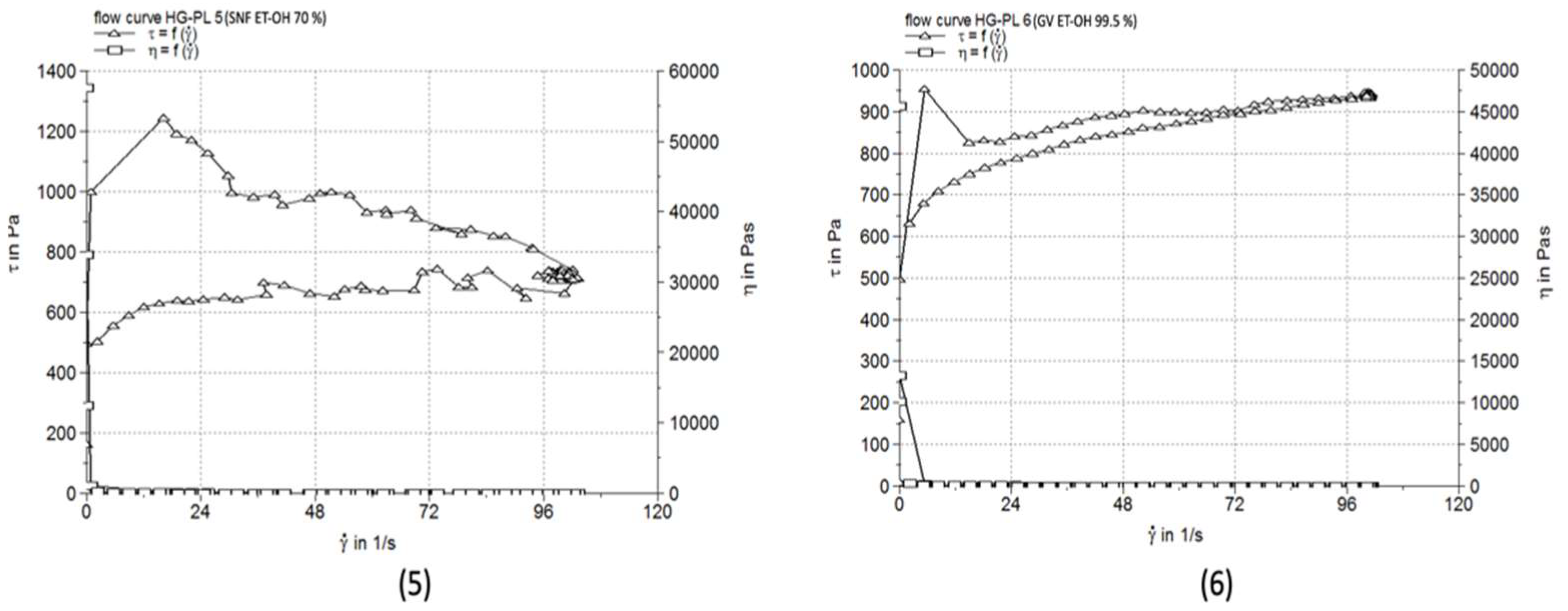
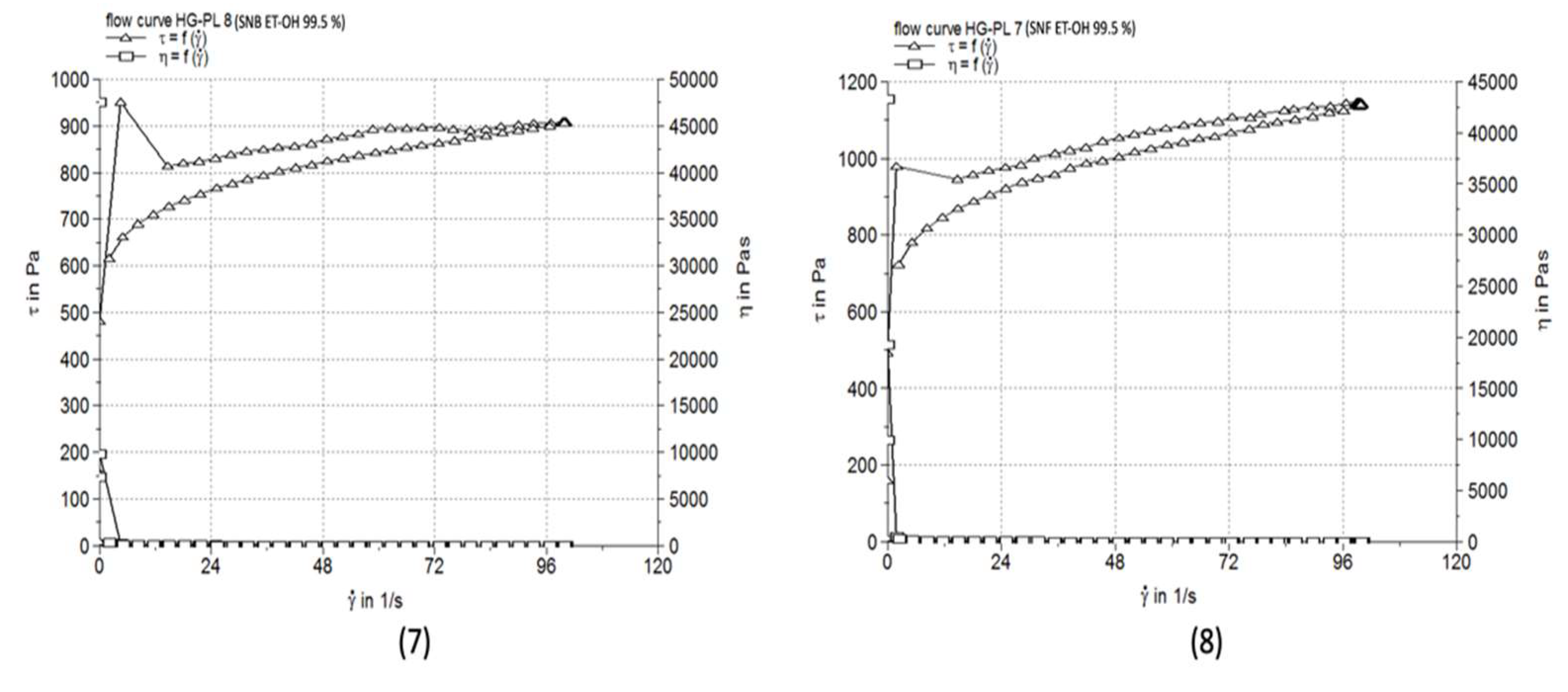
3.5. Comparative pH Evaluation
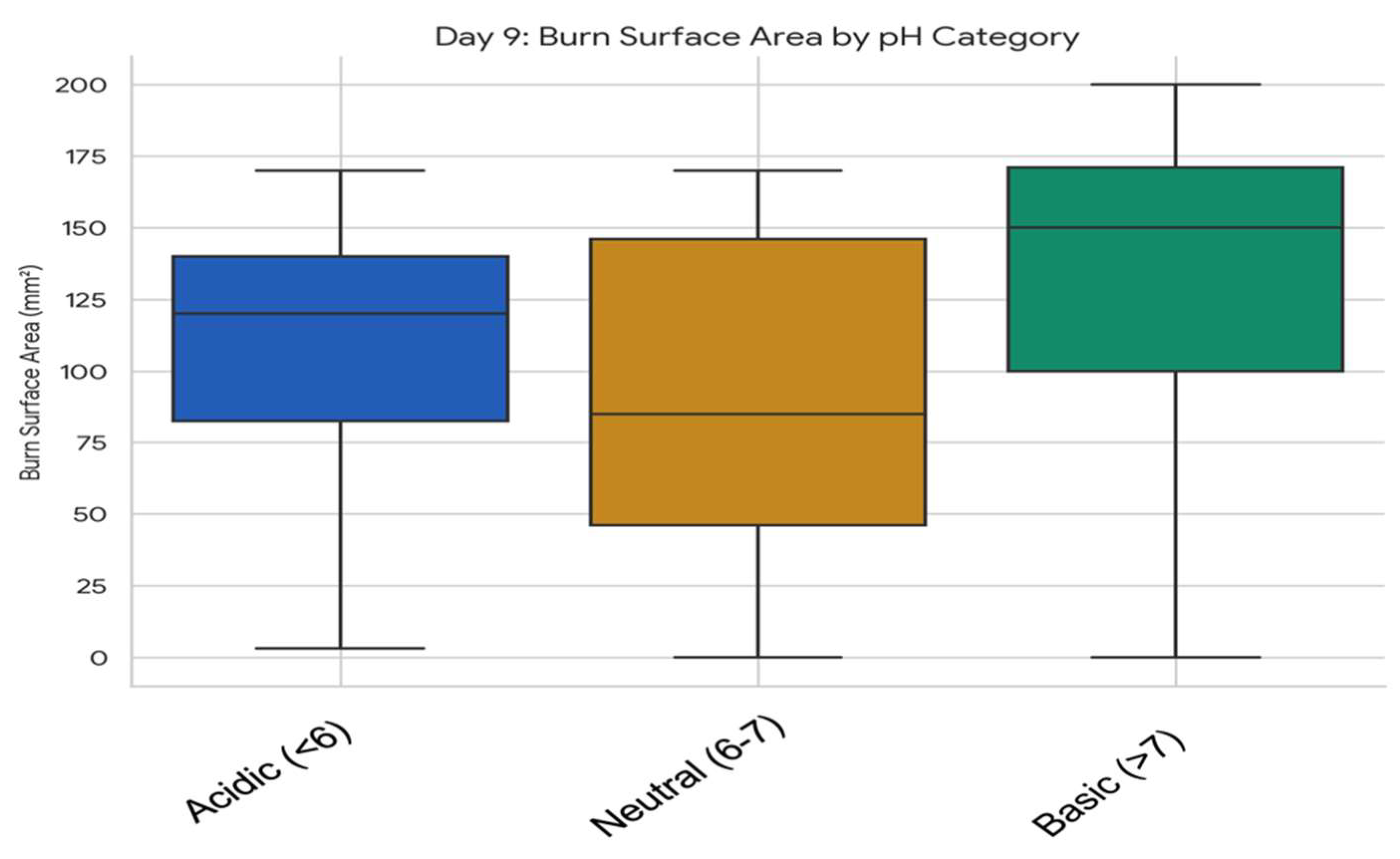
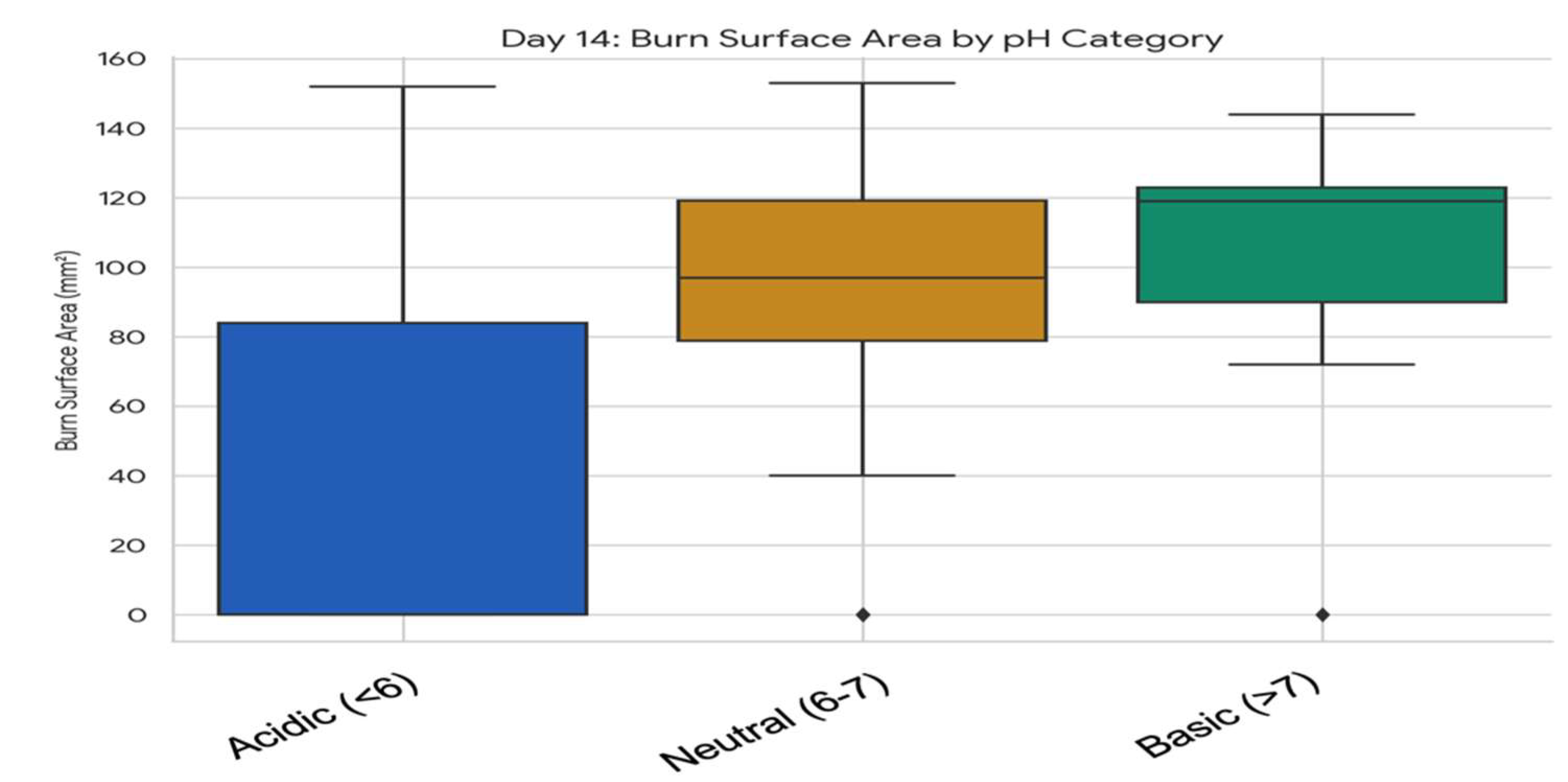
3.5.1. Association Between Wound pH Category and Burn Surface Area at Specific Timepoints
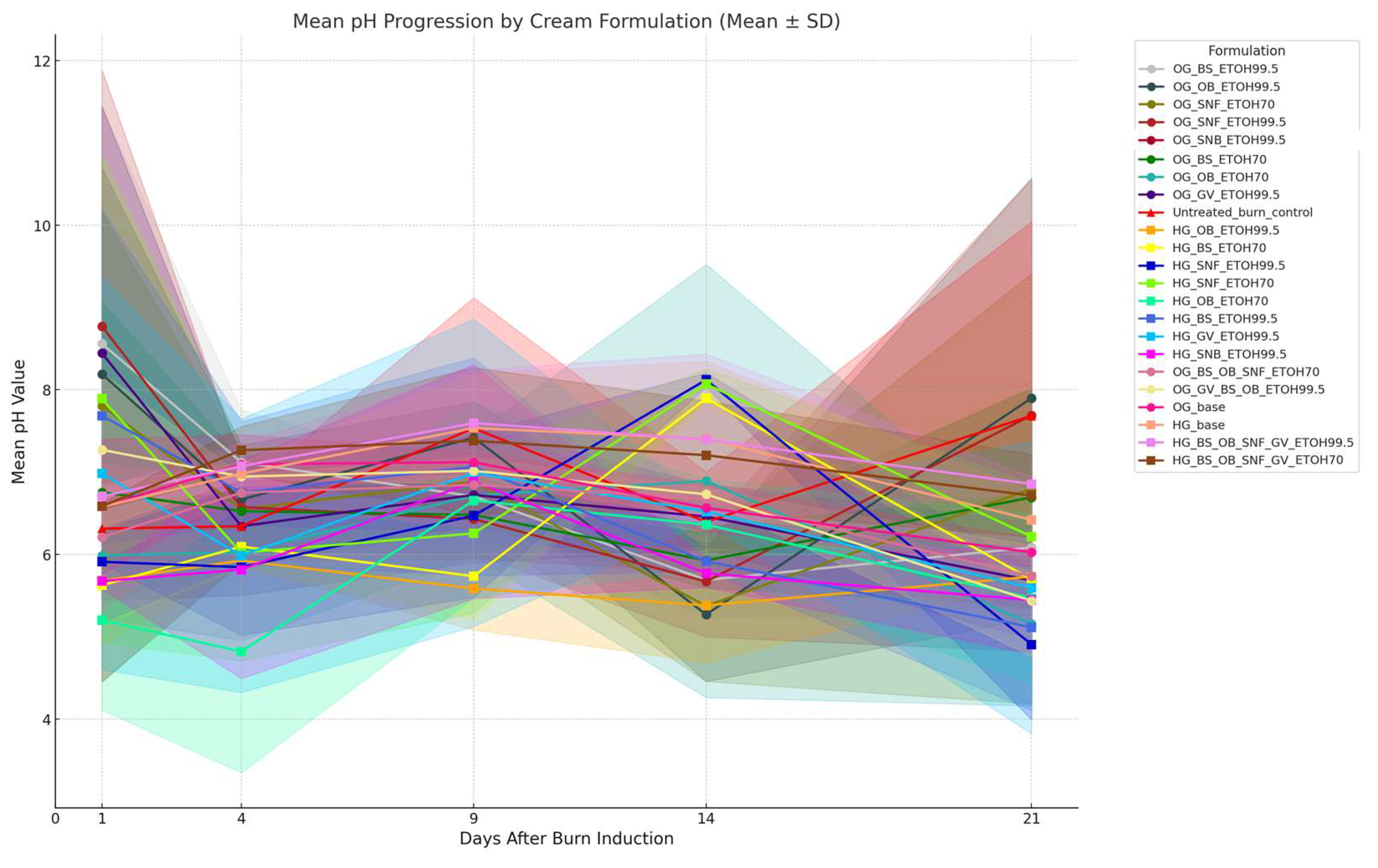
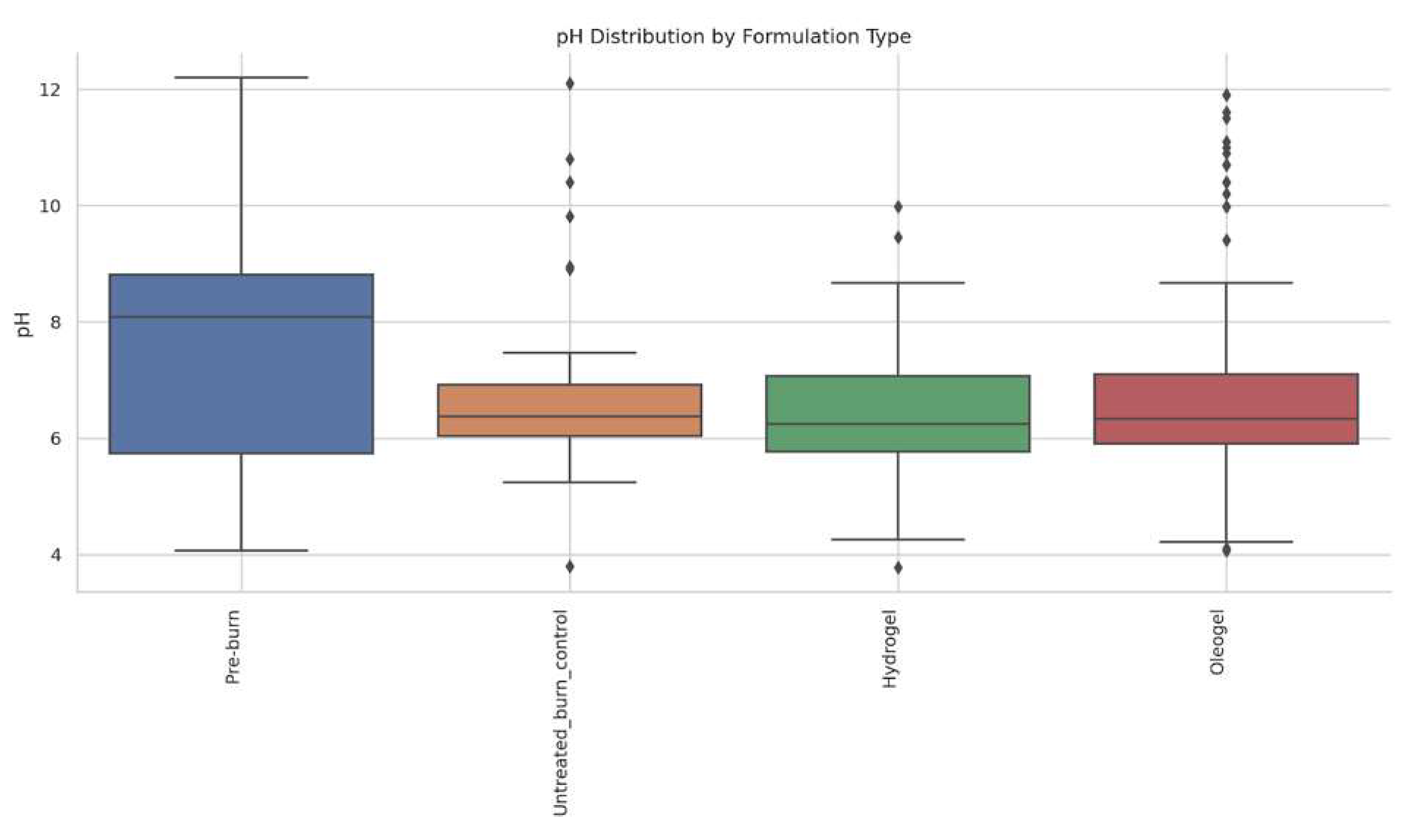
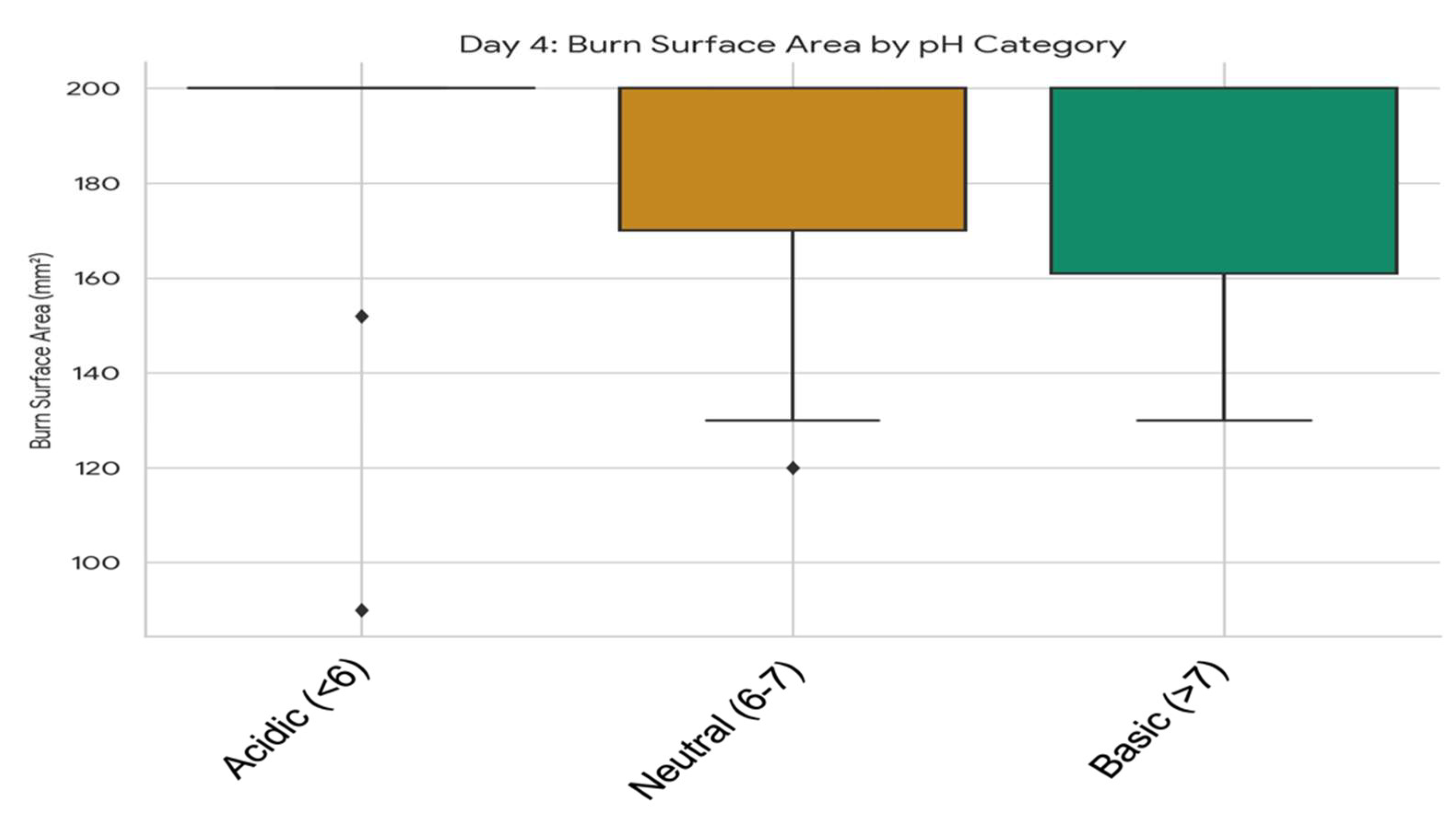
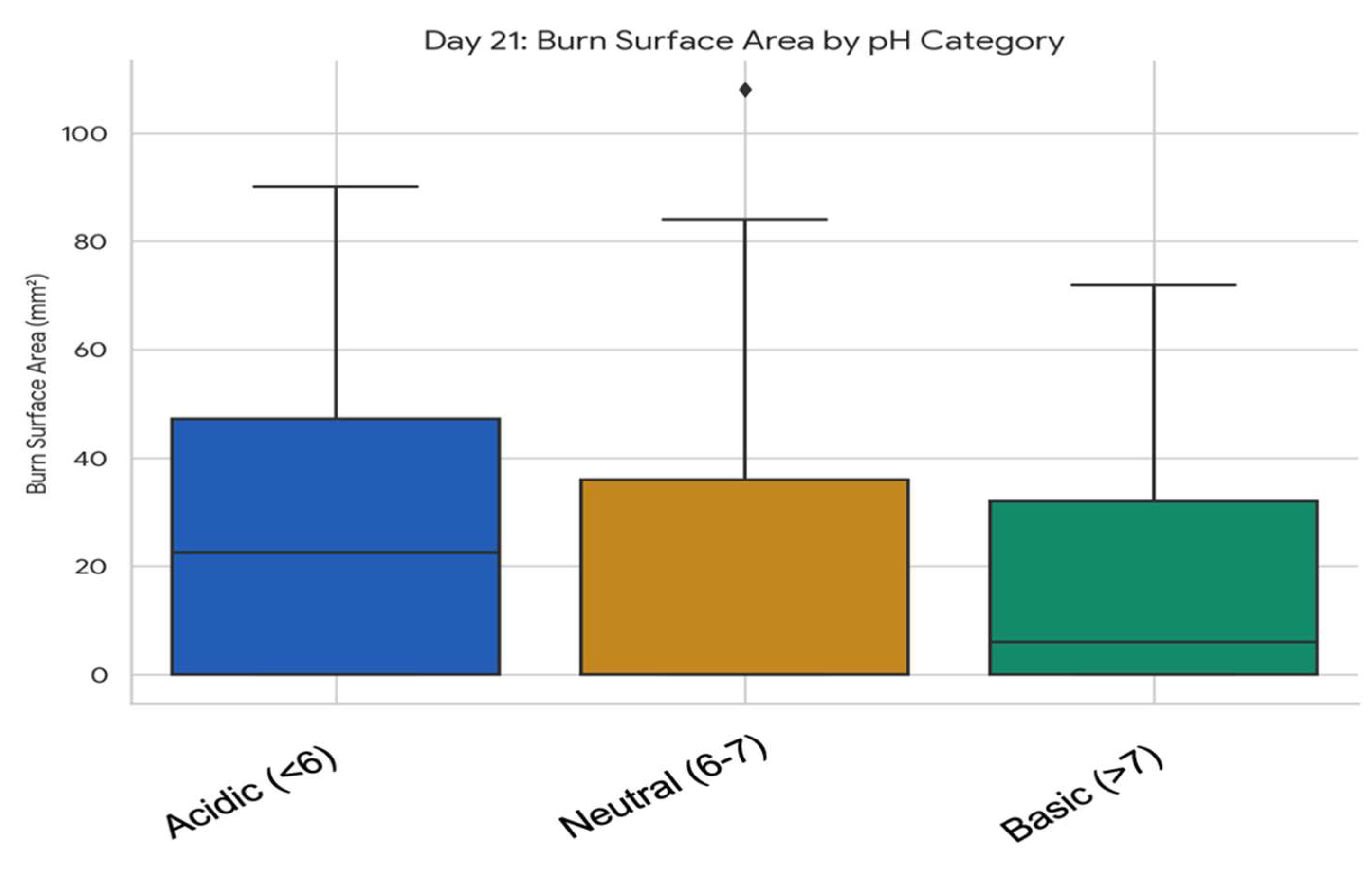

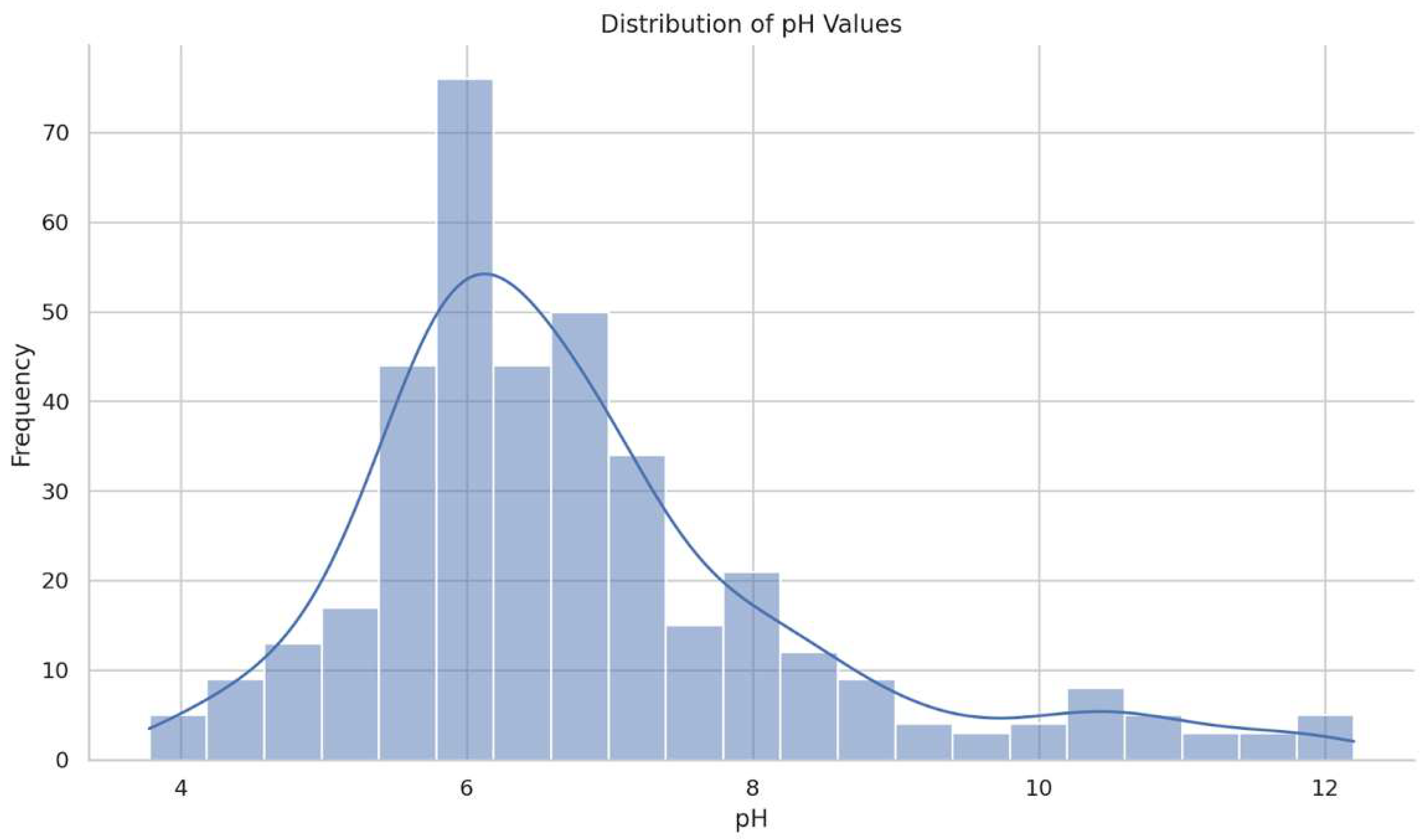
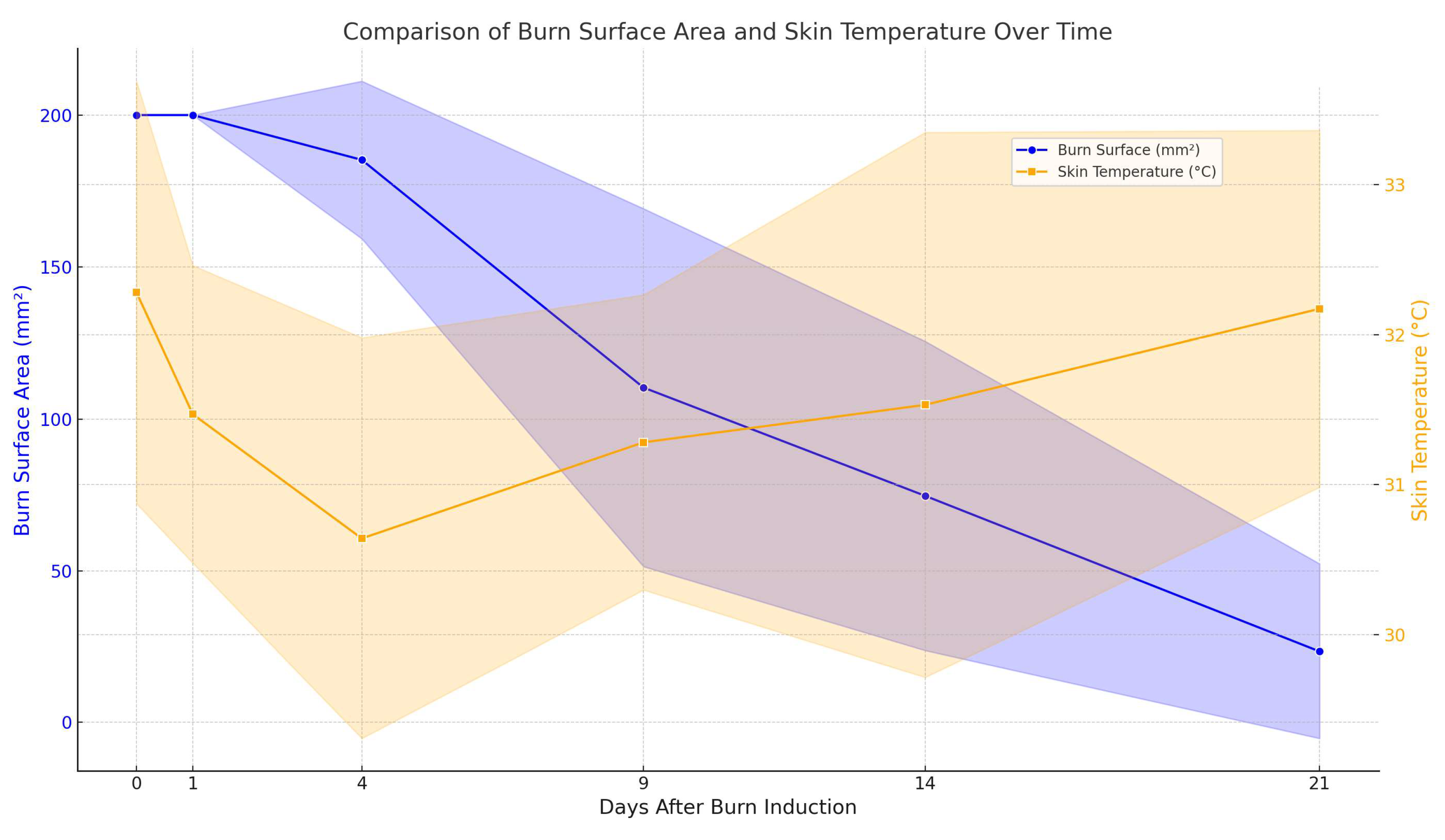
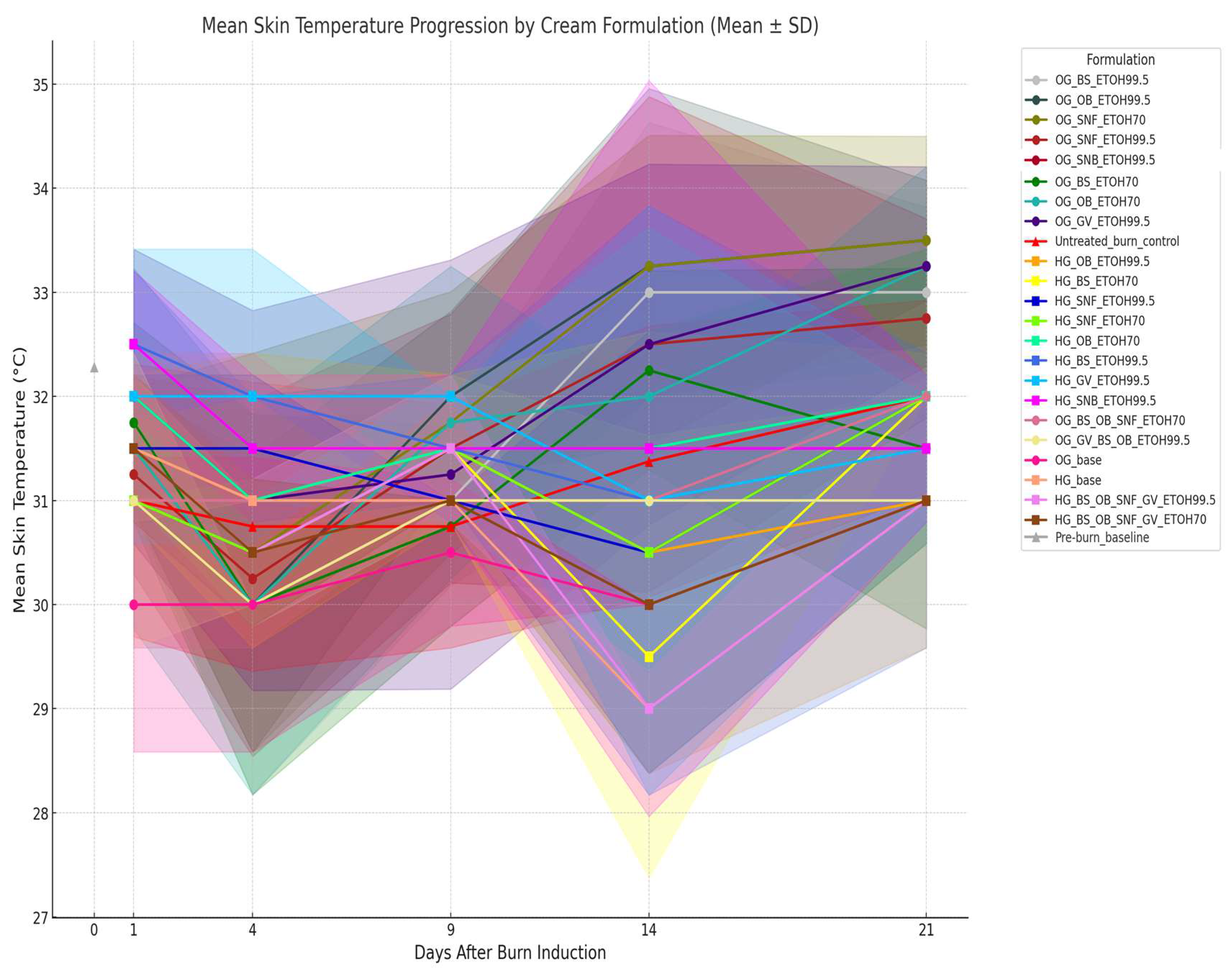
3.5.2. Comparative Analysis of Skin Surface pH and Temperature Dynamics in Relation to Plant Extracts and Formulation Types
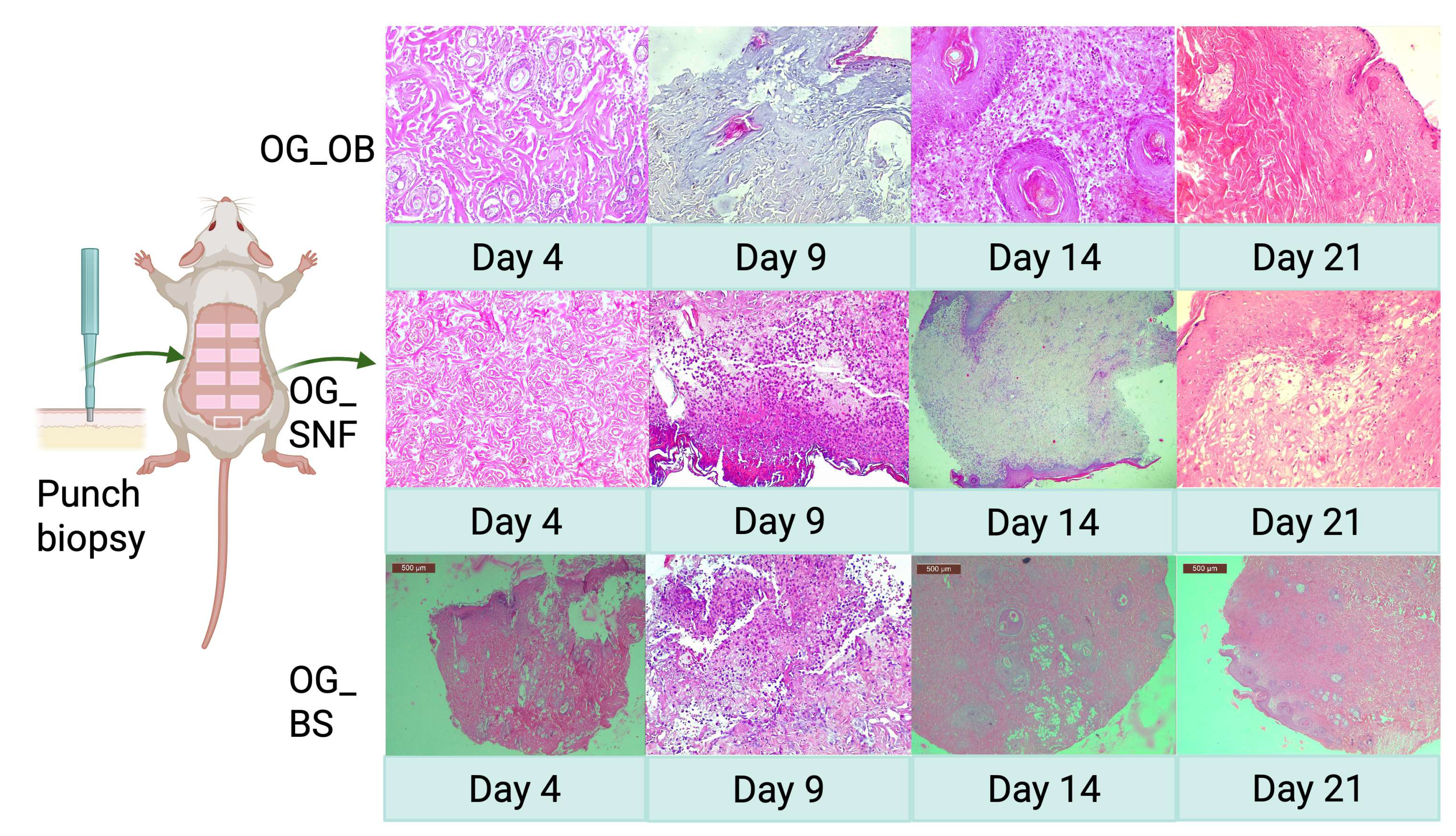
3.6. Clinical and Histological Evaluation of Burn Healing
3.6.1. Impact of pH Variations in Hydrogel Formulations on Vascularization, Epithelialization, and Inflammation
3.6.2. Correlation Between Formulation pH, Rheological Characteristics, and Healing Progression
4. Discussion
Correlation of Rheological Assessment in Literature Topical Formulation
Clinical Significance of pH and Temperature Monitoring in Effective Wound Healing Management
Limitations and Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Blank-OG-OO | Blank Oleogel Olive Oil formulation |
| BS | Boswellia serrata |
| Compritol 888 ATO | Glyceryl dibehenate excipient |
| DEGEE | Diethylene glycol monoethyl ether |
| GV | Galium verum |
| HG | Hydrogel |
| LC-MS | Liquid chromatography-mass spectrometry |
| LogP | Logarithm of partition coefficient |
| OC | Ocimum basilicum |
| OG | Oleogel |
| pH | Potential of hydrogen |
| PVP-I | Liposome polyvinyl-pyrrolidone-iodine |
| RAPID-3D | Rat Printed Induction Device - 3D |
| SD | Standard deviation |
| SNB | Sambucus nigra brunch bark |
| SNF | Sambucus nigra flower |
| SFO | Sunflower oil |
| TA | Topical antimicrobial |
References
- Okur, M.E.; Karantas, I.D.; Şenyiğit, Z.; Üstündağ Okur, N.; Siafaka, P.I. Recent trends on wound management: new therapeutic choices based on polymeric carriers. Asian J. Pharm. Sci. 2020, 15, 661–684. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, H.N.; Hardman, M.J. Wound healing: cellular mechanisms and pathological outcomes. Open Biol. 2020, 10, 200223. [Google Scholar] [CrossRef]
- Percival, S.L.; McCarty, S.M.; Lipsky, B. Biofilms and wounds: an overview of the evidence. Adv Wound Care 2015, 4, 373–381. [Google Scholar] [CrossRef]
- Gethin, G. The Significance of Surface pH in Chronic Wounds. Wounds UK 2007, 3, 52–56. [Google Scholar]
- Schreml, S.; Szeimies, R.M.; Karrer, S.; Heinlein, J.; Landthaler, M.; Babilas, P. Theimpact of the pH value on skin integrity and cutaneous wound heaking. J Eur Acad Dermatol Venerol. 2010, 24, 373–378. [Google Scholar] [CrossRef] [PubMed]
- Maver, T.; Maver, U.; Stana Kleinschek, K.; Mlinarič Raščan, I.; Smrke, D.M. Advanced therapies of skin injuries. Wien. Klin. Wochenschr. 2015, 127 (Suppl 5), S187–S198. [Google Scholar] [CrossRef] [PubMed]
- Scalamandré, A. Smart Technologies in wound prevention and care. In Innovations and Emerging Technologies in Wound Care; Gefen, A., Ed.; Elsevier: Amsterdam, Netherlands, 2020; pp. 225–244. [Google Scholar]
- Ghasemian, M.; Owlia, S.; Owlia, M.B. Review of anti-inflammatory herbal medicines. Adv Pharmacol Sci. 2016, 2016, 9130979. [Google Scholar] [CrossRef]
- Hamedi, A.; Zarshenas, M.M.; Sohrabpour, M.; Zargaran, A. Herbal medicinal oils in traditional Persian medicine. Pharm Biol. 2013, 51, 1208–18. [Google Scholar] [CrossRef]
- Ajaz, N.; Tiwari, V.; Saxena, V. Advanced topical formulations for wound healing: An overview. Asian J Pharm Sci 2021, 16, 119–136. [Google Scholar] [CrossRef]
- Boateng, J.; Catanzano, O.; Silver, I.A. Advanced therapeutic dressings for effective wound healing—A review. J. Pharm. Sci. 2015, 104, 3653–3680. [Google Scholar] [CrossRef]
- Roșca, O. -J.; Nistor, A.; Coneac, G. -H.; Olariu, I.-V.; Cotan, A.-M.; Racoviceanu, R.; Heredea, E.R.; Ciudoiu, A.; Didea, G.; Lupou, C- M.; et al. Wound Healing Properties of Plant-Based Hydrogel and Oleogel Formulations in a Rat Scald Burn Model. Pharmaceutics 2025, 17, 597. [Google Scholar] [CrossRef]
- . Ghiulai, R.; Avram, S.; Stoian, D.; Pavel, I.Z.; Coricovac, D.; Oprean, C.; Vlase, L.; Farcas, C.; Mioc, M.; Minda, D.; Motoc, A.; Szuhanek, C.; Danciu, C.; Soica, C.; Sima, L. Lemon balm extracts prevent breast cancer progression in vitro and in ovo on chorioallantoic membrane Assay. Evid Based Complement Alternat Med. 2020, 6489159. [Google Scholar] [CrossRef] [PubMed]
- Oniga, I.; Puscas, C.; Silaghi-Dumitrescu, R.; Olah, N.K.; Sevastre, B.; Marica, R.; Marcus, I.; Sevastre-Berghian, A.C.; Benedec, D.; Pop, C.E.; et al. Origanum vulgare ssp. Vulgare: chemical composition and biological studies. Molecules 2018, 23, 2077. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: a free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci Rep. 2017, 7, 42717. [Google Scholar] [CrossRef] [PubMed]
- Pantone LLC. Pantone Matching System® Color Chart; Pantone Inc. Carlstadt, NJ, 2023. [Accessed 2024-05-02]. Available from: https://www.pantone.com/color-systems/pantone-color-systems-explained.
- Wyszecki, G.; Stiles, W.S. Color science: concepts and methods, quantitative data and formulae. Wiley-Interscience: New York, 2000; p. 117. [Google Scholar]
- Sinko, P.J. Martin’s Physical Pharmacy and Pharmaceutical Sciences: Physical Chemical and Biopharmaceutical Principles in the Pharmaceutical Sciences, 7th ed.Wolters Kluwer: Philadelphia, 2017; Volume Chapter 17, pp. 433–466. [Google Scholar]
- European Pharmacopoeia (Ph.Eur.), 11th ed.; Strasbourg, France: Council of Europe; 2023, General Chapter 2.2.1. Clarity and degree of opalescence of liquids.
- The United States Pharmacopeial Convention. The United States Pharmacopoeia and National Formulary USP 43–NF 38. Rockville (MD): The United States Pharmacopeial Convention; 2019, p. 6415.
- Lawless, H.T.; Heymann, H. Sensory evaluation of food: principles and practices, Chapter 10, Descriptive analysis; p. 227–257, 2nd ed.; Springer: New York, 2010. [Google Scholar]
- Stone, H.; Bleibaum, R.N.; Thomas, H.A. Sensory evaluation practices, 5th ed. Academic Press: London, 2020; Chapter 8, Odor evaluation techniques. 177–208. [Google Scholar]
- Meilgaard, M.C.; Civille, G.V.; Carr, B.T. Sensory Evaluation Techniques. 5th ed. Boca Raton: CRC Press; 2016. Chapter 5, Descriptive analysis techniques; p. 141–174.
- Directorate for the Quality of Medicines; Healthcare of the Council of Europe. Measurement of Consistency by Penetrometry. In European Pharmacopoeia; 11th ed.; Council of Europe: Strasbourg, France, 2022; p. p 360. [Google Scholar]
- Parente, M.E.; Ochoa Andrade, A.; Ares, G.; Russo, F.; Jiménez-Kairuz, Á. Bioadhesive hydrogels for cosmetic applications. Int J Cosmet Sci. 2015, 37, 511–518. [Google Scholar] [CrossRef] [PubMed]
- Directorate for the Quality of Medicines; Healthcare of the Council of Europe. Potentiometric Determination of pH. European Pharmacopeia; 11th edition; EDQM Council of Europe: Strasbourg, France, 2022. [Google Scholar]
- NC3Rs. Revision of the ARRIVE Guidelines. https://nc3rs.org.uk/our-portfolio/revision-arrive-guidelines (accessed accessed Jan 1, 2025).
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate the solubility and pemeability in drug discovery and development settings. Adv Drug Deliv Rev. 2001, 46, 3–26. [Google Scholar] [CrossRef]
- Bos, J.D.; Meinardi, M.M. The 500 Dalton rule for the skin penetration of chemical compounds and drugs. Exp Dermatol. 2000, 9, 165–169. [Google Scholar] [CrossRef]
- Schreml, S.; Szeimies, R.M.; Karrer, S.; Heinlin, J.; Landthaler, M.; Babilas, P. The impact of the pH value on skin integrity and cutaneous wound healing. J. Eur Acad. Dermatol. Venereol. 2010, 24, 373–378. [Google Scholar] [CrossRef]
- Wilkinson, H.N.; Hardman, M.J. Wound healing: cellular mechanism and pathological outcomes. Open Biol. 2020, 10, 200223. [Google Scholar] [CrossRef]
- Percival, S.L.; McCarty, S.M.; Lipsky, B. Biofilms and wounds: An overview of the evidence. Adv. Wound Care, 2015; 4, 373–381. [Google Scholar] [CrossRef]
- Gethin, G. The Significance of Surface pH in Chronic Wounds. Wounds UK 2007, 3, 52–56. [Google Scholar]
- Chen, Y.; Quan, P.; Liu, X.; Wang, M.; Fang, L. Novel Chemical Permeation enhancers for Transdermal Drug Delivery. Asian J. Pharm Sci. 2018, 13, 51–64. [Google Scholar] [CrossRef]
- Pecoraro, B.; Tutone, M.; Hoffman, E.; Hutter, V.; Almerico, AM.; Traynor, M. Predicting skin permeability by means of computational approaches: reliability and caveats in pharmaceutical studies. J.Chem Inf Model. 2019, 59, 968–981. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.; Iliopoulos, F.; Caspers, P.J.; Puppes, G.J.; Lane, M.E. In vitro – in vivo correlation in dermal delivery: the role of excipients. Pharmaceutics 2021, 13, 542. [Google Scholar] [CrossRef]
- . Iliopoulos, F.; Caspers, PJ.; Puppels, G.J.; Jane, M.E. Franz cell diffusion testing and quantitative confocal Raman spectroscopy: in vitro-in vivo correlation. Pharmaceutics 2020, 12, 887. [Google Scholar] [CrossRef] [PubMed]
- Mewis, J.; Wagner, N.J. Thixotropy. Adv Colloid Interface Sci. 2009, 147–148, 214–227. [Google Scholar] [CrossRef]
- Barroso, N.G.; Okuro, P.K.; Ribeiro, A.P. B.; Cunha, R.L. Tailoring properties of mixed-component oleogels: wax and monoglyceride interactions towards flaxseed oil structuring. Gels 2020, 6, 5. [Google Scholar] [CrossRef]
- Martín-Alfonso, M.A.; Rubio-Valle, J.F.; Hinestroza, J.P.; Martín-Alfonso, J.E. Impact of vegetable oil type on the rheological and tribological behavior of montmorillonite-based oleogels. Gels 2022, 8, 504. [Google Scholar] [CrossRef]
- Sánchez, R.; Franco, J.M.; Delgado, M.A.; Valencia, C.; Gallegos, C. Rheology of oleogels based on sorbitan and glycerol monostearates and vegetable oils for lubricating applications. Grasas Aceites 2011, 62, 328–336; [Google Scholar] [CrossRef]
- Chaibundit, C.; Ricardo, N.M.; Muryn, C.A.; Madec, M.B. , Yeates, S.G., Booth, C. Effect of ethanol on the gelation of aqueous solutions of Pluronic F127. J. Colloid Interface Sci. 2010, 351, 190–196. [Google Scholar] [CrossRef]
- Fakhari, A.; Corcoran, M.; Schwarz, A. Thermogelling properties of purified Poloxamer 407. Heliiyon 2017, 3, e00390. [Google Scholar] [CrossRef]
- Schneider, L.A.; Korber, A.; Grabbe, S.; Dissemond, J. Influence of pH on wound-healing: a new perspective for wound-therapy? Arch. Dermatol. Res. 2007, 298, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Tang, N.; Zheng, Y.; Jiang, X. , Zhou, C.; Jin, H.; Jin, K., Wu, W., Haick, H. Wearable sensors and systems for wound healing-related pH and temperature detection. Micromachines 2021, 12, 430. [Google Scholar] [CrossRef]
- Ono, S.; Imai, R.; Ida, Y.; Shibata, D.; Komiya, T.; Matsumura, H. Increased wound pH as an indicator of local wound infection in second degree burns. Burns 2015, 41, 820–824. [Google Scholar] [CrossRef]
- Watters, C.; Yuan, T.T.; Rumbaugh, K.P. Beneficial and deleterious bacterial-host interactions in chronic wound pathophysiology. Chronic Wound Care Manag. Res. 2015, 2, 53–62. [Google Scholar] [CrossRef]
- Martínez-Jiménez, M.A.; Aguilar-García, J.; Valdés-Rodríguez, R. Metlich-Medlich, M.A.; Dietsch, L.J.P. Gaitán-Gaona, F.I. Kolosovas-Machuca; González, F.J. Sánchez-Aguilar, J.M. Local Use of insulin in wounds of diabetic patients: higher temperature, fibrosis, and angiogenesis. Plast. Reconstr. Surg. 2013, 132, 1015e–1019e. [Google Scholar] [CrossRef] [PubMed]
- Nakagami, G.; Sanada, H.; Iizaka, S.; Kadono, T.; Higashino, T.; Koyanagi, H.; Haga, N. Predicting Delayed Pressure Ulcer Healing Using Thermography: A Prospective Cohort Study. J. Wound Care 2010, 19, 465–472. [Google Scholar] [CrossRef]
- Wilmore, D.W.; Aulick, L.H.; Mason, A.D., Jr.; Pruitt, B.A., Jr. Influence of the Burn Wound on Local and Systemic Responses to Injury. Ann. Surg. 1977, 186, 444. [Google Scholar] [CrossRef]
- Wijlens, A.M.; Holloway, S.; Bus, S.A.; van Netten, J.J. An Explorative Study on the Validity of Various Definitions of a 2.2 °C Temperature Threshold as Warning Signal for Impending Diabetic Foot Ulceration. Int. Wound J. 2017, 14, 1346–1351. [Google Scholar] [CrossRef]
- Armstrong, D.G.; Holtz-Neiderer, K.; Wendel, C.; Mohler, M.J.; Kimbriel, H.R.; Lavery, L.A. Skin temperature monitoring reduces the risk for diabetic foot ulceration in high-risk patients. Am. J. Med. 2007, 120, 1042–1046. [Google Scholar] [CrossRef]
- Heberlé, G.; Dos Santos, M.A.; Magri, S. Cosmetic formulations containing blueberry extracts (Vaccinium myrtillus L.). TOJSAT 2012, 2, 1–6. [Google Scholar]
- Da-Lozzo, E.J.; Moledo, R.C.; Faraco, C.D.; Ortolani-Machado, C.F.; Bresolin, T.M.; Silveira, J.L. Curcumin/Xanthan-Galactomannan hydrogels: Rheological analysis and biocompatibility. Carbohydr. Polym. 2013, 93, 279–284. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.M.; Yosipovitch, G. Skin pH: from basic science to basic skin care. Acta Derm. Venereol. 2013, 93, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Power, G.; Moore, Z.; O’Connor, T. Measurement of pH, exudate composition and temperature in wound healing: A systematic review. J. Wound Care 2017, 26, 381–397. [Google Scholar] [CrossRef] [PubMed]

| Qualitative description (original) | Suggested Pantone reference |
|---|---|
| Pearly white | Pantone 11-0601 |
| Straw yellow | Pantone 13-0922 |
| White | Pantone 11-0601 |
| Dull-yellow | Pantone 13-0850 |
| Orange | Pantone 16-1364 |
| Green-moss | Pantone 17-0530 |
| Brown | Pantone 18-0930 |
| Pale brown | Pantone 15-1213 |
| Brownish-dark | Pantone 19-1015 |
| Brownish-green | Pantone 18-0435 |
| Colorless | Pantone Transparent |
| Term used | Definition | Example formulations |
|---|---|---|
| Transparent | Completely clear, no turbidity | Blank-HG |
| Translucent | Slightly cloudy but still clear | HG-PL 3–8 |
| Slightly opaque | Mild turbidity, partially opaque | Blank-OG-SFO, HG-PL 1, HG-PL 2 |
| Opaque | Completely opaque, no transparency | OG 1–8, Blank-OG-OO, Blank-OG-IPM, Blank-OG-DEGEE |
| Original odor description | Improved standardized description |
|---|---|
| Sunflower oil characteristic | Mild vegetal, characteristic of sunflower oil |
| Olive oil characteristic | Mild vegetal, characteristic of olive oil |
| Isopropyl myristate characteristic | Neutral, characteristic of IPM |
| DEGEE characteristic | Slightly chemical, neutral odor |
| Specific, slightly aromatic | Mild herbal aroma |
| Specific aromatic | Herbal, fresh aroma |
| Specific aromatic (brownish) | Earthy, herbal aroma |
| Specific aromatic (brown-dark) | Woody, earthy aroma |
| Odorless | Odorless (neutral) |
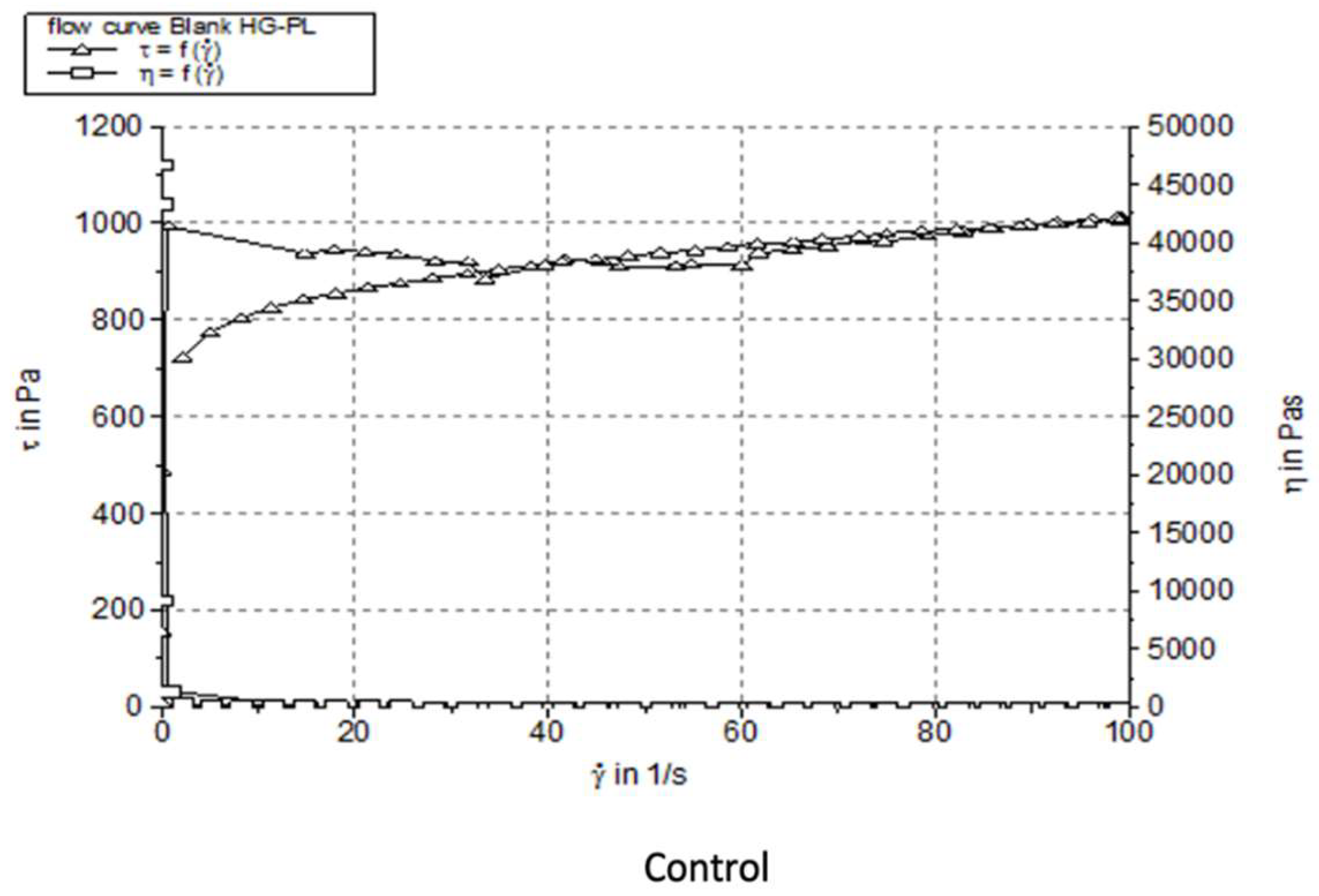
| Formulation | Viscosiy (Pa·s) | Thixotropy (Pa/s) | Penetration depth (mm) |
|---|---|---|---|
| Blank-OG-SFO | 1.071733±0.014 | 8019 | 370.0±7.24 |
| Blank-OG-OO | 0.799833±0.009 | 5845 | 321.0±8.07 |
| Blank-OG-IPM | 1.3042±0.041 | 7367 | 339.0±3.65 |
| Blank-OG-DEGEE | 1.217±0.081 | 54860 | 330.0±6.41 |
| OG 1 | 2.583±0.084 | 10850 | 107.0±5.64 |
| OG 2 | 2.586±0.066 | 6977 | 106.7±5.03 |
| OG 3 | 0.640±0.041 | 2900 | 256.3±4.73 |
| OG 4 | 1.185±0.182 | 17370 | 113.0±2.28 |
| OG 5 | 0.867±0.043 | 7717 | 103.7±1.53 |
| OG 6 | 2.062±0.091 | 17300 | 118.0±2.14 |
| OG 7 | 2.457±0.081 | 15910 | 113.7±1.53 |
| OG 8 | 1.645±0.068 | 13710 | 130.0±2.66 |
| Blank HG-PL | 10.179 ± 0.027 | 9932 | 126.3±1.58 |
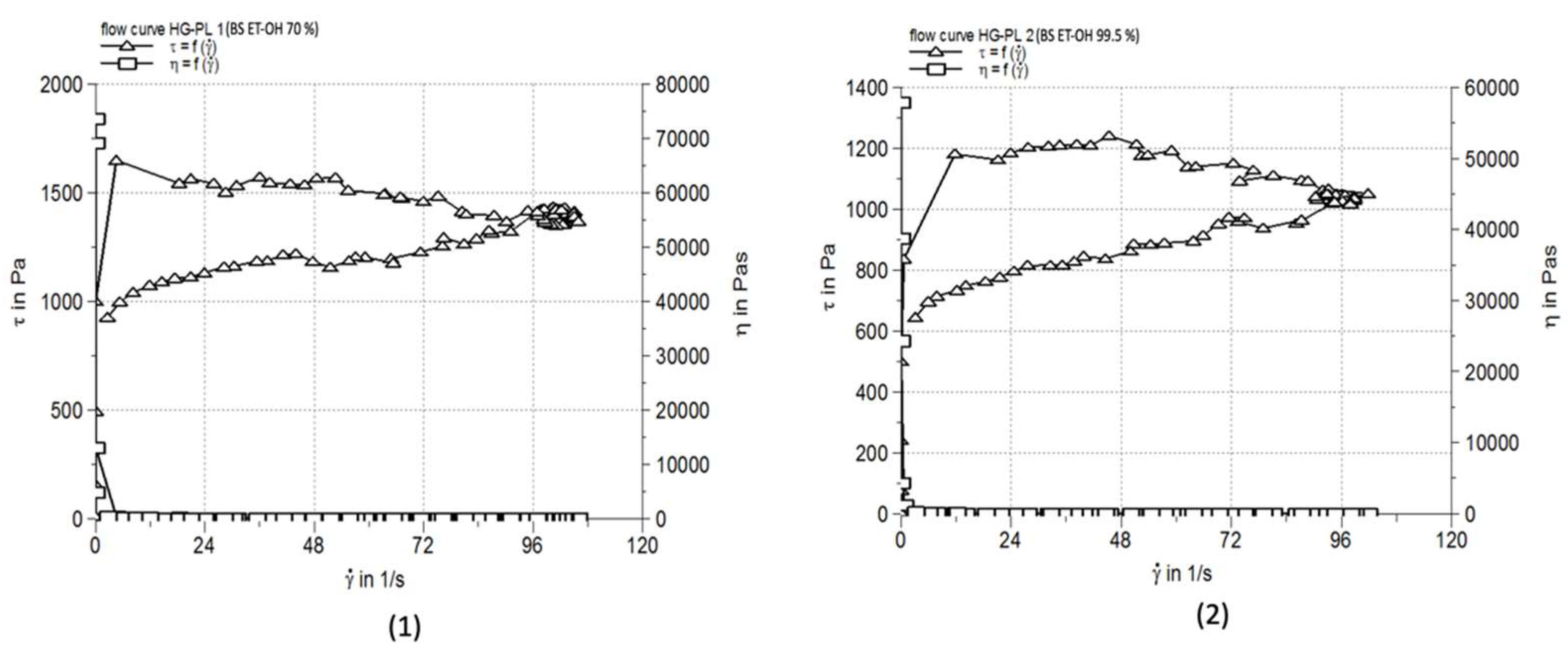
| HG-PL 1 | 13.855±0.415 | 94340 | 84.7±3.05 |
| HG-PL 2 | 10.839±0.275 | 88420 | 85.3±2.52 |
| HG-PL 3 | 11.389±0.097 | 17410 | 97.7±1.15 |
| HG-PL 4 | 5.723±0.028 | 30040 | 99.3±1.53 |
| HG-PL 5 | 7.274±0.187 | 77680 | 119.7±0.58 |
| HG-PL 6 | 9.341±0.055 | 14740 | 113.3±2.08 |
| HG-PL 7 | 11.467±0.059 | 15360 | 112.0±1.05 |
| HG-PL 8 | 9.073±0.019 | 10470 | 117.3±2.52 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





