1. Introduction
The Crimean-Congo Haemorrhagic Fever Virus (CCHFV) is an emerging zoonotic pathogen. Crimean-Congo Haemorrhagic Fever (CCHF) is an expanding public health concern, classified as a priority disease by the World Health Organisation (WHO) since the disease is associated with high case fatality risk and the absence of effective vaccines and a specific treatment [
1,
2,
3]. Historically concentrated in Africa, Asia, and parts of Eastern and Southern Europe [
4], CCHFV has been progressively expanding into new areas, raising awareness about potential establishment in previously unaffected regions of Europe [
5,
6,
7,
8].
Humans may be exposed to CCHFV either through tick bites or by direct contact with infected animal tissues and fluids [
9]. Livestock handlers, slaughterhouse workers, and agricultural and healthcare workers are at higher risk of CCHFV infection [
10].
Although infection is usually asymptomatic and may circulate unnoticed in wild and domestic animals, the case fatality risk may range from 5% to 40% in humans, with the possibility of reaching 80% in some nosocomial settings [
2,
11,
12]. The incubation period lasts from 2 to 14 days, varying depending on the mode of viral transmission and load, with the possibility of lasting between 2 and 7 days after tick-bite and 10 to 14 days if the infection occurs by blood transfusion [
13]. Symptoms and clinical signs include, among other, high fever, nausea, vomiting, headache, diarrhoea, myalgia, hypotension, haemorrhage (petechiae, ecchymoses, epistaxis, hematuria, melena) and, ultimately, multiorgan failure [
2,
11]. As previously mentioned, no vaccine or specific effective antiviral treatment is available [
1]. As a tick-borne disease, with
Hyalomma spp. as main vectors, its distribution is closely linked to the ecology and dispersal of the latter. Ground-feeding migratory birds play an essential role in the dispersal and importation of
Hyalomma spp. ticks, especially
Hyalomma marginatum complex, into Europe during their migration season [
12]. This phenomenon facilitates the introduction of
Hyalomma spp. into new environments, potentially accelerating the spread of CCHFV in Europe [
14]. Moreover, it is expected that climatic factors, such as temperature and precipitation, may be helpful predictors of CCHF once climatic variability influences the distribution of ticks [
10,
15].
This review investigates the expansion of CCHF in Europe, highlighting the role of Hyalomma spp. as vectors of CCHFV and the contribution of migratory birds to their introduction and dispersal across the continent. It also provides a descriptive inventory of bird species previously reported as hosts of Hyalomma spp. in Europe and explores the role of climate change in the dispersal of Hyalomma ticks in this region.
2. Materials and Methods
A rapid literature search was conducted from January to March 2025 across the PubMed, Google Scholar, CAB Abstracts, and ScienceDirect databases.
The inclusion of articles was limited to those published in peer-reviewed/academic journals or conference proceedings, written in English or Spanish, and that addressed the following topics: (1) the occurrence of CCHFV and CCHF in Europe; (2) the life cycle and ecology of Hyalomma ticks as vectors of CCHFV in Europe; (3) the occurrence of Hyalomma spp. and CCHFV-positive Hyalomma ticks in migratory birds in Europe over the last 17 years (studies with data collection starting in 2008); and (4) the impact of climate change on the expansion of Hyalomma spp. in Europe were included. Additionally, studies describing migratory patterns of bird species known to host Hyalomma spp., as identified in the selected literature, were included.
Search keywords included, among other variations, “Crimean-Congo Haemorrhagic Fever Virus” OR “CCHFV” OR “CCHF”; “Crimean-Congo Haemorrhagic Fever Virus” OR “CCHFV” AND “Europe”; “Hyalomma” AND “Europe”; “Migratory birds” AND “CCHFV” AND “Hyalomma”; “Crimean-Congo Haemorrhagic Fever Virus” AND “Hyalomma”; “Climate change” AND “Hyalomma” OR “CCHFV” were considered as search terms. The removal of duplicates across journals and databases was then carried out.
A total of 290 articles were initially selected based on their titles and abstracts. After full-text screening, 18 studies assessing the occurrence of Hyalomma species in migratory birds and the detection of CCHFV-positive ticks on these hosts in Europe were retained. All these studies were published after 2012 and covered sampling periods ranging from 2008 to 2022. In this selection, studies were excluded if they were not conducted in Europe, lacked a clear distinction of European findings, or failed to specify the bird species reported to be infested with Hyalomma.
Furthermore, noting that some articles were selected simultaneously to discuss different topics, 25 articles were included to provide an overview of the migratory patterns of bird species reported to have been infested by Hyalomma spp. in Europe, and 16 articles were included to discuss the impact of climate change on Hyalomma spp. expansion; 14 articles were included to examine the occurrence of CCHFV and CCHF in Europe; 13 articles were included to discuss the role of Hyalomma spp. as vectors of CCHFV and the occurrence of Hyalomma species in Europe; and 7 articles were included to provide an introduction to the theme regarding the role of migratory birds in the dispersal of Hyalomma spp. in Europe. Complementary data from the European Centre for Disease Prevention and Control (ECDC) and BirdLife Datazone were also consulted.
3. Crimean-Congo Haemorrhagic Fever Virus (CCHFV) and Its Evidence in Europe
The CCHFV belongs to the genus
Orthonairovirus of the family Nairoviridae and order Bunyavirales [
1,
2]. The emergence of CCHF in new non-endemic regions is primarily attributed to international animal trade, human travel, the transportation of infected ticks by migratory birds, and climate change [
16].
Hyalomma spp. ticks are the primary vectors of CCHFV, and their distribution correlates with the global distribution of CCHF [
11]. Although evidence for active CCHFV transmission by non-
Hyalomma ticks is limited, the virus has also been detected in non-
Hyalomma ticks of the genera
Amblyomma,
Boophilus,
Dermacentor,
Haemaphysalis, and
Rhipicephalus. However, their role in natural transmission cycles or in maintaining CCHFV foci remains uncertain [
17].
According to the model developed by Okely et al. [
18], CCHFV exhibited high environmental suitability across Southern and Central Europe, namely in Western Mediterranean countries.
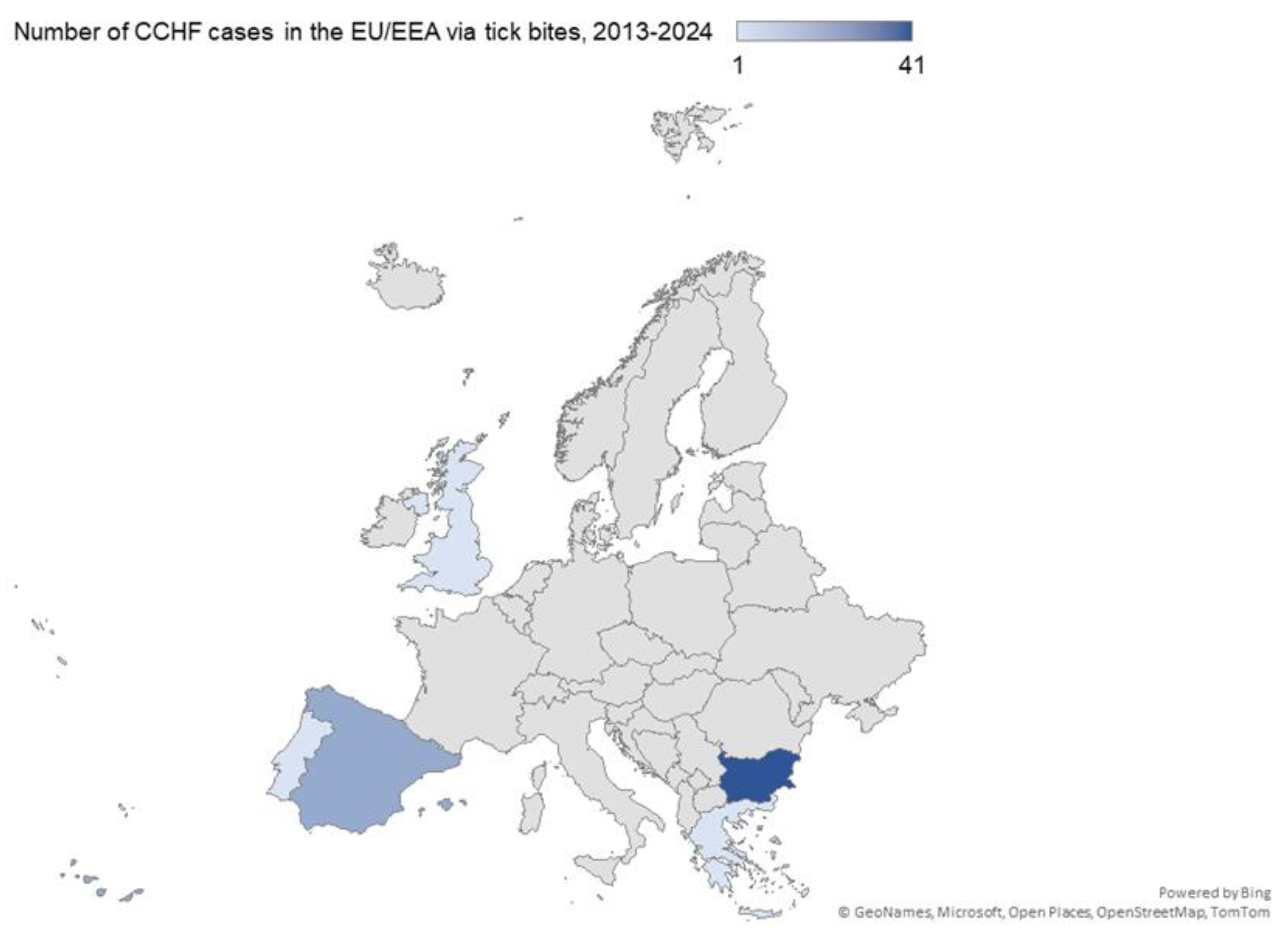
Currently, CCHF is considered endemic in Southwestern Europe [
3]. Since 2013, human cases have been detected in Bulgaria (n = 41 cases), Spain (n = 17 cases), Greece (n = 1 case), Portugal (n = 1 case), and the United Kingdom (n = 1 case) [
19] (
Figure 1). From these cases, seven and six deaths were registered in Bulgaria and Spain, respectively [
19]. In Portugal, the only reported death since 2013 associated with CCHF occurred in 2024 [
20]. Moreover, CCHF is endemic in Turkey, particularly in the Central Anatolian region [
11].
Good indicators of CCHFV occurrence in specific areas comprise the status of antibodies to CCHFV in the animal populations of the corresponding region [
21]. For instance, sheep seroconvert to CCHFV and are considered suitable sentinels for monitoring the virus and its circulation in new non-endemic regions [
22]. Additionally, antibodies against CCHFV have been detected in several wild and domestic animals, such as cattle, swine, horses, donkeys, lagomorphs, and rodents [
22]. Moreover, to assess the risk of exposure to CCHFV in the Iberian Peninsula, the seroprevalence of CCHFV has already been studied in populations of red deer (
Cervus elaphus), wild boar (
Sus scrofa), and roe deer (
Capreolus capreolus) [
1,
23,
24]. Furthermore, unlike most avian species, magpies (
Pica pica) and ostriches (
Struthio camelus) display CCHFV antibodies [
9].
Given that the distribution of CCHFV closely follows that of its primary vector, Hyalomma spp., understanding their ecology, dispersal patterns, and role in viral transmission is essential for assessing the risk of CCHF expansion in Europe.
4. Hyalomma Ticks in Europe
The genus
Hyalomma comprises more than 20 species [
7]. The natural distribution of
Hyalomma species is confined to the continents of Asia, Africa, and Europe [
25]. The distribution of
Hyalomma spp. has been expanding since the first recorded evidence of
Hyalomma marginatum into Europe in the late 20th century [
26].
The majority of
Hyalomma spp. have a three-host cycle, with the exception of
H. marginatum complex, which are diphasic, with the larvae and nymph taking their meals on the same host [
26,
27].
Hyalomma marginatum sensu lato (s.l.) includes, among other species,
Hyalomma marginatum sensu stricto (s.s.) and
Hyalomma rufipes [
14].
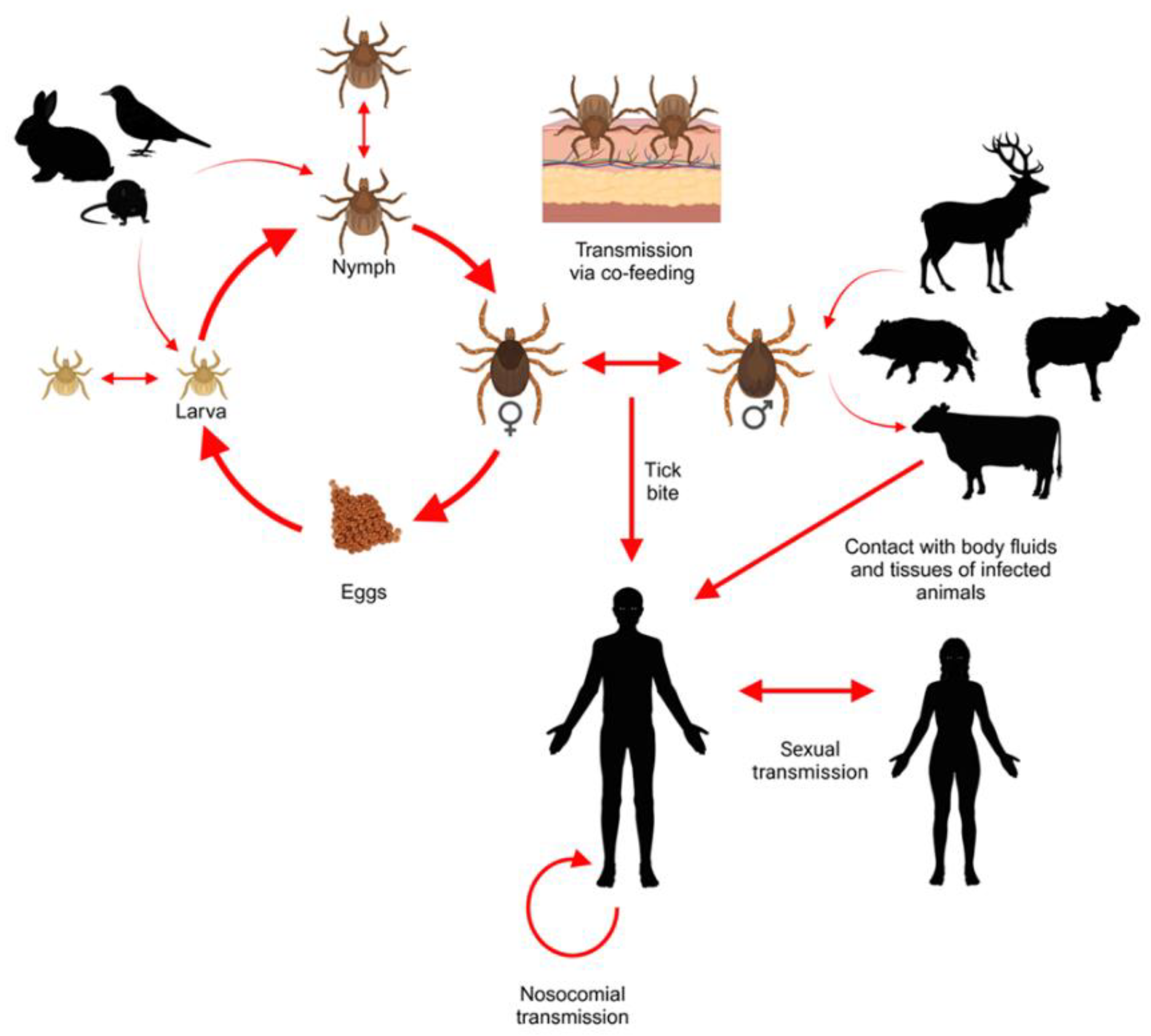
Hyalomma ticks can become infected by CCHFV when feeding on an infected host (
Figure 2). Immature ticks prefer to feed on rodentia, lagomorpha, and aves, while adults infest large ungulates, with a lower yet notable preference for lagomorpha and suidae [
28].
Regarding the mechanism of CCHFV infection of
Hyalomma marginatum, initial viral replication occurs in the intestinal epithelium following ingestion, with subsequent dissemination to other tissues. The highest viral loads are typically observed in the reproductive organs and salivary glands [
29]. Following replication and dissemination, the virus can be transmitted both vertically and horizontally, including via co-feeding [
29]. Co-feeding transmission occurs when infected ticks introduce the virus into the host’s skin or bloodstream during feeding, enabling non-infected ticks feeding in close proximity on the same host to acquire the virus, thereby increasing the overall probability of transmission [
28,
29]. Given that small mammals tend to develop prolonged viremia, the feeding preference of
Hyalomma spp. for these hosts may result in high tick burdens on key reservoir species, facilitating sustained viral circulation [
28].
Hyalomma marginatum s.s. may pose a natural reservoir of CCHFV once ticks carry the virus throughout the life stages [
29].
Hyalomma marginatum is primarily distributed in Southern Europe, Northern Africa and parts of Asia [
30]. In Europe,
H. marginatum is endemic in the Mediterranean regions and Balkan countries, where established populations are present. However, it is also occasionally found in Central Europe [
14]. Besides CCHFV,
H. marginatum can transmit diverse tick-borne pathogens, such as spotted fever rickettsiae to humans [
14],
Anaplasma spp. [
31] and
Theileria annulata to cattle
, and
Babesia caballi and
Theileria equi to horses [
14].
Immature
H. marginatum ticks feed on the same host, such as hares, hedgehogs, rodents, and ground-resident birds, in contrast with adult forms, which prefer large hosts like cattle, horses, and, sometimes, humans [
14,
32].
Hyalomma marginatum is commonly found in passerine migratory birds. Jameson et al. [
33] reported that up to 21% of birds migrating from Africa to the United Kingdom were infested with
H. marginatum nymphs. Consequently, it is predicted that every year a high number of immature ticks are passively transported by migrating birds from Africa and Southern Europe into or over Central Europe [
34].
Hyalomma rufipes is also one of the vectors of CCHFV [
26]. In April 2020, Rudolf et al. [
35] obtained one live specimen of an adult
Hyalomma rufipes tick in the Czech Republic that might have moulted during the autumn-spring period and overwintered in an adult stage, a circumstance which can support the assumption of a future establishment of
Hyalomma spp. ticks in Central Europe.
Along with H. marginatum, Hyalomma lusitanicum is endemic in the Iberian Peninsula [
36]. Hyalomma lusitanicum is a vector of CCHFV, but it is also suspected to transmit Theileria equi, T. annulata, Babesia pecorum, Anaplasma phagocytophilum, Borrelia burgdorferi, B. lusitaniae, and Coxiella burnetti, although these species remain poorly understood in terms of their vectorial ability for these pathogens [
26]. The detection of CCHFV genetic material in adult H. lusitanicum ticks collected from red deer in Spain may suggest the possibility of the virus circulating in this country [
5].
5. The Role of Migratory Birds in the Dispersal of Hyalomma spp. in Europe
Migratory birds play a key role in the passive transport of
Hyalomma spp. from Africa and Southern Europe to the northern breeding areas, as millions of birds migrate between these continents, breeding in the northern hemisphere during summer and returning to warmer regions of Africa in autumn to the wintering locations, thus contributing to intercontinental dispersal of several pathogens and vectors [
9,
37,
38]. The role of migratory birds in the spread of tick-borne pathogens, including CCHFV, has been studied and highlighted [
39,
40]. In stopover sites, engorged ticks can detach from their bird host and, under favourable conditions, colonise new habitats [
39].
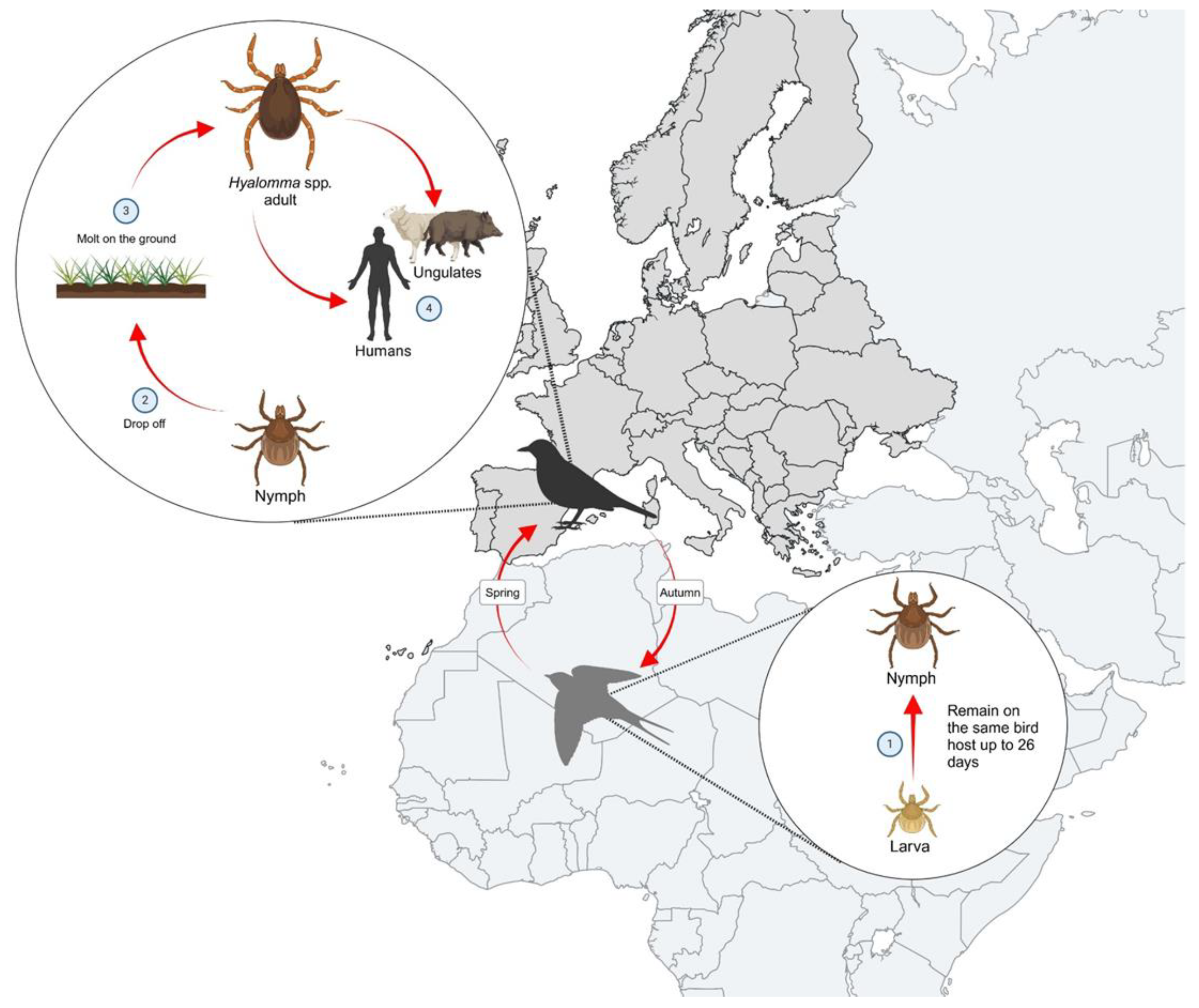
Immature
Hyalomma ticks can remain attached to the primary host for up to 26 days, enabling migratory birds to transport ticks over long distances [
22,
32,
40].
Figure 3.
Life cycle of Hyalomma ticks in migratory birds and their role in tick dispersal to Europe. Created in BioRender®.
Figure 3.
Life cycle of Hyalomma ticks in migratory birds and their role in tick dispersal to Europe. Created in BioRender®.
Viremia does not develop in most passerine birds [
40]. Understanding the contribution of migratory birds in the dispersal of pathogens plays a crucial part in assessing the risk of spread and introducing emerging diseases and pathogens [
40]. Findings of ticks belonging to
Hyalomma spp.,
H. marginatum, and
H. rufipes, from migratory birds in the last seventeen years, are shown in
Table 1,
Table 2 and
Table 3.
5.1. Acrocephalus spp.
The great reed warbler (
Acrocephalus arundinaceus), the Sedge
Warbler (
Acrocephalus schoenobaenus), and the common reed warbler (
Acrocephalus scirpaceus) are migratory birds that prefer wet habitats, travelling between their European breeding grounds and African wintering quarters [
53].
The great reed warbler inhabits reed vegetation habitats with water in Europe and Western Paleartic, preferring canals, ponds, shallow lakes and fish ponds [
54]. Individuals of the great reed warbler were previously reported as carriers of
Hyalomma spp. ticks in Turkey (n = 6 ticks in 3 birds) [
41] and in Italy (n = 1 tick in a bird) [
22]. More specifically,
H. marginatum was recovered from a great reed warbler in Czech Republic (n = 8 ticks) [
32], in Spain (n = 7 ticks) [
50], in Greece (n = 1 tick) [
27], and in Slovakia (n = 1 tick) [
32]. More recently, 14
H. rufipes ticks were sampled from two great reed warblers in Malta [
51] and in Italy (n = 3 ticks) [
40]. Furthermore, a Turkish study already reported a CCHFV-positive
Hyalomma spp. nymph collected from a great reed warbler [
41].
The sedge warbler (
Acrocephalus schoenobaenus) is found in diverse types of low, dense vegetation, often near to water or moist depressions and in Western Europe, its egg-laying mainly begins in late April, while in Central Europe it starts from early May [
55]. Nonspecific species of
Hyalomma spp. ticks were previously recovered from sedge warblers in Italy by Mancuso et al. [
40] (n= 20 ticks) and by De Liberato et al. [
22] (n = 7 ticks), in Spain (n = 5 ticks) [
43], in Hungary (n = 3 infested birds) [
42], and in the United Kingdom (n = 1 tick in a bird) [
33]. In contrast
, H. marginatum have been reported in sedge warblers in Slovakia (n = 10 ticks in 7 birds) [
32], in Malta (n = 2 ticks in a bird) [
51], and in Greece (n = 1 tick in a bird) [
27]. Individuals of sedge warbler were also found to be infested by
H. rufipes in the African-Western Palearctic region (n = 53 ticks) [
5], in Malta (n = 12 ticks in 6 birds) [
51], in Italy (n = 2 ticks) [
40], and in Greece (n = 1 tick) [
27]. In a Maltese study, the infestation of
Hyalomma spp. was detected in three sedge warblers (n = 4 ticks), but it was not possible to determine whether the tick species was
H. marginatum or
H. rufipes [
51].
The Eurasian reed warbler (
Acrocephalus scirpaceus) is a trans Saharan migratory species that breeds from May until August or September in Central and Western Europe, and winters in Africa, primarily in tropical Africa [
56,
57]. Previous monitoring suggests that their migratory behaviour may be changing, and some birds now remain on the Iberian Peninsula over winter instead of crossing the Sahara [
58].
Fourteen ticks of
Hyalomma spp. were previously sampled from an Eurasian reed warbler in the Netherlands [
44]. Also, in Italy,
Hyalomma spp. (n = 2 ticks) were collected from an individual of this species [
40].
H. marginatum ticks were found to be infesting Eurasian reed warblers in Spain (n = 10 ticks in 5 birds) [
50], in Slovakia (n = 3 ticks in 2 birds) [
32], and in Czech Republic (n = 1 tick in a bird) [
32]. In Italy (n = 5 ticks) [
40], in Malta (n = 3 ticks in a bird) [
51], and in a study carried out in the African-Western Palearctic region (n = 1 tick) [
5],
H. rufipes was collected from Eurasian reed warblers.
Like other
Acrocephalus warblers, the marsh warbler (
Acrocephalus palustris) is among the most frequent long-distance passerine migrants along the eastern route [
53]. Their breeding season takes place between the second half of May and July [
55]. In the spring migratory season of 2012, a study carried out in Czech Republic found a bird belonging to
Acrocephalus palustris infested by
H. marginatum [
32].
5.2. Anthus trivialis
The tree pipit (
Anthus trivialis) is a migratory bird inhabiting predominantly the temperate regions of Eurasia [
59].
Individuals of tree pipit have been previously reported to be infested by nonspecific
Hyalomma spp. in a research study conducted in the African-Western Palearctic region (n = 3 ticks) [
5] and in four Italian studies. In Italy, a study published in 2013 found six
Hyalomma spp. ticks in three birds [
45]. Other Italian studies detected
Hyalomma spp. ticks in tree pipits sampled in the period between 2013-2014 (n = 8 ticks in 4 birds) [
22], between 2016-2017 (n = 3 ticks) [
46], and in the period between 2017 and 2019 (n = 34 ticks) [
40].
Hyalomma rufipes ticks were recovered from tree pipit birds in a study carried out in the African-Western Palearctic region (n = 4 ticks) [
5] and in Italy (n = 3 ticks) [
40].
5.3. Carduelis spp.
The European goldfinches (
Carduelis carduelis) are widely distributed across Europe, Central Asia and the Northern tip of Africa, inhabiting diverse habitats, from forests and wetlands to grasslands and urban areas, breeding between April and early August [
55].
Hyalomma marginatum was reportedly collected from a European goldfinch bird (n = 1 tick) in Italy [
40]. Non-defined species of
Hyalomma spp. were identified in a European goldfinch (n = 1 tick) in Italy [
40].
Furthermore
, H. marginatum was recovered from a citril finch (
Carduelis citronella) (n = 2 ticks), a partial short-distance migrant and an altitudinal migrant, in Spain [
50,
55].
5.4. Curruca spp.
Common whitethroat (
Curruca communis) is a long-distance migrant that overwinters in sub-Saharan Africa, breeding primarily from April to July [
55,
60].
The occurrence of non-defined
Hyalomma spp. ticks in common whitethroat individuals have been significant. In Italy, Mancini et al. [
45], De Liberato et al. [
22] and Battisti et al. [
46] collected 18, 55 and 24 ticks, respectively. Another Italian study highlighted the infestation of common whitethroats by undefined
Hyalomma spp. ticks (n = 109 ticks) [
40]. In Spain, England et al. [
43] recovered five. In the United Kingdom, four ticks were detected by Jameson et al. [
33]. Also, in Hungary, Keve et al. [
42] found a bird infested by
Hyalomma spp.
Mancuso et al. [
40] also recovered
H. marginatum (n = 2 ticks) and
H. rufipes (n = 52 ticks) from common whitethroats. Hoffman et al. [
5] found six
H. marginatum in a sampling from the common whitethroat.
Hyalomma marginatum was also described in Malta (n = 1 tick) [
51]. Similarly, the latter two mentioned studies also identified
H. rufipes as infesting ticks of common whitethroats (n = 56 ticks and n = 19 ticks, respectively) [
5,
51]. Additionally, Battisti et al. [
46], in Italy, also obtained five
Hyalomma rufipes ticks from common whitethroats.
Additionally,
H. rufipes was collected from eastern subalpine warblers (
Curruca cantillans) (n = 5 ticks) in Italy [
40] and from a western subalpine warbler (
Curruca iberiae) (n = 1 tick) bird in Malta [
51].
Hyalomma spp. ticks were also reported in eastern subalpine warbler (
Curruca cantillans) birds in Italy by De Liberato et al. [
22] and by Battisti et al. [
46].
5.5. Erithracus rubecula
The European robin (
Erithracus rubecula) (
Figure 4) is a widespread migrating songbird species, breeding from April to July in Eurasia, varying by region [
61]. The species inhabits forests, thickets, and urban areas, requiring shade, cover, and bare ground [
55].
European robins were classified as vectors of
Hyalomma spp. ticks in studies carried out in France [
52], in Italy [
40], and in the African-Western Palearctic region, more specifically in Greece [
5].
Mancuso et al. [
40], Vial et al. [
52], and Hoffman et al. [
5] identified
H. marginatum obtained from European robins (n = 22 ticks, n = 2 ticks in 2 birds, and n = 2 ticks in a bird, respectively). Mancuso et al. [
40] also recovered three non-defined
Hyalomma spp.. Additionally, in Italy, ten ticks of
H. rufipes were collected from European robins [
46].
5.6. Ficedula spp.
Pied flycatcher (
Ficedula hypoleuca) inhabits semi-open areas, breeding across a vast area of the Western Palearctic region and wintering in West Africa [
62].
In a Spanish study published in 2016,
H. marginatum was reported as being recovered from a pied flycatcher bird (n = 1 tick) [
50]. Meanwhile,
H. rufipes ticks were collected from pied flycatchers in Italy (n = 52 ticks) [
40], in a study carried out in the African-Western Palearctic region (n = 19 ticks) [
5], and in Malta (n = 6 ticks in 2 birds) [
51]. In Italy, four studies have described nonspecific
Hyalomma spp. ticks collected from pied flycatchers. Mancini et al. [
45] have recovered two ticks in two birds, De Liberato et al. [
22] have recovered eight ticks, and, more recently, Battisti et al. [
46] obtained six ticks, and Mancuso et al. [
40] have collected 138 ticks from pied flycatchers. Also in Hungary,
Hyalomma spp. were detected in an infested bird [
42].
Meanwhile, individuals of collared flycatcher (
Ficedula albicollis) are migratory birds previously identified as carriers of
H. rufipes in a study conducted in the African-Western Palearctic region (n = 8 ticks) [
5], in two Italian studies (n = 1 ticks and n = 4 ticks, respectively) [
40,
46], and in a Maltese study (n = 1 tick) [
51]. Nonspecific
Hyalomma spp. ticks were also detected in pied flycatcher birds in Italy (n = 27 ticks) [
40].
5.7. Hippolais spp.
Icterine warbler (
Hippolais icterina) is a migratory bird, breeding from the end of May to July, wintering in Africa [
55].
Hyalomma marginatum was obtained from an icterine warbler in a previous study conducted in Italy (n = 1 tick) [
40]. Meanwhile, in the same study, 25
H. rufipes ticks were recovered from icterine warblers [
40]. Hoffman et al. [
5] also detected five
H. rufipes ticks from the icterine warbler. Moreover, non-defined
Hyalomma spp. were described as infesting icterine warbler
s in three Italian studies. The first of the studies sampled three ticks [
22]. Later, Battisti et al. [
46] obtained two
Hyalomma spp. ticks and, more recently, Mancuso et al. [
40] recovered sixty-seven.
Additionally, the melodious warbler (
Hippolais polyglottal) (n = 1 bird) was also described as carrier of
Hyalomma spp. (n = 2 ticks) in Italy [
45].
5.8. Hirundo rustica
The barn swallow (
Hirundo rustica) is migratory, breeding from May to August, with the European birds wintering in sub-Saharan Africa and in southern and western Europe [
55].
Barn swallows were previously reported as carriers of
H. rufipes in Italy by Mancuso et al. [
40] and in Malta by Hornok et al. [
51] (n = 1 tick and n = 1 tick, respectively). Also in Italy, Battisti et al. [
46] obtained an indistinguishable
Hyalomma spp. tick from a barn swallow individual.
5.9. Lanius spp.
Three CCHFV-positive
H. marginatum nymphs were already obtained from woodchat shrike (
Lanius senator) in a Greek study [
4].
Woodchat shrike (
Lanius senator) is adapted to open landscapes, widely distributed from the Iberian Peninsula to Western Turkey, preferring to breed in semi-open, dry grassland habitats with scattered shrubs [
63,
64].
Non-specified
Hyalomma spp. were previously reported in woodchat shrikes in three Italian studies (n = 1 tick [
45], n = 2 ticks [
22], and n = 12 ticks [
40].
Hoffman et al. [
5] described the occurrence of
H. marginatum (n = 2 ticks) and
H. rufipes (n = 45 ticks) in woodchat shrikes. Other studies also collected
H. rufipes from woodchat shrikes. Among them, two Italian studies [
40,
48]. Mancuso et al. [
40] identified ten ticks. A Maltese study [
51] found two ticks collected from a woodchat shrike.
Furthermore, in Turkey, a red-backed shrike (
Lanius collurio) was reported by Leblebicioglu et al. [
41] to carry
Hyalomma spp. (n = 1 tick).
5.10. Luscinia megarhynchos
The common nightingale (
Luscinia megarhynchos) is a migratory bird that winters in Africa [
65].
Hyalomma marginatum ticks were recovered from common nightingales in Czech Republic in 2010 (n = 4 ticks in a bird) and in 2012 (n = 1 tick in a bird) [
32]. Also, a Spanish study [
50], a study in the African-Western Palearctic region [
5], and an Italian one [
40] sampled
H. marginatum from common nightingale (n = 8 ticks, n = 5 ticks, n = 2 ticks, respectively). Regarding the occurrence of
H. rufipes in individuals of common nightingale, it was reported the sampling of 14 ticks collected in a study carried out in the African-Western Palearctic region [
5] and 10 ticks in Italy [
40].
Furthermore, non-defined
Hyalomma spp. were sampled in three Italian studies. Mancini et al. [
45] detected 25 ticks in a total of six birds, De Liberato et al. [
22] identified six ticks in two birds, and, more recently, Mancuso et al. [
40] collected 15 ticks from common nightingale individuals.
Also, a thrush nightingale (
Luscinia luscinia) individual was reported in Turkey as carrying an undefined
Hyalomma spp. [
41].
5.11. Motacilla flava
The western yellow wagtail (
Motacilla flava) is a common Paleartic passerine, breeding in this region between March and May, and wintering in Africa and Southern Asia [
66].
Western yellow wagtails were described carrying
H. rufipes in Italy (n = 11 ticks) [
40], in a study in the African-Western Palearctic region (n = 2 ticks) [
5], and in Malta (n = 1 tick in a bird) [
51]. In Italy, three studies detected
Hyalomma spp., although not defining the species. Mancini et al. [
45] recovered two
Hyalomma spp. ticks in a western yellow wagtail, De Liberato et al. [
22] collected nine ticks in two birds, and Mancuso et al. [
40] detected 13
Hyalomma spp. in the same bird species.
5.12. Muscicapa striata
The spotted flycatcher (
Muscicapa striata) is a migratory bird, breeding in Europe from mid-May to mid-August and wintering in sub-Saharan Africa [
55].
Mancuso et al. [
40] identified a
H. marginatum infesting a spotted flycatcher bird in Italy. Meanwhile, the same study also recovered 39
H. rufipes ticks from the same species of birds [
40].
Hyalomma rufipes were also described by Battisti et al. [
46] (n = 2 ticks) and by Hoffman et al. [
5] (n = 4 ticks).
In Italy, Mancini et al. [
45] have identified a
Hyalomma spp. tick in a spotted flycatcher, similarly to De Liberato et al. [
22]. Also in Italy, Mancuso et al. [
40] recovered 83 ticks from spotted flycatchers.
5.13. Oenanthe spp.
The black-eared wheatear (
Oenanthe hispanica) is a passerine species associated with warm areas in the Mediterranean region, where it breeds [
67].
The black-eared wheatear has been described as a carrier of
Hyalomma spp. in Italy. Mancini et al. [
45] reported the occurrence of a tick in a bird of this species, similarly to the findings of De Liberato et al. [
22]. Meanwhile, Mancuso et al. [
40] found four ticks of
Hyalomma spp. (the species was not defined) and, more specifically, two ticks of
H. rufipes infesting black-eared wheatear. The same study recovered a CCHFV-positive larva of
H. rufipes from a black-eared wheatear in May 2018 [
40].
The northern wheatear (
Oenanthe oenanthe) is a mountain generalist and open grassland species that primarily breeds in Southern Europe, migrating approximately 3500 km to its wintering grounds in sub-Saharan Africa [
68].
Hoffman et al. [
5] sampled six
H. marginatum and 21
H. rufipes ticks from northern wheatear individuals. Also, two Italian studies reported the infestation of northern wheatear birds by
H. rufipes (n = 1 tick and n = 7 ticks) [
40,
46]. In the same study, 59 ticks of
Hyalomma spp., which species was not defined, were collected from these birds [
40]. Similarly, De Liberato et al. [
22] and Battisti et al. [
46] found
Hyalomma spp. in northern wheatears (n = 2 ticks in 2 birds and n = 1 tick, respectively).
5.14. Oriolus oriolus
The Eurasian golden oriole (
Oriolus oriolus) is a long-distance migrant that winters on the south Sahara regions [
69].
Hyalomma marginatum was previously described in Eurasian golden oriole birds in Spain [
50], Greece [
27], and Italy [
40]. In Spain, Palomar et al. [
50] reported two ticks in a bird, while Wallménius et al. [
27] and Mancuso et al. [
40] detected a tick, each (in Greece and in Italy, respectively).
Hyalomma rufipes ticks were also detected in Eurasian golden orioles by Hoffman et al. [
5] (n = 30 ticks) and by Mancuso et al. [
40] (n = 28 ticks). Eurasian golden orioles were also described as carriers of
Hyalomma spp. (non-defined) in Italy by De Liberato et al. [
22] (n = 6 ticks in 4 birds) and by Mancuso et al. [
40] (n = 34 ticks).
5.15. Otus scops
The Eurasian scops-owl (
Otus scops) is the only long-distance migrating European owl, wintering in south Sahara [
70].
Mancuso et al. [
40] have detected
H. marginatum (n = 1 tick) in an Eurasian scops-owl in Italy. The same study also described another
Hyalomma spp. tick, which species is not defined. Similarly, an Italian study [
22] found two ticks of unspecific Hyalomma spp. in an Eurasian scops-owl.
5.16. Parus major
The great tit (
Parus major) (
Figure 5) is generally a resident bird, but with seasonal altitudinal movements and partial eruptions [
55].
In Spain, Palomar et al. [
50] reported the presence of four
H. marginatum ticks in five great tit. Meanwhile, in Italy, a
Hyalomma spp. tick was found infesting a great tit individual [
40].
5.17. Phoenicurus spp.
The common redstart (
Phoenicurus phoenicurus) is a migratory species that breeds from late-April to mid-July, with earlier breeding in Southern Europe and later May to late-June in northern Finland [
5][].
Common redstarts have been reported to be carriers of
H. marginatum in studies carried out in Italy (n = 4 ticks) [
40], in the African-Western Palearctic region (n = 2 ticks) [
5], and in Greece (n = 1 tick) [
27]. Also,
H. rufipes ticks were sampled from this bird species by Mancuso et al. [
40] (n = 65 ticks), by Hoffman et al. [
5] (n = 29 ticks), by Battisti et al. [
46] (n = 12 ticks), by Hornok et al. [
51] (n = 5 ticks in 2 birds), and, more recently, by Mancuso et al. [
40].
Non-specific
Hyalomma spp. ticks were obtained from common redstarts in Turkey (n = 3 ticks) [
41], in the United Kingdom (n = 1 tick) [
33], and in Italy [
22,
40,
45,
46]. In Italy, more specifically, Mancini et al. [
45] sampled two ticks, De Liberato et al. [
22] detected 49, Battisti et al. [
46] obtained nine ticks, and Mancuso et al. [
40] recovered 122 ticks from
Phoenicurus phoenicurus birds.
Additionally, two black redstarts (
Phoenicurus ochruros) were also described as carrying
H. marginatum in Spain [
50] and
H. rufipes in Italy [
40], respectively. Also in Italy,
H. rufipes (n = 1 tick) and non-defined
Hyalomma spp. ticks (n = 3 ticks) were collected from black redstarts by Battisti et al. [
46]. Their breeding populations in Iberia, southern and central France, Italy, the Balkans, and central Turkey are mostly sedentary, with mountain-dwelling individuals moving to lower elevations during winter. In contrast, populations from northern Europe migrate southwest towards, among others, the Balearic Islands and Spain [
55].
5.18. Phylloscopus spp.
The wood warbler (
Phylloscopus sibilatrix) is a migratory species which breeds in mid to high latitudes from Western Europe to Western Asia, wintering near the equator in Central and West Africa [
71]. After leaving their wintering grounds in March, these bird species arrive at their breeding grounds in May [
71].
Mancuso et al. [
40] described the infestation of
H. marginatum (n = 2 ticks) in Italy. Meanwhile,
H. rufipes are more extensively reported as infesting ticks of wood warblers by Mancuso et al. [
40] (n = 83 ticks), by Battisti et al. [
46] (n = 1 tick), and by Mancuso et al. [
40] in Italy. Hoffman et al. [
5] collected
H. rufipes from samples recovered from wood warblers in the African-Western Palearctic region (n = 21 ticks). Similar findings were obtained by Hornok et al. [
51] in Malta (n = 9 ticks in 4 birds).
Moreover, De Liberato et al. [
22] (n = 26 ticks in 17 birds), Battisti et al. [
46] (n = 7 ticks], Mancuso et al. [
40] (n = 159 ticks), and Mancuso et al. [
48] reported indistinguishable
Hyalomma spp. ticks sampled from wood warbler in Italy.
The willow warbler (
Phylloscopus trochilus) is a migratory songbird, breeding in Northern Europe and Asia and wintering in sub-Saharan Africa [
72]. Hornok et al. [
51] collected
H. marginatum (n = 2 ticks a bird) and
H. rufipes (n = 4 ticks in 2 birds) from willow warblers in Malta [
51]. Additionally, in Italy, Mancuso et al. [
40] identified 11
H. rufipes. Also, in Italy, Mancini et al. [
40] sampled a tick of non-specific
Hyalomma spp., similar to De Liberato et al. [
22] and to Battisti et al. [
46] findings. Moreover, Mancuso et al. [
40] obtained 24 non-defined
Hyalomma spp. ticks from willow warblers.
5.19. Prunella modularis
The dunnock (
Prunella modularis) breeds throughout the temperate zones and marginally in the subarctic and boreal regions of the Palearctic [
73].
Dunnock birds were previously identified as carriers of
H. marginatum and undistinguishable
Hyalomma spp. in Italy and France [
40,
46,
52]. Mancuso et al. [
40] and Vial et al. [
52] recovered four and two
H. marginatum ticks, respectively. Additionally, Battisti et al. [
46] obtained a non-defined
Hyalomma spp. tick from this bird species.
5.20. Saxicola rubetra
In April 2017, an Italian study [
40] found a
H. rufipes nymph positive to CCHFV collected from a whinchat (
Saxicola rubetra).
The whinchat breeds from Western Europe into Western Asia, spending the winter in the humid zone of sub-Saharan Africa [
74]. The infestation of
Hyalomma spp. ticks in these individuals is extensively reported. Hoffman et al. [
5] revealed the infestation by 123
H. rufipes ticks. In Italy, 65 and seven
H. rufipes ticks were sampled from whinchats by Battisti et al. [
46] and by Mancuso et al. [
40], respectively. Mancuso et al. [
48] also detected
H. rufipes.
Non-defined
Hyalomma spp. ticks were sampled from whinchats by Mancuso et al. [
40] (n = 214 ticks), by De Liberato et al. [
22] (n = 41 ticks in 22 birds), by Mancini et al. [
45] (n = 20 ticks), by Battisti et al. [
46] (n = 13 ticks), and by Hoffman et al. [
5] (n = 8 ticks).
5.21. Sylvia spp.
The Eurasian blackcap (
Sylvia atricapilla) is sedentary on islands, partially migratory in the Mediterranean and Western Europe, and migrates long distances in the north and east [
55].
Hyalomma rufipes were collected from Eurasian blackcaps by Hoffman et al. [
5] (n = 4 ticks) in the African-Western Palearctic region, and by Vial et al. [
52] (n = 2 ticks in 2 birds) in France.
Hyalomma spp. (n = 2 ticks in 2 birds) were reported in Italy [
45].
Individuals of garden warbler (
Sylvia borin), a long-distance migrant, were previously described to be infested with
H. rufipes by Mancuso et al. [
40] (n = 10 ticks) and by Hoffman et al. [
5] (n = 1 tick). Unspecific
Hyalomma spp. ticks were sampled from garden warblers in Italy, by De Liberato et al. [
22] (n = 1 tick) and Mancuso et al. [
40] (n = 11 ticks), and in the Netherlands by Heylen et al. [
49] (n = 1 tick).
5.22. Turdus spp.
The Eurasian blackbird (
Turdus merula) (
Figure 6) is a ubiquitous species and, depending on latitude, sedentary, partially and fully migratory [
55,
75]. Birds belonging to this species were found to be infested by
H. marginatum (n = 31 ticks in 11 birds) [
50]. In Italy, Mancini et al. [
45] identified four
Hyalomma spp. in four birds.
Song thrush (
Turdus philomelos) breeds in Scandinavia and northwestern Russia and spends the winter in the Mediterranean basin [
76]. A previous Italian study [
40] sampled
H. marginatum from song thrushes (n = 57 ticks). The same Italian study also detected
H. rufipes (n = 2 ticks) and other
Hyalomma spp. (n = 28 ticks) in this bird species [
40]. Also, in Italy, Battisti et al. [
46] obtained one H
yalomma rufipes and three
Hyalomma spp. ticks from song thrushes.
5.23. Upupa epops
Northern populations of the common hoopoe (
Upupa epops) are fully migratory, while others are either only partially migratory or sedentary [
55].
Hyalomma rufipes has been reported in common hoopoe birds in a study conducted in the African-Western Palearctic region [
5] (n = 2 ticks) and in Malta [
51] (n = 1 tick in a bird). De Liberato et al. [
22] and Mancuso et al. [
40] revealed the occurrence of
Hyalomma spp. in common hoopoe individuals analysed in Italy (n = 1 tick and n = 2 ticks, respectively).
6. Climate Change and Its Effects on the Expansion of Hyalomma spp.
Climate change is considered the main factor behind the observed expansion of
Hyalomma marginatum, [
6].
Factors such as temperature, precipitation, and air currents influence the seasonal migration routes of migratory birds, as well as the time of attachment of ticks to birds and the distance over which the feeding immature ticks are transported [
39]. Moreover, climate and weather changes determine the time of birds’ stay in their wintering grounds and the course of seasonal migrations [
39], and impact the moulting from nymphs to adults [
44].
In several regions of Europe, spring temperatures are generally considered insufficient to support the moulting of
Hyalomma spp. nymphs into adults [
22]. Nevertheless, recent reports have documented successful moulting events in newly colonised areas, indicating that
Hyalomma spp. may adapt to changing climatic conditions or benefit from localised microclimates [
7] In Southern Europe and Northern Africa, the summer rainfall and evapotranspiration regulate the
Hyalomma marginatum populations [
77]. In Eastern Europe and the Caucasus, warmer autumns are considered ideal conditions for
Hyalomma marginatum and are associated with decreased mortality rates [
77].
Areas where the annual cumulative temperatures reach between 3000-4000°C and the water vapour deficit is below 15 hPa are favorable for the distribution of
Hyalomma marginatum [
77,
78]. Moreover, established populations of
Hyalomma marginatum occur in areas where the average cumulative temperatures during September and December are around 800°C [
77,
79]. Thus, warm autumns can lead to nymph populations to moult into adults and taking into account that adults are more cold-resistant in comparison to nymphs, autumn temperatures are connected with lower winter mortality [
77].
The timing of first arrival for many migratory bird species is closely linked to average monthly temperatures, with warmer conditions often associated with earlier arrivals. As temperatures rise, suitable breeding and stopover habitats are also expected to shift northward. Conversely, approximately one-eighth of bird species are projected to face a high risk of extinction in the coming decades [
80].
There is growing interest in creating maps that delineate areas where the risk of ticks or tick-borne pathogens is likely to occur [
81], with several studies modelling the distribution of
Hyalomma spp. and the potential expansion of CCHF under climate change scenarios. A retrospective study revealed changes in the life cycle of
Hyalomma marginatum along a latitudinal gradient, with more significant changes between 1901–1922 and 1989–2009 [
15]. Climate modelling carried out by Estrada-Peña et al. [
15] showed that warmer temperatures and lower humidity have increased the tick’s development and survival rates, particularly in regions where the tick has been historically present. Their model results [
15] predicted that large regions in the Atlantic domain could experience a 60%-80% increase in development rates and over 80% in survival rates during the developmental and questing stages. In turn, Gale et al. [
82] projected that, by 2075–2084, the number of European regions meeting the moulting threshold for
Hyalomma marginatum nymphs (≥8 °C for 15 consecutive days) will increase relative to 2005–2014, particularly in coastal areas during March and expanding inland by April. Despite this, the frequency of annual CCHFV incursions via immature ticks on migratory birds (e.g., Common Quail, Tree Pipit, Willow Warbler, Northern Wheatear) is expected to remain stable, with reduced risk in Central and Southern Europe but increased risk in the North [
82]. More recent projections by Okely et al. [
18] suggest a possible northward expansion of
Hyalomma rufipes into Germany and the UK. Similarly, Gillingham et al. [
77] identified regions in the UK with suitable temperatures for
H. marginatum nymphal moulting between 2000–2019, although autumn conditions remain suboptimal for population establishment. The model developed by Fanelli et al. [
83] predicts areas at risk of further CCHFV expansion, such as Italy and France, but highlights a still low risk of CCHFV entry and exposure in most Western European countries. Moreover, in comparison with Cuadrado-Matías et al. [
23], the predictions carried out by Fanelli et al. [
83] show a more expansive distribution of favourable CCHF occurrence conditions in the Iberian Peninsula.
As climate change persists, predictive models incorporating data regarding
Hyalomma spp. habitat suitability, host distribution and temperature project a continued expansion of CCHFV into Central and Northern Europe, with a medium risk of CCHFV introduction in France, Italy and Germany [
8].
7. Conclusions
The Crimean-Congo Hemorrhagic Fever (CCHF) poses a significant public health threat due to its high mortality rate, lack of effective treatments or vaccines, and expanding geographic distribution. Understanding the population dynamics of Hyalomma ticks and studying the eco-epidemiology of CCHF is crucial to preventing this emerging disease in Europe. Variation in the prevalence and intensity of Hyalomma spp. infestation among migratory bird species may indicate differential host susceptibility or distinct migratory strategies that modulate exposure timing and risk, as previously proposed by several authors. Additionally, identifying knowledge gaps, such as the specific influence of bird migration routes and the effectiveness of different surveillance strategies, is essential for controlling this underestimated disease in Europe. Moreover, it has been established that future climate change scenarios may contribute to habitat suitability and the spread of CCHFV northward. Furthermore, public awareness regarding CCHFV, mainly among healthcare workers, veterinarians, hunters, and farmers, should be promoted. The need for continuous projections and adaptation of monitoring strategies as the climate changes is highlighted. Monitoring vectors and suitable sentinels is crucial for assessing the risk of introducing CCHFV in non-endemic areas, predicting possible outbreaks, implementing complementary surveillance strategies, and designing action plans. To conclude, it is essential to strengthen collaboration between European countries for disease monitoring and response.
Supplementary Materials
The following supporting information can be downloaded at [x], Table S1: Detection of Hyalomma spp. in European migratory birds (2008-2025).
Author Contributions
Conceptualization, M. Alves Rodrigues.; writing—original draft preparation, M. Alves Rodrigues; writing—review and editing, M. Alves Rodrigues, P. Lesiczka, M.C. Fontes, L. Cardoso, and A.C. Coelho.; supervision, P. Lesiczka, M.C. Fontes, L. Cardoso, and A.C. Coelho; funding acquisition, M. Alves Rodrigues. All authors have read and agreed to the published version of the manuscript.
Funding
The APC was funded by M. Alves Rodrigues.
Institutional Review Board Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Baz-Flores, S.; Herraiz, C.; Peralbo-Moreno, A.; Barral, M.; Arnal, M.C.; Balseiro, A.; Ruiz-Fons, F. Mapping the risk of exposure to Crimean-Congo haemorrhagic fever virus in the Iberian Peninsula using Eurasian wild boar (Sus scrofa) as a model. Ticks Tick Borne Dis 2024, 15, 102281. [Google Scholar] [CrossRef] [PubMed]
- D’Addiego, J.; Wand, N.; Afrough, B.; Fletcher, T.; Kurosaki, Y.; Leblebicioglu, H.; Hewson, R. Recovery of complete genome sequences of Crimean-Congo haemorrhagic fever virus (CCHFV) directly from clinical samples: A comparative study between targeted enrichment and metagenomic approaches. J. Virol. Methods 2024, 323, 114833. [Google Scholar] [CrossRef]
- Juanes, H.M. L.; López-Bernus, A.; Vicente, B.; Alonso-Sardón, M.; Alonso, B.R.; Ulerio, J.P.; Belhassen-García, M. Crimean-Congo haemorrhagic fever virus screening among African individuals in Spain: Lessons to learn. Travel Med Infect Dis 2025, 102814. [Google Scholar] [CrossRef]
- Lindeborg, M.; Barboutis, C.; Ehrenborg, C.; Fransson, T.; Jaenson, T.G.; Lindgren, P.E.; Olsen, B. Migratory birds, ticks, and Crimean-Congo hemorrhagic fever virus. Emerg. Infect. Dis 2012, 18, 2095. [Google Scholar] [CrossRef]
- Hoffman, T.; Carra, L.G.; Öhagen, P.; Fransson, T.; Barboutis, C.; Piacentini, D.; Olsen, B. Association between guilds of birds in the African-Western Palaearctic region and the tick species Hyalomma rufipes, one of the main vectors of Crimean-Congo hemorrhagic fever virus. One Health 2021, 13, 100349. [Google Scholar] [CrossRef]
- Moroșan, Ș.; Cozma, A.P.; Dascălu, M.A.; Crivei, L.A. Impact of recent and future climate change on vectorborne diseases: viruses analyses. Lucr. ştiinţ., Ser. Med. Vet. [CrossRef]
- Sesmero-García, C.; Cabanero-Navalon, M.D.; Garcia-Bustos, V. The Importance and Impact of Francisella-like Endosymbionts in Hyalomma Ticks in the Era of Climate Change. Diversity 2023, 15, 562. [Google Scholar] [CrossRef]
- Petit, M.J.; Johnson, N.; Mansfield, K.L. Vectorial dynamics underpinning current and future tick-borne virus emergence in Europe. J Gen Virol 2024, 105, 002041. [Google Scholar] [CrossRef]
- Celina, S.S.; Italiya, J.; Tekkara, A.O.; Černý, J. Crimean-Congo haemorrhagic fever virus in ticks, domestic, and wild animals. Front. Vet. Sci 2025, 11, 1513123. [Google Scholar] [CrossRef]
- Harris, J.; Munasinghe, T.; Tubbs, H.; Anyamba, A. (2022, May). In Predicting Crimean-Congo Hemorrhagic Fever Outbreaks via Multivariate Time-Series Classification of Climate Data. In Proceedings of the 6th International Conference on Medical and Health Informatics 2022, Virtual Event Japan, May 13 - 15, Association for Computing Machinery, New York, United States; 2022; pp. 215–218. [Google Scholar] [CrossRef]
- Duygu, F.; Sari, T.; Kaya, T.; Tavsan, O.; Naci, M. The relationship between crimean-congo hemorrhagic fever and climate: Does climate affect the number of patients? Acta Clin. Croat 2018, 57, 443. [Google Scholar] [CrossRef]
- Fanelli, A.; Buonavoglia, D. Risk of Crimean Congo haemorrhagic fever virus (CCHFV) introduction and spread in CCHF-free countries in southern and Western Europe: A semi-quantitative risk assessment. One Health 2021, 13, 100290. [Google Scholar] [CrossRef]
- Greene, L.; Uwishema, O.; Nicholas, A.; Kapoor, A.; Berjaoui, C.; Adamolekun, E.; Onyeaka, H. Crimean-Congo haemorrhagic fever during the COVID-19 pandemic in Africa: efforts, recommendations and challenges at hand. AfJEM 2022, 12, 117–120. [Google Scholar] [CrossRef] [PubMed]
- Celina, S.S.; Černý, J.; Samy, A.M. Mapping the potential distribution of the principal vector of Crimean-Congo haemorrhagic fever virus Hyalomma marginatum in the Old World. PLoS Negl Trop Dis 2023, 17, e0010855. [Google Scholar] [CrossRef]
- Estrada-Peña A, de la Fuente J, Latapia T, Ortega C The Impact of Climate Trends on a Tick Affecting Public Health: A Retrospective Modeling Approach for Hyalomma marginatum (Ixodidae). PLoS ONE, 0125. [CrossRef]
- Malik, Y.S.; Arun Prince Milton, A.; Ghatak, S.; Ghosh, S. Neglected Bird-Associated Viral Zoonotic Infections. In: Role of Birds in Transmitting Zoonotic Pathogens. Livestock Diseases and Management, Springer, Singapore, 2021, pp. 101–112. [CrossRef]
- Nasirian, H. Ticks infected with Crimean-Congo hemorrhagic fever virus (CCHFV): A decision approach systematic review and meta-analysis regarding their role as vectors. Travel Med Infect Di 2022, 47, 102309. [Google Scholar] [CrossRef]
- Okely, M.; Anan, R.; Gad-Allah, S.; Samy, A.M. Mapping the environmental suitability of etiological agent and tick vectors of Crimean-Congo hemorrhagic fever. Acta Trop 2020, 203, 105319. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control. (2024). Crimean-Congo haemorrhagic fever: Surveillance – Cases in the EU since 2013. Available online: https://www.ecdc.europa.eu/en/crimean-congo-haemorrhagic-fever/surveillance/cases-eu-since-2013 (accessed on 6th March 2025).
- Zé-Zé, L.; Nunes, C.; Sousa, M.; de Sousa, R.; Gomes, C.; Santos, A.S.; Alves, M.J. Fatal Case of Crimean-Congo Hemorrhagic Fever, Portugal, 2024. Emerg. Infect. Dis 2025, 31, 139. [Google Scholar] [CrossRef]
- Schuster, I.; Mertens, M.; Mrenoshki, S.; Staubach, C.; Mertens, C.; Brüning, F.; Groschup, M.H. Sheep and goats as indicator animals for the circulation of CCHFV in the environment. Exp Appl Acarol 2016, 68, 337–346. [Google Scholar] [CrossRef]
- De Liberato, C.; Frontoso, R.; Magliano, A.; Montemaggiori, A.; Autorino, G.L.; Sala, M.; Scicluna, M.T. Moni-toring for the possible introduction of Crimean-Congo haemorrhagic fever virus in Italy based on tick sampling on migratory birds and serological survey of sheep flocks. Prev. Vet. Med 2018, 149, 47–52. [Google Scholar] [CrossRef]
- Cuadrado-Matías, R.; Cardoso, B.; Sas, M.A.; García-Bocanegra, I.; Schuster, I.; González-Barrio, D.; Ruiz-Fons, F. Red deer reveal spatial risks of Crimean-Congo haemorrhagic fever virus infection. Transbound Emerg Dis 2022, 69, e630–e645. [Google Scholar] [CrossRef]
- Cevidanes, A.; Barandika, J.F.; Aduriz, G.; Hurtado, A.; García-Pérez, A.L.; Barral, M. Exposure to Crimean-Congo Hemorrhagic Fever Virus in Wild Ungulates in the Basque Country, Northern Iberian Peninsula. Transbound Emerg Dis 2024, 2024, 8553577. [Google Scholar] [CrossRef]
- Kumar, B.; Manjunathachar, H.V.; Ghosh, S. A review on Hyalomma species infestations on human and animals and progress on management strategies. Heliyon 2020, 6. [Google Scholar] [CrossRef]
- Bonnet, S.I.; Bertagnoli, S.; Falchi, A.; Figoni, J.; Fite, J.; Hoch, T.; Vial, L. An update of evidence for pathogen transmission by ticks of the genus Hyalomma. Pathogens 2023, 12, 513. [Google Scholar] [CrossRef] [PubMed]
- Wallménius, K.; Barboutis, C.; Fransson, T.; Jaenson, T.G.; Lindgren, P.E.; Nyström, F.; Nilsson, K. Spotted fever Rickettsia species in Hyalomma and Ixodes ticks infesting migratory birds in the European Mediterranean area. Parasites & Vectors 2014, 7, 1–20. [Google Scholar] [CrossRef]
- Spengler, J.R.; Estrada-Peña, A. Host preferences support the prominent role of Hyalomma ticks in the ecology of Crimean-Congo hemorrhagic fever. PLoS Neglect Trop D 2018, 12, e0006248. [Google Scholar] [CrossRef]
- Fillâtre, P.; Revest, M.; Tattevin, E.P. Crimean-Congo hemorrhagic fever: An update. Med Mal Infect 2019, 49, 574–585. [Google Scholar] [CrossRef]
- Grandi, G.; Chitimia-Dobler, L.; Choklikitumnuey, P.; Strube, C.; Springer, A.; Albihn, A.; Omazic, A. First records of adult Hyalomma marginatum and H. rufipes ticks (Acari: Ixodidae) in Sweden. Ticks Tick Borne Dis 2020, 11, 101403. [Google Scholar] [CrossRef]
- Bakheit, M.; Latif, A.; Vatansever, Z.; Seitzer, U.; Ahmed, J. The huge risks due to Hyalomma ticks. In Arthropods As Vectors of Emerging Diseases; Mehlhorn, H.; Ed. B: Springer, 2012. [Google Scholar] [CrossRef]
- Capek, M.; Literak, I.; Kocianova, E.; Sychra, O.; Najer, T.; Trnka, A.; Kverek, P. Ticks of the Hyalomma marginatum complex transported by migratory birds into Central Europe. Ticks and Tick-borne Diseases 2014, 5, 489–493. [Google Scholar] [CrossRef]
- Jameson, L.J.; Morgan, P.J.; Medlock, J.M.; Watola, G.; Vaux, A.G. Importation of Hyalomma marginatum, vector of Crimean-Congo haemorrhagic fever virus, into the United Kingdom by migratory birds. Ticks Tick Borne Dis 2012, 3, 95–99. [Google Scholar] [CrossRef]
- Chitimia-Dobler, L.; Schaper, S.; Rieß, R.; Bitterwolf, K.; Frangoulidis, D.; Bestehorn, M.; Dobler, G. Imported Hyalomma ticks in Germany in 2018. Parasites & vectors 2019, 12, 1–9. [Google Scholar] [CrossRef]
- Rudolf, I.; Vojtíšek, J.; Kejíková, R.; Šikutová, S.; Mendel, J.; Hubálek, Z.; Estrada-Peña, A. (2021). Probable overwintering of adult Hyalomma rufipes in Central Europe. Ticks Tick Borne Dis. 1017. [Google Scholar] [CrossRef]
- Cuadrado-Matías, R.; Moraga-Fernández, A.; Peralbo-Moreno, A.; Negredo, A.I.; Sánchez-Seco, M.P.; Ruiz-Fons, F. Crimean–Congo haemorrhagic fever virus in questing non-Hyalomma spp. ticks in Northwest Spain, 2021. Zoonoses Public Hlth 2024, 71, 578–583. [Google Scholar] [CrossRef]
- Duscher, G.G.; Hodžić, A.; Hufnagl, P.; Wille-Piazzai, W.; Schötta, A.M.; Markowicz, M.A.; Allerberger, F. Adult Hyalomma marginatum tick positive for Rickettsia aeschlimannii in Austria, October 2018. Eurosurveillance 2018, 23, 1800595. [Google Scholar] [CrossRef]
- Estrada-Peña, A.; D’Amico, G.; Fernández-Ruiz, N. Modelling the potential spread of Hyalomma marginatum ticks in Europe by migratory birds. Int J Parasitol. [CrossRef]
- Buczek, A.M.; Buczek, W.; Buczek, A.; Bartosik, K. (2020). The potential role of migratory birds in the rapid spread of ticks and tick-borne pathogens in the changing climatic and environmental conditions in Europe. Int. J. Environ. Res. Public Health 2021, 17, 2117. [Google Scholar] [CrossRef]
- Mancuso, E.; Toma, L.; Pascucci, I.; d’Alessio, S.G.; Marini, V.; Quaglia, M.; Monaco, F. Direct and indirect role of migratory birds in spreading CCHFV and WNV: a multidisciplinary study on three stop-over islands in Italy. Pathogens 2022, 11, 1056. [Google Scholar] [CrossRef]
- Leblebicioglu, H.; Eroglu, C.; Erciyas-Yavuz, K.; Hokelek, M.; Acici, M.; Yilmaz, H. Role of migratory birds in spreading Crimean-Congo hemorrhagic fever, Turkey. Emerg. Infect. Dis 2014, 20, 1331. [Google Scholar] [CrossRef]
- Keve, G.; Csörgő, T.; Benke, A.; Huber, A.; Mórocz, A.; Németh, Á.; Hornok, S. Ornithological and molecular evidence of a reproducing Hyalomma rufipes population under continental climate in Europe. Front. Vet. Sci 2023, 10, 1147186. [Google Scholar] [CrossRef]
- England, M.E.; Phipps, P.; Medlock, J.M.; Atkinson, P.M.; Atkinson, B.; Hewson, R.; Gale, P. Hyalomma ticks on northward migrating birds in southern Spain: Implications for the risk of entry of Crimean-Congo haemorrhagic fever virus to Great Britain. J Vector Ecol 2016, 41, 128–134. [Google Scholar] [CrossRef]
- Uiterwijk, M.; Ibáñez-Justicia, A.; van de Vossenberg, B.; Jacobs, F.; Overgaauw, P.; Nijsse, R.; Sprong, H. Imported Hyalomma ticks in the Netherlands 2018–2020. Parasites & Vectors 2021, 14, 244. [Google Scholar] [CrossRef]
- Mancini, F.; Toma, L.; Ciervo, A.; Di Luca, M.; Faggioni, G.; Lista, F.; Rezza, G. Virus investigation in ticks from migratory birds in Italy. New Microbiol 2013, 36, 433–434. [Google Scholar]
- Battisti, E.; Urach, K.; Hodžić, A.; Fusani, L.; Hufnagl, P.; Felsberger, G.; Duscher, G.G. Zoonotic pathogens in ticks from migratory birds, Italy. Emerg. Infect. Dis 2020, 26, 2986. [Google Scholar] [CrossRef]
- Foley-Fisher, M.; Phipps, P.; Medlock, J.M.; Atkinson, P.; Atkinson, B.; Hewson, R.; Gale, P. Ticks on northward migrating birds in southern Spain during Spring, 2011. J Vector Ecol 2012, 37, 478–480. [Google Scholar] [CrossRef]
- Mancuso, E.; Di Domenico, M.; Di Gialleonardo, L.; Menegon, M.; Toma, L.; Di Luca, M.; Monaco, F. Tick species diversity and molecular identification of spotted fever group Rickettsiae collected from migratory birds arriving from Africa. Mi-croorganisms 2023, 11, 2036. [Google Scholar] [CrossRef]
- Heylen, D.; Fonville, M.; Docters van Leeuwen, A.; Stroo, A.; Duisterwinkel, M.; Van Wieren, S.; Sprong, H. Pathogen communities of songbird-derived ticks in Europe’s low countries. Parasites & Vectors 2017, 10, 1–12. [Google Scholar] [CrossRef]
- Palomar, A.M.; Portillo, A.; Mazuelas, D.; Roncero, L.; Arizaga, J.; Crespo, A.; Oteo, J.A. Molecular analysis of Crimean-Congo hemorrhagic fever virus and Rickettsia in Hyalomma marginatum ticks removed from patients (Spain) and birds (Spain and Morocco), 2009–2015. Ticks Tick Borne Dis 2016, 7, 983–987. [Google Scholar] [CrossRef]
- Hornok, S.; Cutajar, B.; Takács, N.; Galea, N.; Attard, D.; Coleiro, C.; Kontschán, J. On the way between Africa and Europe: molecular taxonomy of ticks collected from birds in Malta. Ticks Tick Borne Dis 2022, 13, 102001. [Google Scholar] [CrossRef]
- Vial, L.; Stachurski, F.; Leblond, A.; Huber, K.; Vourc’h, G.; René-Martellet, M.; Estrada-Peña, A. Strong evidence for the presence of the tick Hyalomma marginatum Koch, 1844 in southern continental France. Ticks Tick Borne Dis 2016, 7, 1162–1167. [Google Scholar] [CrossRef]
- Stępniewska, K.; Ożarowska, A.; Busse, P.; Bobrek, R.; Zehtindjiev, P.; Ilieva, M.; Meissner, W. Autumn migration strategy of juvenile great reed warblers Acrocephalus arundinaceus on the eastern European flyway: A spatiotemporal pattern of accumulation and utilisation of energy stores. Eur. Zool. J 2020, 87, 537–551. [Google Scholar] [CrossRef]
- Mérő, T.O.; Žuljević, A.; Kolykhanova, O.; Lengyel, S. Reuse of nests in the Great Reed Warbler Acrocephalus arundinaceus: A behavior to save time and energy and to deter nest parasites? Ecol Evol 2022, 12, e9452. [Google Scholar] [CrossRef]
- BirdLife International. (2024). BirdLife Data Zone. Available online: https://datazone.birdlife.org/ (accessed on 16th March 2025).
- Halupka, L.; Dyrcz, A.; Borowiec, M. Climate change affects breeding of reed warblers Acrocephalus scirpaceus. J. Avian Biol 2008, 39, 95–100. [Google Scholar] [CrossRef]
- Ientile, R.; Tuliozi, B.; Campobello, D.; Borghi, S.; Sala, L.; Dal Zotto, M.; Massa, B. Analysis of prey composition in Eurasian Reed Warblers’ Acrocephalus scirpaceus droppings at four breeding sites in Italy. Diversity 2022, 14, 1134. [Google Scholar] [CrossRef]
- Sætre, C.L. C.; Eroukhmanoff, F.; Rönkä, K.; Kluen, E.; Thorogood, R.; Torrance, J.; Tørresen, O.K. A chromosome-level genome assembly of the reed warbler (Acrocephalus scirpaceus). Genome Biol Evol 2021, 13, evab212. [Google Scholar] [CrossRef]
- Tumendemberel, U.; Byambadash, S.E.; Sramko, G.; Batsukh, T. A phylogenetic study of the Mongolian Tree Pipit (Anthus trivialis, Linnaeus, 1758) population based on mitochondrial DNA (mtDNA) genes. Proc. Mong. Acad. Sci. 2024, 26–30. [Google Scholar] [CrossRef]
- Briedis, M.; Wong, J.B.; Adamík, P.; Lislevand, T.; Funts, K.; Hromádka, M.; Hahn, S. Seasonal variation in migration routes of Common Whitethroat Curruca communis. J Ornithol 2024, 1–10. [Google Scholar] [CrossRef]
- Karpińska, O.; Kamionka-Kanclerska, K.; Neubauer, G.; Rowiński, P. Characteristics and selection of nest sites of the flexible cavity-nester, the European robin Erithacus rubecula, in the temperate primeval forest (Białowieża National Park, Poland). Eur Zool J 2022, 89, 1–14. [Google Scholar] [CrossRef]
- Fourcade, J.M.; Fontanilles, P.; Demongin, L. Fuel management, stopover duration and potential flight range of pied flycatcher Ficedula hypoleuca staying in South-West France during autumn migration. J Ornithol 2022, 163, 61–70. [Google Scholar] [CrossRef]
- Nasuelli, M.; Ilahiane, L.; Boano, G.; Cucco, M.; Galimberti, A.; Pavia, M.; Pellegrino, I. Phylogeography of Lanius senator in its breeding range: conflicts between alpha taxonomy, subspecies distribution and genetics. Eur Zool J 2022, 89, 941–956. [Google Scholar] [CrossRef]
- Bloche, D.A.; Sapir, N. Breeding performance and nest-site selection of Woodchat Shrikes Lanius senator near the southern edge of their breeding distribution. J Ornithol 2024, 165, 691–701. [Google Scholar] [CrossRef]
- Villarán, A. Ruiseñor común–Luscinia megarhynchos (Brehm, 1831). CSIC – MNCN. [CrossRef]
- Ergen, A.G.; Kefelioğlu, H. Directional preferences of Yellow Wagtail (Motacilla flava) (Linnaeus, 1758) subspecies migrating through Kızılırmak Delta in spring. Bio Di Con 2019, 12, 9–14. [Google Scholar] [CrossRef]
- Brambilla, M.; Fulco, E.; Gustin, M.; Celada, C. Habitat preferences of the threatened Black-eared Wheatear Oenanthe hispanica in southern Italy. Bird Study 2013, 60, 432–435. [Google Scholar] [CrossRef]
- Sander, M.M.; Jähnig, S.; Lisovski, S.; Mermillon, C.; Alba, R.; Rosselli, D.; Chamberlain, D. High nest failure but better nestling quality for early breeders in an alpine population of Northern Wheatear (Oenanthe oenanthe). Ibis 2023, 165, 125–141. [Google Scholar] [CrossRef]
- Dolenec, Z. Dates of arrival of the Eurasian golden oriole (Oriolus oriolus L.) in decidious forest in relation to increase of local air temperature in NW Croatia. Sumar List. [CrossRef]
- Barriocanal, C.; Robson, D.; Gargallo, G. Migration of the Eurasian scops-owl (Otus scops) over the Western Mediterranean. AIRO 2021, 29, 15–22. [Google Scholar]
- Jarrett, C.; Powell, L.L.; Claire, T.T. R.; Tchoumbou, M.; Helm, B. Moult of overwintering Wood Warblers Phyl-loscopus sibilatrix in an annual-cycle perspective. J Ornithol 2021, 162, 645–653. [Google Scholar] [CrossRef]
- Matantseva, M.V.; Simonov, S.A. Two-and three-dimensional territories and territory overlap of Willow Warblers Phylloscopus trochilus in Arctic forests. Polar Biol 2023, 46, 881–893. [Google Scholar] [CrossRef]
- Harnos, A.; Fehérvári, P.; Piross, I.S.; Ágh, N.; Karcza, Z.; Konrád, K.; Csörgő, T. Exploratory analyses of migration timing and morphometrics of the Dunnock (Prunella modularis). Ornis Hungarica 2016, 24, 127–144. [Google Scholar] [CrossRef]
- Stanbury, A.J.; Tománková, I.; Teuten, E.L.; Douglas, D.J. No evidence that declining Whinchat Saxicola rubetra are currently limited by the availability of apparently suitable breeding habitat within the UK uplands. J Ornithol 2022, 163, 273–283. [Google Scholar] [CrossRef]
- Mohring, B.; Brischoux, F.; Angelier, F. Vineyards, but not cities, are associated with lower presence of a generalist bird, the Common Blackbird (Turdus merula), in Western France. Avian Res 2021, 12, 1–11. [Google Scholar] [CrossRef]
- Redlisiak, M.; Remisiewicz, M.; Mazur, A. Sex-specific differences in spring migration timing of Song Thrush Turdus philomelos at the Baltic coast in relation to temperatures on the wintering grounds. Eur Zool J 2021, 88, 191–203. [Google Scholar] [CrossRef]
- Gillingham, E.L.; Medlock, J.M.; Macintyre, H.; Phalkey, R. Modelling the current and future temperature suitability of the UK for the vector Hyalomma marginatum (Acari: Ixodidae). Ticks Tick Borne Dis 2023, 14, 102112. [Google Scholar] [CrossRef]
- Estrada-Peña, A.; Martínez Avilés, M.; Muñoz Reoyo, M.J. A population model to describe the distribution and seasonal dynamics of the tick Hyalomma marginatum in the Mediterranean Basin. Transbound Emerg Dis 2011, 58, 213–223. [Google Scholar] [CrossRef]
- Gray, J.S.; Dautel, H.; Estrada-Peña, A.; Kahl, O.; Lindgren, E. Effects of climate change on ticks and tick-borne diseases in Europe. Interdiscip Perspect Infect Dis 2009, 2009, 593232. [Google Scholar] [CrossRef]
- Li, X.; Liu, Y.; Zhu, Y. The Effects of Climate Change on Birds and Approaches to Response. IOP Conf. Ser. Earth. Environ. Sci. 2022, 1011, 012054. [Google Scholar] [CrossRef]
- Estrada-Peña, A.; de la Fuente, J. The ecology of ticks and epidemiology of tick-borne viral diseases. Antivir Res 2014, 108, 104–128. [Google Scholar] [CrossRef]
- Gale, P.; Stephenson, B.; Brouwer, A.; Martinez, M.; De la Torre, A.; Bosch, J.; Muñoz, M.J. Impact of climate change on risk of incursion of Crimean-Congo haemorrhagic fever virus in livestock in Europe through migratory birds. J Appl Microbiol 2012, 112, 246–257. [Google Scholar] [CrossRef] [PubMed]
- Fanelli, A.; Schnitzler, J.C.; De Nardi, M.; Donachie, A.; Capua, I.; Lanave, G.; Tizzani, P. Epidemic intelligence data of Crimean-Congo haemorrhagic fever, European Region, 2012 to 2022: a new opportunity for risk mapping of neglected diseases. Eurosurveillance 2023, 28, 2200542. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).