One sentence Summary
Cells targeted by EVs release secondary effector vesicles that account for the histocompatibility and disease specificity of MSC EV treatments.
1. Introduction
1.1. The Central Idea Here Is That Recipient Cells Administered Primary Extracellular Vesicles (EVs) Can Induce Further Recipient Production of Secondary EVs That Generate the Final Detected Biological Event Mediated by the Targeted Effector Cells
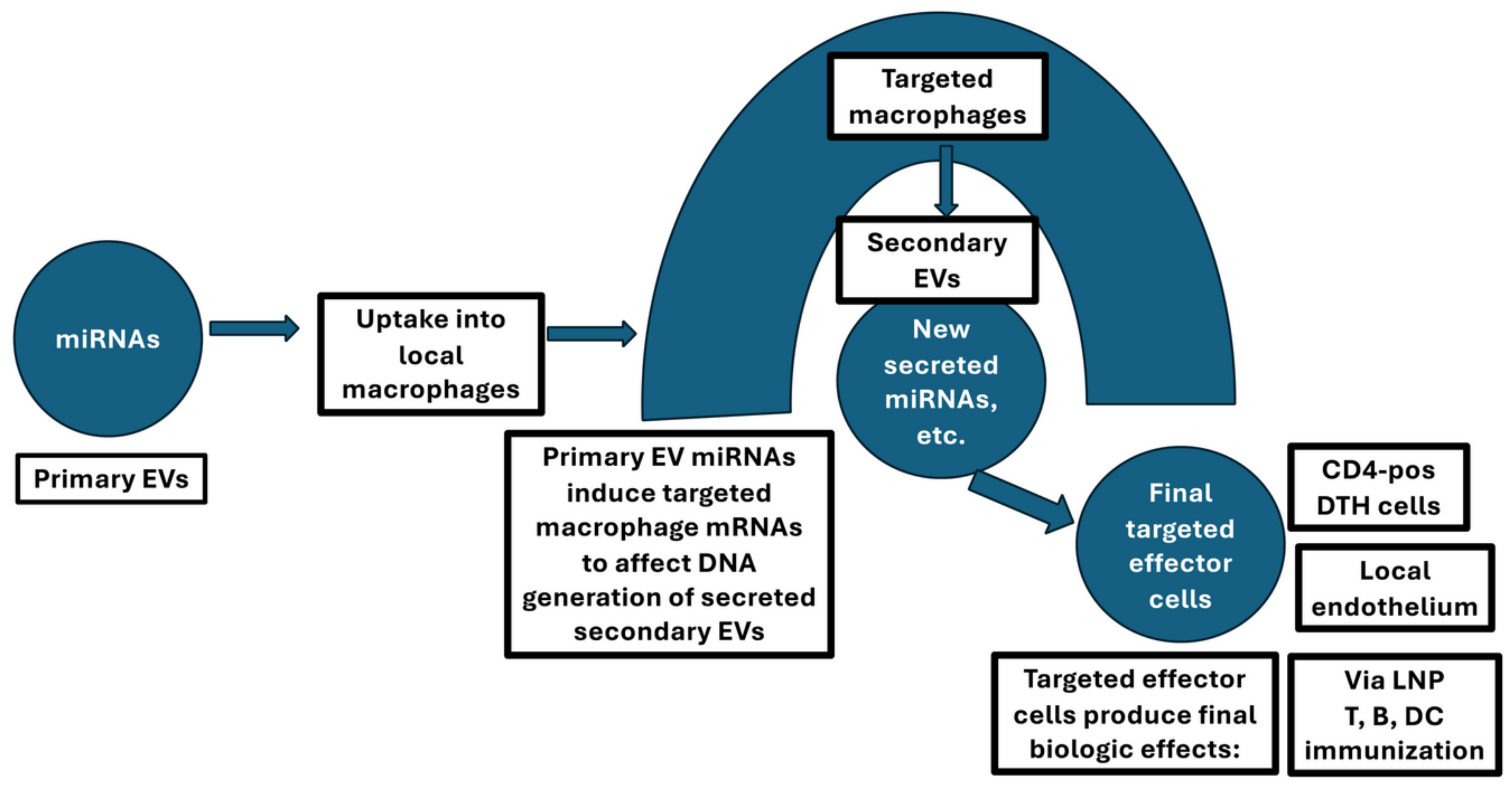
Presented here is a new view that EVs produced by initially activated cells induce other cells to produce new secondary EVs crucially active in the recipients to produce the final detected biological event (
Figure 1). Thus, in some instances, when primary cell-derived EVs act on targeted cells, these are activated by the transferred miRNAs (micro [small] RNAs) or other cargoes to generate new secondary EVs. This usually features the involvement of targeted selected local cells, often of the greater monocytic family like macrophages (Macs), but not exclusively, to generate the new secondary EVs. Illustrated in
Figure 1 are the three instances given here as examples (lower right rectangles). First, CD8
+ suppressor T cell-derived EVs, induced by systemic high-dose antigen-induced immunologic tolerogenesis, act via antigen-presenting Macsto affect CD4
+ effector cells (Bryniarski et al.; Nazimek et al.). This is described further in our literature (Nazimek et al.; Ptak et al.; Nazimek et al.; Wąsik et al.; Bryniarski et al.; Nazimek et al.; Tsuji et al.). Second, mesenchymal stromal cell (MSC)-derived EVs act on M2-type Macs to generate secondary EVs that heal microvascular abnormalities in spinal cord injury (SCI); and Third, by analogy, vaccine mRNA (messenger RNA) delivered lipid nanoparticles (LNPs) act via host phagocytic cells such as Macs to generate effector RNA-containing EVs.
In some instances, when primary EVs act on targeted cells, they are stimulated by the transferred miRNAs or other cargoes to generate new secondary EVs. This usually features the involvement of the targeted cell DNA in generating secondary EVs that are then secreted to act on the final target cells to mediate the principal in vivo biological activity, illustrated by the three instances given here as examples (lower-right rectangles). First, one instance involves suppressor CD8+ T cell-derived primary EVs stimulated by high antigen dose tolerogenesis. These then act on local macrophages to induce their generation of secondary EVs, which then influence the final targeted Th1 effector T cells of DTH. Then, these secondary EV-targeted cells alter T cell activation and subsequent cytokine production to participate in tolerance-mediated inhibition of delayed-type hypersensitivity (DTH), including the suppression of the release of INF-γ. Second, EVs derived from MSCs can aggregate in the lung to form primary EVs, or these primary EVs injected intravenously, promote maturation of M2 macrophages at the site of spinal cord injury (SCI) for production of TGF-β. Consequently, there is primary EV activation of these M2 Macs to produce secondary EVs that then target the local microvasculature to produce TGF-β receptors. Furthermore, the microendothelial cells are induced to produce mRNA and subsequent proteins of vascular adhesion molecules to enable healing of the disturbed vascular permeability of SCI. A third instance, by analogy, is that LNP mRNA vaccines acting like primary EVs are taken up by local macrophages at the site of injection to produce secondary EVs for their transfer of mRNAs and miRNAs to the final targeted distant antigen-presenting, T, and B cells to generate a vaccine-induced immune response.
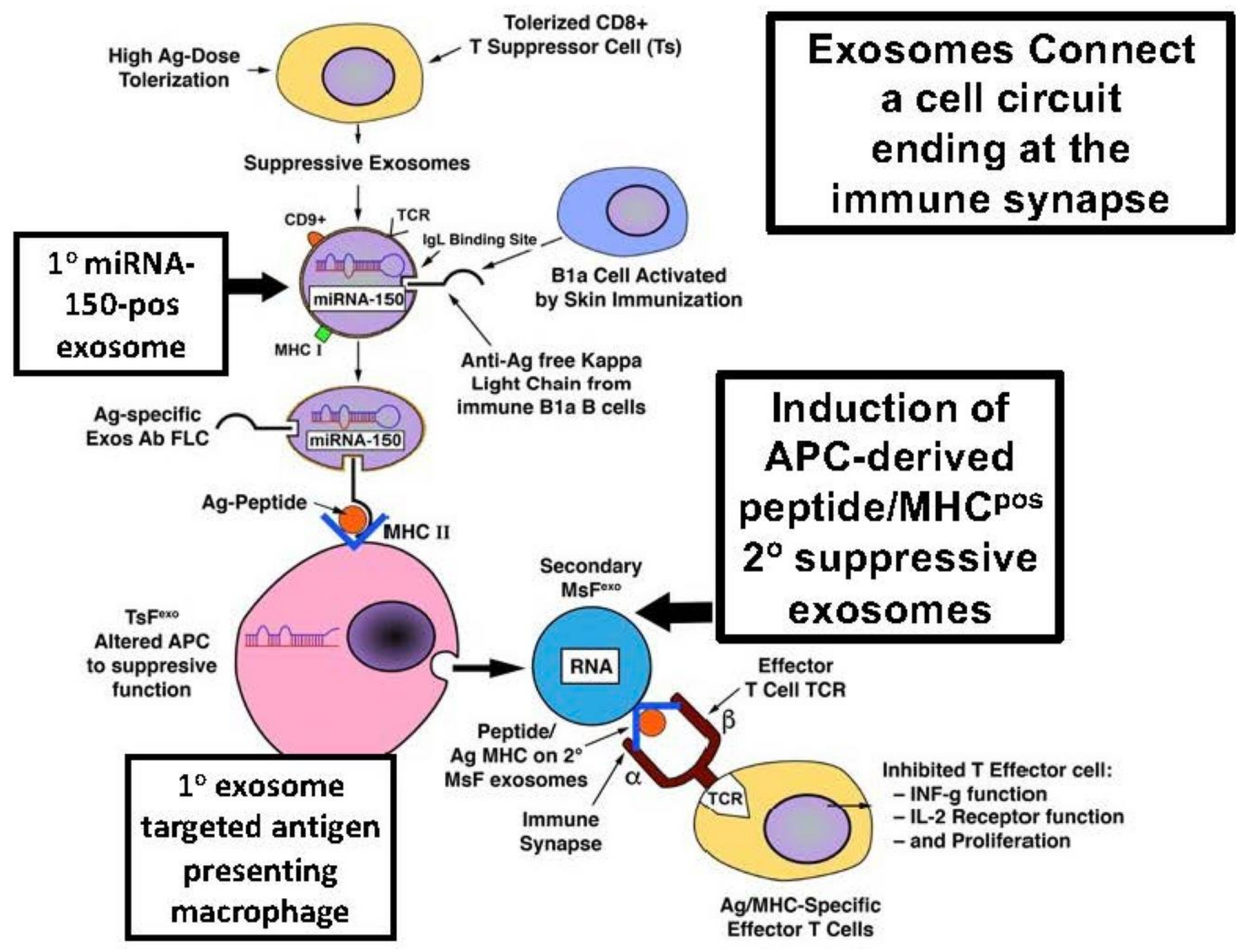
1.2. In Vivo Regulation at the Immune Synapse of a Circuit of Primary EVs Induce Active Secondary EVs Among a Series of Antigen Presenting Cell (APC)-Connected T Cells
In a system of high antigen dose-induced immunological tolerance mediated by generated CD8
+ suppressive T cell-derived EVs (Bryniarski et al.; Nazimek et al.) targeted APC (Macs) are stimulated to produce secondary inhibitory EVs that act on the targeted effector CD4
+ Th1 cells (Nazimek et al.) (
Figure 2). Induced secondary EVs act at the immune synapse by interacting with the APC and targeted T cell surface membranes (Nazimek et al.). This is accomplished by APC-derived secondary suppressive EVs expressing surface antigen-peptide/major histocompatibility complex (MHC) complexes that specifically bind to the antigen-specific surface peptide-specific Ag/MHC T cell receptors (TCRs) of the affected Th1 effector cells (Nazimek et al.) (Figure). This example fulfils the central hypothesis put forth here that primarily administered EVs can induce recipient cells to produce secondary EVs that mediate the final detected biological event.
1.3. Role of miRNA-150 in the Activity of the Primary Stimulating Release of Suppressive Secondary Mac-EVs
This in vivo biological effect depends on miRNA-150 carried by primary EVs released by original CD8
+ suppressive T cells (Ts) (Bryniarski et al.; Ptak et al.; Nazimek et al.; Wąsik et al.; Bryniarski et al.). This EV-transferred miRNA-150 alters the targeted Mac-APCs to generate secondary suppressive EVs (Bryniarski et al.), which act at the immune synapse formed between the binding of the Mac-APC-surface antigen (Ag) peptide-MHC on the secondary EVs binding to specific surface TCR of the CD4
+ effector Th1 cells (Nazimek et al.). This inhibits their activities, including the production of pro-inflammatory cytokines such as INF-γ (interferon-gamma) (Tsuji et al.) (
Figure 2).
Firstly, inducing activity in Mac-APCs mediated by transferred primary Ts EVs depends on the transfer of the contained miRNA-150, as shown by three experimental protocols in
Figure 1, which specifically block this effect (Bryniarski et al.). Second, such secondary suppressive EVs do not develop when there is an attempt to form primary Ts EVs with genetically miRNA-150-negative CD8 T cells (Bryniarski et al.). Third, and most convincingly, the suppressive activity of primary EVs raised in miRNA-150 deficient animals was restored by specifically adding miRNA-150 (Bryniarski et al.). Taken together, these results confirmed that miRNA-150 transferred by primary Ts EVs induces targeted APCs to release secondary Mac EVs that suppress the effector T cells (
Figure 2).
1.4. General Characteristics of the Secondary APC-Produced Inhibitory EVs
Secondary APC-produced inhibitory EVs were also found in the supernatants of Mac-APCs treated with suppressive EVs from CD8
+ antigen-tolerised cells. Furthermore, this EV activity was pelleted by ultracentrifugation at 100,000 ×
g (Nazimek et al.). The assay system co-transferred potential regulatory Mac-APC EVs with effector CD4
+ indicator cells to mediate murine cutaneous delayed-type hypersensitivity (DTH). This was measured as the ear skin swelling elicited at 24–48 h after intravenous (IV) adoptive transfer of EV-treated CD4
+ DTH effector cells to naïve recipients (Nazimek et al.). Inhibition of the transferred DTH required co-culture for 24–48 h. This suggested that the generation of secondary suppressive EVs required internal cellular processes in the Mac-APCs (
Figure 2).
It is my opinion that electron microscopy showed that the pelleted Mac-APC-derived EVs resembled exosomes, but this was not established. Flow cytometry showed the surface expression of different tetraspanins (Nazimek et al.). These data further identified these secondary EVs as being different from the original CD8
+ T cell-derived primary EVs. These primary T cell-derived suppressive EVs expressed only surface tetraspanin CD9, whilst the secondary Mac-APC EVs displayed CD9 and CD81. Importantly, unlike the primary EVs, these secondary EVs also expressed I-A MHC Class II molecules expected of Mac-APC-derived EVs that usually participate in presentation of peptide antigens to the T cells (Nazimek et al.) (
Figure 2).
1.5. In Vitro Activity of Suppressive Secondary Mac APC EVs in a Human Clonal Assay System
Since miRNAs are conserved among species, the nucleotide sequence of mouse primary Ts EV-enclosed miRNA-150 (mmu-miRNA-150-5p), which induces Mac APC to release secondary suppressive Mac-APC-EVs, has the same nucleotide composition and sequence order as human hsa-miRNA-150-5p (according to miRbase). This allowed testing of the murine primary Ts EVs in an in vitro human immune synapse system that involves clonal Raji B cells as secondary-acting APC and human clonal Jurkat T cells as the corresponding T cells (Nazimek et al.).
Green fluorescent protein substitution for CD81 tetraspanin allowed the identification of Raji B cell APC-derived secondary EVs stimulated by primary murine Ts EV-enclosing miRNA-150. Employing these CD81
+ green-tagged Raji B cells as peptide/MHC-expressing APC showed that their derived green-tagged EVs bound to a specific TCR on the Jurkat T cell surface at the immune synapse (Nazimek et al.). Furthermore, green fluorescent material was transferred from Raji B cell APC to Jurkat T cells (Nazimek et al.). These activities only occur if the Raji B cell APCs contains miRNA-150 derived from prior incubation with primary Ts EVs (Nazimek et al.). It was concluded that the primary miRNA-150-positive Ts-derived EVs induced Raji B cell APCs to release secondary suppressive EVs that acted at the immune synapse, specifically with the surface-expressed TCRs of the Jurkat T cells to then enable their inhibition of T cell effector functions (
Figure 2).
1.6. Antibody Treatments Determine the Role of Secondary Mac-APC-EVs Surface Molecules in the Immune Synapse with the Corresponding T Cells
The experiments presented thus far focused on cell-to-cell interactions between APC-derived secondary EVs and T cells at the immune synapse. We then tested whether secondary Mac-APC-derived EVs had characteristics similar to those of the parental Mac-APCs.
Since specific antibody treatment of the secondary Mac-APC-EVs showed that binding to the TCRs of Jurkat T cells depended on the surface expression of CD9 and MHC class II (Nazimek et al.; Nazimek et al.), we studied whether relevant specific antibody-treated secondary Mac-APC-EVs affected functional interactions with the targeted Th1 DTH effector cells. A murine monoclonal TCR Th1 anti-ovalbumin (OVA) system, OT-II, was used (Nazimek et al.). The experimental groups used EVs from OVA-pulsed OT-II-derived Macs. Experimentally, these were incubated with anti-OVA-323 peptide IgG antibodies directed against the crucial OVA peptide complexed in MHC, which is recognised by the TCRs of OT-II Th1 cells.
After ultracentrifugation to wash away free antibodies, the antibody-treated secondary Mac-OVA-EVs were used for in vitro treatment of DTH effector CD4+ anti-OVA Th1 effector cells from OT-II OVA immune mice. T cells treated with Mac-OVA-EVs incubated with anti-OVA-323 peptide IgG antibody and untreated control EVs were adoptively transferred to OVA antigen-ear-challenged naïve recipients to elicit cutaneous DTH. Remarkably, incubation of OT-II murine OVA-secondary Mac-EVs with anti-OVA-323 IgG antibodies caused a five-fold augmentation in their inhibition of the in vivo ear swelling activity of the DTH effector T cells, compared with DTH effector T cells treated with OVA-Mac-EVs from OT-II mouse Macs not treated with these peptide-specific monoclonal antibodies (Nazimek et al.).
In a second protocol, in contrast to the in vivo results of modifying in vitro adoptive transfers of DTH effector activity as described above, a separate wholly in vivo approach was employed. This involved systemic IV injection of OT-II mouse OVA-secondary Mac-EVs, either alone or following preincubation with anti-OVA-323 IgG antibodies, into actively OVA-immunised mice at the 24 h peak of their elicited DTH responses. Remarkably, this also resulted in a five-fold augmentation in the suppression of active in vivo DTH, which was subsequently manifested at 48, 96 and 120 h. after intradermal skin challenge with OVA antigen in the ear (Nazimek et al.). It was concluded that OVA-peptide/MHC complexes were present on the secondary Mac-derived EV, such that when bound by appropriate anti-peptide antibodies augmented their suppression of OT-II DTH effector T cells in vivo (
Figure 2).
1.7. The Mechanism of Anti-Antigen Peptide IgG Antibody Augmentation of OVA-Secondary Mac-EVs Inhibition Involves Their Aggregation into Polymeric Activators of Targeted T Cell TCRs
The surprising findings detailed above led to experiments to uncover the mechanism underlying the enhancing effect of anti-OVA-323 IgG antibodies on the suppressive activity of OT-II mouse OVA-Mac-EVs from OVA-exposed MACs incubated with CD4
+ anti-OVA monoclonal Th1 effector T cells (Nazimek et al.) (
Figure 2).
Importantly, anti-OVA peptide antibody-induced augmented suppression resulted from the aggregation of secondary OVA-Mac-EVs (Nazimek et al.). This likely leads to their polymeric binding to the surface TCRs at the immune synapse with targeted DTH mediating anti-OVA Th1 cells. Taken together, these in vitro and in vivo studies showed that aggregation of primary miRNA-150 containing Ts EVs induced secondary Mac-APC EVs that influenced interactions with TCR on the targeted Th1 cells at the immune synapse. In summary, the augmented suppression of elicited cutaneous DTH was generated by the monoclonal antibody anti-peptide Ag-peptide binding to secondary Mac-APC EVs acting as triggers of the TCR at the immune synapse (Figure).
2. Other Circuits Involving Primary Administered MSC EVs That Might Induce Secondary EVs Active in the Recipients
In many other instances, systemic administration of MSCs in clinical disease models and in actual diseases can result in the prolonged reversal of abnormalities. These beneficial effects can be nutritive, trophic, reparative, anti-inflammatory and anti-immunological, as well as able to heal abnormalities in the microvasculature (Askenase; Pittenger et al.). These positive effects of administered MSCs are due to their release of EVs (Nazimek et al.).
Accordingly, the therapeutic effects of MSCs can be replaced by the systemic transfer of their secreted EVs in a variety of experimental and clinical diseases and injuries as well as in many inflammatory, fibrotic and degenerative disease models and clinical entities (Askenase; Pittenger et al.; Manzoor et al.; Varderidou-Minasian and Lorenowicz; Racchetti and Meldolesi). Prominent among these are myocardial and vascular diseases (Fu et al.), ischemic injury of the kidney (Li et al.) and liver (Wu et al.) and several autoimmune diseases (Wang et al.). Further, the therapeutic effects of MSCs in severe respiratory failure have evolved from MSCs to EVs (Abraham and Krasnodembskaya). Similarly, MSC therapy for central nervous system pathologies has been replaced by EVs released in vivo (Askenase). EVs tend to replace MSCs in many instances (Fu et al.; Li et al.; Wu et al.; Wang et al.; Abraham and Krasnodembskaya; Askenase); however, they have not yet fully replaced them. Overall, this is a rapidly evolving field that still needs full toxicity testing and comprehensive quantitative studies to determine whether MSC-derived EVs are non-toxic and are truly superior to their parental MSCs when employed as clinical treatments for human diseases.
These MSC EVs are easy to enrich and purify, are much less dangerous than MSCs, as there is no possibility of conversion to a neoplastic phenotype (Miura et al.; Rosland et al.; Li et al.; Hill et al.), can easily be stored as freeze-dried powder (Sivanantham and Jin), are able to escape the lung entrapment that MSCs suffer from (Nakazaki et al.), and are naturally able to cross biological barriers, such as the nervous system blood-brain barrier (Hajnik et al.; Rak et al.; Tai et al.). The fact that MSC EVs were clinically active in such a wide variety of instances, seeming to have specificity for multiple specific instances, led to the hypothesis that there might be induction of secondary EVs by MSC EV targeted local cells that are appropriate to each specific instance.
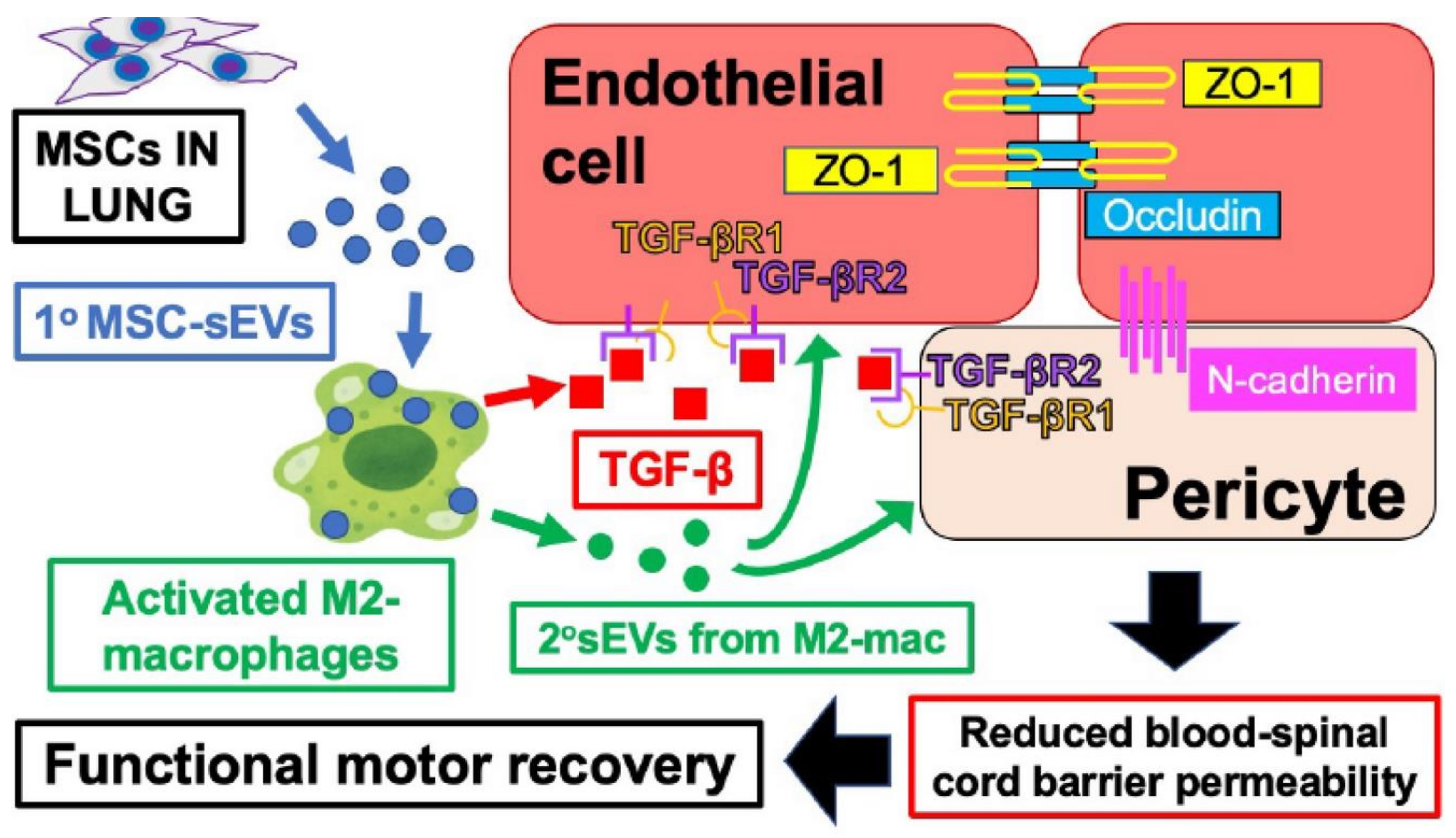
Primary and Induced Secondary EVs in MSC Healing of SCI
We performed these experiments using MSCs administered to a rat model of spinal cord injury (SCI). It has been shown that the healing effects are due to EVs produced in vivo by MSCs aggregated in the lung or can be achieved by IV injection alone of MSC-derived EVs that initially and specifically promote M2 Mac polarisation at the site of SCI (Nakazaki et al.) (Figure). There is increased expression of M2 markers on these M2 Macs and TGF-β (transforming growth factor-beta) production (Nakazaki et al.).
It turns out that the administered MSC-derived EVs were acting primarily. The primary EV-mediated process induces altered M2 Macs at the SCI site to produce secondary EVs, which then positively affect gene expression in the surrounding microendothelial cells, improving vascular permeability (Nakazaki et al.). Thus, secreted Mac2-derived secondary EVs go on to target gene expression in the local capillary endothelium to mediate responsiveness to the released TGF-β via upregulation by expression of TGF-β receptors. In contrast and crucially, in these SCI sites containing localised M2 Mac recipients of internally labelled primary MSC-derived EVs, the affected microvascular cell recipients showed no labelling of the initial MCC EVs (Nakazaki et al.) (
Figure 3).
Importantly, these locally released secondary M2-Mac EVs also induce a reduction in disturbed vascular permeability, restoring normal blood–brain function (Nakazaki et al.). Thus, following the IV injection of blue dye, there was a significant reduction in the extravasation of the dye into the affected area of the SCI. Further, this reduced local vascular permeability is accompanied by the expression of particular vascular proteins that promote an increase in tight adherent junctions in the affected capillary endothelial and pericyte cells. Specifically, they express mRNAs encoding four vascular adherent junction proteins: endothelial occludin, claudin-5, ZO-1 and pericyte N-cadherin (Nakazaki et al.) (
Figure 3). Thus, in summary, these data show that MSC-EVs acting in a primary manner specifically targeting local M2 Macs particularly at the site of SCI to produce secondary EVs targeting microvascular cells and thus are responsible for healing of the SCI (Figure). This is another example, in a completely different system, involving the common use of MSC EVs in disease models, which also fulfils our central hypothesis that administered primary EVs induce recipient cells (again Macs) to produce secondary EVs that mediate the final biological event.
3. A Model System Employing EV-Like Artificial LNPs That Imitates Immunisations Against COVID-19 Viral Infection Is Analogous to the Action of Primary EVs
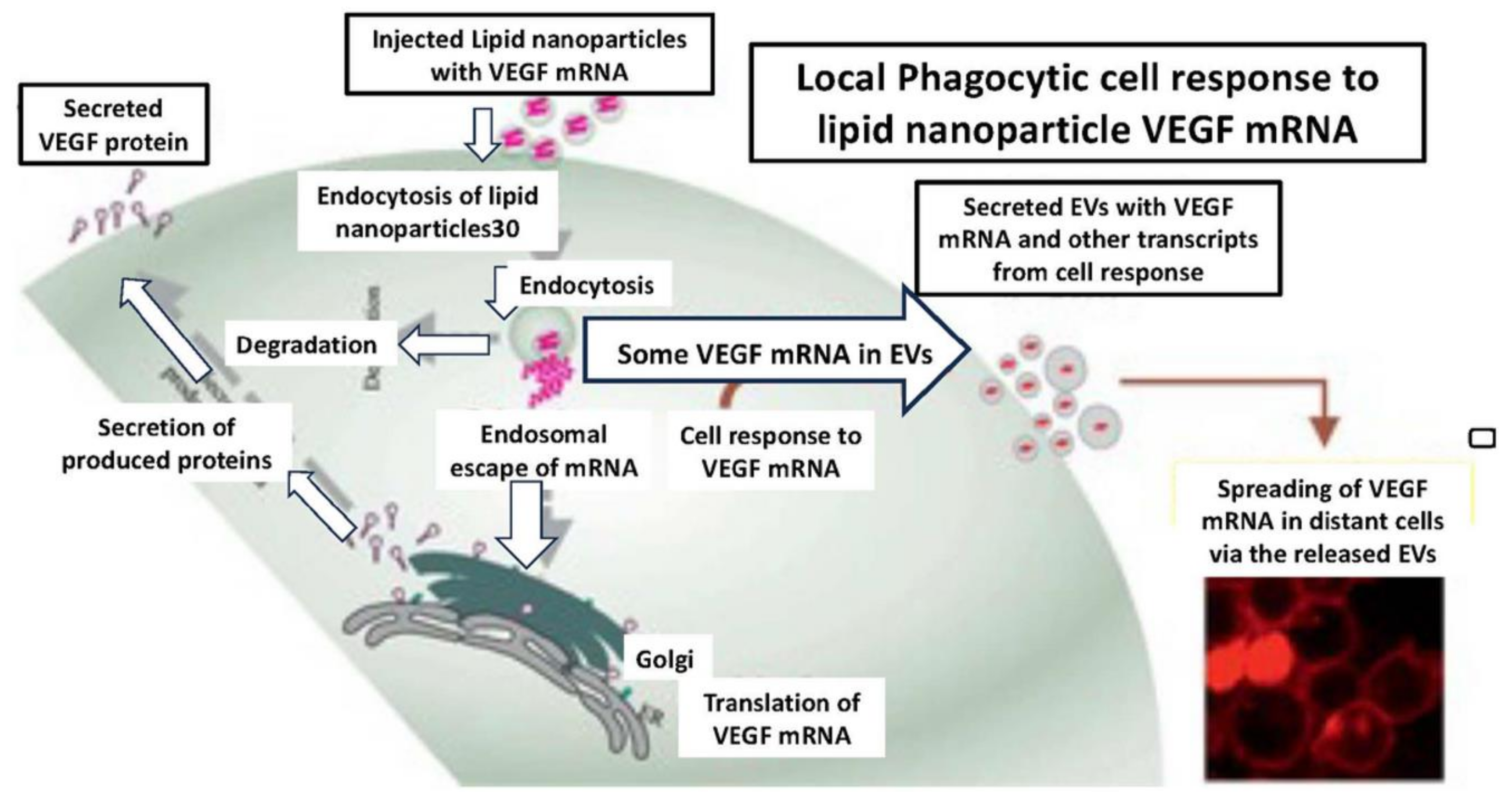
There are many systems in which it is worthwhile exploring the possibility that primary EVs induce as yet unstudied secondary EVs. There is a common system analogous to that of primary-like EV generation of secondary EVs in the extensive use of mRNA LNPs for vaccine-induced immune protection against COVID-19. Such LNP-mediated processes have been employed in many instances, beginning with the induction of potent immune responses to SARS-CoV-2 viral spikes and nucleocapsid proteins (Hajnik et al.; Rak et al.; Tai et al.). Similar to antigen-specific suppressive EVs and healing EVs from MSC, subcutaneous immunisation with such LNPs also acts through uptake by recipient Macs (Wang et al.). (Yang et al.) and can induce similar secondary EVs (Maugeri et al.). Here, for the sake of the principal arguments, it was considered that such LNP are analogous to primary EVs, although, of course, most of their composition is completely different.
As a model system, an extensive study was conducted on the immune consequences of vaccination with vascular endothelial growth factor (VEGF)-encoding mRNA LNPs (Nawaz et al.) (
Figure 4). The use of VEGF mRNA instead of COVID-19 spike protein mRNA enabled the molecular tracking of results. This showed uptake of these LNPs by local phagocytic cells, likely tissue Macs, which were then activated for the generation and secretion of several subsets of secondary EVs, upon which the immunisation may depend, at least in part (Nawaz et al.). Thus, this immunisation induced local Macs to produce VEGF-encoding mRNA EVs with subsequent wide tissue distribution resulting in strong stimulation of B and T cell immune responses (Nawaz et al.) (
Figure 4).
Therefore, these VEGF mRNA LNPs, analogous to primary EVs, were locally transformed by phagocytic Macs at the site of immunisation to produce secondary-like EVs. These act as functional extensions of this protective vaccine response, enabling systemic distribution and resulting in immunisation that could even possibly involve additional tertiary EV transfers by other APCs and affected B and T cells. Indeed, these LNPs acting like primary EVs could successfully deliver their VEGF-A mRNA, translatable into significant copies of VEGF protein in distant immunologic, epithelial, endothelial and cardiac progenitor cells (Nawaz et al.) as functional extensions of this immune response (
Figure 4).
Summary
Our central hypothesis that primary EVs induce targeted cells to generate and then produce different secondary EVs to mediate the final biological event is confirmed by the examples presented (
Figure 1). We compared different but similar instances. In the first, primary EVs from high antigen dose immune tolerance induced CD8
+ suppressor T cells activated recipient Mac APCs, generating production of secondary inhibitory EVs (
Figure 2). In the second presented system, a model of SCI, a different but similar process occurs in which administered primary MSC EVs target local tissue M2 Macs in the spinal cord to release secondary EVs that subsequently affect the local endothelium to mediate healing (
Figure 3).
Particularly regarding the transfer of MSCs and their derived EVs, these proposed concepts can explain what appears to be the lack of tissue histocompatibility matching in MSC recipients. Moreover, they can also explain the seeming “disease specificity” of these treatments, resulting in the healing of so many diverse injuries and a wide variety of diseases. Additionally, the worldwide use of COVID-19 mRNA vaccination with EV-like LNPs supports these postulated concepts (
Figure 4). We propose that there are many other instances in which primary EVs induce secondary effects via inducing secondary EVs (Figure). Indeed, as predicted here, a healing model of central nervous system stroke demonstrated the astrocytic release of healing secondary EVs induced by primary EVs from miR-133b-overexpressing MSCs (Xin et al.).
5. Discussion
5.1. Introduction
During immune tolerance induced by suppressor T cells, the generated primary EVs act on companion-targeted APC Macs, inducing them to release biologically active secondary EVs (Figure). Surprisingly, a different process underlying the repair of SCI by MSCs replaced by their secreted secondary EVs was similar (Figure). These instances involve primary EVs targeting local Macs to generate secondary EVs that mediate biological alterations in the affected local final targeted cells (
Figure 1,
Figure 2 and
Figure 3). We do not claim that all EV effector activities involve processing from primary to secondary-acting EVs, nor that local Macs or TGF-β are routinely involved, but only that some do. In terms of the central ideas that are being proposed here (
Figure 3), suppressor T cell EVs in high-dose antigen-generated immune tolerance and the different system of MSC-primary EVs used for treatment of SCI are surprisingly similar.
5.2. Rarity of Demonstrations of Primary EVs Leading to Generation of Secondary EVs
The rarity of demonstrations described thus far of primary EVs leading to the generation of secondary EVs can be explained by practical factors. Most studies on the biological effects of primary EVs require a large number of targeted secondary cells to obtain sufficient amounts of potentially generated secondary EVs. Such primary EV-targeted recipient secondary cell products are usually needed in small volumes to detect activities using currently available assays that are relatively insensitive. Thus, there is little opportunity to culture great numbers of the cells targeted by the primary EVs to obtain enough supernatant material to test for the possible induction of secondary EVs.
5.3. Primary EVs Produced by CD8+ Suppressor T Cells Target Their Associated Mac APCs
In studies involving primary EVs produced by CD8
+ suppressor T cells of animals undergoing immune tolerance by injections of large amounts of antigen, early studies found that their target was Mac APCs accompanying the seemingly final affected T cells (Figure). Considering the conditions for testing the postulated concepts, large amounts of primary EV-affected targeted Mac-APCs were cultured. This enabled testing for the production of secondary EVs that mediated the final targeting of the affected Ag-peptide/MHC-specific TCR of Th1 effector CD4
+ DTH T cells at the immune synapse (
Figure 2).
5.4. Similarity of Primary EV-Induced Secondary EVs in MSC Healing of SCI
Published experiments on primary MSC therapy administered to rats with SCI showed that their produced healing EVs affected target-selected secondary-acting M2 Macs at the site of injury (
Figure 3). These SCI M2 Macs produce secondary EVs that affect gene expression in the surrounding microvasculature that contributes to neurovascular healing. Vascular permeability is suppressed by the expression of induced mRNAs and the consequent production of essential vascular junction proteins. Therefore, comparing the targeting of primary effector EVs from CD8
+ suppressor T cells in high antigen dose-induced immune tolerance and the other seemingly quite different system of MSC-derived primary EVs in the treatment of SCI shows that these diverse systems are surprisingly similar in terms of the central ideas that are being proposed here.
5.5. Lack of Tissue Histocompatability Matching in Successful Treatment with MSCs or Their EVs
Remarkably, MSCs and their secreted EVs do not require histocompatible tissue matching, in contrast to many organ and cell treatments. Ordinarily, unmatched donor sources activate the recipient’s immune system to reject the transferred cells. Initial MSC therapies, and eventually with their secreted primary EVs, involved harvest and transfer from autologous sources (Perico et al.), but eventually it was noted that the donors could be allogeneic of the same species (Klyushnenkova et al.; Ryan et al.; Qi et al.) or even EVs from xenogeneic donors could suffice (Chen et al.; Liu et al.; Zhang et al.; Gregorius et al.).
We concluded that, in this case, the MSC and their EV did not require tissue matching. This phenomenon could arise from several properties of MSCs, such as low immunogenicity, stimulation of immune tolerance, induction of immune modulation, or active specific immune suppression. However, the lack of tissue matching for successful treatment with MSCs or their EVs remains an unresolved mystery. Now, the data presented here offer a solution that recipient secondary EVs produced by local cells of the greater Mac family can provide strictly tissue-matched EVs.
5.6. MSC-Derived EVs Stimulate Healing Subpopulations of the Mac Family That Then Release “Disease-Specific” Secondary EVs
Thus, we postulated that some MSC-derived primary EVs induce local members of the Mac family among lesional tissue cells to generate an appropriate recipient cell response through the production of selected healing secondary EVs. Additionally, we postulate that, in some instances, generation of primary to secondary or even tertiary, and even further EV pathways of connectivity could be involved. This is analogous to neuromediator vesicle transfers of single neurotransmitters at synapses between axons and EV connections in the nervous system, which have not been previously reported (Rajendran et al.; Schnatz et al.; Gassama and Favereaux).
Many Mac subpopulations may be involved; however, there seems to be a specific pattern. There are numerous instances of primary EVs preferentially targeting local Macs, causing their differentiation into M2-type Macs rather than the M1 proinflammatory Macs (Wang et al.; Rana et al.). As noted here, there is subsequent release of pro-healing M2 Mac-derived secondary EVs as the principal effectors of the actions of primary EVs derived from MSC-induced tissue processes that, therefore, seem to mediate “disease specific” effects. Thus, primary to secondary, often tissue processes of local lesional M2 Macs mediated by treatments with MSC-derived EVs can account for both the unmatched MHC cell interactions and the seeming “disease specific” effects of MSC-derived EV treatments in a variety of injury, pathology and disease-specific instances.
5.7. Other Examples of the Induction of Secondary Active EVs by Recipient Cells Stimulated with Primary Suppressive EVs
We postulate that there may be many systems in which it would be worthwhile to explore the possibility that primary EVs induce unstudied secondary EVs. We also describe how current LNP mRNA vaccines are analogous to the primary EV generation of secondary EVs. This is due to the immune responses induced by the mRNA-containing LNP vaccines employed to protect against COVID-19. This idea comes from an extensive study of the immune consequences of subcutaneous vaccination with VEGF mRNA-encoding LNPs. This enabled molecular tracking, and a wide tissue distribution was observed in the tested animals, stimulating immune B and T cells (Wang et al.) (Figure). This result seemed to be due to the transformation into secondary EVs, which then acted as functional extensions that could even have been involved in tertiary EV transfers to other immune cells.
Several systems are currently being explored for mRNA vaccination. This suggests that LNPs acting through recipient Macs induce a similar induction of secondary EVs (Al Fayez et al.; Matarazzo and Bettencourt). Thus, EV-like LNPs, which affect the local Mac family consisting of activated monocytes, Macs and dendritic cells, may lead to the secondary production of mRNA-containing EVs with subsequent synthesis of the encoded protein in locally affected Macs to drive the adaptive immune response (Ndeupen et al.; Breda et al.; Zhu et al.) including EVs as bioinspired nanocarriers for RNA delivery (Amiri et al.).
6. Overall Summary
Induction of Secondary Active EVs by Recipient Cells Stimulated with Primary EVs
Treatment of a great variety of disease models with MSCs and their released EVs can be achieved in many diverse instances, including those from allogenic and even xenogeneic sources. This apparent lack of histocompatibility matching is addressed here, offering a new hypothesis based on published data. The action of primary EVs across tissue histocompatibility barriers is postulated to be triggered by host secondary cells that produce tissue-matched EVs.
Successful treatment with MSC or their derived EVs can be achieved for various injuries and diseases that, according to current concepts, seem impossible. This new hypothesis explains how a multimodal mechanism can be readily accomplished. It is proposed that these diverse treatments do not act directly through the transferred MSCs or their derived EVs but indirectly through the transferred primary EVs activating appropriate subsets of recipient cells participating in each pathological condition by producing suitable secondary EVs. Such cells are thought to be present in the local host cellular response, varying according to involved specific problems, and are often, but not exclusively, among Mac subpopulations. This work should be of interest to persons in all biological and clinical sciences, certainly pertaining to immunology, as EVs are made by virtually all cells of all species (Breda et al.) and likely participate to some extent in all cellular interactions.
Author Contributions
Dr. Philip W. Askenase alone contributed to this manuscript in its entirety.
Funding
Dr. Philip W. Askenase was supported by NIH grants: AI-07074, AI-076366 and AI-59801.
Acknowledgments
The author is indebted to Katarzyna Nazimek, Krzysztof Bryniarski and Jefferey Kocsis and their collaborators, who performed most of the experiments described, and to Jordan Pober, Fredrick Naftolin and Ivana Kawakova, who provided important reviews and advice concerning the manuscript. Philip W. Askenase was supported by NIH grants: AI-07074, AI-076366 and AI-59801.
>Disclosure:
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organisations or those of the publisher, editors and reviewers. Any product that may be evaluated in this article or claims to be made by the manufacturer is not guaranteed or endorsed by the publisher.
Ethics Statement
All animal experiments were reviewed and approved by the Yale Medical Animal Care Committee.
Conflicts of Interest
The author declares no conflicts of interest. The original data contributions presented in this study are included in the references; further inquiries can be directed to the corresponding authors of these publications.
Abbreviations
| APC |
antigen presenting cell |
| COVID-19 |
coronavirus Sars-CoV-2-induced clinical processes |
| DTH |
delayed-type hypersensitivity |
| EV |
extracellular vesicle |
| INF-γ |
interferon-gamma |
| IV |
intravenous |
| miRNA |
micro (small) RNA |
| mRNA |
messenger RNA |
| Mac |
macrophage |
| MSCs |
mesenchymal stromal cells |
| MHC |
major histocompatibility complex |
| OVA |
ovalbumin |
| TCR |
T cell receptors |
| TGF-β |
transforming growth factor-beta |
| Ts |
T cell suppressive |
| VEGF |
vascular endothelial growth factor |
References
- Abraham, A., and A. Krasnodembskaya. 2020. “Mesenchymal Stem Cell-Derived Extracellular Vesicles for the Treatment of Acute Respiratory Distress Syndrome.” Clinical and Translational Medicine 9, no. 1: 28–38.
- Al Fayez, N., M. S. Nassar, A. A. Alshehri, et al. 2023. “Recent Advancement in mRNA Vaccine Development and Applications.” Pharmaceutics 15, no. 7: 1972.
- Amiri, A., R. Bagherifar, E. Ansari Dezfouli, S. H. Kiaie, R. Jafari, and R. Ramezani. 2022. “Exosomes as Bio-Inspired Nanocarriers for RNA Delivery: Preparation and Applications.” Journal of Translational Medicine 20, no. 1: 125.
- Askenase, P. W. 2020. “COVID-19 Therapy With Mesenchymal Stromal Cells (MSC) and Convalescent Plasma Must Consider Exosome Involvement: Do the Exosomes in Convalescent Plasma Antagonize the Weak Immune Antibodies?” Journal of Extracellular Vesicles 10, no. 1: e12004.
- Askenase, P. W. 2022. “Recommendation: Treatment of Clinical Long Covid Encephalopathies With Nasally-Administered Mesenchymal Stromal Cell Extracellular Vesicle Exosomes.” Frontiers in Nanotechnology 4: 987117.
- Breda, L., T. E. Papp, M. P. Triebwasser, et al. 2023. “In Vivo Hematopoietic Stem Cell Modification by mRNA Delivery.” Science 381, no. 6656: 436–443.
- Bryniarski, K., K. Nazimek, W. Ptak, T. Groot Kormelink, and P. W. Askenase. 2020. “Orally Administered T and B Cell Antigen-Specific Suppressor Exosomes Deliver miRNA-150 to Inhibit DTH via Their Surface Antibody Light Chains Binding Antigen Peptides in MHC on APC Targeted Cells.” International Journal of Molecular Sciences 21, no. 15: 5540.
- Bryniarski, K., W. Ptak, A. Jayakumar, K. Püllmann, et al. 2013. “Antigen-Specific, Antibody-Coated, Exosome-Like Nanovesicles Deliver Suppressor T-Cell microRNA-150 to Effector T Cells to Inhibit Contact Sensitivity.” Journal of Allergy and Clinical Immunology 132, no. 1: 170–181.
- Chen, K. H., C. H. Chen, C. G. Wallace, et al. 2016. “Intravenous Administration of Xenogenic Adipose-Derived Mesenchymal Stem Cells (ADMSC) and ADMSC-Derived Exosomes Markedly Reduced Brain Infarct Volume and Preserved Neurological Function in Rat After Acute Ischemic Stroke.” Oncotarget 7, no. 46: 74537–74556.
- Fu, S., Y. Zhang, Y. Li, L. Luo, Y. Zhao, and Y. Yao. 2020. “Extracellular Vesicles in Cardiovascular Diseases.” Cell Death Discovery 6: 68.
- Gassama, Y., and A. Favereaux. 2020. “Emerging Roles of Extracellular Vesicles in the central Nervous System: Physiology, Pathology, and Therapeutic Perspectives.” Frontiers in Cellular Neuroscience 15: 626043.
- Gregorius, J., C. Wang, O. Stambouli, et al. 2021. “Small Extracellular Vesicles Obtained From Hypoxic Mesenchymal Stromal Cells Have Unique Characteristics That Promote Cerebral Angiogenesis, Brain Remodeling and Neurological Recovery After Focal Cerebral Ischemia in Mice.” Basic Research in Cardiology 116, no. 1: 40.
- Hajnik, R. L., J. A. Plante, Y. Liang, et al. 2022. “Dual Spike and Nucleocapsid mRNA Vaccination Confer Protection Against SARS-CoV-2 Omicron and Delta Variants in Preclinical Models.” Science Translational Medicine 14, no. 662: eabq1945.
- Hill, B. S., A. Pelagalli, N. Passaro, and A. Zannetti. 2017. “Tumor-Educated Mesenchymal Stem Cells Promote Pro-Metastatic Phenotype.” Oncotarget 8, no. 42: 73296–73311.
- Klyushnenkova, E., J. D. Mosca, V. Zernetkina, et al. 2005. “T Cell Responses to Allogeneic Human Mesenchymal Stem Cells: Immunogenicity, Tolerance, and Suppression.” Journal of Biomedical Science 12, no. 1: 47–57.
- Li, Q., H. Hisha, T. Takaki, et al. 2010. “Transformation Potential of Bone Marrow Stromal Cells Into Undifferentiated High-Grade Pleomorphic Sarcoma.” Journal of Cancer Research and Clinical Oncology 136: 829–838.
- Li, X. Q., J. F. Liu, H. Liu, and Y. Meng. 2022. “Extracellular Vesicles for Ischemia/Reperfusion Injury-Induced Acute Kidney Injury: A Systematic Review and Meta-analysis of Data From Animal Models.” Systematic Reviews 11, no. 1: 197.
- Liu, H., Z. Liang, F. Wang, et al. 2019. “Exosomes From Mesenchymal Stromal Cells Reduce Murine Colonic Inflammation via a Macrophage-Dependent Mechanism.” JCI Insight 4, no. 24: e131273.
- Manzoor, T., A. Saleem, N. Farooq, et al. 2023. “Extracellular Vesicles Derived From Mesenchymal Stem Cells—A Novel Therapeutic Tool in Infectious Diseases.” Inflammation and Regeneration 43, no. 1: 17.
- Matarazzo, L., and P. J. G. Bettencourt. 2023. “mRNA Vaccines: A New Opportunity for Malaria, Tuberculosis and HIV.” Frontiers in Immunology 14: 1172691.
- Maugeri, M., M. Nawaz, A. Papadimitriou, et al. 2019. “Linkage Between Endosomal Escape of LNP-mRNA and Loading Into EVs for Transport to Other Cells.” Nature Communications 10: 4333.
- Miura, M., Y. Miura, H. M. Padilla-Nash, et al. 2006. “Accumulated Chromosomal Instability in Murine Bone Marrow Mesenchymal Stem Cells Leads to Malignant Transformation.” Stem Cells 24, no. 4: 1095–1103.
- Nakazaki, M., T. Morita, K. L. Lankford, P. W. Askenase, and J. D. Kocsis. 2021. “Extracellular Vesicles Released by Systemically Delivered MSCs Target M2 Macrophages That Upregulate TGF-β Linked to Microvascular Stabilization and Functional Recovery in Spinal Cord Injury.” Journal of Extracellular Vesicles 10, no. 11: e12137.
- Nawaz, M., S. Heydarkhan-Hagvall, B. Tangruksa, et al. 2023. “Lipid Nanoparticles Deliver the Therapeutic VEGFA mRNA In Vitro and In Vivo and Transform Extracellular Vesicles for Their Functional Extensions.” Advanced Science (Weinh) 10, no. 12: e2206187.
- Nazimek, K., E. Bustos-Morán, N. Blas-Rus, et al. 2021. “Antibodies Enhance miRNA-150-Dependent Suppressive Activity of MHC Class II-Positive Extracellular Vesicles in Mouse Delayed-Type Hypersensitivity.” Pharmaceuticals (Basel) 14, no. 8: 734.
- Nazimek, K., E. Bustos-Morán, N. Blas-Rus, et al. 2021. “Antibodies Enhance miRNA-150-Dependent Suppressive Activity of MHC Class II-Positive Extracellular Vesicles in Mouse Delayed-Type Hypersensitivity.” Pharmaceuticals (Basel) 14, no. 8: 734.
- Nazimek, K., M. Ptak, W. Ptak, P. W. Askenase, and K. Bryniarski. 2016. “Macrophages Affected With Exosome-Carried miRNA-150 Release Secondary Vesicles to Inhibit the Proliferation of Effector T Lymphocytes of Mouse Contact and Delayed-Type Hypersensitivity.” European Journal of Immunology 46, no. Suppl. 1: 854–855.
- Nazimek, K., W. Ptak, B. Nowak, M. Ptak, P. W. Askenase, and K. Bryniarski. 2015. “Macrophages Play an Essential Role in Antigen-Specific Immune Suppression Mediated by CD8+ T Cell-Derived Exosomes.” Immunology 146, no. 1: 23–32.
- Ndeupen, S., Z. Qin, S. Jacobsen, A. Bouteau, H. Estanbouli, and B. Z. Igyártó. 2021. “The mRNA-LNP Platform’s Lipid Nanoparticle Component Used in Preclinical Vaccine Studies Is Highly Inflammatory.” Iscience 24: 103479.
- Perico, N., F. Casiraghi, M. Introna, et al. 2011. “Autologous Mesenchymal Stromal Cells and Kidney Transplantation: A Pilot Study of Safety and Clinical Feasibility.” Clinical Journal of the American Society of Nephrology 6, no. 2: 412–422.
- Pittenger, M. F., D. E. Discher, B. M. Péault, D. G. Phinney, J. M. Hare, and A. I. Caplan. 2019. “Mesenchymal Stem Cell Perspective: Cell Biology to Clinical Progress.” NPJ Regenerative Medicine 4: 22.
- Ptak, W., K. Nazimek, P. W. Askenase, and K. Bryniarski. 2015. “From a Mysterious Supernatant Entity to miRNA-150 in Antigen-Specific Exosomes: History of Hapten-Specific T Suppressor Factor.” Archivum Immunolgiae Et Therapiae Experimentalis (Warsz) 63, no. 5: 345–356.
- Qi, X., J. Zhang, H. Yuan, et al. 2016. “Exosomes Secreted by Human-Induced Pluripotent Stem Cell-Derived Mesenchymal Stem Cells Repair Critical-Sized Bone Defects Through Enhanced Angiogenesis and Osteogenesis in Osteoporotic Rats.” International Journal of Biological Sciences 12, no. 7: 836–849.
- Racchetti, G., and J. Meldolesi. 2021. “Extracellular Vesicles of Mesenchymal Stem Cells: Therapeutic Properties Discovered With Extraordinary Success.” Biomedicines 9, no. 6: 667.
- Rajendran, L., J. Bali, M. M. Barr, et al. 2014. “Emerging Roles of Extracellular Vesicles in the Nervous System.” Journal of Neuroscience 34, no. 46: 15482–15489.
- Rak, A., I. Isakova-Sivak, and L. Rudenko. 2023. “Overview of Nucleocapsid-Targeting Vaccines Against COVID-19.” Vaccines (Basel) 11, no. 12: 1810.
- Rana, N., S. Suliman, N. Al-Sharabi, and K. Mustafa. 2022. “Extracellular Vesicles Derived From Primed Mesenchymal Stromal Cells Loaded on Biphasic Calcium Phosphate Biomaterial Exhibit Enhanced Macrophage Polarization.” Cells 11, no. 3: 470.
- Rosland, G. V., A. Svendsen, A. Torsvik, et al. 2009. “Long-term Cultures of Bone Marrow-Derived Human Mesenchymal Stem Cells Frequently Undergo Spontaneous Malignant Transformation.” Cancer Research 69, no. 13: 5331–5339.
- Ryan, J. M., F. P. Barry, J. M. Murphy, and B. P. Mahon. 2005. “Mesenchymal Stem Cells Avoid Allogeneic Rejection.” Journal of Inflammation (Lond) 2: 8.
- Schnatz, A., C. Müller, A. Brahmer, and E. M. Krämer-Albers. 2021. “Extracellular Vesicles in Neural Cell Interaction and CNS Homeostasis.” FASEB BioAdvances 3, no. 8: 577–592.
- Sivanantham, A., and Y. Jin. 2022. “Impact of Storage Conditions on EV Integrity/Surface Markers and Cargos.” Life (Basel) 12, no. 5: 697.
- Tai, W., S. Feng, B. Chai, et al. 2023. “An mRNA-Based T-Cell-Inducing Antigen Strengthens COVID-19 Vaccine Against SARS-CoV-2 Variants.” Nature Communications 14: 2962.
- Tsuji, R. F., M. Szczepanik, I. Kawikova, et al. 2002. “B Cell-Dependent T Cell Responses: IgM Antibodies Are Required to Elicit Contact Sensitivity.” Journal of Experimental Medicine 196, no. 10: 1277–1290.
- Varderidou-Minasian, S., and M. J. Lorenowicz. 2020. “Mesenchymal Stromal/Stem Cell-Derived Extracellular Vesicles in Tissue Repair: Challenges and Opportunities.” Theranostics 10, no. 13: 5979–5997.
- Wang, J., Y. Ding, K. Chong, et al. 2024. “Recent Advances in Lipid Nanoparticles and Their Safety Concerns for mRNA Delivery.” Vaccines (Basel) 12, no. 10: 1148.
- Wang, J., J. Xia, R. Huang, et al. 2020. “Mesenchymal Stem Cell-Derived Extracellular Vesicles Alter Disease Outcomes via Endorsement of Macrophage Polarization.” Stem Cell Research & Therapy 11, no. 1: 424.
- Wang, J. H., X. L. Liu, J. M. Sun, J. H. Yang, D. H. Xu, and S. S. Yan. 2020. “Role of Mesenchymal Stem Cell Derived Extracellular Vesicles in Autoimmunity: A Systematic Review.” World Journal of Stem Cells 12, no. 8: 879–896.
- Wąsik, M., K. Nazimek, B. Nowak, P. W. Askenase, and K. Bryniarski. 2019. “Delayed-Type Hypersensitivity Underlying Casein Allergy Is Suppressed by Extracellular Vesicles Carrying miRNA-150.” Nutrients 11, no. 4: 907.
- Wu, R., X. Fan, Y. Wang, et al. 2022. “Mesenchymal Stem Cell Derived Extracellular Vesicles in Liver Immunity and Therapy.” Frontiers in Immunology 13: 833878.
- Xin, H., F. Wang, Y. Li, et al. 2017. “Secondary Release of Exosomes from Astrocytes Contributes to the Increase in Neural Plasticity and Improvement of Functional Recovery after Stroke in Rats Treated with Exosomes Harvested From MicroRNA 133b-Overexpressing Multipotent Mesenchymal Stromal Cells.” Cell Transplantation 26, no. 2: 243–257.
- Yang, L., L. Gong, P. Wang, et al. 2022. “Recent Advances in Lipid Nanoparticles for Delivery of mRNA.” Pharmaceutics 14, no. 12: 2682.
- Zhang, S., K. Y. W. Teo, S. J. Chuah, R. C. Lai, S. K. Lim, and W. S. Toh. 2019. “MSC Exosomes Alleviate Temporomandibular Joint Osteoarthritis by Attenuating Inflammation and Restoring Matrix Homeostasis.” Biomaterials 200: 35–47.
- Zhu, M., X. Tian, X. Song, et al. 2012. “Nanoparticle-Induced Exosomes Target Antigen-Presenting Cells to Initiate Th1-Type Immune Activation.” Small 24: 2841–2848.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).