1. Introduction
Plastic waste generated from the use of tanks for the storage of fuels, lubricants, additives, and other petroleum-derived products represents an increasingly pressing yet underexplored environmental challenge. While specific data on such waste streams are often not disaggregated, it is estimated that a significant proportion of the more than 460 million metric tons of plastics used annually is associated with the energy and transportation sectors, including high-density polyethylene (HDPE) containers for petroleum derivatives. This figure is projected to exceed 1,231 million metric tons by 2060 [
7].
Among these applications, HDPE tanks used for diesel storage are notable for their chemical resistance, durability, low weight, and ease of manufacturing. They are commonly employed in agriculture, mining, and transportation, with capacities ranging from 200 to over 5,000 liters. In Chile, these tanks are regulated under Decree No. 160 [
16] issued by the Superintendence of Electricity and Fuels. However, after up to two decades of contact with diesel fuel, their recycling becomes highly complex due to the adsorption and diffusion of hydrocarbons into the polymer matrix, compounded by long-term environmental weathering [
23].
Conventional cleaning methods for these tanks typically involve washing with water and detergents, which generates hydrocarbon-laden wastewater requiring subsequent treatment. This merely transfers the contamination from the plastic to the effluent, posing additional environmental risks.
Diesel fuel is a complex mixture comprising hundreds to thousands of compounds, including aromatic hydrocarbons (15–40%), linear alkanes (25–50%), and naphthenes (20–40%), with carbon numbers between C9 and C22 [
5]. Among these, aromatic compounds such as toluene are particularly persistent and problematic, and are considered representative of this fraction [
11]. These substances are chemically stable and tend to accumulate in plastic matrices.
Weathering, driven by prolonged exposure to environmental factors such as UV radiation, atmospheric oxygen, temperature fluctuations, and humidity, induces substantial chemical transformations in diesel residues adhered to HDPE surfaces [
2,
8]. These changes include progressive increases in viscosity, reductions in aqueous solubility, and declines in bioavailability [
10]. For example, photochemical weathering of petroleum at 5 °C has been reported to increase its viscosity more than sixteenfold and decrease its water-soluble fraction by a factor of seven compared to petroleum at 30 °C, with direct implications for its dispersion and treatability [
22].
Under the temperate, semi-arid climate of Santiago, Chile—characterized by high seasonal solar radiation—significant changes in the chemical structure of diesel residues on exposed HDPE tanks begin to manifest within 6 to 12 months. During this period, lighter aliphatic hydrocarbons (C10–C14) tend to evaporate, while heavier components (C15–C22) and aromatic compounds undergo partial oxidation, yielding intermediate products such as carboxylic acids and alcohols. Between 12 and 24 months, the chemical profile of the adhered contaminants diverges markedly from that of fresh diesel, becoming dominated by recalcitrant residues, oxidized polar fragments, and low-volatility compounds, thereby altering their environmental behavior and microbial interactions [
1].
Moreover, the simultaneous exposure of HDPE to diesel and sunlight may trigger photoxidative processes within the polymer itself, modifying its surface topography, oxidation state, and affinity for microbial colonization and enzymatic action [
4]. This combined effect has direct implications for fungal adhesion and metabolism in bioremediation settings involving Aspergillus flavus.
Diesel-contaminated plastic waste also poses environmental and human health risks due to emissions of volatile organic compounds and the potential for leaching into soils and aquatic systems. Bioremediation emerges as a viable, cost-effective, and environmentally sustainable alternative to traditional physico-chemical treatment methods [
21].
In particular, filamentous fungi such as white-rot fungi (WRF) are noted for their ability to penetrate solid matrices, secrete ligninolytic enzymes, and degrade petroleum hydrocarbons [
4]. The concept of fungal-based biocleaning involves using fungal strains to degrade organic contaminants directly on solid surfaces, with minimal water use. This method reduces the generation of contaminated effluents and water consumption, although it presents technological challenges such as maintaining suitable environmental conditions, preserving surface moisture, and selecting strains capable of effective colonization on weathered and contaminated substrates.
Fungal genera such as Aspergillus and Penicillium—both from the Aspergillaceae family—are recognized for their resilience to extreme environmental conditions and their versatility in industrial and bioremediation applications. However, their application in closed-system biocleaning remains unreported [
12,
18].
In the context of fungal biodegradation studies, radial growth rate has been widely used as a kinetic parameter to assess colonization capacity on various substrates [
13,
15]. Additionally, the formation of halos surrounding the fungal colonies has been recognized as a complementary indicator of metabolic activity, likely associated with the diffusion of extracellular enzymes or secondary metabolites, thus offering insights into their biochemical potential for surface degradation [
14].
Since petroleum derivatives such as diesel undergo physicochemical transformations during weathering that alter their toxicity and bioavailability, the development of fungal-based biocleaning technologies must begin by evaluating microbial strains capable of colonizing and growing on contaminated plastics under these conditions. Accordingly, this study aims to assess the growth capacity of the filamentous fungus Aspergillus flavus on HDPE surfaces retrieved from discarded diesel storage tanks contaminated with diesel fuel weathered for more than two years. The study also includes a comparative evaluation on HDPE surfaces contaminated with fresh diesel (less than two months of exposure), to identify the environmental and experimental conditions that enhance fungal adaptation and inform potential biocleaning strategies.
2. Materials and Methods
2.1. Study Objective and Experimental Design
This study aimed to evaluate the ability of the filamentous fungus Aspergillus flavus to colonize and grow on high-density polyethylene (HDPE) surfaces contaminated with diesel fuel. Two types of contamination were considered: naturally weathered diesel (exposed for over two years) and non-weathered diesel (exposed for less than two months). Experiments were conducted under controlled laboratory conditions, assessing fungal growth at three temperature ranges to determine thermal influence. Radial growth rates were employed as a kinetic metric, following methodologies described by [
14,
15], serving as indicators of performance in bioremediation processes.
2.2. Chemical Characterization of Diesel Fuel in Study Samples
2.2.1. Virgin Diesel
Samples of virgin diesel were collected directly from service stations in the Metropolitan Region of Santiago, Chile. Sampling was performed using sterilized and labeled 1-liter amber glass containers, which were stored at 4 °C until analysis. The samples were diluted 1:10 with HPLC-grade n-hexane and analyzed via gas chromatography-mass spectrometry (GC-MS) using an Agilent 7890B GC system coupled with a 5977B MSD detector and an HP-5MS UI column (30 m × 0.25 mm × 0.25 µm). The oven temperature gradient was set between 40 °C and 300 °C. Compound identification was conducted with reference to the NIST library, focusing on the relative quantification of n-alkanes, aromatic hydrocarbons, naphthenes, and heavy fractions.
2.2.2. Weathered Diesel
For the analysis of weathered diesel, HDPE tanks with exactly four years of continuous environmental exposure were selected from industrial and agricultural disposal sites in the Metropolitan Region. Contaminated plastic fragments (approximately 5 g) were collected from the internal walls of the tanks and stored in sealed bags at 4 °C. Residue extraction was performed using solid-phase microextraction (SPME) with DVB/CAR/PDMS fibers (Supelco, USA). Samples were heated to 60 °C for 30 minutes in sealed vials, and thermal desorption was conducted in the injector of the same GC-MS system (Agilent 7890B GC coupled with a 5977B MSD detector and an HP-5MS UI column).
2.3. Preparation of HDPE Samples
Two types of contaminated plastic matrices were selected for experimental evaluation. The first group consisted of six samples extracted from the internal side walls of three grey HDPE tanks, each with a capacity of 1000 liters. These tanks had been in service for seven years and had undergone at least four years of environmental exposure without subsequent intervention. The second group comprised six samples from three white HDPE tanks of the same brand, capacity, and service duration but with significantly less environmental exposure, under two months, referred to as non-weathered.
Both tank types exhibited visible diesel fuel residues, remained intact (without perforations), and had functional closure systems. All were retrieved from authorized industrial disposal sites in the Metropolitan Region of Santiago, Chile. Sampling focused on the lower third of the internal side walls, where higher accumulation and adherence of hydrocarbon contaminants were observed.
Additionally, six control samples were obtained from a new, uncontaminated grey HDPE diesel fuel tank of identical characteristics. All samples were processed in triplicate. In total, 18 sets of three circular plastic discs, each 65 mm in diameter, were prepared using a carbon steel hole saw attached to an industrial drill, ensuring uniformity in dimensions and exposed area.
Prior to inoculation with Aspergillus flavus, all samples underwent a microbiological decontamination protocol using ultraviolet (UV) radiation, with 48-hour exposure on each side. This procedure, based on previous studies [
20,
24], aimed to eliminate pre-existing microorganisms and ensure that observed growth was exclusively attributable to the inoculated strain.
2.4. Preparation of Culture Medium
For the cultivation of Aspergillus flavus, potato dextrose agar (PDA) medium was used, prepared from a standardized commercial formulation (Difco™ PDA, BD Diagnostics, USA, Cat. No. 213400). A total of 39 g of dehydrated powder was dissolved in 1 liter of distilled water and sterilized in an autoclave at 121 °C for 20 minutes. Once cooled to 50 °C, the medium was applied with a sterile brush onto the surface of the plastic discs, ensuring a thin and homogeneous coating. The plastic discs were then placed in sterile 10 cm diameter Petri dishes for incubation.
2.5. Inoculation and Incubation
The strain used in this study was Aspergillus flavus, selected for its recognized capacity to metabolize hydrocarbons and its tolerance to adverse environmental conditions [
19]). This strain was obtained from the fungal bank of the Renewable Energies and Waste Laboratory (LERR-UC) at the Pontificia Universidad Católica de Chile and maintained under refrigeration until experimental use. The genetic sequence of the strain employed in this study has been published in [
6].
The selection of incubation temperatures (20 °C, 25 °C, and 30 °C) was based on the known thermal tolerance of Aspergillus flavus, which ranges approximately between 12 °C and 48 °C, with a general physiological optimum near 37 °C under laboratory conditions. Additionally, the production of secondary metabolites such as aflatoxins is higher between 25 °C and 33 °C, with aflatoxin B1 (AFB1) peaking at 33 °C and AFB2 at temperatures around 25–30 °C, provided favorable water activity conditions exist [
3,
17].
However, this study prioritized an environmental applicability approach. Considering that fungal-based biocleaning technologies could be implemented in industrial, agricultural, or exposed disposal environments, it was deemed essential to evaluate the efficacy of the fungus at temperatures compatible with ambient conditions, without the need for artificial thermal control. In this context, temperatures of 20 °C and 25 °C were included to represent real-world usage scenarios, allowing exploration of the potential of A. flavus in sustainable and low-energy decontamination systems, even operating below its optimal toxin production range.
Inoculation involved depositing a portion of active A. flavus mycelium at the center of each plastic sample previously coated with PDA medium. The samples were placed in sterile glass Petri dishes and incubated in a forced convection incubator (Memmert IF55, Memmert GmbH, Germany), programmed at three experimental temperatures: 20 ± 1 °C, 25 ± 1 °C, and 30 ± 1 °C. These conditions were selected based on literature highlighting their influence on enzymatic activity and mycelial expansion in fungal bioremediation processes [
4,
10]. The incubation period extended for 15 days, maintaining constant relative humidity and internal ventilation within the equipment.
2.6. Monitoring and Evaluation of Fungal Growth
Digital images were captured every three days to monitor the progression of fungal colonization. The method described by Khan [
14] was employed to measure the radial diameter of mycelial growth, thereby estimating the growth rate as a kinetic parameter of the biocleaning process. Images were analyzed using ImageJ v1.54 software (NIH, USA), calibrated with a physical scale printed on each Petri dish. The radial diameter of the mycelium was measured along perpendicular axes, obtaining an average per sample.
3. Results
3.1. Composition of Virgin Diesel Fuel
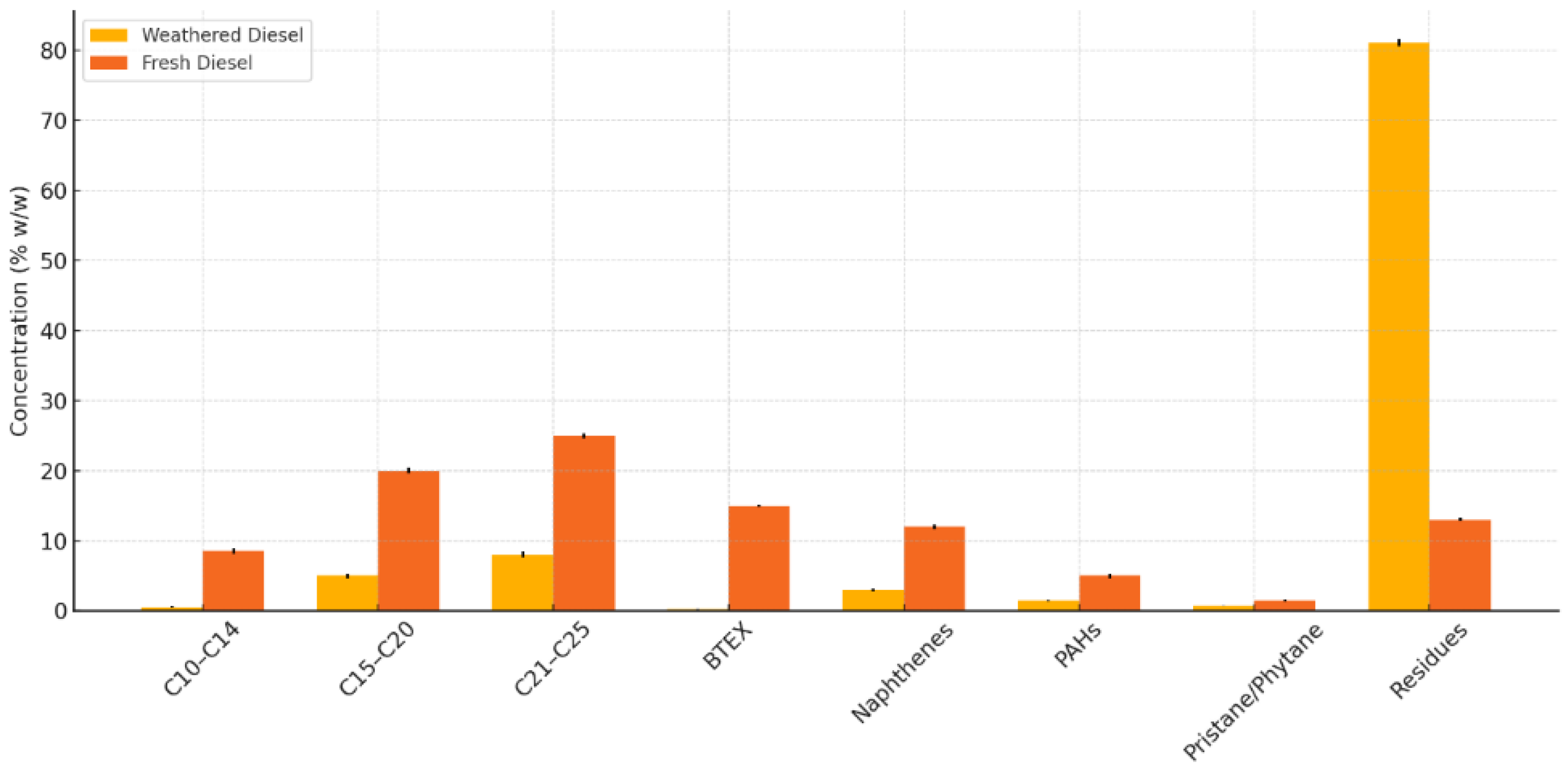
Gas chromatographic analysis of virgin diesel fuel collected from service stations in Santiago revealed a characteristic chemical profile dominated by mid- and long-chain n-alkanes, aromatic hydrocarbons, and naphthenes. The most abundant components were n-alkanes ranging from C15 to C25, collectively accounting for over 45% of the total mass. Mono- and polycyclic aromatic hydrocarbons (including BTEX and trace levels of PAHs) were also detected, representing approximately 20% of the composition. An additional 13% of the sample mass consisted of unidentified residues, including heavier compounds and fuel additives. These data are summarized in
Table 1 and graphically represented in
Figure 1.
3.2. Composition of Weathered Diesel Fuel Extracted from HDPE Fuel Container Surfaces
The diesel fuel extracted from HDPE plastic fragments exhibited a significantly altered composition due to prolonged weathering (4 years). A marked reduction in volatile compounds (C10–C14) and light aromatic hydrocarbons was observed, while the proportion of heavier n-alkanes (C21–C25) and unidentified residuals increased substantially, accounting for over 80% of the sample. These results indicate extensive loss of components through evaporation, oxidation, and both chemical and biological transformation over time. Detailed results are presented in
Table 2 and compared to virgin diesel fuel composition in
Figure 1.
3.3. Fungal Growth Dynamics on Plastic Substrates
The analysis of Aspergillus flavus growth revealed effective colonization across all evaluated HDPE plastic substrates, both in the presence and absence of weathered diesel fuel residues. Throughout the incubation period, a steady increase in colonized area by fungal hyphae was observed, along with peripheral halo formation, reaching over 300 mm² in radial expansion and up to 3,000 mm² in halo extension, depending on the experimental conditions. These results suggest a suitable physiological adaptation of the fungus to the tested substrates.
3.3.1. Comparative Fungal Behavior at 30 °C According to Plastic Substrate Type
At 30 °C, marked differences in
Aspergillus flavus behavior were observed depending on the type of plastic matrix and its contamination status. The weathered and diesel-contaminated grey HDPE, as shown in
Table 3, proved to be the most favorable substrate, reaching the highest values of mycelial expansion (colony area averaging up to 355.8 mm² ± 125.13) and halo formation (up to 3,109.66 mm²). This suggests a positive synergy between contaminant weathering and thermal conditions.
In contrast, the non-weathered, diesel-contaminated white HDPE, shown in
Table 4, exhibited the most limited fungal growth, with average colony areas of just 20.1 mm² ± 14.16 and inconsistent or minimal halo formation. These results indicate a likely combination of toxicity from fresh diesel and a possible inhibitory effect of additives in the white HDPE matrix.
The control group, represented by uncontaminated grey HDPE (
Table 5), displayed an intermediate growth pattern, with more stable mycelial expansion and moderate colony areas (average of 103.15 mm² ± 41.69). Interestingly, halo formation in the control samples reached values comparable to those observed on weathered diesel-contaminated plastic, suggesting strong metabolic activity even in the absence of hydrocarbons, likely supported by the nutritional contribution of the PDA medium.
3.3.2. Comparison of Fungal Behavior at 25 °C by Plastic Matrix Type
At 25 °C, the behavior of Aspergillus flavus followed the same trend observed at 30 °C, although with a general reduction in colony growth and halo formation areas. The grey HDPE plastic contaminated and weathered for more than two years, as shown in
Table 6, remained the most favorable substrate, with mean colony areas reaching up to 74.19 mm² ± 25.08 and halo extensions up to 2,623.59 mm². These results confirm that diesel weathering reduces its toxicity and enhances the fungus’s metabolic activity, even at temperatures close to ambient.
The white HDPE plastic contaminated with non-weathered diesel (less than two months of exposure), as presented in
Table 7, again showed limited fungal growth, with mean colony areas ranging from 23.79 mm² to 38.17 mm², and halo formation being sporadic and highly variable (some exceeding 1,000 mm², others completely absent). This heterogeneity suggests that the presence of fresh hydrocarbons and possibly plastic additives in the white HDPE continued to negatively impact fungal colonization.
The control samples (uncontaminated grey HDPE), shown in
Table 8, exhibited moderate and consistent growth. Colonies reached mean areas of up to 55.14 mm² ± 13.24, with halos exceeding 1,400 mm² in some replicates. While these values were lower than those observed at 30 °C, they indicate that the substrate supports active fungal metabolism even in the absence of hydrocarbons, confirming its suitability as an experimental reference baseline.
3.3.3. Comparison of Fungal Behavior at 20 °C According to Plastic Matrix Type
At 20 °C, the behavior of Aspergillus flavus followed the same pattern observed at higher temperatures, although with an overall reduction in mycelial growth rate and halo formation. The weathered and diesel-contaminated gray plastic (see
Table 9) remained the most favorable matrix, reaching an average colony area of 78.39 mm² ± 23.36 and halo areas up to 1,640.63 mm² ± 1,044.26. Although these figures were lower than those observed at 25 °C and 30 °C, they confirm that the weathered contaminant continues to offer conditions compatible with fungal activity, even under less favorable thermal conditions.
The unweathered, diesel-contaminated white plastic (see
Table 10) once again showed the most limited growth. Average colony areas ranged from 19.24 mm² to 33.85 mm², with halos absent in several replicates or very small, with maximum values around 871.23 mm². The data dispersion and limited halo formation reinforce the hypothesis that both the composition of fresh diesel and the additives present in white HDPE negatively impact
A. flavus colonization capacity.
In the case of the control (uncontaminated gray plastic, see
Table 11), fungal growth was moderate and relatively homogeneous, with average colony areas of 42.30 mm² ± 24.32 and halo areas reaching up to 1,538.96 mm² ± 949.21. Although these values were lower than those recorded at higher temperatures, they indicate that the fungus maintains a stable growth capacity, confirming the utility of this matrix as a comparative reference under varying thermal conditions.
3.3.4. Analysis of Experimental Variability Across Treatments
To complement the evaluation of Aspergillus flavus behavior on the different plastic matrices, coefficients of variation (CV) were calculated for colony area in each experimental combination (matrix × temperature). This allowed for quantification of relative data dispersion and the identification of patterns of homogeneity or heterogeneity among replicates.
At 30 °C, the treatment involving weathered and diesel-contaminated gray plastic exhibited a CV of 35.2%, indicating moderate variability among replicates, likely attributable to microenvironmental differences or heterogeneity in the aged plastic substrate. In contrast, the unweathered diesel-contaminated white plastic presented a high CV of 58.3%, reflecting considerable data dispersion and a potential sensitivity of the fungus to this specific matrix. The control treatment—uncontaminated gray plastic—showed an intermediate CV of 40.4%.
At 25 °C, a similar trend was observed: weathered diesel-contaminated gray plastic maintained moderate variability (CV = 33.8%), while the white unweathered plastic again demonstrated high variability (CV = 55.1%), reinforcing its erratic behavior. The control group displayed the lowest CV in this series (24.0%), indicating greater consistency among replicates.
At 20 °C, the pattern was again consistent: the CV was 29.8% for weathered diesel-contaminated gray plastic, 50.2% for unweathered diesel-contaminated white plastic, and 34.2% for the control. These values suggest that even under less favorable thermal conditions, the weathered and contaminated matrix promotes more predictable fungal growth compared to fresh diesel-contaminated white plastic.
These findings indicate that experimental variability is influenced not only by incubation temperature but also by the matrix type and the degree of contaminant weathering. The most heterogeneous conditions were consistently associated with the white plastic containing fresh diesel, whereas weathered or uncontaminated substrates offered more stable environments for A. flavus growth.
The coefficient of variation (CV) is a statistical metric that expresses the relative dispersion of a dataset with respect to its mean, enabling the comparison of variability across experimental conditions with differing absolute magnitudes. It is calculated as the ratio of the standard deviation to the mean, expressed as a percentage. In microbiological studies, CV values below 30% are typically interpreted as indicative of high experimental homogeneity, whereas values between 30% and 50% represent acceptable variability. CVs exceeding 50% reflect substantial data dispersion, often associated with complex interactions or non-uniform responses by the organism under study.
In the present work, the highest CV values were systematically observed in treatments involving non-weathered, diesel-contaminated white plastic, consistently exceeding 50% at all tested temperatures. This finding suggests a combination of physiological sensitivity of the fungus to fresh diesel and potential interference from physicochemical characteristics of the white polymer, such as the presence of antioxidant additives or pigments, which may hinder mycelial colonization in a heterogeneous manner across replicates. These results reinforce the importance of considering both the composition of the contaminant and the plastic substrate in the design of fungus-based biocleaning strategies.
3.3.5. Influence of Incubation Temperature on Fungal Colonization
Incubation temperature had a decisive impact on the behavior of Aspergillus flavus, affecting both the mycelial growth area and the formation of visible extracellular metabolites (halos). In general, a trend of increasing mycelial development was observed as incubation temperature rose from 20 °C to 30 °C, particularly on substrates containing weathered diesel.
In the grey plastic matrices contaminated with weathered diesel, increasing temperature led to progressive increases in the average colony area—from 78.39 mm² at 20 °C, to 74.19 mm² at 25 °C, and up to 355.8 mm² at 30 °C. Similarly, halo expansion followed this trend, reaching a maximum area of 3,109.7 mm² at 30 °C. These results suggest a positive synergy between elevated thermal conditions and the presence of partially degraded contaminants, which were likely more bioavailable as carbon sources.
In contrast, white plastic samples contaminated with non-weathered diesel exhibited severely limited growth at all temperatures. This matrix proved the least favorable for A. flavus, with consistently low colony areas (typically under 50 mm²) and erratic halo formation. The lack of diesel weathering, combined with the potential inhibitory effects of HDPE additives typical in fuel storage tanks, may have created chemically adverse conditions for both mycelial colonization and enzymatic expression.
The control samples (uncontaminated grey plastic) displayed moderate and stable growth. Although the average colony area increased with temperature (from 42.3 mm² to 103.15 mm²), halo formation was variable but significant, indicating metabolic activity even in the absence of hydrocarbons. This highlights the capacity of PDA medium to support baseline fungal growth, as well as the influence of thermal gradients on fungal physiology.
In summary, incubation temperature acts as a critical modulating factor, but its effects depend strongly on the substrate type and the weathering status of the contaminant. A temperature of 30 °C was consistently the most favorable for A. flavus growth in the presence of weathered diesel, whereas temperature effects were less pronounced or even adverse in matrices containing fresh diesel.
3.3.6. Temporal Evolution of Mycelial Growth and Halo Formation
The temporal analysis of Aspergillus flavus growth on different plastic matrices revealed distinct patterns based on substrate and incubation temperature. Overall, a progressive increase in total colony and halo areas was observed over the 17-day incubation period, although with notable differences among treatments.
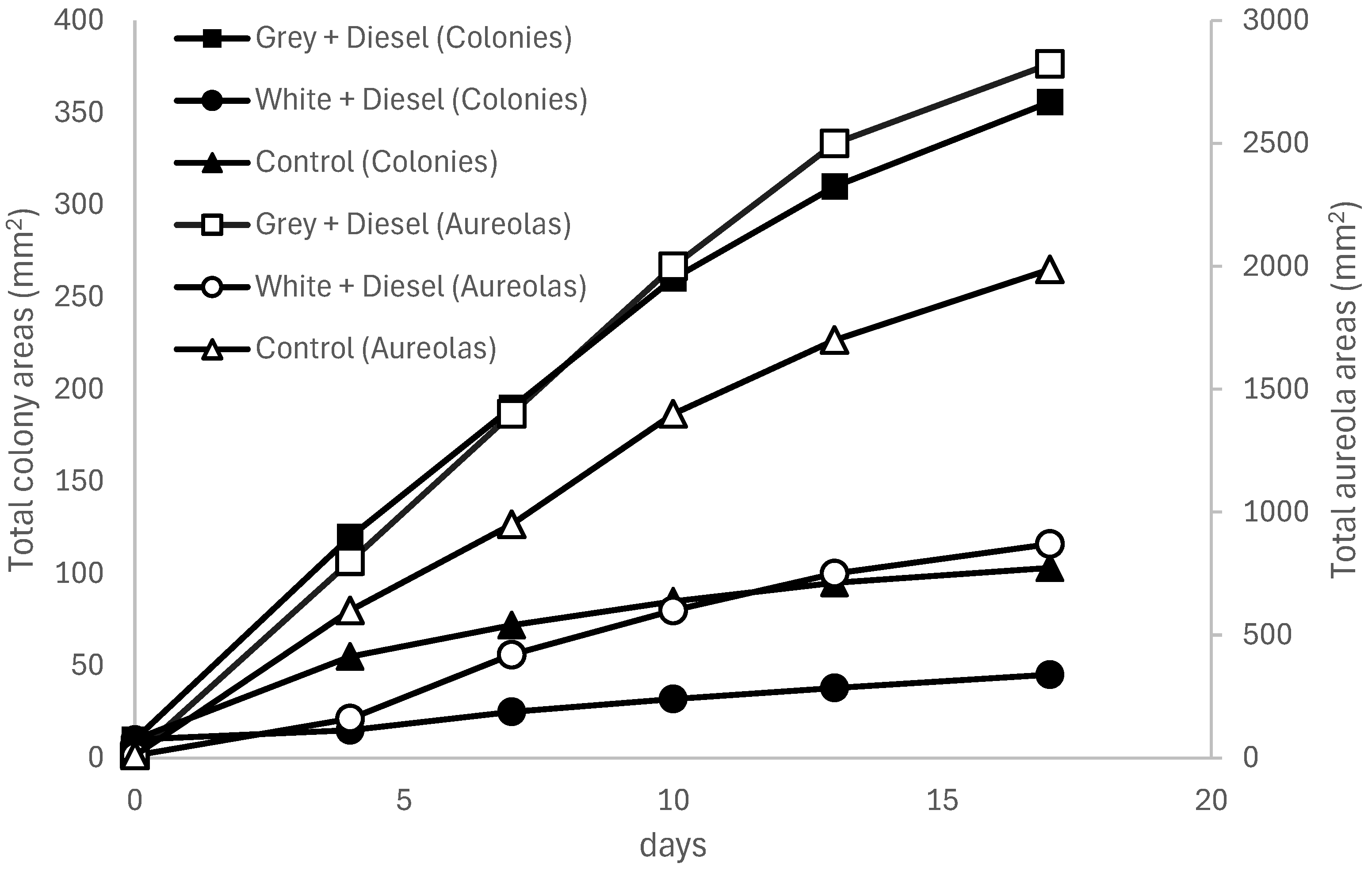
At 30 °C (see
Figure 2), the grey plastic matrix contaminated with weathered diesel demonstrated the most pronounced and sustained progression over time, both in radial mycelial expansion and in the formation of diffusible extracellular metabolites. Colony areas increased in a near-linear fashion from 10 mm² on day 0 to 355.8 mm² by day 17, while halo areas reached up to 2,823.4 mm² during the same period. This pattern reflects highly favorable conditions for fungal growth and metabolism, with relatively low measurement errors across time points, suggesting robust reproducibility among replicates.
At 25 °C (see
Figure 3), the same substrate exhibited effective growth, albeit with a slight reduction in expansion rate, particularly during the final days of the experiment. Colony areas reached 190 mm² and halos 1,437.97 mm², indicating a deceleration compared to the values observed at 30 °C. This behavior suggests that, although the temperature still supports significant activity, the metabolic performance of the fungus is partially constrained.
In contrast, the white plastic matrices contaminated with non-weathered diesel exhibited the lowest fungal development under all conditions, with slower growth and greater data dispersion, as reflected by the standard errors (
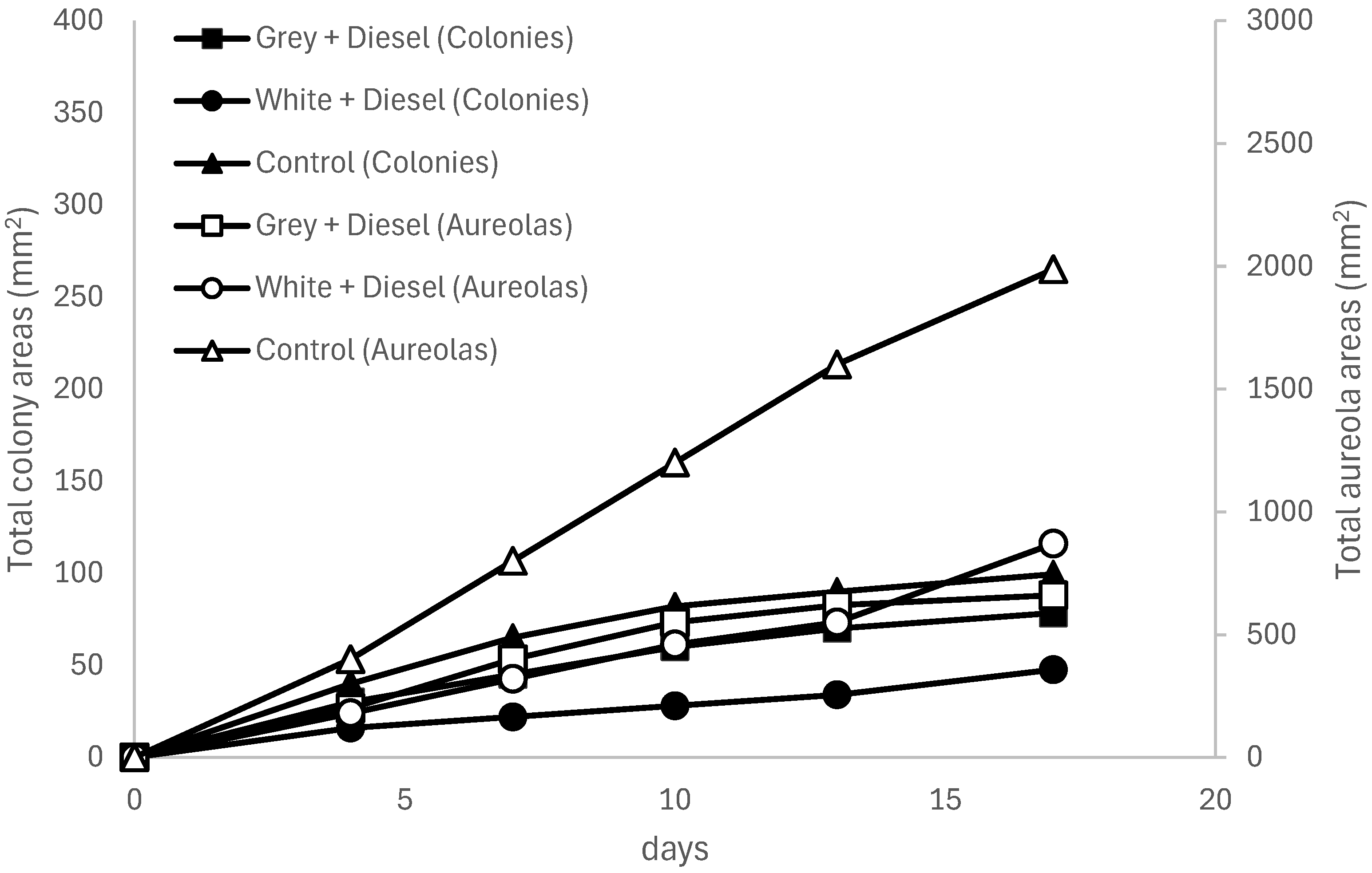
Table 13). At 20 °C and 25 °C (see
Figure 3 and
Figure 4), increases in area were low and erratic, with colony sizes barely exceeding 45 mm² and halos showing high variability and little sustained expression over time. This dynamic supports the hypothesis that both the lack of contaminant weathering and the nature of the white polymer (possibly containing inhibitory additives) negatively impacted the colonization and metabolism of
A. flavus.
In turn, the uncontaminated grey plastic (control) exhibited progressive and more stable growth, particularly in the formation of halos. At 20 °C and 25 °C (see
Figure 3 and
Figure 4), these reached total areas of 1,987.5 mm² and 1,538.96 mm², respectively, while colony areas approached 100 mm², with lower relative variability. This suggests that although the absence of diesel does not stimulate fungal metabolism as a carbon source, the plastic substrate alone does not significantly inhibit its growth.
Overall, these results highlight that the fungal growth of A. flavus is influenced not only by the availability of contaminants as a substrate but also by factors such as the prior weathering of diesel, incubation temperature, and the composition of the base polymer. The incubation temperature of 30 °C was clearly the most favorable, particularly when combined with weathered grey plastic matrices, thereby establishing a useful framework for future applied studies on fungal-based biocleaning.
Table 15.
Standard errors of the mean in cultures incubated at 20 °C.
Table 15.
Standard errors of the mean in cultures incubated at 20 °C.
| Day |
Grey + Diesel (Colonies Error) |
Grey + Diesel (Halos Error) |
White + Diesel (Colonies Error) |
White + Diesel (Halos Error) |
Control (Grey) (Colonies Error) |
Control (Grey) (Halos Error) |
| 0 |
3.0 |
3.0 |
5.0 |
5.0 |
3.5 |
3.5 |
| 4 |
8.0 |
80.0 |
5.0 |
45.0 |
9.0 |
110.0 |
| 7 |
12.0 |
130.0 |
8.0 |
90.0 |
13.0 |
160.0 |
| 10 |
16.0 |
180.0 |
11.0 |
130.0 |
17.0 |
220.0 |
| 13 |
18.0 |
220.0 |
14.0 |
160.0 |
21.0 |
290.0 |
| 17 |
21.0 |
240.0 |
16.0 |
185.0 |
24.0 |
310.0 |
4. Qualitative Analysis of Photographic Images of Fungal Cultures
The qualitative analysis of the images captured throughout the experiment revealed marked morphological differences in the colonization patterns of Aspergillus flavus, depending on the plastic matrix and the incubation temperature.
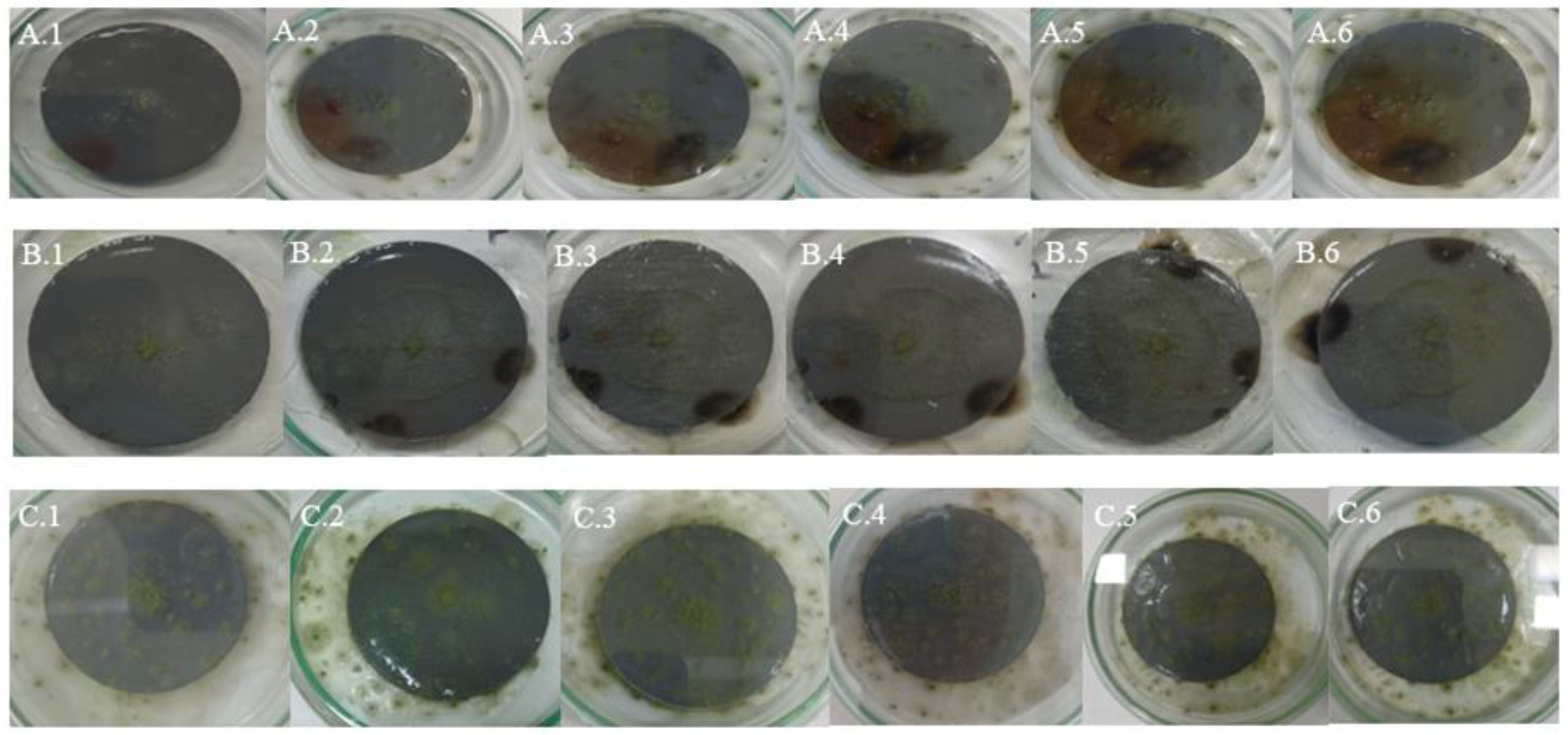
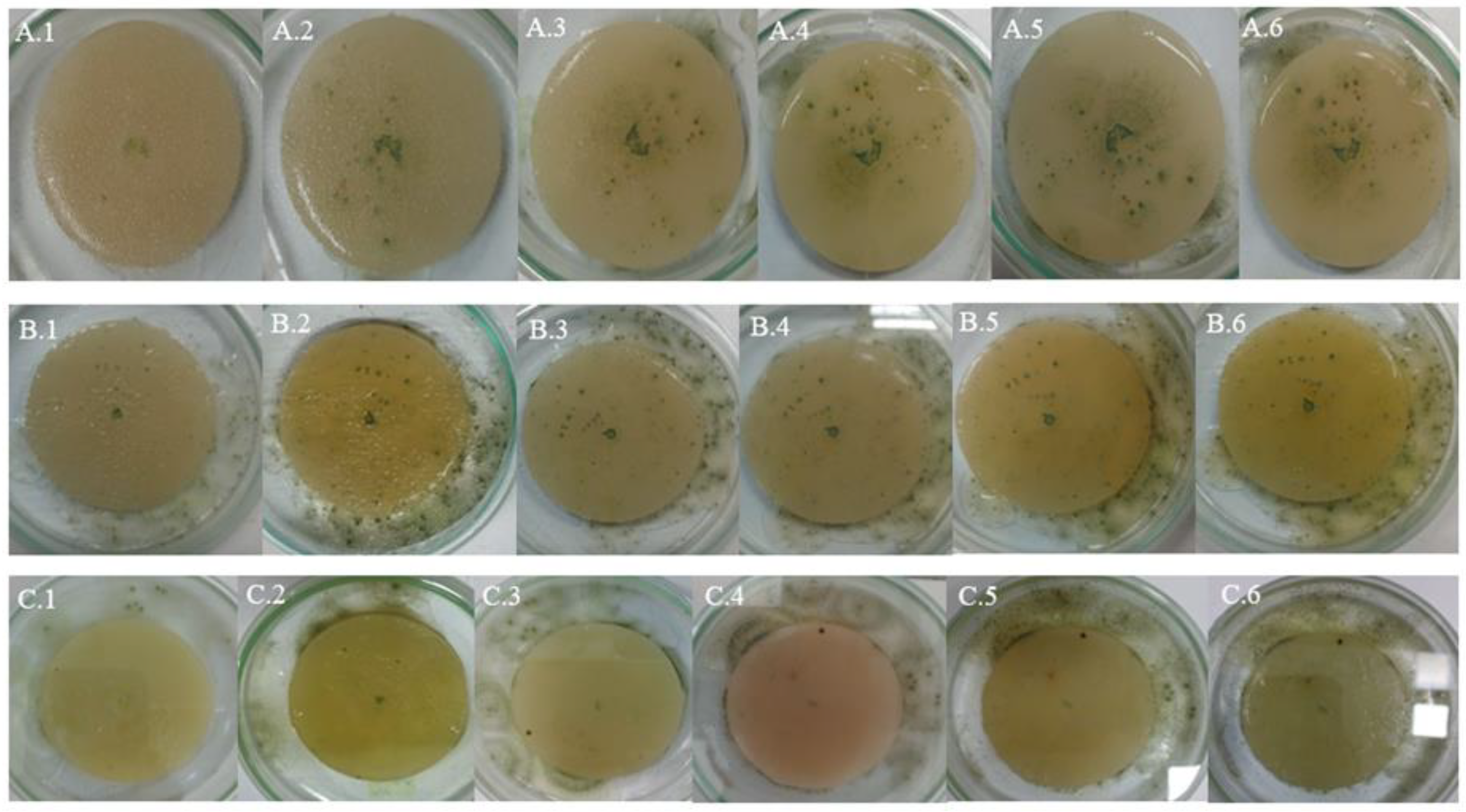
4.1. Samples of Grey Plastic Contaminated with Weathered Diesel Fuel
In samples of grey plastic contaminated with weathered diesel fuel, the colonies exhibited a continuous and homogeneous radial expansion pattern across all evaluated temperatures. At 20 °C, colonies displayed well-defined edges and halos of low to moderate peripheral intensity (
Figure 5A), consistent with the average halo area of 660.4 mm² recorded under this condition (
Table 9). At 25 °C (
Figure 6B), the mycelium appeared denser and the halos expanded significantly, reflecting the increases quantified in the average halo values (
Table 6).
At 30 °C (
Figure 6C), the highest degree of mycelial expansion was observed. Colonies presented a compact and regular structure, with broad, diffuse, and well-distributed halos, in line with the maximum halo areas exceeding 3,000 mm² recorded under this condition (
Table 3). These observations support the quantitative results and suggest that the fungus not only tolerates but adapts favorably to the presence of the contaminant in this matrix, particularly under elevated thermal conditions.
4.2. White Plastic Contaminated with Non-Weathered Diesel Fuel
In samples of white plastic contaminated with non-weathered diesel fuel,
Aspergillus flavus exhibited markedly more irregular and fragmented growth compared to other matrices. At 20 °C (
Figure 7A), colonies showed diffuse edges and discontinuous expansion, consistent with the low radial growth values observed (average of 47.68 mm², see
Table 10) and with the absence or minimal development of peripheral halos.
At 25 °C (
Figure 7B), a slight increase in mycelial density was observed, although irregularity at the colony margins persisted, in line with the high variability recorded in growth areas (
Table 7). At 30 °C (
Figure 7C), the pattern was even more restricted: colonies were small, with poorly defined edges and low cohesiveness, while halos, when present, were limited in size, with averages below 300 mm² (
Table 4).
These visual observations reinforce the hypothesis that this matrix offers less favorable conditions for fungal development, even in the presence of the contaminant. This may be attributed to specific physicochemical characteristics of the white plastic that hinder adhesion, metabolite diffusion, or access to hydrocarbons, in addition to potential inhibitory effects of the fresh, non-weathered diesel fuel stored in the tank.
4.3. Grey Plastic Without Contamination Used as Control
In the control samples, consisting of new, uncontaminated grey plastic, Aspergillus flavus exhibited a more orderly and symmetrical growth pattern compared to contaminated white plastic, although the overall expansion was less than on contaminated grey plastic. At 20 °C (Figure 8A), colonies showed uniform radial growth with well-defined edges, while halos were less intense and smaller in size than those observed on contaminated grey plastic.
At 25 °C (Figure 8B), a slight increase in mycelial density and halo extension was recorded, consistent with the growth increases observed in the quantitative halo area data (
Table 8). At 30 °C (Figure 8C), some replicates showed broader expansion and more compact structures, although not all reached the level of dispersion and coverage observed in contaminated samples, indicating some metabolic limitation in the absence of weathered hydrocarbon.
These observations confirm that while PDA medium supports the growth of A. flavus on uncontaminated plastic surfaces, the presence of contaminants from weathered diesel fuel on HDPE tank surfaces stimulates more vigorous mycelial development, particularly on compatible matrices like grey plastic.
5. Conclusions
The results of this study confirm the potential of
Aspergillus flavus as an efficient fungal agent for colonizing and growing on high-density polyethylene (HDPE) surfaces contaminated with diesel fuel. The most notable mycelial development was observed on grey plastic samples with diesel residues that had undergone natural weathering for more than two years under temperate environmental conditions, such as those found in Santiago, Chile. Incubation at 30 °C resulted in a significant increase in both biomass and the formation of diffusible metabolites, suggesting a synergistic interaction between contaminant aging and incubation temperature. These findings are consistent with the observations of Bai [
4], who highlight the thermal sensitivity of fungal metabolism in bioremediation contexts.
Moreover, the presence of weathered diesel-derived contaminants did not inhibit
A. flavus growth; rather, it appeared to stimulate metabolic activity, likely due to the increased availability of higher molecular weight oxidized compounds, which are more readily metabolized by the fungus's enzymatic machinery. This behavior aligns with previous reports describing
A. flavus as a microorganism with a high capacity for adaptation and degradation of recalcitrant organic pollutants [
14,
18].
The methodology employed—based on kinetic analysis of radial growth and quantification of halo formation—proved robust for assessing the dynamics of fungal colonization. As previously noted by Houbraken [
12], these metrics are useful for characterizing fungal metabolic activity and its responses to various environmental conditions or contaminated substrates, providing a replicable framework for future studies.
A noteworthy observation from this study was the limited colonization of white plastic samples recently contaminated with diesel (<2 months of exposure). This behavior may be explained by the higher proportion of volatile and toxic compounds, such as BTEX, present in fresh diesel, along with the higher concentration of plastic additives (e.g., antioxidants and inorganic pigments) that have not yet degraded through weathering. These factors appear to exert a stronger inhibitory effect than the contaminant itself, suggesting that the efficiency of bioremediation depends not only on the fungus and the hydrocarbon but also on the properties of the polymer substrate and its degree of environmental aging.
Overall, this study provides robust experimental evidence supporting the feasibility of using Aspergillus flavus in fungal-based cleaning strategies for diesel-contaminated plastic waste. The findings lay the groundwork for developing sustainable and scalable technologies that address the urgent need for eco-friendly and cost-effective solutions in managing waste from industrial and agricultural activities.
Funding
This study was supported by internal funding from the Laboratory of Renewable Energy and Waste, Pontificia Universidad Católica de Chile.
Data Availability Statement
The data supporting the findings of this study are included within the article.
Acknowledgments
The authors would like to express their special thanks to undergraduate student Rosario Andrea Gaete Díaz, from the Department of Chemical and Bioprocess Engineering, School of Engineering, Pontificia Universidad Católica de Chile, for her valuable contribution to data collection and assistance with experimental setups. During the preparation of this manuscript, the authors used ChatGPT (OpenAI, version April 2024) for the purposes of grammar and spelling corrections in English. The authors have reviewed and edited the output and take full responsibility for the content of this publication.
Conflicts of Interest
The funders had no role in the design of the study; in the collection, analysis, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Abbreviations
The following abbreviations are used in this manuscript:
| HPDE |
High-Density Polyethylene |
| PDA |
Potato Dextrose Agar |
| CV |
Coefficient of Variation |
| BTEX |
Benzene, Toluene, Ethylbenzene, and Xylenes |
| PAHs |
Polycyclic Aromatic Hydrocarbons |
| GC-MS |
Gas Chromatography–Mass Spectrometry |
| SPME |
Solid Phase Microextraction |
| UV |
Ultraviolet |
| AFB1 |
Aflatoxin B1 |
| AFB2 |
Aflatoxin B2 |
| WRF |
White-Rot Fungi |
| A. flavus |
Aspergillus flavus |
References
- Alexandrino, G.L.; Malmborg, J.; Augusto, F.; Christensen, J.H. Investigating weathering in light diesel oils using comprehensive two-dimensional gas chromatography-High resolution mass for spectrometry and pixel-based analysis: Possibilities and limitations. Journal of Chromatography A 2019, 1591, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Almeida, C. M. R. , Mucha, A. P., & Bordalo, A. A. (2013). Microbial communities in estuarine sediments: Response to petroleum hydrocarbons and metals. Science of the Total Environment, 454–455, 20–29.
- Al-Zaban, M.I. Impacts of Temperature and Water Activity Interactions on Growth, Aflatoxin B1 Production and Expression of Major Biosynthetic Genes of AFB1 in Aspergillus flavus Isolates. Microorganisms 2023, 11. [Google Scholar] [CrossRef] [PubMed]
- Bai, X. , Guo, F., Li, Y., & Liu, X. (2024). Biodegradation of petroleum hydrocarbons using white rot fungi: Mechanisms and environmental applications. Journal of Hazardous Materials, 457, 131759.
- Bacha, J. D., Freel, J., Gibbs, L., Gibbs, J., Hemighaus, G., Hoekman, S. K., ... & Yost, D. (2007). Diesel fuels technical review (5th ed.). Chevron Products Company.
- Caceres-Zambrano, J.Z.; Rodríguez-Córdova, L.A.; Sáez-Navarrete, C.A.; Rives, Y.C. Biodegradation capabilities of filamentous fungi in high-concentration heavy crude oil environments. Arch. Microbiol. 2024, 206, 19. [Google Scholar] [CrossRef] [PubMed]
- Center for Sustainable Systems. (2023). Plastic production and disposal factsheet (Pub. No. CSS05-06). University of Michigan. https://css.umich.edu/publications/factsheets/materials-factsheets/plastic-production-and-disposal-factsheet.
- Chen, Q., Liu, Y., Li, J., et al. (2023). Weathering processes of oil spill and their influence on environmental behavior. Marine Pollution Bulletin, 192, 114952.
- Erdmann, M.; Kleinbub, S.; Wachtendorf, V.; Schutter, J.D.; Niebergall, U.; Boehning, M.; Koerdt, A. Photo-oxidation of PE-HD affecting polymer/fuel interaction and bacterial attachment. Npj Materials Degradation 2020, 4. [Google Scholar] [CrossRef]
- Freeman, D. , Reddy, C. M., & Valentine, D. L. (2023). Photochemical weathering of petroleum in cold waters. Environmental Science & Technology Letters, 10(1), 25–30.
- Guo, H., Wang, C., Yang, X., et al. (2021). Composition and combustion behavior of diesel fuel. Fuel, 290, 120033.
- Houbraken, J., et al. (2014). Diversity and importance of Aspergillus species in industrial applications. Studies in Mycology, 78, 141–173.
- Khan, A. A. , et al. (2022a). Bioremediation of petroleum hydrocarbons using microbial consortia: An overview. Environmental Technology & Innovation, 25, 102125.
- Khan, A. A. , et al. (2022b). Radial growth kinetics of fungal strains in petroleum-contaminated media. Mycological Progress, 21(2), 137–145.
- Liu, X., et al. (2022). Characterization of fungal growth kinetics for bioremediation performance. Ecotoxicology and Environmental Safety, 241, 113784.
- Ministry of Economy, Development and Reconstruction, Chile. (2009). Supreme Decree No. 160: Approves the Safety Regulation for the Facilities and Operations of Production and Refining, Transportation, Storage, Distribution, and Supply of Liquid Fuels. Superintendence of Electricity and Fuels (SEC). https://www.sec.cl/sitioweb/transparencia_activa/julio2010/Decreto_160.
- Norlia, M.; Jinap, S.; Nor-Khaizura, M.A.R.; Radu, S.; John, J.M.; Rahman, M.A.H.; Peter, M.L.; Sharif, Z. Modelling the effect of temperature and water activity on the growth rate of Aspergillus flavus and aflatoxin production in peanut meal extract agar. International Journal of Food Microbiology 2020, 335. [Google Scholar] [CrossRef] [PubMed]
- Nrior, R. R. , & Odokuma, L. O. (2017). Bioremediation of petroleum-contaminated environments using fungal isolates. Journal of Bioremediation & Biodegradation, 8(4), 403.
- Nrior, R. R. , Uzochukwu, S. C., & Uzochukwu, C. I. Biodegradation of petroleum oils by fungi isolated from oil palm fruit and mechanic village. Journal of Environmental Science and Technolog 2017, 10(2), 85–94. [Google Scholar]
- Scott, J. E. , et al. (2022). Accelerated degradation of plastics in marine environments due to TiO₂ additives. Marine Pollution Bulletin, 180, 113779.
- Soghandi, B. , & Salimi, F. Bioremediation of diesel-contaminated soil by autochthonous microorganisms. Environmental Science and Pollution Research 2023, 30, 6235. [Google Scholar] [CrossRef]
- Ülker, E. et al. Physical and chemical transformations of weathered oil and implications for response. Journal of Environmental Management 315, 115087.
- Valenzuela, J. , et al. (2021). Problemáticas ambientales derivadas del uso de plásticos contaminados con hidrocarburos. Revista Chilena de Medio Ambiente, 14(2), 33–45.
- Wei, L. , et al. Enhancing durability of wood-plastic composites via coextrusion techniques. Composite Structures 2022, 275, 1145. [Google Scholar]
Figure 1.
Comparison of virgin and weathered diesel composition.
Figure 1.
Comparison of virgin and weathered diesel composition.
Figure 2.
Temporal evolution of fungal colony and halo areas at 30 °C.
Figure 2.
Temporal evolution of fungal colony and halo areas at 30 °C.
Figure 3.
Temporal evolution of fungal colony and halo areas at 25 °C.
Figure 3.
Temporal evolution of fungal colony and halo areas at 25 °C.
Figure 4.
Temporal evolution of fungal colony and halo areas at 20 °C.
Figure 4.
Temporal evolution of fungal colony and halo areas at 20 °C.
Figure 5.
Growth of Aspergillus flavus on grey plastic samples contaminated with weathered diesel fuel at 20 °C (A), 25 °C (B), and 30 °C (C).
Figure 5.
Growth of Aspergillus flavus on grey plastic samples contaminated with weathered diesel fuel at 20 °C (A), 25 °C (B), and 30 °C (C).
Figure 6.
Growth of Aspergillus flavus on white plastic samples contaminated with non-weathered diesel fuel at 20 °C (A), 25 °C (B), and 30 °C (C).
Figure 6.
Growth of Aspergillus flavus on white plastic samples contaminated with non-weathered diesel fuel at 20 °C (A), 25 °C (B), and 30 °C (C).
Figure 7.
Growth of Aspergillus flavus on uncontaminated grey plastic samples at 20 °C (A), 25 °C (B), and 30 °C (C).
Figure 7.
Growth of Aspergillus flavus on uncontaminated grey plastic samples at 20 °C (A), 25 °C (B), and 30 °C (C).
Table 1.
Estimated chemical composition of virgin diesel fuel samples based on GC-MS analysis.
Table 1.
Estimated chemical composition of virgin diesel fuel samples based on GC-MS analysis.
| Component |
Estimated Concentration (% w/w) |
Estimated Error (%) |
| n-Alkanes C10–C14 |
8.5 |
0.38 |
| n-Alkanes C15–C20 |
20 |
0.42 |
| n-Alkanes C21–C25 |
25 |
0.39 |
| Aromatic hydrocarbons (BTEX) |
15 |
0.16 |
| Naphthenes |
12 |
0.31 |
| Polycyclic aromatic hydrocarbons (PAHs) |
5 |
0.32 |
| Pristane and phytane |
1.5 |
0.19 |
| Unidentified fraction(residuals) |
13 |
0.24 |
Table 2.
Chemical composition of weathered diesel fuel extracted from HDPE plastic containers.
Table 2.
Chemical composition of weathered diesel fuel extracted from HDPE plastic containers.
| Component |
Estimated Concentration (% w/w) |
Estimated Error (%) |
| n-Alkanes C10–C14 |
0.5 |
0.1 |
| n-Alkanes C15–C20 |
5.0 |
0.3 |
| n-Alkanes C21–C25 |
8.0 |
0.4 |
| Aromatic hydrocarbons (BTEX) |
0.2 |
0.05 |
| Naphthenes |
3.0 |
0.2 |
| Polycyclic aromatic hydrocarbons (PAHs) |
1.5 |
0.1 |
| Pristane and phytane |
0.7 |
0.05 |
| Unidentified fraction(residuals) |
81.1 |
0.5 |
Table 3.
Total colony and halo areas on samples of weathered HDPE grey plastic contaminated with diesel fuel, incubated at 30 °C.
Table 3.
Total colony and halo areas on samples of weathered HDPE grey plastic contaminated with diesel fuel, incubated at 30 °C.
| Sample |
Total Colony Area (mm²) |
Mean ± Standard Deviation (mm²) |
Total Halo Area (mm²) |
Mean ± Standard Deviation (mm²) |
| 1 |
180.02 |
168.01 ± 22.3 |
1,251.82 |
1,647.17 ± 463.91 |
| 142.29 |
1,531.82 |
| 181.73 |
2,157.87 |
| 2 |
199.54 |
154.62 ± 63.01 |
1,249.35 |
1,632.86 ± 470.5 |
| 82.60 |
1,491.35 |
| 181.73 |
2,157.87 |
| 3 |
301.33 |
275.51 ± 30.16 |
2,111.98 |
2,363.83 ± 488.25 |
| 282.84 |
2,926.58 |
| 242.36 |
2,052.92 |
| 4 |
254.36 |
269.97 ± 14.44 |
1,764.81 |
2,347.39 ± 580.89 |
| 282.84 |
2,926.58 |
| 272.72 |
2,350.79 |
| 5 |
497.67 |
355.8 ± 125.13 |
2,949.17 |
2,823.45 ± 365.65 |
| 261.19 |
2,411.53 |
| 308.52 |
3,109.66 |
| 6 |
497.67 |
355.8 ± 125.13 |
2,949.17 |
2,823.45 ± 365.65 |
| 261.19 |
2,411.53 |
| 308.52 |
3,109.66 |
Table 4.
Total colony and halo areas on samples of unweathered white HDPE plastic contaminated with diesel fuel, incubated at 30 °C.
Table 4.
Total colony and halo areas on samples of unweathered white HDPE plastic contaminated with diesel fuel, incubated at 30 °C.
| Sample |
Total Colony Area (mm²) |
Mean ± Standard Deviation (mm²) |
Total Halo Area (mm²) |
Mean ± Standard Deviation (mm²) |
| 1 |
13.86 |
11.72 ± 4.12 |
201.26 |
— |
| 14.33 |
67.06 |
| 6.97 |
— |
| 2 |
22.41 |
18.54 ± 9.16 |
109.10 |
257.85 ± 311.29 |
| 25.13 |
615.61 |
| 8.08 |
48.85 |
| 3 |
22.41 |
18.31 ± 9.55 |
109.10 |
273.85 ± 296.04 |
| 25.13 |
615.61 |
| 7.40 |
96.84 |
| 4 |
22.41 |
18.31 ± 9.55 |
109.10 |
273.85 ± 296.04 |
| 25.13 |
615.61 |
| 7.40 |
96.84 |
| 5 |
11.56 |
18.23 ± 15.3 |
1,105.35 |
786.62 ± 597.94 |
| 35.74 |
1,157.67 |
| 7.40 |
96.84 |
| 6 |
16.42 |
20.1 ± 14.16 |
1,417.43 |
1,036.23 ± 454.27 |
| 35.74 |
1,157.67 |
| 8.15 |
533.58 |
Table 5.
Total colony and halo areas on unweathered and uncontaminated (control) grey HDPE plastic samples, incubated at 30 °C.
Table 5.
Total colony and halo areas on unweathered and uncontaminated (control) grey HDPE plastic samples, incubated at 30 °C.
| Sample |
Total Colony Area (mm²) |
Mean ± Standard Deviation (mm²) |
Total Halo Area (mm²) |
Mean ± Standard Deviation (mm²) |
| 1 |
157.09 |
101.07 ± 56.03 |
1,932.32 |
— |
| 45.04 |
1,924.41 |
| 101.08 |
1,233.77 |
| 2 |
157.09 |
108.89 ± 61.16 |
1,932.32 |
1,788.58 ± 178.13 |
| 40.09 |
1,844.13 |
| 129.49 |
1,589.29 |
| 3 |
133.79 |
101.12 ± 52.90 |
2,421.59 |
1,951.67 ± 426.44 |
| 40.09 |
1,844.13 |
| 129.49 |
1,589.29 |
| 4 |
133.79 |
103.15 ± 41.69 |
2,421.59 |
2,068.26 ± 320.96 |
| 55.67 |
1,988.48 |
| 119.99 |
1,794.72 |
| 5 |
133.79 |
103.15 ± 41.69 |
2,421.59 |
2,068.26 ± 320.96 |
| 55.67 |
1,988.48 |
| 119.99 |
1,794.72 |
| 6 |
133.79 |
103.15 ± 41.69 |
3,089.46 |
2,290.89 ± 698.34 |
| 55.67 |
1,988.48 |
| 119.99 |
1,794.72 |
Table 6.
Total colony and halo areas on weathered grey HDPE plastic contaminated with diesel fuel, incubated at 25 °C.
Table 6.
Total colony and halo areas on weathered grey HDPE plastic contaminated with diesel fuel, incubated at 25 °C.
| Sample |
Total Colony Area (mm²) |
Mean & Standard Deviation |
Total Halo Area (mm²) |
Mean & Standard Deviation |
| 1 |
49.26 |
43.88 ± 6.87 |
544.31 |
754.65 ± 188.26 |
| 46.25 |
812.32 |
| 36.14 |
907.34 |
| 2 |
62.38 |
64.01 ± 15.76 |
544.31 |
1,640.63 ± 1,044.26 |
| 80.53 |
2,623.59 |
| 49.14 |
1,754.00 |
| 3 |
41.63 |
47.88 ± 8.18 |
515.06 |
1,344.78 ± 723.93 |
| 57.14 |
1,847.70 |
| 44.87 |
1,671.58 |
| 4 |
41.63 |
47.88 ± 8.18 |
515.06 |
1,344.78 ± 723.93 |
| 57.14 |
1,847.70 |
| 44.87 |
1,671.58 |
| 5 |
103.05 |
72.19 ± 27.19 |
1,163.20 |
1,437.97 ± 260.99 |
| 51.72 |
1,468.13 |
| 61.79 |
1,682.57 |
| 6 |
103.05 |
74.19 ± 25.08 |
1,163.20 |
1,437.97 ± 260.99 |
| 57.72 |
1,468.13 |
| 61.79 |
1,682.57 |
Table 7.
Total colony and halo areas on unweathered white HDPE plastic contaminated with diesel fuel, incubated at 25 °C.
Table 7.
Total colony and halo areas on unweathered white HDPE plastic contaminated with diesel fuel, incubated at 25 °C.
| Sample |
Total Colony Area (mm²) |
Mean ± Standard Deviation |
Total Halo Area (mm²) |
Mean ± Standard Deviation |
| 1 |
25.0030.9520.93 |
25.63 ± 5.04 |
- |
- |
| 2 |
18.16 |
23.79 ± 7.49 |
933.551 |
781.98 ± 214.36 |
| 32.29 |
630.407 |
| 20.93 |
- |
| 3 |
18.16 |
35.55 ± 19.23 |
933.551 |
598.39 ± 352.26 |
| 32.29 |
630.407 |
| 56.20 |
231.224 |
| 4 |
16.26 |
34.49 ± 17.11 |
1,241.946 |
1,039.12 ± 728.00 |
| 37.00 |
1,644.198 |
| 50.20 |
231.224 |
| 5 |
21.31 |
38.17 ± 17.47 |
568.543 |
814.66 ± 737.94 |
| 37.00 |
1,644.198 |
| 56.20 |
231.224 |
| 6 |
21.31 |
45.24 ± 26.37 |
568.543 |
870.22 ± 984.13 |
| 73.52 |
1,969.881 |
| 40.90 |
72.246 |
Table 8.
Total colony and halo areas on unweathered, uncontaminated gray plastic (control), incubated at 25 °C.
Table 8.
Total colony and halo areas on unweathered, uncontaminated gray plastic (control), incubated at 25 °C.
| Sample |
Total Colony Area (mm²) |
Mean ± Standard Deviation |
Total Halo Area (mm²) |
Mean ± Standard Deviation |
| 1 |
14.88 |
42.3 ± 24.32 |
239.93 |
- |
| 61.28 |
670.153 |
| 50.74 |
850.99 |
| 2 |
14.88 |
42.3 ± 24.32 |
239.93 |
587.02 ± 313.9 |
| 61.28 |
670.153 |
| 50.74 |
850.99 |
| 3 |
16.63 |
54.21 ± 32.73 |
824.422 |
1,538.96 ± 949.21 |
| 69.53 |
1,176.458 |
| 76.48 |
2,616.005 |
| 4 |
18.22 |
53.86 ± 31.24 |
855.301 |
1,628.69 ± 899.63 |
| 66.87 |
1,414.778 |
| 76.48 |
2,616.005 |
| 5 |
40.78 |
55.14 ± 13.24 |
1,186.99 |
1,268.75 ± 126.77 |
| 66.87 |
1,414.778 |
| 57.77 |
1,204.467 |
| 6 |
22.81 |
49.15 ± 23.26 |
1,068.336 |
1,229.19 ± 174.54 |
| 66.87 |
1,414.778 |
| 57.77 |
1,204.467 |
| |
|
|
|
|
Table 9.
Total colony and halo area on weathered and diesel-contaminated gray plastic samples, incubated at 20 °C.
Table 9.
Total colony and halo area on weathered and diesel-contaminated gray plastic samples, incubated at 20 °C.
| Sample |
Total Colony Area (mm²) |
Mean ± Standard Deviation |
Total Halo Area (mm²) |
Mean ± Standard Deviation |
| 1 |
24.97 |
29.31 ± 7.82 |
420.508 |
314.65 ± 173.9 |
| 38.34 |
409.49 |
| 24.63 |
113.946 |
| 2 |
152.58 |
119.42 ± 56.37 |
1,042.766 |
735.88 ± 428.1 |
| 151.33 |
918.058 |
| 54.34 |
246.824 |
| 3 |
66.90 |
90.86 ± 52.75 |
712.788 |
625.89 ± 343.95 |
| 151.33 |
918.058 |
| 54.34 |
246.824 |
| 4 |
66.90 |
78.39 ± 23.36 |
712.788 |
660.4 ± 152.58 |
| 105.27 |
779.895 |
| 62.99 |
488.528 |
| 5 |
66.90 |
78.39 ± 23.36 |
712.788 |
660.4 ± 152.58 |
| 105.27 |
779.895 |
| 62.99 |
488.528 |
| 6 |
66.90 |
78.39 ± 23.36 |
712.788 |
660.4 ± 152.58 |
| 105.27 |
779.895 |
| 62.99 |
488.528 |
Table 10.
Total colony and halo area on unweathered and diesel-contaminated white plastic samples, incubated at 20 °C.
Table 10.
Total colony and halo area on unweathered and diesel-contaminated white plastic samples, incubated at 20 °C.
| Sample |
Total Colony Area (mm²) |
Mean ± Standard Deviation |
Total Halo Area (mm²) |
Mean ± Standard Deviation |
| 1 |
7.52 |
14.78 ± 10.97 |
- |
- |
| 9.42 |
- |
| 27.39 |
- |
| 2 |
16.09 |
33.92 ± 20.04 |
234.52 |
157.17 ± 133.89 |
| 30.05 |
234.42 |
| 55.61 |
2.56 |
| 3 |
18.51 |
53.27 ± 30.78 |
207.93 |
- |
| 64.20 |
- |
| 77.10 |
- |
| 4 |
21.59 |
54.29 ± 29.05 |
396.51 |
- |
| 64.20 |
- |
| 77.10 |
- |
| 5 |
18.89 |
46.59 ± 24.28 |
375.60 |
498.91 ± 174.39 |
| 64.20 |
- |
| 56.69 |
622.22 |
| 6 |
22.14 |
47.68 ± 22.43 |
431.45 |
526.83 ± 134.9 |
| 64.20 |
- |
| 56.69 |
622.22 |
Table 11.
Total colony and halo area on unweathered and uncontaminated gray plastic samples (control group), incubated at 20 °C.
Table 11.
Total colony and halo area on unweathered and uncontaminated gray plastic samples (control group), incubated at 20 °C.
| Sample |
Total Colony Area (mm²) |
Mean ± Standard Deviation |
Total Halo Area (mm²) |
Mean ± Standard Deviation |
| 1 |
12.96 |
24.24 ± 10.51 |
382.262 |
- |
| 25.98 |
495.779 |
| 33.77 |
403.472 |
| 2 |
100.36 |
69.55 ± 26.68 |
1,118.552 |
1,024.85 ± 86.90 |
| 54.46 |
946.923 |
| 53.83 |
1,009.068 |
| 3 |
100.36 |
69.55 ± 26.68 |
1,118.552 |
1,024.85 ± 86.90 |
| 54.46 |
946.923 |
| 53.83 |
1,009.068 |
| 4 |
242.04 |
128.11 ± 100.07 |
2,067.664 |
1,568.5 ± 570.31 |
| 54.46 |
946.923 |
| 87.84 |
1,690.927 |
| 5 |
133.28 |
95.48 ± 34.63 |
2,173.285 |
1,896.73 ± 248.84 |
| 65.30 |
1,825.986 |
| 87.84 |
1,690.927 |
| 6 |
133.28 |
99.47 ± 33.99 |
2,173.285 |
1,987.47 ± 174.92 |
| 65.30 |
1,825.986 |
| 99.82 |
1,963.15 |
Table 12.
Coefficients of Variation (CV%) by Plastic Matrix and Temperature.
Table 12.
Coefficients of Variation (CV%) by Plastic Matrix and Temperature.
| Temperature (°C) |
Matrix Type |
CV (%) |
| 20 |
Grey + Diesel |
29.8 |
| 20 |
White + Diesel |
50.2 |
| 20 |
Control (Grey) |
34.2 |
| 25 |
Grey + Diesel |
33.8 |
| 25 |
White + Diesel |
55.1 |
| 25 |
Control (Grey) |
24.0 |
| 30 |
Grey + Diesel |
35.2 |
| 30 |
White + Diesel |
58.3 |
| 30 |
Control (Grey) |
40.4 |
Table 13.
Standard errors of the mean in cultures incubated at 30 °C.
Table 13.
Standard errors of the mean in cultures incubated at 30 °C.
| Day |
Grey + Diesel (Colonies Error) |
Grey + Diesel (Halos Error) |
White + Diesel (Colonies Error) |
White + Diesel (Halos Error) |
Control(Colonies Error) |
Control (Halos Error) |
| 0 |
3.0 |
3.0 |
5.0 |
5.0 |
3.5 |
3.5 |
| 4 |
36.0 |
240.0 |
7.5 |
80.0 |
19.25 |
210.0 |
| 7 |
57.0 |
420.0 |
12.5 |
210.0 |
25.2 |
332.5 |
| 10 |
78.0 |
600.0 |
16.0 |
300.0 |
29.75 |
490.0 |
| 13 |
93.0 |
750.0 |
19.0 |
375.0 |
33.25 |
595.0 |
| 17 |
106.74 |
847.02 |
22.6 |
435.1 |
36.09 |
695.63 |
Table 14.
Standard Errors of the Mean in Cultures Incubated at 25 °C.
Table 14.
Standard Errors of the Mean in Cultures Incubated at 25 °C.
| Day |
Grey + Diesel (Colonies Error) |
Grey + Diesel (Halos Error) |
White + Diesel (Colonies Error) |
White + Diesel (Halos Error) |
Control (Colonies Error) |
Control (Halos Error) |
| 0 |
3.0 |
3.0 |
5.0 |
5.0 |
3.5 |
3.5 |
| 4 |
12.0 |
150.0 |
6.0 |
50.0 |
10.0 |
120.0 |
| 7 |
18.0 |
255.0 |
10.0 |
110.0 |
15.0 |
195.0 |
| 10 |
24.0 |
320.0 |
13.0 |
180.0 |
19.0 |
270.0 |
| 13 |
29.0 |
380.0 |
15.0 |
250.0 |
23.0 |
340.0 |
| 17 |
32.0 |
410.0 |
18.0 |
295.0 |
27.0 |
380.0 |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).