Submitted:
01 May 2025
Posted:
08 May 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Methodology
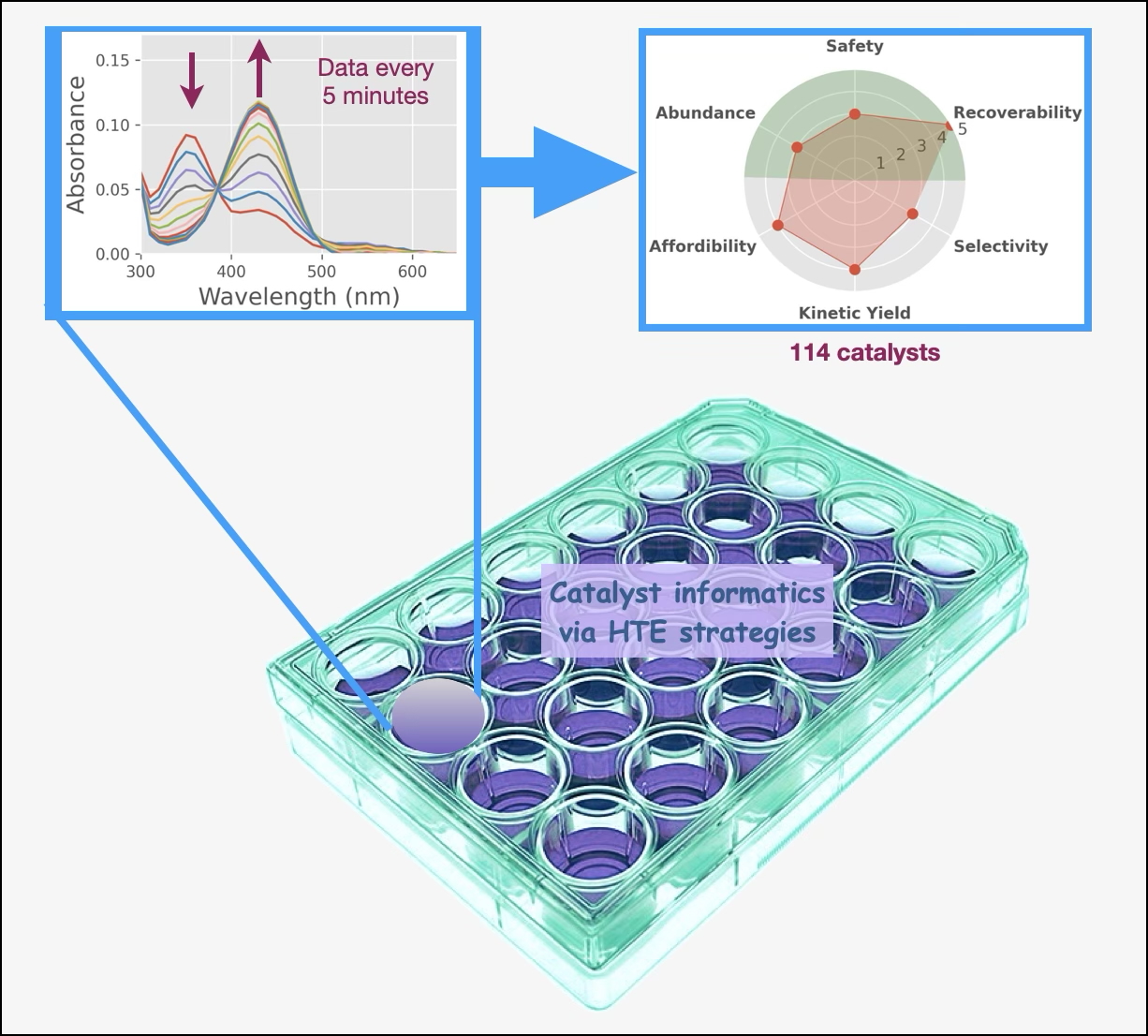
Assay Preparation for Data Collection
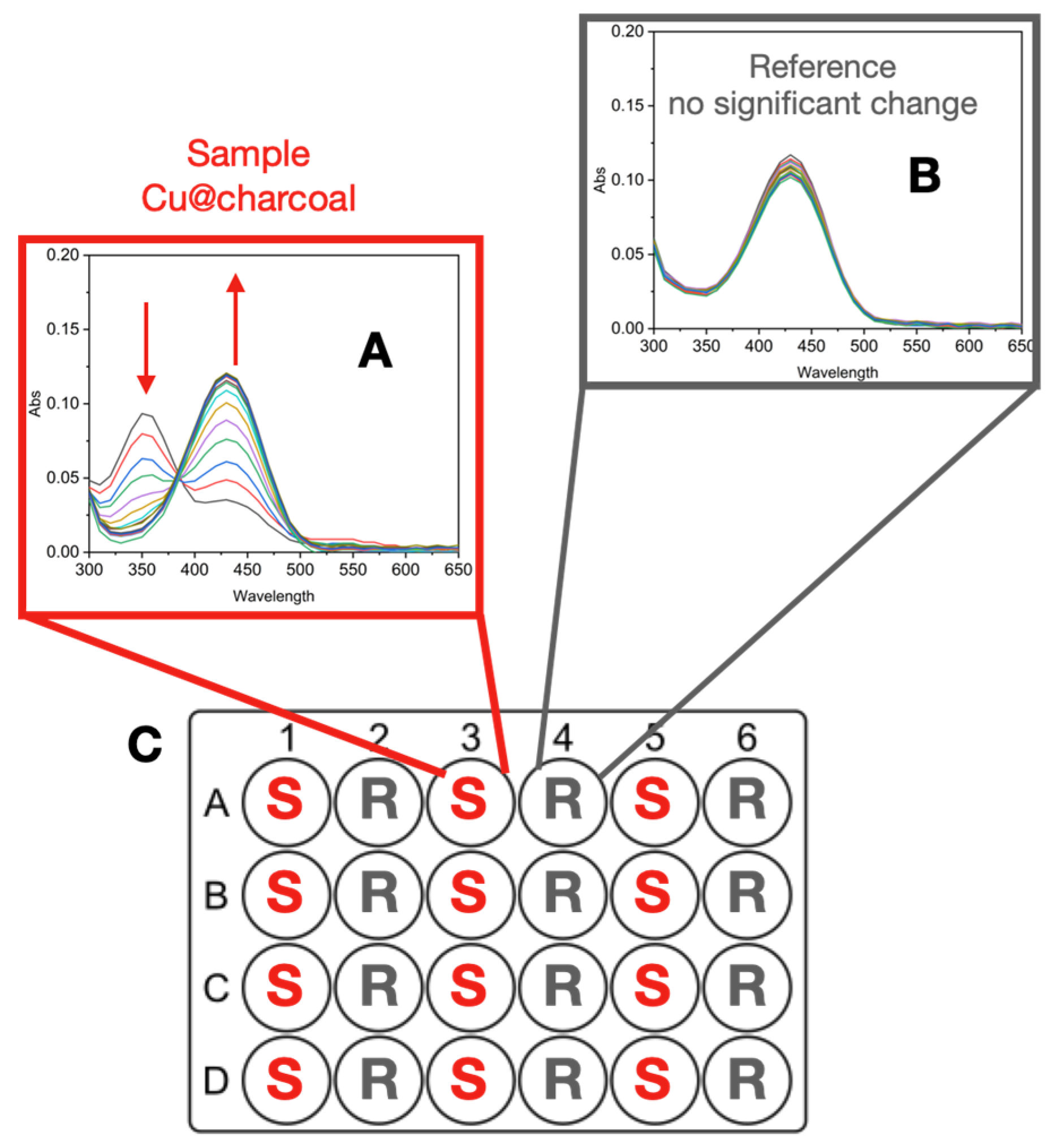
Well Plate Set-Up
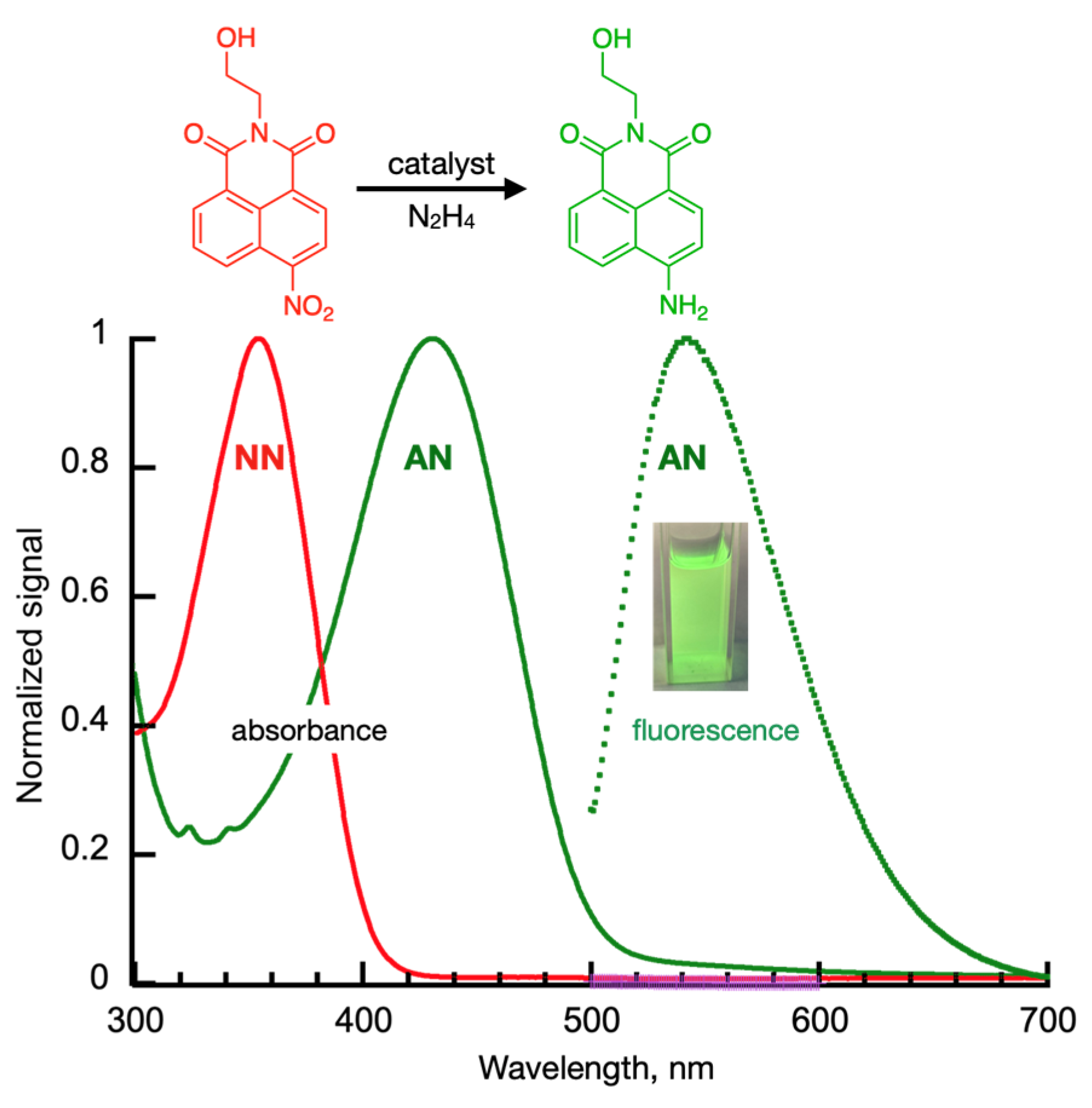
Initial Processing of Spectroscopic Data
Parameter Database
- Material abundance was evaluated based on the available supply of the material. The information was adapted from the American Chemical Society: “the periodic table’s endangered elements”[21].
- The affordability of the material was evaluated based on the material price per gram which was obtained from Sigma Aldrich on September 10, 2021, or estimated for material prepared in our laboratories. This estimate was usually four times the cost of precursors.
- Recoverability is the first step towards re-useability. Recoverability was ranked based on the material’s solubility in the reaction solvent (H2O), with heterogenous catalysts scoring higher on our scale, and unrecoverable soluble catalysts scoring low.
- Safety was measured as the inverse of each materials’ relative hazard score. The National Fire Protection Association (NFPA) evaluates materials’ hazard levels based on health, flammability, and instability. Each hazard factor was ranked from 0 to 4 based on severity, where a higher ranking indicated a greater hazard level. Overall, the hazard levels were added up and the inverse value was guided the level of relative safety.
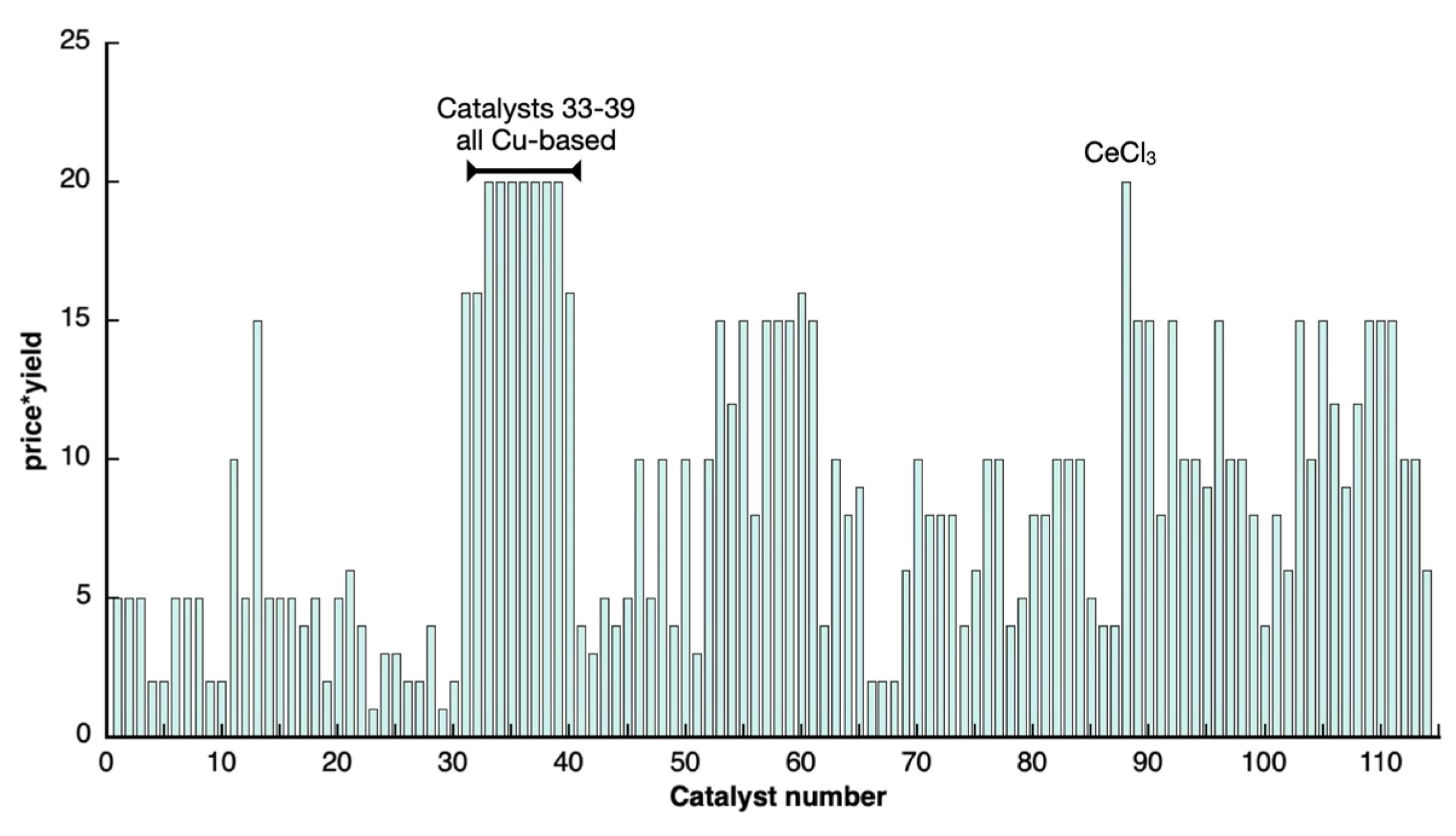
Materials Tested
3. Results and Discussion
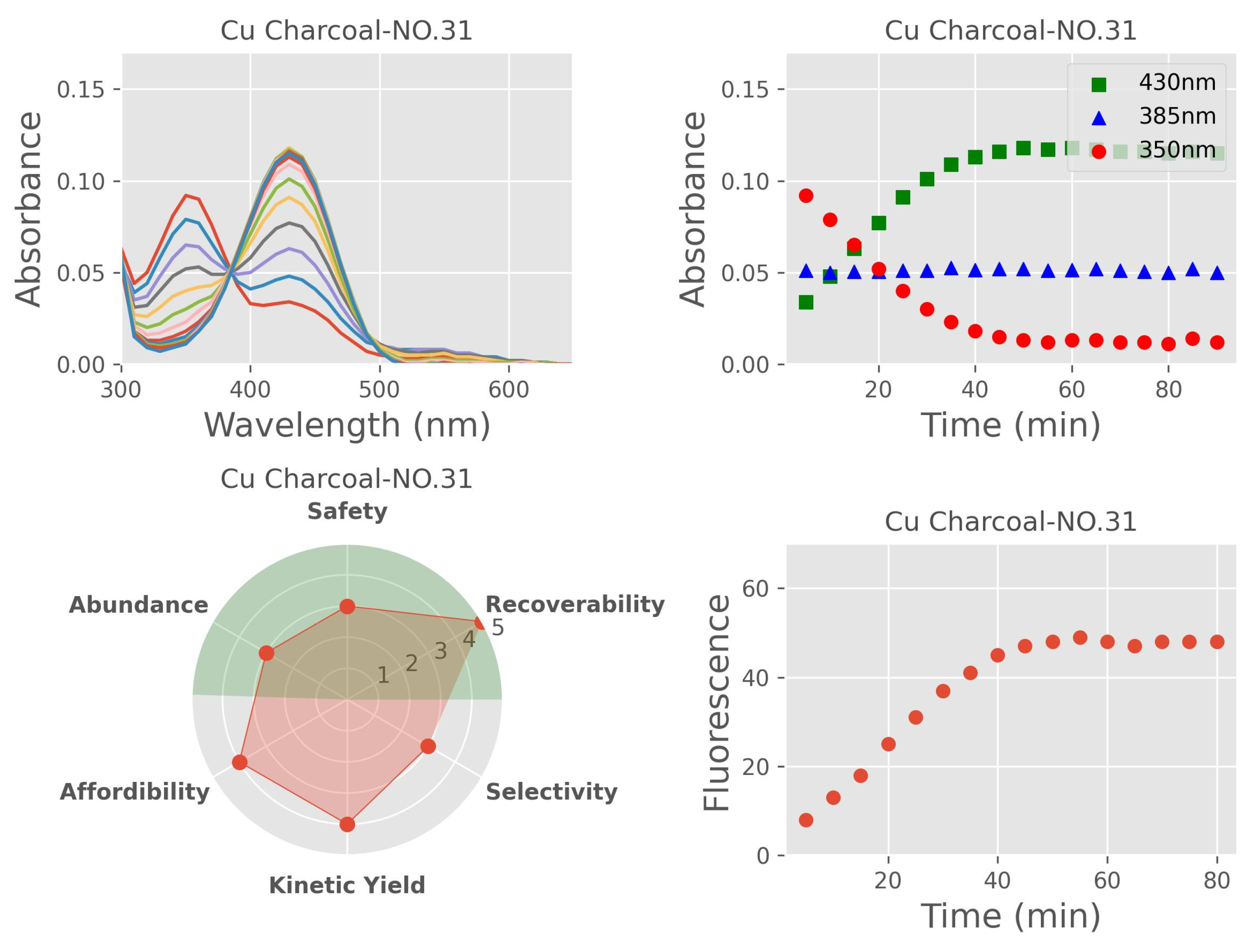
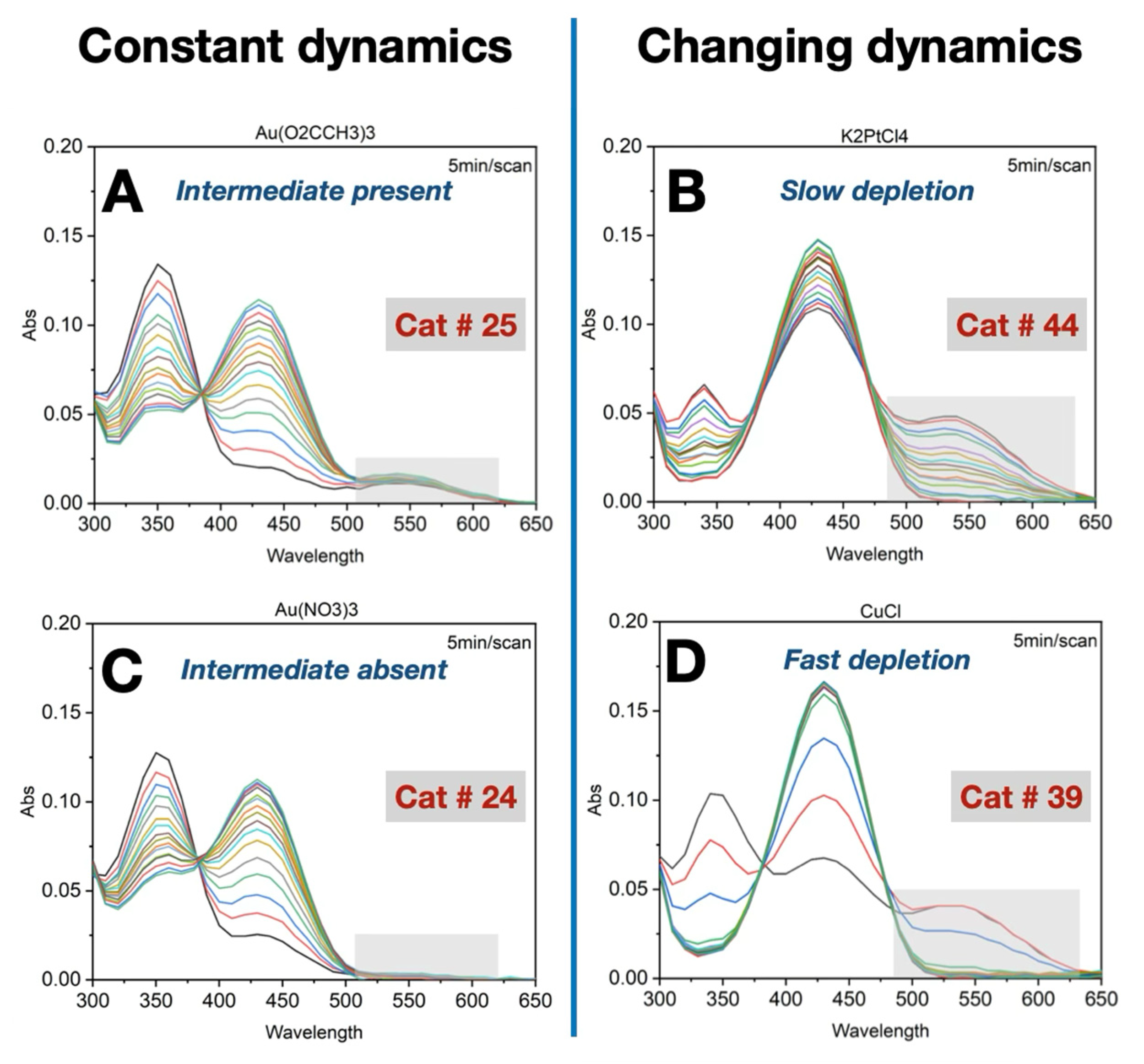
Importance of Time-Course Analysis in Catalyst Discovery
- A constant amount of intermediate was maintained throughout the reaction.
- In some cases, the intermediate was undetectable.
- A significant accumulation of the intermediate occurred at the beginning of the reaction, followed by a slow depletion rate.
- In other cases, the intermediate also accumulated early but was followed by a rapid depletion rate.
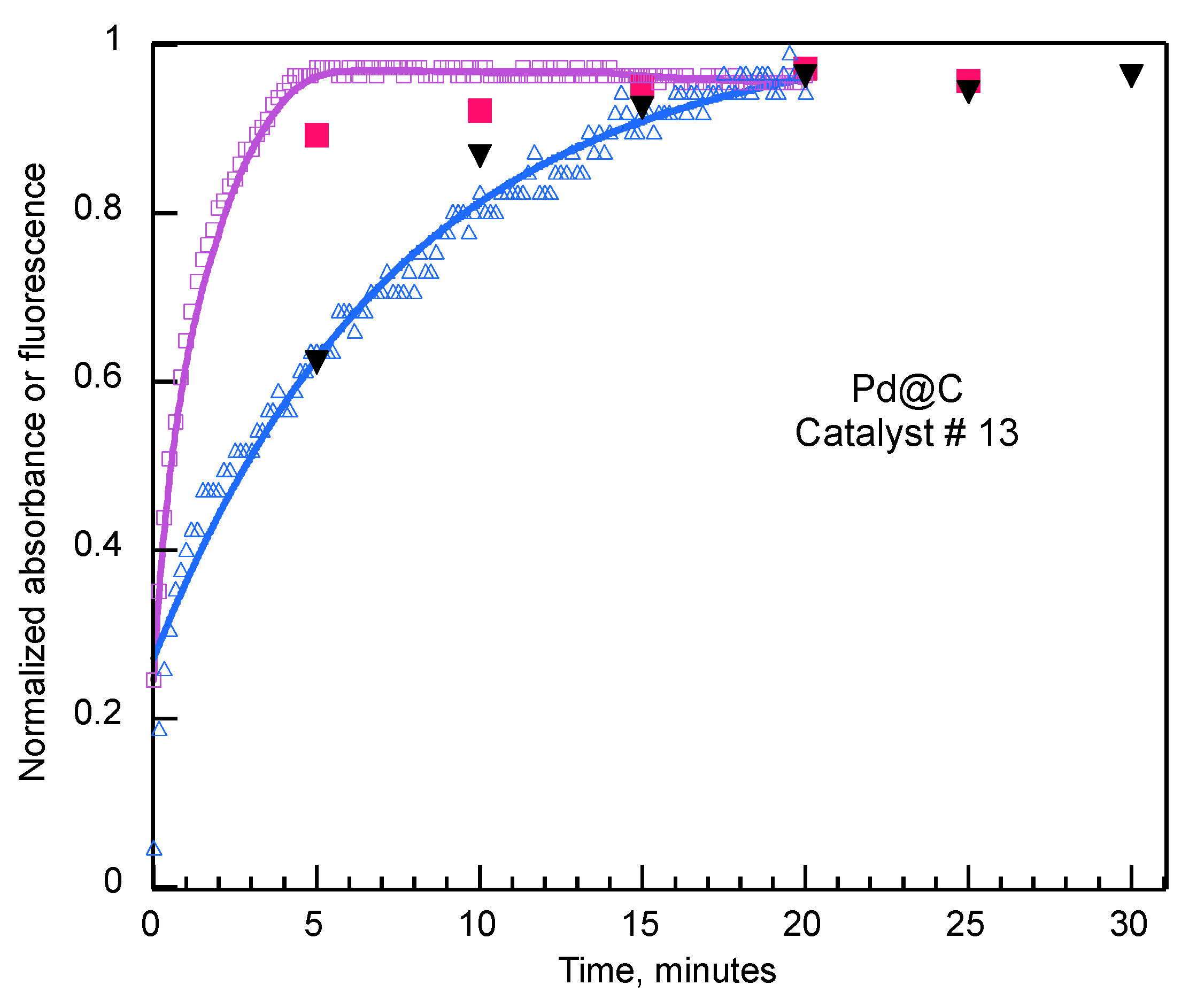
Fast Kinetics
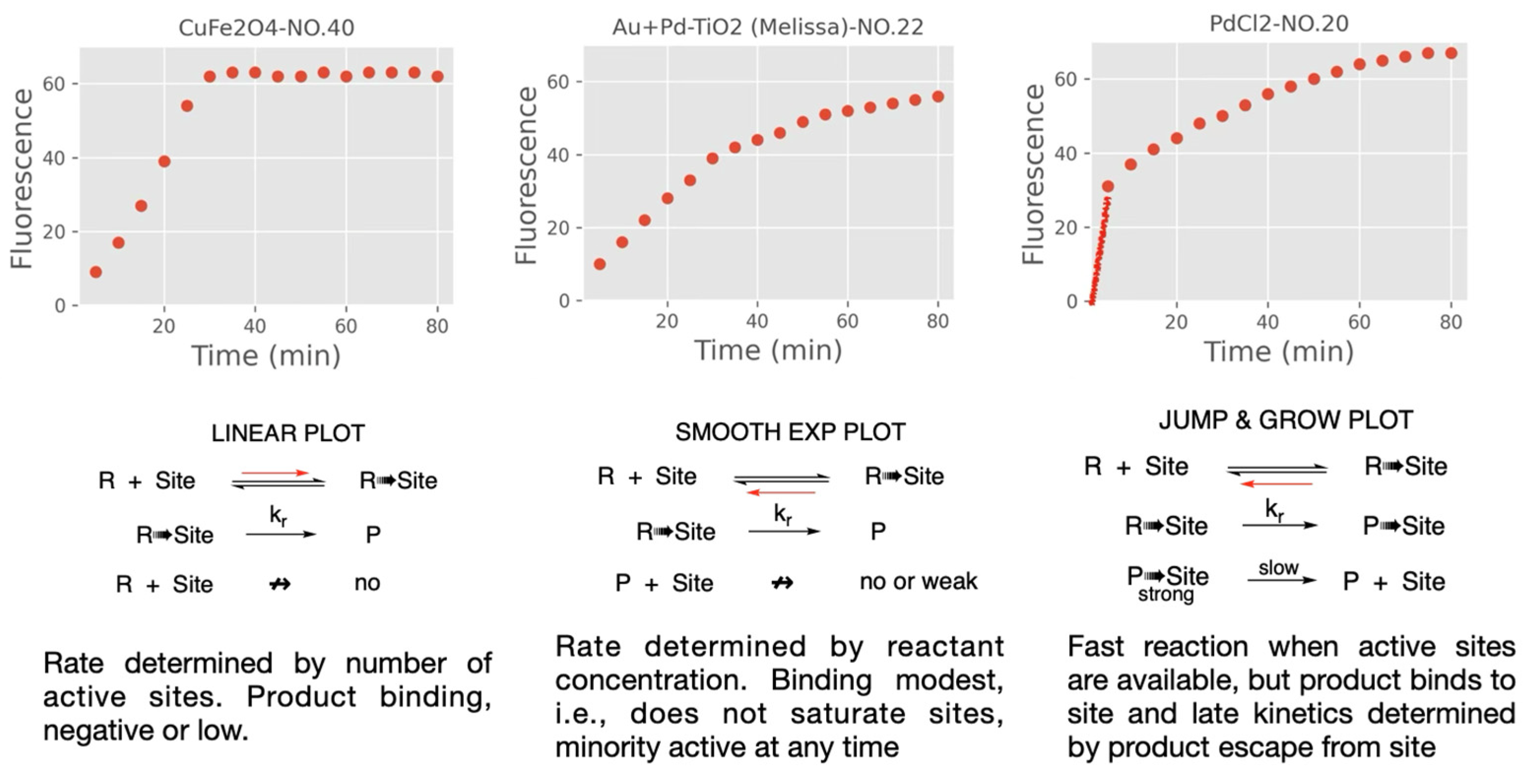
Kinetic Models for Heterogeneous Catalysts
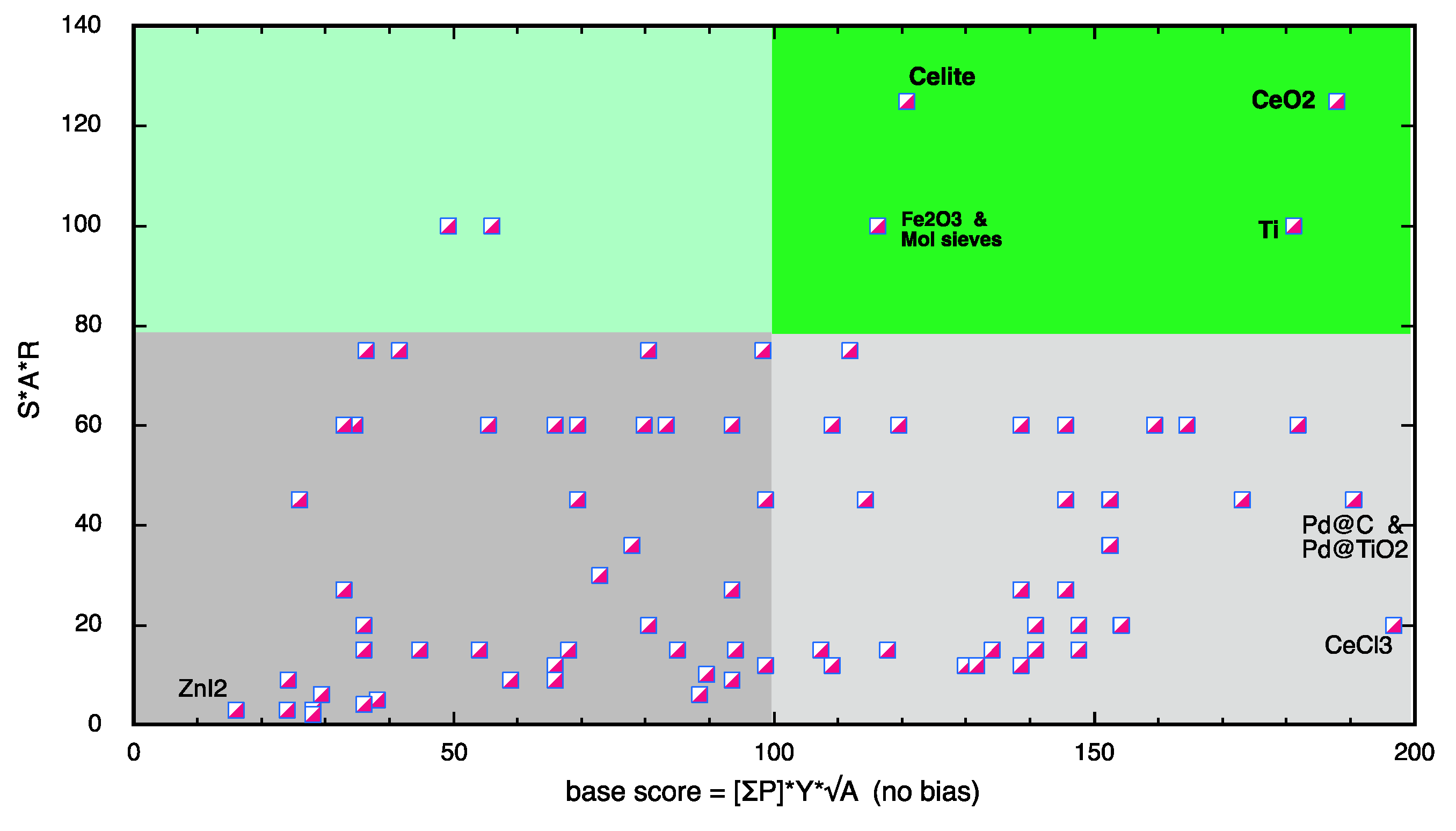
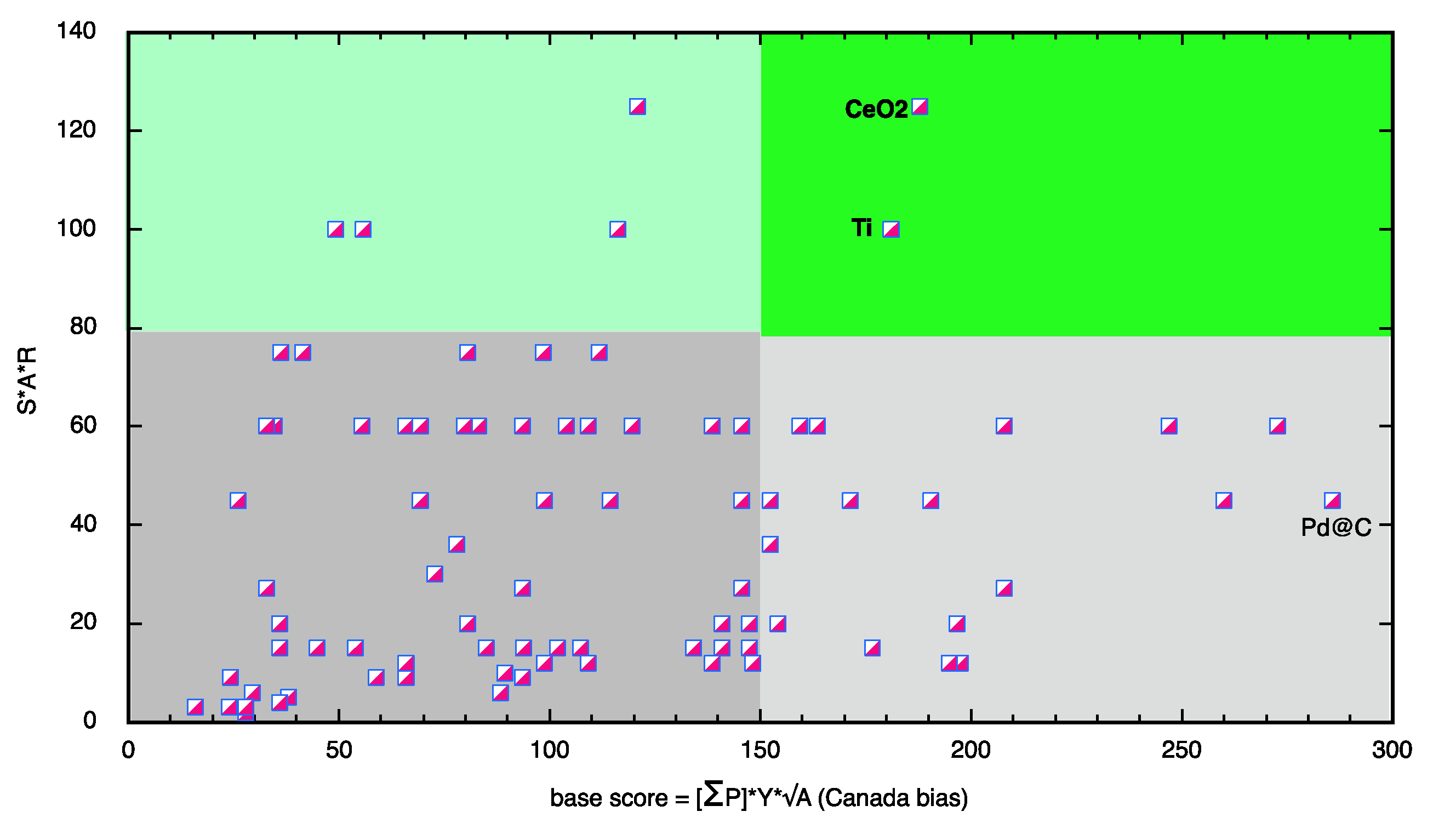
Scoring System
4. Conclusions
Supplementary Materials
References
- Trunschke, A. Prospects and challenges for autonomous catalyst discovery viewed from an experimental perspective. Catal. Sci. Tech. 2022, 12, 3650–3669. [Google Scholar] [CrossRef]
- Kearnes, S.M.; Maser, M.R.; Wleklinski, M.; Kast, A.; Doyle, A.G.; Dreher, S.D.; Hawkins, J.M.; Jensen, K.F.; Coley, C.W. The Open Reaction Database. J. Am. Chem. Soc. 2021, 143, 18820–18826. [Google Scholar] [CrossRef] [PubMed]
- Melián-Cabrera, I. Catalytic Materials: Concepts to Understand the Pathway to Implementation. Ind. Eng. Chem. Res. 2021, 60, 18545–18559. [Google Scholar] [CrossRef]
- Tang, M.; Yuan, W.; Ou, Y.; Li, G.; You, R.; Li, S.; Yang, H.; Zhang, Z.; Wang, Y. Recent Progresses on Structural Reconstruction of Nanosized Metal Catalysts via Controlled-Atmosphere Transmission Electron Microscopy: A Review. ACS Catalysis 2020, 10, 14419–14450. [Google Scholar] [CrossRef]
- Costa, P.; Sandrin, D.; Scaiano, J.C. Real-time fluorescence imaging of a heterogeneously catalysed Suzuki–Miyaura reaction. Nature Catal. 2020, 3, 427–437. [Google Scholar] [CrossRef]
- Ouyang, R.; Liu, J.-X.; Li, W.-X. Atomistic Theory of Ostwald Ripening and Disintegration of Supported Metal Particles under Reaction Conditions. J. Am. Chem. Soc. 2013, 135, 1760–1771. [Google Scholar] [CrossRef]
- Moulijn, J.A.; Pérez-Ramı́rez, J.; Berger, R.J.; Hamminga, G.; Mul, G.; Kapteijn, F. High-throughput experimentation in catalyst testing and in kinetic studies for heterogeneous catalysis. Catalysis Today 2003, 81, 457–471. [Google Scholar] [CrossRef]
- Takahashi, K.; Takahashi, L.; Nishimura, S.; Fujima, J.; Ohyama, J. The Rise of Catalysts Informatics. In Crystalline Metal Oxide Catalysts, Ueda, W., Ed.; Springer Nature Singapore: Singapore, 2022; pp. 349–371. [Google Scholar]
- Murbach, G.; Biyani, S.A.; Thompson, D.H. High Throughput Experimentation as a Tool to Guide the Microwave Assisted Catalytic Amidation of Aryl Amines with Aryl Acids. Eur. J. Org. Chem. 2025, 28, e202400764. [Google Scholar] [CrossRef]
- Mennen, S.M.; Alhambra, C.; Allen, C.L.; Barberis, M.; Berritt, S.; Brandt, T.A.; Campbell, A.D.; Castañón, J.; Cherney, A.H.; Christensen, M.; et al. The Evolution of High-Throughput Experimentation in Pharmaceutical Development and Perspectives on the Future. Org. Proc. Res. Dev. 2019, 23, 1213–1242. [Google Scholar] [CrossRef]
- Isbrandt, E.S.; Sullivan, R.J.; Newman, S.G. High Throughput Strategies for the Discovery and Optimization of Catalytic Reactions. Angew. Chem. Int. Ed. 2019, 58, 7180–7191. [Google Scholar] [CrossRef]
- Benavides-Hernández, J.; Dumeignil, F. From Characterization to Discovery: Artificial Intelligence, Machine Learning and High-Throughput Experiments for Heterogeneous Catalyst Design. ACS Catalysis 2024, 14, 11749–11779. [Google Scholar] [CrossRef]
- Suh, C.; Sieg, S.C.; Heying, M.J.; Oliver, J.H.; Maier, W.F.; Rajan, K. Visualization of High-Dimensional Combinatorial Catalysis Data. J. Comb. Chem. 2009, 11, 385–392. [Google Scholar] [CrossRef]
- Shevlin, M. Practical High-Throughput Experimentation for Chemists. ACS Med. Chem. Lett. 2017, 8, 601–607. [Google Scholar] [CrossRef] [PubMed]
- Christensen, M.; Yunker, L.P.E.; Adedeji, F.; Häse, F.; Roch, L.M.; Gensch, T.; dos Passos Gomes, G.; Zepel, T.; Sigman, M.S.; Aspuru-Guzik, A.; et al. Data-science driven autonomous process optimization. Comm. Chem. 2021, 4, 112. [Google Scholar] [CrossRef] [PubMed]
- Shevlin, M.; Friedfeld, M.R.; Sheng, H.; Pierson, N.A.; Hoyt, J.M.; Campeau, L.-C.; Chirik, P.J. Nickel-Catalyzed Asymmetric Alkene Hydrogenation of α,β-Unsaturated Esters: High-Throughput Experimentation-Enabled Reaction Discovery, Optimization, and Mechanistic Elucidation. J. Am. Chem. Soc. 2016, 138, 3562–3569. [Google Scholar] [CrossRef]
- Herrera, B.T.; Pilicer, S.L.; Anslyn, E.V.; Joyce, L.A.; Wolf, C. Optical Analysis of Reaction Yield and Enantiomeric Excess: A New Paradigm Ready for Prime Time. J. Am. Chem. Soc. 2018, 140, 10385–10401. [Google Scholar] [CrossRef]
- Wang, B.; Bourgonje, C.R.; Scaiano, J.C. Fiber-glass supported catalysis: real-time, high-resolution visualization of active palladium catalytic centers during the reduction of nitro compounds. Catal. Sci. Tech. 2023, 13, 1021–1031. [Google Scholar] [CrossRef]
- Kumari, R.; R, V.; Sunil, D.; Ningthoujam, R.S.; Pandey, B.N.; Kulkarni, S.D.; Varadavenkatesan, T.; Venkatachalam, G.; Anil Kumar, N. A Nitronaphthalimide Probe for Fluorescence Imaging of Hypoxia in Cancer Cells. J. Fluores. 2021, 31, 1665–1673. [Google Scholar] [CrossRef]
- Carrillo, A.I.; Stamplecoskie, K.G.; Marin, M.L.; Scaiano, J.C. ’From the mole to the molecule’: ruthenium catalyzed nitroarene reduction studied with ’bench’, high-throughput and single molecule fluorescence techniques. Cat. Sci. Tech. 2014, 4, 1989–1996. [Google Scholar] [CrossRef]
- American Chemical Society. Green Chemistry: Endangered Elements. Available online: (accessed on August 29, 2023).
- Wang, B.; Durantini, J.; Nie, J.; Lanterna, A.E.; Scaiano, J.C. Heterogeneous Photocatalytic Click Chemistry. J. Am. Chem. Soc. 2016, 138, 13127–13130. [Google Scholar] [CrossRef]
- Hainer, A.S.; Hodgins, J.S.; Sandre, V.; Vallieres, M.; Lanterna, A.E.; Scaiano, J.C. Photocatalytic Hydrogen Generation Using Metal-Decorated TiO2: Sacrificial Donors vs True Water Splitting. ACS Energ. Lett. 2018, 3, 542–545. [Google Scholar] [CrossRef]
- Elhage, A.; Lanterna, A.E.; Scaiano, J.C. Catalytic farming: reaction rotation extends catalyst performance. Chem. Sci. 2019, 10, 1419–1425. [Google Scholar] [CrossRef]
- Gawargy, T.A.; Costa, P.; Lanterna, A.E.; Scaiano, J.C. Photochemical benzylic radical arylation promoted by supported Pd nanostructures. Org. Biomol. Chem. 2020, 18, 6047–6052. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Lanterna, A.E.; Scaiano, J.C. Mechanistic Insights on the Semihydrogenation of Alkynes over Different Nanostructured Photocatalysts. ACS Catalysis 2021, 11, 4230–4238. [Google Scholar] [CrossRef]
- da Silva, D.R.C.; Mapukata, S.; Currie, S.; Kitos, A.A.; Lanterna, A.E.; Nyokong, T.; Scaiano, J.C. Fibrous TiO2 Alternatives for Semiconductor-Based Catalysts for Photocatalytic Water Remediation Involving Organic Contaminants. ACS Omega 2023, 8, 21585–21593. [Google Scholar] [CrossRef]
- Vivier, L.; Duprez, D. Ceria-Based Solid Catalysts for Organic Chemistry. ChemSusChem 2010, 3, 654–678. [Google Scholar] [CrossRef]
- Yan, B.; Cho, K. CatScore: A High-Efficiency Evaluation Metric for Asymmetric Catalyst Design. 2023. [Google Scholar] [CrossRef]

| Score† ➔ Parameter ↓ |
1 (least favorable) |
2 | 3 | 4 | 5 (most favorable) |
|---|---|---|---|---|---|
| (S) Safety (NFPA score) | 8-7 | 6-5 | 4-3 | 2-1 | 0 |
| Selectivity | Intermediate persists at reaction endpoint (550 nm band remain at 80 min) | NA | Intermediate formed then disappears by reaction end point. 550 nm band gone at 80 min | NA | Intermediate does not form across the reaction profile. No 550 nm signal |
| Reaction rate | Less than 25% yield in 80 min | 25 ≤%yield≤ 60 in 80 min | 60 ≤%yield≤ 100 in 80 min | Plateaus to completion in 80 min | Plateaus to completion in < 20 min |
| ($)Affordability | > $100 | 60 ≤ $ ≤ 100 | 30 ≤ $ ≤ 60 | 30 ≤ $ ≤ 10 | < $10 |
| (A) Supply & Abundance | 0 score | NA | 1, 2 score | NA | 3 score |
| (R)Recoverability | 0 score | NA | 1 score | NA | 2 & 3 scores |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





