Submitted:
06 May 2025
Posted:
08 May 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
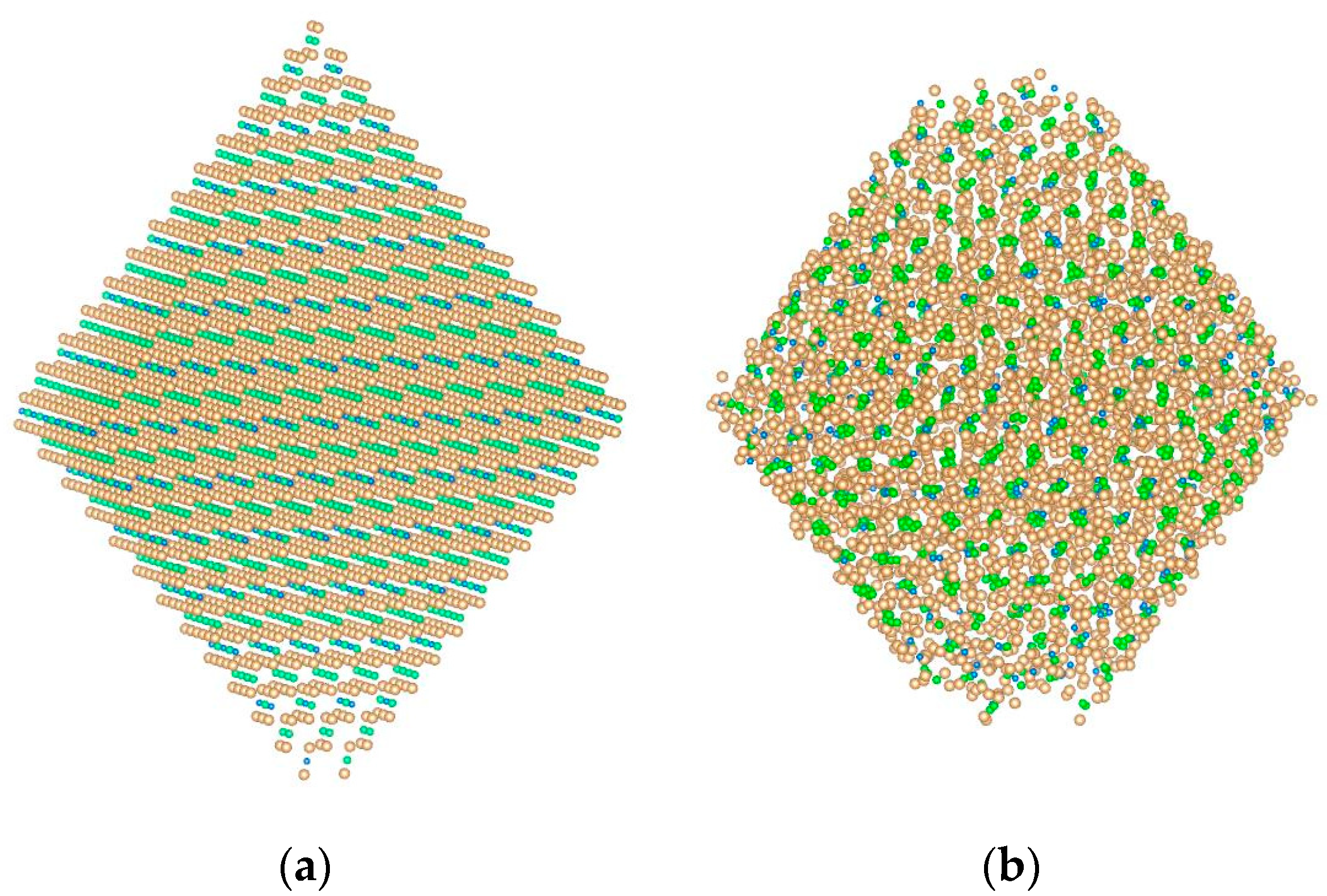
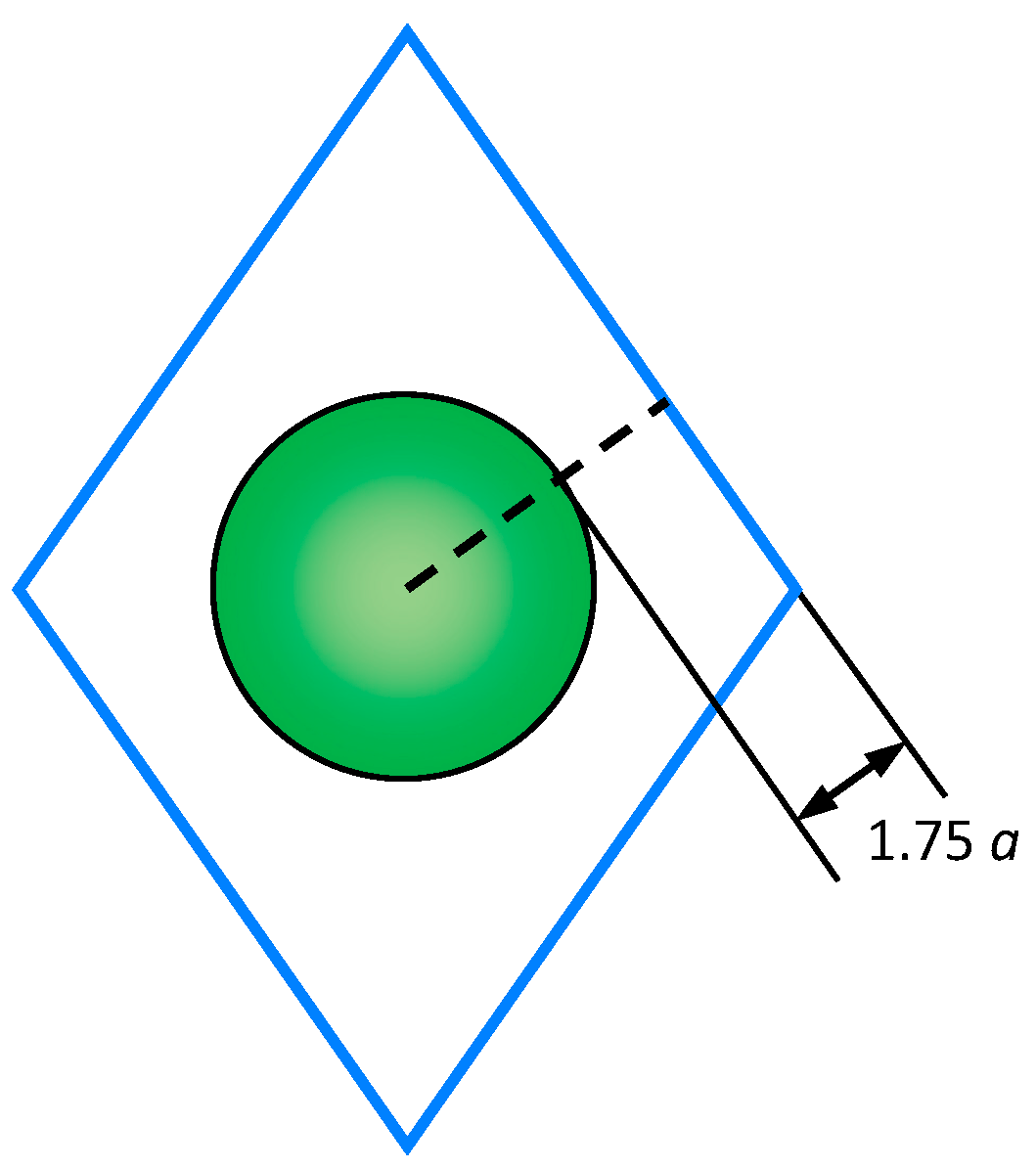
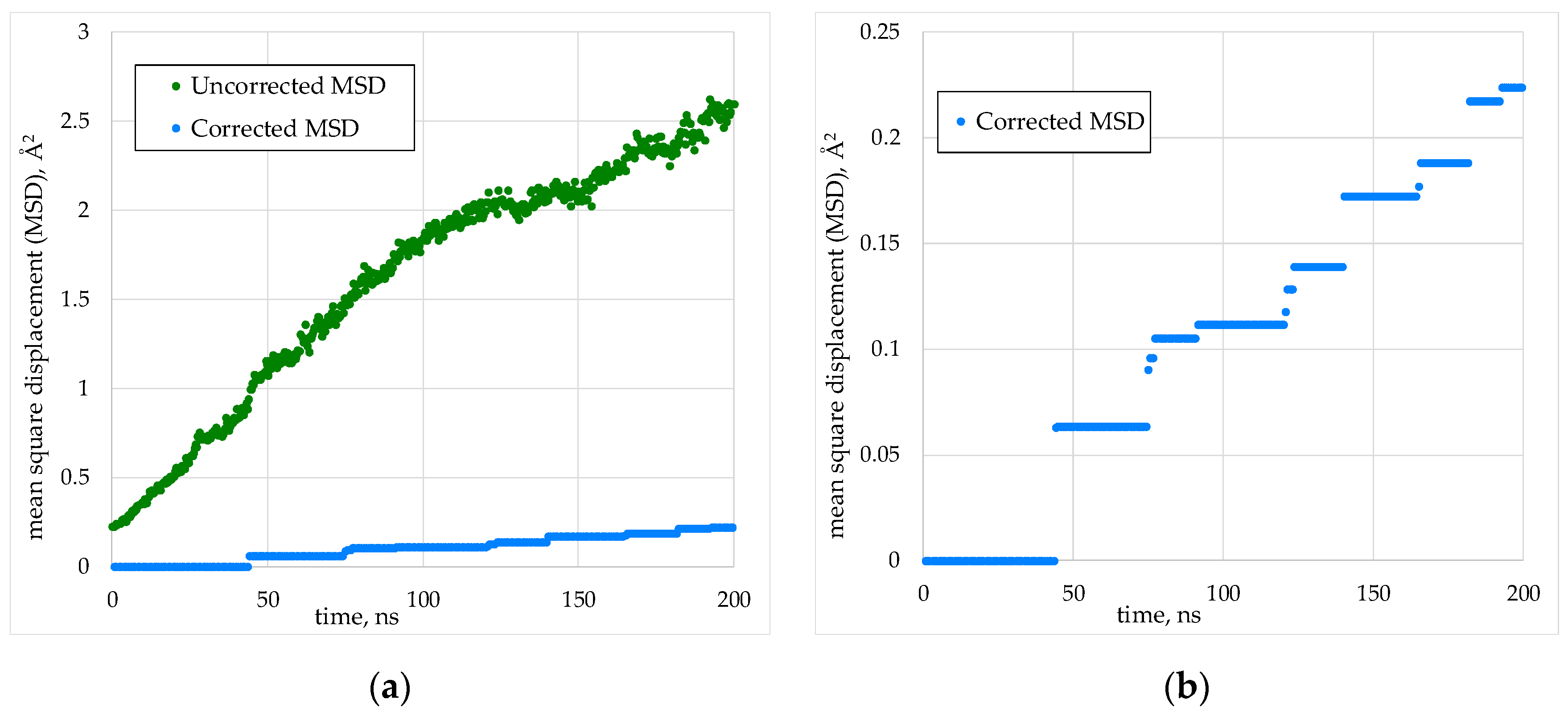
2. Modeling Methodology
3. Results and Discussion
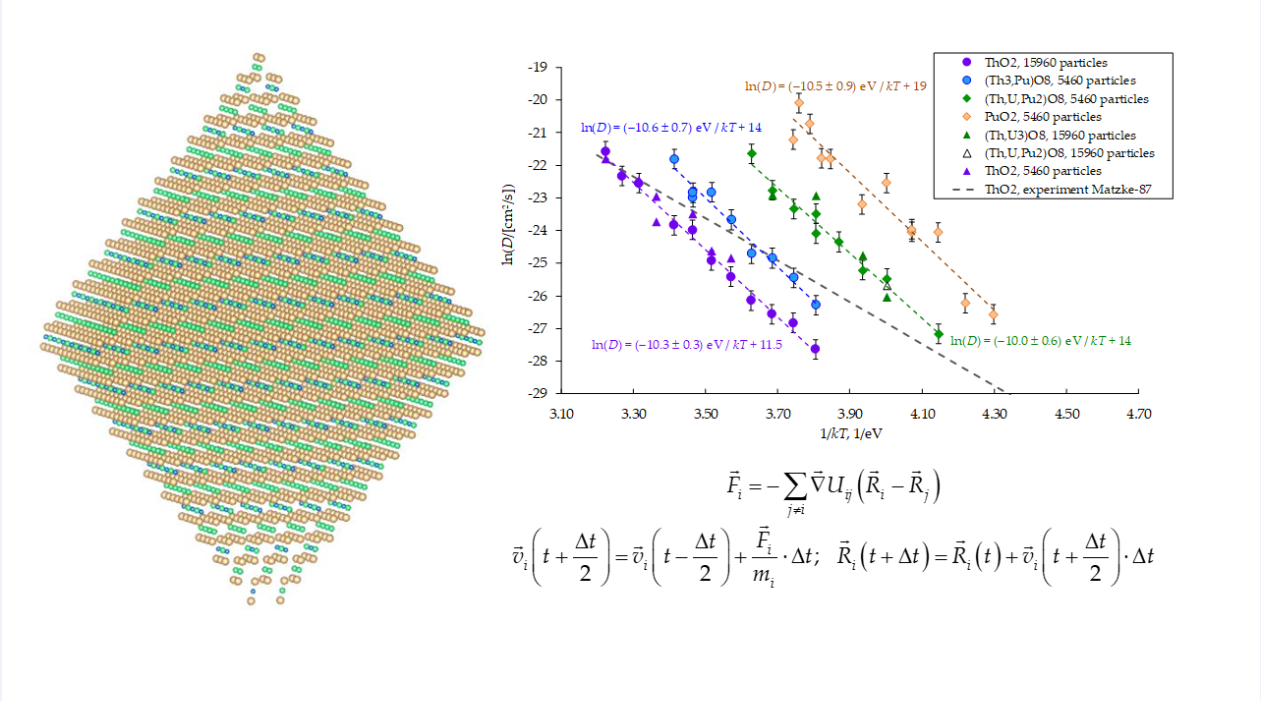
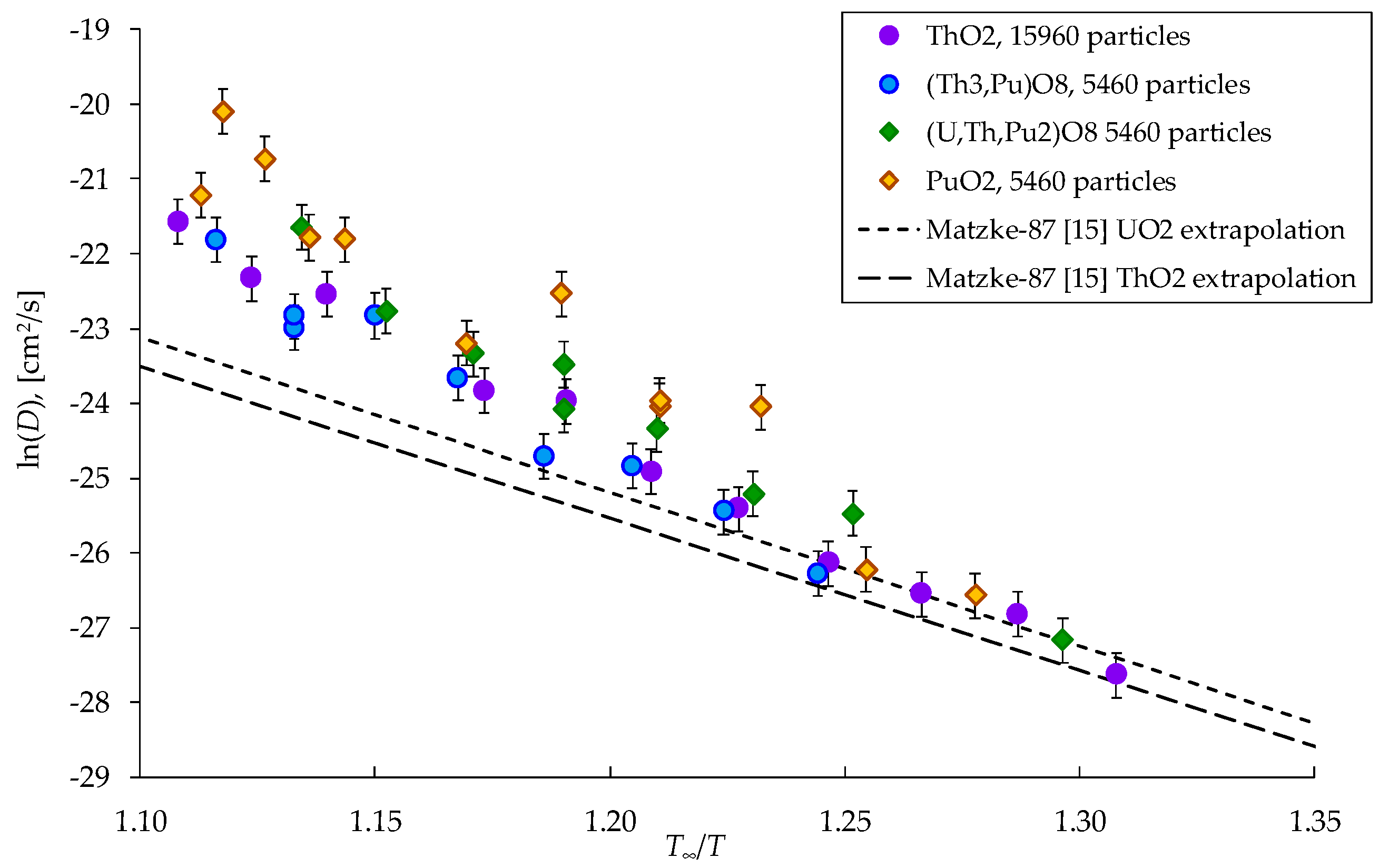
3.1. Cation Diffusion Coefficients
3.2. Activation Energies of Cation Diffusion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Carbajo, J.J.; Yoder, G.L.; Popov, S.G.; Ivanov, V.K. A Review of the Thermophysical Properties of MOX and UO₂ Fuels. J. Nucl. Mater. 2001, 299, 181–198. [Google Scholar] [CrossRef]
- Parrish, R.; Aitkaliyeva, A. A Review of Microstructural Features in Fast Reactor Mixed Oxide Fuels. J. Nucl. Mater. 2018, 510, 644–660. [Google Scholar] [CrossRef]
- Ghosh, P.S.; Kuganathan, N.; Galvin, C.O.T.; Arya, A.; Dey, G.K.; Dutta, B.K.; Grimes, R.W. Melting Behavior of (Th,U)O₂ and (Th,Pu)O₂ Mixed Oxides. J. Nucl. Mater. 2016, 479, 112–122. [Google Scholar] [CrossRef]
- Meyer, M.K.; Fielding, R.; Gan, J. Fuel development for gas-cooled fast reactors. J. Nucl. Mater. 2007, 371, 281–287. [Google Scholar] [CrossRef]
- Crawford, D.C.; Porter, D.L.; Hayes, S.L. Fuels for Sodium-Cooled Fast Reactors: US Perspective. J. Nucl. Mater. 2007, 371, 202–231. [Google Scholar] [CrossRef]
- Matthews, R.B.; Chidester, K.M.; Hoth, C.W.; Mason, R.E.; Petty, R.L. Fabrication and Testing of Uranium Nitride Fuel for Space Power Reactors. J. Nucl. Mater. 1988, 151, 345–351. [Google Scholar] [CrossRef]
- Matzke, Hj. Science of Advanced LMFBR Fuels; North-Holland: Amsterdam, The Netherlands, 1986. [Google Scholar]
- Godinho, J.R.A.; Piazolo, S.; Stennett, M.C.; Hyatt, N.C. Sintering of CaF₂ Pellets as Nuclear Fuel Analog for Surface Stability Experiments. J. Nucl. Mater. 2011, 419, 46–51. [Google Scholar] [CrossRef]
- Idriss, H. Surface Reactions of Uranium Oxide Powder, Thin Films and Single Crystals. Surf. Sci. Rep. 2010, 65, 67–109. [Google Scholar] [CrossRef]
- Potashnikov, S.I.; Boyarchenkov, A.S.; Nekrasov, K.A.; Kupryazhkin, A.Ya. High-Precision Molecular Dynamics Simulation of UO₂–PuO₂: Pair Potentials Comparison in UO₂. J. Nucl. Mater. 2011, 419, 217–225. [Google Scholar] [CrossRef]
- Murch, G.E.; Catlow, C.R.A. Oxygen Diffusion in UO₂, ThO₂ and PuO₂: A Review. J. Chem. Soc. Faraday Trans. 1987, 83, 1157–1169. [Google Scholar] [CrossRef]
- Potashnikov, S.I.; Boyarchenkov, A.S.; Nekrasov, K.A.; Kupryazhkin, A.Ya. High-Precision Molecular Dynamics Simulation of UO₂–PuO₂: Anion Self-Diffusion in UO₂. J. Nucl. Mater. 2013, 433, 215–224. [Google Scholar] [CrossRef]
- Matzke, Hj. Lattice Disorder and Metal Self-Diffusion in Non-Stoichiometric UO₂ and (U, Pu)O₂. J. Phys. Colloq. 1973, 34, 317–325. [Google Scholar] [CrossRef]
- Matzke, Hj. Atomic Transport Properties in UO₂ and Mixed Oxides (U, Pu)O₂. J. Chem. Soc. Faraday Trans. 2 1987, 83, 1121–1142. [Google Scholar] [CrossRef]
- Ronchi, C.; Sheindlin, M.; Staicu, D.; Kinoshita, M. Effect of Burn-Up on the Thermal Conductivity of Uranium Dioxide up to 100,000 MWdt⁻¹. J. Nucl. Mater. 2004, 327, 58–76. [Google Scholar] [CrossRef]
- Govers, K.; Lemehov, S.E.; Hou, M.; Verwerft, M. Comparison of Interatomic Potentials for UO₂. Part I: Static Calculations. J. Nucl. Mater. 2007, 366, 161–177. [Google Scholar] [CrossRef]
- Govers, K.; Lemehov, S.E.; Hou, M.; Verwerft, M. Comparison of Interatomic Potentials for UO₂: Part II: Molecular Dynamics Simulations. J. Nucl. Mater. 2008, 376, 66–77. [Google Scholar] [CrossRef]
- Balboa, H.; Van Brutzel, L.; Chartier, A.; Le Bouar, Y. Assessment of Empirical Potential for MOX Nuclear Fuels and Thermomechanical Properties. J. Nucl. Mater. 2017, 495, 67–79. [Google Scholar] [CrossRef]
- Shi, H.; Chu, M.; Zhang, P. Optical Properties of UO₂ and PuO₂. J. Nucl. Mater. 2010, 400, 151–156. [Google Scholar] [CrossRef]
- Peng-Fei, S.; Zhen-Hong, D.; Xiao-Ling, Z.; Yin-Chang, Z. Electronic Structure and Optical Properties in Uranium Dioxide: The First Principle Calculations. Chin. Phys. Lett. 2015, 32, 077101. [Google Scholar] [CrossRef]
- Boudjemline, A.; Louail, L.; Islam, M.M.; Diawara, B. Dependence of Pressure on Elastic, Electronic and Optical Properties of CeO₂ and ThO₂: A First Principles Study. Comput. Mater. Sci. 2011, 50, 2280–2286. [Google Scholar] [CrossRef]
- Li, Y. A Universal COMB Potential for the Whole Composition Range of the Uranium-Oxygen System. J. Nucl. Mater. 2019, 513, 102–111. [Google Scholar] [CrossRef]
- Phillpot, S.R.; Antony, A.C.; Shi, L.; Fullarton, M.L.; Liang, T.; Sinnott, S.B.; Zhang, Y.; Biner, S.B. Charge Optimized Many Body (COMB) Potentials for Simulation of Nuclear Fuel and Clad. Comput. Mater. Sci. 2018, 148, 231–241. [Google Scholar] [CrossRef]
- Li, Y.; Liang, T.; Sinnott, S.B.; Phillpot, S.R. A Charge-Optimized Many-Body Potential for the U–UO₂–O₂ System. J. Phys. Condens. Matter 2013, 25, 505401. [Google Scholar] [CrossRef] [PubMed]
- Ryzhkov, M.V.; Kupryazhkin, A.Ya. First-Principles Study of Electronic Structure and Insulating Properties of Uranium and Plutonium Dioxides. J. Nucl. Mater. 2009, 384, 226–232. [Google Scholar] [CrossRef]
- Ryzhkov, M.V.; Kovalenko, M.A.; Kupryazhkin, A.Ya.; Gupta, S.K. Transformation of Electron Density Distribution Induced by the Cation Point Defects in Uranium Dioxide. J. Radioanal. Nucl. Chem. 2020, 325, 253–262. [Google Scholar] [CrossRef]
- Boyarchenkov, A.S.; Nekrasov, K.A.; Kupryazhkin, A.Ya.; Gupta, S.K. A Novel Empirical Potential for High-Temperature Molecular Dynamics Simulation of ThO₂ and MOX Nuclear Fuel Crystals. AIP Conf. Proc. 2020, 2313, 030064. [Google Scholar] [CrossRef]
- Brutzel, L.V.; Rarivomanantsoa, M.; Ghaleb, D. Displacement Cascade Initiated with the Realistic Energy of the Recoil Nucleus in UO₂ Matrix by Molecular Dynamics Simulation. J. Nucl. Mater. 2006, 354, 28–35. [Google Scholar] [CrossRef]
- Martin, G.; Garcia, P.; Brutzel, L.V.; Dorado, B.; Maillard, S. Effect of the Cascade Energy on Defect Production in Uranium Dioxide. Nucl. Instrum. Methods Phys. Res. B 2011, 269, 1727–1731. [Google Scholar] [CrossRef]
- Balboa, H.; Van Brutzel, L.; Chartier, A.; Le Bouar, Y. Damage Characterization of (U,Pu)O₂ Under Irradiation by Molecular Dynamics Simulations. J. Nucl. Mater. 2018, 512, 440–451. [Google Scholar] [CrossRef]
- Devanathan, R. Molecular Dynamics Simulation of Fission Fragment Damage in Nuclear Fuel and Surrogate Material. MRS Adv. 2017, 2, 1225–1236. [Google Scholar] [CrossRef]
- Yablinsky, C.A.; Devanathan, R.; Pakarinen, J.; Gan, J.; Severin, D.; Trautmann, C.; Allen, T.R. Characterization of Swift Heavy Ion Irradiation Damage in Ceria. J. Mater. Res. 2015, 30, 1473–1484. [Google Scholar] [CrossRef]
- Kovalenko, M.A.; Kupryazhkin, A.Ya. Mechanisms of Exchange and Anion Frenkel Diffusion in Uranium Dioxide: Molecular Dynamics Study. J. Nucl. Mater. 2019, 522, 255–264. [Google Scholar] [CrossRef]
- Boyarchenkov, A.S.; Potashnikov, S.I.; Nekrasov, K.A.; Kupryazhkin, A.Ya. Molecular Dynamics Simulation of UO₂ Nanocrystals Melting Under Isolated and Periodic Boundary Conditions. J. Nucl. Mater. 2012, 427, 311–322. [Google Scholar] [CrossRef]
- Singh, S.; Sonvane, Y.; Nekrasov, K.A.; Boyarchenkov, A.S.; Kupryazhkin, A.Ya.; Gajjar, P.N.; Gupta, S.K. Ab-Initio Investigation of Crystal Structure and Pressure Induced Phase Transition in ThO₂ and PuO₂. Mater. Today Commun. 2021, 28, 102579. [Google Scholar] [CrossRef]
- Kim, K.C.; Olander, D.R. Oxygen Diffusion in UO₂₋ₓ. J. Nucl. Mater. 1981, 102, 192–199. [Google Scholar] [CrossRef]
- Basak, C.; Sengupta, A.; Kamath, H. Classical Molecular Dynamics Simulation of UO₂ to Predict Thermophysical Properties. J. Alloys Compd. 2003, 360, 210–216. [Google Scholar] [CrossRef]
- Morelon, N.-D.; Ghaleb, D.; Delaye, J.-M.; Brutzel, L.V. A New Empirical Potential for Simulating the Formation of Defects and Their Mobility in Uranium Dioxide. Philos. Mag. 2003, 83, 1533–1555. [Google Scholar] [CrossRef]
- Yakub, E.; Ronchi, C.; Staicu, D. Computer Simulation of Defects Formation and Equilibrium in Non-Stoichiometric Uranium Dioxide. J. Nucl. Mater. 2009, 389, 119–129. [Google Scholar] [CrossRef]
- Vincent-Aublant, E.; Delaye, J.-M.; Van Brutzel, L. Self-Diffusion Near Symmetrical Tilt Grain Boundaries in UO₂ Matrix: A Molecular Dynamics Simulation Study. J. Nucl. Mater. 2009, 392, 114–122. [Google Scholar] [CrossRef]
- Arima, T.; Yoshida, K.; Idemitsu, K.; Inagaki, Y.; Sato, I. Molecular Dynamics Analysis of Diffusion of Uranium and Oxygen Ions in Uranium Dioxide. IOP Conf. Ser. Mater. Sci. Eng. 2010, 9, 012003. [Google Scholar] [CrossRef]
- Desai, T.G.; Millett, P.; Tonks, M.; Wolf, D. Atomistic Simulations of Void Migration Under Thermal Gradient in UO₂. Acta Mater. 2010, 58, 330–339. [Google Scholar] [CrossRef]
- Boyarchenkov, A.S.; Potashnikov, S.I.; Nekrasov, K.A.; Kupryazhkin, A.Ya. Investigation of Cation Self-Diffusion Mechanisms in UO₂±ₓ Using Molecular Dynamics. J. Nucl. Mater. 2013, 442, 148–161. [Google Scholar] [CrossRef]
- Pitskhelaury, S.; Seitov, D.; Nekrasov, K.; Boyarchenkov, A.; Kupryazhkin, A.; Gupta, S.K. Influence of the Superionic Transition on the Diffusion of Cations in ThO₂ Nanocrystals: A Molecular Dynamics Simulation. Mater. Today Proc. 2023, in press. [CrossRef]
- Momma, K.; Izumi, F. VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data. J. Appl. Crystallogr. 2011, 44, 1272–1276. [Google Scholar] [CrossRef]
- Seitov, D.D.; Pitskhelaury, S.S.; Nekrasov, K.A.; Boyarchenkov, A.S.; Kupryazhkin, A.Ya. A Mechanism of Cation Diffusion in ThO₂ Nanocrystal Bulk: A Molecular Dynamic Simulation. AIP Conf. Proc. 2022, 2466, 030040. [Google Scholar] [CrossRef]
- Cooper, M.W.D.; Rushton, M.J.D.; Grimes, R.W. A Many-Body Potential Approach to Modelling the Thermomechanical Properties of Actinide Oxides. J. Phys. Condens. Matter 2014, 26, 105401. [Google Scholar] [CrossRef]
- Verlet, L. Computer "Experiments" on Classical Fluids. I. Thermodynamical Properties of Lennard-Jones Molecules. Phys. Rev. 1967, 159, 98–103. [Google Scholar] [CrossRef]
- Berendsen, H.J.C.; Postma, J.P.M.; van Gunsteren, W.F.; DiNola, A.; Haak, J.R. Molecular Dynamics with Coupling to an External Bath. J. Chem. Phys. 1984, 81, 3684–3690. [Google Scholar] [CrossRef]
- Bussi, G.; Donadio, D.; Parrinello, M. Canonical Sampling Through Velocity Rescaling. J. Chem. Phys. 2007, 126, 014101. [Google Scholar] [CrossRef]
- Nosé, S. A Unified Formulation of the Constant Temperature Molecular Dynamics Methods. J. Chem. Phys. 1984, 81, 511–519. [Google Scholar] [CrossRef]
- Hoover, W.G. Canonical Dynamics: Equilibrium Phase-Space Distributions. Phys. Rev. A 1985, 31, 1695–1697. [Google Scholar] [CrossRef] [PubMed]
- Nekrasov, K.A.; Boyarchenkov, A.S.; Kupryazhkin, A.Ya.; Gupta, S.K. The melting mechanisms of UO2 nanocrystals: A molecular dynamics simulation. AIP Conf. Proc. 2019, 2142, 020001. [Google Scholar] [CrossRef]
- Vanfleet, R.R.; Mochel, J.M. Thermodynamics of Melting and Freezing in Small Particles. Surf. Sci. 1995, 341, 40–50. [Google Scholar] [CrossRef]
- Pavlov, T.R.; Wangle, T.; Wenman, M.R.; Tyrpekl, V.; Vlahovic, L.; Robba, D.; Van Uffelen, P.; Konings, R.J.M.; Grimes, R.W. High Temperature Measurements and Condensed Matter Analysis of the Thermo-Physical Properties of ThO₂. Sci. Rep. 2018, 8, 5038. [Google Scholar] [CrossRef] [PubMed]
- De Bruycker, F.; Boboridis, K.; Manara, D.; Pöml, P.; Rini, M.; Konings, R.J.M. Reassessing the Melting Temperature of PuO₂. Mater. Today 2010, 13, 52–55. [Google Scholar] [CrossRef]
- Epstein, L.F. Ideal Solution Behavior and Heats of Fusion from the UO₂-PuO₂ Phase Diagram. J. Nucl. Mater. 1967, 22, 340–348. [Google Scholar] [CrossRef]
- Fink, J.K.; Sofu, T.; Ley, H. International Nuclear Safety Center Database on Thermophysical Properties of Reactor Materials. Int. J. Thermophys. 1999, 20, 279–287. [Google Scholar] [CrossRef]
- Dorado, B.; Andersson, D.A.; Stanek, C.R.; Bertolus, M.; Uberuaga, B.P.; Martin, G.; Freyss, M.; Garcia, P. First-Principles Calculations of Uranium Diffusion in Uranium Dioxide. Phys. Rev. B 2012, 86, 035110. [Google Scholar] [CrossRef]
- Gupta, F.; Brillant, G.; Pasturel, A. Correlation Effects and Energetics of Point Defects in Uranium Dioxide: A First Principle Investigation. Philos. Mag. 2007, 87, 2561–2569. [Google Scholar] [CrossRef]
- Crocombette, J.P.; Jollet, F.; Nga, L.T.; Petit, T. Plane-Wave Pseudopotential Study of Point Defects in Uranium Dioxide. Phys. Rev. B 2001, 64, 104107. [Google Scholar] [CrossRef]
- Freyss, M.; Petit, T.; Crocombette, J.P. Point Defects in Uranium Dioxide: Ab Initio Pseudopotential Approach in the Generalized Gradient Approximation. J. Nucl. Mater. 2005, 347, 44–51. [Google Scholar] [CrossRef]
- Freyss, M.; Vergnet, N.; Petit, T. Ab Initio Modeling of the Behavior of Helium and Xenon in Actinide Dioxide Nuclear Fuels. J. Nucl. Mater. 2006, 352, 144–150. [Google Scholar] [CrossRef]
- Moxon, S.; Skelton, J.; Joshua, S.T.; Flitcroft, J.; Togo, A.; Cooke, D.J.; Da Silva, E.L.; Harker, R.M.; Storr, M.T.; Parker, S.C. Structural Dynamics of Schottky and Frenkel Defects in ThO₂: A Density-Functional Theory Study. J. Mater. Chem. A 2022, 10, 1861–1875. [Google Scholar] [CrossRef]
- Yun, Y.; Oppeneer, P.M.; Kim, H.; Park, K. Defect Energetics and Xe Diffusion in UO₂ and ThO₂. Acta Mater. 2009, 57, 1655–1659. [Google Scholar] [CrossRef]
- Dorado, B.; Jomard, G.; Freyss, M.; Bertolus, M. Stability of Oxygen Point Defects in UO₂ by First-Principles DFT+U Calculations: Occupation Matrix Control and Jahn-Teller Distortion. Phys. Rev. B 2010, 82, 035114. [Google Scholar] [CrossRef]
- Iwasawa, M.; Chen, Y.; Kaneta, Y.; Ohnuma, T.; Geng, H.Y.; Kinoshita, M. First-Principles Calculation of Point Defects in Uranium Dioxide. Mater. Trans. 2006, 47, 2651–2657. [Google Scholar] [CrossRef]
- Nerikar, P.; Watanabe, T.; Tulenko, J.S.; Phillpot, S.R.; Sinnott, S.B. Energetics of Intrinsic Point Defects in Uranium Dioxide from Electronic-Structure Calculations. J. Nucl. Mater. 2009, 384, 61–69. [Google Scholar] [CrossRef]
- Dorado, B.; Freyss, M.; Martin, G. GGA+U Study of the Incorporation of Iodine in Uranium Dioxide. Eur. Phys. J. B 2009, 69, 203–209. [Google Scholar] [CrossRef]
- Wang, L.F.; Sun, B.; Liu, H.F.; Lin, D.Y.; Song, H.F. Thermodynamics and Kinetics of Intrinsic Point Defects in Plutonium Dioxides. J. Nucl. Mater. 2019, 526, 151762. [Google Scholar] [CrossRef]
- Nakamura, H.; Machida, M. A First-Principles Study on Point Defects in Plutonium Dioxide. Prog. Nucl. Sci. Technol. 2018, 5, 132–135. [Google Scholar] [CrossRef]
- Singh, S.; Sonvane, Y.; Nekrasov, K.A.; Kupryazhkin, A.Ya.; Gajjar, P.N.; Gupta, S.K. A First Principles Investigation of Defect Energetics and Diffusion in Actinide Dioxides. J. Nucl. Mater. 2024, 591, 154901. [Google Scholar] [CrossRef]
- Lu, Y.; Yang, Y.; Zhang, P. Charge States of Point Defects in Plutonium Oxide: A First-Principles Study. J. Alloys Compd. 2015, 649, 544–552. [Google Scholar] [CrossRef]
- Tian, X.; Gao, T.; Lu, C.; Shang, J.; Xiao, H. First Principle Study of the Behavior of Helium in Plutonium Dioxide. Eur. Phys. J. B 2013, 86, 179. [Google Scholar] [CrossRef]
- Adamson, M.G.; Aitken, E.A.; Caputi, R.W. Experimental and thermodynamic evaluation of the melting behavior of irradiated oxide fuels. J. Nucl. Mater. 1985, 130, 245–252. [Google Scholar] [CrossRef]
- Yun, Y.; Eriksson, O.; Oppeneer, P.M. First-Principles Study of Helium Behavior in Nuclear Fuel Materials. Preprints 2011. Available online: https://www.researchgate.net/publication/287350512_Firstprinciples_study_of_helium_behavior_in_nuclear_fuel_materials (accessed on 04 May 2025).
- Lu, Y.; Yang, Y.; Zhang, P. Thermodynamic Properties and Structural Stability of Thorium Dioxide. J. Phys. Condens. Matter 2012, 24, 225801. [Google Scholar] [CrossRef] [PubMed]
- Murphy, S.T.; Cooper, M.W.D.; Grimes, R.W. Point Defects and Non-Stoichiometry in Thoria. Solid State Ionics 2014, 267, 80–87. [Google Scholar] [CrossRef]
- Konings, R.J.M.; Beneš, O. The Heat Capacity of NpO₂ at High Temperatures: The Effect of Oxygen Frenkel Pair Formation. J. Phys. Chem. Solids 2013, 74, 653–655. [Google Scholar] [CrossRef]
- Hutchings, M.T. High-Temperature Studies of UO₂ and ThO₂ Using Neutron Scattering Techniques. J. Chem. Soc. Faraday Trans. 2 1987, 83, 1083–1103. [Google Scholar] [CrossRef]
- Matthews, J.R. Technological Problems and the Future of Research on the Basic Properties of Actinide Oxides. J. Chem. Soc. Faraday Trans. 2 1987, 83, 1273–1285. [Google Scholar] [CrossRef]
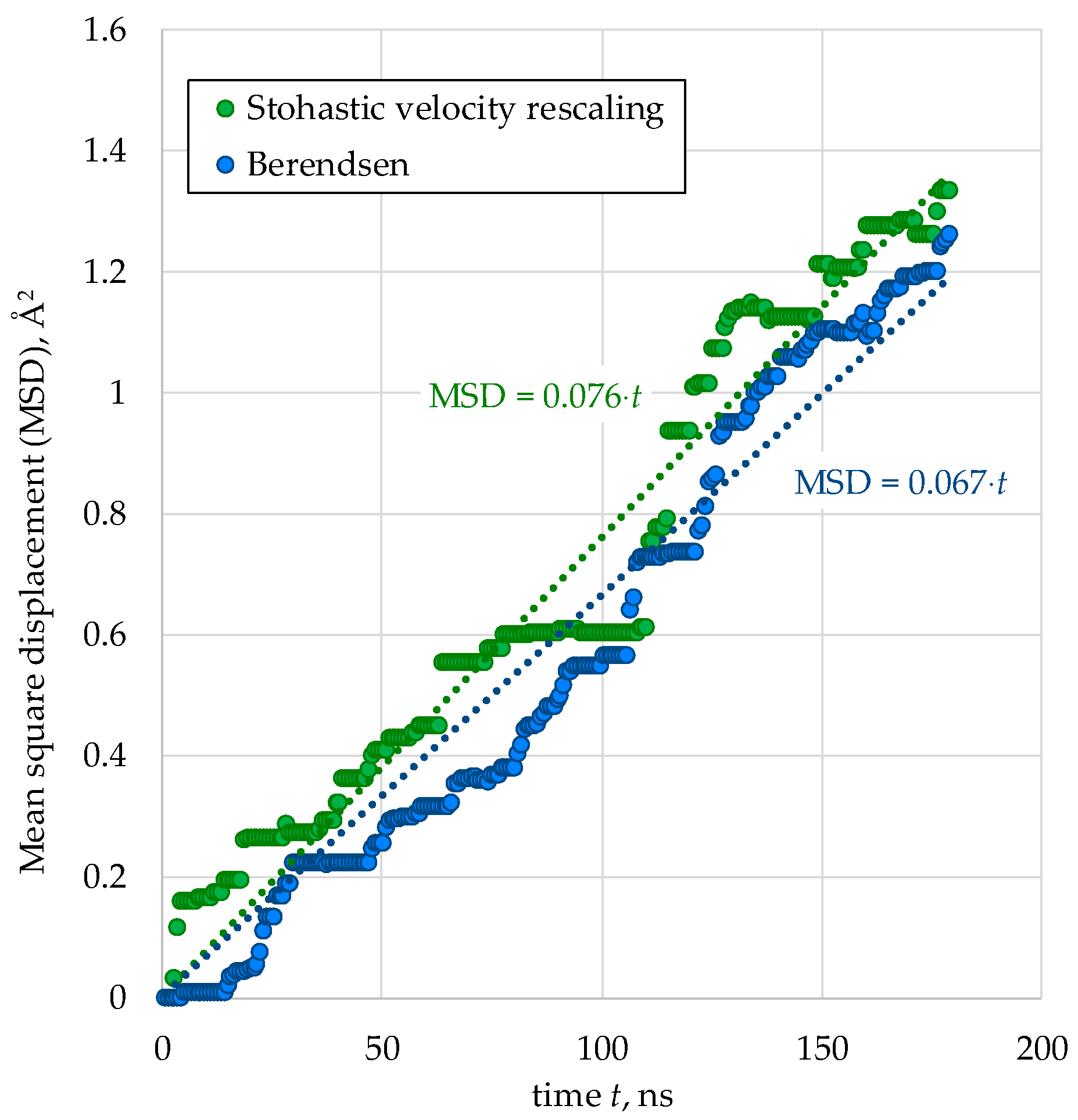
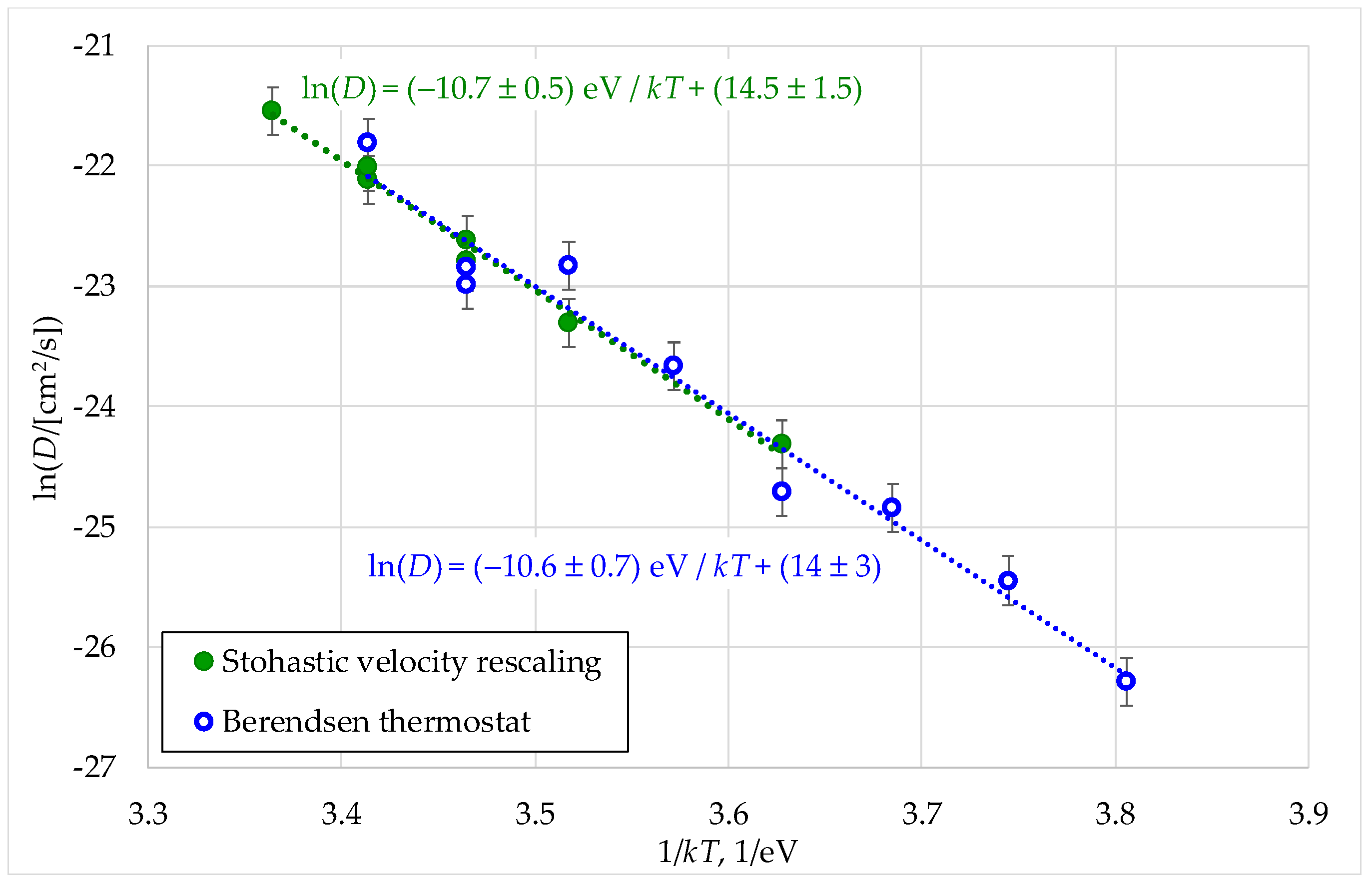
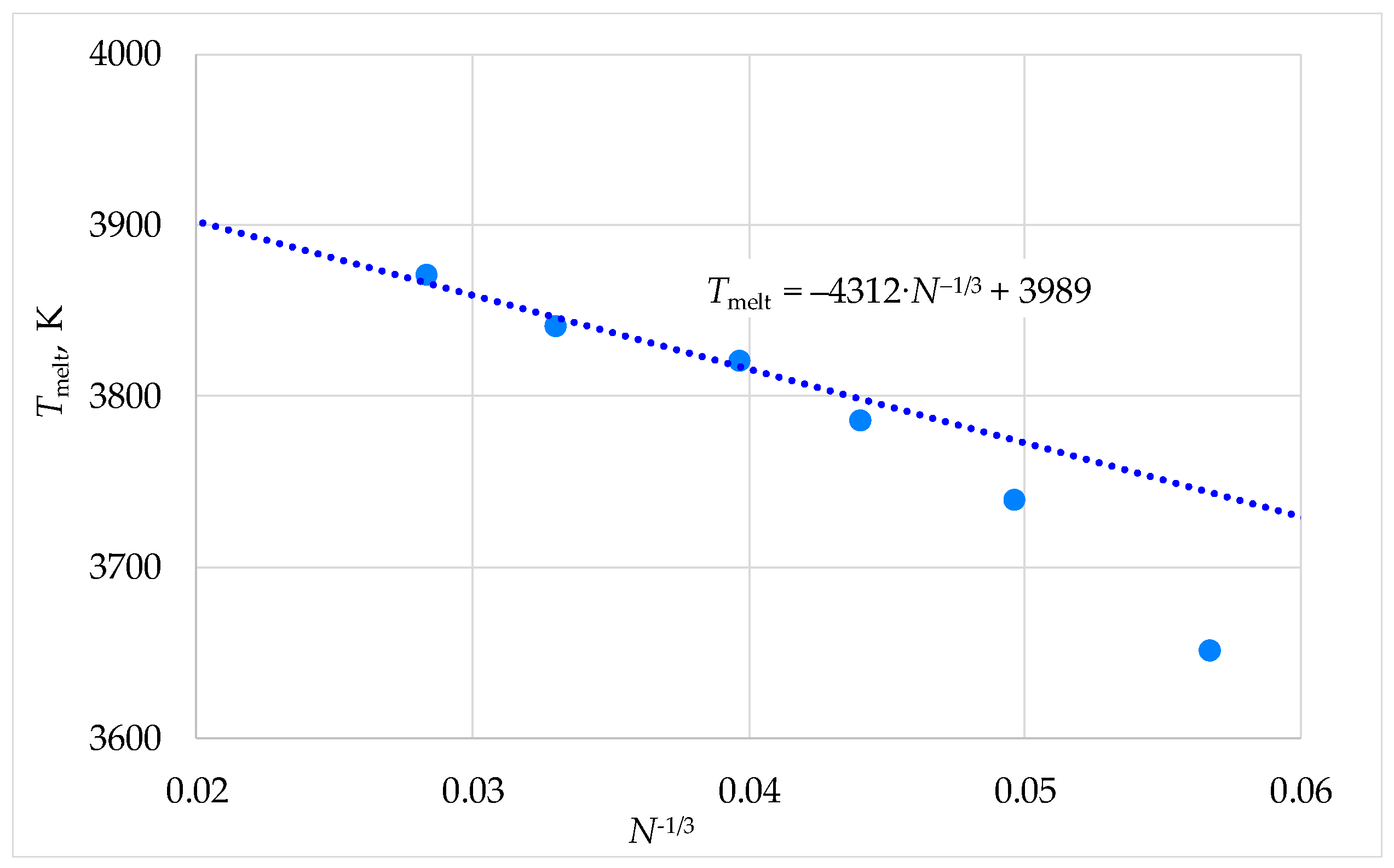
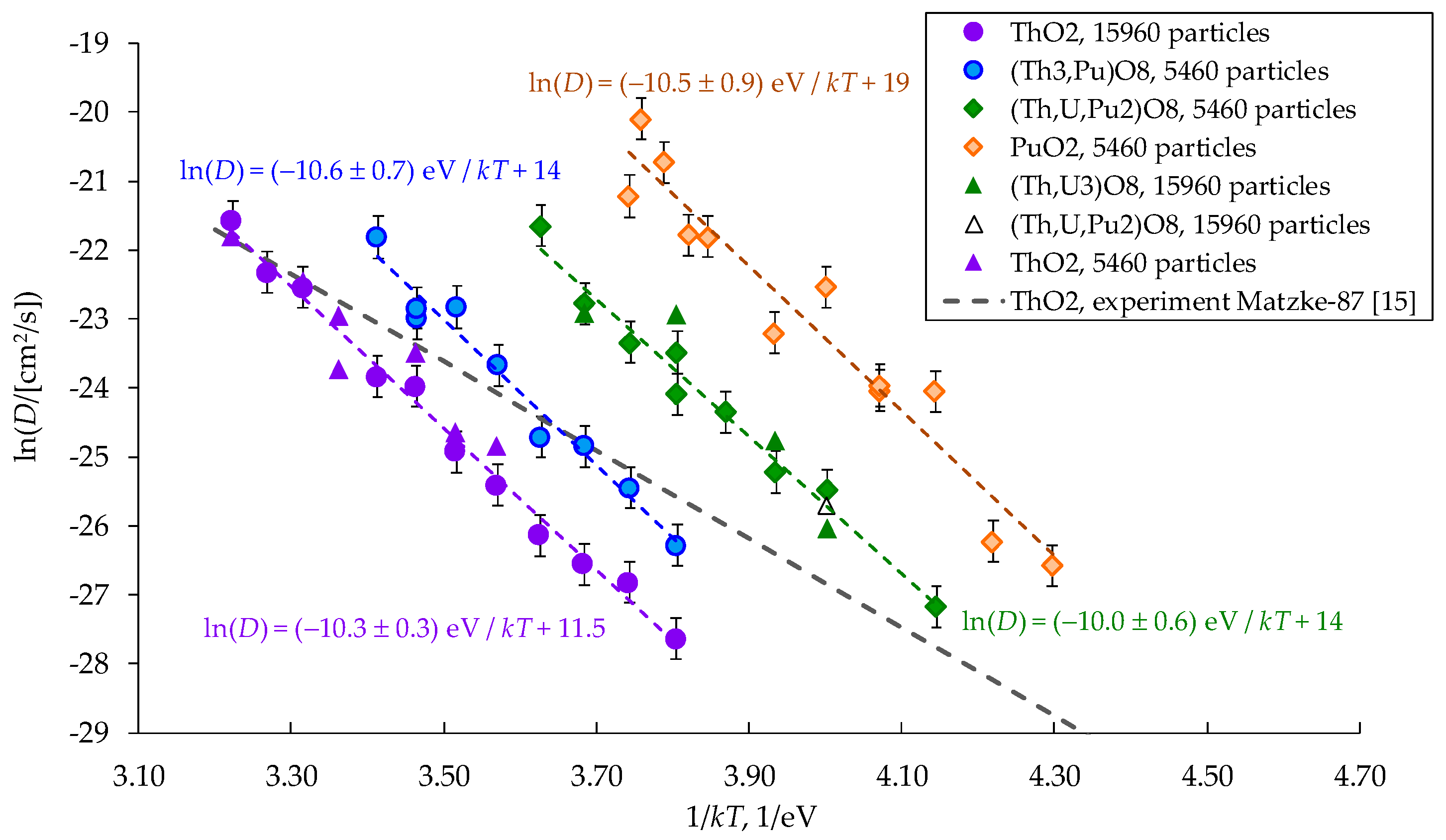
| Compound | T∞, K, present work | T∞, K, experiment |
| ThO2 | 3990 | 3665 ± 70 K [55] |
| (Th0.75Pu0.25)O2 | 3795 | – |
| (Th0.25U0.25Pu0.5)O2 | 3630 | – |
| PuO2 | 3450 | 3017 ± 28 K [56] |
| Compound | L, kJ/mol | γ, J/m2 |
| ThO2 | 37.1 | 4.40 |
| (Th0.75Pu0.25)O2 | 45.3 | 4.33 |
| (Th0.25U0.25Pu0.5)O2 | 65.1 | 3.94 |
| PuO2 | 68.2 | 4.20 |
| Mechanism of disor dering | Formation energy, eV | ||
| ThO2 | UO2 | PuO2 | |
| Unbound intersti tial anion and anion vacancy (EAF) | 4.5, MOX-07 [44] 6.8, GGA [77] 5.0, GGA [78] 9.8, GGA [65] 9.5, GGA [72] 2.3-4.7, exp [12] 4.42, exp [79] |
4.1, MOX-07 [30] 5.9, CRG [30] 4.0, GGA+U [60] 3.6, GGA [60] 4.5, GGA [65] 3.95, GGA+U [68] 5.8, GGA+U [66] 3.3, GGA+U [59] 3.6, GGA [63] 3.5±0.5, exp [14] 4.6, exp [80] |
3.9, this work 3.9, MOX-07 [30] 5.5, CRG [30] 5.5, CRG [70] 3.48, LDA+U [73] 5.3, GGA [63] 4.4, GGA, [76] 4.6, GGA+U, [71] 4.2, GGA [72] 9.8, GGA+U [74] 2.7-2.9, exp [12] |
| Classic Schottky trio V4− + 2V2+ (ESh) | 12.7, MOX-07 [44] 8.2, GGA [77] 8.05, GGA [78] 20.6, GGA, [65] |
8.9, this work 9.7, MOX-07 [30] 10.9, CRG [30] 7.2, GGA+U [60] 5.2, GGA [60] 7.2, GGA [65] 7.6, GGA+U [68] 6.0, GGA+U [59] 5.6, GGA [63] 6-7, exp [14] |
9.5, MOX-07 [30] 10.0, CRG [30] 10.4, CRG [70] 7.5, LDA+U [73] 9.1, GGA [63] 7.1, GGA [76] 6.09, GGA+U [71] 14.9, GGA+U [74] |
| Bound Schottky trio (V4−⋅2V2+)111 | 6.9, MOX-07 [44] 5.4, GGA [64] 4.5, GGA [78] 4.6, GGA [72] |
4.8, this work 4.8, MOX-07 [30] 5.0, CRG [30] 3.6, GGA [72] |
5.0, MOX-07 [30] 4.8, CRG [30] 4.8, CRG [70] 3.6, GGA [72] |
| Partially bound Schottky trio V4−⋅V2++V2+ | 9.7, MOX-07 [44] | 6.7, this work | 7.0, this work |
| Migration of a single cation vacancy V4− (height of potential barrier EM) | 5.45, this work 4.5, GGA [65] 5.7, GGA [72] |
3.1, GGA [65] 4.2, GGA+U [81] 3.6, GGA+U [59] 5.4, GGA [72] 2.4, exp [14] |
4.5, this work 3.4, CRG [70] 5.8, GGA [72] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





