1. Introduction
Since receiving FDA approval in 1995, oral bisphosphonates (BP) have been extensively used for a variety of medical indications over the past 16 years. These drugs are predominantly prescribed to treat and prevent osteoporosis in at-risk populations [
1,
2,
3,
4]. Additionally, Bisphosphonates are used in the management of patients with bone metastases from various cancers, including primary gastrointestinal tumors, and are also being explored for the prevention of skeletal complications in cancer patients [
1,
2,
5,
6]. Recent studies have associated these drugs with a reduced risk of breast cancer [
1,
7]. Bisphosphonates possess the ability activate both adaptive and innate immune responses, suppress tumor angiogenesis, invasion, and cell adhesion, thus influencing overall tumor progression [
8]. Nitrogen-containing bisphosphonates interfere with the mevalonate pathway, leading to the inhibition of protein prenylation, which is believed to affect cancer cell growth and metastasis [
8,
9]. Furthermore, bisphosphonates have been shown To suppress colorectal cancer development in an experimental model of ulcerative colitis. Recent evidence also suggests that these drugs can reduce proliferation and induce apoptosis of colon cancer cells [
8]. In the past 2 years, several studies have evaluated the association between oral bisphosphonate use and the risk of colorectal cancer, yielding conflicting results. The definition of bisphosphonate exposure varied among these studies, limiting the generalizability of their findings to routine clinical practice. This meta-analysis aims to further assess the existing evidence on the association between oral bisphosphonate use and colorectal cancer risk, and quantitatively synthesize the findings through a meta-analysis based on a standardized definition of bisphosphonate exposure.
2. Materials and Methods
We adhered to a standardized methodology [
10] and present the findings in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [
11]. Meta-analysis is registered in PROSPERO n.1035664.
2.1. Search Strategy
To identify all pertinent papers, in English or other European languages, examining the risk of intestinal cancer associated with bisphosphonate (BP) therapy in well-defined observational studies, we performed a comprehensive and systematic search using PubMed, Embase and Scopus as search engine from January 1, 1966 until 5 April, 2025 using the MESH terms diphosphonates AND epidemiology combined with a free text search on the words: ’observational study’ OR ’case control study’ with the following filter: studies of humans (for the complete search strategy, see supplementary material). In addition, reference lists of all included papers and their “related articles” were scrutinized to disclose additional literature on the topic.
2.2. Inclusion/Exclusion Criteria
The full texts of potentially eligible articles were independently reviewed by two authors (E.A.G., and F.F.). We considered articles that were epidemiologic studies examining any intestinal cancer outcome, including colorectal, colon, or rectal cancer, in populations of any ethnicity, gender or age in all countries and settings.
This meta-analysis includes studies that fulfilled the following criteria:
1) Randomized controlled trials, prospective or retrospective cohort studies, nested case-control studies or population-based case-control studies;
2) The primary outcome was the incidence of colorectal, colon, or rectal cancer;
3) The study reported relative risks (RR), odds ratios (OR), or hazard ratios (HR) along with 95% confidence intervals were reported or enough data to calculate them;
4) Bisphosphonate exposure was defined as having at least one documented prescription or dispensation prescription of BPs or pharmacy record or reported in interview in at least the last two weeks.
Papers focused on cancer mortality and review articles were excluded. In instances of duplicate publications, the most recent study with the longest follow-up of patients was considered.
2.3. Data Extraction
Data extraction was performed independently by 2 authors (E.A.G. and F.F.). Disagreements were resolved by consensus. The included papers were reviewed in detail for data on number of patients studied, calendar year of publication, number of colon and rectum cancers observed in the cohort, expected numbers in a matched background population, with relative Confidence Intervals at 95% (95% C.I.s).
We selected the ORs or HRs that provided the highest level of adjustment for potential confounders for inclusion in the primary analysis.
2.4. Quality Assessment
The quality of the studies was independently evaluated by two authors (E.A.G. and F.F.) using the 9-star Newcastle-Ottawa Scale (NOS) [
12,
13]. The NOS evaluates study quality based on three criteria: selection, comparability, and either exposure (for case-control studies) or outcome (for cohort studies). A maximum of four points is awarded for selection, two for comparability, and three for the assessment of exposure or outcome. A total score of nine points on the NOS indicates the highest methodological quality of the study.
2.5. Statistical Analysis
We presented the extracted random-effects estimates by means of forest plots displaying the association between BP and CRC.
If OR/HR of CRC were reported without 95% C.Is, the interval was calculated assuming a gaussian distribution of cases.
Meta-analysis used the Restricted Maximum Likelihood [
14,
15,
16] (REML) for estimating variance components in mixed-effects models to estimate the between-study variance. The influence of the use of BP over time was assessed with the REML method.
Subsequently, pooled ORs with 95% CIs were calculated. Depending on the heterogeneity test, either a fixed or a random effects model was applied. To test the null hypothesis, the two-tailed probability level was set at 0.05. All analyses were conducted with Stata 18 (StataCorp. 2023. Stata Statistical Software: Release 18, College Station, TX: StataCorp LLC.), and R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing, Vienna, Austria, 2024;
https://www.R-project.org).
2.6. Ethics Statement
This meta-analysis was conducted using data extracted from previously published population-based cohort studies and case-control studies. All included studies had obtained the necessary ethical approval from their respective institutional review boards, as documented in the original publications.
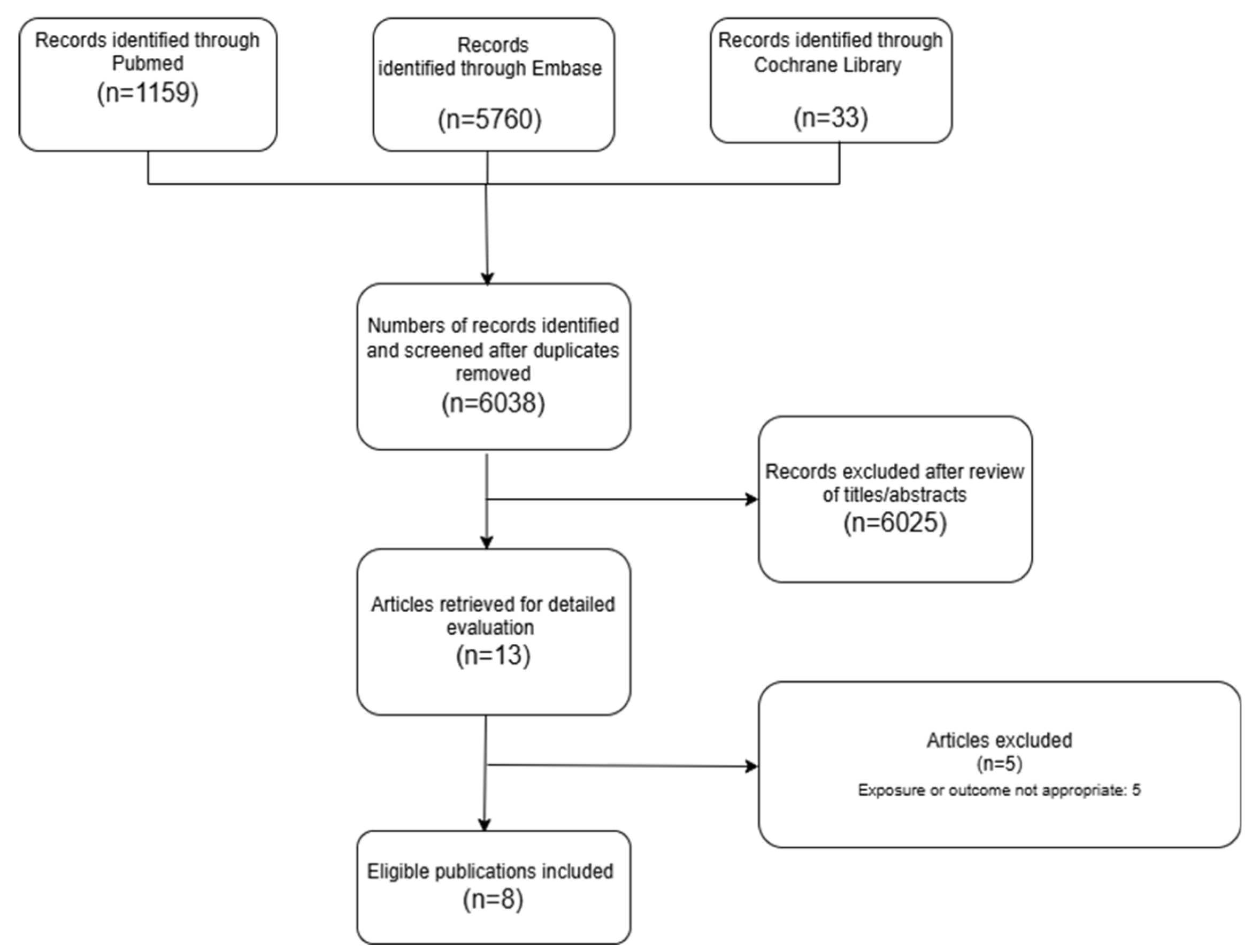
Figure 1.
PRISMA selection progress.
Figure 1.
PRISMA selection progress.
3. Results
3.1. Literature Search
The detailed steps of our literature search are shown in the supplementary material. Briefly, we identified 8 relevant articles [
2,
6,
17,
18,
19,
20,
21,
22] focusing on BP exposure and gastrointestinal cancers, that were included in the meta-analysis.
3.2. Study Characteristics
Eight studies published between 2010 and 2020 examined the relationship between oral bisphosphonates and the risk of colorectal cancer (CRC). The detailed study characteristics are presented in
Table 1.
There were three nested case-control studies; two were population-based case-control studies and three were nationwide retrospective cohort studies. The studies were judged to warrant 7 on the 9-star Newcastle-Ottawa Scale (See supplementary Tables S1, and S2). The details of the quality assessment for each study are shown in supplementary materials. Regarding exposure assessment, some studies, such as those by Green et al. [
18], Ibanez et al. [
19], Vinogradova et al. [
21] and Vogtmann et al. [
22], considered the number of written bisphosphonate prescriptions, while others, including Choi et al. [
17], Rennert et al. [
20], and Vestergaard [
2], used pharmacy records of such prescriptions. Passarelli et al. employed an interview-based approach, where participants were asked to bring in all medications taken in the previous weeks.
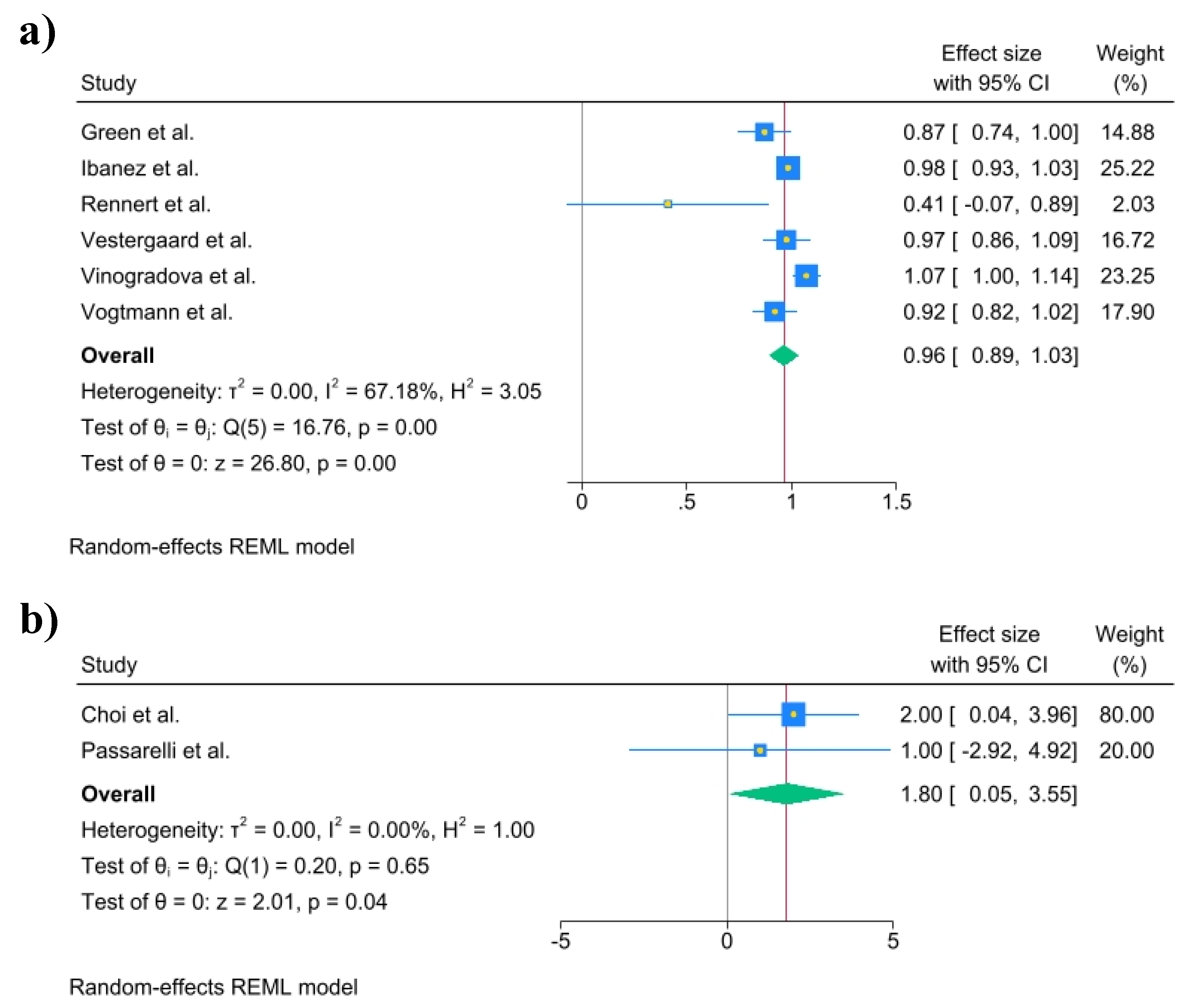
The multivariate-adjusted odds ratios (ORs) for colorectal cancer (CRC) risk for each individual study, as well as for all studies combined, comparing any bisphosphonate exposure with no exposure, are presented in
Figure 2a and 2b. The pooled joint OR was 0.96 (95% C.I., 0.89 to 1.03), Heterogeneity: τ
2 = 0.01, I
2 = 67.18%, H
2 = 3.05, Test of qi = qj: Q(4) = 16.76, p = 0.00, test of q = 0: z = 26.8, p = 0.00. The pooled joint HR in cohort studies was 1.80 (95% C.I., 0.05 to 3.55), Heterogeneity: τ
2 = 0.00, I
2 = 0.00%, H
2 = 1.00, Test of qi = qj: Q(1) = 0.20, p = 0.65, Test of q = 0: z = 2.01, p = 0.04.
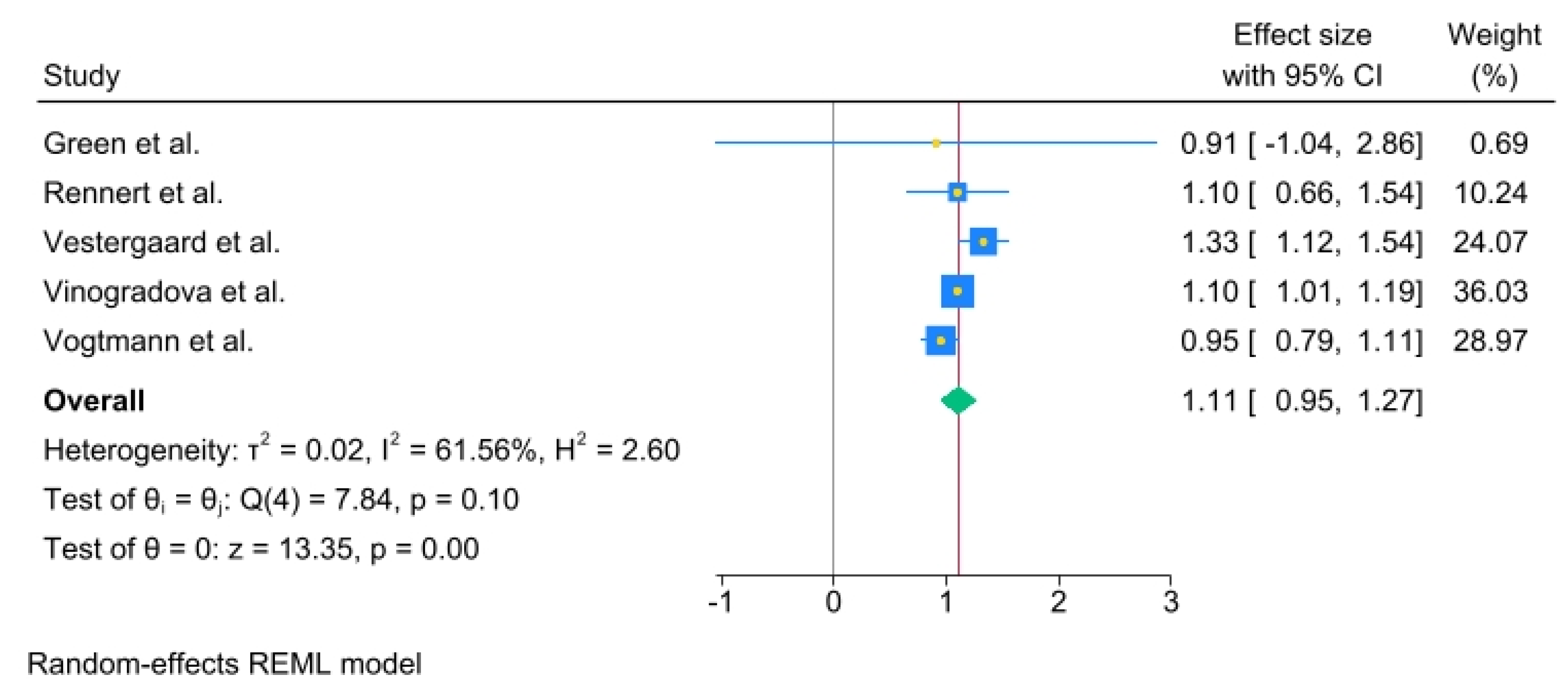
3.3. Duration of Exposure Analysis
The odds ratios (ORs) for each study and the pooled ORs for categories of use duration: less than 1 year, from 1 to 3 years, and more than 3 years were analysed. Less than 1 year of use of oral BPs yielded an OR= 1.11, (95% C.I., 0.95 to 1.27), Heterogeneity: τ
2 = 0.02, I
2 = 61.56%, H
2 = 2.60, Test of qi = qj: Q(4) = 7.84, p = 0.10 Test of q = 0: z = 13.35, p = 0.00 (
Figure 3).
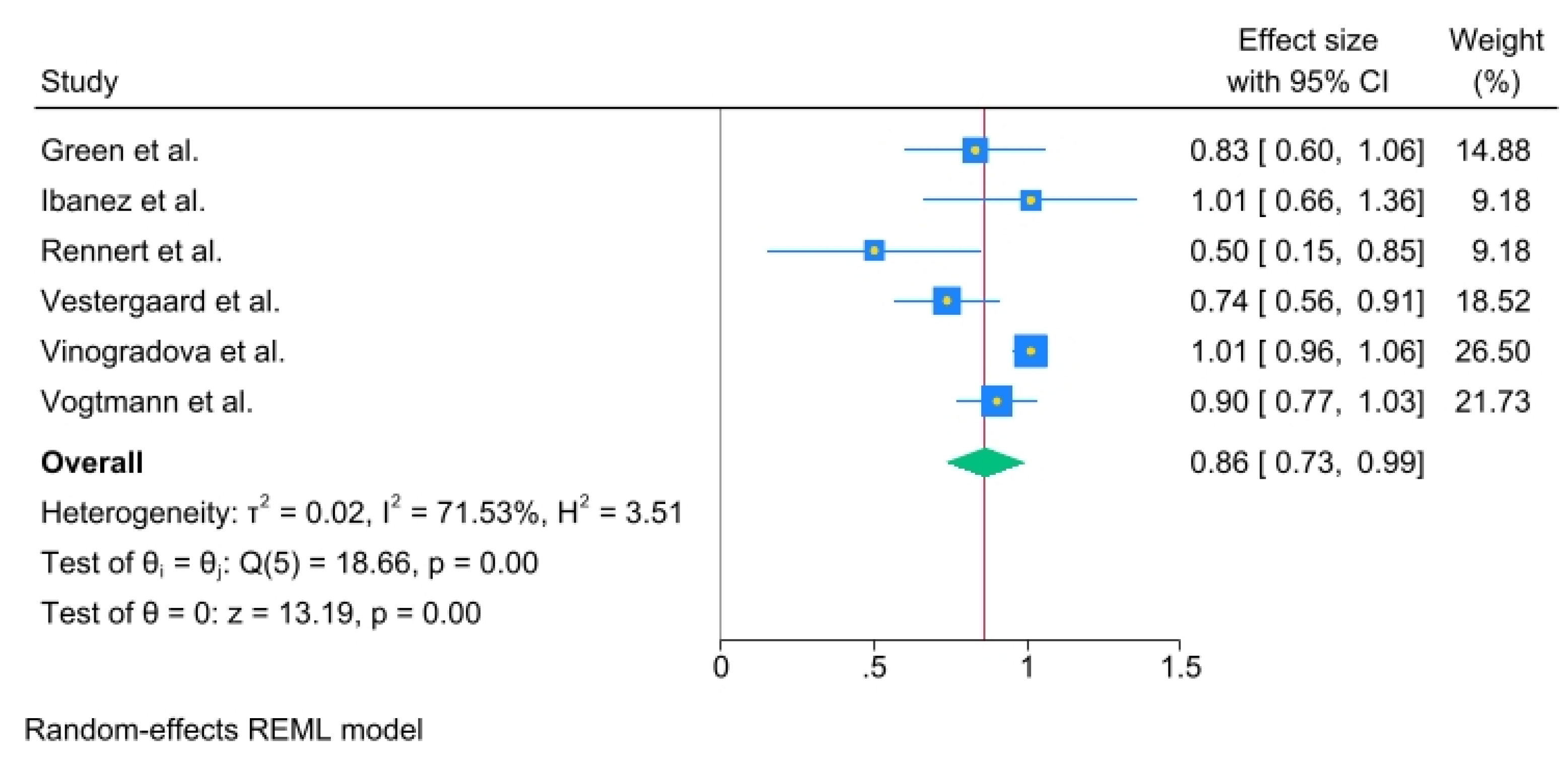
A significant association was noted between 1-3 years (OR, 0.86; 95% C.I., 0.73 to 0.99), Heterogeneity: τ
2 = 0.02, I
2 = 71.53%, H
2 = 3.51, Test of qi = qj: Q(5) = 18.66, p = 0.00, Test of q = 0: z = 13.19, p = 0.00 (
Figure 4).
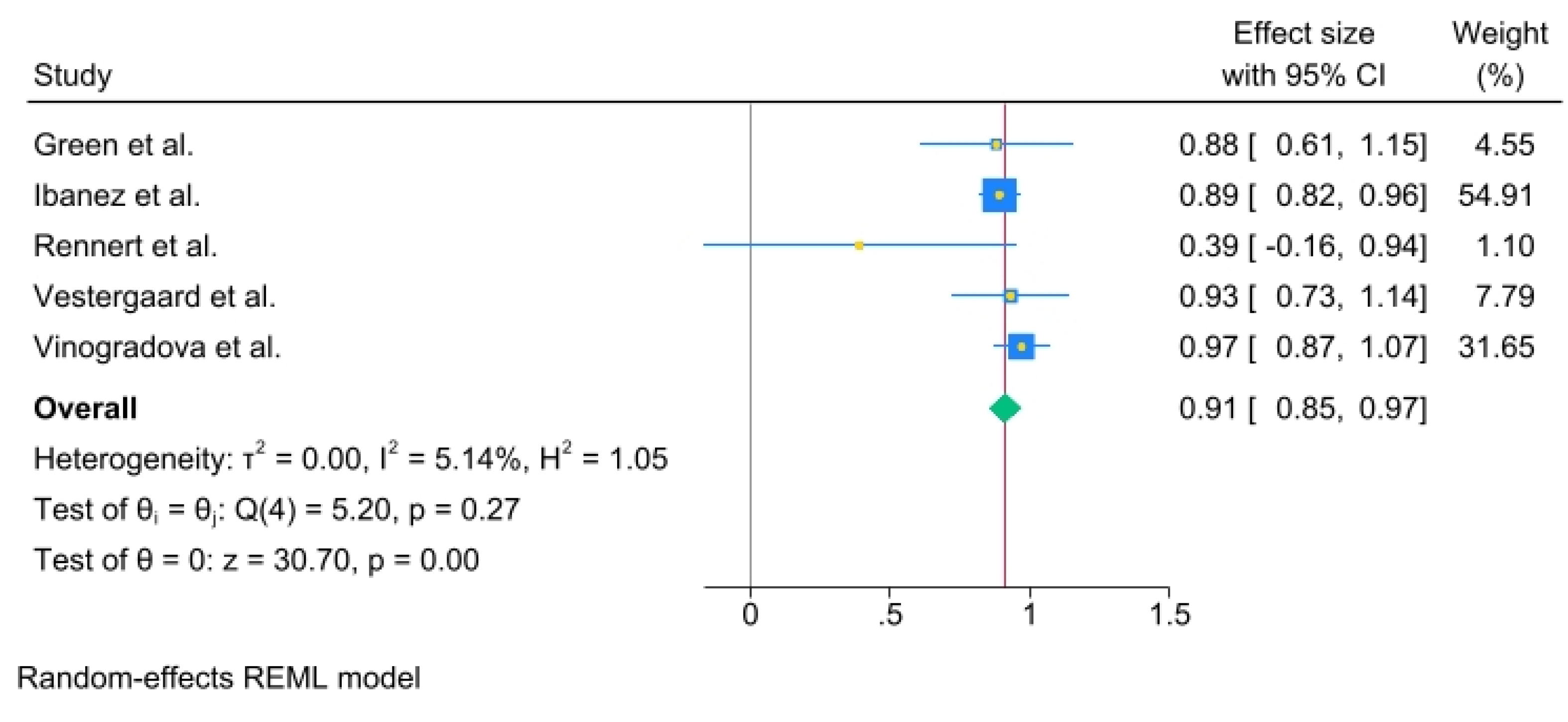
Results for more than 3 years of use were: (OR, 0.91; 95% C.I., 0.85 to 0.97) Heterogeneity: τ
2 = 0.00, I
2 = 5.14%, H
2 = 1.05, Test of qi = qj: Q(4) = 5.20, p = 0.27, Test of q = 0: z = 30.70, p = 0.00 (
Figure 5).
3.4. Heterogeneity and Publication Bias
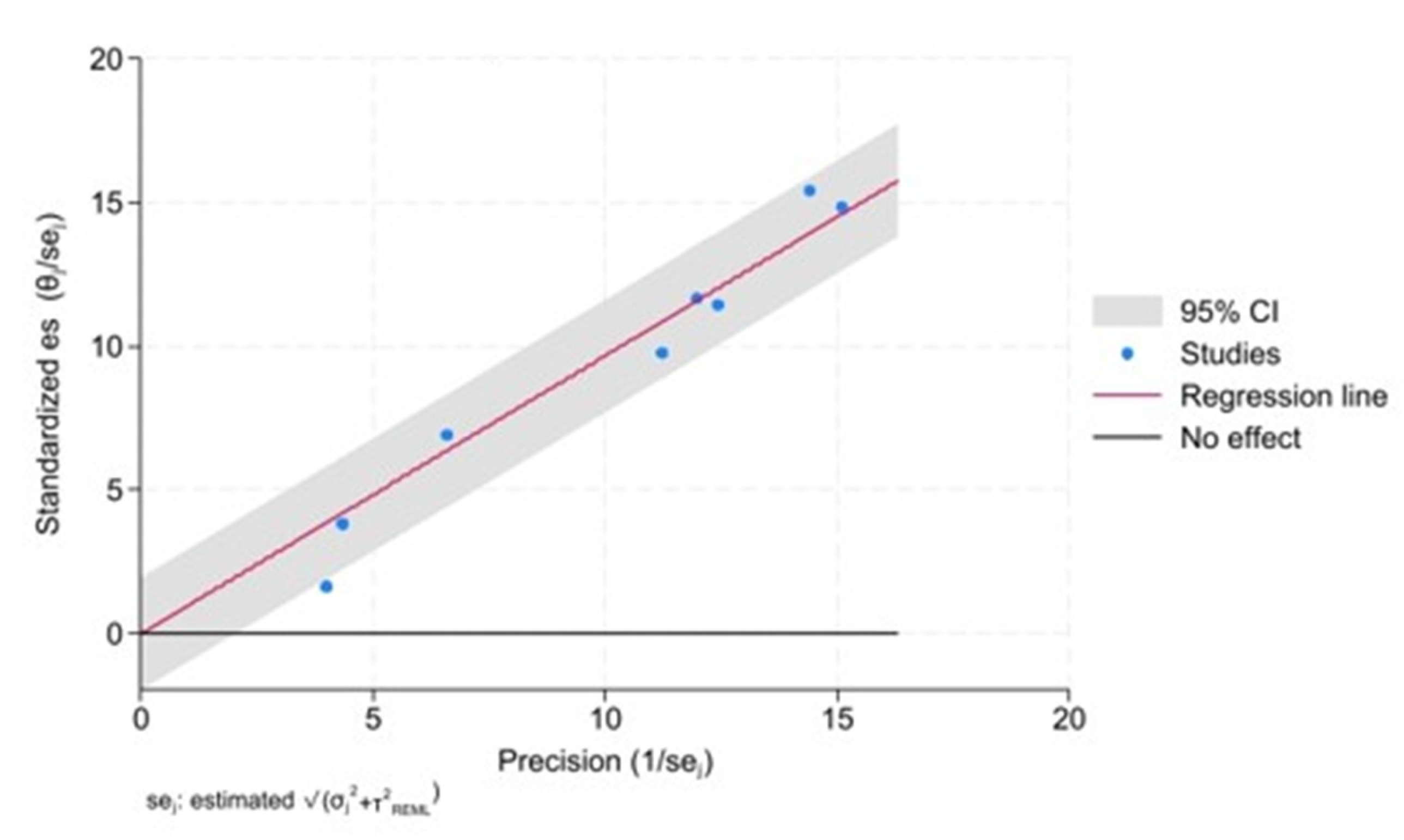
The Galbraith plot depicts the assessment of heterogeneity [
23,
24,
25,
26] and potential publication bias across the included studies. In order to estimate the origin of the heterogeneity we considered whether the quality was a problem in the Included Studies and Publication Bias assessment.
This plot can help visualize the extent of heterogeneity and identify any potential outlier studies that may be contributing to the observed heterogeneity (
Figure 6).
Each study is represented as a point, where the x-axis denotes the precision (defined as the inverse of the standard error), and the y-axis indicates the standardized effect size. The red regression line represents the overall summary effect estimated from the random-effects model, and the grey shaded area corresponds to the confidence interval at 95% around regression line. The horizontal black line at y=0 corresponds to the line of no effect, serving as a reference.
To further assess potential publication bias, we conducted the Egger regression test [
11,
27], which yielded a p-value of 0.08. This Egger test showed no statistically significant evidence of publication bias (p = 0.058). Additionally, the ORs and 95% C.I.s for any use of BPs and CRC did not change substantially with the
“trim-and-fill method
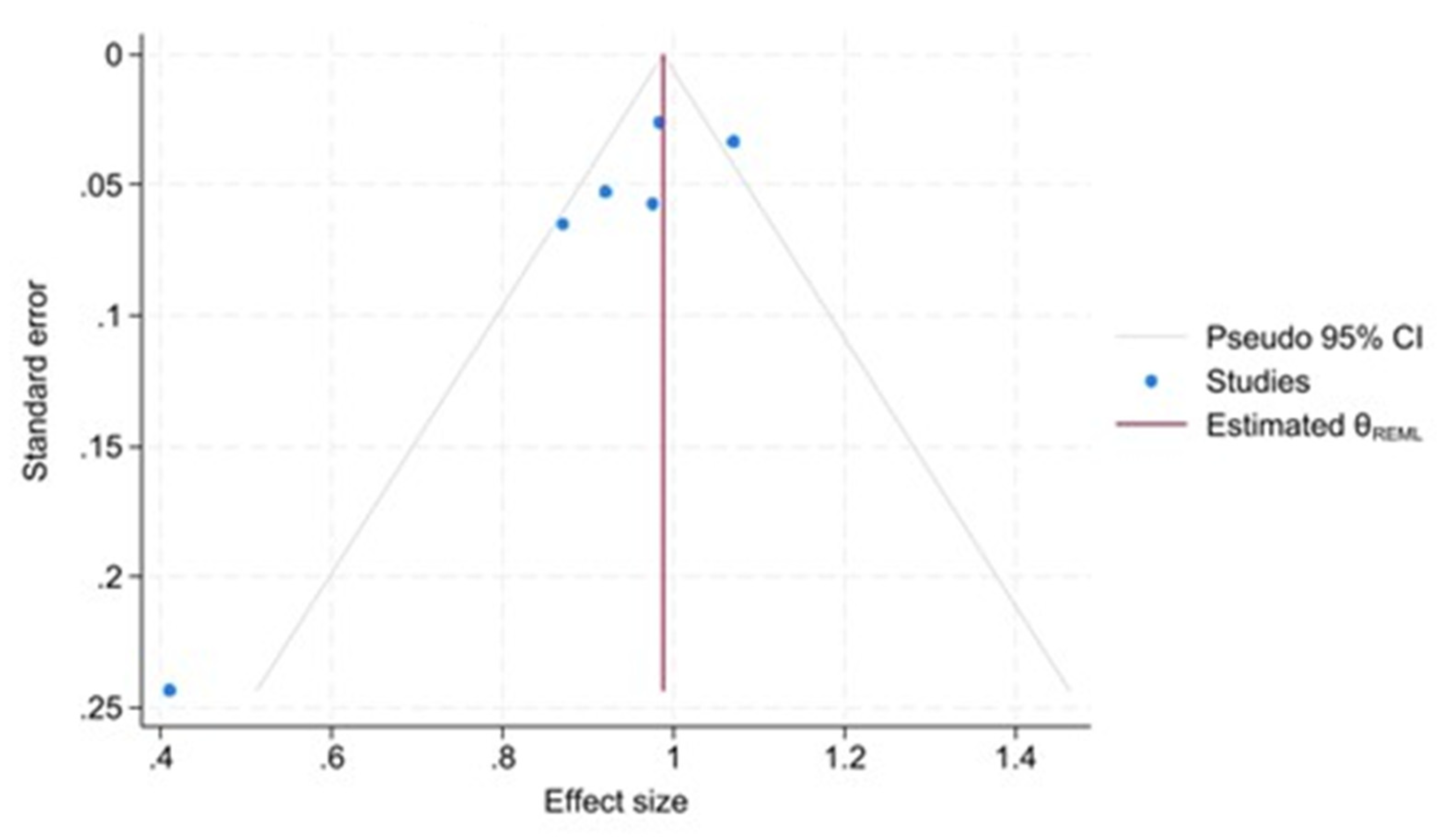
” 26 of publication bias adjustment, suggesting that publication bias is not a major concern in this meta-analysis. A funnel plot for the publication bias [
28,
29] is also shown in
Figure 7, which provides a visual representation of the potential asymmetry in the study effect sizes.
Each blue dot represents a single study, plotted with its effect size on the x-axis and its standard error on the y-axis. The vertical red line represents the overall effect estimate derived from a random-effects model using restricted maximum likelihood (REML). The pseudo 95% confidence interval region, shown as grey diagonal lines, outlines the expected spread of studies in the absence of bias or between-study heterogeneity.
4. Discussion
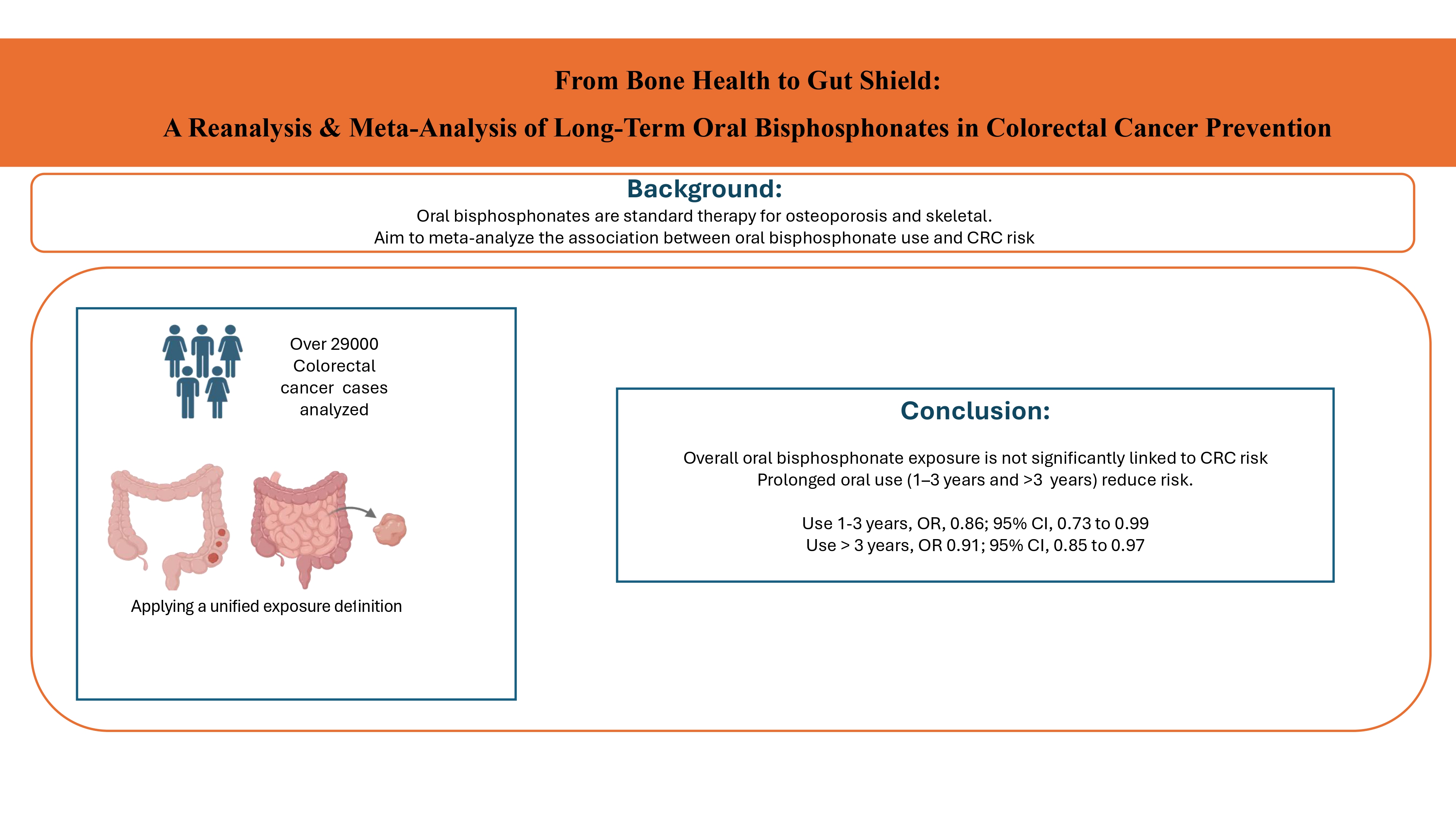
The current meta-analysis summarizes the results of eight large epidemiologic studies, including a total of 29,169 colorectal cancer cases. By incorporating data from a wide range of well-designed observational studies, this comprehensive analysis provides important insights into the potential association between bisphosphonate use and the risk of developing CRC.
This meta-analysis shows that overall, bisphosphonate exposure is not significantly associated with the risk of colorectal cancer. On the contrary, prolonged use for between 1-3 years and over 3 years seems to have a protective effect against colorectal cancer.
In their primary studies, Choi et al. [
17], Ibanez-Sanz et al. [
19], Passarelli et al. [
6] and Vogtmann et al. [
22] all reported no statistically significant association between bisphosphonate use and CRC. Similarly, Green et al. [
18] and Vestergaard [
2] found a borderline, not significant risk reduction of CRC with the use of oral treatment whereas Rennert reported a significant risk reduction of CRC.
Interestingly, the nationwide retrospective cohort studies, Choi et al. [
17], Passarelli et al. [
6] and Vestergaard [
2] showed an overall not statistically significant association with CRC.
Bisphosphonates are synthetic compounds categorized into older non-nitrogen-containing and newer nitrogen-containing types. These drugs have exhibited concentration-dependent direct anti-cancer effects against various malignancies, including colorectal cancer, through mechanisms including the inhibition of angiogenesis, prevention of tumor progression, induction of tumor cell apoptosis, and suppression of metastasis [
5,
18]. Bisphosphonates have been shown to inhibit the growth, proliferation, and metastasis of colorectal cancer cells, and may also work synergistically with chemotherapeutic agents to enhance their anti-cancer effects [
18,
22,
30]. The anti-cancer activities of bisphosphonates are believed to stem from their ability to disrupt the mevalonate pathway, which is crucial for cancer cell growth and metastasis, as well as their capacity to inhibit angiogenesis and tumour cell adhesion, and promote apoptosis [
8]. However, the precise mechanisms by which bisphosphonates may reduce the risk of colorectal cancer require further investigation, and additional research is needed to confirm these findings and determine the optimal use of bisphosphonates for cancer prevention. Although experimental studies have demonstrated the ability of amino bisphosphonates to promote apoptosis and suppress growth and angiogenic factors, it remains unclear whether they play a role in the primary prevention of cancer [
5,
9].
Bisphosphonate treatment has also been associated with adverse outcomes such as osteonecrosis of the jaw and atypical femoral fractures. Osteonecrosis of the jaw has mainly been observed in patients with particular risk factors, including the use of high-dose intravenous bisphosphonate therapy, exposure lasting more than 1 year, concurrent cancer and anticancer treatments, preexisting dental conditions, dental implants, and smoking [
1,
2,
3,
4,
17]. Although the absolute risk is low, prolonged bisphosphonate use for over 5 years can lead to a modest risk of atypical femoral fractures in younger women. Further prospective studies are required to identify the subgroups at highest risk. Additional research is also needed to evaluate the long-term adverse effects and risks associated with extended bisphosphonate therapy and to determine whether the therapeutic benefits truly outweigh the associated risks. While the overall risk of these adverse events is relatively low, it is important for healthcare providers to carefully evaluate the potential risks and benefits of bisphosphonate therapy for each individual patient, especially those with additional risk factors. Ongoing monitoring and appropriate management strategies are crucial to minimize the likelihood of these complications and optimize the safety and efficacy of bisphosphonate treatment.
Given the increasing global burden of colorectal cancer and the well-established safety profile of bisphosphonates for their approved indications, further high-quality research, including randomized controlled trials, is imperative to definitively evaluate their potential as a chemo preventive strategy.
Meta-analysis is a crucial tool that helps clarify the reasons behind discrepancies in trial results, enhances research and editorial standards by highlighting the strengths and weaknesses of the existing body of evidence, and provides practitioners with an objective perspective on the research literature [
31,
32]. The present meta-analysis offers several advantages. First, we employed a consistent definition of exposure, reanalyzing the data based on this standardized definition before pooling it for the analysis. Second, the large number of total cases and controls enhances the statistical power of the analysis. Thirdly, we used Restricted maximum likelihood methods to analyse our data to obtain the maximal likelihood estimate of variance components to maximize the precision of the data. Fourthly, we performed subgroup analysis based on the duration of bisphosphonate exposure to explore potential effect modifications. Fifthly, we used data with the greatest adjustment.
This meta-analysis has different limitations that warrant consideration. Firstly, as an observational study design, it is inherently limited in its ability to fully account for potential confounding factors present in the included studies. Although the primary studies attempted to adjust for relevant confounding variables, the possibility of residual or unknown confounding factors influencing the observed findings cannot be entirely eliminated, necessitating some caution in the interpretation of the results.Furthermore, while major publication bias was not detected in this analysis, the potential for such bias cannot be entirely ruled out. Small studies with null results are generally less likely to be published, while small studies with larger effects have a higher chance of being published, potentially introducing publication bias. The Egger regression test is generally considered more robust than the rank correlation test for evaluating small study effects, but both have limited power, unless significant bias is present, and the number of studies included studies is sufficiently large. Therefore, the results of this meta-analysis should be interpreted while bearing these important limitations in mind.
5. Conclusions
The findings from this meta-analysis suggest that prolonged use of oral bisphosphonates may be associated with a reduced risk of colorectal cancer. However, this potential protective effect was not observed for less than 1 year of bisphosphonate use. Further in-depth longitudinal studies are necessary to consider all potential confounding factors should be considered, different types and methods of bisphosphonate use examined, and the effects of cumulative dose and duration of use on the risk of both colorectal and esophageal cancer evaluated. This approach would provide more accurate estimates and a clearer understanding of the potential role of bisphosphonates in gastrointestinal carcinogenesis. Ultimately, the results of these observational studies will need to be validated through large, well-conducted randomized clinical trials or secondary analysis of previous randomized trials on oral bisphosphonates and osteoporosis, with long-term clinical follow-up to definitively assess the relationship between bisphosphonate use and gastrointestinal cancer risk.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org, Table S1: Quality Assessment for Studies Using the Newcastle-Ottawa Quality Assessment Scale: Case-Control Studies.; Table S2: Quality Assessment for Studies Using the Newcastle-Ottawa Quality Assessment Scale: Cohort Studies.
Author Contributions
Conceptualization, E.A.G., C.G., P.P. and F.F.; methodology, E.A.G., C.G., P.P. and F.F.; software, E.A.G. and F.F.; validation, E.A.G., C.G., P.P., F.F. and R.D.; formal analysis, E.A.G., and F.F.; investigation, E.A.G., C.G., P.P. and F.F.; resources, X.X.; data curation, E.A.G., and F.F.; writing—original draft preparation, E.A.G., C.G. and F.F.; writing—review and editing, E.A.G., C.G., P.P., F.F and R.D.; visualization, E.A.G., F.F. and R.D.; supervision, E.A.G. and R.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Italian Ministry of Health. Ricerca Corrente 2025.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data, analytic methods, and study materials used in this research will be made available to other researchers upon request.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| BP |
Bisphosphonate |
| CRC |
Colorectal Cancer |
| FDA |
Food and Drug Administration |
Search Strategy
PUBMED
(“Diphosphonates”[Mesh] OR diphosphonates[All Fields] OR diphosphonate[All Fields] OR “Bisphosphonates”[All Fields] OR “Alendronate”[Mesh] OR alendronate[All Fields] OR “Clodronic acid”[Mesh] OR clodronic acid[All Fields] OR clodronate[All Fields] OR “Etidronic acid”[Mesh] OR etidronic acid[All Fields] OR etidronate[All Fields] OR “Ibandronic acid”[Substance Name] OR ibandronic acid[All Fields] OR ibandronate[All Fields] OR Minodronate[All Fields] OR “YM 529”[Substance Name] OR “YM 529”[All Fields] OR Neridronate[All Fields] OR “6-amino-1-hydroxyhexane-1,1-diphosphonate”[Substance Name] OR “6-amino-1-hydroxyhexane-1,1-diphosphonate”[All Fields] OR Olpadronic acid[Substance Name] OR olpadronic acid[All Fields] OR olpadronate[All Fields] OR Pamidronate[Substance Name] OR pamidronate[All Fields] OR Risedronic acid[Substance Name] OR risedronic acid[All Fields] OR risedronate[All Fields] OR Tiludronic acid[Substance Name] OR tiludronic acid[All Fields] OR tiludronate[All Fields] OR Zoledronic acid[Substance Name] OR zoledronic acid[All Fields] OR zoledronate[All Fields] ) AND ( “Observational studies”[All Fields] OR “Cohort studies”[All Fields] OR “Case control studies”[All Fields] OR “case-control studies”[All Fields] OR “Case referent studies”[All Fields] OR “case-referent studies”[All Fields] ) AND Humans[Mesh]
EMBASE
(’bisphosphonate’/exp OR ’bisphosphonate’ OR ’diphosphonate’/exp OR ’diphosphonate’ OR ’alendronate’/exp OR ’alendronate’ OR ’clodronate’/exp OR ’clodronate’ OR ’etidronate’/exp OR ’etidronate’ OR ’ibandronate’/exp OR ’ibandronate’ OR ’minodronate’/exp OR ’minodronate’ OR ’neridronate’/exp OR ’neridronate’ OR ’olpadronate’/exp OR ’olpadronate’ OR ’pamidronate’/exp OR ’pamidronate’ OR ’risedronate’/exp OR ’risedronate’ OR ’tiludronate’/exp OR ’tiludronate’ OR ’zoledronate’/exp OR ’zoledronate’) AND (’observational study’/exp OR ’observational study’ OR ’cohort analysis’/exp OR ’cohort analysis’ OR ’case control study’/exp OR ’case control study’ OR ’case referent study’) AND [humans]/lim
COCHRANE LIBRARY
diphosphonate OR diphosphonates OR bisphosphonates OR alendronate OR clodronic acid OR clodronate OR etidronic acid OR etidronate OR ibandronic acid OR ibandronate OR minodronate OR neridronate OR “6-amino-1-hydroxyhexane-1,1-diphosphonate” OR olpadronic acid OR olpadronate OR pamidronate OR risedronic acid OR risedronate OR tiludronic acid OR tiludronate OR zoledronic acid OR zoledronate
References
- Newcomb, P.A.; Trentham-Dietz, A.; Hampton, J.M. Bisphosphonates for osteoporosis treatment are associated with reduced breast cancer risk. Br J Cancer 2010, 102, 799–802. [Google Scholar] [CrossRef] [PubMed]
- Vestergaard, P. Occurrence of gastrointestinal cancer in users of bisphosphonates and other antiresorptive drugs against osteoporosis. Calcif Tissue Int. 2011, 89, 434–41. [Google Scholar] [CrossRef]
- Watts, N.B.; Bilezikian, J.P.; Camacho, P.M.; Greenspan, S.L.; Harris, S.T.; Hodgson, S.F.; Kleerekoper, M.; Luckey, M.M.; McClung, M.R.; Pollack, R.P.; Petak, S.M. American Association of Clinical Endocrinologists Medical Guidelines for Clinical Practice for the diagnosis and treatment of postmenopausal osteoporosis: executive summary of recommendations. Endocr Pract. 2010, 16, 1016–9. [Google Scholar] [CrossRef]
- Composton, J.; Cooper, A.; Cooper, C.; Francis, R.; Kanis, J.A.; Marsh, D.; McCloskey, E.V.; Reid, D.M.; Selby, P.; Wilkins, M.; National Osteoporosis Guideline Group (NOGG). Guidelines for the diagnosis and management of osteoporosis in postmenopausal women and men from the age of 50 years in the UK. Maturitas 2009, 62, 105–8. [Google Scholar] [CrossRef] [PubMed]
- Heras, P.; Karagiannis, S.; Kritikos, K.; Hatzopoulos, A.; Mitsibounas, D. Ibandronate is effective in preventing skeletal events in patients with bone metastases from colorectal cancer. Eur J Cancer Care (Engl). 2007, 16, 539–42. [Google Scholar] [CrossRef]
- Passarelli, M.N.; Newcomb, P.A.; LaCroix, A.Z.; Lane, D.S.; Ho, G.Y.F.; Chlebowski, R.T. Oral bisphosphonate use and colorectal cancer incidence in the Women’s Health Initiative. J Bone Miner Res. 2013, 28, 2043–8. [Google Scholar] [CrossRef] [PubMed]
- Grant, M.; Mlineritsch, B.; Schippinger, W.; Luschin-Ebengreuth, G.; Pöstlberger, S.; Menzel, C.; Jakesz, R.; Seifert, M. , Hubalek, M.; Bjelic-Radisic, V.; Samonigg, H., Tausch, C:; Eidtmann, H.; Steger, G.; Kwasny, W.; Dubsky, P.; Fridrik, M.; Fitzal, F.; Stierer, M.; Rücklinger, E.; Greil, R.; ABCSG-12 Trial Investigators; Marth, C. Endocrine therapy plus zoledronic acid in premenopausal breast cancer. N Engl J Med. 2009, 360, 679–91. [Google Scholar]
- Sewing, L.; Steinberg, F.; Schmidt, H.; Göke, R. The bisphosphonate zoledronic acid inhibits the growth of HCT-116 colon carcinoma cells and induces tumor cell apoptosis. Apoptosis 2008, 13, 782–9. [Google Scholar] [CrossRef]
- Luckman, S.P.; Hughes, D.E.; Coxon, F.P.; Graham, R.; Russell, G.; Rogers, M.J. Nitrogen-containing bisphosphonates inhibit the mevalonate pathway and prevent post-translational prenylation of GTP-binding proteins, including Ras. J Bone Miner Res. 1998, 13, 581–9. [Google Scholar] [CrossRef]
- Higgins, J.P.T., Green, S. Cochrane handbook for systematic reviews of interventions. Version 5.1.0. www.handbook.Cochrane.Org.
- Moher, D.; Liberati, A. , Tetzlaff, J.; Altman, D.G., PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Wells, G.A., Shea, B., O’Connell, D., Peterson, J.; Welch, V., Losos, M., Tugwell, P. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
- Stang, A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010, 25, 603–5. [Google Scholar] [CrossRef] [PubMed]
- Corbeil, R.R.; Searle, S.R. Restricted Maximum Likelihood (REML) Estimation of Variance Components in the Mixed Model. Taylor & Francis 1976, 8, 31–38. [Google Scholar]
- Van Arendok, J.A.; Tier, B.; Bink, C.; Bovenhuis, H. Restricted maximum likelihood analysis of linkage between genetic markers and quantitative trait loci for a granddaughter design. J Dairy Sci. 1998, 81, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.P.; Graser, H.U. Estimating Variance Components in a Class of Mixed Models by Restricted Maximum Likelihood. J Dairy Sci. 1986, 69, 1156–1165. [Google Scholar] [CrossRef]
- Choi, D. , Choi, S.; Chang, J.; Park, S.M. Exposure to oral bisphosphonates and risk of gastrointestinal cancer. Osteoroporos Int. 2020, 31, 775–782. [Google Scholar] [CrossRef]
- Green, J.; Czanner, G.; Reeves, G.; Watson, J.; Wise, L.; Beral, V. Oral bisphosphonates and risk of cancer of oesophagus, stomach, and colorectum: case-control analysis within a UK primary care cohort. BMJ 2010, 341, c4444. [Google Scholar] [CrossRef]
- Ibáñez-Sanz, G.; Guinó, E.; Pontes, C.; Morros, R.; de la Peña-Negro, L.C.; Quijada-Manuitt, M.; Moreno, V. Risk of colorectal cancer in users of bisphosphonates: analysis of population-based electronic health records. Eur J Epidemiol. 2020, 35, 37–48. [Google Scholar] [CrossRef]
- Rennert, G.; Pinchev, M.; Rennert, H.S.; Gruber, S.B. Use of bisphosphonates and reduced risk of colorectal cancer. J Clin Oncol. 2011, 29, 1146–50. [Google Scholar] [CrossRef]
- Vinogradova, Y.; Coupland, C.; Hippisisle-Cox, J. Exposure to bisphosphonates and risk of gastrointestinal cancers: series of nested case-control studies with QResearch and CPRD data. BMJ 2013, 346, f114. [Google Scholar] [CrossRef]
- Vogtmann, E.; Corley, D.A.; Almers, L.M.; Cardwell, C.R.; Murray, L.J.; Abnet, C.C. Oral bisphosphonates and colorectal cancer. Sci Rep. 2017, 7, 44177. [Google Scholar] [CrossRef]
- Higgins, J.P.; Thompson, S.G. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002, 21, 1539–58. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Thompson, S.G.; Deeks, J.J.; Altman, D.D. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–60. [Google Scholar] [CrossRef] [PubMed]
- Begg, C.B.; Mazumdar, M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994, 50, 1088–101. [Google Scholar] [CrossRef]
- Duval, S.; Tweedie, R. Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics 2000, 56, 455–63. [Google Scholar] [CrossRef]
- Egger, M.; Smith, G.D.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–34. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.C.; Sutton, A.J.; Ioannidis, J.P.A.; Terrin, N.; Jones, D.R.; lau, J.; Carpenter, J.; Rücker, G.; Harbord, R.M.; Schmid, C.H.; Tetzlaff, J.; Deeks, J.J.; Peters, J.; Macaskill, P.; Schwarzer, G.; Duval, S.; Altman, D.G.; Moher, D.; Higgins, J.P.T. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ 2011, 343, d4002. [Google Scholar] [CrossRef]
- Duval, S.; Tweedie, R. Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics 2000, 56, 455–63. [Google Scholar] [CrossRef]
- Khalili, H.; Huang, E.S.; Ogino, S.; Fuchs, C.S.; Chan, A.T. A prospective study of bisphosphonate use and risk of colorectal cancer. J Clin Oncol. 2012, 30, 3229–33. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thompson, S.G. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002, 21, 1539–58. [Google Scholar] [CrossRef]
- Stroup, D.F.; Berlin, J.A.; Morton, S.C.; Olkin, I.; Williamson, G.D.; Rennie, D.; et al. Meta-Analysis of Observational Studies in Epidemiology A Proposal for Reporting Because Of Pressure For Timely. Available at: http://www.wesleyan.edu.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).