Submitted:
04 May 2025
Posted:
07 May 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Experimental Conditions
2.2. Plant Growth and Experiment Setup
2.3. Harvest and Data Collection
2.3.1. Shoot Traits
2.3.2. Root Traits
2.3.3. Root Biotic Traits
2.4. Statistical Analysis
3. Results
3.1. Variation of Traits Among Wheat PS Lines
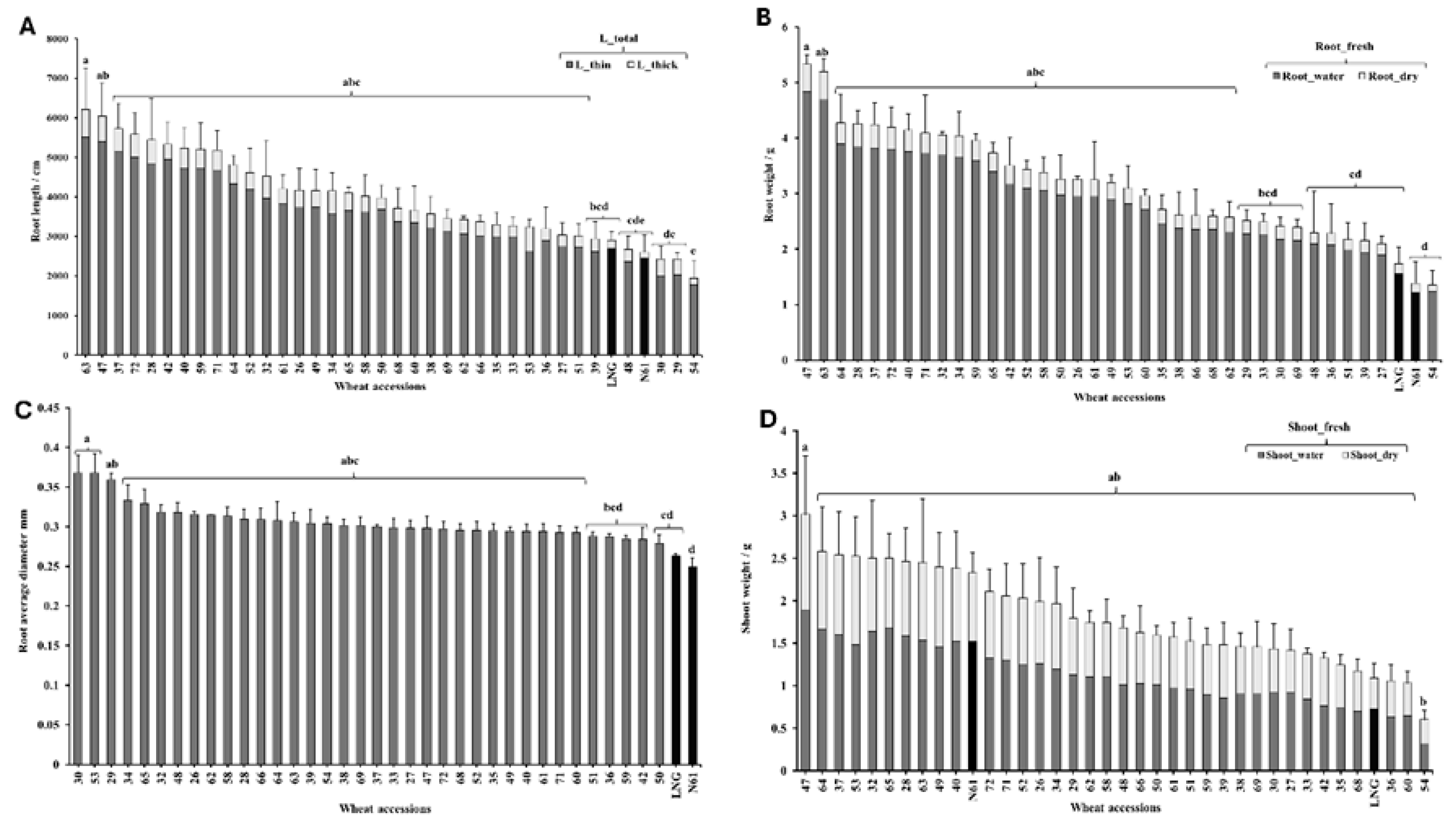
3.1.1. Root Traits
3.1.2. Shoot Traits
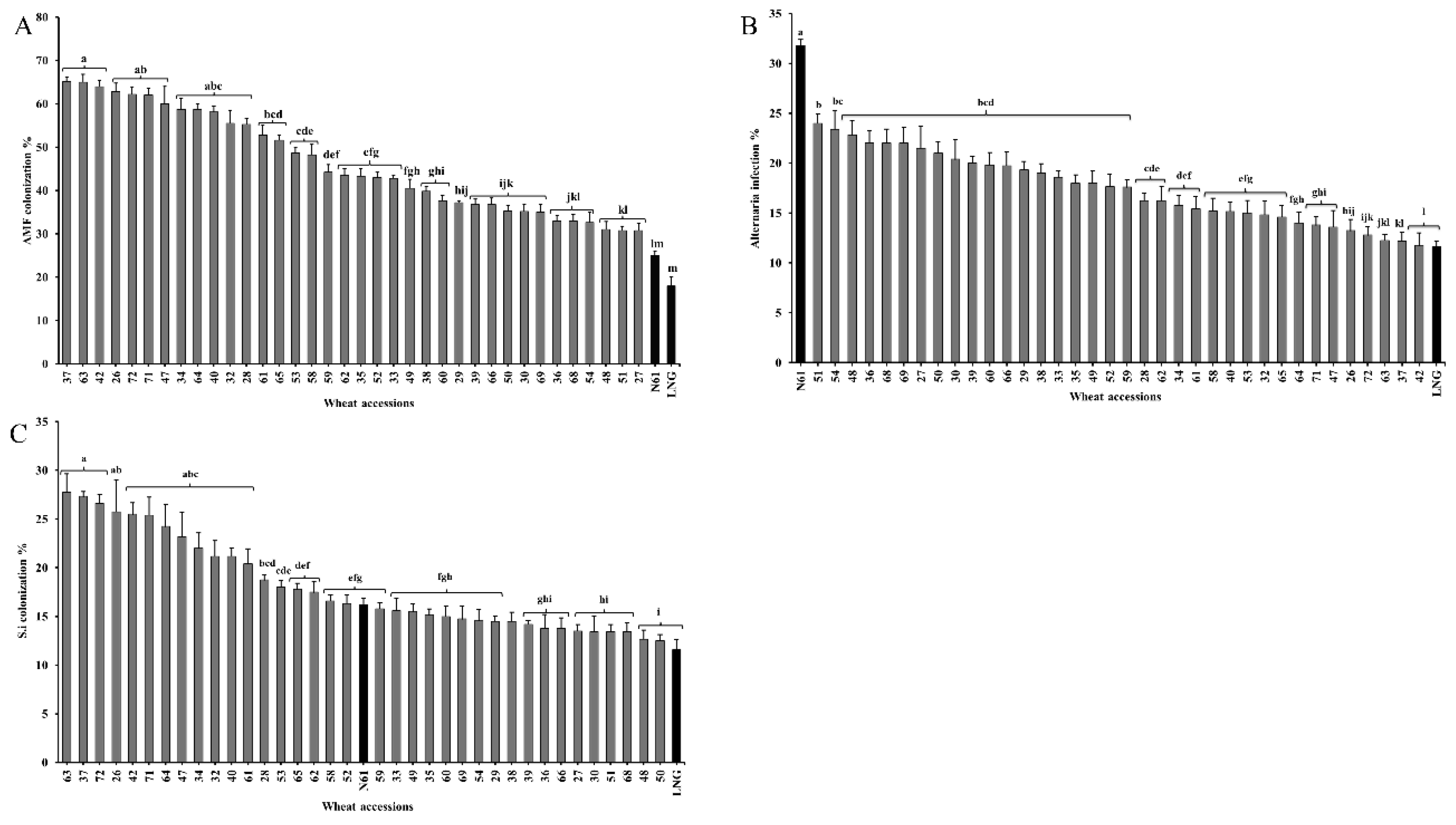
3.1.3. Biotic Traits
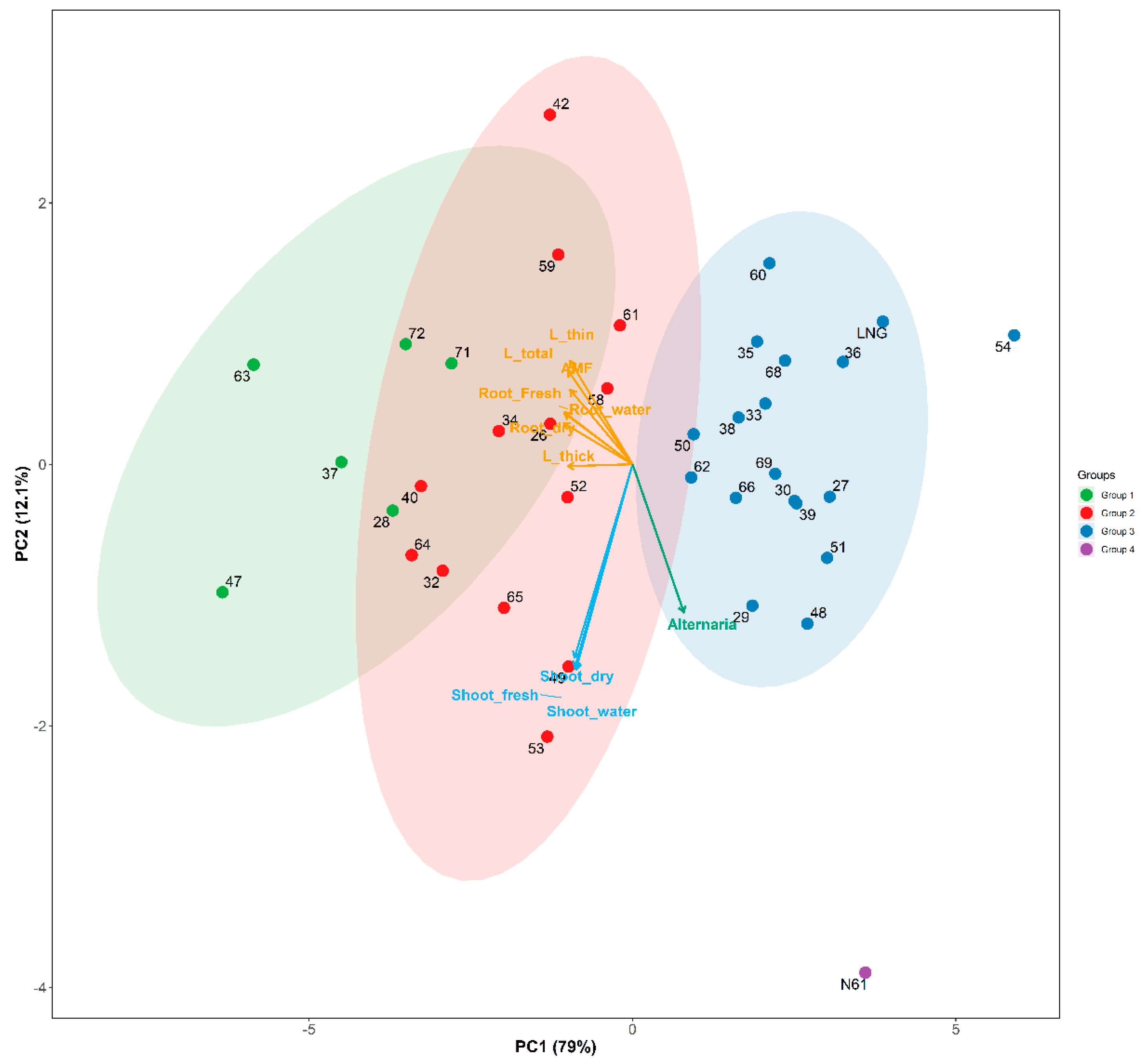
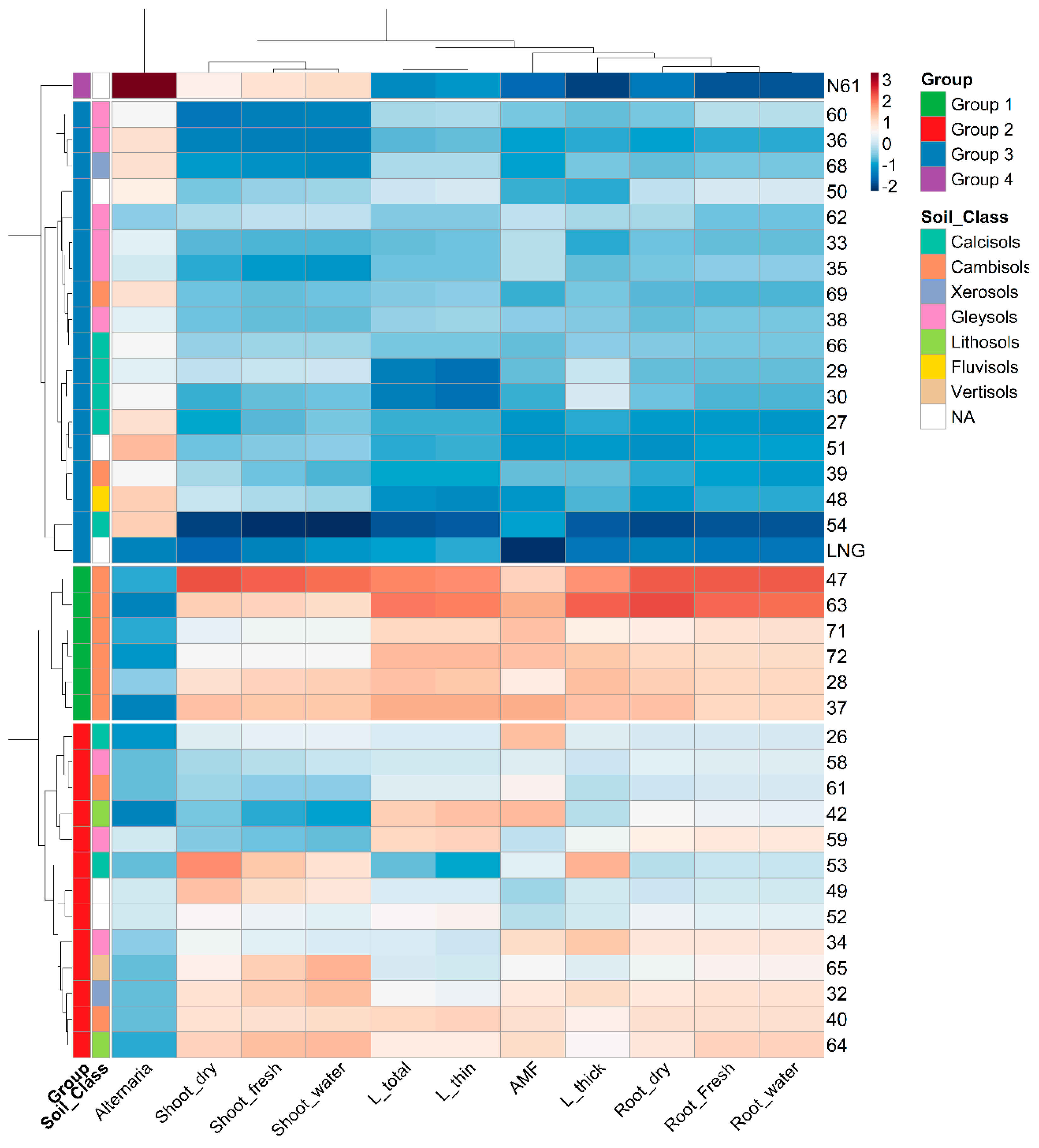
3.1.4. Association Among Wheat Accession Traits
3.2. Wheat Accession Grouping and Traits Association
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Ethical Compliance
References
- Calvin, K.; Dasgupta, D.; Krinner, G.; Mukherji, A.; Thorne, P.W.; Trisos, C.; Romero, J.; Aldunce, P.; Barrett, K.; Blanco, G.; et al. IPCC, 2023: Climate Change 2023: Synthesis Report. Contribution of Working Groups I, II and III to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change [Core Writing Team, H. Lee and J. Romero (Eds.)]. IPCC, Geneva, Switzerland.; First.; Intergovernmental Panel on Climate Change (IPCC), 2023;
- Constas, M.A.; d’Errico, M.; Hoddinott, J.F.; Pietrelli, R. Resilient Food Systems – A Proposed Analytical Strategy for Empirical Applications Available online: https://ageconsearch.umn.edu/record/319840 (accessed on 28 March 2025).
- Reif, J.C.; Zhang, P.; Dreisigacker, S.; Warburton, M.L.; van Ginkel, M.; Hoisington, D.; Bohn, M.; Melchinger, A.E. Wheat Genetic Diversity Trends during Domestication and Breeding. Theor Appl Genet 2005, 110, 859–864. [CrossRef]
- Maeght, J.-L.; Rewald, B.; Pierret, A. How to Study Deep Roots—and Why It Matters. Front Plant Sci 2013, 4, 299. [CrossRef]
- Pierret, A.; Maeght, J.-L.; Clément, C.; Montoroi, J.-P.; Hartmann, C.; Gonkhamdee, S. Understanding Deep Roots and Their Functions in Ecosystems: An Advocacy for More Unconventional Research. Annals of Botany 2016, 118, 621–635. [CrossRef]
- Ober, E.S.; Alahmad, S.; Cockram, J.; Forestan, C.; Hickey, L.T.; Kant, J.; Maccaferri, M.; Marr, E.; Milner, M.; Pinto, F.; et al. Wheat Root Systems as a Breeding Target for Climate Resilience. Theor Appl Genet 2021, 134, 1645–1662. [CrossRef]
- Jaster-Keller, J.; Müller, M.E.H.; El-Khatib, A.H.; Lorenz, N.; Bahlmann, A.; Mülow-Stollin, U.; Bunzel, M.; Scheibenzuber, S.; Rychlik, M.; von der Waydbrink, G.; et al. Root Uptake and Metabolization of Alternaria Toxins by Winter Wheat Plants Using a Hydroponic System. Mycotoxin Res 2023, 39, 109–126. [CrossRef]
- Qiao, X.; Sun, T.; Lei, J.; Xiao, L.; Xue, L.; Zhang, H.; Jia, J.; Bei, S. Frontiers | Arbuscular Mycorrhizal Fungi Contribute to Wheat Yield in an Agroforestry System with Different Tree Ages.. [CrossRef]
- Crespo-Herrera, L.A.; Govindan, V.; Stangoulis, J.; Hao, Y.; Singh, R.P. QTL Mapping of Grain Zn and Fe Concentrations in Two Hexaploid Wheat RIL Populations with Ample Transgressive Segregation. Front. Plant Sci. 2017, 8. [CrossRef]
- Elbashir, A.A.E.; Gorafi, Y.S.A.; Tahir, I.S.A.; Kim, J.-S.; Tsujimoto, H. Wheat Multiple Synthetic Derivatives: A New Source for Heat Stress Tolerance Adaptive Traits. Breed. Sci. 2017, 67, 248–256. [CrossRef]
- Itam, M.O.; Mega, R.; Gorafi, Y.S.A.; Yamasaki, Y.; Tahir, I.S.A.; Akashi, K.; Tsujimoto, H. Genomic Analysis for Heat and Combined Heat–Drought Resilience in Bread Wheat under Field Conditions. Theor Appl Genet 2022, 135, 337–350. [CrossRef]
- Mahjoob, M.M.M.; Chen, T.-S.; Gorafi, Y.S.A.; Yamasaki, Y.; Kamal, N.M.; Abdelrahman, M.; Iwata, H.; Matsuoka, Y.; Tahir, I.S.A.; Tsujimoto, H. Traits to Differentiate Lineages and Subspecies of Aegilops Tauschii, the D Genome Progenitor Species of Bread Wheat. Diversity 2021, 13, 217. [CrossRef]
- Soil Atlas of Asia; Jones, A., Caon, L., Yigini, Y., Verbeke, I., Konyushkova, M., Vargas, R., Viatkin, K., Michéli, E., European Commission, Eds.; Publications Office: Luxembourg, 2024; ISBN 978-92-68-03116-2.
- Mauer, O.; Palátová, E. Mountain Ash (Sorbus Aucuparia L.) Root System Morphogenesis. J. For. Sci. 2002, 48, 342–350. [CrossRef]
- Wang, H.; Hu, Z.; Huang, K.; Han, Y.; Zhao, A.; Han, H.; Song, L.; Fan, C.; Li, R.; Xin, M.; et al. Three Genomes Differentially Contribute to the Seedling Lateral Root Number in Allohexaploid Wheat: Evidence from Phenotype Evolution and Gene Expression. The Plant Journal 2018, 95, 976–987. [CrossRef]
- Mohammedali, A.Kh.H.; Gorafi, Y.S.A.; Kamal, N.M.; Tahir, I.S.A.; Tsujimoto, H.; Taniguchi, T. Wheat Root Traits and Endophytic Fungal Association Differ with Ploidy. under_review 2025.
- FAO Fertilizer Use by Crop in the Islamic Republic of Iran Available online: https://openknowledge.fao.org/server/api/core/bitstreams/a59738f1-92fc-4bac-bbd9-00e6eadb0483/content (accessed on 30 April 2025).
- Martín, M.; Rubio, A.; Remesal, E.; Cano, C.; Bago, A. Application of the Ultimate Arbuscular Mycorrhizal Inoculant MYCOGEL® in Japan: Results and Prospects.
- Phillips, J.M.; Hayman, D.S. Improved Procedures for Clearing Roots and Staining Parasitic and Vesicular-Arbuscular Mycorrhizal Fungi for Rapid Assessment of Infection. Transactions of the British Mycological Society 1970, 55, 158-IN18. [CrossRef]
- McGONIGLE, T.P.; Miller, M.H.; Evans, D.G.; Fairchild, G.L.; Swan, J.A. A New Method Which Gives an Objective Measure of Colonization of Roots by Vesicular—Arbuscular Mycorrhizal Fungi. New Phytologist 1990, 115, 495–501. [CrossRef]
- Smith, F.A. Plant Roots. Growth, Activity and Interaction with Soils. Annals of Botany 2007, 100, 151–152. [CrossRef]
- Lynch, J.P. Root Phenotypes for Improved Nutrient Capture: An Underexploited Opportunity for Global Agriculture. New Phytologist 2019, 223, 548–564. [CrossRef]
- Hafeez, A.; Ali, S.; Javed, M.A.; Iqbal, R.; Khan, M.N.; ÇIĞ, F.; Sabagh, A.E.; Abujamel, T.; Harakeh, S.; Ercisli, S.; et al. Breeding for Water-Use Efficiency in Wheat: Progress, Challenges and Prospects. Mol Biol Rep 2024, 51, 429. [CrossRef]
- Chen, S.; Long, L.; Sun, X.; Parsons, D.; Zhou, Z. Responsive Root Traits and Mitigating Strategies for Wheat Production under Single or Combined Abiotic Stress. European Journal of Agronomy 2025, 162, 127393. [CrossRef]
- Lynch, J.P. Harnessing Root Architecture to Address Global Challenges. The Plant Journal 2022, 109, 415–431. [CrossRef]
- Cheng, S.; Zou, Y.-N.; Kuča, K.; Hashem, A.; Abd_Allah, E.F.; Wu, Q.-S. Elucidating the Mechanisms Underlying Enhanced Drought Tolerance in Plants Mediated by Arbuscular Mycorrhizal Fungi. Front. Microbiol. 2021, 12. [CrossRef]
- Hosseini, F.; Mosaddeghi, M.R.; Zarebanadkouki, M. Enhancing Rhizosphere Soil Water Retention in Wheat through Colonization with Endophytic Fungus Serendipita Indica. Rhizosphere 2023, 26, 100709. [CrossRef]
- Duan, H.-X.; Luo, C.-L.; Zhu, Y.; Zhao, L.; Wang, J.; Wang, W.; Xiong, Y.-C. Arbuscular Mycorrhizal Fungus Activates Wheat Physiology for Higher Reproductive Allocation under Drought Stress in Primitive and Modern Wheat. European Journal of Agronomy 2024, 161, 127376. [CrossRef]
- Tkacz, A.; Pini, F.; Turner, T.R.; Bestion, E.; Simmonds, J.; Howell, P.; Greenland, A.; Cheema, J.; Emms, D.M.; Uauy, C.; et al. Agricultural Selection of Wheat Has Been Shaped by Plant-Microbe Interactions. Front. Microbiol. 2020, 11, 132. [CrossRef]
- Lattacher, A.; Le Gall, S.; Rothfuss, Y.; Gao, C.; Harings, M.; Pagel, H.; Giraud, M.; Alahmad, S.; Hickey, L.T.; Kandeler, E.; et al. Rooting for Microbes: Impact of Root Architecture on the Microbial Community and Function in Top- and Subsoil. Plant and Soil 2025, 1–19. [CrossRef]
- Klimek-Kopyra, A.; Kulig, B.; Głąb, T.; Zając, T.; Skowera, B.; Kopcińska, J. EFFECT OF PLANT INTERCROPPING AND SOIL TYPE ON SPECIFIC ROOT LENGTH. ROMANIAN AGRICULTURAL RESEARCH 2015.
- Schneider, F.; Don, A. Root-Restricting Layers in German Agricultural Soils. Part I: Extent and Cause. Plant and Soil 2019, 442, 433–451. [CrossRef]
- Labidi, S.; Calonne, M.; Ben Jeddi, F.; Debiane, D.; Rezgui, S.; Laruelle, F.; Tisserant, B.; Grandmougin-Ferjani, A.; Lounès-Hadj Sahraoui, A. Calcareous Impact on Arbuscular Mycorrhizal Fungus Development and on Lipid Peroxidation in Monoxenic Roots. Phytochemistry 2011, 72, 2335–2341. [CrossRef]
- Sediqui, N.; Amin, M.W.; Dawlatzai, N.; Gulab, G.; Poyesh, D.S.; Terada, N.; Sanada, A.; Kamata, A.; Koshio, K. Elucidation of Shoot and Root Growth, Physiological Responses, and Quality Traits of Tomato (Solanum Lycopersicon L.) Exposed to Elevated Calcium Carbonate Concentrations. Horticulturae 2024, 10, 573. [CrossRef]
- Bektas, H.; Hohn, C.E.; Waines, J.G. Characteristics of the Root System in the Diploid Genome Donors of Hexaploid Wheat (Triticum Aestivum L.). Genet Resour Crop Evol 2017, 64, 1641–1650. [CrossRef]
- Li, A.; Liu, D.; Yang, W.; Kishii, M.; Mao, L. Synthetic Hexaploid Wheat: Yesterday, Today, and Tomorrow. Engineering 2018, 4, 552–558. [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).