Submitted:
05 May 2025
Posted:
07 May 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
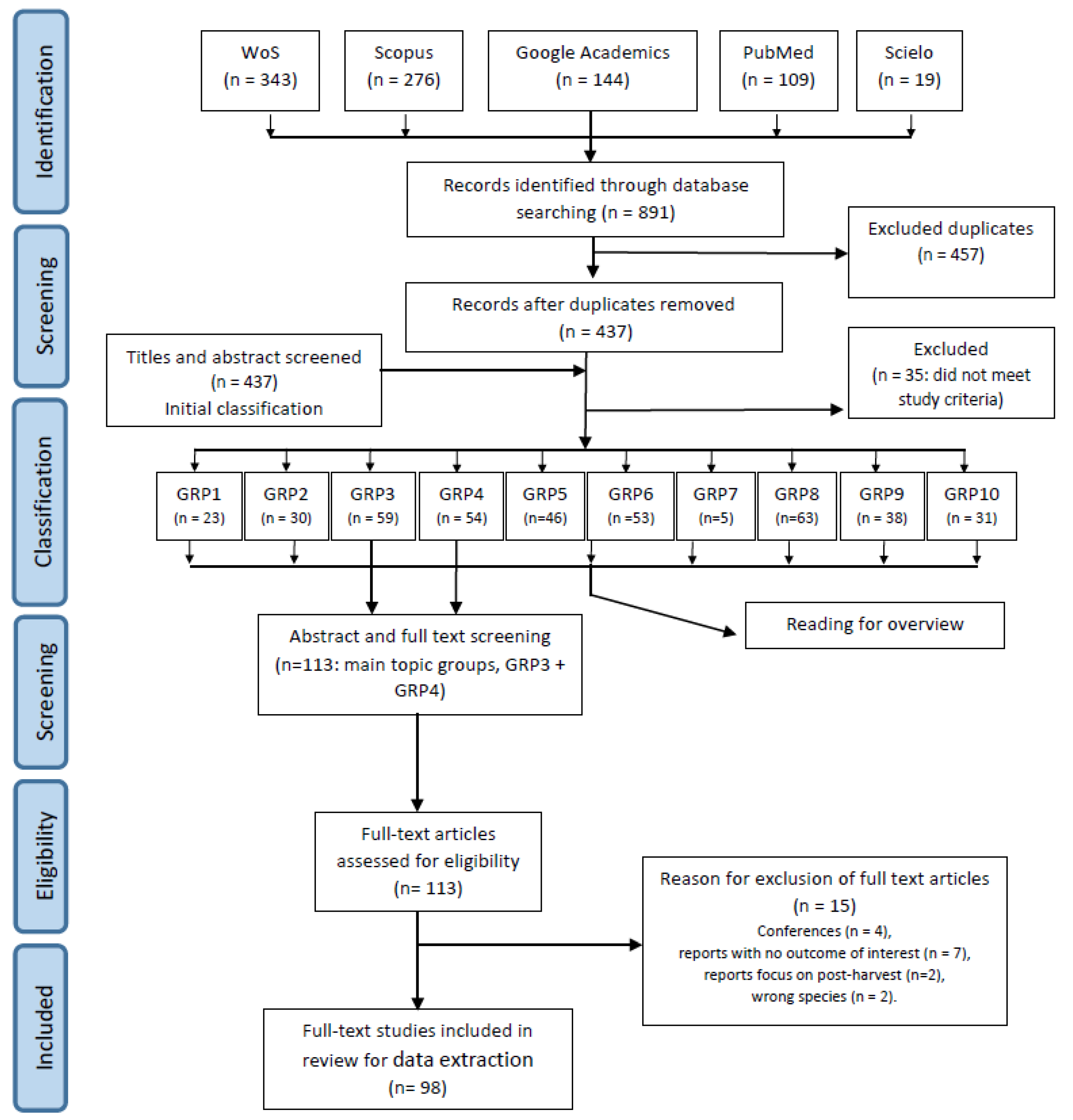
2.1. Step 1: Information Sources and Search Strategy
2.2. Step 2: Initial Classification of Records and First Data Collection
2.3. Step 3: Eligibility Criteria for Full-Text Review
2.4. Step 4: Charting Data
- Article identifiers: authors, year of publication, country, and title.
- Target species: plant species, variety, and disease (A. a. general, ABS, or post-harvest losses)
- Substance Information: group, common (commercial) name, scientific (substance) name, additional information.
- Experiment information: type (I, II; when two different types of experiments were used in the same study), concentration, and additional information (field experiments: yes/no).
- Main result: text (the results explained in the text), MIC (Minimum Inhibitory Concentration), MGI (Mycelial Growth Inhibition), EC50% (Concentration causing 50% growth inhibition), effectiveness (Type I, Type II).
- Conclusion: text.
- Interest: importance (goes from 1 to 5, and reflects the closeness to the main topic), reliability scale (goes from 1 to 3, low, medium, high; and reflects the quality and reproducibility of experiments).
2.5. Step 5: Collating, Summarizing, and Reporting the Results
3. Results
3.1. Search in Databases
3.2. Initial Classification
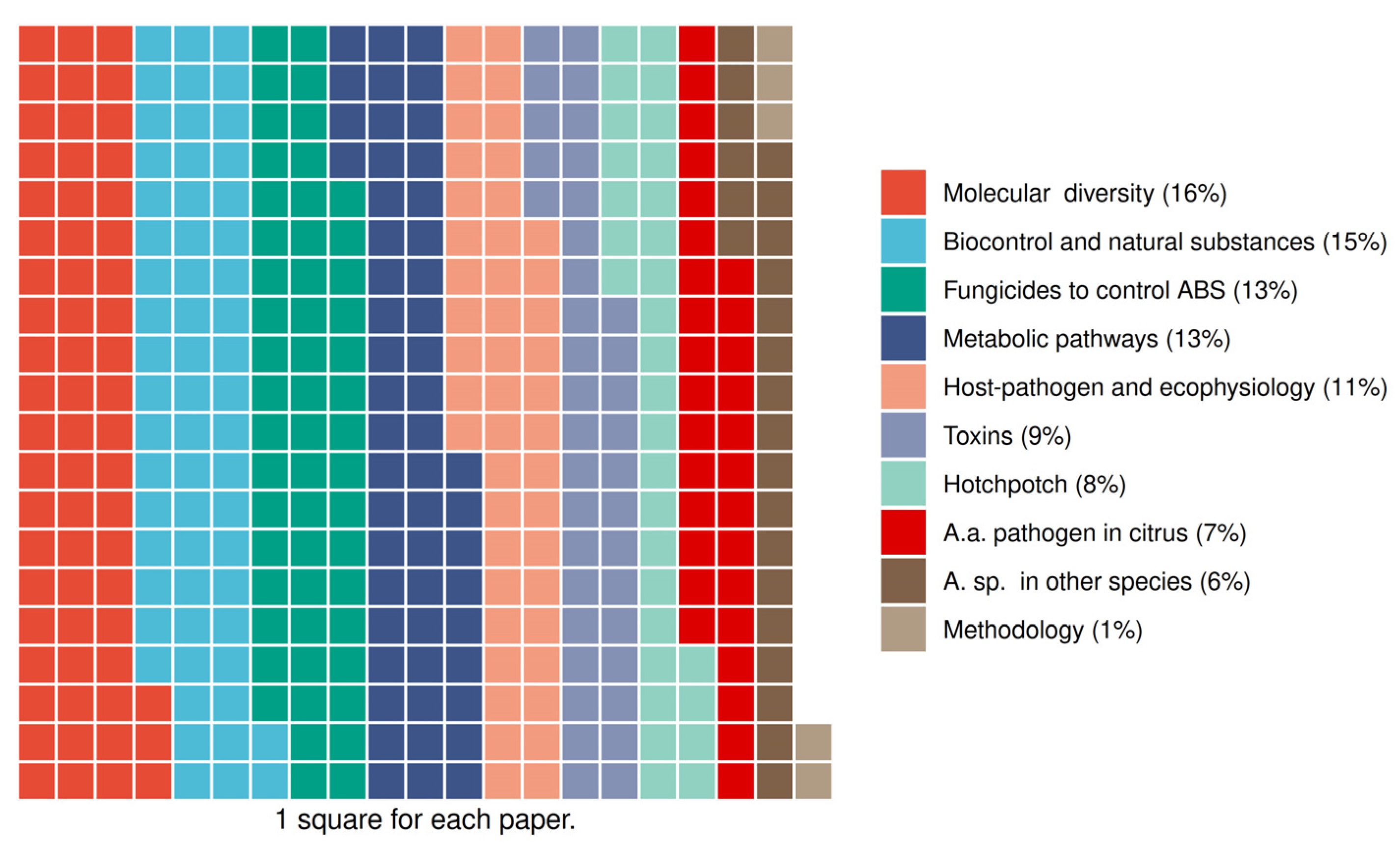
3.3. Data Extraction for the Main Topic (Groups 3 and 4)
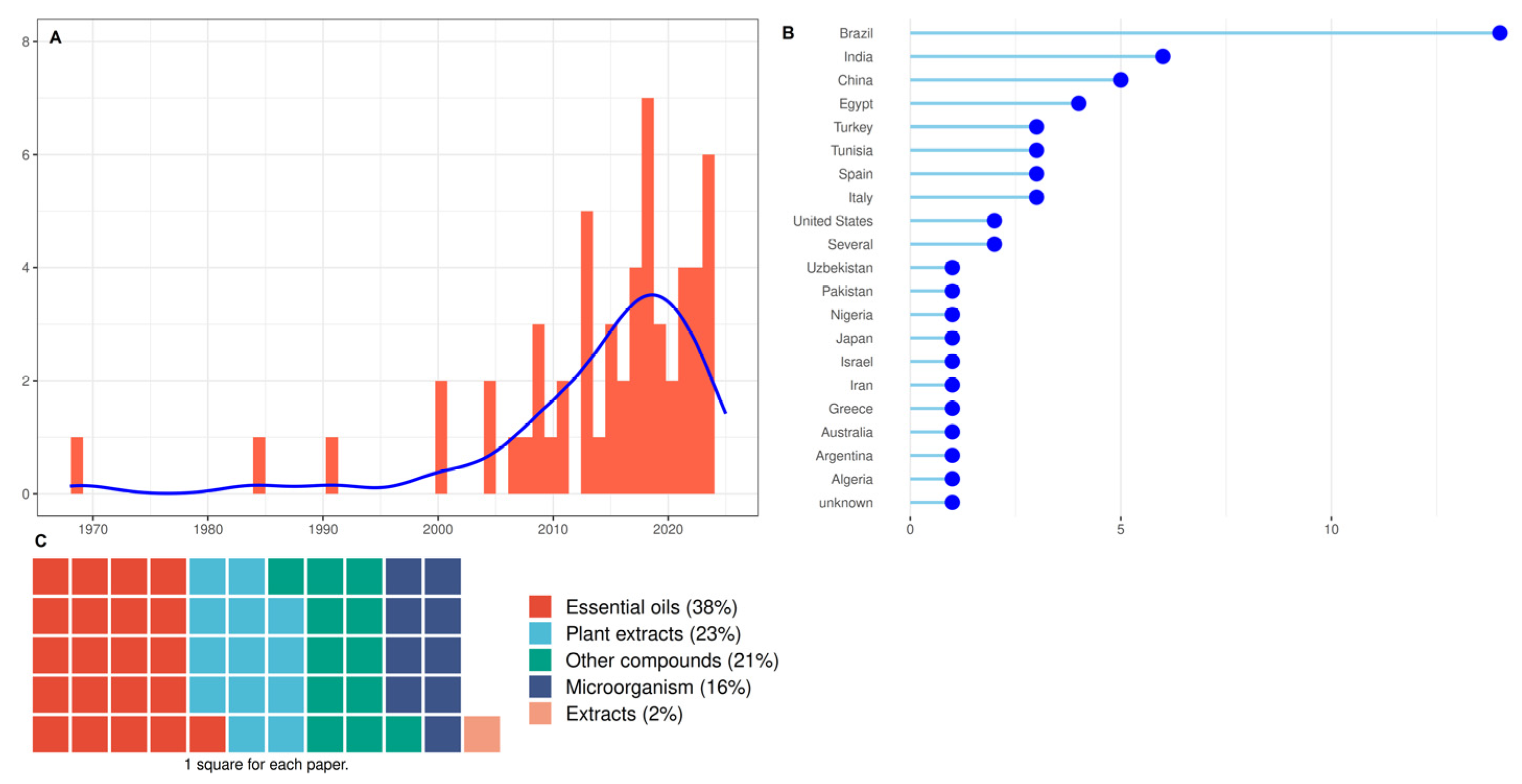
3.4. Studies Characteristics of Group 3: Biocontrol and Natural Substances
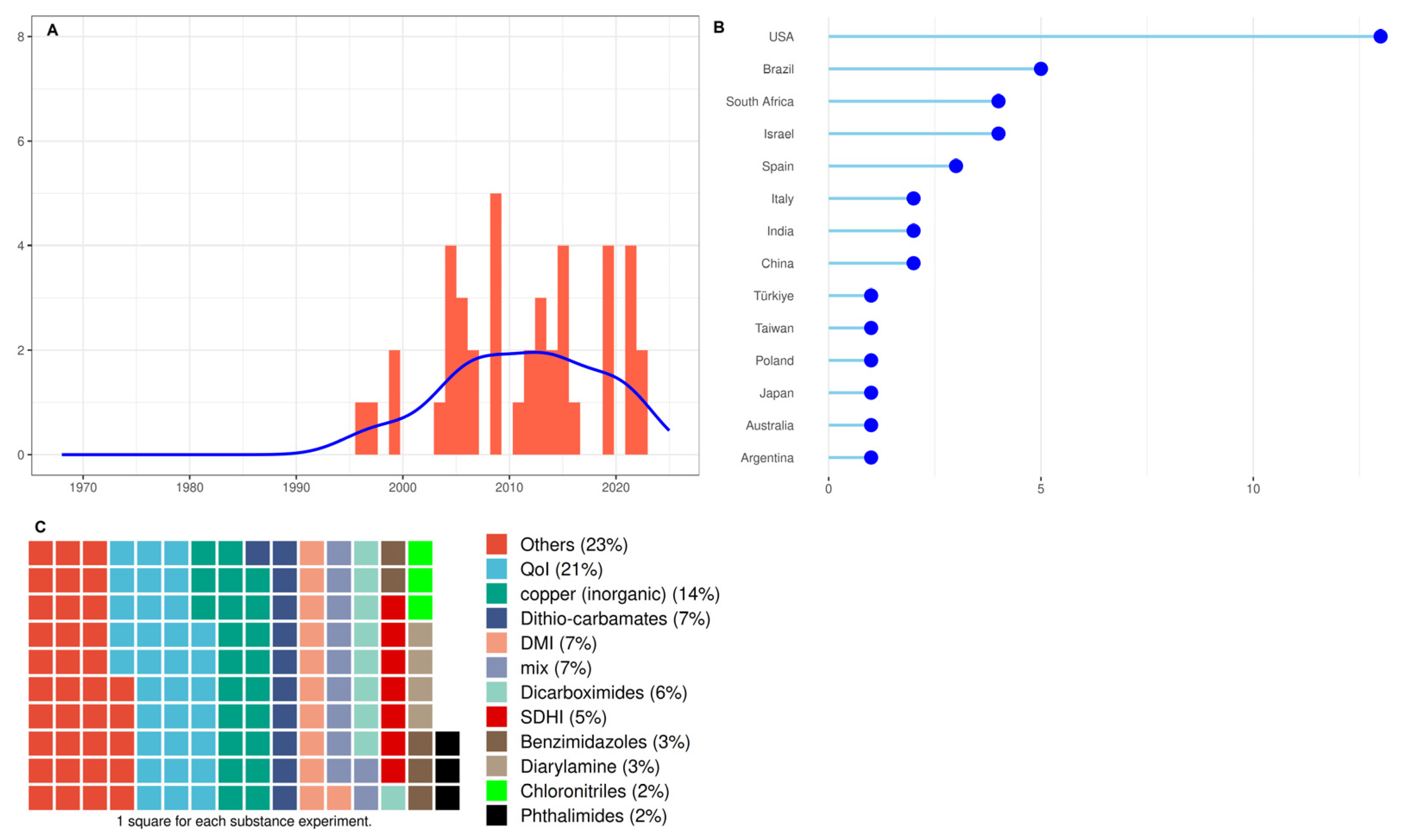
3.4. Studies Characteristics of Group 4: Fungicides to Control ABS
4. Discussion
4.1. Natural Substances to Control ABS
4.1.1. Methodologies Used to Test the Antifungal Activity of Natural Substances
4.1.2. Biocontrol and Natural Substances to Control ABS
- - Microorganisms to Control ABS
- - Essential oils to control ABS
- - Plant extracts to control ABS
- - Other compounds to control ABS
4.1.3. Summary of the Findings of the Biological Control and Natural Substances Group and Why These Substances Are Rarely Used in Field Conditions
4.2. Fungicides to Control ABS
4.2.1. Methodologies Used to Test the Antifungal Activity of Fungicides
4.2.2. Fungicides to Control ABS
- - Copper (Inorganic) Group
- Dithiocarbamates group and other MSCA
- - DeMethylation Inhibitors (DMI) imidazoles and triazoles group
- - Quinone outside Inhibitors (QoI) group
- - Benzimidazoles, Diarylamine and Dicarboximides group
- - Succinate-dehydrogenase inhibitors (SDHI) group
- - Others (not classified in previous groups)
4.2.3. Summary of the Findings of the Fungicides Group to Control ABS and Why They Are Currently Failing to Control the Disease in the Field
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ABS | Alternaria Brown Spot |
| ACT | Alternaria Citri Toxin |
| AUDPC | Area under the disease progress curve |
| CEO | Citrus essential oil |
| DMI | DeMethylation Inhibitors |
| EC50 | Concentration causing 50% growth inhibition |
| EO: | Essential oil |
| FRAC | Fungicide Resistance Action Committee |
| MBC | Methyl Benzimidazole Carbamates |
| MGA | Mycelial Growth Assay |
| MGI | Mycelial Growth Inhibition |
| MGR | Mycelial growth rate |
| MIC | Minimum Inhibitory Concentration |
| MSCA: | Multi-Site Contact Activity |
| QoI | Quinone outside Inhibitors |
| SDHI | Succinate-dehydrogenase inhibitors |
References
- Whiteside, J.O. Alternaria-Recognition, Prevention and Control of Alternaria Brown Spot on Dancy Tangerines and Minneola Tangelos. Citrus Industry Magazine 1986, 67, 44–47.
- Pacheco, F.A. de, Camilla de Andrade, Martelli, Ivan Bortolato, Polydoro, Denis Augusto, Schinor, Evandro Henrique, Pio, Rose Mary, Kupper, Kátia Cristina, Azevedo Resistance and Susceptibility of Mandarins and Their Hybrids to Alternaria Alternata. Scientia Agricola 2012, 69, 386–392. [CrossRef]
- Reis, R.F.; Almeida, T.F. de; Stuchi, E.S.; Goes, A. de Susceptibility of Citrus Species to Alternaria Alternata, the Causal Agent of the Alternaria Brown Spot. Scientia Horticulturae 2007, 113, 336–342. [CrossRef]
- Arlotta, C.; Ciacciulli, A.; Strano, M.C.; Cafaro, V.; Salonia, F.; Caruso, P.; Licciardello, C.; Russo, G.; Smith, M.W.; Cuenca, J.; et al. Disease Resistant Citrus Breeding Using Newly Developed High Resolution Melting and CAPS Protocols for Alternaria Brown Spot Marker Assisted Selection. AGRONOMY-BASEL 2020, 10. [CrossRef]
- de Souza, M.C.; Stuchi, E.S.; de Goes, A. Evaluation of Tangerine Hybrid Resistance to Alternaria Alternata. Scientia Horticulturae 2009, 123, 1–4.
- Hu, J.; Liu, R.; Wang, X.; Zhou, N.; Hong, Q.; Yao, T.; Li, T.; Jiang, D.; Cao, L.; Li, H. Evaluation of Citrus Germplasm Resistance to Alternaria Alternata. Journal of Fruit Science 2015, 32, 672–680.
- Solel, Z.; Kimchi, M. Susceptibility and Resistance of Citrus Genotypes to Alternaria Alternata Pv. Citri. JOURNAL OF PHYTOPATHOLOGY-PHYTOPATHOLOGISCHE ZEITSCHRIFT 1997, 145, 389–391. [CrossRef]
- Llorens, E.; Scalschi, L.; Fernández-Crespo, E.; Lapeña, L.; García-Agustín, P. Hexanoic Acid Provides Long-Lasting Protection in “Fortune” Mandarin against Alternaria Alternata. Physiological and Molecular Plant Pathology 2015, 91, 38-45-38–45. [CrossRef]
- Felipini, R.B.; Brito, R.A.S.; Azevedo, F.A.; Massola, N.S. Alternaria Alternata f. Sp. Citri Tangerine Pathotype Induces Reactive Oxygen Species Accumulation in Susceptible Citrus Leaves. Physiological and Molecular Plant Pathology 2023, 126.
- Kimura, N.; Tsuge, T. Gene Cluster Involved in Melanin Biosynthesis of the Filamentous Fungus Alternaria Alternata. J Bacteriol 1993, 175, 4427–4435. [CrossRef]
- Ajiro, N.; Miyamoto, Y.; Masunaka, A.; Tsuge, T.; Yamamoto, M.; Ohtani, K.; Fukumoto, T.; Gomi, K.; Peever, T.L.; Izumi, Y.; et al. Role of the Host-Selective ACT-Toxin Synthesis Gene ACTTS2 Encoding an Enoyl-Reductase in Pathogenicity of the Tangerine Pathotype of Alternaria Alternata. Phytopathology 2010, 100, 120–126. [CrossRef]
- Badal, J.; Cuenca, F.; Zornoza, C.; Jiménez, J.G.; Peris, A.; Armengol, J.; Civera, A.V.; Alfaro-Lassala, F. Conocimientos Sobre La Epidemiología de Alternaria Alternata Pv. Citri y Su Utilización En El Diseño de Estrategias de Control de La Mancha Marrón de Los Cítricos. Phytoma España: La revista profesional de sanidad vegetal 2004, 112–116.
- Timmer, L.W.; Peever, T.L.; Solel, Z.; Akimitsu, K. Alternaria Diseases of Citrus - Novel Pathosystems. Phytopathologia Mediterranea 2003, 42, 99–112.
- Agostini, J.; Bushong, P.; Timmer, L. Greenhouse Evaluation of Products That Induce Host Resistance for Control of Scab, Melanose, and Alternaria Brown Spot of Citrus. PLANT DISEASE 2003, 87, 69–74. [CrossRef]
- Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V. Cochrane Handbook for Systematic Reviews of Interventions. Hoboken: Wiley 2019.
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E. Declaración PRISMA 2020: Una Guía Actualizada Para La Publicación de Revisiones Sistemáticas. Revista española de cardiología 2021, 74, 790–799.
- R Core Team R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2024;
- RStudio Team RStudio: Integrated Development Environment for R; RStudio, PBC: Boston, MA, 2025;
- Wickham, H.; Chang, W.; Henry, L.; Pedersen, T.L.; Takahashi, K.; Wilke, C.; Woo, K.; Yutani, H.; Dunnington, D. Ggplot2: Create Elegant Data Visualisations Using the Grammar of Graphics; 2023;
- Rudis, B.; Gandy, D. Waffle: Create Waffle Chart Visualizations; 2023;
- Pinto, K.M.S.; Melo, P.A.F.R. de; Nascimento, L.C. do; Cortez, M.I.G.M.; Aires, A.A. de C.; Mondego, J.M.; Lima, R.P.; Silva, E.C. da; Mesquita, M.L.R.; Lemos, R.N.S. de Biological Potential of Extracts of Caatinga Plants in the Control of Alternaria Alternata f. Sp. Citri in Citrus. Journal of Agricultural Science (Toronto) 2018, 10, 116–125. [CrossRef]
- Soylu, E.M.; Kose, F. Antifungal Activities of Essential Oils Against Citrus Black Rot Disease Agent Alternaria Alternata. Journal of Essential Oil Bearing Plants 2015, 18, 894-903-894–903. [CrossRef]
- Carvalho, D.D.C.; Alves, E.; Camargos, R.B.; Oliveira, D.F.; Scolforo, J.R.S.; de Carvalhod, D.A.; Batista, T.R.S. Plant Extracts to Control Alternaria Alternata in Murcott Tangor Fruits. Revista Iberoamericana De Micologia 2011, 28, 173-178-173–178. [CrossRef]
- de Azevedo, F.A.; Devite, F.T.; Bastianel, M.; Schinor, E.H.; Da Conceição, P.M. Citrus Essential Oils as an Alternative Method of Control of the Fungus Alternaria Alternata (Fr.: Fr.) Keissler 2023.
- Tullio, V.; Nostro, A.; Mandras, N.; Dugo, P.; Banche, G.; Cannatelli, M.A.; Cuffini, A.M.; Alonzo, V.; Carlone, N.A. Antifungal Activity of Essential Oils against Filamentous Fungi Determined by Broth Microdilution and Vapour Contact Methods. Journal of Applied Microbiology 2007, 102, 1544-1550-1544–1550. [CrossRef]
- Fokkema, N.J. Fungal Antagonisms in the Phyllosphere. Annals of Applied Biology 1978, 89, 115–119. [CrossRef]
- Dilantha Fernando, W.G.; Linderman, R.G. Inhibition of Phytophthora Vignae and Stem and Root Rot of Cowpea by Soil Bacteria. Biological Agriculture & Horticulture 1995, 12, 1–14. [CrossRef]
- Tozlu, E.; Kotan, M.S.; Tekiner, N.; Dikbas, N.; Kotan, R. Biological Control of Postharvest Spoilage in Fresh Mandarins (Citrus Reticulata Blanco) Fruits Using Bacteria During Storage. Erwerbs-Obstbau 2019, 61, 157-164-157–164. [CrossRef]
- Perina, F.J.; Lage de Andrade, C.C.; Moreira, S.I.; Nery, E.M.; Ogoshi, C.; Alves, E. Cinnamomun Zeylanicum Oil and Trans-Cinnamaldehyde against Alternaria Brown Spot in Tangerine: Direct Effects and Induced Resistance. PHYTOPARASITICA 2019, 47, 575–589. [CrossRef]
- Camargos, R.B.; Perina, F.J.; Carvalho, D.D.C.; Alves, E.; Mascarello, A.; Chiaradia-Delatorre, L.D.; Yunes, R.A.; Nunes, R.J.; Oliveira, D.F. CHALCONES TO CONTROL Alternaria Alternata IN MURCOTT TANGOR FRUITS. Bioscience Journal 2016, 32, 1512-1521-1512–1521.
- Demartelaere, A.C.F.; do Nascimento, L.C.; Abraão, P.C.; Gomes, R.S.S.; Marinho, C.O.; Nunes, M.C. Alternatives in the Control of Alternaria Brown Spot in ‘Dancy’ Tangerine. Summa Phytopathologica 2018, 44, 164-169-164–169. [CrossRef]
- Lamine, M.; Hamdi, Z.; Zemni, H.; Rahali, F.Z.; Melki, I.; Mliki, A.; Gargouri, M. From Residue to Resource: The Recovery of High-Added Values Compounds through an Integral Green Valorization of Citrus Residual Biomass. Sustainable Chemistry and Pharmacy 2024, 37. [CrossRef]
- Badawy, M.E.I.; Abdelgaleil, S.A.M. Composition and Antimicrobial Activity of Essential Oils Isolated from Egyptian Plants against Plant Pathogenic Bacteria and Fungi. Industrial Crops and Products 2014, 52, 776–782. [CrossRef]
- Perina, F.J.; Amaral, D.C.; Fernandes, R.S.; Labory, C.R.G.; Teixeira, G.A.; Alves, E. Thymus Vulgaris Essential Oil and Thymol against Alternaria Alternata (Fr.) Keissler: Effects on Growth, Viability, Early Infection and Cellular Mode of Action. Pest Management Science 2015, 71, 1371-1378-1371–1378. [CrossRef]
- Papoutsis, K.; Vuong, Q.V.; Tesoriero, L.; Pristijono, P.; Stathopoulos, C.E.; Gkountina, S.; Lidbetter, F.; Bowyer, M.C.; Scarlett, C.J.; Golding, J.B. Microwave Irradiation Enhances the in Vitro Antifungal Activity of Citrus By-Product Aqueous Extracts against Alternaria Alternata. International Journal of Food Science and Technology 2018, 53, 1510-1517-1510–1517. [CrossRef]
- Saks, Y.; Barkaigolan, R. ALOE VERA GEL ACTIVITY AGAINST PLANT-PATHOGENIC FUNGI. Postharvest Biology and Technology 1995, 6, 159–165.
- Porcino, L.C., Mirelly M.,. Oliveira, Valdeir de S.,. da Silva, Hilderlande F.,. de Souza, Mileny dos S.,. do Nascimento Essential Oils in the Management of Alternaria Alternata f. Sp. Citri in ‘Dancy’ Tangerine Fruits. Revista Caatinga 2023, 36, 291–299.
- Affes, T.G.; Lasram, S.; Hammami, M.; Yeddes, W.; Wannes, W.A.; Khammassi, S.; Ben Hmida, N.L.; Nasraoui, B.; Tounsi, M.S. In Vitro Antifungal Potential of Peel Essential Oils from Different Citrus Species on Alternaria Alternata. Trends in Phytochemical Research 2022, 6, 214-223-214–223. [CrossRef]
- Camargos, R.B.; Perina, F.J.; Carvalho, D.D.C.; Alves, E.; Mascarello, A.; Chiaradia-Delatorre, L.D.; Yunes, R.A.; Nunes, R.J.; Oliveira, D.F. Chalcones to Control Alternaria Alternata in Murcott Tangor Fruits; [Chalconas No Controle de Alternaria Alternata Em Frutos de Tangor Murcote]. Bioscience Journal 2016, 32, 1512–1521.
- Demartelaere, A.C.F.; Nascimento, L.C.; Almeida, L.C.; Vargas, C.S.; Porcino, M.M.; Clemente, P.A. Using Caesalpinia Ferrea Extract the Management of Alternaria Brown Spot in Tangerine Seedlings “Dancy.” Revista Brasileira de Plantas Medicinais 2021, 19, 58–66.
- Pinto, K.M.S.; Melo, P.A.F.R. de; Mondego, J.M.; Nascimento, L.C. do; Cortez, M.I.M.M.; Aires, A.A. de C.; Anjos Neto, A.P. dos; Medeiros, R.L.S. de; Araujo, J.R.G.; Silva, H.F. da Plant Extracts Enhancers of Defense Response in Ponkan Mandarin Seedlings against Alternaria Alternate f. Spp. Citri Infection. African Journal of Agricultural Research 2018, 13, 650–656. [CrossRef]
- Cirvilleri, G.; Bonaccorsi, A.; Scuderi, G.; Scortichini, M. Potential Biological Control Activity and Genetic Diversity of Pseudomonas Syringae Pv. Syringae Strains. Journal of Phytopathology 2006, 154, 654–666.
- Khalil, M.S.A.; El-Gamal, N.G.; El-Mougy, N.S.; Abdel-Kader, M.M. Occurrence of Citrus Brown and Black Spot Diseases and Their Control Using Pre-Harvest Approaches. Bioscience Journal 2022, 38. [CrossRef]
- Wilson, C.; Chalutz, E.; Wilson, C.L.; McLaughlin, R.J.; McLaughin, R.J. Biological Control of Post-Harvest Rot on Fruits by Applying New Strains of Pichia Guilliermondii (Anamorph Candida Guilliermondii).
- Zhang, R.; Yu, J.; Yin, X.; Ren, X.; Kong, Q. Biocontrol of Postharvest Decay on Cherry Tomatoes by Recombinant Strain GS115/CEC and Its Possible Mechanism. Food Biotechnology 2018, 32, 163–177.
- Riera, N.; Handique, U.; Zhang, Y.Z.; Dewdney, M.M.; Wang, N.A. Characterization of Antimicrobial-Producing Beneficial Bacteria Isolated from Huanglongbing Escape Citrus Trees. Frontiers in Microbiology 2017, 8, 2415–2415. [CrossRef]
- Ferreira, F.V.; Herrmann-Andrade, A.M.; Calabrese, C.D.; Bello, F.; Vazquez, D.; Musumeci, M.A. Effectiveness of Trichoderma Strains Isolated from the Rhizosphere of Citrus Tree to Control Alternaria Alternata, Colletotrichum Gloeosporioides and Penicillium Digitatum A21 Resistant to Pyrimethanil in Post-Harvest Oranges (Citrus Sinensis L. (Osbeck)). Journal of Applied Microbiology 2020, 129, 712-727-712–727. [CrossRef]
- Scuderi, G.; Bonaccorsi, A.; Panebianco, S.; Vitale, A.; Polizzi, G.; Cirvilleri, G. Some Strains of Burkholderia Gladioli Are Potential Candidates for Postharvest Biocontrol of Fungal Rots in Citrus and Apple Fruits. Journal of Plant Pathology 2009, 91, 207-213-207–213.
- Turaeva, B.I.; Soliev, A.B.; Karimov, H.K.; Azimova, N.S.Q.; Kutlieva, G.J.; Khamidova, K.M.; Zuxritdinova, N.Y. Disease Causing Phytopathogenic Micromycetes in Citrus in Uzbekistan. Pakistan Journal of Phytopathology 2021, 33, 383–393. [CrossRef]
- Lu, Q.; Liu, J.; Tu, C.; Li, J.; Lei, C.; Guo, Q.; Zhang, Z.; Qin, W. In Vitro Antibacterial Activity of 34 Plant Essential Oils against Alternaria Alternata. E3S Web of Conferences 2019, 136.
- Aslam, M.F.; Irshad, G.; Naz, F.; Khan, M.A. Evaluation of the Antifungal Activity of Essential Oils against Alternaria Alternata Causing Fruit Rot of Eriobotrya Japonica. Turkish Journal of Biochemistry 2022, 47, 511–521.
- Ajayi-Moses, O.B.; Ogidi, C.O.; Akinyele, B.J. Bioactivity of Citrus Essential Oils (CEOs) against Microorganisms Associated with Spoilage of Some Fruits. Chemical and Biological Technologies in Agriculture 2019, 6, 22–22. [CrossRef]
- Shukla, A.C.; Shahi, S.K.; Anupam Dikshit, A.D. Epicarp of Citrus Sinensis: A Potential Source of Natural Pesticide. Indian Phytopathology 2000, 53, 468–471.
- Raina, P.K. In Vitro Fungitoxicity of Citrus Sinensis Essential Oil to Compost-Based Weed Fungi of Agaricus Bisporus. Mushroom Research 2004, 13, 82–83.
- Singh, P.; Shukla, R.; Prakash, B.; Kumar, A.; Singh, S.; Mishra, P.K.; Dubey, N.K. Chemical Profile, Antifungal, Antiaflatoxigenic and Antioxidant Activity of Citrus Maxima Burm. and Citrus Sinensis (L.) Osbeck Essential Oils and Their Cyclic Monoterpene, DL-Limonene. Food and Chemical Toxicology 2010, 48, 1734-1740-1734–1740. [CrossRef]
- Hamdani, F.Z.; Allem, R. Antifungal Properties of Leaf Essential Oils of Citrus against Alternaria Alternata and Penicillium Sp in Vitro. Phytotherapie 2017, 15, 263-266-263–266. [CrossRef]
- Gomes, M. de S.; Cardoso, M. das G.; Souza, P.E. de; Machado, S.M.F.; Silva, L.F.; Teixeira, M.L.; Andrade, J. de; Miranda, C.A.S.F. de; Andrade, M.A. Multivariate Analysis of the Essential Oil Components of the Genus Citrus and Their Antifungal Activity. Cientifica (Jaboticabal) 2013, 41, 111–121.
- de Lima, C.B.; Assumpcao Rentschler, L.L.; Bueno, J.T.; Boaventura, A.C. Plant Extracts and Essential Oils on the Control of Alternaria Alternata, Alternaria Dauci and on the Germination and Emergence of Carrot Seeds (Daucus Carota L.). CIENCIA RURAL 2016, 46, 764–770. [CrossRef]
- Shafique, S.; Shafique, S.; Ahmed, A. Ecofriendly Response of Citrus Peels to Alternaria Leaf Spots of Tomato: Exclusive Role of Peel Phenolics. International Journal of Agriculture and Biology 2013, 15, 1236–1242.
- Triaca, T.; Cavião, H.C.; Pansera, M.R.; Venturin, L.; Sartori, V.C. Detection of Antifungal Activity of Plant Extracts on Alternaria Citrus. Summa Phytopathologica 2018, 44, 185-188-185–188. [CrossRef]
- Campos, V.A.; Perina, F.J.; Alves, E.; Sartorelli, J.; Moura, A.M.; Oliveira, D.F. Anadenanthera Colubrina (Vell.) Brenan Produces Steroidal Substances That Are Active against Alternaria Alternata (Fr.) Keissler and That May Bind to Oxysterol-Binding Proteins. Pest Management Science 2014, 70, 1815-1822-1815–1822. [CrossRef]
- Hernández, A.; Ruiz-Moyano, S.; Galván, A.I.; Merchán, A.V.; Pérez Nevado, F.; Aranda, E.; Serradilla, M.J.; Córdoba, M.D.G.; Martín, A. Anti-Fungal Activity of Phenolic Sweet Orange Peel Extract for Controlling Fungi Responsible for Post-Harvest Fruit Decay. Fungal Biology 2021, 125, 143-152-143–152. [CrossRef]
- Pontes, F.C.; Abdalla, V.C.P.; Imatomi, M.; Fuentes, L.F.G.; Gualtieri, S.C.J. Antifungal and Antioxidant Activities of Mature Leaves of Myrcia Splendens (Sw.) DC. Brazilian Journal of Biology 2019, 79, 127-132-127–132. [CrossRef]
- Carvalho, D.D.C.; Alves, E.; Barbosa Camargos, R.; Ferreira Oliveira, D.; Soares Scolforo, J.R.; de Carvalho, D.A.; Sâmia Batista, T.R. Plant Extracts to Control Alternaria Alternata in Murcott Tangor Fruits. Revista Iberoamericana de Micologia 2011, 28, 173–178.
- Llorens, E.; Camanes, G.; Lapena, L.; Garcia-Agustin, P. Priming by Hexanoic Acid Induce Activation of Mevalonic and Linolenic Pathways and Promotes the Emission of Plant Volatiles. Frontiers in Plant Science 2016, 7, 495–495. [CrossRef]
- Abdel-Ghafar, R.Y.; Sehim, A.E.; Hamza, Z.K.; El-Nekeety, A.A.; Abdel-Wahhab, M.A. Evaluation of the Antimicrobial, Antioxidant, and Cytotoxicity Against MCF-7 Breast Cell Lines of Biosynthesized Vanadium Nanoparticles. BioNanoScience 2022, 12, 1097–1105.
- Shende, S.S.; Gade, A.K.; Minkina, T.M.; Ingle, P.U.; Rajput, V.D.; Sushkova, S.N.; Mandzhieva, S.S.; Rai, M.; Wong, M.H. Exploring Sustainable Management by Using Green Nano-Silver to Combat Three Post-Harvest Pathogenic Fungi in Crops. Discover Nano 2024, 19.
- Nishimura, S.; Tatano, S.; Gomi, K.; Ohtani, K.; Fukumoto, T.; Akimitsu, K. Chloroplast-Localized Nonspecific Lipid Transfer Protein with Anti-Fungal Activity from Rough Lemon. Physiological and Molecular Plant Pathology 2008, 72, 134-140-134–140. [CrossRef]
- Li, Y.; Fan, L.; Tang, X.-M.; Yang, D.-M.; Hu, J.-H.; Wu, Y.-Z.; Zhan, S.; Yang, D.-C. Synthesis and Antibacterial Activity of C-7 Haloacyl Cephalosporins. Yaoxue Xuebao 2021, 56, 1965-1975-1965–1975. [CrossRef]
- Arslan, D.; Tuccitto, N.; Auditore, A.; Licciardello, A.; Marletta, G.; Riolo, M.; La Spada, F.; Conti Taguali, S.; Calpe, J.; Meca, G.; et al. Chitosan-Based Films Grafted with Citrus Waste-Derived Antifungal Agents: An Innovative and Sustainable Approach to Enhance Post-Harvest Preservation of Citrus Fruit. International Journal of Biological Macromolecules 2024, 264.
- Fungicide Resistance Action Committee FRAC Code List 2024: Fungicide Classification According to Mode of Action 2024.
- Huang ChiaoWen, H.C.; Wu ChaoJung, W.C.; Yang HongRen, Y.H.; Lai SuYu, L.S.; Ni HuiFang, N.H. Physiological Characteristics, Pathogenicity and Fungicide Screening of Citrus Alternaria Brown Spot Disease Caused by Alternaria Alternata. Journal of Taiwan Agricultural Research 2018, 67, 387–400.
- Sharma, R.N.; Gaur, R.B. Management of Post-Harvest Core Rot, Alternaria Alternata in Kinnow, Citrus Deliciosa Fruits. Indian Journal of Plant Protection 2009, 37, 207–210.
- Colturato, A.B.; Paulossi, T.; Venâncio, W.S.; Furtado, E.L. Efficiency and Cost of Chemical Control of Alternaria Brown Spot; [Eficiência e Custo Do Controle Químico Da Mancha de Alternaria Em Tangor Murcote]. Summa Phytopathologica 2009, 35, 210–215.
- Dinesh Singh, D.S.; Thakur, A.K.; Bhagwat, V.R. Effect of Fungicides and Calcium Nitrate on Fruit Drop of Kinnow. Bioved 2005, 16, 47–50.
- Garganese, F.; Sanzani, S.M.; Di Rella, D.; Schena, L.; Ippolito, A. Pre- and Postharvest Application of Alternative Means to Control Alternaria Brown Spot of Citrus. CROP PROTECTION 2019, 121, 73–79. [CrossRef]
- Peres, N.A.; Timmer, L.W. Evaluation of the Alter-Rater Model for Spray Timing for Control of Alternaria Brown Spot on Murcott Tangor in Brazil. Crop Protection 2006, 25, 454-460-454–460. [CrossRef]
- van Zyl, J.G.; Fourie, P.H.; Schutte, G.C. Spray Deposition Assessment and Benchmarks for Control of Alternaria Brown Spot on Mandarin Leaves with Copper Oxychloride. CROP PROTECTION 2013, 46, 80–87. [CrossRef]
- Vicent, A.; Armengol, J.; García-Jiménez, J. Rain Fastness and Persistence of Fungicides for Control of Alternaria Brown Spot of Citrus. Plant Disease 2007, 91, 393–399. [CrossRef]
- Vicent, A.; Armengol, J.; García-Jiménez, J. Protectant Activity of Reduced Concentration Copper Sprays against Alternaria Brown Spot on “Fortune” Mandarin Fruit in Spain. Crop Protection 2009, 28, 1–6.
- Camiletti, B.X.; Lichtemberg, P.S.F.; Paredes, J.A.; Carraro, T.A.; Velascos, J.; Michailides, T.J. Characterization, Pathogenicity, and Fungicide Sensitivity of Alternaria Isolates Associated with Preharvest Fruit Drop in California Citrus. FUNGAL BIOLOGY 2022, 126, 277–289. [CrossRef]
- Miles, A.K.; Willingham, S.L.; Cooke, A.W. Field Evaluation of a Plant Activator, Captan, Chlorothalonil, Copper Hydroxide, Iprodione, Mancozeb and Strobilurins for the Control of Citrus Brown Spot of Mandarin. Australasian Plant Pathology 2005, 34, 63-71-63–71. [CrossRef]
- Mondal, S.N.; da Silva, A.G.; Dewdney, M.M. Resistance to Strobilurin Fungicides in a Population of Alternaria Alternata Causing Alternaria Brown Spot of Citrus. PHYTOPATHOLOGY 2009, 99, S88–S88.
- Solel, Z.; Oren, Y.; Kimchi, M. Control of Alternaria Brown Spot of Minneola Tangelo with Fungicides. CROP PROTECTION 1997, 16, 659–664. [CrossRef]
- Oren, Y.; Solel, Z.; Kimki, M.; Sadovski, A. Controlling Alternaria Alternata in the Citrus Varieties ‘Minneola’and ‘Nova.’ Phytoparasitica 1999, 27, 152–153.
- Reis, R.F.; de Goes, A.; Mondal, S.N.; Shilts, T.; Brentu, F.C.; Timmer, L.W. Effect of Lesion Age, Humidity, and Fungicide Application on Sporulation of Alternaria Alternata, the Cause of Brown Spot of Tangerine. PLANT DISEASE 2006, 90, 1051–1054. [CrossRef]
- Nouhra, G.; Poloni, N.M.; Pereira, F.D.; de Goes, A. Efficiency of Trifloxystrobin and Tebuconazole, in a Commercial Formulation, Associated with Protective Fungicides to Control Alternaria Brown Spot on “Murcott” Tangors. Summa Phytopathologica 2021, 47, 122–125. [CrossRef]
- Mondal, S.; Bhatia, A.; Shilts, T.; Timmer, L. Baseline Sensitivities of Fungal Pathogens of Fruit and Foliage of Citrus to Azoxystrobin, Pyraclostrobin, and Fenbuconazole. PLANT DISEASE 2005, 89, 1186–1194. [CrossRef]
- Jamiołkowska, A. Laboratory Effect of Azoxystrobin (Amistar 250 SC) and Grapefruit Extract (Biosept 33 SL) on Growth of Fungi Colonizing Zucchini Plants; [Laboratoryjna Ocena Wpływu Azoksystrobiny i Ekstraktu z Grejpfruta Na Wzrost Grzybów Wystepujacych Na Cukinii]. Acta Scientiarum Polonorum, Hortorum Cultus 2011, 10, 245–257.
- Chitolina, G.M.; Silva-Junior, G.J.; Feichtenberger, E.; Pereira, R.G.; Amorim, L. Distribution of Alternaria Alternata Isolates with Resistance to Quinone Outside Inhibitor (QoI) Fungicides in Brazilian Orchards of Tangerines and Their Hybrids. CROP PROTECTION 2021, 141. [CrossRef]
- Chitolina, G.M.; Silva-Junior, G.J.; Feichtenberger, E.; Pereira, R.G.; Amorim, L. First Report on Quinone Outside Inhibitor Resistance of Alternaria Alternata Causing Alternaria Brown Spot in Tangerines in São Paulo, Brazil. Plant Health Progress 2019, 20, 94–94. [CrossRef]
- Nicoletta, K.R.; Vega, B.; Dewdney, M.M. Distribution of Azoxystrobin Resistance in Nonpathogenic Alternaria Alternata Isolates of Citrus. PHYTOPATHOLOGY 2015, 105, 101–101.
- Vega, B.; Dewdney, M.M.; Fla State Hort Soc Geographical Distribution of Strobilurin Resistance of Alternaria Alternata, Causal Agent of Alternaria Brown Spot in Florida Citrus Groves.; 2012; Vol. 125, pp. 33–35.
- Vega, B.; Dewdney, M.M. Distribution of Qol Resistance in Populations of Tangerine-Infecting Alternaria Alternata in Florida. PLANT DISEASE 2014, 98, 67–76. [CrossRef]
- Yogev, E.; Sadowsky, A.; Solel, Z.; Oren, Y.; Orbach, Y. The Performance of Potassium Phosphite for Controlling Alternaria Brown Spot of Citrus Fruit. JOURNAL OF PLANT DISEASES AND PROTECTION 2006, 113, 207–213. [CrossRef]
- Mondal, S.N.; Vicent, A.; Reis, R.F.; Timmer, L.W. Efficacy of Pre- and Postinoculation Application of Fungicides to Expanding Young Citrus Leaves for Control of Melanose, Scab, and Alternarial Brown Spot. PLANT DISEASE 2007, 91, 1600–1606. [CrossRef]
- He, M.; Fu, Y.; Ruan, R.; Li, H. Sensitivity Assay of Alternaria Alternata from Citrus in China to Four New Fungicides. Journal of Zhejiang University (Agriculture and Life Sciences) 2016, 42, 535–542.
- Highland, B.H.; Timmer, L.W. The Use of Serenade Biofungicide to Control Foliar Fungal Diseases of Florida Citrus. Proceedings of the Florida State Horticultural Society 2004, 117, 127–130.
- Erkiliç, A.; Canihoş, Y.; Biçici, M.; Kurt, Ş. Iprodione Resistance of Alternaria Alternata f.Sp. Citri Minneola Tangelo Isolates in Turkey; [Türkiye’de Alternaria Alternata f.Sp. Citri’nin Minneola Tangelo İzolatlarinin İprodione’a Dayanikliliklari]. Turkish Journal of Agriculture and Forestry 1999, 23, 1051–1056.
- Solel, Z.; Timmer, L.W.; Kimchi, M. Iprodione Resistance of Alternaria Alternata Pv. Citri from Minneola Tangelo in Israel and Florida. Plant Disease 1996, 80, 291–293.
- Byron, V. Sensitivity of Alternaria Alternata from Citrus to Boscalid and Polymorphism in Iron-Sulfur and in Anchored Membrane Subunits of Succinate Dehydrogenase 2015.
- Nishimura, S.; Tatano, S.; Miyamoto, Y.; Ohtani, K.; Fukumoto, T.; Gomi, K.; Tada, Y.; Ichimura, K.; Akimitsu, K. A Zinc-Binding Citrus Protein Metallothionein Can Act as a Plant Defense Factor by Controlling Host-Selective ACR-Toxin Production. PLANT MOLECULAR BIOLOGY 2013, 81, 1–11. [CrossRef]
- Asanzi, N.M.; Taylor, N.J.; Vahrmeijer, J.T. Can Silicon Be Used to Prevent Alternaria Alternata in Citrus Trees? SA Fruit Journal 2014, 13, 48–51.
- Mvondo-She, M.A.; Gatabazi, A.; Laing, M.D.; Ndhlala, A.R. A Review on the Role of Silicon Treatment in Biotic Stress Mitigation and Citrus Production. AGRONOMY-BASEL 2021, 11. [CrossRef]
- Alayon Luaces, P.; Chabbal, M.D.; Piccoli, A.B.; Yfran Elvira, M.M.; Gaiad, J.E.; Gimenez, L.I. Combination of Treatments with Fungicides and Calcium Nitrate for the Control of Brown Spot (Alternaria Alternata) and Its Effect on the Production of “Murcott” Tangor. RIA, Revista de Investigaciones Agropecuarias 2022, 48, 10–15.
- Vicent, A.; Badal, J.; Asensi, M.; Sanz, N.; Armengol, J.; García-Jiménez, J. Laboratory Evaluation of Citrus Cultivars Susceptibility and Influence of Fruit Size on Fortune Mandarin to Infection by Alternaria Alternata Pv. Citri. EUROPEAN JOURNAL OF PLANT PATHOLOGY 2004, 110, 245–251. [CrossRef]
- Llorens, E.; Fernández-Crespo, E.; Vicedo, B.; Lapeña, L.; García-Agustín, P. Enhancement of the Citrus Immune System Provides Effective Resistance against Alternaria Brown Spot Disease. Journal of Plant Physiology 2013, 170, 146–154. [CrossRef]
- Timmer, L.W.; Darhower, H.M.; Bhatia, A. The Alter-Rater, a New Weather-Based Model for Timing Fungicide Sprays for Alternaria Control; University of Florida Cooperative Extension Service, Institute of Food and …, 2001;
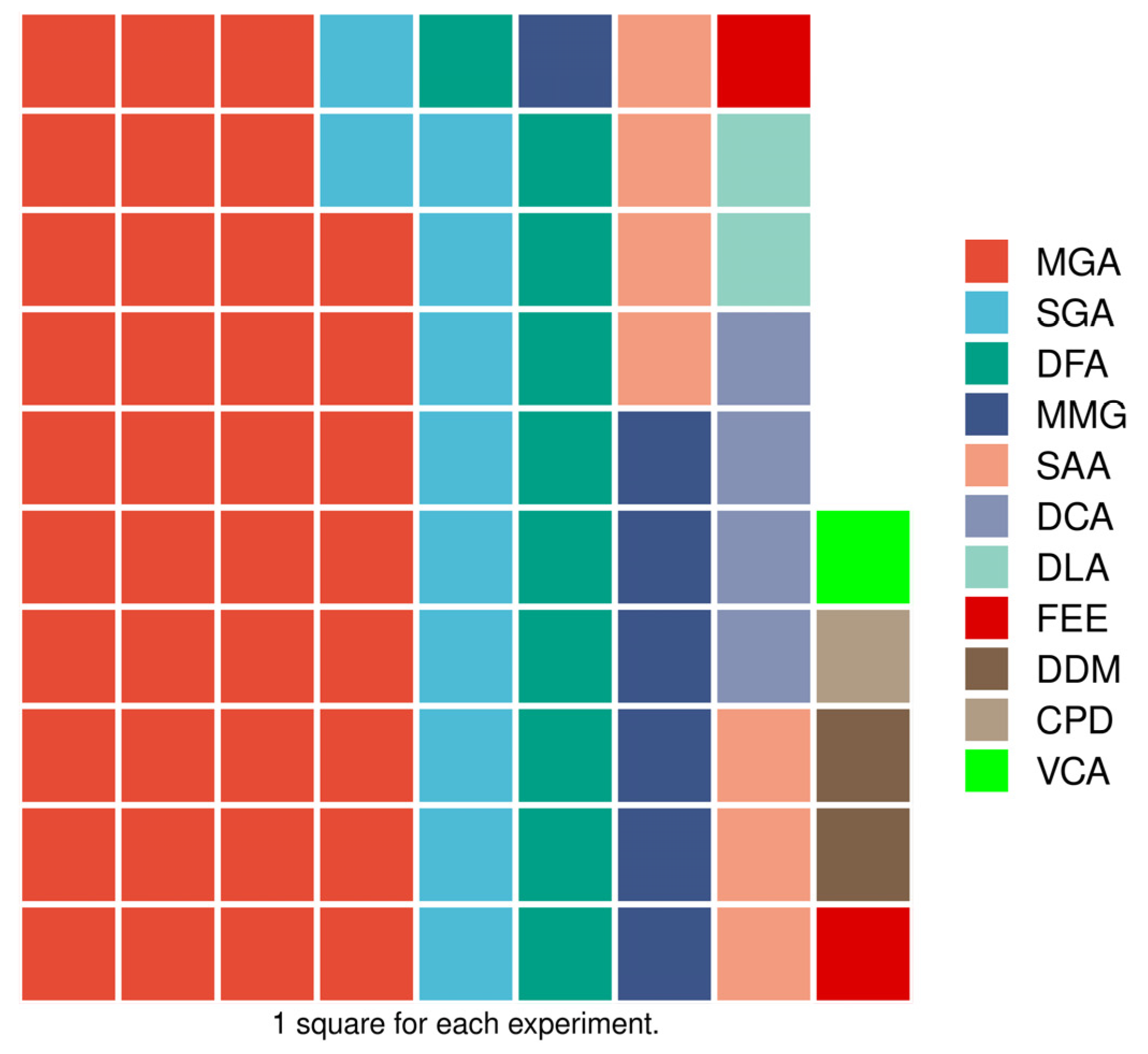
| Database | Specific search string | Published | Doc type | Lang | n |
| WoS | (TI=(Alternaria alternata AND citrus)) OR AB=(Alternaria alternata AND citrus) OR AK=(Alternaria alternata AND citrus) OR KP=(Alternaria alternata AND citrus) | all years | all | Auto | 343 |
| Scopus | TITLE-ABS-KEY ( Alternaria AND alternata AND citrus ) | all years | all | all | 276 |
| Google Academics | allintitle: Alternaria alternata citrus | all years | all | all | 144 |
| PubMed | (Alternaria alternata[Title/Abstract]) AND citrus[Title/Abstract] | all years | all | all | 109 |
| Scielo | All indexes: (Alternaria alternata) AND (citrus) | all years | all | all | 19 |
| Graph . | FRAC Code | Substance | Resist | Lab effectiveness | Field effectiveness | Refs | References |
|---|---|---|---|---|---|---|---|
| copper (inorganic) | MSCA | copper oxychloride | No | low to medium | low to high | 9 | [72,73,74,75,76,77,78,79,80] |
| copper hydroxide | No | low to medium | medium | 5 | [72,79,81,82,83] | ||
| Bordeaux mixture | No | ineffective | not tested | 3 | [72,79,80] | ||
| cuprous oxide | No | high | not tested | 3 | [72,79,80] | ||
| tribasic copper sulfate | No | low | not tested | 1 | [72] | ||
| Dithiocarbamates | MSCA | Mancozeb | No | high | medium to high | 8 | [72,73,74,75,77,79,82,84] |
| Propineb | No | high | medium to high | 3 | [73,74,85] | ||
| Maneb | No | not tested | ineffective | 1 | [84] | ||
| Ferbam | No | ineffective | not tested | 1 | [86] | ||
| Metiram | No | not tested | medium | 1 | [84] | ||
| Chloronitriles | MSCA | Chlorothalonil | No | not tested | ineffective | 2 | [82,84] |
| Phthalimides | MSCA | Captan | No | not tested | contradictory | 2 | [82,84] |
| DeMethylation Inhibitors | DMI | Prochloraz | high | low | 2 | [73,84] | |
| Tebuconazole | medium to high | low | 3 | [81,84,87] | |||
| Difenoconazole | not tested | low | 2 | [74,79] | |||
| Pyrifenox | low to high | not tested | 1 | [85] | |||
| Quinone outside Inhibitors | QoI | Azoxystrobin | Yes | contradictory | medium | 12 | [72,74,81,82,85,88,89,90,91,92,93,94] |
| Pyraclostrobin | Yes | high | high | 12 | [14,74,76,82,83,88,90,91,93,94,95,96] | ||
| Trifloxystrobin | Yes | low | contradictory | 3 | [74,81,82] | ||
| Methoxycrylate | Yes | not tested | medium | 1 | [82] | ||
| Famoxadone | Yes | not tested | not tested | 1 | [96] | ||
| Benzimidazole | MBC | Carbendazim | Yes | low | not tested | 2 | [72,75] |
| Thiophanate methyl | Yes | low | not tested | 2 | [72,73] | ||
| Diarylamine | Fluazinam | No | high | contradictory | 4 | [72,84,97,98] | |
| Dicarboximides | Iprodione | Yes | high | high | 6 | [74,82,84,95,99,100] | |
| Procymidone | Yes | not tested | low | 1 | [84] | ||
| Succinate-dehydrogenase inhibitors | SDHI | Fluopyram | Yes | high | not tested | 1 | [81] |
| Flutolanil | Yes | high | not tested | 1 | [97] | ||
| Thifluzamide | Yes | high | not tested | 1 | [97] | ||
| Boscalid | Yes | medium to low | not tested | 3 | [95,97,101] | ||
| Others | Natamycin | No | high | not tested | 1 | [74] | |
| Metallothionein | No | high | not tested | 1 | [102] | ||
| Silicon | No | medium to low | not tested | 2 | [103,104] | ||
| Calcium nitrate | No | not tested | medium to low | 2 | [75,105] | ||
| Chitosan | No | not tested | medium to low | 1 | [76] | ||
| Salicyl-hydroxamic acid | No | medium to low | not tested | 1 | [83] | ||
| Acibenzolar | No | medium to low | not tested | 2 | [14,82] | ||
| Potassium phosphite | No | ineffective | medium to high | 1 | [95] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





