Submitted:
03 May 2025
Posted:
06 May 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
- i)
- To evaluate the CBW flight dynamics in south-east Romania;
- ii)
- To evaluate the CBW attack on a maize cob in south-east Romania;
- iii)
- To analyze maize grains for aflatoxin content in south-east Romania.
2. Materials and Methods
2.1. Experimental Site
2.2. CBW Flight Monitoring
2.3. Field Trial
2.4. Aflatoxins Analysis
2.5. Statistical Analysis
3. Results
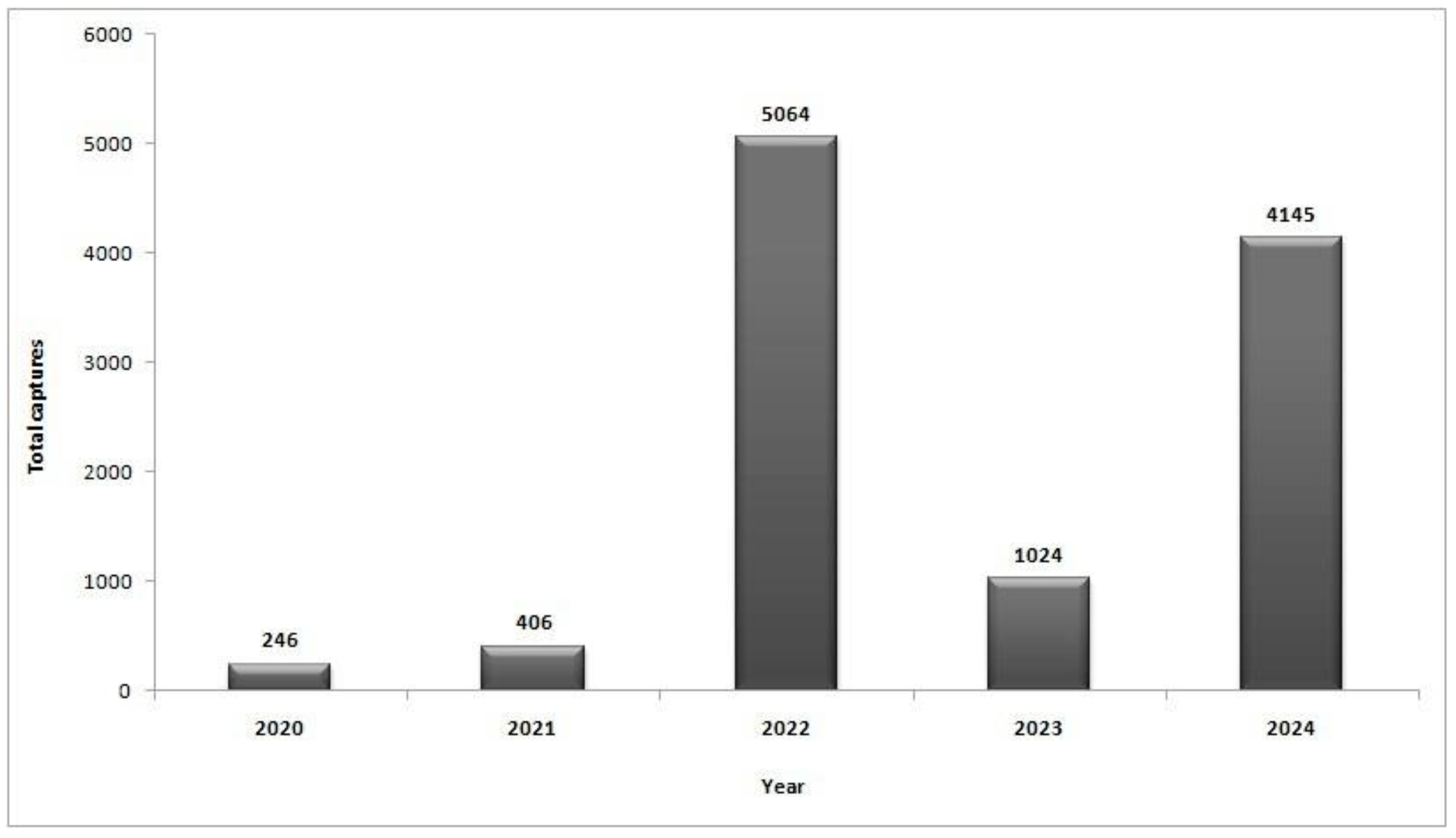
3.1. CBW Flight Dynamics
3.2. CBW Attack Incidence
3.3. CBW Number of Larvae per Plant
3.4. Aflatoxin Level in the Maize Grains
4. Discussions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgements
Conflicts of Interest
References
- Helicoverpa armigera (cotton bollworm). CABI Compendium. Available online: https://www.cabidigitallibrary.org/doi/10.1079/cabicompendium.26757 (accessed on 15 April 2025).
- Pomari-Fernandes, A.; de Freitas Bueno, A.; Sosa-Gómez, D.R. Helicoverpa armigera: current status and future perspectives in Brazil. Current Agric. Sci. and Tech. 2015, 21, 1–7. [Google Scholar] [CrossRef]
- Anderson, C. J.; Tay, W.T.; McGaughran, A.; Gordon, K.; Walsh, T.K. Population structure and gene flow in the global pest, Helicoverpa armigera. Molecular Ec. 2016, 25, 5296–5311. [Google Scholar] [CrossRef] [PubMed]
- Riaz, S.; Johnson, J.B.; Ahmad, M.; Fitt, G.P.; Naiker, M. A review on biological interactions and management of the cotton bollworm, Helicoverpa armigera (Lepidoptera: Noctuidae). Jour. of Appl. Ent. 2021, 145, 467–498. [Google Scholar] [CrossRef]
- Yadav, S.P.S.; Lahutiya, V.; Paudel, P. A review on the biology, ecology, and management tactics of Helicoverpa armigera (Lepidoptera: Noctuidae). Turkish Jour. of Agric.-Food Sci. and Tech. 2022, 10, 2467–2476. [Google Scholar] [CrossRef]
- Czepak, C.; Albernaz, K. C., Vivan, L. M., Guimarães, H. O., & Carvalhais, T. First reported occurrence of Helicoverpa armigera (Hübner)(Lepidoptera: Noctuidae) in Brazil. Pesquisa Agr. Trop. 2013, 43, 110–113. [CrossRef]
- Tay, W.T.; Soria, M.F.; Walsh, T.; Thomazoni, D.; Silvie, P.; Behere, G.T.; Anderson, C.; Downes, S. A brave new world for an Old World pest: Helicoverpa armigera (Lepidoptera: Noctuidae) in Brazil. Plos One 2013, 8, e80134. [Google Scholar] [CrossRef]
- de Freitas Bueno, A.; Sosa-Gómez, D.R. The old world bollworm in the Neotropical region: the experience of Brazilian growers with Helicoverpa armigera. Outlooks on Pest Manag. 2014, 25, 261–264. [Google Scholar] [CrossRef]
- Tembrock, L.R.; Timm, A.E.; Zink, F.A.; Gilligan, T.M. Phylogeography of the recent expansion of Helicoverpa armigera (Lepidoptera: Noctuidae) in South America and the Caribbean Basin. An. of the Entomol. Soc. of America 2019, 112, 388–401. [Google Scholar] [CrossRef]
- Gilligan, T.M.; Goldstein, P.Z.; Timm, A.E.; Farris, R.; Ledezma, L.; Cunningham, A. P. Identification of Heliothine (Lepidoptera: Noctuidae) larvae intercepted at US ports of entry from the New World. Jour. of Econom. Entomol., 2019, 112, 603–615. [Google Scholar] [CrossRef]
- Reisig, D. Helicoverpa zea (bollworm). CABI Compendium 2022, 26776. [Google Scholar] [CrossRef]
- Keszthelyi, S.; Nowinszky, L.; Puskás, J. The growing abundance of Helicoverpa armigera in Hungary and its areal shift estimation. Central Europ. Jour. of Biol. 2013, 8, 756–764. [Google Scholar] [CrossRef]
- Anderson, C. J.; Tay, W.T.; McGaughran, A.; Gordon, K.; Walsh, T. K. Population structure and gene flow in the global pest, Helicoverpa armigera. Molecular Ec. 2016, 25, 5296–5311. [Google Scholar] [CrossRef]
- Jones, C.M.; Parry, H.; Tay, W.T.; Reynolds, D.R.; Chapman, J.W. Movement ecology of pest Helicoverpa: implications for ongoing spread. An. Rev. of Ent. 2019, 64, 277–295. [Google Scholar] [CrossRef] [PubMed]
- Karakantza, E.; Rumbos, C.I.; Cavalaris, C.; Athanassiou, C.G. Different Trap Types Depict Dissimilar Spatio-Temporal Distribution of Helicoverpa armigera (Hübner) (Lepidoptera: Noctuidae) in Cotton Fields. Agronomy 2023, 13, 1256. [Google Scholar] [CrossRef]
- Firempong, S.; Zalucki, M.P. Host plant preferences of populations of Helicoverpa-Armigera (Hubner)(Lepidoptera, Noctuidae) from different geographic locations. Austral. Jour. of Zool. 1989, 37, 665–673. [Google Scholar] [CrossRef]
- Liu, Z.; Li, D.; Gong, P.; Wu, K. Life table studies of the cotton bollworm, Helicoverpa armigera (Hübner)(Lepidoptera: Noctuidae), on different host plants. Environ. Ent. 2004, 33, 1570–1576. [Google Scholar] [CrossRef]
- Talekar, N. S.; Opena, R.T.; Hanson, P. Helicoverpa armigera management: a review of AVRDC's research on host plant resistance in tomato. Crop Prot. 2006, 25, 461–467. [Google Scholar] [CrossRef]
- Hemati, S.A.; Naseri, B.; Nouri Ganbalani, G.; Rafiee Dastjerdi, H.; Golizadeh, A. Effect of different host plants on nutritional indices of the pod borer, Helicoverpa armigera. Jour. of Ins. Sci. 2012, 12, 55. [Google Scholar] [CrossRef]
- Dourado, P.M.; Pantoja-Gomez, L.M.; Horikoshi, R.J.; Carvalho, R.A.; Omoto, C.; Corrêa, A.S.; Kim, H.J.; Martinelli, S.; Head, G.P. Host plant use of Helicoverpa spp. (Lepidoptera: Noctuidae) in the Brazilian agricultural landscape. Pest Manag. Sci. 2021, 77, 780–794. [Google Scholar] [CrossRef]
- Yang, L.; Li, M.; Liu, J.; Zeng, J.; Lu, Y. Long-term expansion of cereal crops promotes regional population increase of polyphagous Helicoverpa armigera. Jour. of Pest Sci. 2024, 98, 131–144. [Google Scholar] [CrossRef]
- Riaz, S.; Johnson, J.B.; Ahmad, M.; Fitt, G.P.; Naiker, M. A review on biological interactions and management of the cotton bollworm, Helicoverpa armigera (Lepidoptera: Noctuidae). Journal of Applied Entomology 2021, 145, 467–498. [Google Scholar] [CrossRef]
- Shanker, R.; Prajapati, M.R.; Singh, R.P.; et al. Isolation, molecular characterization of indigenous Metarhizium anisopliae (Metchnikoff) isolate, using ITS-5.8s rDNA region, and its efficacy against the Helicoverpa armigera (Hubner) (Lepidoptera: Noctuidae). Egypt. J. Biol. Pest Contr. 2023, 33, 23. [Google Scholar] [CrossRef]
- Lopes-da-Silva, M.; Sanches, M.; Stancioli, A.; Alves, G.; Sugayama, R. The Role of Natural and Human-Mediated Pathways for Invasive Agricultural Pests: A Historical Analysis of Cases from Brazil. Agric. Sci. 2014, 5, 634–646. [Google Scholar] [CrossRef]
- Specht, A.; Sosa-Gomez, D.R.; Rios, D.A.M.; Claudino, V.C.M.; Paula-Moraes, S.V.; Malaquias, J.V.; Silva, F.A.M.; Roque-Specht, V.F. Helicoverpa armigera (Hübner)(Lepidoptera: Noctuidae) in Brazil: the big outbreak monitored by light traps. Neotropical Entomology 2021, 50, 53–67. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Plant Health (PLH). Scientific Opinion on the pest categorisation of Helicoverpa armigera (Hübner). EFSA Jour. 2014, 12, 3833. [Google Scholar] [CrossRef]
- Helicoverpa armigera (HELIAR). EPPO Global Database. Available online: https://gd.eppo.int/taxon/HELIAR/datasheet (accessed on 17 April 2025).
- Shimizu, K.; Shimizu, K.; Fujisaki, K. Timing of diapause induction and overwintering success in the cotton bollworm Helicoverpa armigera (Hb.)(Lepidoptera: Noctuidae) under outdoor conditions in temperate Japan. Appl. Ent. and Zool. 2006, 41, 151–159. [Google Scholar] [CrossRef]
- Mironidis, G.K.; Stamopoulos, D.C.; Savopoulou-Soultani, M. Overwintering survival and spring emergence of Helicoverpa armigera (Lepidoptera: Noctuidae) in Northern Greece. Environ. Entomol. 2010, 39, 1068–1084. [Google Scholar] [CrossRef]
- Huang, J.; Li, J. (2015). Effects of climate change on overwintering pupae of the cotton bollworm, Helicoverpa armigera (Hübner)(Lepidoptera: Noctuidae). International Jour. of Biometeorol. 2015, 59, 863–876. [Google Scholar] [CrossRef]
- Jones, C.M.; Parry, H.; Tay, W.T.; Reynolds, D.R.; Chapman, J.W. Movement ecology of pest Helicoverpa: implications for ongoing spread. An. Rev. of Ent. 2019, 64, 277–295. [Google Scholar] [CrossRef]
- Jyothi, P.; Aralimarad, P.; Wali, V.; Dave, S.; Bheemanna, M.; Ashoka, J.; Shivayogiyappa, P.; Lim, K.S.; Chapman, J.W.; Sane, S.P. Evidence for facultative migratory flight behavior in Helicoverpa armigera (Noctuidae: Lepidoptera) in India. PLoS One 2021, 16, e0245665. [Google Scholar] [CrossRef]
- Pinto, F.A.; Mattos, M.V.V.; Silva, F.W.S.; Rocha, S.L.; Elliot, S.L. The Spread of Helicoverpa armigera (Lepidoptera: Noctuidae) and Coexistence with Helicoverpa zea in Southeastern Brazil. Insects 2017, 8, 87. [Google Scholar] [CrossRef]
- Feng, H.Q.; Wu, K.M.; Ni, Y.X.; Cheng, D.F.; Guo, Y.Y. Return migration of Helicoverpa armigera (Lepidoptera: Noctuidae) during autumn in northern China. Bull. of Ent. Res. 2005, 95, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Kriticos, D.J.; Ota, N.; Hutchison, W.D.; Beddow, J., Walsh, T., Tay, W.T.; Borchert, D.M., Paula-Moreas, S.V.; Czepak, C.; Zalucki, M.P. The potential distribution of invading Helicoverpa armigera in North America: is it just a matter of time? PloS One 2015, 10, e0119618. [CrossRef]
- Pedgley, D.E. Windborne migration of Heliothis armigera (Hübner)(Lepidoptera: Noctuidae) to the British Isles. Entomol. Gazette 1985, 36, 15–20. [Google Scholar]
- Torres-Vila, L.M.; Rodrıguez-Molina, M.C.; Lacasa-Plasencia, A.; Bielza-Lino, P. Insecticide resistance of Helicoverpa armigera to endosulfan, carbamates and organophosphates: the Spanish case. Crop Prot. 2002, 21, 1003–1013. [Google Scholar] [CrossRef]
- Srinivas, R.; Udikeri, S.S.; Jayalakshmi, S.K.; Sreeramulu, K. Identification of factors responsible for insecticide resistance in Helicoverpa armigera. Comparative Bioch. and Physiol. Part C: Toxicology & Pharmacol. 2004, 137, 261–269. [Google Scholar] [CrossRef]
- Bues, R.; Bouvier, J.C.; Boudinhon, L. Insecticide resistance and mechanisms of resistance to selected strains of Helicoverpa armigera (Lepidoptera: Noctuidae) in the south of France. Crop Prot. 2005, 24, 814–820. [Google Scholar] [CrossRef]
- Avilla, C.; González-Zamora, J.E. Monitoring resistance of Helicoverpa armigera to different insecticides used in cotton in Spain. Crop Prot. 2010, 29, 100–103. [Google Scholar] [CrossRef]
- Joußen, N.; Heckel, D.G. Resistance mechanisms of Helicoverpa armigera. Advances in Ins. Contr. and Resistance Manag. 2016, 241–261. [Google Scholar] [CrossRef]
- Wang, Q.; Rui, C.; Wang, L.; Nahiyoon, S.A.; Huang, W.; Zhu, J.; Ji, X.; Yang, Q.; Yuan, H.; Cui, L. (2021). Field-evolved resistance to 11 insecticides and the mechanisms involved in Helicoverpa armigera (Lepidoptera: Noctuidae). Pest Manag. Sci. 2021, 77, 5086–5095. [Google Scholar] [CrossRef]
- Stavrakaki, M.; Ilias, A.; Simoglou, K.B.; Mironidis, G.K.; Zimmer, C.T.; Souza, D.; Roditakis, E. Revision of Helicoverpa armigera insecticide resistance status in Greece. Crop Prot. 2024, 175, 106446. [Google Scholar] [CrossRef]
- Richardson, E.B.; Troczka, B.J.; Gutbrod, O.; Davies, T.E. Nauen, R. Diamide resistance: 10 years of lessons from lepidopteran pests. Journal of Pest Sci. 2020, 93, 911–928. [Google Scholar] [CrossRef]
- Abbade-Neto, D.; Amado, D.; Pereira, R.M.; Basso, M.; Spineli-Silva, S.; Gonçalves, T.M.; Corrêa, A.S.; Omoto, C. First Report of Helicoverpa armigera (Lepidoptera: Noctuidae) Resistance to Flubendiamide in Brazil: Genetic Basis and Mechanisms of the Resistance. Agronomy 2022, 12, 1664. [Google Scholar] [CrossRef]
- Amado, D.; Koch, E.L.; Cordeiro, E.M.; Araújo, W.A.; Garcia, A.A.; Heckel, D.G.; Montejo-Kovacevich, G.; North, H.L.; Corrêa, A.S. Jiggins, C.D.; Omoto, C. The genetic architecture of resistance to flubendiamide insecticide in Helicoverpa armigera (Hübner). PloS One 2025, 20, e0318154. [Google Scholar]
- Wu, K. Monitoring and management strategy for Helicoverpa armigera resistance to Bt cotton in China. Jour. of Inverteb. Path. 2007, 95, 220–223. [Google Scholar] [CrossRef] [PubMed]
- Downes, S.; Mahon, R. Successes and challenges of managing resistance in Helicoverpa armigera to Bt cotton in Australia. GM crops & food 2012, 3, 228–234. [Google Scholar] [CrossRef]
- Downes, S.; Kriticos, D.; Parry, H.; Paull, C.; Schellhorn, N.; Zalucki, M.P. A perspective on management of Helicoverpa armigera: transgenic Bt cotton, IPM, and landscapes. Pest Manag. Sci. 2017, 73, 485–492. [Google Scholar] [CrossRef] [PubMed]
- Windus, L.C.; Jones, A.M.; Downes, S.; Walsh, T.; Knight, K.; Kinkema, M. Hear NPV susceptibility in Helicoverpa armigera and Helicoverpa punctigera strains resistant to Bt toxins Cry1Ac, Cry2Ab, and Vip3Aa. Journal of Invertebr. Path. 2021, 183, 107598. [Google Scholar] [CrossRef]
- Shahid, M.R.; Farooq, M.; Shakeel, M.; Ashraf, M.; Zia, Z.U.; Ahmad, S.; Mahmood, A. Need for growing non-Bt cotton refugia to overcome Bt resistance problem in targeted larvae of the cotton bollworms, Helicoverpa armigera and Pectinophora gossypiella. Egyptian Journal of Biol. Pest Contr. 2021, 31, 1–8. [Google Scholar] [CrossRef]
- Tang, J.; Lu, J.; Zhang, C.; Yu, S.; Ding, Z.; Soe, E.T.; Liang, G. The evaluation of resistance risk to Cry2Ab and cross-resistance to other insecticides in Helicoverpa armigera. Journal of Pest Sci. 2024, 97, 173–184. [Google Scholar] [CrossRef]
- Darvas, B.; Bánáti, H.; Takács, E.; Lauber, É.; Szécsi, Á.; Székács; A. (2011). Relationships of Helicoverpa armigera, Ostrinia nubilalis and Fusarium verticillioides on MON 810 maize. Insects 2011, 2, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Niculina, N.G.; Cotună, O.; Sărățeanu, V.; Durău, V.; Claudia, C.; Suba, T. Research regarding the relationship among the pests Ostrinia nubilalis, Helicoverpa armigera and the fungi Fusarium verticillioides, Aspergillus flavus in corn in the climatic conditions from Lovrin (Timiș County). Res. Jour. of Agric. Sci. 2019, 51, 282–291. Available online: https://rjas.ro/paper_detail/3022 (accessed on 19 April 2025).
- Miller, J.D. Mycotoxins in small grains and maize: old problems, new challenges. Food Add. and Contam. 2008, 25, 219–230. [Google Scholar] [CrossRef] [PubMed]
- de Galarreta, J.I.R.; Butrón, A.; Ortiz-Barredo, A.; Malvar, R.A.; Ordás, A.; Landa, A.; Revilla, P. Mycotoxins in maize grains grown in organic and conventional agriculture. Food Contr. 2015, 52, 98–102. [Google Scholar] [CrossRef]
- Palumbo, R.; Gonçalves, A.; Gkrillas, A.; Logrieco, A.; Dorne, J.L.; Dall'Asta, C.; Venâncio, A.; Battilani, P. Mycotoxins in maize. Phytopat. Med. 2020, 59, 5–28. [Google Scholar] [CrossRef]
- Payne, G.A.; Widstrom, N.W. Aflatoxin in maize. Critical Rev. in Pl. Sci. 1992, 10, 423–440. [Google Scholar] [CrossRef]
- Kos, J.; Mastilović, J.; Hajnal, E.J.; Šarić, B. Natural occurrence of aflatoxins in maize harvested in Serbia during 2009–2012. Food Contr. 2013, 34, 31–34. [Google Scholar] [CrossRef]
- Wu, F.; Stacy, S.L.; Kensler, T.W. Global risk assessment of aflatoxins in maize and peanuts: are regulatory standards adequately protective? Toxicol. Sci. 2013, 135, 251–259. [Google Scholar] [CrossRef]
- Okun, D.O.; Khamis, F.M.; Muluvi, G.M.; Ngeranwa, J.J.; Ombura, F.O.; Yongo, M.O.; Kenya, E. U; Distribution of indigenous strains of atoxigenic and toxigenic Aspergillus flavus and Aspergillus parasiticus in maize and peanuts agro-ecological zones of Kenya. Agric. & Food Sec. 2015, 4, 1–10. [Google Scholar] [CrossRef]
- Wu, F. Global impacts of aflatoxin in maize: trade and human health. World Mycotox. Jour. 2015, 8, 137–142. [Google Scholar] [CrossRef]
- Kilonzo, R.M.; Imungi, J.K.; Muiru, W.M.; Lamuka, P.O.; Njage, P.M.K. Household dietary exposure to aflatoxins from maize and maize products in Kenya. Food Add. & Contamin.: Part A 2014, 31, 2055–2062. [Google Scholar] [CrossRef]
- Kibwana, M.; Kimbokota, F.; Christopher, R.; Mmongoyo, J.A. Aflatoxins in stored maize, maize flours, and stiff porridge consumed in schools: A case study of Dodoma region, Tanzania. Food Contr. 2023, 146, 109519. [Google Scholar] [CrossRef]
- Garrido, N.S.; Iha, M.H.; Santos Ortolani, M.R.; Duarte Fávaro, R.M. Occurrence of aflatoxins M1 and M2 in milk commercialized in Ribeirão Preto-SP, Brazil. Food Add. & Contamin. 2003, 20, 70–73. [Google Scholar] [CrossRef]
- Prandini, A.; Tansini, G.; Sigolo, S.; Filippi, L.; Laporta, M.; Piva, G. On the occurrence of aflatoxin M1 in milk and dairy products. Food and Chem. Toxicol. 2009, 47, 984–991. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, S.Z.; Jinap, S.; Pirouz, A.A.; Faizal, A.A. Aflatoxin M1 in milk and dairy products, occurrence and recent challenges: A review. Trends in Food Sci. & Tech. 2015, 46, 110–119. [Google Scholar] [CrossRef]
- Fountain, J.C.; Scully, B.T.; Ni, X.; Kemerait, R.C. Lee; R.D., Chen, Z.Y., Guo, B. Environmental influences on maize-Aspergillus flavus interactions and aflatoxin production. Frontiers in Microbiol. 2014, 5, 40. [Google Scholar] [CrossRef]
- Milićević, D.; Petronijević, R.; Petrović, Z.; Đjinović-Stojanović, J.; Jovanović, J.; Baltić, T.; Janković, S. (2019). Impact of climate change on aflatoxin M1 contamination of raw milk with special focus on climate conditions in Serbia. Journal of the Sci. of Food and Agr. 2019, 99, 5202–5210. [Google Scholar] [CrossRef]
- Molnár, K.; Rácz, C.; Dövényi-Nagy, T.; Bakó, K.; Pusztahelyi, T.; Kovács, S.; Adácsi, C.; Pócsi, I.; Dobos, A. The Effect of Environmental Factors on Mould Counts and AFB1 Toxin Production by Aspergillus flavus in Maize. Toxins 2023, 15, 227. [Google Scholar] [CrossRef]
- EUR-Lex. Document 02006R1881-20140701: Commission Regulation (EC) No 1881/2006 of 19 December 2006 setting maximum levels for certain contaminants in foodstuffs. Available online: https://eur-lex.europa.eu/eli/reg/2006/1881/2014-07-01 (accessed on 22 April 2025).
- Marete, G.N.; Kanja, L.W.; Mbaria, J.M.; Okumu, M.O.; Ateku, P.A.; Korhonen, H.; Joutsjoki, V. Effects of the Use of Good Agricultural Practices on Aflatoxin Levels in Maize Grown in Nandi County, Kenya. Sci 2020, 2, 85. [Google Scholar] [CrossRef]
- Xu, F.; Baker, R.C.; Whitaker, T.B.; Luo, H.; Zhao, Y.; Stevenson, A.; Boesch, C.J.; Zhang, G. Review of good agricultural practices for smallholder maize farmers to minimise aflatoxin contamination. World Mycotox. Jour. 2022, 15, 171–186. [Google Scholar] [CrossRef]
- Robens, J.; Cardwell, K. The costs of mycotoxin management to the USA: management of aflatoxins in the United States. Jour. of Toxicol.: Toxin Rev. 2003, 22, 139–152. [Google Scholar] [CrossRef]
- Umar, A.; Bhatti, H.S.; Honey, S.F. A call for aflatoxin control in Asia. CABI Agric. and Biosci. 2023, 4, 27. [Google Scholar] [CrossRef]
- Ministry of Agriculture and Rural Development Romania. Available online: https://madr.ro/culturi-de-camp/cereale/porumb.html (accessed on 23 April 2025).
- Eurostat database. Available online: https://ec.europa.eu/eurostat/data/database (accessed on 23 April 2025).
- Dinca, V.M.; Trocinescu, B.; Stamule, S.; Bunea, M.; Dinu, V. Opportunities and challenges for managers within the East-European agriculture sector: Case study on Romania. E&M Ec. and Manag. 2024, 27, 121–134. [Google Scholar] [CrossRef]
- Toader, M.; Georgescu, E.; Ion, V.; Cionga, C.; Radu, C.; Epure, L.I. Bășa, A.G. Pests of maize crops and integrated control strategy in Romania. Sci. Pap. Series A. Agr. 2024, 67, 717–724. Available online: https://agronomyjournal.usamv.ro/index.php/scientific-papers/past-issues?id=1802 (accessed on 23 April 2025).
- Partal, E. Contescu, L.E.; Paraschivu; M., Sălceanu; C., Oltenacu; C.V. Impact of crop rotation and soil management practices on weeding and soil water dynamics in maize crop in southern Romania. An. of the Univ. of Craiova-Agric. Montanol. Cadastre Series 2024, 54, 228–234. [Google Scholar] [CrossRef]
- Georgescu, E.; Toader, M.; Canǎ, L.; Horhocea, D.; Manole, T.; Zaharia, R.; Rîşnoveanu, L. Researches concerning the effectiveness of the maize foliar treatment compared with seeds treatment for chemical control of the maize leaf weevil (Tanymecus dilaticollis Gyll) in the South-East of Romania. Rom. Agric. Res. 2021, 38, 357–369. Available online: https://new.incda-fundulea.ro/index.php?option=com_content&view=article&id=24&Itemid=230&lang=en (accessed on 24 April 2025). [CrossRef]
- Traşcă, F.; Traşcă, G.; Podea, M.M.; Ghiorghe, C.; Dinuță, C.I.; Gheorhe, R.M.; Georgescu, E.I. Damages and integrated control possibilities of wire worms in corn crops, in the area of subcarpatic hills. Ann. of NARDI Fundulea [Analele Institutului Național de Cercetare-Dezvoltare Agricolă Fundulea] 2021, 89, 1–10. Available online: https://www.incda-fundulea.ro/anale/anale89.html (accessed on 24 April 2025). (In Romanian). Available online: https://www.incda-fundulea.ro/anale/anale89.html (accessed on 24 April 2025).
- Pintilie, P.L.; Trotuș, E.; Tălmaciu, N.; Irimia, L.M.; Herea, M.; Mocanu, I.; Amarghioalei, R.G.; Popa, L.D.; Tălmaciu, M. European Corn Borer (Ostrinia nubilalis Hbn.) Bioecology in Eastern Romania. Insects 2023, 14, 738. [Google Scholar] [CrossRef]
- Amarghioalei, R.-G.; Tălmaciu, N.; Herea, M.; Mocanu, I.; Pintilie, P.-L.; Pintilie, A.-S.; Trotuș, E.; Tălmaciu, M. Chemical Control of Western Corn Rootworm (Diabrotica virgifera virgifera Le Conte, Coleoptera: Chrysomelidae) in Eastern Romania. Insects 2025, 16, 293. [Google Scholar] [CrossRef]
- Popov, C.; Bărbulescu, A. 50 years of scientific activity in field protection domain against diseases and pests. Ann. of NARDI Fundulea [Analele Institutului Național de Cercetare-Dezvoltare Agricolă Fundulea] 2007, 75, 371–404. Available online: https://www.incda-fundulea.ro/anale/anale75.html (accessed on 24 April 2025).
- Roşca, I. Research regarding interaction of Mon810 biotech corn on the Helicoverpa armigera in Romania. Sci. Pap., UASVM Bucharest, Series A 2009, 53, 403–411. Available online: https://www.researchgate.net/profile/Andreea-Cosoveanu/publication/273885821_ANTIFUNGAL_ACTIVITY_OF_MACROALGAE_EXTRACTS/links/6787ea3e1afb4e11f5e7f555/ANTIFUNGAL-ACTIVITY-OF-MACROALGAE-EXTRACTS.pdf#page=404 (accessed on 24 April 2025).
- Pintilie, P.L.; Amarghioalei, R.G.; Trotuş, E.; Buburuz, A.A.; Isticioaia, S.F.; Leonte, A.; Popa, L.D. Helicoverpa armigera Hbn. (Lepidoptera: Noctuidae) a pest of agricultural crops in eastern Romania (Helicoverpa armigera Hbn. (Lepidoptera: Noctuidae) un dăunător al culturilor agricole din estul României). Conferencs: Scientific Problems in field crops area-realisations and perspectives (Probleme științifice în domeniul culturilor de câmp-realizări și perspective), 2024, 300-313. Available online: https://ibn.idsi.md/vizualizare_articol/215052 (accessed on 24 April 2025).
- Cotună, O.; Aflatoxins or „cocktail” of mycotoxins from maize grains (Aflatoxinele sau „cocktailul” de micotoxine din boabele de porumb). Farmer journal (Revista Fermierului) 2023. Available online: https://www.revistafermierului.ro/din-revista/protectia-plantelor/item/5589-aflatoxinele-sau-cocktailul-de-micotoxine-din-boabele-de-porumb.html (accessed on 25.04.2025).
- Smeu, I.; Casian, H. Food safety perspectives and management on total aflatoxin levels in 2019 Romanian maize (Zea mays L.) samples. Conference paper: ISB-INMA TEH' 2020, Agricultural and mechanical engineering, Bucharest, Romania, 30 October 2020. Jubilee edition 2020, 176-180. Available online: https://www.cabidigitallibrary.org/doi/full/10.5555/20210291247 (accessed on 24 April 2025).
- Dragomir, V.; Ioan Sebastian, B.; Alina, B.; Victor, P.; Tanasă, L.; Horhocea, D. An overview of global maize market compared to Romanian production. Rom. Agric. Res. 2022, 39, 535–544. Available online: https://new.incda-fundulea.ro/index.php?option=com_content&view=article&id=94&Itemid=229&lang=en (accessed on 24 April 2025). [CrossRef]
- National Agricultural Research and Development Institute, General Information. Available online: https://www.incda-fundulea.ro/informatii_en.htm (accessed on 26 April 2025).
- Busuioc, A.; Giorgi, F.; Bi, X.; Ionita, M. Comparison of regional climate model and statistical downscaling simulations of different winter precipitation change scenarios over Romania. Theor. Appl. Climatol. 2006, 86, 101–123. [Google Scholar] [CrossRef]
- Ionita, M.; Nagavciuc, V. The year with too much summer in the eastern part of Europe. Weather. [CrossRef]
- Csalomon, Cotton bollworm – Helicoverpa (Heliothis) armigera Hbn. Available online: https://www.csalomontraps.com/4listbylatinname/helicoverpaarmigera.htm (accessed on 27 April 2025).
- Horhocea, D., Martura, T., Iordan, H. L., Bãduț, C., Ciocãzanu, I. Felix, a new semi-late maize hybrid released by the NARDI Fundulea. Ann. of NARDI Fundulea [Analele Institutului Național de Cercetare-Dezvoltare Agricolă Fundulea] 2019, 87, 57–80. Available online: https://www.incda-fundulea.ro/anale/anale87.html (accessed on 27 April 2025).
- Horhocea, D.; Petcu, E.; Iordan, H.; Ciontu, C. Evaluation of New Maize Genotypes for Seed Yield Potential and Stability. Rom. Agric. Res. 2024, 41, 489–496. Available online: https://new.incda-fundulea.ro/index.php?option=com_content&view=article&id=685&Itemid=861&lang=en (accessed on 27 April 2025). [CrossRef]
- Spanjer, M.C.; Rensen, P.M.; Scholten, J.M. LC–MS/MS multi-method for mycotoxins after single extraction, with validation data for peanut, pistachio, wheat, maize, cornflakes, raisins and figs. Food Add. and Cont. 2008, 25, 472–489. [Google Scholar] [CrossRef]
- Gylling Data Management Inc. ARM 2022® GDM Software, Revision 9.2022.5 October 25, 2022 (B = 28627); Gylling Data Management Inc.: Brookings, SD, USA, 2022. [Google Scholar] [CrossRef]
- Shrestha, S. Effects of climate change on agricultural insect pests. Acta Sci. Agric. 2019, 3, 74–80. [Google Scholar] [CrossRef]
- Skendžić, S.; Zovko, M.; Živković, I.P.; Lešić, V.; Lemić, D. The Impact of Climate Change on Agricultural Insect Pests. Insects 2021, 12, 440. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, B.; Yu, H.; Zhang, H.; He, Z.; Zhuo, Z. The Effects of Global Climate Warming on the Developmental Parameters of Helicoverpa armigera (Hübner, 1808) (Lepidoptera: Noctuidae). Insects 2024, 15, 888. [Google Scholar] [CrossRef]
- Zeng, J.; Liu, Y.; Zhang, H., Liu, J.; Jiang, Y.; Wyckhuys, K.A.; Wu, K. Global warming modifies long-distance migration of an agricultural insect pest. J. Pest. Sci. 2020, 93, 569–581. [CrossRef]
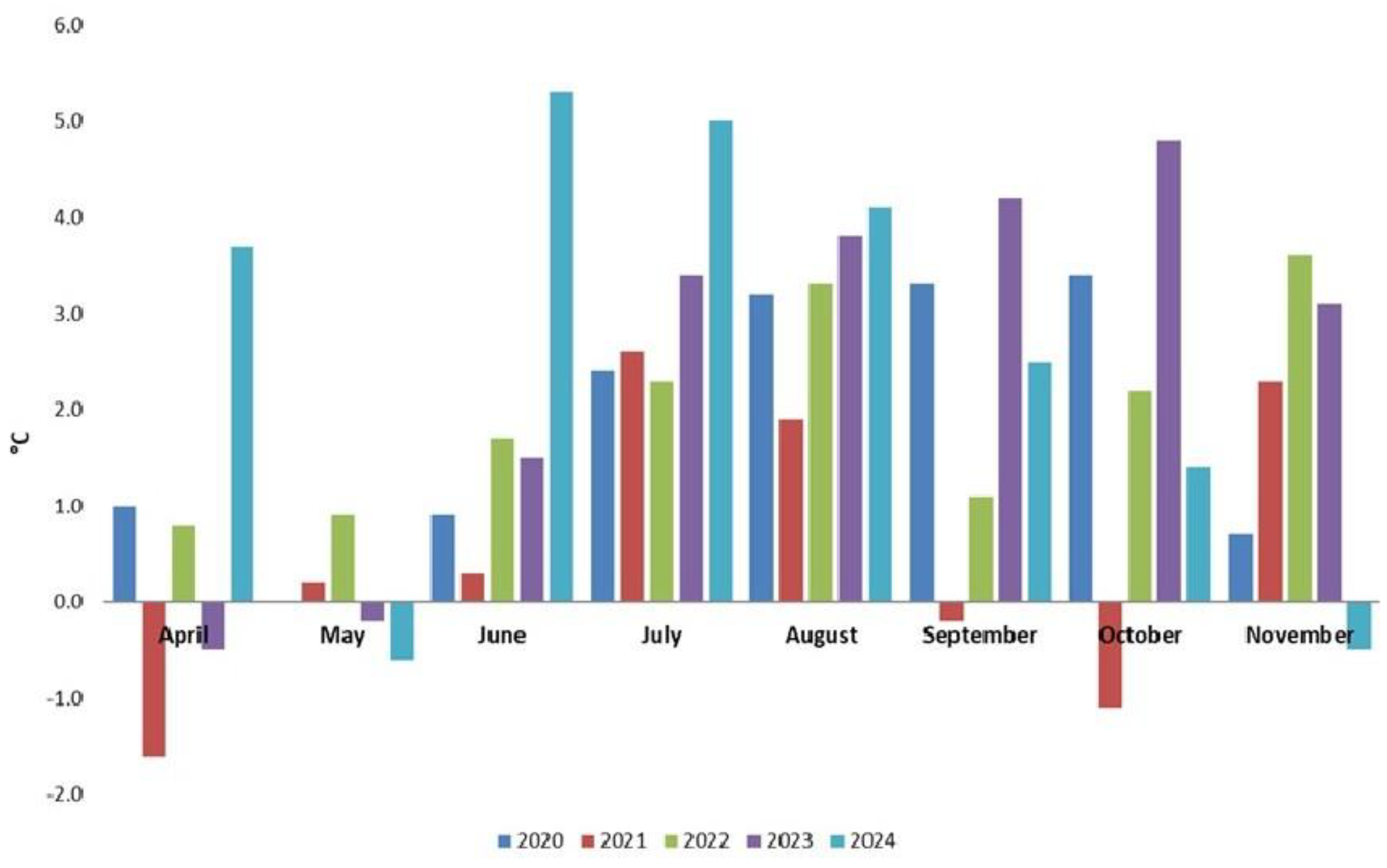
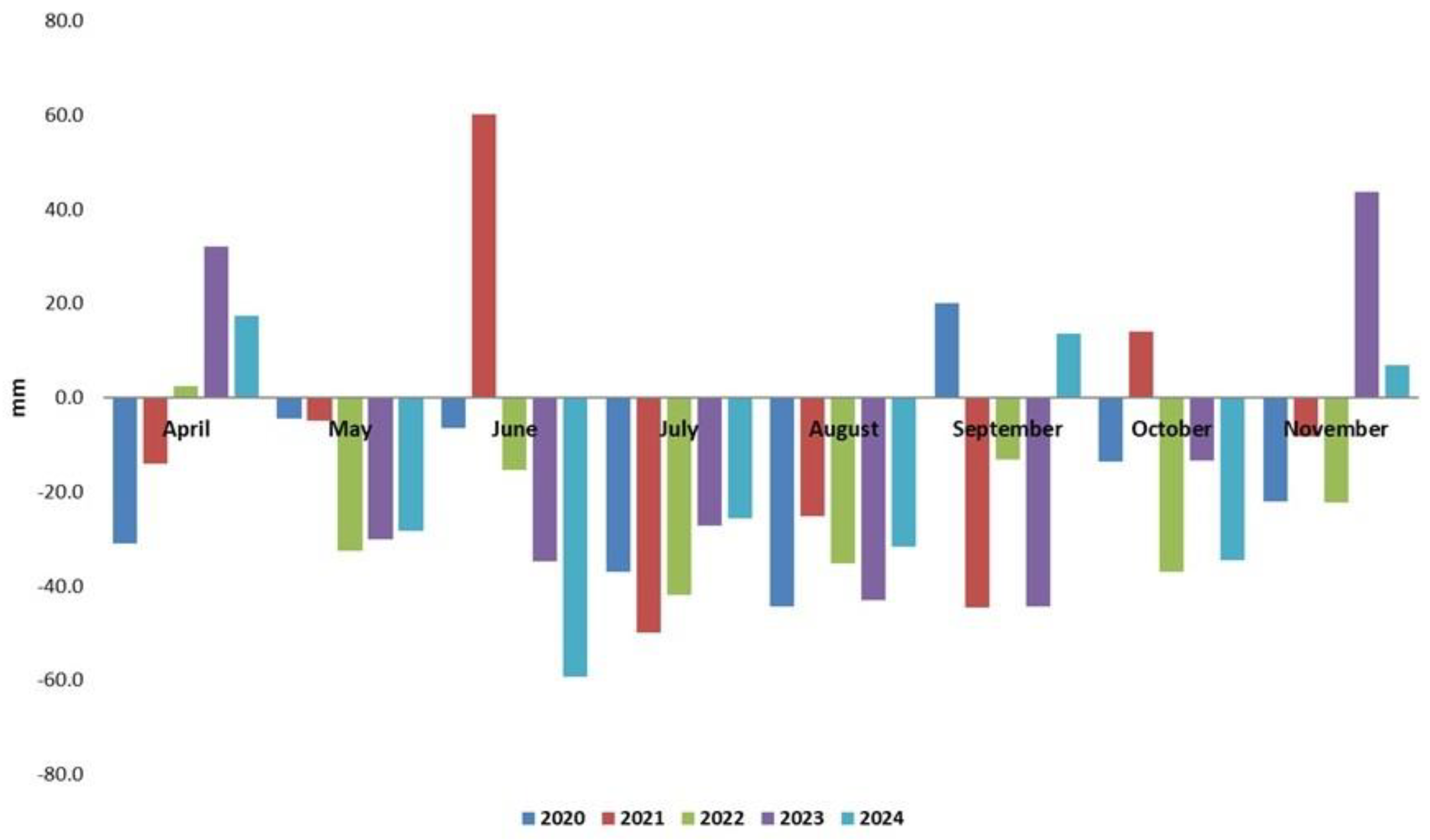
| Year | The temperature 50-year average (°C) |
Rainfalls 50 years average (mm) |
| April | 11.3 | 45.1 |
| May | 17.0 | 62.5 |
| June | 20.8 | 74.9 |
| July | 22.7 | 71.1 |
| August | 22.3 | 49.7 |
| September | 17.5 | 48.5 |
| October | 11.3 | 42.3 |
| November | 5.4 | 42.0 |
| Year | Sowing | Emergence | Harvest |
|---|---|---|---|
| 2020 | 14 April | 28 April | 17 September |
| 2021 | 7 May | 15 May | 5 October |
| 2022 | 3 May | 10 May | 4 October |
| 2023 | 5 May | 12 May | 22 September |
| 2024 | 14 May | 21 May | 31 October |
| Variant | Maize Hybrid name |
Company | FAO group |
|---|---|---|---|
| 1 | KWS Adonisio | KWS | 300-349 |
| 2 | P9415 | Corteva | 300-349 |
| 3 | Magnus | NARDI Fundulea | 350-399 |
| 4 | P9944 | Corteva | 350-399 |
| 5 | DKC 4031 | Dekalb | 350-399 |
| 6 | KWS Kashmir | KWS | 350-399 |
| 7 | KWS Inteligens | KWS | 400-449 |
| 8 | DKC 5110 | Dekalb | 400-449 |
| 9 | P0450 | Corteva | 400-449 |
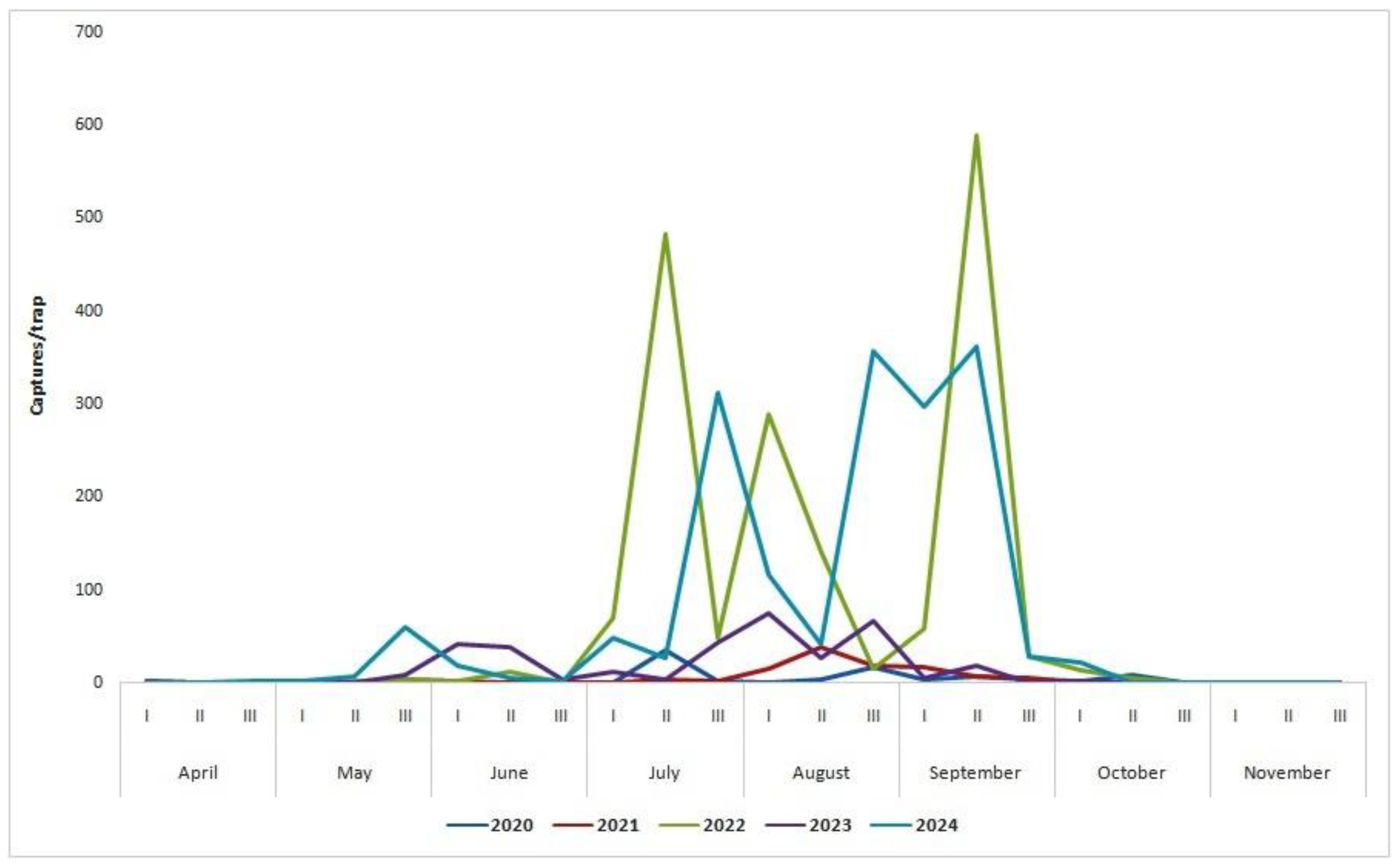
| Month | Year 2020 |
Year 2021 |
Year 2022 |
Year 2023 |
Year 2024 |
|
|---|---|---|---|---|---|---|
| Number of captures/trap | ||||||
| April | I | 0 | 0 | 1.0 | 1.0 | 0 |
| II | 0 | 0 | 0.5 | 0 | 0 | |
| III | 0 | 0 | 0 | 0.5 | 2.3 | |
| May | I | 0 | 0 | 0 | 0 | 1.3 |
| II | 0 | 0 | 1.7 | 0,7 | 7.0 | |
| III | 0 | 4.0 | 3.7 | 8.3 | 60.0 | |
| June | I | 1,0 | 1.0 | 1.3 | 42.3 | 18.0 |
| II | 0 | 0.3 | 11.3 | 38.0 | 5.0 | |
| III | 0 | 0.7 | 0 | 2.7 | 1.0 | |
| July | I | 0 | 0 | 69.3 | 12.0 | 49.0 |
| II | 36.0 | 3.3 | 483.3 | 3.7 | 27.3 | |
| III | 2.0 | 1.7 | 48.0 | 44.3 | 311.7 | |
| August | I | 0 | 14.3 | 288.0 | 76.0 | 116.0 |
| II | 4.0 | 38.3 | 140.3 | 26.7 | 42.0 | |
| III | 17.0 | 18.7 | 14.3 | 68.0 | 358.0 | |
| September | I | 3.0 | 17.0 | 58.7 | 4.3 | 296.7 |
| II | 6.0 | 6.7 | 589.0 | 19.0 | 362.3 | |
| III | 4.0 | 4.3 | 27.7 | 0 | 28.3 | |
| October | I | 1.0 | 0 | 14.0 | 1.0 | 21.0 |
| II | 8.0 | 0 | 4.3 | 0.7 | 0.7 | |
| III | 0 | 0 | 0 | 0 | 0.3 | |
| November | I | 0 | 0 | 0 | 0.3 | 0 |
| II | 0 | 0 | 0.7 | 0 | 0 | |
| III | 0 | 0 | 0 | 0 | 0 | |
| Year | First capture |
Last capture |
| 2020 | 1 June | 13 October |
| 2021 | 26 May | 21 September |
| 2022 | 7 April | 13 November |
| 2023 | 6 April | 10 November |
| 2024 | 27 April | 22 October |
| Variant | Maize hybrid |
18 July | 23 July | 2 August | 13 September | 4 October |
|---|---|---|---|---|---|---|
| Attack incidence (%) | ||||||
| 1 | KWS Adonisio | 0±0a | 0±0a | 48.75±4.79a | 100±0a | 100±0a |
| 2 | P9415 | 0±0a | 0±0a | 53.75±4.79a | 100±0a | 100±0a |
| 3 | Magnus | 0±0a | 0±0a | 53.75±4.79a | 100±0a | 100±0a |
| 4 | P9944 | 0±0a | 0±0a | 50.00±8.16a | 100±0a | 100±0a |
| 5 | DKC 4031 | 0±0a | 0±0a | 43.75±6.29a | 100±0a | 100±0a |
| 6 | KWS Kashmir | 0±0a | 0±0a | 45.00±4.08a | 100±0a | 100±0a |
| 7 | KWS Inteligens | 0±0a | 0±0a | 47.50±6.45a | 100±0a | 100±0a |
| 8 | DKC 5110 | 0±0a | 0±0a | 43.75±6.29a | 100±0a | 100±0a |
| 9 | P0450 | 0±0a | 0±0a | 50.00±4.28a | 100±0a | 100±0a |
| Tukey's HSD (P=0.05) | 0 | 0 | 11.152 | 0 | 0 | |
| Standard deviation (SD) | 0 | 0 | 4.640 | 0 | 0 | |
| Variation coeficient (CV) | 0 | 0 | 9.57 | 0 | 0 | |
| Variant | Maize hybrid |
18 July | 23 July | 2 August | 13 September | 4 October |
|---|---|---|---|---|---|---|
| Larvae/cob | ||||||
| 1 | KWS Adonisio | 0±0a | 0±0a | 0.34±0.09a | 0.99±0.09a | 0±0a |
| 2 | P9415 | 0±0a | 0±0a | 0.38±0.03a | 0.96±0.03a | 0±0a |
| 3 | Magnus | 0±0a | 0±0a | 0.38±0.05a | 1.01±0.03a | 0±0a |
| 4 | P9944 | 0±0a | 0±0a | 0.35±0.07a | 0.95±0.09a | 0±0a |
| 5 | DKC 4031 | 0±0a | 0±0a | 0.34±0.05a | 0.98±0.06a | 0±0a |
| 6 | KWS Kashmir | 0±0a | 0±0a | 0.34±0.03a | 0.89±0.03a | 0±0a |
| 7 | KWS Inteligens | 0±0a | 0±0a | 0.39±0.05a | 0.96±0.06a | 0±0a |
| 8 | DKC 5110 | 0±0a | 0±0a | 0.34±0.05a | 0.99±0.03a | 0±0a |
| 9 | P0450 | 0±0a | 0±0a | 0.39±0.03a | 0.93±0.06a | 0±0a |
| Tukey's HSD (P=0.05) | 0 | 0 | 0.110 | 0.136 | 0 | |
| Standard deviation (SD) | 0 | 0 | 0.046 | 0.057 | 0 | |
| Variation coeficient (CV) | 0 | 0 | 12.79 | 5.89 | 0 | |
| Variant | Maize hybrid |
Aflatoxins (µ/kg) | |||
|---|---|---|---|---|---|
| B1 | B2 | G1 | G2 | ||
| 1 | KWS Adonisio | 205.06±56.43 | 8.99±1.15 | 13.66±2.80 | 0±0 |
| 2 | P9415 | 356.49±98.10 | 14.08±1.80 | 5.17±1.06 | 0±0 |
| 3 | Magnus | 419.67±115.49 | 13.80±1.77 | 18.71±3.83 | 0±0 |
| 4 | P9944 | 204.00±56.14 | 6.06±0.78 | 2.09±0.43 | 0±0 |
| 5 | DKC 4031 | 198.54±54.64 | 11.01±1.41 | 32.80±6.72 | 0±0 |
| 6 | KWS Kashmir | 39.73±10.84 | 3.08±0.39 | 30.01±6.15 | 0±0 |
| 7 | KWS Inteligens | 76.56±21.07 | 3.66±0.47 | 15.70±3.22 | 0±0 |
| 8 | DKC 5110 | 315.50±86.83 | 30.96±3.96 | 16.81±3.44 | 0±0 |
| 9 | P0450 | 81.17±22.34 | 4.69±0.59 | 57.99±11.88 | 0±0 |
| Variant | Maize hybrid |
Total aflatoxins (µ/kg) (B1+B2+G1+G2) |
|---|---|---|
| 1 | KWS Adonisio | 229.87±7.98 |
| 2 | P9415 | 379.11±117.05 |
| 3 | Magnus | 455.50±140.66 |
| 4 | P9944 | 213.60±65.96 |
| 5 | DKC 4031 | 245.00±75.66 |
| 6 | KWS Kashmir | 73.20±22.60 |
| 7 | KWS Inteligens | 96.80±29.89 |
| 8 | DKC 5110 | 370.66±114.46 |
| 9 | P0450 | 144.87±44.74 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





