1. Introduction
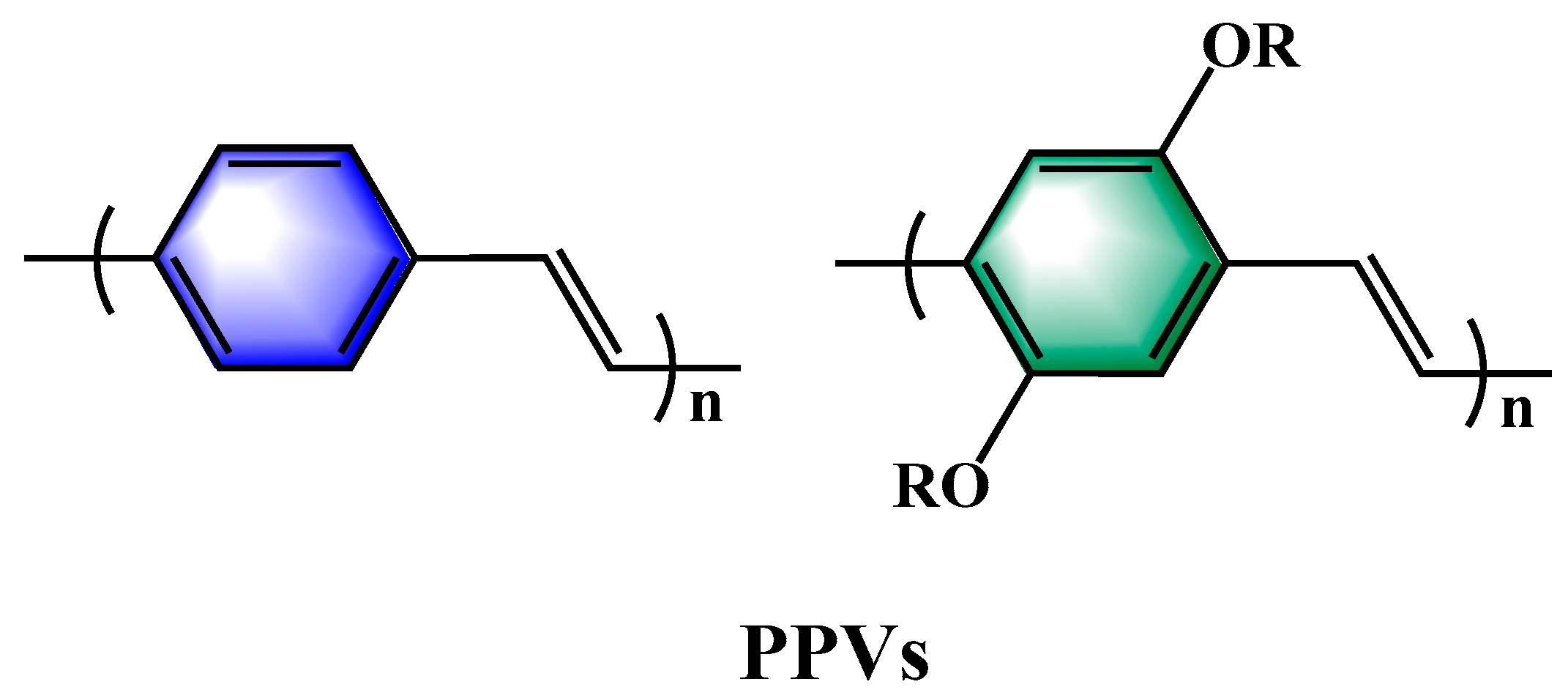
Although PPVs have many advantages as important polymers, their simple and efficient synthesis methods have always been a major challenge for researchers [
1], and as a rigid rod polymer, unsubstituted PPVs are insoluble in water and most solvents, so the development of soluble PPVs has always been the goal of researchers [
2]. Although PPV is water-insoluble, its precursors can be chemically modified to improve their water solubility [
1].
Figure 1.
Chemical structures of PPV and its derivatives.
Figure 1.
Chemical structures of PPV and its derivatives.
In terms of molecular chemical structure, PPVs themselves are excellent two-photon fluorescent materials, and although the research work on the development and design of fluorescent probes is scarce, there are some developments. It has been widely used in the design of metal ions, enzymes, ROS, Aβ-amyloid, and DNA fluorescent probes, as well as in photodynamic therapy (PDT) [
3,
4,
5,
6,
7,
8,
9]. Despite these developments, there is still a huge scope for the development of PPV-derived fluorescent dyes and probes with better performance, especially in terms of improving the water solubility of molecules and redshifting the fluorescence emission wavelength [
10,
11,
12,
13].
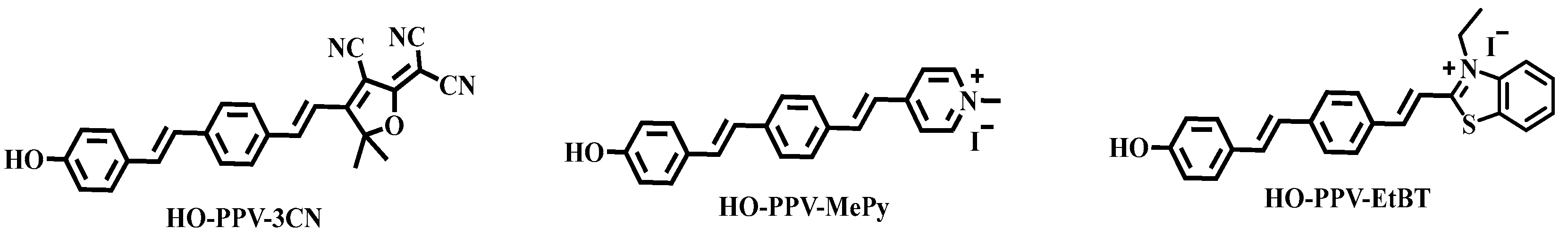
In this chapter, near infrared two-photon fluorescent dyes are derived through structural modification with PPV as the core backbone. A series of small molecule fluorescent dyes with improved water solubility with strong push-pull structure (named HO-PPV-X,
Figure 2.) were designed by inserting electron-donating hydroxyl groups (-OH) and various electron-withdrawing groups (neutral and cationic fragments) at both ends of the PPV core fragment. The final three dyes, HO-PPV-3CN, HO-PPV-MePy and HO-PPV-EtBT, were successfully applied to two-photon imaging in live cells, and the imaging results showed that HO-PPV-3CN was mainly distributed in the cytoplasm, HO-PPV-MePy was distributed in the whole cell, and HO-PPV-EtBT could be concentrated in the nucleus. The different cellular localization of the three fluorochromes gives them the potential for the development of fluorescent probes and diagnostic reagents with different suborganelle localization functions. Among them, HO-PPV-EtBT itself can serve as a new near-infrared two-photon fluorescence probe to locate atomic nuclei [
14].
2. Materials and Methods
2.1. Materials
The probes HO-PPV-3CN, HO-PPV-MePy, and HO-PPV-EtBT are all custom-synthesized and commercially available.
The reagents used in organic synthesis include: 4-Hydroxybenzaldehyde(CAS 123-08-0), TBSCl(CAS 18162-48-6), Imidazole(CAS 288-32-4),4-Cyanobenzyl bromide(CAS 17201-43-3), Lithium diisopropylamide,LDA,CAS 4111-54-0), DIBALH(CAS 1191-15-7), TBAF(CAS 429-41-4),3-Hydroxy-3-methyl-2-butanone(CAS 115-22-0), Malononitrile(CAS 109-77-3),Ammonium acetate(CAS 631-61-8), Sodium ethoxide(CAS 141-52-6), Potassium tert-butoxide(CAS 865-47-4), 2-Methylbenzothiazole(CAS 120-75-2), Iodoethane(CAS 75-03-6), all reagents were of analytical grade.
Roswell Park Memorial Institute (RPMI) -1640 culture medium was purchased from Sigma Aldrich (St. Louis, MO, USA). 3-4 (4,5-dimethyl-2-thiazole) -2,5-diphenyltetrazolium bromide (MTT) was purchased from Beijing Boaotoda Technology Co., Ltd. Pancreatic enzyme cell digestion solution (0.25% trypsin, containing phenol red), Hoechst 33342, Mito Tracker Green, Lyso Tracker Green, and ER Tracker Green were purchased from Shanghai Biyuntian Biotechnology Co., Ltd. Penicillin and streptomycin were purchased from Beijing Soleibao Technology Co., Ltd. Fetal bovine serum was purchased from Lanzhou Rongye Biotechnology Co., Ltd.
2.2. Instrumentation
UV spectrophotometer: TU-1901,Persee
Fluorescence spectrophotometer: LS55, PerkinElmer, USA
PH meter: PB-10, Sartorius, Germany
Microplate Reader: Infinite M200,TECAN,Switzerland
Cell culture incubator: Thermo Scientific
Laser confocal microscope: STELLARIS 5, CLSM, Leica Co., Ltd. Germany
2.3. Design and Synthesis of PPV Fluorescent Dyes
PPV and its derivatives have excellent photoluminescence efficiency and can achieve high quantum yield luminescence through conjugated π - electron structures. This characteristic enables it to generate strong and stable fluorescence signals during intracellular imaging, significantly improving imaging sensitivity and signal-to-noise ratio. For example, the derivative of PPV, MEH-PPV (poly [2-methoxy-5- (2-ethylhexoxy) -1,4-phenylene])[
14], exhibits excellent fluorescence emission performance in the visible light range and is suitable for live cell imaging. PPV materials can be chemically modified (such as introducing hydrophilic groups or biomolecules) to improve their water solubility and biocompatibility, reducing their toxicity to cells.
The chemical structure of PPV can be customized through modification to achieve precise control of its fluorescence properties. For example, by adjusting the emission wavelength and introducing different substituents (such as alkoxy and halogen atoms), the fluorescence emission wavelength of PPV can be tuned from the visible light region (about 500 nm) to the near-infrared region (about 700-900 nm), reducing self fluorescence interference in biological tissues and improving imaging depth.
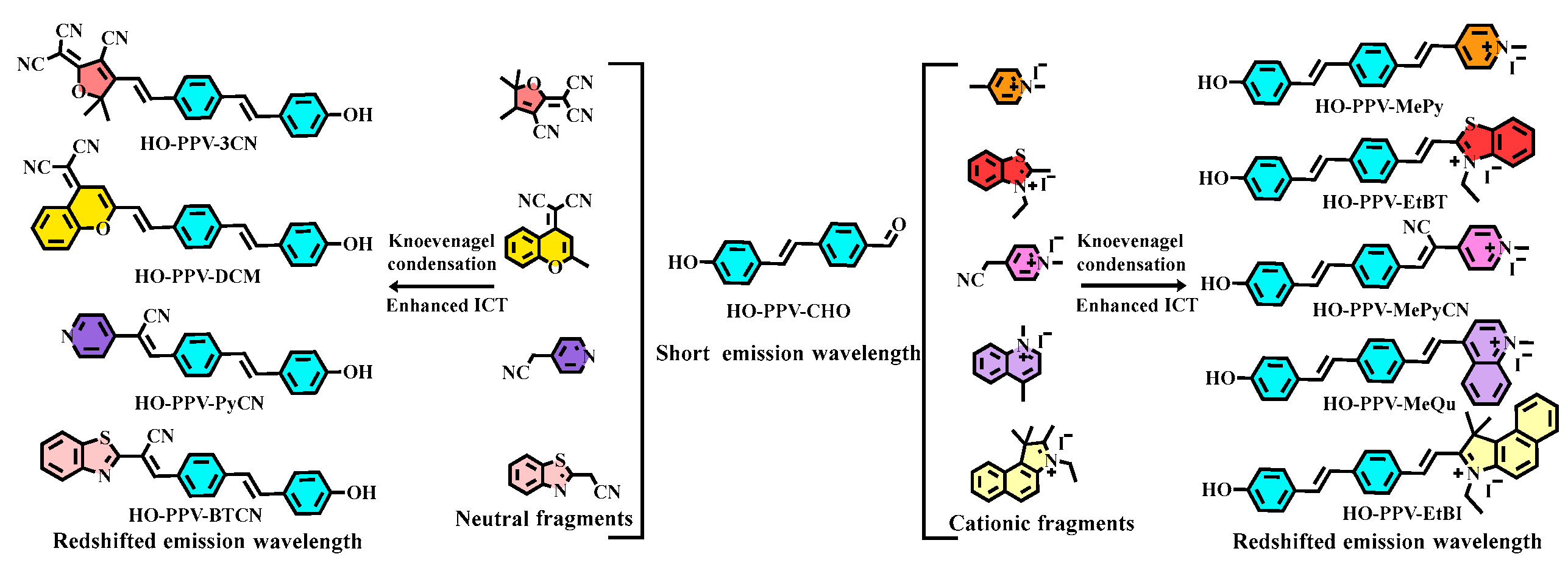
This article focuses on the design, synthesis, and potential application exploration of near-infrared two-photon fluorescent dyes with a new poly (p-phenylene vinylene) (PPV) skeleton structure. Firstly, the core fragment HO-PPV-CHO of PPV structure was constructed using the method described in reference [
15]. Then, based on this fragment, the classical Knoevenagel condensation reaction was attempted to introduce various neutral and cationic electron withdrawing groups, in order to increase the push-pull effect of the entire conjugated fluorescent dye molecule, enhance the ICT effect, and shift the fluorescence emission wavelength to the red. Through continuous synthetic exploration, three fluorescent probes, HO-PPV-3CN, HO-PPV MePy, and HO-PPV EtBT, were synthesized(
Scheme 1.).
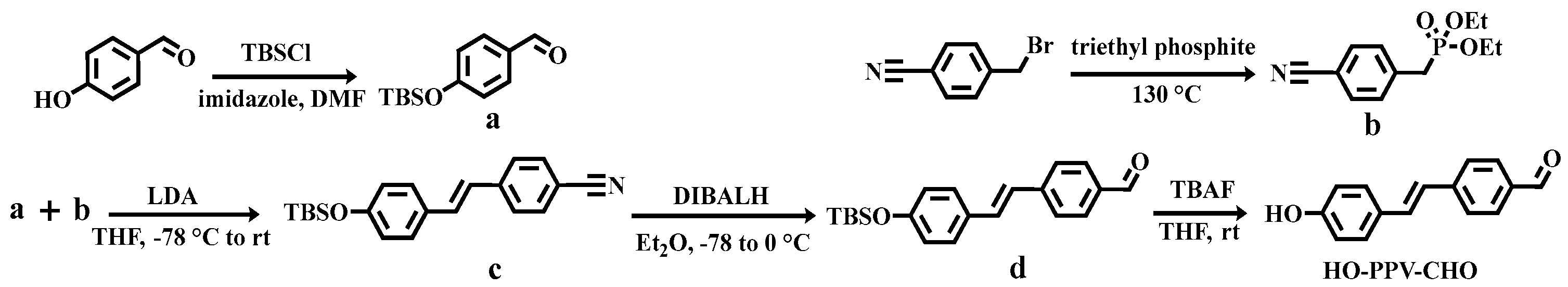
Firstly, the intermediate HO-PPV-CHO was synthesized using the method reported in reference [
14]. In short, p-hydroxybenzaldehyde (compound a) protected by tert butyldimethylsilyl (TBS) and diethyl (4-cyanobenzyl) phosphate (compound b) were reacted with strong base lithium diisopropylamide (LDA) at low temperatures (-78 ℃ to room temperature) to construct an olefin double bond through the classical Wittig Horner Emmons reaction, resulting in compound c. Then, using diisobutylaluminum hydride (DIBALH) at low temperatures (-78 ℃ to 0 ℃), the cyanide group in compound c was reduced to an aldehyde group, resulting in compound d. Finally, TBS protection was removed using tetrabutylammonium fluoride (TBAF) to obtain the intermediate HO-PPV-CHO (
Scheme 2.).
Next, we will explore the synthesis methods and results of the PPV series fluorescent dyes (HO-PPV-3CN, HO-PPV-MePy, HO-PPV-EtBT) derived from HO-PPV-CHO.
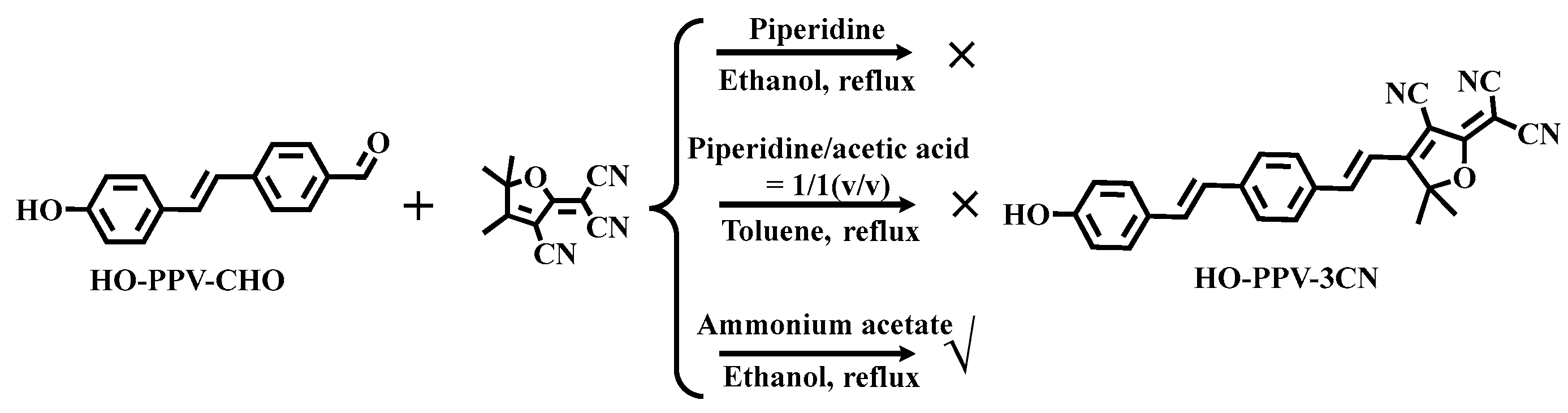
The synthesis of fluorescent dye HO-PPV-3CN: Firstly, we attempted to use pyridine catalysis and ethanol as the solvent for direct reflux. TLC monitoring showed that the reaction could hardly occur; Further attempts were made to use the salt produced in situ by piperidine and acetic acid for catalysis, with toluene as the solvent for reflux, but the target product still could not be obtained; Finally, using ammonium acetate as a catalyst and ethanol as a solvent, the reaction is carried out under reflux conditions for 7-8 hours. After cooling, natural crystallization can precipitate a black precipitate, which is the target molecule HO-PPV-3CN(
Scheme 3.).
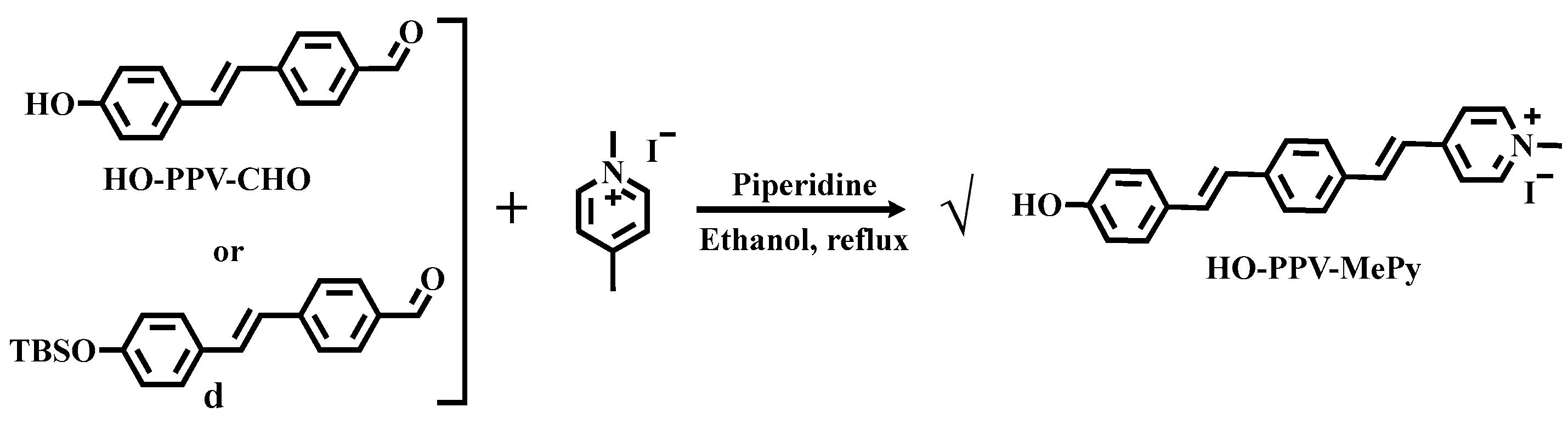
The synthesis of fluorescent dye HO-PPV-MePy: The synthesis method of this target molecule is very clear, that is, using pyridine catalysis and ethanol as the solvent reflux conditions, the target molecule can be successfully obtained. However, during the synthesis process, it was found that the target molecule can also be directly obtained by reacting with 1,4-dimethylpyridine salt using aldehyde without removing TBS protection under these conditions. Compared with the two methods, it is obvious that the latter is simpler, and it has been observed that the reaction effect is better, with fewer by-products in the reaction system(
Scheme 4.).
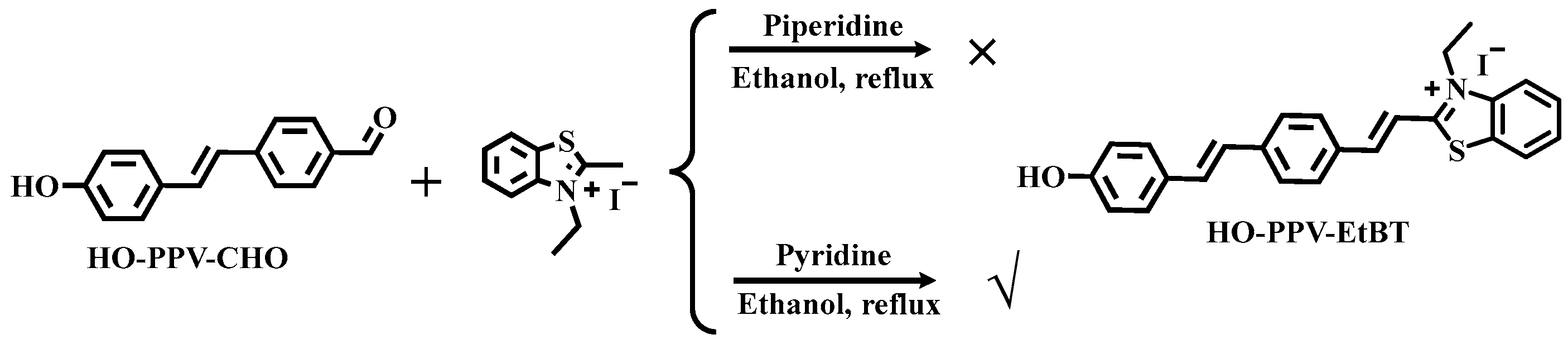
The synthesis of fluorescent dye HO-PPV-EtBT: Firstly, we attempted to use pyridine catalysis and reflux ethanol as a solvent overnight. The reaction system turned dark purple, and TLC monitoring showed that the raw material HO-PPV-CHO was completely consumed. However, the reaction system was very complex, with many by-products and no clear target point. Try replacing the weaker base with pyridine catalysis. After refluxing in ethanol for 7-8 hours, a large amount of brownish red precipitate appears. After cooling to room temperature, filter and wash the solid three times with cold ethanol. Then air dry naturally and dry in a vacuum drying oven at 60 ℃ for 6 hours to obtain the target molecule(
Scheme 5.).
In summary, after the above synthesis exploration, a total of 3 molecules were successfully synthesized, purified, and obtained in quantities suitable for subsequent performance testing, namely: HO-PPV-3CN、HO-PPV-MePy、HO-PPV-EtBT.
2.4. General Procedures for Spectral Research
Prepare stock solutions of 1 mM HO-PPV-3CN, HO-PPV-MePy, and HO-PPV-EtBT fluorescent dyes in analytical pure DMSO. Before spectral measurement, prepare the corresponding test solution by diluting the high concentration reserve solution fresh. The spectral performance testing in different solvents was carried out according to the following procedure: 30 μ L of fluorescent dye stock solution (1 mM) was added to a 4 mL test tube, and then diluted with 2.97 mL of the corresponding solvent to a total volume of 3 mL. The final tested fluorescent dye concentration was 10 μ M. The excitation wavelength, spectral acquisition range, and measurement slit width are determined based on the instrument performance, fluorescence excitation spectrum, and actual spectral emission, and remain consistent within the group.
2.5. Determination of Absolute Fluorescence Quantum Yield
The absolute fluorescence quantum yields (Φ) of three probes in different solvents were obtained using an integrating sphere on a FLS 920 fluorescence spectrophotometer (Edinburgh Instruments, UK). The excitation wavelength is set to 610 nm, and the collected fluorescence emission band is 625-780 nm. The width of the slit and the final concentration of the test sample depend on the strength of the test signal. The final absolute fluorescence quantum yield was automatically simulated by specific computer software, and the results are summarized in the table
2.6. Cell Culture
HeLa and HepG2 cells were purchased from the Chinese Academy of Sciences Shanghai Institute of Biochemistry and Cell Biology. Cells were cultured in RPMI-1640 medium containing 10% fetal bovine serum (FBS), 100U/mL streptomycin, 100U/mL penicillin, 2g/L NaHCO3, or DMEM medium containing 10% fetal bovine serum (FBS), 100U/mL streptomycin, 100U/mL penicillin, 3.7g/L NaHCO3. Cultivate in a 37 ℃ incubator containing 5% CO2. Experiments were conducted using cells in the exponential growth phase.
2.7. Cytotoxic Assay
MTT assay was used to determine the cytotoxicity of probes on HeLa, HepG2 cells. 100pL cells (density 6x104 cells/mL) were seeded onto a 96 well plate and incubated overnight in a 5% CO2 incubator at 37 ° C. Remove the old culture medium, add fresh culture medium, and incubate with different concentrations (0、1.25、2.5、5、10、20、25、30、40 μM) of probes in fresh culture medium for 24 hours. Subsequently, remove the old culture medium and incubate with 10 pL MTT (5 mg/mL) and 90 pL culture medium for 4 hours. Finally, remove the MTT containing culture medium, add 100 μ L DMSO to each well, shake, dissolve with formazan crystals, and measure the absorbance value of each well at 570m using an enzyme-linked immunosorbent assay (ELISA) reader. All experiments were repeated three times independently.
2.8. Cell Imaging
Two photon imaging of HeLa and HepG2 cells using fluorescent dyes HO-PPV-3CN, HO-PPV-MePy, and HO-PPV-EtBT was performed on a laser scanning confocal microscope (upright) (Olympus FV1000 MPE, 100x oil objective); Single photon imaging of HeLa and HepG2 cells using HO-PPV-MePy was performed on a laser confocal microscope (inverted) - Olympus FV3000 (40x air objective).
4. Discussion
The design, synthesis, and application of near-infrared two-photon fluorescent dyes derived from PPV have been the focus of this study. The insertion of electron-donating hydroxyl groups (-OH) and various electron-withdrawing groups at both ends of the PPV core fragment has led to the creation of three novel dyes: HO-PPV-3CN, HO-PPV-MePy, and HO-PPV-EtBT. These dyes exhibit improved water solubility and strong push-pull structures, contributing to their unique optical properties.
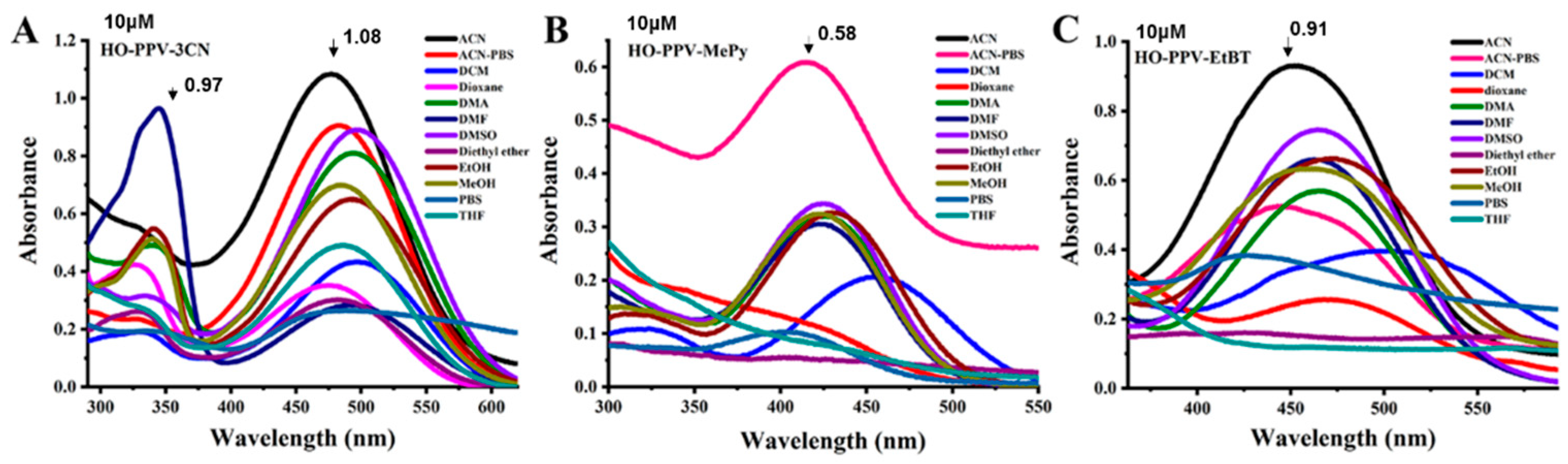
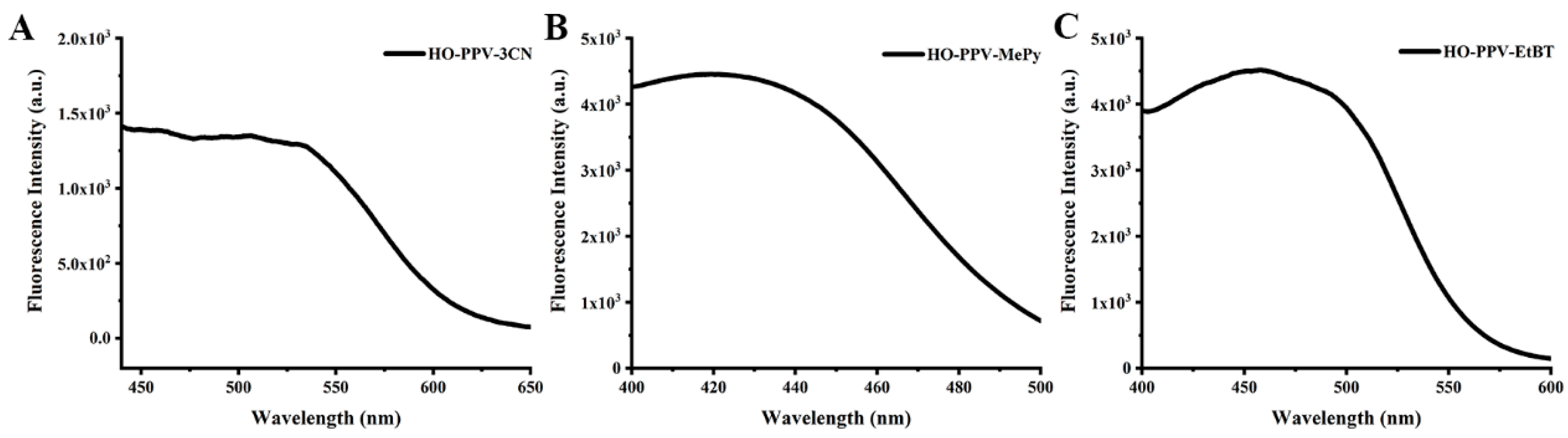
The spectral analysis revealed that HO-PPV-3CN, HO-PPV-MePy, and HO-PPV-EtBT display distinct absorption and emission characteristics in various solvents. Notably, HO-PPV-3CN demonstrated dual absorption peaks in the ultraviolet-visible range, with significant solvent-dependent shifts in its emission wavelength. This suggests a strong influence of solvent polarity on the fluorescence emission spectrum of HO-PPV-3CN. In contrast, HO-PPV-EtBT showed the least variation in its emission spectrum across different solvents, indicating greater stability in its optical properties.
The cellular staining experiments using these dyes provided intriguing results. HO-PPV-3CN predominantly stained the cytoplasm, suggesting its potential use for cytoplasm-specific imaging. HO-PPV-MePy stained the entire cell, encompassing both cytoplasm and nucleus, as further confirmed by single-photon imaging. Most notably, HO-PPV-EtBT demonstrated selective staining of the nucleus, positioning it as a promising candidate for nucleus-localized near-infrared two-photon fluorescent probes.
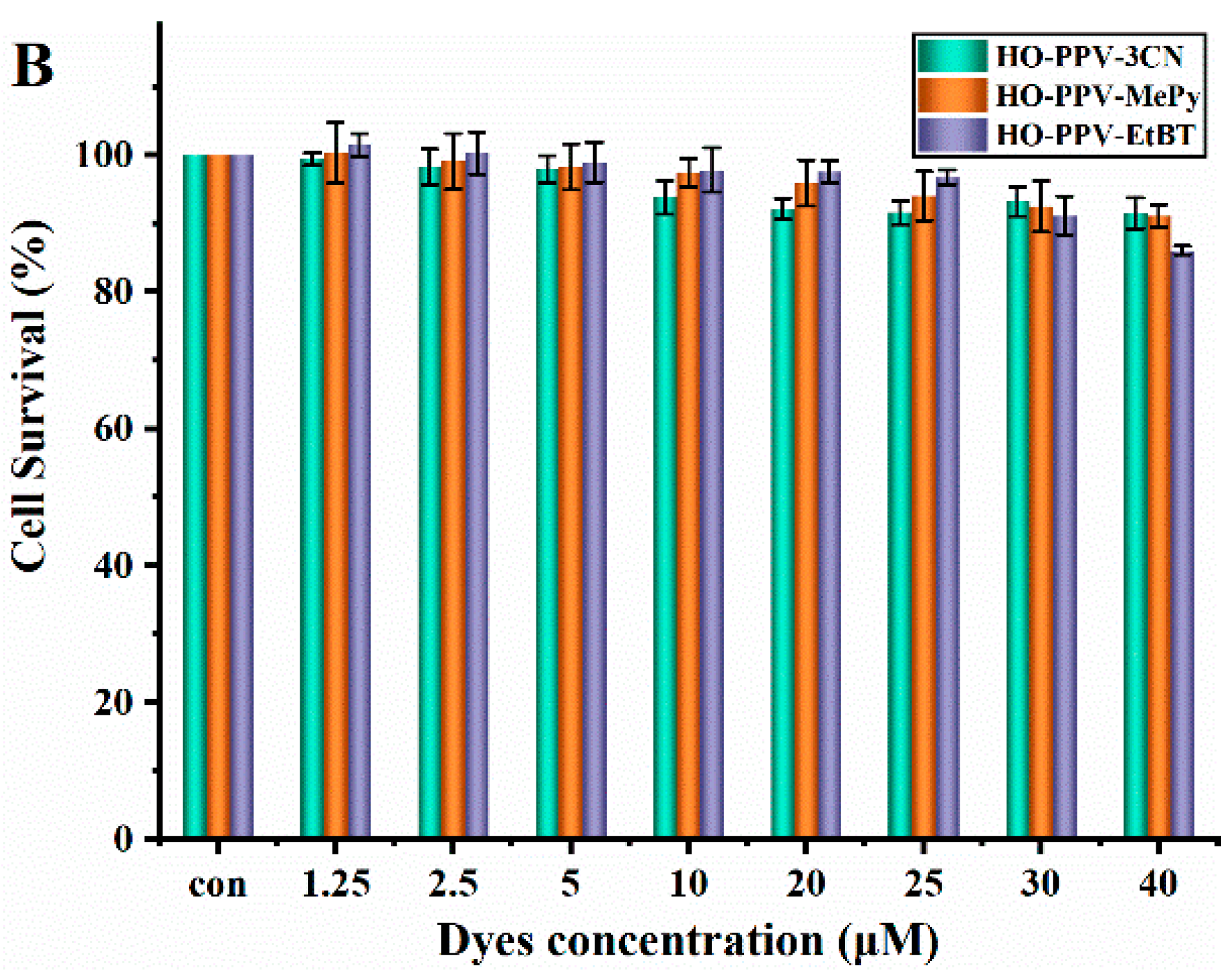
The cytotoxicity assessments using the MTT assay ensured the biocompatibility of these dyes, particularly at lower concentrations, which is crucial for their potential applications in biological imaging. The selective staining patterns observed in live-cell imaging experiments underscore the potential of these PPV-derived dyes for subcellular localization studies.
When compared to existing literature, the PPV-based dyes developed in this study offer several advantages. Their improved water solubility broadens the scope of applications in aqueous environments, such as biological samples. Moreover, the near-infrared emission wavelengths place them within a range that minimizes autofluorescence and maximizes tissue penetration, making them suitable for deep-tissue imaging. However, further studies are needed to fully understand the mechanisms underlying their subcellular localization and to explore their potential applications in disease diagnosis and therapy.
Figure 2.
Chemical structures of HO-PPV-3CN, HO-PPV-MePy, HO-PPV-EtBT.
Figure 2.
Chemical structures of HO-PPV-3CN, HO-PPV-MePy, HO-PPV-EtBT.
Scheme 1.
Design of PPV two-photon fluorescent dyes based on ICT mechanism.
Scheme 1.
Design of PPV two-photon fluorescent dyes based on ICT mechanism.
Scheme 2.
Synthesis route of intermediate HO-PPV-CHO.
Scheme 2.
Synthesis route of intermediate HO-PPV-CHO.
Scheme 3.
Synthesis route of fluorescent dye HO-PPV-3CN.
Scheme 3.
Synthesis route of fluorescent dye HO-PPV-3CN.
Scheme 4.
Synthesis route of fluorescent dye HO-PPV-MePy.
Scheme 4.
Synthesis route of fluorescent dye HO-PPV-MePy.
Scheme 5.
Synthesis route of fluorescent dye HO-PPV-EtBT.
Scheme 5.
Synthesis route of fluorescent dye HO-PPV-EtBT.
Figure 3.
Uv-vis absorption spectra of fluorescent dyes HO-PPV-3CN, HO-PPV-MePy and HO-PPV-EtBT (Concentration=10 μ m) in different solvents at 25℃, slit width: 10/10 nm.
Figure 3.
Uv-vis absorption spectra of fluorescent dyes HO-PPV-3CN, HO-PPV-MePy and HO-PPV-EtBT (Concentration=10 μ m) in different solvents at 25℃, slit width: 10/10 nm.
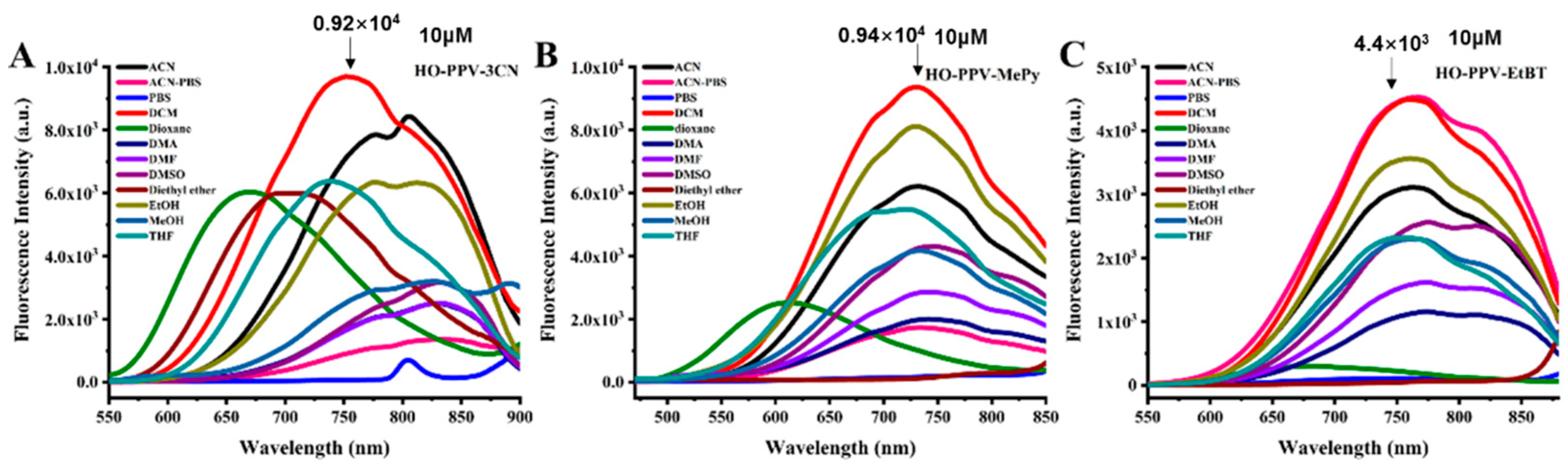
Figure 4.
Fluorescence excitation spectra of fluorescent dyes HO-PPV-3CN, HO-PPV-MePy and HO-PPV-EtBT (λex = 440 nm, 380 nm, 380 nm, Concentration=10 μ m) in Acetonitrile-PBS (1:1, v/v, pH =7.4, 10 mM) solvents at 25℃, slit width: 10/10 nm.
Figure 4.
Fluorescence excitation spectra of fluorescent dyes HO-PPV-3CN, HO-PPV-MePy and HO-PPV-EtBT (λex = 440 nm, 380 nm, 380 nm, Concentration=10 μ m) in Acetonitrile-PBS (1:1, v/v, pH =7.4, 10 mM) solvents at 25℃, slit width: 10/10 nm.
Figure 5.
Fluorescence emission spectra of fluorescent dyes HO-PPV-3CN, HO-PPV-MePy and HO-PPV-EtBT(λex = 550 nm, 470 nm, 550 nm, Concentration=10 μ m) in different solvents at 25℃, slit width: 10/10 nm.
Figure 5.
Fluorescence emission spectra of fluorescent dyes HO-PPV-3CN, HO-PPV-MePy and HO-PPV-EtBT(λex = 550 nm, 470 nm, 550 nm, Concentration=10 μ m) in different solvents at 25℃, slit width: 10/10 nm.
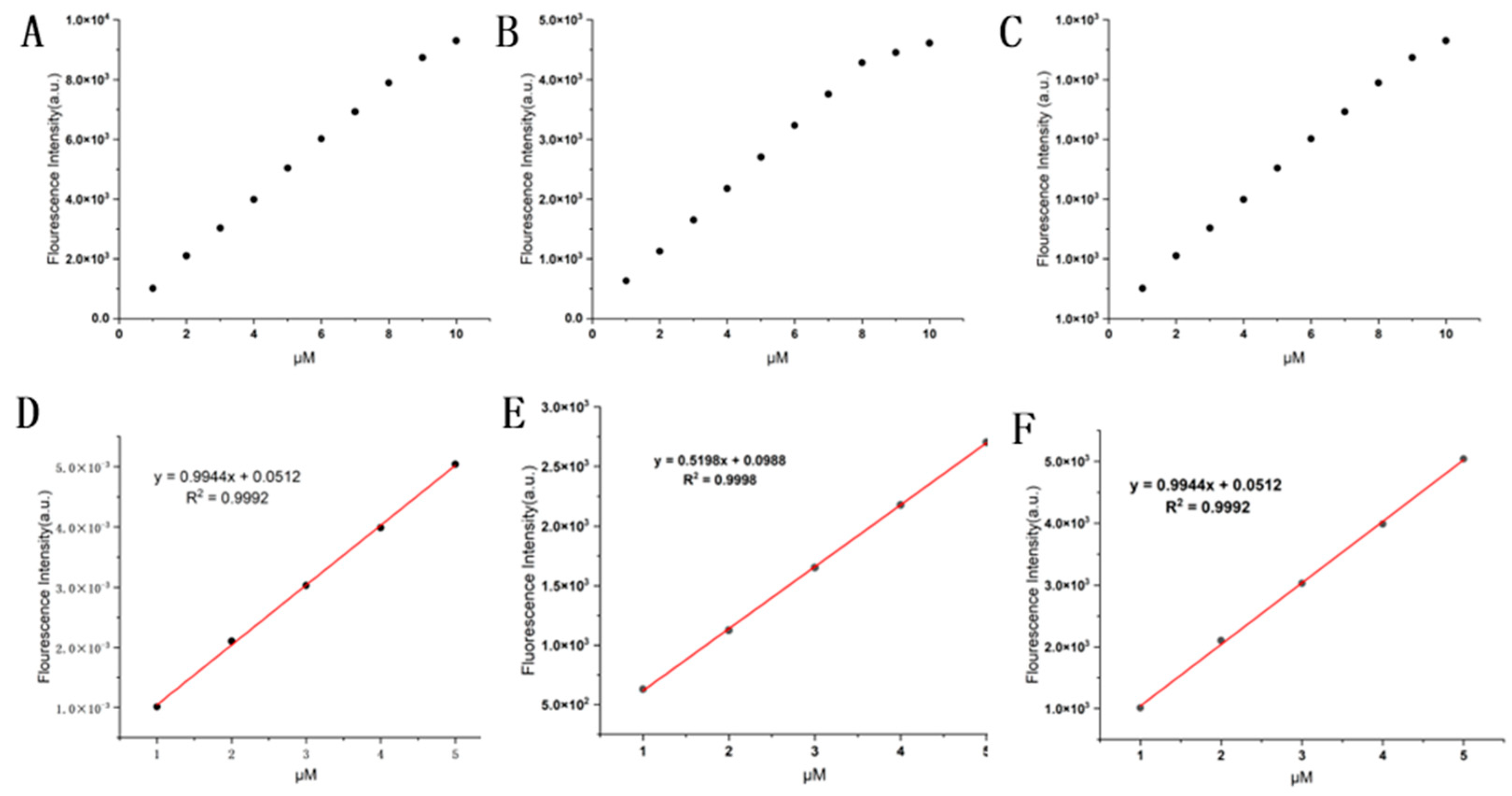
Figure 6.
Concentration-dependence of HO-PPV-3CN, HO-PPV-MePy, HO-PPV-EtBT (A) The fluorescence intensity (λex/λem = 550 nm/750 nm) of probe HO-PPV-3CN (1, 2, 3, 4, 5, 6, 7, 8, 9, 10 μM) in DCM at 25 °C. , slit width: 10/10 nm. (B) The fluorescence intensity (λex/λem = 445 nm/730 nm) of probe HO-PPV-MePy (1, 2, 3, 4, 5, 6, 7, 8, 9, 10 μM) in DCM at 25 °C, slit width: 10/10 nm. (C) The fluorescence intensity (λex/λem = 550 nm/770 nm) of probe HO-PPV-EtBT (1, 2, 3, 4, 5, 6, 7, 8, 9, 10 μM) in DCM at 25 °C, slit width: 10/10 nm. (D)The linear relationship of fluorescence intensity of HO-PPV-3CN (1-5 μM). λex/λem = 550 nm/750 nm in DCM at 25 °C, slit width: 10/10 nm.(E) The linear relationship of fluorescence intensity of HO-PPV-MePy (1-5 μM). λex/λem = 445 nm/730 nm in DCM at 25 °C, slit width: 10/10 nm. (F) The linear relationship of fluorescence intensity of HO-PPV-EtBT (1-5 μM). λex/λem = 550 nm/770 nm in DCM at 25 °C, slit width: 10/10 nm.
Figure 6.
Concentration-dependence of HO-PPV-3CN, HO-PPV-MePy, HO-PPV-EtBT (A) The fluorescence intensity (λex/λem = 550 nm/750 nm) of probe HO-PPV-3CN (1, 2, 3, 4, 5, 6, 7, 8, 9, 10 μM) in DCM at 25 °C. , slit width: 10/10 nm. (B) The fluorescence intensity (λex/λem = 445 nm/730 nm) of probe HO-PPV-MePy (1, 2, 3, 4, 5, 6, 7, 8, 9, 10 μM) in DCM at 25 °C, slit width: 10/10 nm. (C) The fluorescence intensity (λex/λem = 550 nm/770 nm) of probe HO-PPV-EtBT (1, 2, 3, 4, 5, 6, 7, 8, 9, 10 μM) in DCM at 25 °C, slit width: 10/10 nm. (D)The linear relationship of fluorescence intensity of HO-PPV-3CN (1-5 μM). λex/λem = 550 nm/750 nm in DCM at 25 °C, slit width: 10/10 nm.(E) The linear relationship of fluorescence intensity of HO-PPV-MePy (1-5 μM). λex/λem = 445 nm/730 nm in DCM at 25 °C, slit width: 10/10 nm. (F) The linear relationship of fluorescence intensity of HO-PPV-EtBT (1-5 μM). λex/λem = 550 nm/770 nm in DCM at 25 °C, slit width: 10/10 nm.
Figure 7.
Cell viabilities of HeLa (A) and HepG2 (B) cells treated with three dyes for 24 h.
Figure 7.
Cell viabilities of HeLa (A) and HepG2 (B) cells treated with three dyes for 24 h.
Figure 8.
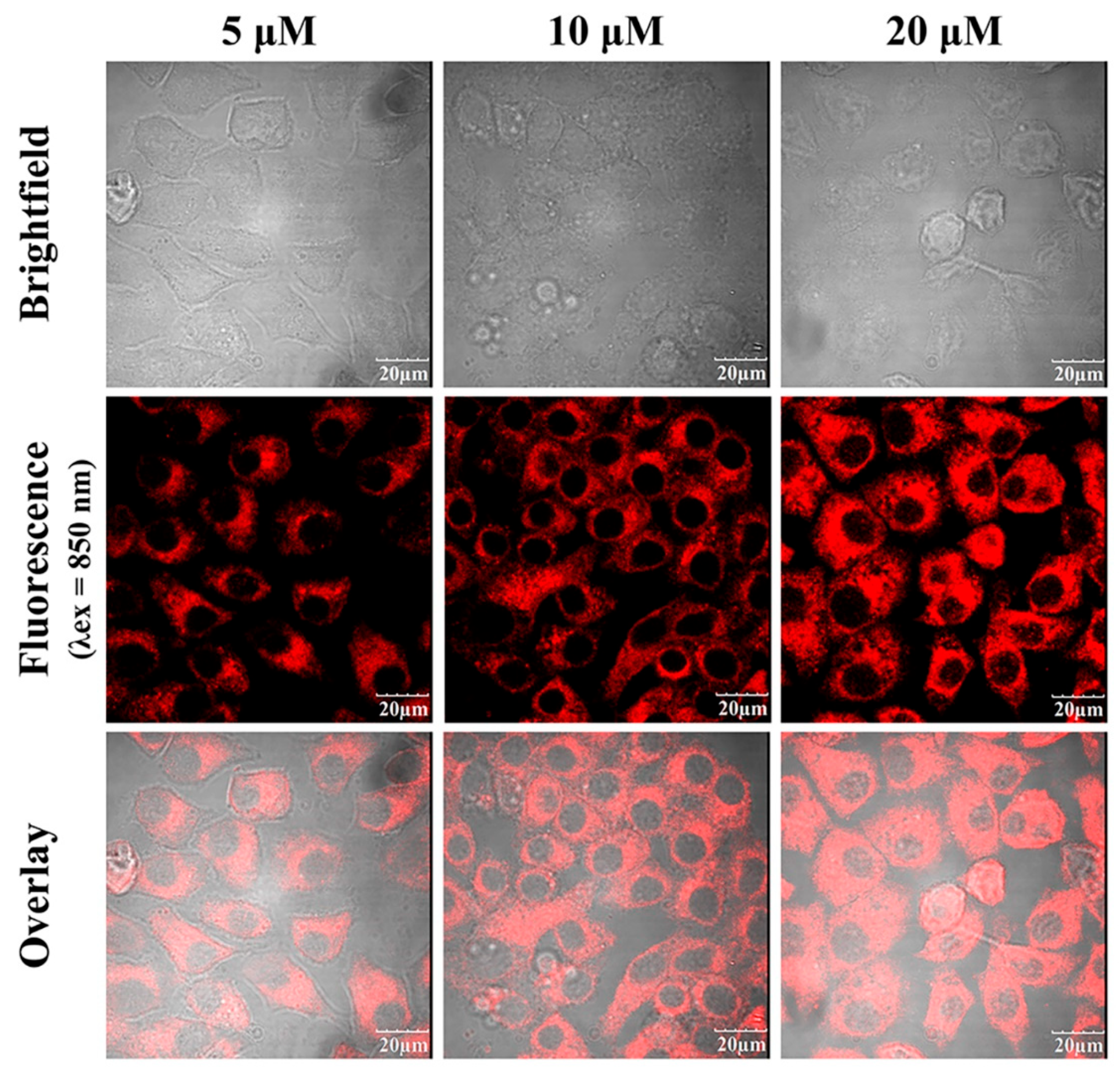
Two-photon confocal fluorescence images of HeLa cells stained with different concentrations of the fluorescent dye HO-PPV-3CN (5, 10 and 20 μM). From left to right: different HO-PPV-3CN concentrations. From top to bottom: bright field images, fluorescence images, and overlay images. (λex = 850 nm, red channel emission wavelength, λex≥575 nm) Scale bar: 20 μm.
Figure 8.
Two-photon confocal fluorescence images of HeLa cells stained with different concentrations of the fluorescent dye HO-PPV-3CN (5, 10 and 20 μM). From left to right: different HO-PPV-3CN concentrations. From top to bottom: bright field images, fluorescence images, and overlay images. (λex = 850 nm, red channel emission wavelength, λex≥575 nm) Scale bar: 20 μm.
Figure 9.
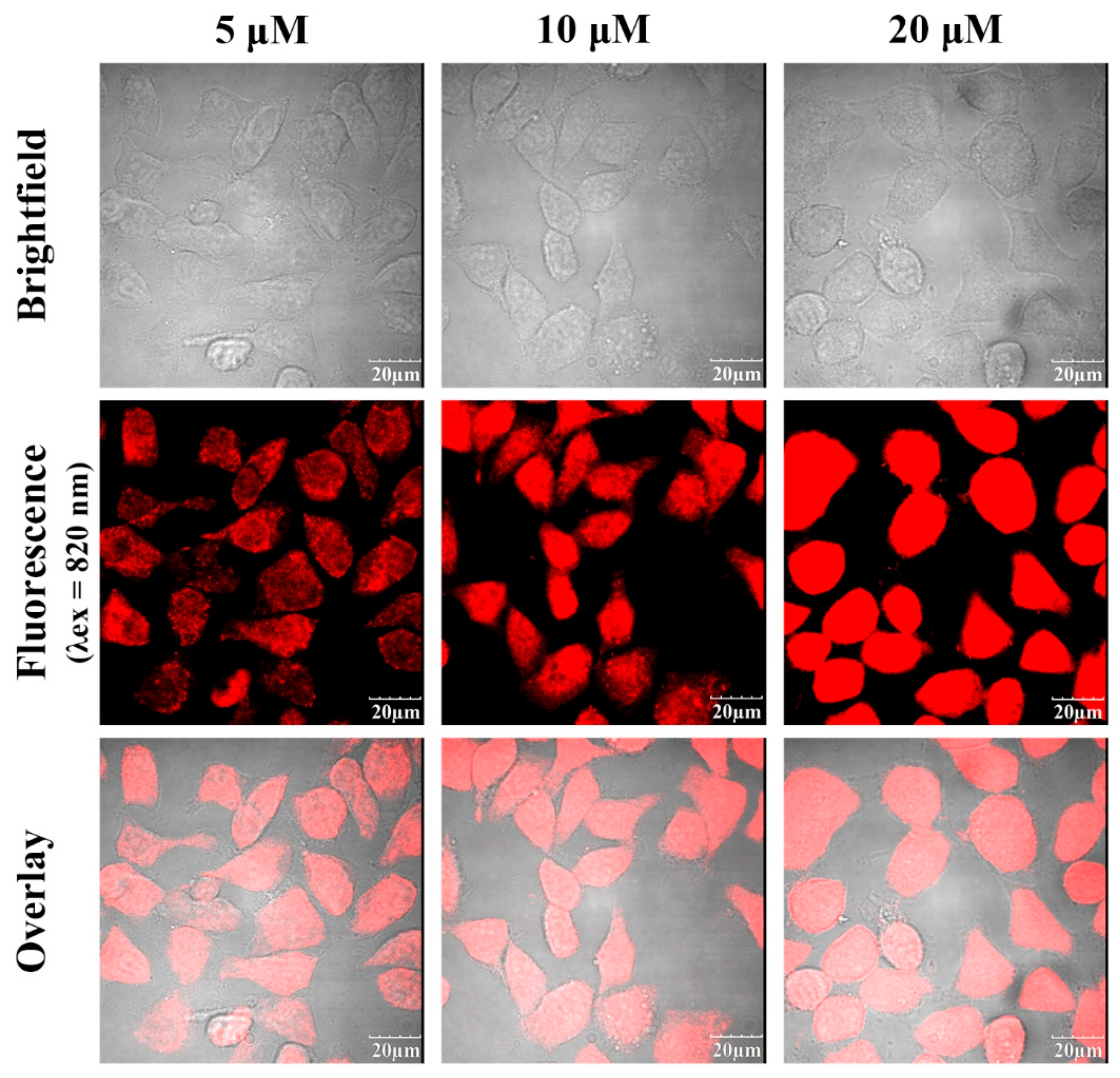
Two-photon confocal fluorescence images of HeLa cells stained with different concentrations of the fluorescent dye HO-PPV-MePy (5, 10 and 20 μM). From left to right: different HO-PPV-MePy concentrations. From top to bottom: bright field images, fluorescence images, and overlay images. (λex = 820 nm, red channel emission wavelength, λex≥575 nm) Scale bar: 20 μm.
Figure 9.
Two-photon confocal fluorescence images of HeLa cells stained with different concentrations of the fluorescent dye HO-PPV-MePy (5, 10 and 20 μM). From left to right: different HO-PPV-MePy concentrations. From top to bottom: bright field images, fluorescence images, and overlay images. (λex = 820 nm, red channel emission wavelength, λex≥575 nm) Scale bar: 20 μm.
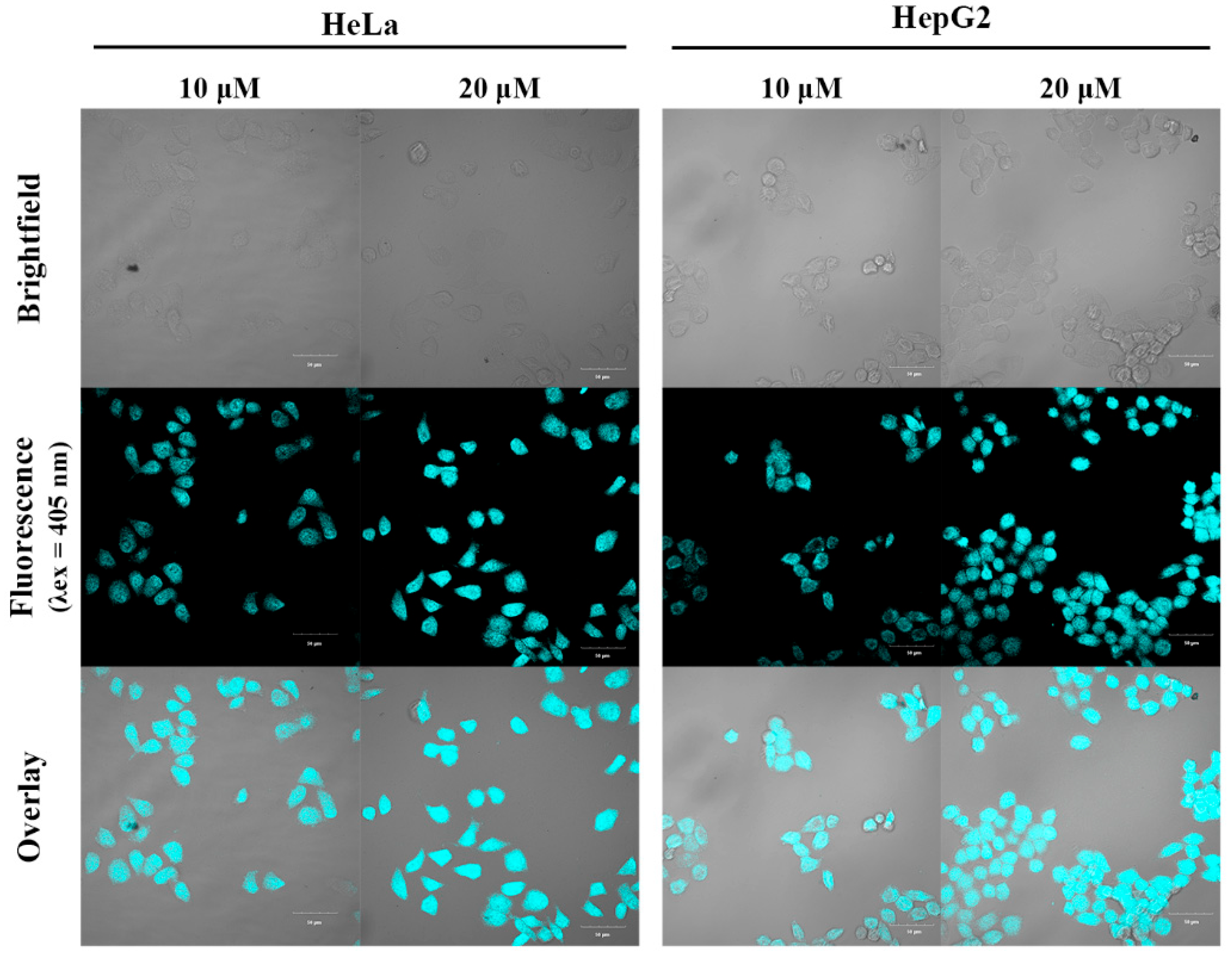
Figure 10.
One-photon confocal fluorescence images of HeLa and HepG2 cells stained with different concentrations of the fluorescent dye HO-PPV-MePy (10 and 20 μM). From left to right: different HO-PPV-MePy concentrations with HeLa and HepG2 cells, respectively. From top to bottom: bright field images, pseudocolored fluorescent images, and overlay images. (λex = 405 nm, red channel emission wavelength, λem = 700 -740 nm) Scale bar: 50 μm.
Figure 10.
One-photon confocal fluorescence images of HeLa and HepG2 cells stained with different concentrations of the fluorescent dye HO-PPV-MePy (10 and 20 μM). From left to right: different HO-PPV-MePy concentrations with HeLa and HepG2 cells, respectively. From top to bottom: bright field images, pseudocolored fluorescent images, and overlay images. (λex = 405 nm, red channel emission wavelength, λem = 700 -740 nm) Scale bar: 50 μm.
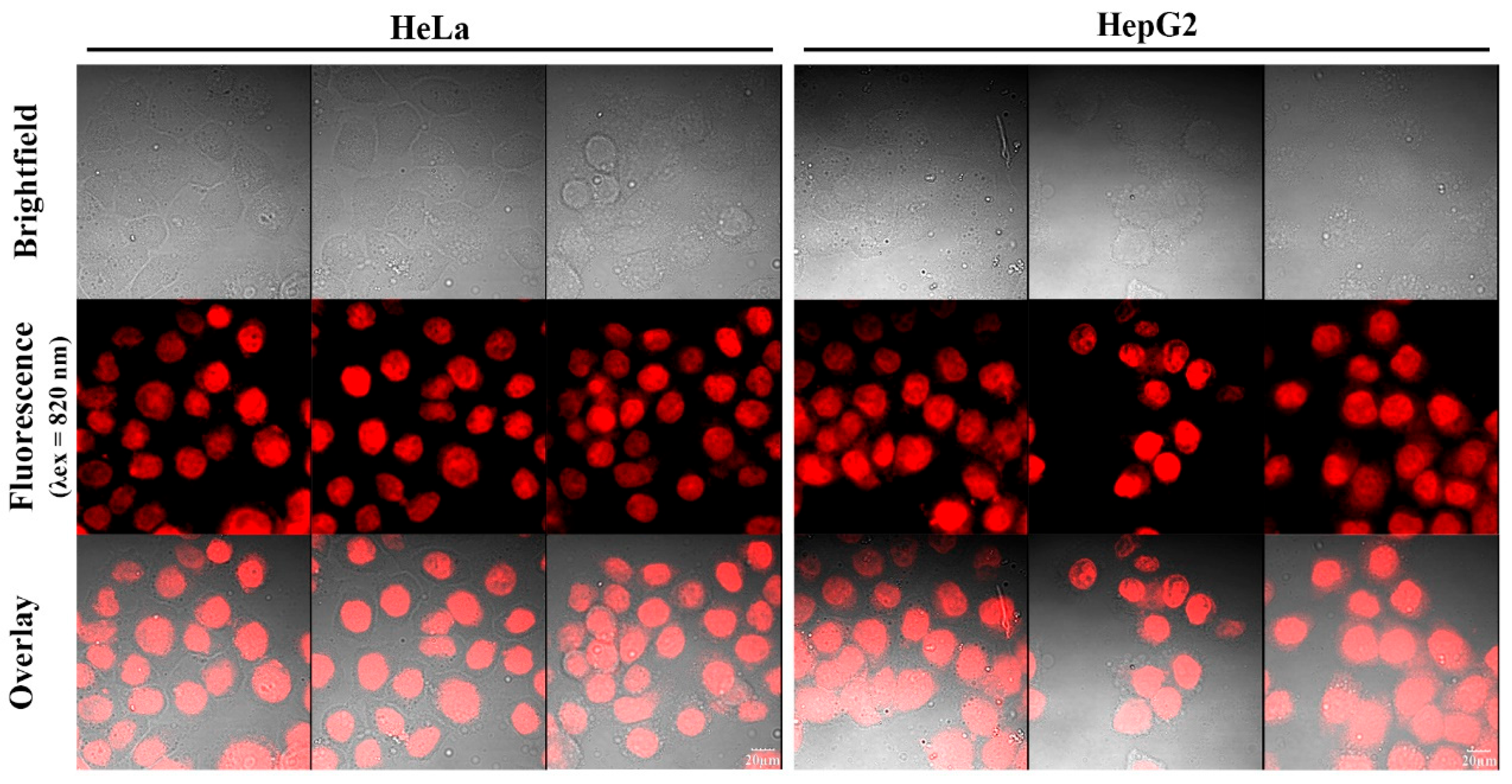
Figure 11.
Two-photon confocal fluorescence images of HeLa and HepG2 cells stained with different concentrations of the fluorescent dye HO-PPV-EtBT (5, 10 and 20 μM). From left to right: HeLa and HepG2 cells. From top to bottom: bright field image, fluorescence image, and superimposed image. (λex = 820 nm, red channel emission wavelength, λex≥575 nm) Scale bar: 20 μm.
Figure 11.
Two-photon confocal fluorescence images of HeLa and HepG2 cells stained with different concentrations of the fluorescent dye HO-PPV-EtBT (5, 10 and 20 μM). From left to right: HeLa and HepG2 cells. From top to bottom: bright field image, fluorescence image, and superimposed image. (λex = 820 nm, red channel emission wavelength, λex≥575 nm) Scale bar: 20 μm.
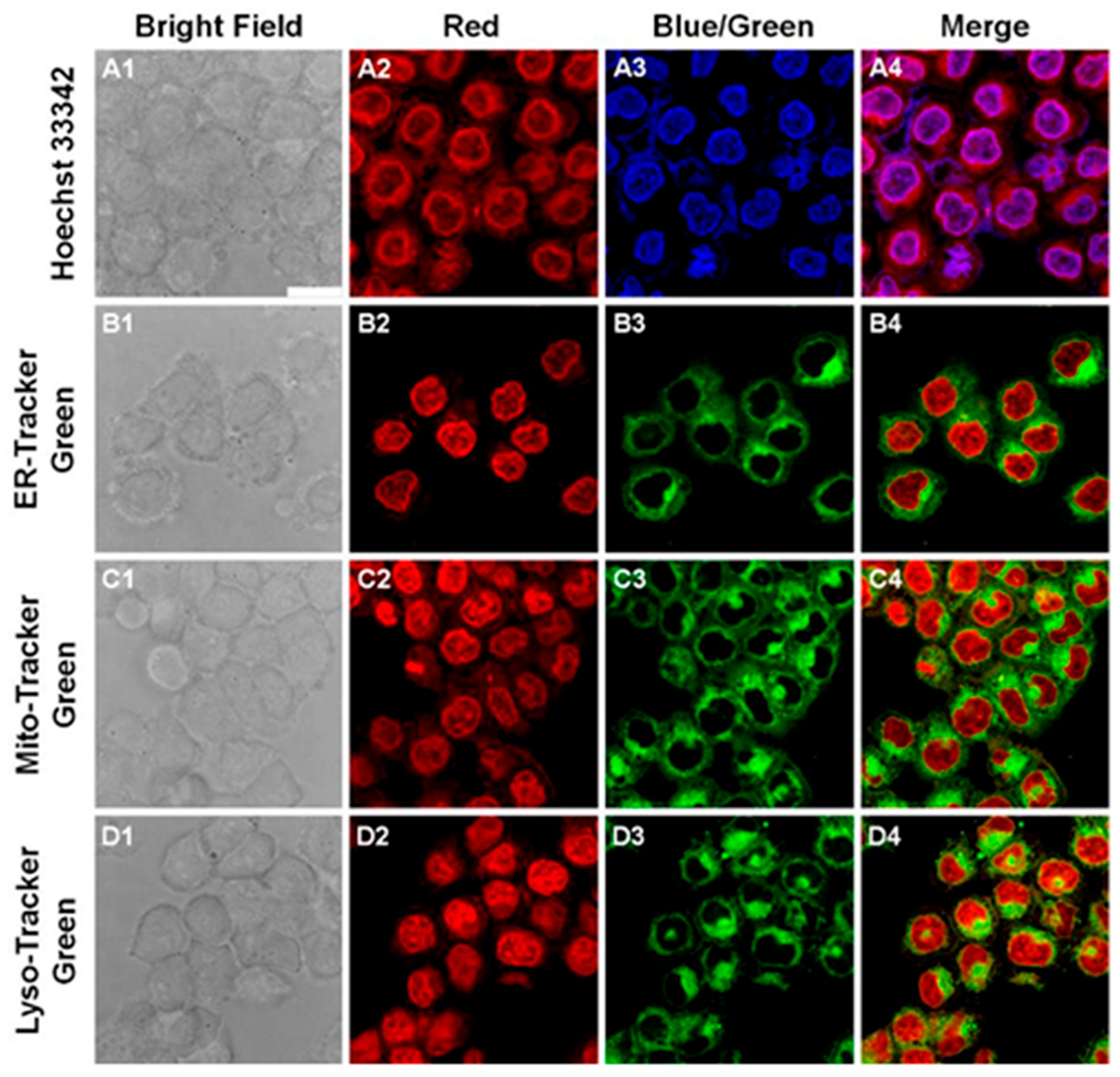
Figure 12.
Subcellular organelle colocalization of HO-PPV-EtBT. Confocal fluorescence images of HepG2 cells co-stained with HO-PPV-EtBT (10 μM) and Hoechst 33342 (A1-A4), ER-Tracker Green (B1-B4), Mito Tracker Green (C1-C4) or Lyso-Tracker Green (D1-D4) for 30 min respectively. HO-PPV-EtBT: λex/λem = 575 nm/730 ± 25 nm; Hoechst 33342: λex/λem = 405 nm/445 ± 25 nm; ER-Tracker Green, Mito-Tracker Green, Lyso-Tracker Green: λex/λem = 488 nm/525 ± 25 nm. Scale bar: 25 μm.
Figure 12.
Subcellular organelle colocalization of HO-PPV-EtBT. Confocal fluorescence images of HepG2 cells co-stained with HO-PPV-EtBT (10 μM) and Hoechst 33342 (A1-A4), ER-Tracker Green (B1-B4), Mito Tracker Green (C1-C4) or Lyso-Tracker Green (D1-D4) for 30 min respectively. HO-PPV-EtBT: λex/λem = 575 nm/730 ± 25 nm; Hoechst 33342: λex/λem = 405 nm/445 ± 25 nm; ER-Tracker Green, Mito-Tracker Green, Lyso-Tracker Green: λex/λem = 488 nm/525 ± 25 nm. Scale bar: 25 μm.
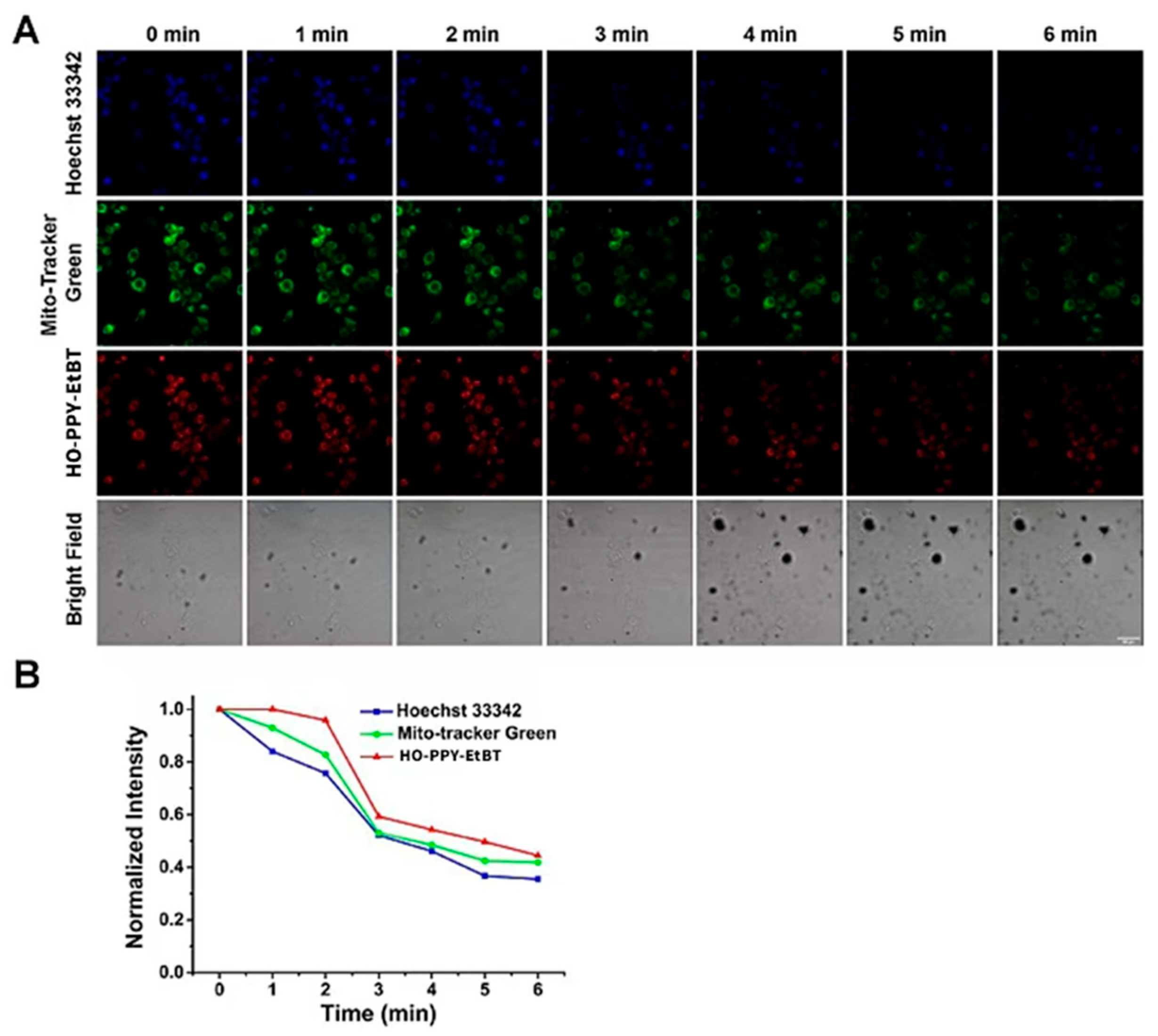
Figure 13.
The photostability experiments of HO-PPV-EtBT, Hoechst 33342, and Mito Tracker Green HepG2 cells were co stained with Hoechst 33342 (1 μ M), Mito Tracker Green (500 nM), and HO-PPV-EtBT (10 µ M) for 30 minutes, and then images at different time points were obtained by continuous scanning in chronological order. From left to right: 0 1 2 3 4 5. 6 minutes. From top to bottom, they are the blue channel, green channel, red channel, and bright field. Ruler: 50 μ m.
Figure 13.
The photostability experiments of HO-PPV-EtBT, Hoechst 33342, and Mito Tracker Green HepG2 cells were co stained with Hoechst 33342 (1 μ M), Mito Tracker Green (500 nM), and HO-PPV-EtBT (10 µ M) for 30 minutes, and then images at different time points were obtained by continuous scanning in chronological order. From left to right: 0 1 2 3 4 5. 6 minutes. From top to bottom, they are the blue channel, green channel, red channel, and bright field. Ruler: 50 μ m.