Submitted:
01 May 2025
Posted:
02 May 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. General Remarks
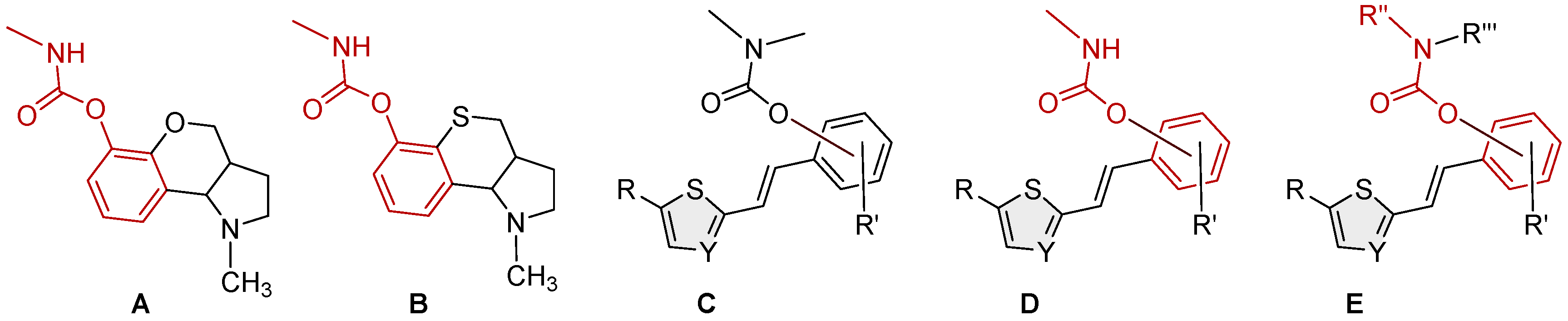
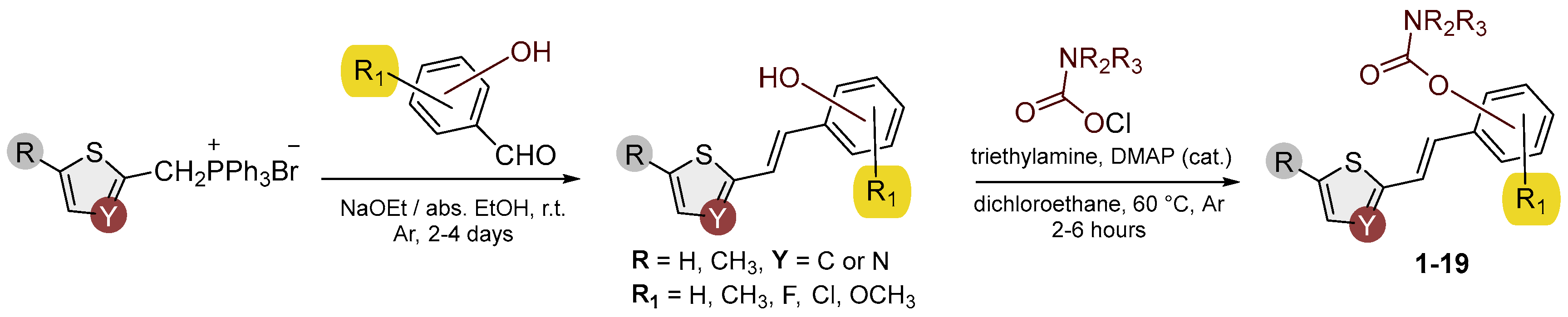
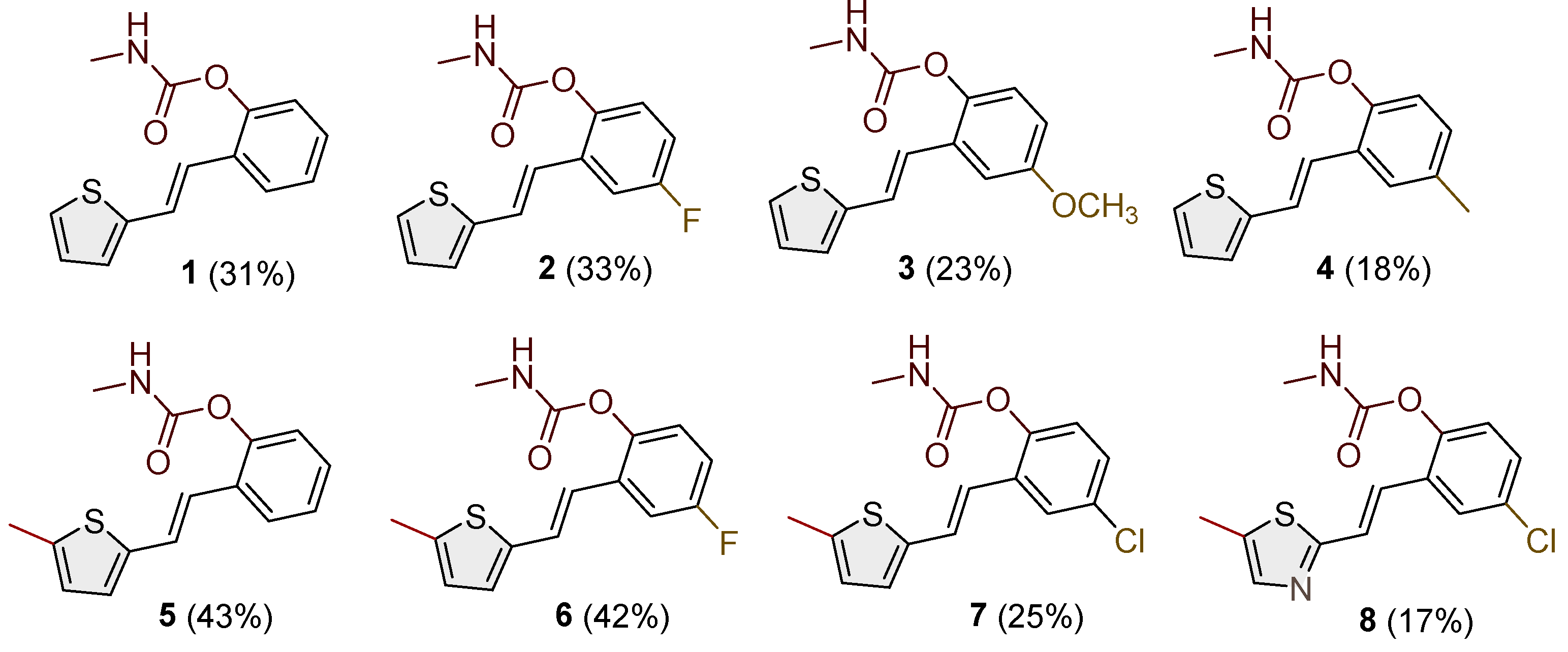
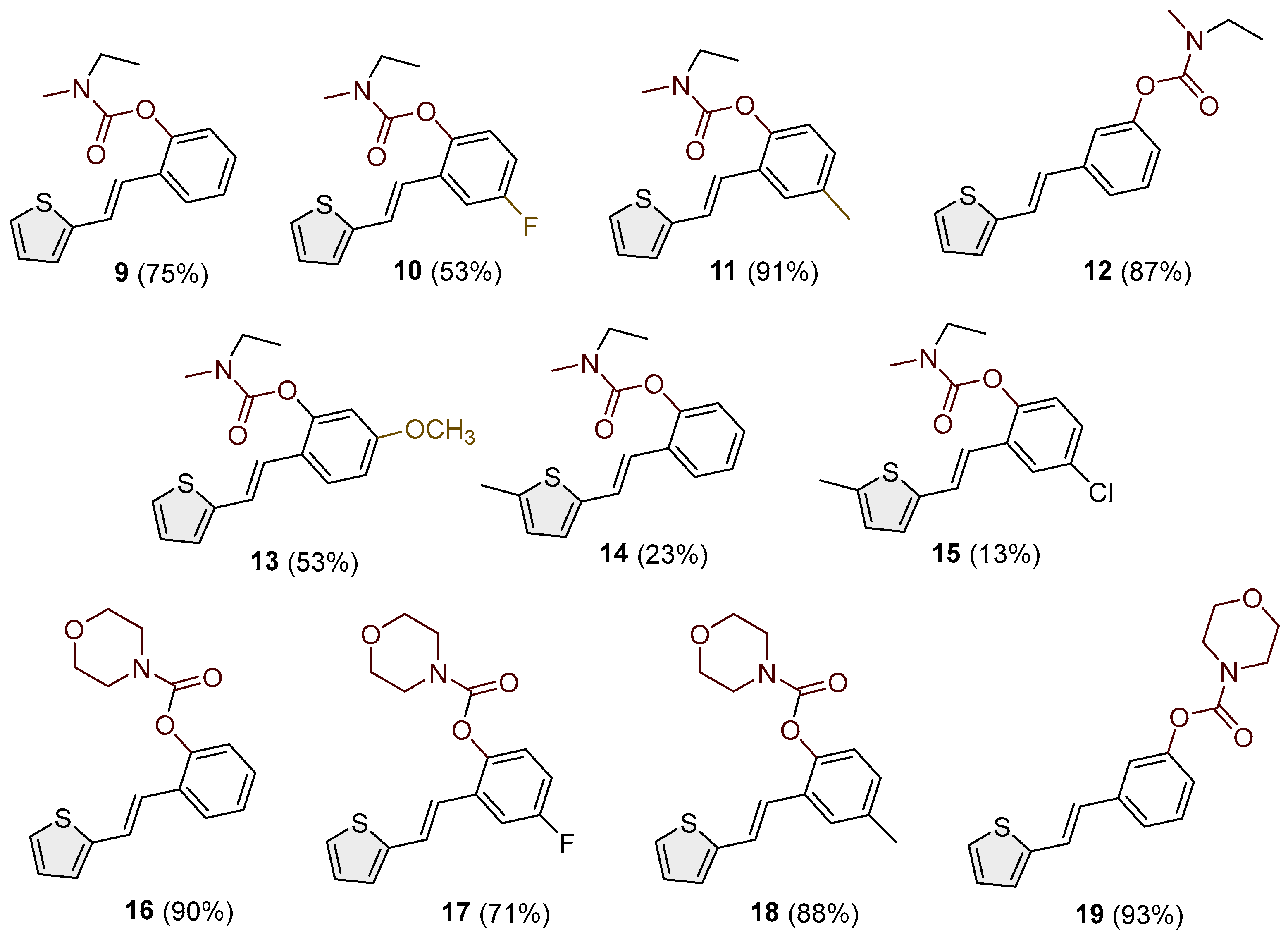
2.2. Synthesis of Heterostilbene Carbamates 1–19
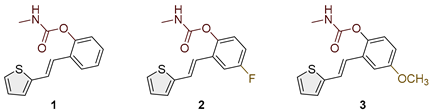
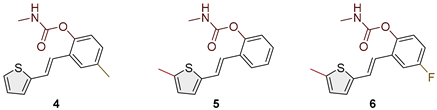
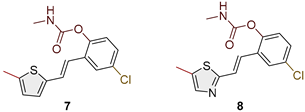
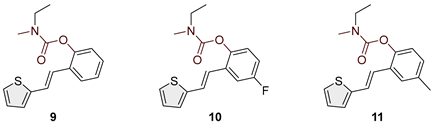
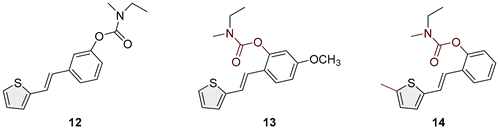
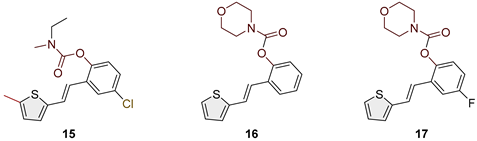
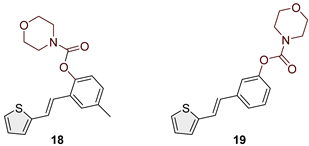
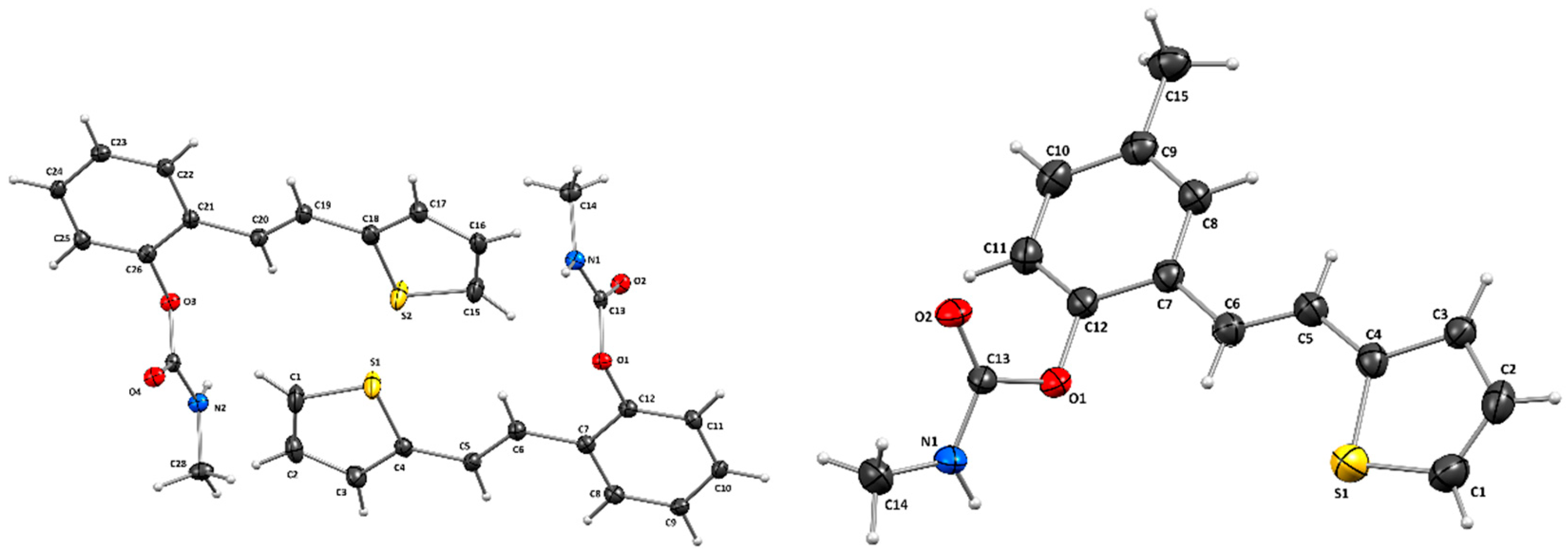
2.3. X-Ray Crystallography
2.4. In Vitro ChE Activity Assay
2.5. Anti-Inflammatory Activity
2.6. Computational Details
3. Results and Discussion
3.1. Synthesis and Characterization of New Heterostilbene Carbamates 1–19
3.2. Crystal Structure of Carbamates 1 and 4
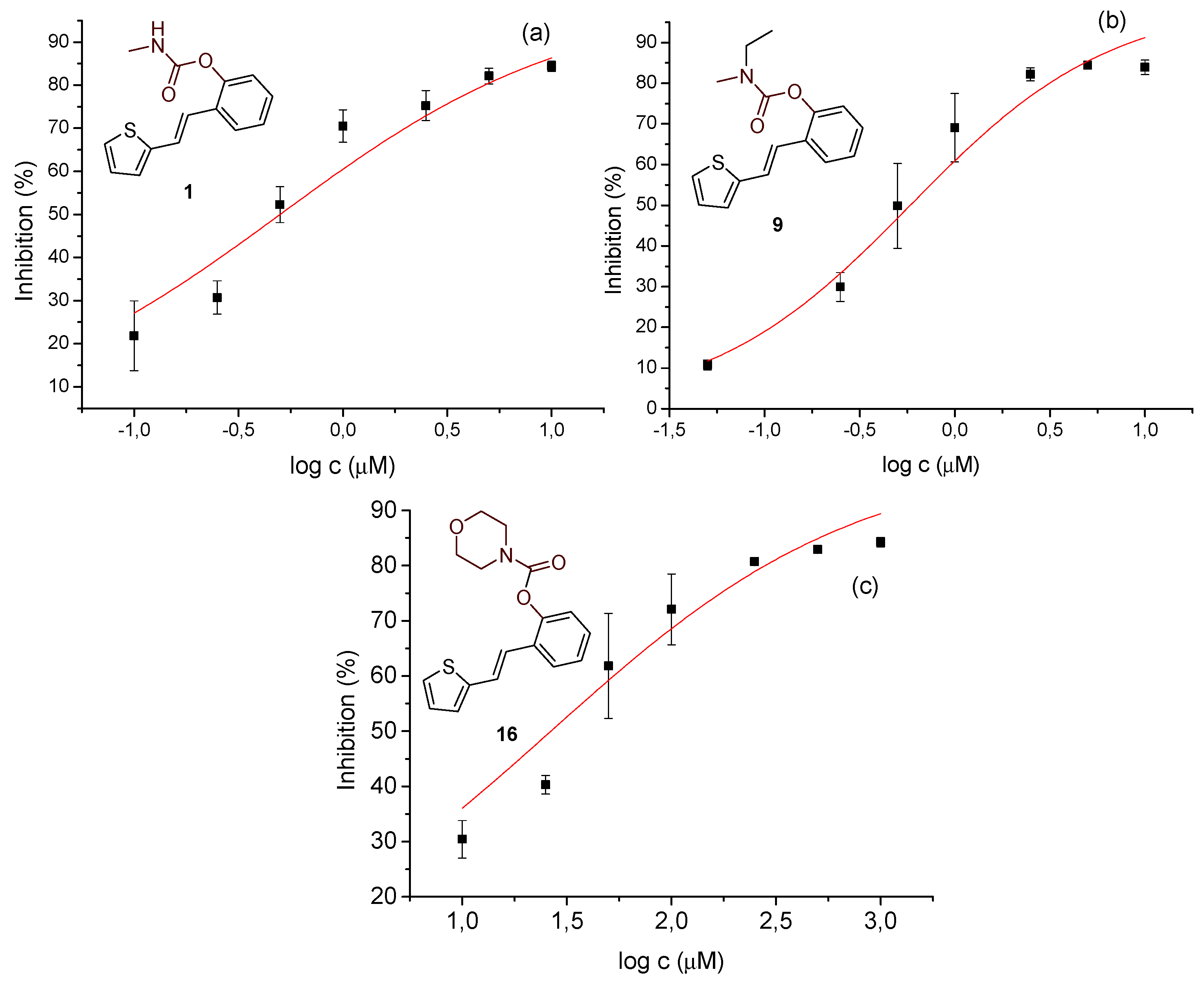
3.3. Cholinesterase Inhibition of Heterostilbene Carbamates 1–19
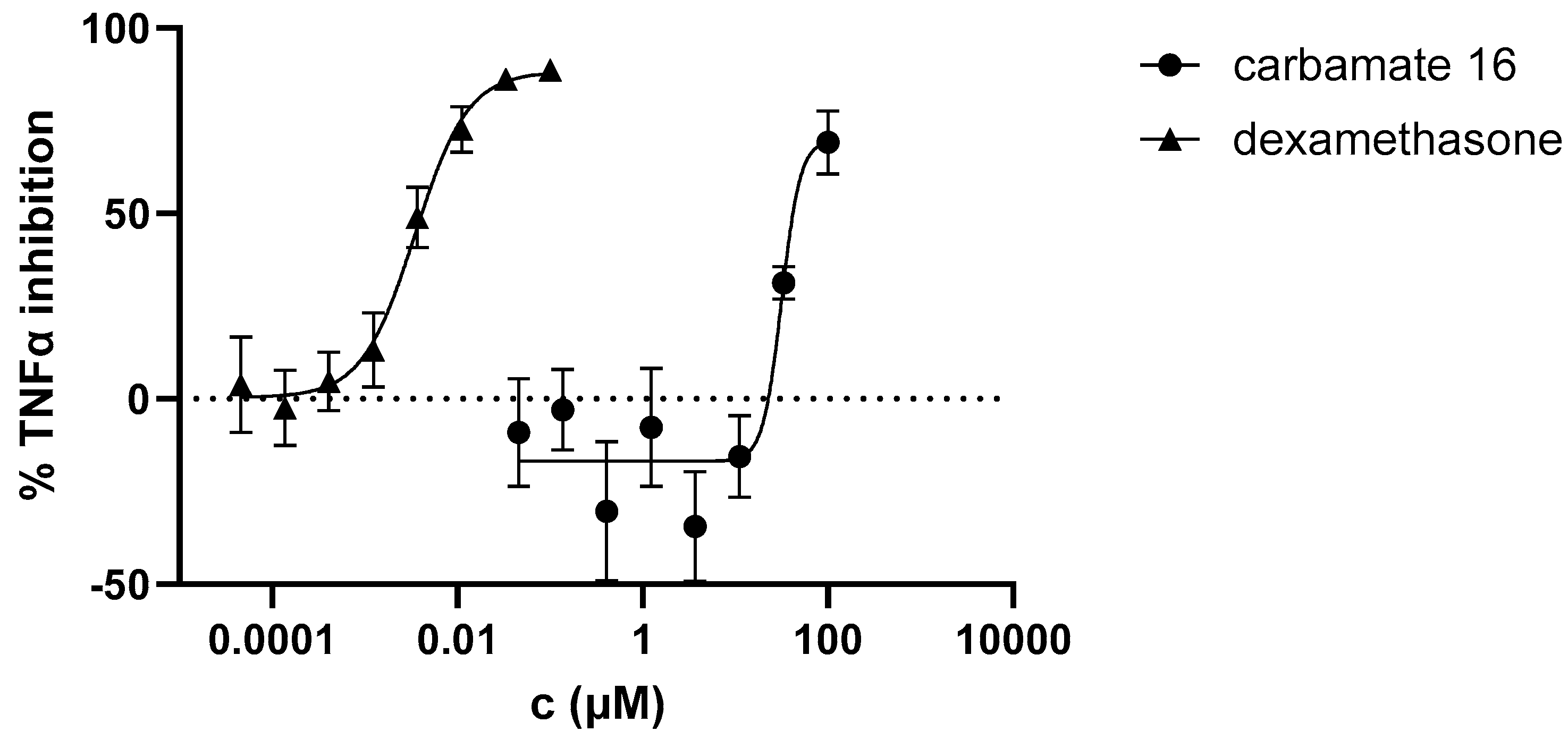
3.4. Anti-Inflammatory activity of heterostilbene carbamates 1–19
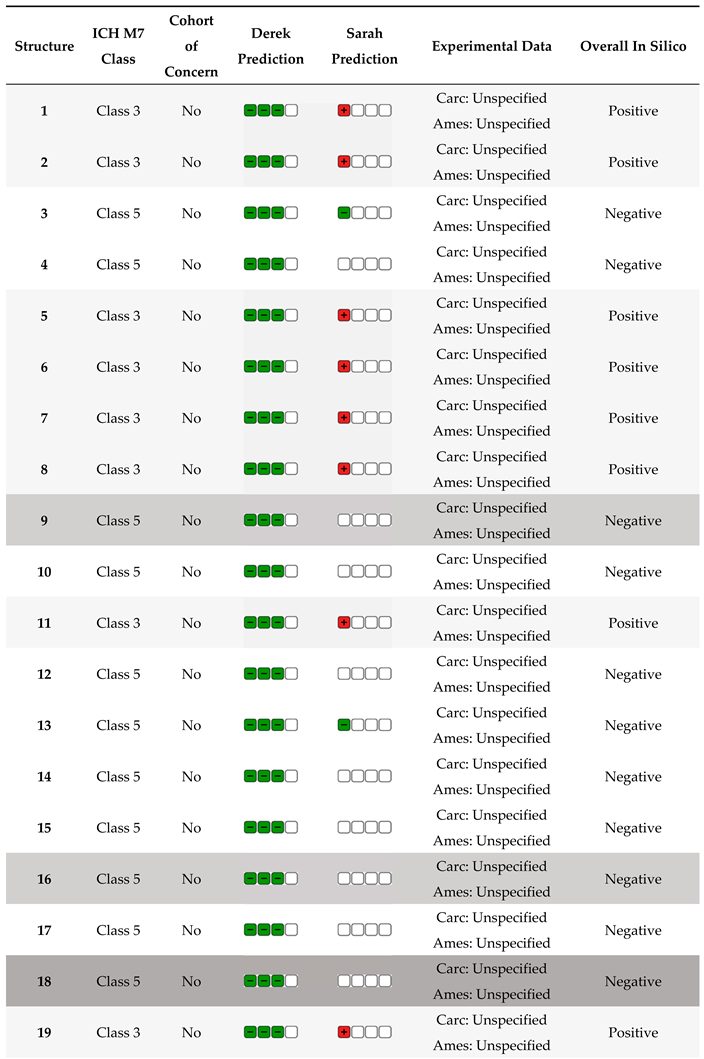
3.5. ADME(T) properties and genotoxicity - ICH (M7) Q(SAR)
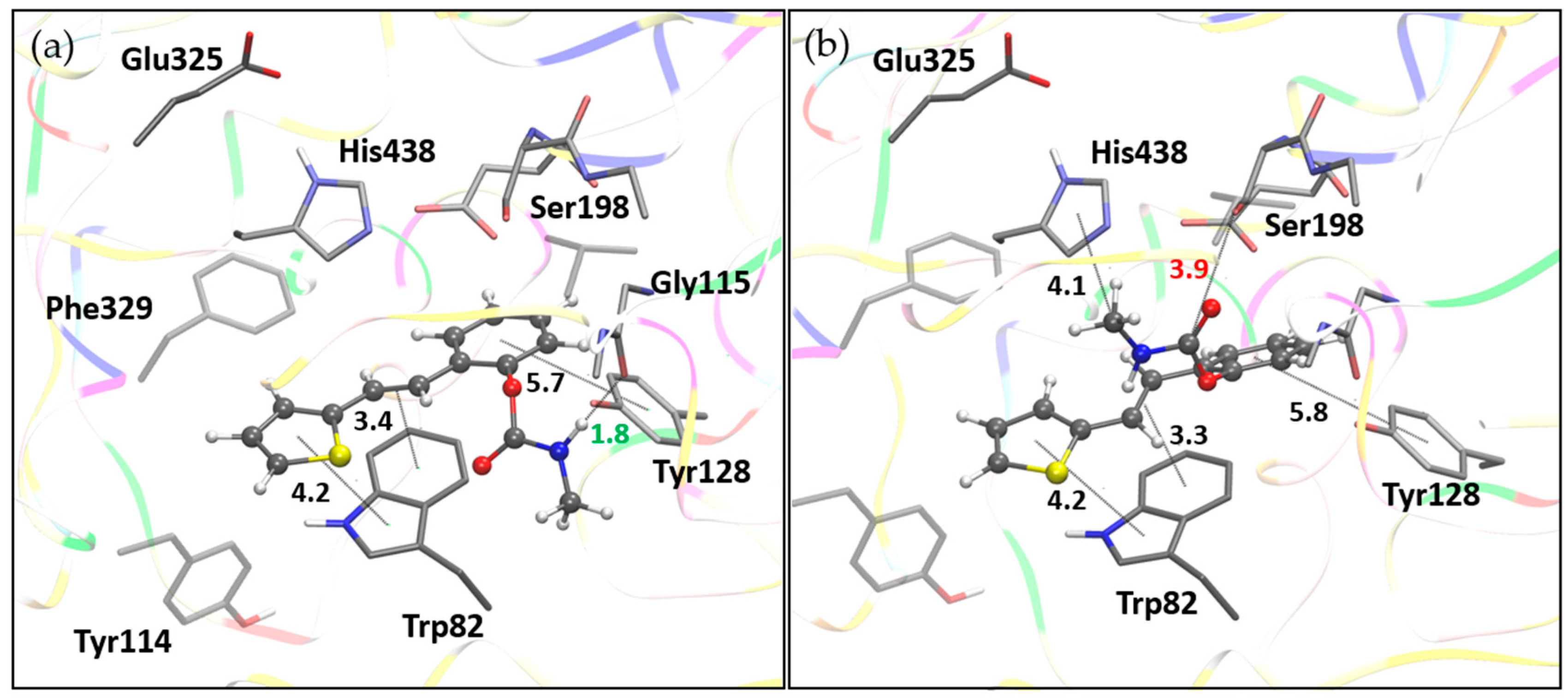
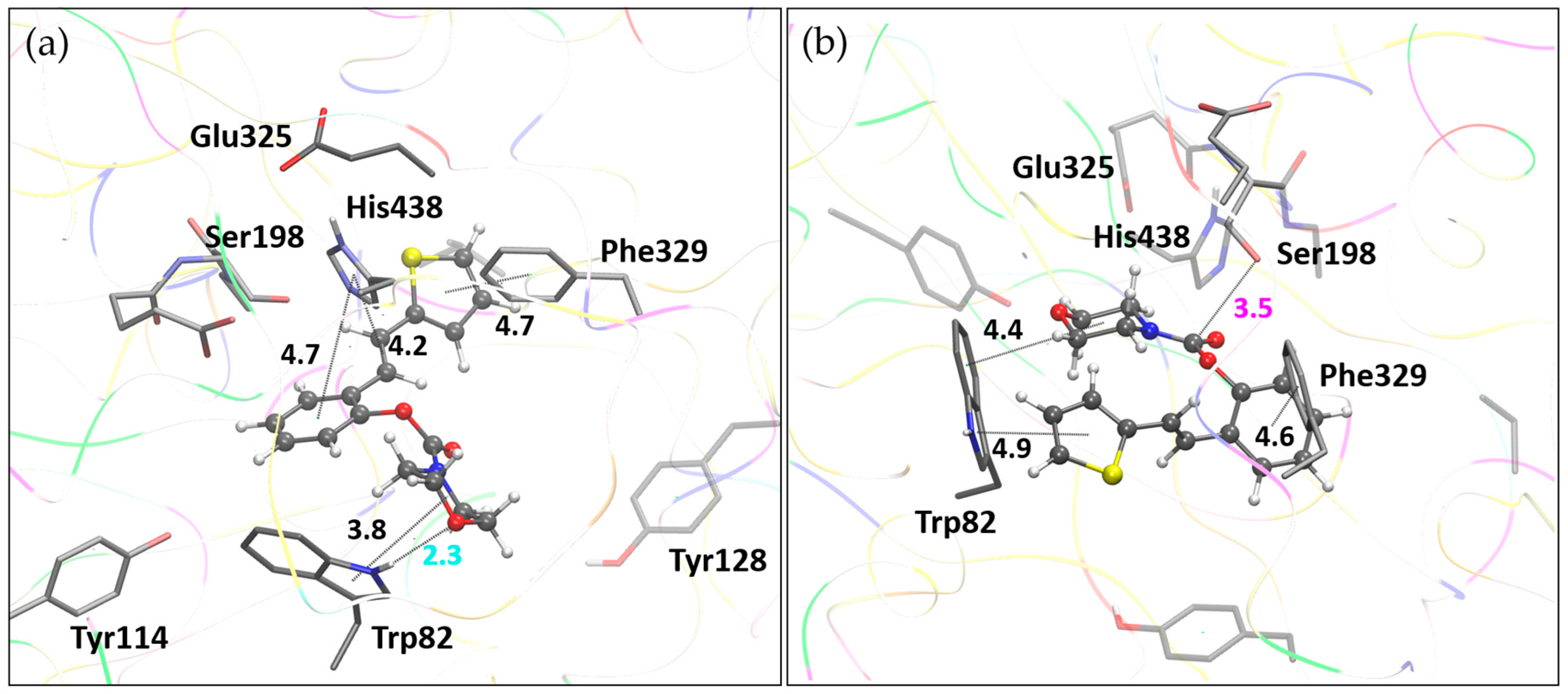
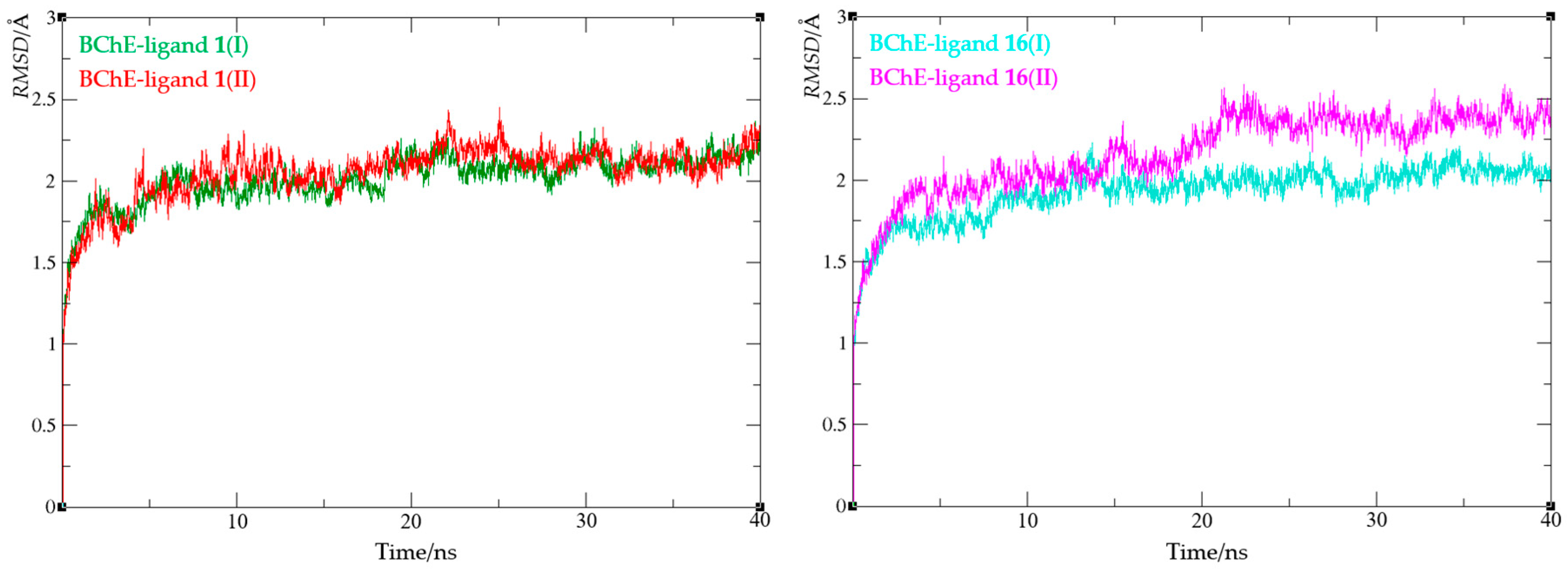
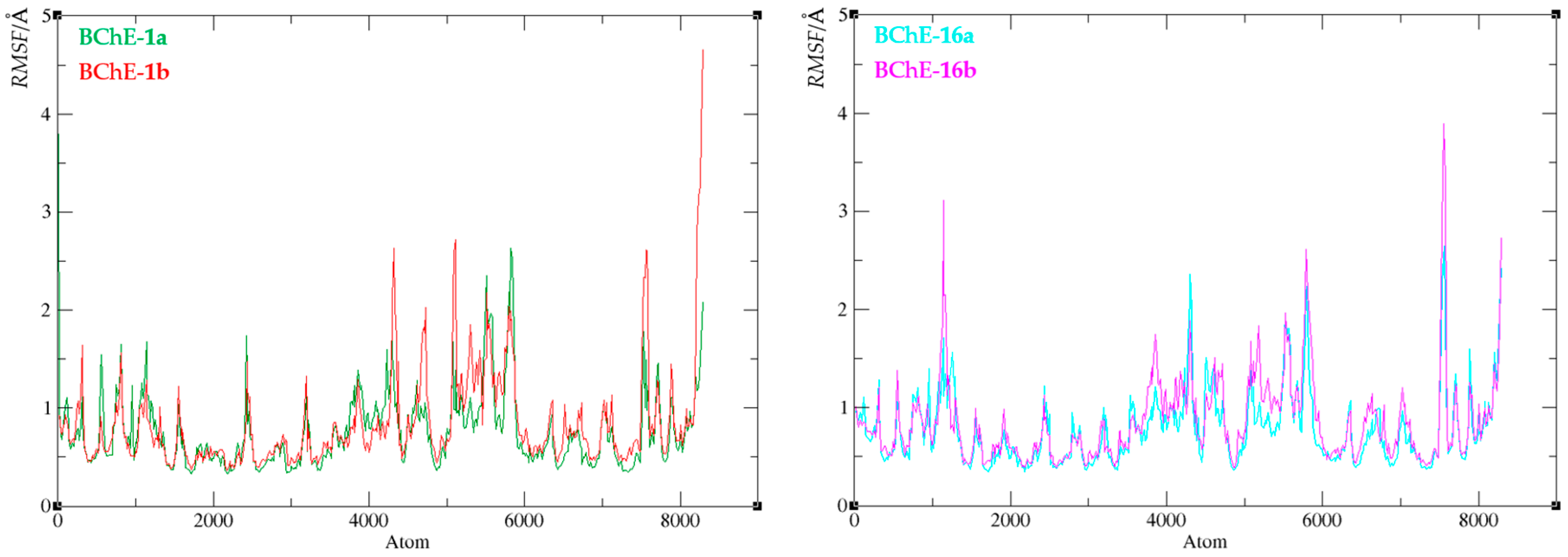
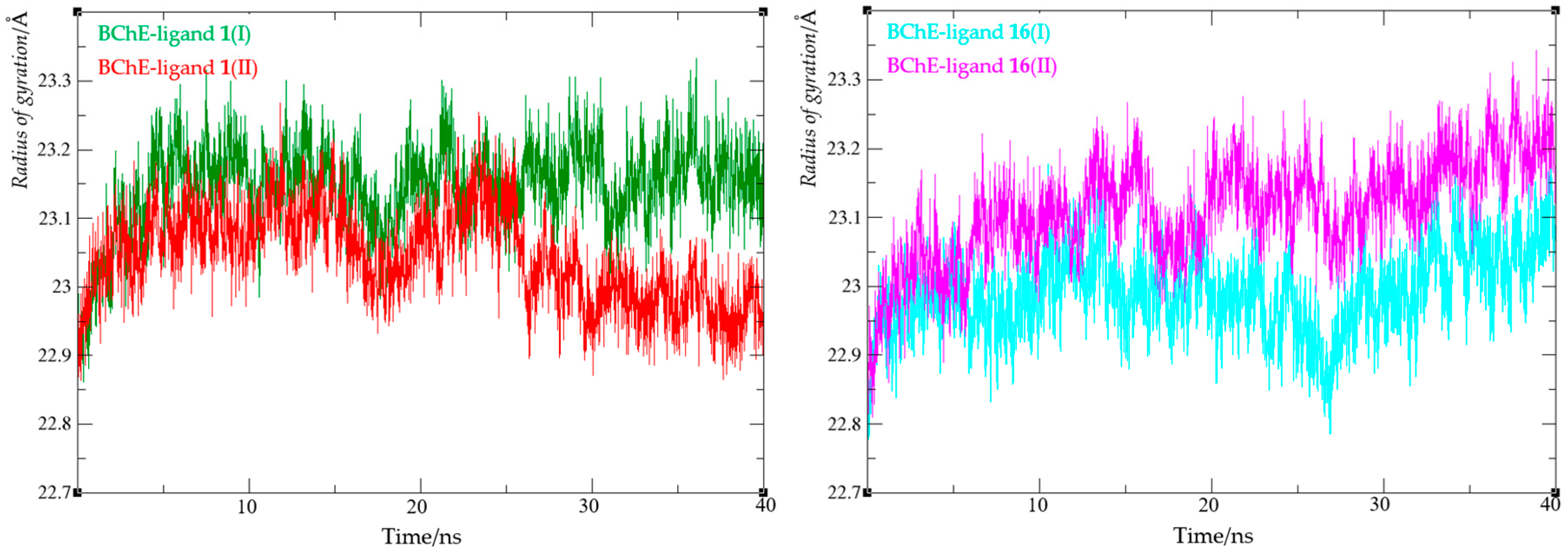
3.6. Computational Study
3. Conclusions
Supplementary Materials
Author Contributions
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sussman, J.L.; Harel, M.; Frolow, F.; Oefner, C.; Goldman, A.; Toker, L.; Silman, I. A Structural Model of Butyrylcholinesterase. Proc. Natl. Acad. Sci. USA 1997, 94, 3398–3403. [Google Scholar]
- Santos, A.L.; Carvalho, M.M.; Silva, D.F.; Amado, F.; Oliveira, C.R.; Pereira, C.M. Dual Inhibition of Acetylcholinesterase and Butyrylcholinesterase by Phenserine Improves Cognitive Function in Alzheimer's Disease. J. Alzheimers Dis. 2009, 16, 767–774. [Google Scholar] [CrossRef]
- Sugimoto, H.; Ogura, H.; Arai, Y.; Hashimoto, Y. The Role of Butyrylcholinesterase in Alzheimer's Disease and Its Potential as a Therapeutic Target. Neurobiol. Aging 2001, 22, 471–478. [Google Scholar]
- Caccamo, A.; Oddo, S.; Sugarman, M.C.; LaFerla, F.M. Molecular Targets for Alzheimer's Disease Therapy: Amyloid-Beta Aggregation and Cholinergic Dysfunction. Neurobiol. Aging 2010, 31, 987–999. [Google Scholar] [CrossRef]
- Vila, J.L.; Medina, M.; Correa, J.; Romero, L. Butyrylcholinesterase Inhibitors as New Drugs for Alzheimer's Disease. J. Med. Chem. 2001, 44, 2022–2027. [Google Scholar]
- Pereira, R.M.; Marques, L.A.; Silva, D.F.; Oliveira, C.R.; Pereira, C.M. Phenserine: A Selective Butyrylcholinesterase Inhibitor. Curr. Alzheimer Res. 2002, 4, 1–11. [Google Scholar]
- Röcken, C.; Bartels, M.; Jessberger, S.; Stühmer, W.; Alzheimer, C. The Pharmacological Profile of Rivastigmine as a Selective Inhibitor of Acetylcholinesterase and Butyrylcholinesterase. Neurochem. Int. 2003, 43, 315–323. [Google Scholar]
- Giacobini, E. Cholinesterase Inhibitors: From the Bench to the Bedside. Neurochem. Int. 2004, 45, 1–10. [Google Scholar] [CrossRef]
- Hasselbalch, S.G.; Knudsen, G.M.; Jakobsen, J.; Høgh-Rasmussen, E.; Holm, S.; Paulson, O.B. The Role of Acetylcholine and Acetylcholinesterase Inhibitors in Parkinson’s Disease. J. Clin. Neurosci. 2007, 14, 610–615. [Google Scholar] [CrossRef]
- Ghosh, A.K.; Brindisi, M. Organic Carbamates in Drug Design and Medicinal Chemistry. J. Med. Chem. 2015, 58, 2895–2940. [Google Scholar] [CrossRef]
- Anand, P.; Singh, B. A Review on Cholinesterase Inhibitors. Arch. Pharm. Res. 2013, 36, 375–399. [Google Scholar] [CrossRef] [PubMed]
- Bolognesi, M.L.; Bartolini, M.; Cavalli, A.; Andrisano, V.; Rosini, M.; Minarini, A.; Melchiorre, C. Design, Synthesis, and Biological Evaluation of Conformationally Restricted Rivastigmine Analogues. J. Med. Chem. 2004, 47, 5945–5952. [Google Scholar] [CrossRef]
- Sterling, J.; Herzig, Y.; Goren, T.; Finkelstein, N.; Lerner, D.; Goldenberg, W.; Miskolczi, I.; Molnar, S.; Rantal, F.; Tamas, T.; Toth, G.; Zagyva, A.; Zekany, A.; Finberg, J.; Lavian, G.; Gross, A.; Friedman, R.; Razin, M.; Huang, W.; Krais, B.; Chorev, M.; Youdim, M.B.; Weinstock, M. Novel Dual Inhibitors of AChE and MAO Derived from Hydroxy Aminoindan and Phenethylamine as Potential Treatment for Alzheimer's Disease. J. Med. Chem. 2002, 45, 5260–5279. [Google Scholar] [CrossRef] [PubMed]
- Ucar, G.; Gokhan, N.; Yesilada, A.; Bilgin, A.A. 1-N-Substituted Thiocarbamoyl-3-Phenyl-5-Thienyl-2-Pyrazolines: Novel Cholinesterase and Selective Monoamine Oxidase B Inhibitors for the Treatment of Parkinson’s and Alzheimer’s Diseases. Neurosci. Lett. 2005, 38, 327–331. [Google Scholar] [CrossRef]
- Toda, N.; Tago, K.; Marumoto, S.; Takami, K.; Ori, M.; Yamada, N.; Koyama, K.; Naruto, S.; Abe, K.; Yamazaki, R.; Hara, T.; Aoyagi, A.; Abe, Y.; Kaneko, T.; Kogen, H. A Conformational Restriction Approach to the Development of Dual Inhibitors of Acetylcholinesterase and Serotonin Transporter. Bioorg. Med. Chem. 2003, 11, 4389–4415. [Google Scholar] [CrossRef]
- Krátký, M.; Štěpánková, Š.; Vorčáková, K.; Švarcová, M.; Vinšová, J. Novel Cholinesterase Inhibitors Based on O-Aromatic N,N-Disubstituted Carbamates and Thiocarbamates. Molecules 2016, 21, 191. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, Y.; Wang, Y.; Li, X.; Wang, S.; Wang, Z. Recent Advances on Carbamate-Based Cholinesterase Inhibitors as Potential Multifunctional Agents Against Alzheimer's Disease. Eur. J. Med. Chem. 2022, 240, 114606. [Google Scholar] [CrossRef] [PubMed]
- Yanovsky, I.; Finkin-Groner, E.; Zaikin, A.; Lerman, L.; Shalom, H.; Zeeli, S.; Weill, T.; Ginsburg, I.; Nudelman, A.; Weinstock, M. Carbamate Derivatives of Indolines as Cholinesterase Inhibitors and Antioxidants for the Treatment of Alzheimer’s Disease. J. Med. Chem. 2012, 55, 10700–10715. [Google Scholar] [CrossRef]
- Sviben, M.; Odak, I.; Barić, D.; Mlakić, M.; Horváth, O.; Fodor, L.; Roca, S.; Šagud, I.; Škorić, I. Resveratrol-Based Carbamates as Selective Butyrylcholinesterase Inhibitors: Design, Synthesis, Computational Study and Biometal Complexation Capability. Molecules 2025, 30, 316. [Google Scholar] [CrossRef]
- Ljubić, A.; Vušak, V.; Cingesar, I.K.; Vrsaljko, D.; Šalić, A.; Škorić, I. Application of natural deep eutectic solvents in the continuous process for synthesis of resveratrol analogues by the Wittig reaction. J. Flow Chem. 2025. [Google Scholar] [CrossRef]
- Mlakić, M.; Odak, I.; Barić, D.; Talić, S.; Šagud, I.; Štefanić, Z.; Molčanov, K.; Lasić, Z.; Kovačević, B.; Škorić, I. New Resveratrol Analogs as Improved Biologically Active Structures: Design, Synthesis and Computational Modeling. Bioorg. Chem. 2024, 143, 106965. [Google Scholar] [CrossRef] [PubMed]
- Mlakić, M.; Rajić, L.; Ljubić, A.; Vušak, V.; Zelić, B.; Gojun, M.; Odak, I.; Čule, I.; Šagud, I.; Šalić, A.; Škorić, I. Synthesis of New Heterocyclic Resveratrol Analogues in Milli- and Microreactors: Intensification of The Wittig Reaction. J. Flow Chem. 2022, 12, 429–440. [Google Scholar] [CrossRef]
- Mlakić, M.; Talić, S.; Odak, I.; Barić, D.; Šagud, I.; Škorić, I. Cholinesterase Inhibition and Antioxidative Capacity of New Heteroaromatic Resveratrol Analogs: Synthesis and Physico–Chemical Properties. Int. J. Mol. Sci. 2024, 25, 7401. [Google Scholar] [CrossRef] [PubMed]
- Rigaku OD. CrysAlis PRO. Rigaku Corporation, Wrocław, Poland, 2024.
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. Olex2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Crystallogr. C 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Spek, A.L. Structure Validation in Chemical Crystallography. Acta Crystallogr. D 2009, 65, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Macrae, C.F.; Edgington, P.R.; McCabe, P.; Pidcock, E.; Shields, G.P.; Taylor, R.; Towler, M.; van de Streek, J. Mercury: Visualization and Analysis of Crystal Structures. J. Appl. Cryst. 2006, 39, 453–457. [Google Scholar] [CrossRef]
- Ellman, G.L.; Courtney, K.D.; Andres, V.; Featherstone, R.M. A New and Rapid Colorimetric Determination of Acetylcholinesterase Activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Mlakić, M.; Faraho, I.; Odak, I.; Kovačević, B.; Raspudić, A.; Šagud, I.; Bosnar, M.; Škorić, I.; Barić, D. Cholinesterase Inhibitory and Anti-Inflammatory Activity of the Naphtho- and Thienobenzo-Triazole Photoproducts: Experimental and Computational Study. Int. J. Mol. Sci. 2023, 24, 14676. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision C01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated Docking with Selective Receptor Flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef]
- Coquelle, N.; Colletier, J.P. Crystal Structure of Human Butyrylcholinesterase in Complex with 2-{1-[4-(12-Amino-3-chloro-6,7,10,11-tetrahydro-7,11-methanocycloocta[b]quinolin-9-yl)butyl]-1H-1,2,3-triazol-4-yl}-N-[4-hydroxy-3-methoxybenzyl]acetamide. PDB 7AIY. Available online: https://doi.org/10.2210/pdb7aiy/pdb.
- Case, D.A.; Ben-Shalom, I.Y.; Brozell, S.R.; Cerutti, D.S.; Cheatham, T.E. III; Cruzeiro, V.W.D.; Darden, T.A.; Duke, R.E.; Ghoreishi, D.; Gilson, M.K.; et al. AMBER 2016, University of California, San Francisco, 2016.
- Maier, J.A.; Martinez, C.; Kasavajhala, K.; Wickstrom, L.; Hauser, K.E.; Simmerling, C. ff14SB: Improving the Accuracy of Protein Side Chain and Backbone Parameters. J. Chem. Theory Comput. 2015, 11, 3696–3713. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wolf, R.M.; Caldwell, J.W.; Kollman, P.A.; Case, D.A. Development and Testing of a General Amber Force Field. J. Comput. Chem. 2004, 25, 1157–1174. [Google Scholar] [CrossRef] [PubMed]
- Hasselgren, C.; Bercu, J.; Cayley, A.; Cross, K.; Glowienke, S.; Kruhlak, N.; Muster, W.; Nicolette, J.; Vijayaraj Reddy, M.; Saiakhov, R.; Dobo, K. Management of Pharmaceutical ICH M7 (Q)SAR Predictions – The Impact of Model Updates. Regul. Toxicol. Pharmacol. 2020, 118, 104807. [Google Scholar] [CrossRef] [PubMed]
- Vrbanac, J.; Slauter, R. ADME in Drug Discovery. In A Comprehensive Guide to Toxicology in Nonclinical Drug Development, 2nd ed.; Faqi, A.S., Ed.; Academic Press: Cambridge, MA, USA, 2017; pp. 39–67. [Google Scholar]
- Pires, D.E.V.; Blundell, T.L.; Ascher, D.B. pkCSM: Predicting Small-Molecule Pharmacokinetic Properties Using Graph-Based Signatures. J. Med. Chem. 2015, 58, 4066–4072. [Google Scholar] [CrossRef]
- AI-DrugLab, Singapore Management University. Available online: https://ai-druglab.smu.edu/admetresult (accessed April/May 2025).
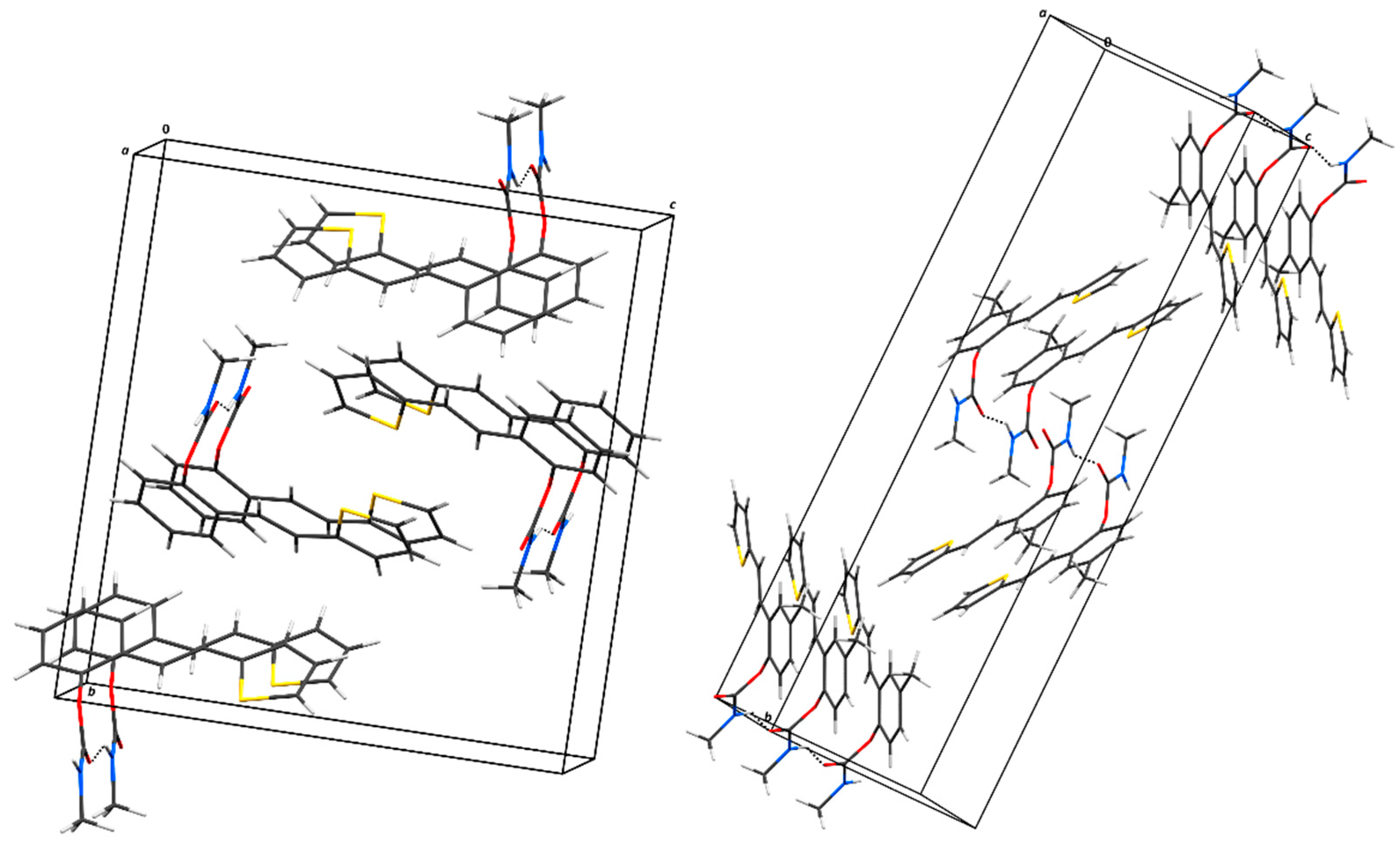
| Compound | 1 | 4 |
| Empirical formula | C14H13NO2S | C15H15NO2S |
| Formula wt. / g mol-1 | 259.31 | 273.34 |
| Crystal dimensions / mm | 0.3 x 0.03 x 0.02 | 0.1 × 0.05 × 0.02 |
| Space group | P21 | P21/c |
| a / Å | 5.07910(10) | 5.12806(17) |
| b / Å | 16.6438(2) | 30.1746(7) |
| c / Å | 15.4486(2) | 9.2923(4) |
| α / ° | 90 | 90 |
| β / ° | 98.0250(10) | 105.137(4) |
| γ / ° | 90 | 90 |
| Z | 4 | 4 |
| V / Å3 | 1293.17(3) | 1387.97(8) |
| Dcalc / g cm-3 | 1.332 | 1.308 |
| μ / mm-1 | 2.171 | 2.049 |
| Θ range / ° | 3.93 – 80.03 | 5.12 – 75.85 |
| T(K) | 104(6) | 293(2) |
| Radiation wavelength | 1.54184 (CuKα) | 1.54184 (CuKα) |
| Diffractometer type | XtaLAB Synergy, Dualflex, HyPix |
XtaLAB Synergy, Dualflex, HyPix |
| Range of h, k, l | –6 > h > 6; –21 > k > 21; –19 > l > 19 |
–6 > h > 6; –26 > k > 37; –11 > l > 11 |
| Reflections collected | 17951 | 25937 |
| Independent reflections | 5485 | 2990 |
| Observed reflections (I ≥ 2σ) |
5146 | 2337 |
| Rint | 0.0776 | 0.0899 |
| R (F) | 0.0508 | 0.0743 |
| Rw (F2) | 0.1369 | 0.2130 |
| No. of parameters, restraints | 335, 1 | 181, 0 |
| Goodness of fit | 1.039 | 1.054 |
| Δρmax, Δρmin (eÅ–3) | 0.48; –0.64 | 0.66; –0.51 |
| D–H / Å | H···A / Å | D···A / Å | D–H···A / º | Symm. op. on A | |
| Compound 1 | |||||
| N1–H1A∙∙∙O2 | 0.84 | 2.11 | 2.824(4) | 143 | –1+x, y, z |
| N2–H2A∙∙∙O4 | 0.93 | 2.10 | 2.821(4) | 134 | 1+x, y, z |
| C6–H6∙∙∙S1 | 0.95 | 2.73 | 3.156(5) | 108 | x, y, z |
| C6–H6∙∙∙O1 | 0.95 | 2.46 | 2.828(5) | 103 | x, y, z |
| C10–H10∙∙∙O4 | 0.95 | 2.57 | 3.477(5) | 160 | 1+x, y, 1+z |
| C16–H16∙∙∙O2 | 0.95 | 2.60 | 3.397(5) | 142 | x, y, z |
| C20–H20∙∙∙S2 | 0.95 | 2.73 | 3.158(3) | 108 | x, y, z |
| C20–H20∙∙∙O3 | 0.95 | 2.46 | 2.821(4) | 103 | x, y, z |
| Compound 4 | |||||
| N1–H1A∙∙∙O2 | 0.86 | 2.14 | 2.892(3) | 145.4 | –1+x, y, z |
| C6–H6∙∙∙S1 | 0.93 | 2.83 | 3.210(4) | 106 | x, y, z |
| C6–H6∙∙∙O1 | 0.93 | 2.45 | 2.800(4) | 103 | x, y, z |
| π···π | Cga···Cg / Å |
αb / º | βc / º | Cg···plane(Cg2) / Å | Offset/ Å |
Symm. |
| Compound 1 | ||||||
| S1→C4∙∙∙S1→C4 | 5.079(3) | 0.0(3) | 50.0 | 3.264(2) | 3.891 | –1+x, y, z |
| S1→C4∙∙∙S2→C18 | 4.246(3) | 6.1(2) | 33.7 | 3.751(2) | 2.355 | x, y, z |
| C7→C12∙∙∙C7→C12 | 5.079(2) | 0.0(17) | 50.4 | 3.2388(15) | 3.912 | –1+x, y, z |
| C7→C12∙∙∙C21→C26 | 5.521(2) | 0.78(17) | 58.4 | 2.8750(15) | 4.703 | 1+x, y, z |
| S2→C18∙∙∙S1→C4 | 4.246(3) | 6.1(2) | 27.9 | 3.5336(19) | 1.989 | x, y, z |
| S2→C18∙∙∙S2→C18 | 5.079(3) | 0.0(2) | 52.6 | 3.0846(19) | 4.035 | –1+x, y, z |
| C21→C26∙∙∙C7→C12 | 5.521(2) | 0.78(17) | 58.6 | 2.8916(15) | 4.713 | –1+x, y, –1+z |
| C21→C26∙∙∙C21→C26 | 5.079(2) | 0.03(17) | 49.6 | 3.2914(15) | 3.868 | –1+x, y, z |
| Compound 4 | ||||||
| S1→C4∙∙∙S1→C4 | 5.128(2) | 0.00(19) | 54.6 | 2.9734(16) | 4.178 | –1+x, y, z |
| S1→C4∙∙∙C7→C12 | 5.269(2) | 8.71(18) | 41.0 | 3.4207(16) | 3.458 | –1+x, y, –1+z |
| C7→C12∙∙∙C7→C12 | 5.128(2) | 0.00(16) | 46.1 | 3.5526(14) | 3.698 | –1+x, y, z |
| C–H···π | H···Cg / Å |
γa / º | C–H···Cg / Å | C···Cg / Å | Symm. Operation on Cg |
| C15–H15A∙∙∙C7→C12 | 2.68 | 11.24 | 171 | 3.627(4) | 1+x, y, z |
| Compound | BChE | |
|---|---|---|
| % Inhibition* | IC50/μM | |
| 1 | 84.37±1.18 (10) | 0.500 |
| 2 | 84.66±3.20 (100) | 3.740 |
| 3 | 82.15±2.00 (500) | 149.7 |
| 4 | 85.38±2.29 (10) | 0.913 |
| 5 | 74.79±2.36 (5) | 1.102 |
| 6 | 74.00±3.20 (50) | 14.053 |
| 7 | 69.80±1.15 (100) | 30.403 |
| 8 | 87.21±0.47 (500) | 59.108 |
| 9 | 83.91±1.77 (10) | 0.583 |
| 10 | 83.48±1.49 (27) | 3.081 |
| 11 | 82.75±1.57 (10) | 1.541 |
| 12 | 83.19±0.84 (50) | 1.067 |
| 13 | 84.41±2.29 (25) | 1.883 |
| 14 | 70.00±0.80 (250) | 90.001 |
| 16 | 84.25±0.82 (1) | 0.0265 |
| 17 | 82.28±2.44 (25) | 0.645 |
| 18 | 87.48±1.31 (25) | 0.430 |
| 19 | 79.67±2.51 (50) | 2.045 |
| Galantamine | 90.10±3.40 (4.5) | 7.90 |
| Property | Model Name | 9 | 16 | 18 | Bambuterol | Rivastigmine | Unit |
| Absorption | Water solubility | -4.358 | -4.272 | -4.547 | log mol/L | ||
| Caco2 | 1.907 | 2.117 | 1.962 | log Papp in 10−6 cm/s | |||
| Intestinal absorption | 90.33 | 91.775 | 92.085 | % Absorbed | |||
| Skin permeability | -2.693 | -2.743 | -2.757 | log Kp | |||
| P−glycoprotein substrate | No | No | Yes | ||||
| P−glycoprotein I inhibitor | No | Yes | Yes | ||||
| P−glycoprotein II inhibitor | No | No | No | ||||
| Distribution | VDss (human) | 0.4 | 0.298 | 0.324 | 0.401 | 0.451 | log L/kg |
| Fraction unbound | 0.022 | 0.033 | 0.025 | − | − | Fu | |
| BBB permeability | 0.343 | 0.315 | 0.261 | 0.707 | 0.968 | log BB | |
| CNS permeability | -0.979 | -1.069 | -1.063 | −1.95 | −0.801 | log PS | |
| Metabolism | CYP2D6 substrate | No | No | No | |||
| CYP3A4 substrate | Yes | Yes | Yes | ||||
| CYP1A2 inhibitor | Yes | Yes | Yes | ||||
| CYP2C19 inhibitor | Yes | Yes | Yes | ||||
| CYP2C9 inhibitor | Yes | No | Yes | ||||
| CYP2D6 inhibitor | No | No | No | ||||
| CYP3A4 inhibitor | No | No | No | ||||
| Excretion | Total clearance | 0.138 | 0.373 | 0.262 | log ml/min/kg | ||
| Renal OCT2 substrate | Yes | No | No | Yes/No | |||
| Toxicity | AMES toxicity | No | No | No | Yes/No | ||
| Max. tolerated dose | 0.292 | -0.157 | -0.249 | log mg/kg/day | |||
| hERG I inhibitor | No | No | No | ||||
| hERG II inhibitor | Yes | Yes | Yes | ||||
| Oral rat acute toxicity (LD50) | 2.648 | 2.618 | 2.656 | mol/kg | |||
| Oral rat chronic toxicity (LOAEL) | 1.896 | 1.281 | 1.34 | log mg/kg_bw/day | |||
| Hepatotoxicity | Yes | No | No | ||||
| Skin sensitization | No | No | No | ||||
| T. pyriformis toxicity | 1.812 | 1.465 | 1.542 | log ug/L | |||
| Minnow toxicity | 0.214 | -0.487 | -0.62 | log mM |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





