Submitted:
30 April 2025
Posted:
02 May 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
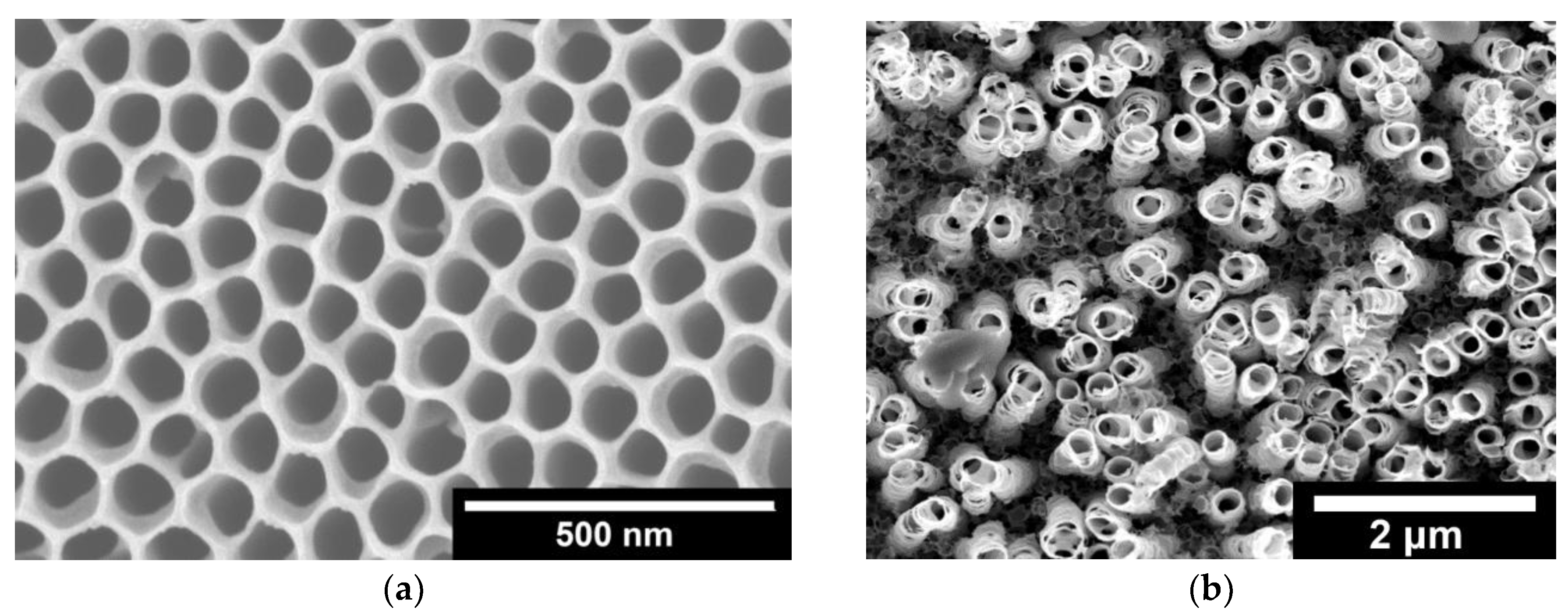
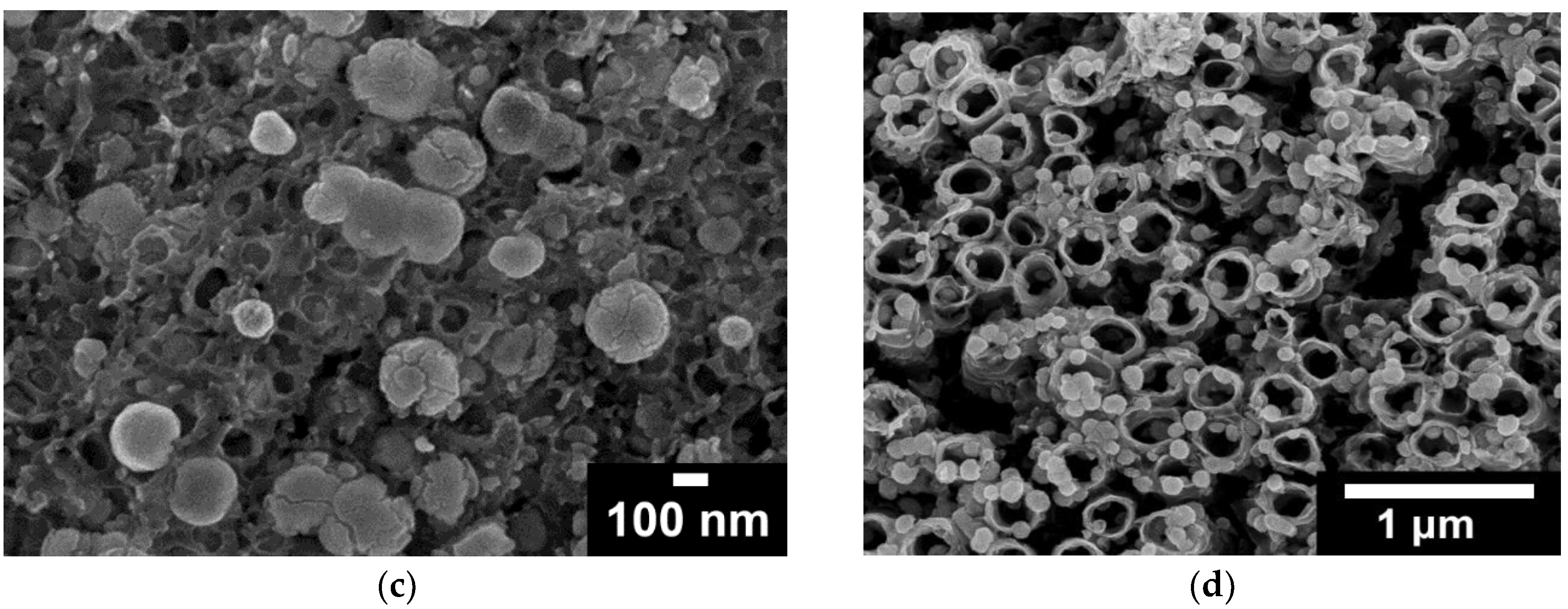
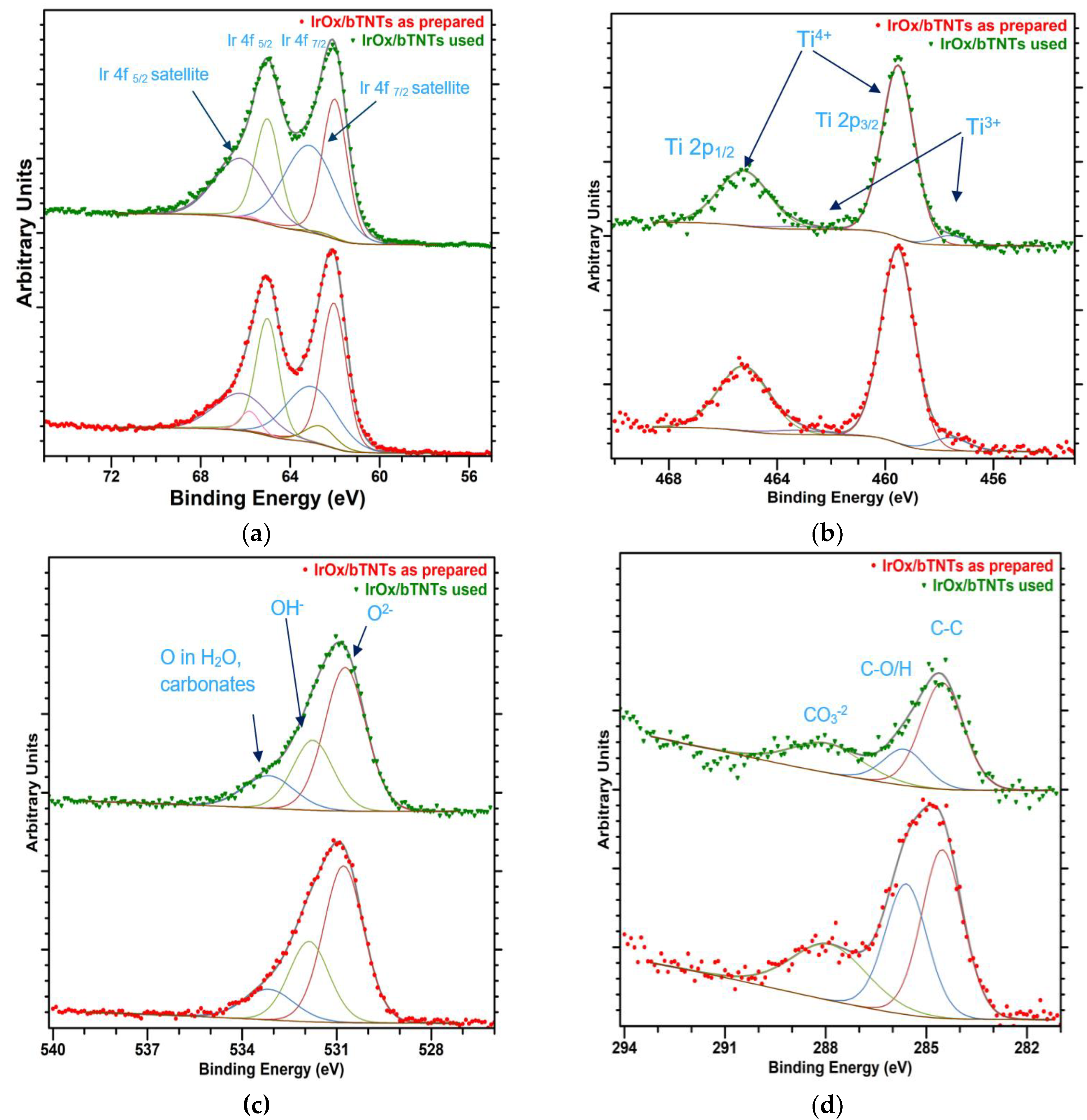
2.1. Microscopic (SEM) and Spectroscopic (EDS, ICP-MS, XPS) Analysis
2.2. Electrochemical Characterization
2.2.1. Surface Electrochemistry of the Catalysts
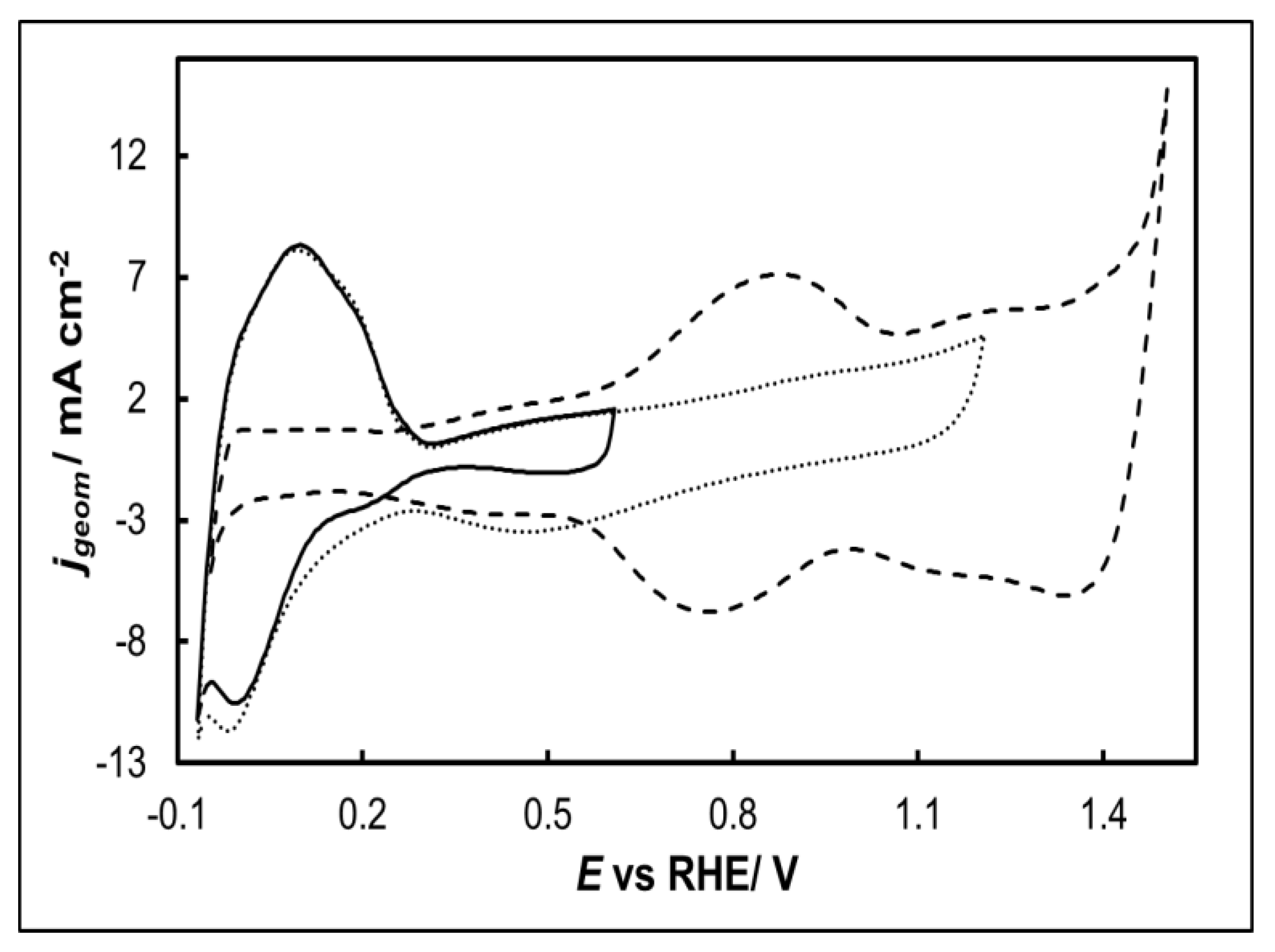
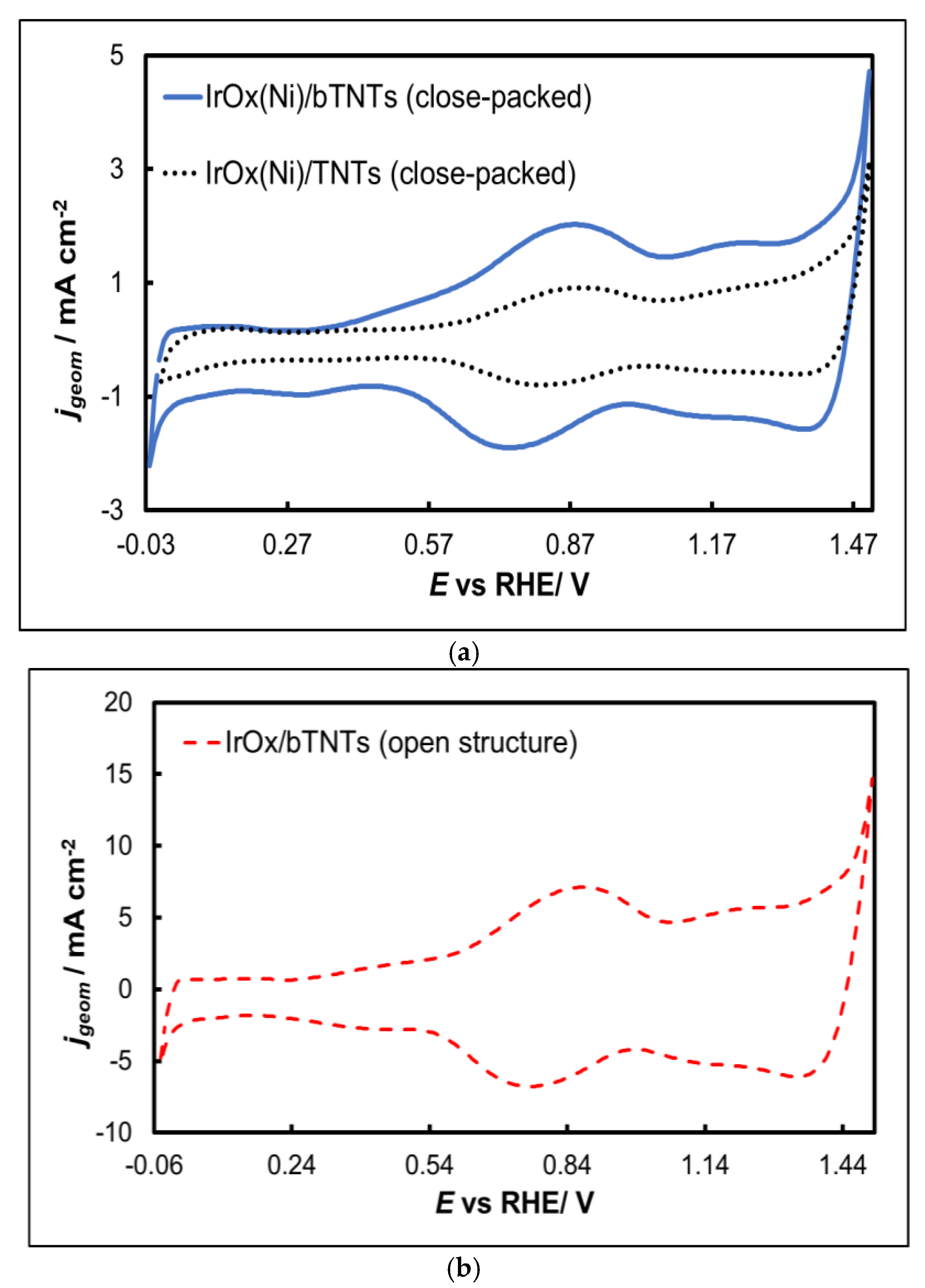
- 0.0 VRHE to +0.6 VRHE, corresponding to the potential range between the onset of hydrogen evolution and the end of Ir double layer potential region, for the electrochemical dissolution of any surface uncovered/unreacted Ni [68,69] and the formation of an Ir(Ni) mixed core-skin structure [57] in those samples that contain Ni, until only the peaks attributed to the adsorption and desorption of an under-potentially deposited hydrogen (UPD-H) layer on metallic Ir could be clearly recorded, as depicted indicatively in Figure 4 for the case of open-structure Ir/bTNTs. The electrochemical surface area (ECSA) of metallic Ir is related to the charge associated with the adsorption of a H monolayer on Ir; the former could be calculated from the voltammograms of Figure 4 as 21.8, 49.4 and 239 cm2Ir cm-2 for the close-packed Ir(Ni)/TNTs, the close-packed Ir(Ni)/bTNTs and the open-structure Ir/bTNTs, respectively [13,56,70], in line with the increase in IrO2 coating roughness/particle dispersion depicted in Figure 2, Figure 1c and Figure 1d respectively that correspond to these three different electrode types.
- 0.0 VRHE to +1.5 VRHE, for the electrochemical anodization of metallic Ir to different oxidation states (IV, V) in order to form stable, porous 3D-IrOx which also extends to the interior of the material [71,72,73]. In Figure 5 the electrochemistry due to anodically generated IrOx appears above +1.0 VRHE [59,74,75]. From the CVs, the charge corresponding to Ir oxides, which is representative of the electroactive surface area available for OER [76,77,78], was calculated as 16.6, 26 and 80 mC cm-2 for the close-packed IrOx(Ni)/TNT and IrOx(Ni)/bTNTs, as well as the open-structure IrOx/bTNTs, respectively (again, in line with coating roughness/particle dispersion shown in the SEM micrographs of Figure 1 and Figure 2).
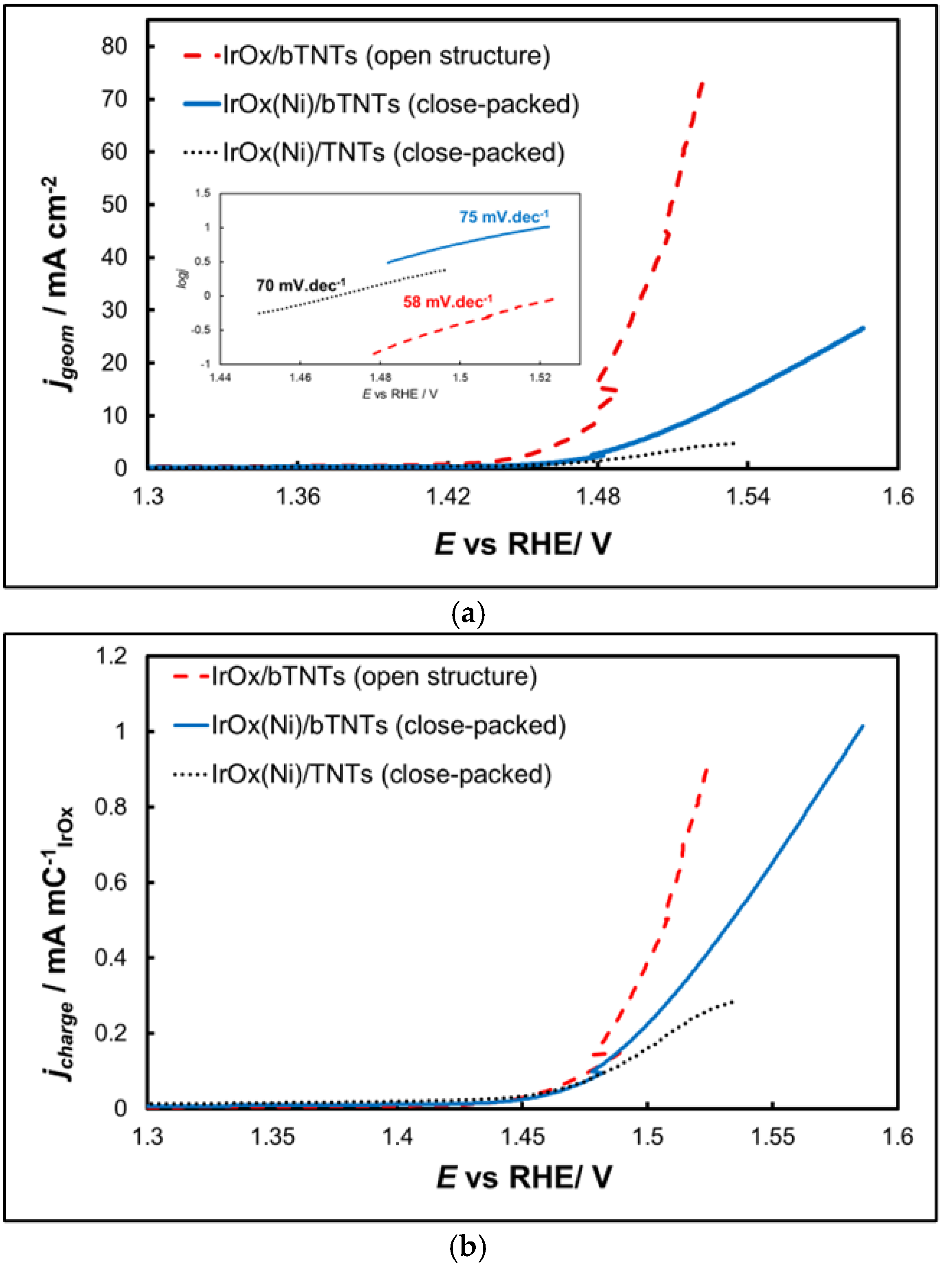
2.2.2. Oxygen Evolution Reaction
2.2.3. Electrochemical Impedance Spectroscopy (EIS)
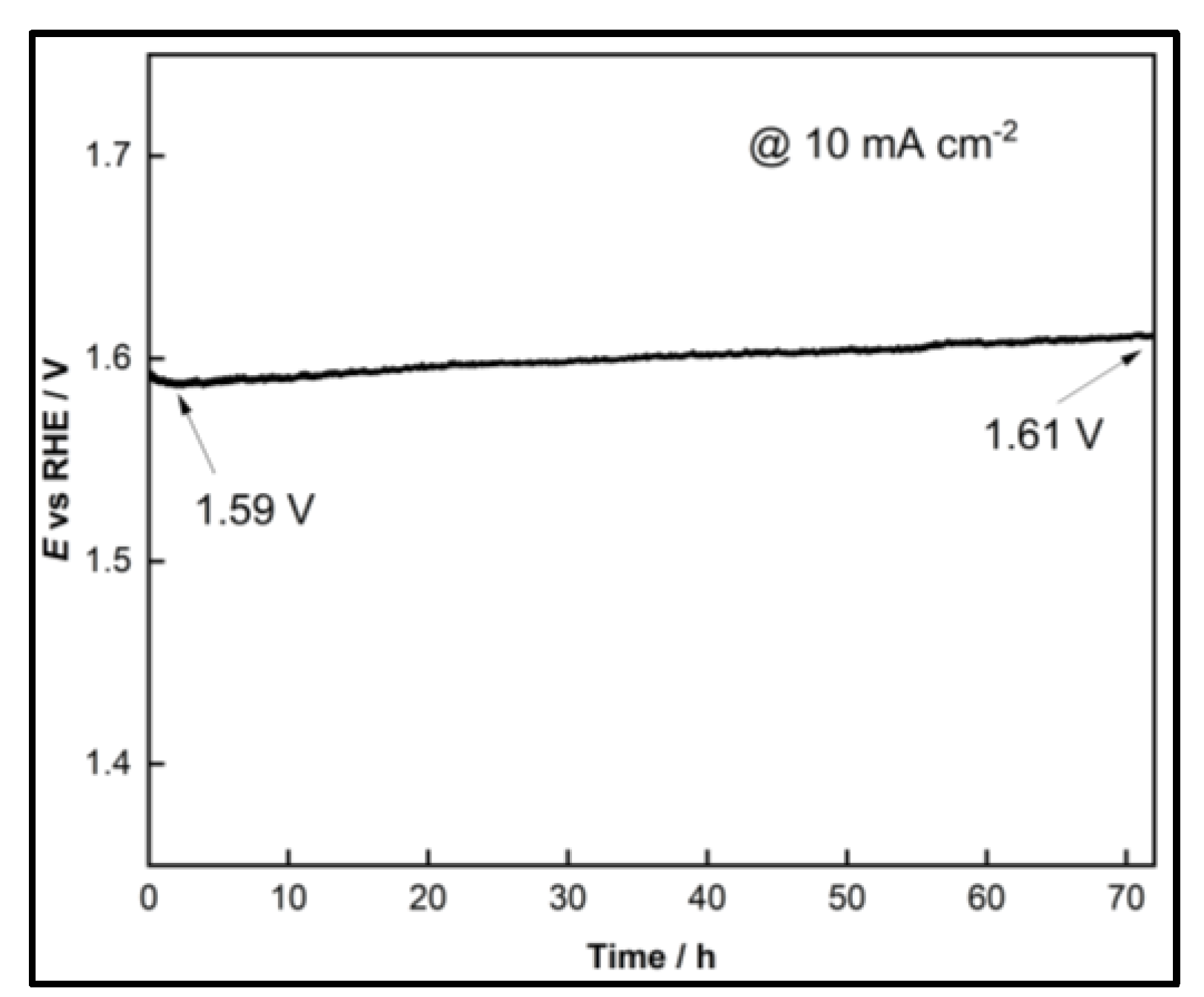
2.2.4. Stability Testing
3. Discussion
3.1. Catalytic Electrode Morphology
3.2. Catalytic Electrode Performance
4. Materials and Methods
4.1. Preparation of IrO2 Catalysts Supported on TNTs and bTNTs
4.1.1. Preparation of Open-Structure and Close-Packed TNTs and bTNTs Substates
4.1.2. Preparation of IrOx(Ni)/TNTs and bTNTs
4.2. Electrochemical Setup and Procedures
4.3. Microscopic and Spectroscopic Characterization
5. Conclusions
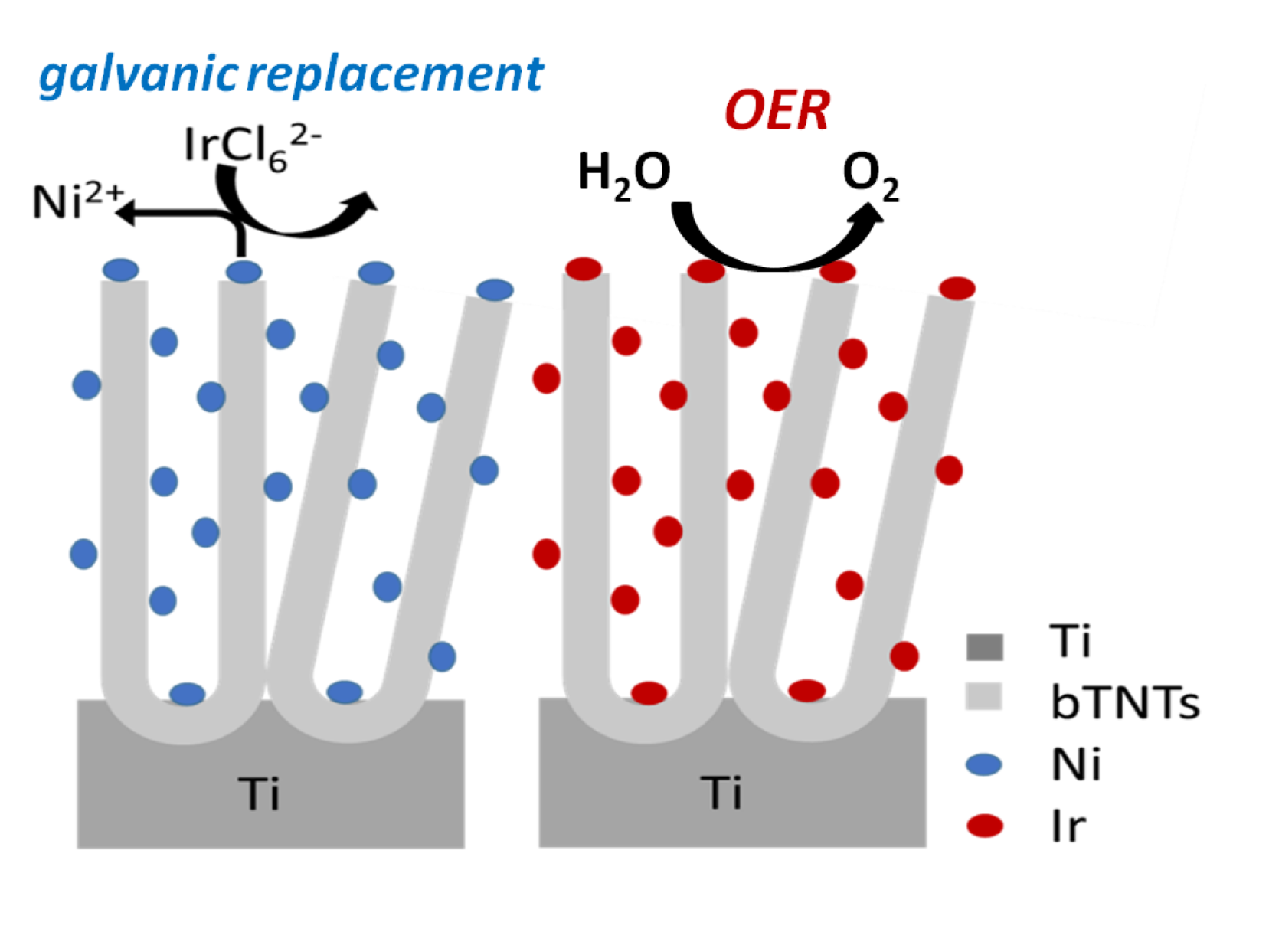
- Electrodeposition of sacrificial Ni on conducting bTNTs and its subsequent galvanic replacement by Ir resulted (depending on substrate type) into Ir particles (<100 nm) for open-structure bTNTs or into larger aggregates for close-packed, bTNTs; in the case of open-structure bTNTs these particles were highly dispersed (some residing inside the nanotubes), thus increasing the electroactive area while at the same time retaining an open electrode structure.
- For the semiconducting TNTs, an increase in the charge of electrodeposited Ni was necessary to rectify their original low electrical conductivity and resulting in the filling of the nanotubes for Ni deposits to act also as a current collector. The eventual formation of a continuous Ir(Ni) film on the surface following galvanic replacement, resulted in a significant decrease in the electroactive surface area.
- The open-structure IrOx(Ni)/bTNTs (more precisely, IrOx/bTNTs since no Ni has been detected after the galvanic replacement/metal exhange process), exhibited an enhanced activity towards the OER. This can be attributed to the higher surface area of the support, higher Ir dispersion and catalytic activity (due to IrO2-bTNT interactions) as well as less pore clogging during O2 evolution. An overpotential of η=240 mV at 10 mA cm-2 and a mass-specific current density of 258 mA mgIr-1 at η=300 mV has been recorded, rendering them comparable or better than similar electrodes reported in the literature. Furthermore, the optimized electrodes, when tested for prolonged periods of time under OER conditions, were characterized by good short-term (72 h) stability.
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| TNTs | Titania Nanotubes |
| bTNTS | Titania Black Nanotubes |
| CV | Cyclic Voltammogram/Voltammetry |
| LSV | Linear Sweep Voltammogram/Voltammetry |
| EIS | Electrochemical Impedance Spectroscopy |
| ICP-MS | Inductively Coupled Plasma Mass Spectrometry |
| SEM | Scanning Electron Microscopy |
| EDS | Energy Dispersive Spectroscopy |
| XPS | X-ray Photoelectron Spectroscopy |
References
- Duby, P. The History of Progress in Dimensionally Stable Anodes. JOM 1993, 45, 41–43. [Google Scholar] [CrossRef]
- Pavlović, M.G.; Dekanski, A. On the Use of Platinized and Activated Titanium Anodes in Some Electrodeposition Processes. Journal of Solid State Electrochemistry 1997, 1, 208–214. [Google Scholar] [CrossRef]
- Zhang, W.; Ghali, E.; Houlachi, G. Review of Oxide Coated Catalytic Titanium Anodes Performance for Metal Electrowinning. Hydrometallurgy 2017, 169, 456–467. [Google Scholar] [CrossRef]
- Choi, H.; Kim, O.H.; Kim, M.; Choe, H.; Cho, Y.H.; Sung, Y.E. Next-Generation Polymer-Electrolyte-Membrane Fuel Cells Using Titanium Foam as Gas Diffusion Layer. ACS Appl Mater Interfaces 2014, 6, 7665–7671. [Google Scholar] [CrossRef]
- Yasutake, M.; Kawachino, D.; Noda, Z.; Matsuda, J.; Lyth, S.M.; Ito, K.; Hayashi, A.; Sasaki, K. Catalyst-Integrated Gas Diffusion Electrodes for Polymer Electrolyte Membrane Water Electrolysis: Porous Titanium Sheets with Nanostructured TiO 2 Surfaces Decorated with Ir Electrocatalysts. J Electrochem Soc 2020, 167, 124523. [Google Scholar] [CrossRef]
- Cherevko, S.; Geiger, S.; Kasian, O.; Kulyk, N.; Grote, J.-P.; Savan, A.; Shrestha, B.R.; Merzlikin, S.; Breitbach, B.; Ludwig, A.; et al. Oxygen and Hydrogen Evolution Reactions on Ru, RuO 2, Ir, and IrO 2 Thin Film Electrodes in Acidic and Alkaline Electrolytes: A Comparative Study on Activity and Stability. Catal Today 2016, 262, 170–180. [Google Scholar] [CrossRef]
- Alia, S.M.; Rasimick, B.; Ngo, C.; Neyerlin, K.C.; Kocha, S.S.; Pylypenko, S.; Xu, H.; Pivovar, B.S. Activity and Durability of Iridium Nanoparticles in the Oxygen Evolution Reaction. J Electrochem Soc 2016, 163, F3105–F3112. [Google Scholar] [CrossRef]
- Liang, Q.; Brocks, G.; Bieberle-Hütter, A. Oxygen Evolution Reaction (OER) Mechanism under Alkaline and Acidic Conditions. JPhys Energy 2021, 3. [Google Scholar] [CrossRef]
- Ledendecker, M.; Geiger, S.; Hengge, K.; Lim, J.; Cherevko, S.; Mingers, A.M.; Göhl, D.; Fortunato, G.V.; Jalalpoor, D.; Schüth, F.; et al. Towards Maximized Utilization of Iridium for the Acidic Oxygen Evolution Reaction. Nano Res 2019, 12, 2275–2280. [Google Scholar] [CrossRef]
- Oh, H.S.; Nong, H.N.; Reier, T.; Bergmann, A.; Gliech, M.; Ferreira De Araújo, J.; Willinger, E.; Schlögl, R.; Teschner, D.; Strasser, P. Electrochemical Catalyst-Support Effects and Their Stabilizing Role for IrOx Nanoparticle Catalysts during the Oxygen Evolution Reaction. J Am Chem Soc 2016, 138, 12552–12563. [Google Scholar] [CrossRef]
- Reier, T.; Teschner, D.; Lunkenbein, T.; Bergmann, A.; Selve, S.; Kraehnert, R.; Schlögl, R.; Strasser, P. Electrocatalytic Oxygen Evolution on Iridium Oxide: Uncovering Catalyst-Substrate Interactions and Active Iridium Oxide Species. J Electrochem Soc 2014, 161, F876–F882. [Google Scholar] [CrossRef]
- Yu, S.; Xie, Z.; Li, K.; Ding, L.; Wang, W.; Yang, G.; Zhang, F.Y. Morphology Engineering of Iridium Electrodes via Modifying Titanium Substrates with Controllable Pillar Structures for Highly Efficient Oxygen Evolution Reaction. Electrochim Acta 2022, 405, 139797. [Google Scholar] [CrossRef]
- Touni, A.; Grammenos, O.A.; Banti, A.; Karfaridis, D.; Prochaska, C.; Lambropoulou, D.; Pavlidou, E.; Sotiropoulos, S. Iridium Oxide-Nickel-Coated Titanium Anodes for the Oxygen Evolution Reaction. Electrochim Acta 2021, 390, 138866. [Google Scholar] [CrossRef]
- Kariman, A.; Marshall, A.T. Improving the Stability of DSA Electrodes by the Addition of TiO 2 Nanoparticles. J Electrochem Soc 2019, 166, E248–E251. [Google Scholar] [CrossRef]
- Oakton, E.; Lebedev, D.; Povia, M.; Abbott, D.F.; Fabbri, E.; Fedorov, A.; Nachtegaal, M.; Copéret, C.; Schmidt, T.J. IrO2-TiO2: A High-Surface-Area, Active, and Stable Electrocatalyst for the Oxygen Evolution Reaction. ACS Catal 2017, 7, 2346–2352. [Google Scholar] [CrossRef]
- Reier, T.; Weidinger, I.; Hildebrandt, P.; Kraehnert, R.; Strasser, P. Electrocatalytic Oxygen Evolution Reaction on Iridium Oxide Model Film Catalysts: Influence of Oxide Type and Catalyst Substrate Interactions. ECS Trans 2013, 58, 39–51. [Google Scholar] [CrossRef]
- Banti, A.; Zafeiridou, C.; Charalampakis, M.; Spyridou, O.N.; Georgieva, J.S.; Binas, V.D.; Mitrousi, E.; Sotiropoulos, S. IrO2 Oxygen Evolution Catalysts Prepared by an Optimized Photodeposition Process on TiO2 Substrates. Molecules 2024, 29. [Google Scholar] [CrossRef]
- Bagheri, S.; Muhd Julkapli, N.; Bee Abd Hamid, S. Titanium Dioxide as a Catalyst Support in Heterogeneous Catalysis. Scientific World Journal 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Hamad, S.; Catlow, C.R.A.; Woodley, S.M.; Lago, S.; Mejías, J.A. Structure and Stability of Small TiO2 Nanoparticles. Journal of Physical Chemistry B 2005, 109, 15741–15748. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, C.; Liu, X.; Gu, F.; Jiang, H.; Shao, W.; Zhang, L.; He, Y. Synthesis and Optical Properties of TiO2 Nanoparticles. Mater Lett 2007, 61, 79–83. [Google Scholar] [CrossRef]
- Viana, M.M.; Soares, V.F.; Mohallem, N.D.S. Synthesis and Characterization of TiO2 Nanoparticles. Ceram Int 2010, 36, 2047–2053. [Google Scholar] [CrossRef]
- Jitputti, J.; Suzuki, Y.; Yoshikawa, S. Synthesis of TiO2 Nanowires and Their Photocatalytic Activity for Hydrogen Evolution. Catal Commun 2008, 9, 1265–1271. [Google Scholar] [CrossRef]
- Wang, G.; Wang, H.; Ling, Y.; Tang, Y.; Yang, X.; Fitzmorris, R.C.; Wang, C.; Zhang, J.Z.; Li, Y. Hydrogen-Treated TiO2 Nanowire Arrays for Photoelectrochemical Water Splitting. Nano Lett 2011, 11, 3026–3033. [Google Scholar] [CrossRef] [PubMed]
- Chuangchote, S.; Jitputti, J.; Sagawa, T.; Yoshikawa, S. Photocatalytic Activity for Hydrogen Evolution of Electrospun TiO 2 Nanofibers. ACS Appl Mater Interfaces 2009, 1, 1140–1143. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Guan, R.; Xie, M.; Dong, P.; Yang, X.; Zhang, J. Advances in Electrospun TiO2 Nanofibers: Design, Construction, and Applications. Chemical Engineering Journal 2022, 431, 134343. [Google Scholar] [CrossRef]
- Chen, F.; Fang, P.; Liu, Z.; Gao, Y.; Liu, Y.; Dai, Y.; Luo, H.; Feng, J. Dimensionality-Dependent Photocatalytic Activity of TiO2-Based Nanostructures: Nanosheets with a Superior Catalytic Property. J Mater Sci 2013, 48, 5171–5179. [Google Scholar] [CrossRef]
- Yu, J.; Fan, J.; Lv, K. Anatase TiO2 Nanosheets with Exposed (001) Facets: Improved Photoelectric Conversion Efficiency in Dye-Sensitized Solar Cells. Nanoscale 2010, 2, 2144–2149. [Google Scholar] [CrossRef]
- Fleischer, C.; Chatzitakis, A.; Norby, T. Intrinsic Photoelectrocatalytic Activity in Oriented, Photonic TiO2 Nanotubes. Mater Sci Semicond Process 2018, 88, 186–191. [Google Scholar] [CrossRef]
- Macak, J.M.; Tsuchiya, H.; Ghicov, A.; Yasuda, K.; Hahn, R.; Bauer, S.; Schmuki, P. TiO2 Nanotubes: Self-Organized Electrochemical Formation, Properties and Applications. Curr Opin Solid State Mater Sci 2007, 11, 3–18. [Google Scholar] [CrossRef]
- Roy, P.; Berger, S.; Schmuki, P. TiO2 Nanotubes: Synthesis and Applications. Angewandte Chemie - International Edition 2011, 50, 2904–2939. [Google Scholar] [CrossRef]
- Mor, G.K.; Varghese, O.K.; Paulose, M.; Shankar, K.; Grimes, C.A. A Review on Highly Ordered, Vertically Oriented TiO2 Nanotube Arrays: Fabrication, Material Properties, and Solar Energy Applications. Solar Energy Materials and Solar Cells 2006, 90, 2011–2075. [Google Scholar] [CrossRef]
- Smith, Y.R.; Ray, R.S.; Carlson, K.; Sarma, B.; Misra, M. Self-Ordered Titanium Dioxide Nanotube Arrays: Anodic Synthesis and Their Photo/Electro-Catalytic Applications. Materials 2013, 6, 2892–2957. [Google Scholar] [CrossRef]
- Tighineanu, A.; Albu, S.P.; Schmuki, P. Conductivity of Anodic TiO2 Nanotubes: Influence of Annealing Conditions. Physica Status Solidi - Rapid Research Letters 2014, 8, 158–162. [Google Scholar] [CrossRef]
- Nah, Y.C.; Paramasivam, I.; Schmuki, P. Doped TiO2 and TiO2 Nanotubes: Synthesis and Applications. ChemPhysChem 2010, 11, 2698–2713. [Google Scholar] [CrossRef]
- Lu, X.; Wang, G.; Zhai, T.; Yu, M.; Gan, J.; Tong, Y.; Li, Y. Hydrogenated TiO 2 Nanotube Arrays for Supercapacitors. Nano Lett 2012, 12, 1690–1696. [Google Scholar] [CrossRef] [PubMed]
- Naldoni, A.; Allieta, M.; Santangelo, S.; Marelli, M.; Fabbri, F.; Cappelli, S.; Bianchi, C.L.; Psaro, R.; Dal Santo, V. Effect of Nature and Location of Defects on Bandgap Narrowing in Black TiO 2 Nanoparticles. J Am Chem Soc 2012, 134, 7600–7603. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Qiu, J.; Ling, M.; Peng, F.; Wood, B.; Zhang, S. Photoelectrochemical Characterization of Hydrogenated TiO2 Nanotubes as Photoanodes for Sensing Applications. ACS Appl Mater Interfaces 2013, 5, 11129–11135. [Google Scholar] [CrossRef] [PubMed]
- Malik, H.; Barrera, K.; Mohanty, S.; Carlson, K. Enhancing Electrochemical Properties of TiO2 Nanotubes via Engineered Defect Laden Crystal Structures. Mater Lett 2020, 273. [Google Scholar] [CrossRef]
- Wu, H.; Xu, C.; Xu, J.; Lu, L.; Fan, Z.; Chen, X.; Song, Y.; Li, D. Enhanced Supercapacitance in Anodic TiO2 Nanotube Films by Hydrogen Plasma Treatment. Nanotechnology 2013, 24. [Google Scholar] [CrossRef]
- Siuzdak, K.; Szkoda, M.; Lisowska-Oleksiak, A.; Karczewski, J.; Ryl, J. Highly Stable Organic-Inorganic Junction Composed of Hydrogenated Titania Nanotubes Infiltrated by a Conducting Polymer. RSC Adv 2016, 6, 33101–33110. [Google Scholar] [CrossRef]
- Li, H.; Chen, J.; Xia, Z.; Xing, J. Microwave-Assisted Preparation of Self-Doped TiO2 Nanotube Arrays for Enhanced Photoelectrochemical Water Splitting. J Mater Chem A Mater 2015, 3, 699–705. [Google Scholar] [CrossRef]
- Andronic, L.; Enesca, A. Black TiO2 Synthesis by Chemical Reduction Methods for Photocatalysis Applications. Front Chem 2020, 8. [Google Scholar] [CrossRef]
- Kang, Q.; Cao, J.; Zhang, Y.; Liu, L.; Xu, H.; Ye, J. Reduced TiO2 Nanotube Arrays for Photoelectrochemical Water Splitting. J Mater Chem A Mater 2013, 1, 5766–5774. [Google Scholar] [CrossRef]
- Liu, X.; Carvalho, P.; Getz, M.N.; Norby, T.; Chatzitakis, A. Black Anatase TiO2 Nanotubes with Tunable Orientation for High Performance Supercapacitors. The Journal of Physical Chemistry C 2019, 123, 21931–21940. [Google Scholar] [CrossRef]
- Touni, A.; Liu, X.; Kang, X.; Papoulia, C.; Pavlidou, E.; Lambropoulou, D.; Tsampas, M.N.; Chatzitakis, A.; Sotiropoulos, S. Methanol Oxidation at Platinum Coated Black Titania Nanotubes and Titanium Felt Electrodes. Molecules 2022, 27. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Song, Y.; Lu, L.; Cheng, C.; Liu, D.; Fang, X.; Chen, X.; Zhu, X.; Li, D. Electrochemically Hydrogenated TiO2 Nanotubes with Improved Photoelectrochemical Water Splitting Performance. Nanoscale Res Lett 2013, 8, 1–7. [Google Scholar] [CrossRef]
- Fabregat-Santiago, F.; Barea, E.M.; Bisquert, J.; Mor, G.K.; Shankar, K.; Grimes, C.A. High Carrier Density and Capacitance in TiO2 Nanotube Arrays Induced by Electrochemical Doping. J Am Chem Soc 2008, 130, 11312–11316. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Zhang, Y. Electrochemically Self-Doped TiO2 Nanotube Arrays for Supercapacitors. Journal of Physical Chemistry C 2014, 118, 5626–5636. [Google Scholar] [CrossRef]
- Zhang, Z.; Hedhili, M.N.; Zhu, H.; Wang, P. Electrochemical Reduction Induced Self-Doping of Ti3+ for Efficient Water Splitting Performance on TiO2 Based Photoelectrodes. Physical Chemistry Chemical Physics 2013, 15, 15637–15644. [Google Scholar] [CrossRef]
- Dehkordi, H.B.; Zhiani, M. A Novel Ir–Ru-Based Nanoparticle Supported on Ordered Electrochemically Synthesized TiO2-Nanotube as a Highly Active and Stable Oxygen Evolution Reaction Catalyst for Water Splitting in Acidic Media. Int J Hydrogen Energy 2023, 48, 33042–33061. [Google Scholar] [CrossRef]
- Genova-Koleva, R.V.; Alcaide, F.; Álvarez, G.; Cabot, P.L.; Grande, H.J.; Martínez-Huerta, M.V.; Miguel, O. Supporting IrO2 and IrRuOx Nanoparticles on TiO2 and Nb-Doped TiO2 Nanotubes as Electrocatalysts for the Oxygen Evolution Reaction. Journal of Energy Chemistry 2019, 34, 227–239. [Google Scholar] [CrossRef]
- Lu, Z.X.; Shi, Y.; Yan, C.F.; Guo, C.Q.; Wang, Z. Da Investigation on IrO2 Supported on Hydrogenated TiO2 Nanotube Array as OER Electro-Catalyst for Water Electrolysis. Int J Hydrogen Energy 2017, 42, 3572–3578. [Google Scholar] [CrossRef]
- Lačnjevac, U.; Vasilić, R.; Dobrota, A.; Đurđić, S.; Tomanec, O.; Zbořil, R.; Mohajernia, S.; Nguyen, N.T.; Skorodumova, N.; Manojlović, D.; et al. High-Performance Hydrogen Evolution Electrocatalysis Using Proton-Intercalated TiO2nanotube Arrays as Interactive Supports for Ir Nanoparticles. J Mater Chem A Mater 2020, 8, 22773–22790. [Google Scholar] [CrossRef]
- Papaderakis, A.; Mintsouli, I.; Georgieva, J.; Sotiropoulos, S. Electrocatalysts Prepared by Galvanic Replacement. Catalysts 2017, 7. [Google Scholar] [CrossRef]
- Touni, A.; Papaderakis, A.; Karfaridis, D.; Vourlias, G.; Sotiropoulos, S. Oxygen Evolution Reaction at IrO2/Ir(Ni) Film Electrodes Prepared by Galvanic Replacement and Anodization: Effect of Precursor Ni Film Thickness. Molecules 2019, 24. [Google Scholar] [CrossRef]
- Papaderakis, A.; Pliatsikas, N.; Patsalas, P.; Tsiplakides, D.; Balomenou, S.; Touni, A.; Sotiropoulos, S. Hydrogen Evolution at Ir-Ni Bimetallic Deposits Prepared by Galvanic Replacement. Journal of Electroanalytical Chemistry 2018, 808, 21–27. [Google Scholar] [CrossRef]
- Papaderakis, A.; Pliatsikas, N.; Prochaska, C.; Vourlias, G.; Patsalas, P.; Tsiplakides, D.; Balomenou, S.; Sotiropoulos, S. Oxygen Evolution at IrO2 Shell-Ir-Ni Core Electrodes Prepared by Galvanic Replacement. Journal of Physical Chemistry C 2016, 120, 19995–20005. [Google Scholar] [CrossRef]
- Papaderakis, A.; Pliatsikas, N.; Prochaska, C.; Papazisi, K.M.; Balomenou, S.P.; Tsiplakides, D.; Patsalas, P.; Sotiropoulos, S. Ternary Pt-Ru-Ni Catalytic Layers for Methanol Electrooxidation Prepared by Electrodeposition and Galvanic Replacement. Front Chem 2014, 2. [Google Scholar] [CrossRef]
- Papaderakis, A.; Matouli, I.; Spyridou, O.N.; Grammenos, A.O.; Banti, A.; Touni, A.; Pliatsikas, N.; Patsalas, P.; Sotiropoulos, S. Ternary IrO2-Pt-Ni Deposits Prepared by Galvanic Replacement as Bifunctional Oxygen Catalysts. Journal of Electroanalytical Chemistry 2020, 877. [Google Scholar] [CrossRef]
- Getz, M.N.; Chatzitakis, A.; Liu, X.; Carvalho, P.A.; Bjørheim, T.S.; Norby, T. Voids in Walls of Mesoporous TiO2 Anatase Nanotubes by Controlled Formation and Annihilation of Protonated Titanium Vacancies. Mater Chem Phys 2020, 239. [Google Scholar] [CrossRef]
- Yan, S.; Chen, Y.; Wang, Z.; Han, A.; Shan, Z.; Yang, X.; Zhu, X. Essential Distinction between One-Step Anodization and Two-Step Anodization of Ti. Mater Res Bull 2017, 95, 444–450. [Google Scholar] [CrossRef]
- Zeng, H.; Li, C.; Dan, Y.; Lu, Y.; Sun, W.; Zhang, S.; Song, Y. A Comparative Study of Two-Step Anodization with One-Step Anodization at Constant Voltage. Nanotechnology 2023, 34. [Google Scholar] [CrossRef]
- Kowalski, D.; Kim, D.; Schmuki, P. TiO2 Nanotubes, Nanochannels and Mesosponge: Self-Organized Formation and Applications. Nano Today 2013, 8, 235–264. [Google Scholar] [CrossRef]
- Valota, A.; LeClere, D.J.; Skeldon, P.; Curioni, M.; Hashimoto, T.; Berger, S.; Kunze, J.; Schmuki, P.; Thompson, G.E. Influence of Water Content on Nanotubular Anodic Titania Formed in Fluoride/Glycerol Electrolytes. Electrochim Acta 2009, 54, 4321–4327. [Google Scholar] [CrossRef]
- Chatzitakis, A.; Papaderakis; Karanasios, N. ; Georgieva, J.; Pavlidou, E.; Litsardakis, G.; Poulios, I.; Sotiropoulos, S. Comparison of the Photoelectrochemical Performance of Particulate and Nanotube TiO2 Photoanodes. Catal Today 2017, 280, 14–20. [Google Scholar] [CrossRef]
- Pfeifer, V.; Jones, T.E.; Velasco Vélez, J.J.; Massué, C.; Arrigo, R.; Teschner, D.; Girgsdies, F.; Scherzer, M.; Greiner, M.T.; Allan, J.; et al. The Electronic Structure of Iridium and Its Oxides. Surface and Interface Analysis 2016, 48, 261–273. [Google Scholar] [CrossRef]
- Freakley, S.J.; Ruiz-Esquius, J.; Morgan, D.J. The X-Ray Photoelectron Spectra of Ir, IrO2 and IrCl3 Revisited. Surface and Interface Analysis 2017, 49, 794–799. [Google Scholar] [CrossRef]
- Tegou, A.; Armyanov, S.; Valova, E.; Steenhaut, O.; Hubin, A.; Kokkinidis, G.; Sotiropoulos, S. Mixed Platinum-Gold Electrocatalysts for Borohydride Oxidation Prepared by the Galvanic Replacement of Nickel Deposits. Journal of Electroanalytical Chemistry 2009, 634, 104–110. [Google Scholar] [CrossRef]
- Mozota, J.; Conway, B.E. Modification of Apparent Electrocatalysis for Anodic Chlorine Evolution on Electrochemically Conditioned Oxide Films at Iridium Anodes. J Electrochem Soc 1981, 128, 2142–2149. [Google Scholar] [CrossRef]
- Mozota, J.; Conway, B.E. Surface and Bulk Processes at Oxidized Iridium Electrodes—I. Monolayer Stage and Transition to Reversible Multilayer Oxide Film Behaviour. Electrochim Acta 1983, 28, 1–8. [Google Scholar] [CrossRef]
- Birss, V.; Myers, R.; Angerstein-Kozlowska, H.; Conway, B.E. Electron Microscopy Study of Formation of Thick Oxide Films on Ir and Ru Electrodes. J Electrochem Soc 1984, 131, 1502–1510. [Google Scholar] [CrossRef]
- Michell, D.; Rand, D.A.J.; Woods, R. Analysis of the Anodic Oxygen Layer on Iridium by X-Ray Emission, Electron Diffraction and Electron Microscopy. J Electroanal Chem Interfacial Electrochem 1977, 84, 117–126. [Google Scholar] [CrossRef]
- Conway, B.E.; Mozota, J. Surface and Bulk Processes at Oxidized Iridium Electrodes—II. Conductivity-Switched Behaviour of Thick Oxide Films. Electrochim Acta 1983, 28, 9–16. [Google Scholar] [CrossRef]
- Juodkazyte, J.; Šebeka, B.; Valsiunas, I.; Juodkazis, K. Iridium Anodic Oxidation to Ir(III) and Ir(IV) Hydrous Oxides. Electroanalysis 2005, 17, 947–952. [Google Scholar] [CrossRef]
- Kötz, R.; Neff, H.; Stucki, S. Anodic Iridium Oxide Films: XPS-Studies of Oxidation State Changes and O2-Evolution. J Electrochem Soc 1984, 131, 72–77. [Google Scholar] [CrossRef]
- Spöri, C.; Briois, P.; Nong, H.N.; Reier, T.; Billard, A.; Kühl, S.; Teschner, D.; Strasser, P. Experimental Activity Descriptors for Iridium-Based Catalysts for the Electrochemical Oxygen Evolution Reaction (OER). ACS Catal 2019, 9, 6653–6663. [Google Scholar] [CrossRef]
- Papaderakis, A.; Tsiplakides, D.; Balomenou, S.; Sotiropoulos, S. Electrochemical Impedance Studies of IrO2 Catalysts for Oxygen Evolution. Journal of Electroanalytical Chemistry 2015, 757, 216–224. [Google Scholar] [CrossRef]
- Reier, T.; Pawolek, Z.; Cherevko, S.; Bruns, M.; Jones, T.; Teschner, D.; Selve, S.; Bergmann, A.; Nong, H.N.; Schlögl, R.; et al. Molecular Insight in Structure and Activity of Highly Efficient, Low-Ir Ir-Ni Oxide Catalysts for Electrochemical Water Splitting (OER). J Am Chem Soc 2015, 137, 13031–13040. [Google Scholar] [CrossRef]
- Hu, J.M.; Zhang, J.Q.; Cao, C.N. Oxygen Evolution Reaction on IrO2-Based DSA® Type Electrodes: Kinetics Analysis of Tafel Lines and EIS. Int J Hydrogen Energy 2004, 29, 791–797. [Google Scholar] [CrossRef]
- De Oliveira-Sousa, A.; Da Silva, M.A.S.; Machado, S.A.S.; Avaca, L.A.; De Lima-Neto, P. Influence of the Preparation Method on the Morphological and Electrochemical Properties of Ti/IrO 2-Coated Electrodes; 2000; Vol. 45.
- Hsu, C.H.; Mansfeld, F. Technical Note: Concerning the Conversion of the Constant Phase Element Parameter Y0 into a Capacitance. CORROSION 2001, 57, 747–748. [Google Scholar] [CrossRef]
- Touni, A.; Liu, X.; Kang, X.; Carvalho, P.A.; Diplas, S.; Both, K.G.; Sotiropoulos, S.; Chatzitakis, A. Galvanic Deposition of Pt Nanoparticles on Black TiO2 Nanotubes for Hydrogen Evolving Cathodes. ChemSusChem 2021, 14, 4993–5003. [Google Scholar] [CrossRef] [PubMed]
- Lončar, A.; Escalera-López, D.; Cherevko, S.; Hodnik, N. Inter-Relationships between Oxygen Evolution and Iridium Dissolution Mechanisms. Angewandte Chemie - International Edition 2022, 61. [Google Scholar] [CrossRef]
- Cao, H.; Zhang, L.; Chen, M. Characteristics of Anodic TiO2 Nanotube Arrays Mediated IrO2 Active Anode in the Oxygen Evolution Reaction. Int J Electrochem Sci 2022, 17, 220461. [Google Scholar] [CrossRef]
- Shi, Y.; Lu, Z.; Guo, L.; Wang, Z.; Guo, C.; Tan, H.; Yan, C. Fabrication of IrO2 Decorated Vertical Aligned Self-Doped TiO2 Nanotube Arrays for Oxygen Evolution in Water Electrolysis. Int J Hydrogen Energy 2018, 43, 9133–9143. [Google Scholar] [CrossRef]
- Lu, Z.X.; Shi, Y.; Yan, C.F.; Guo, C.Q.; Wang, Z. Da Investigation on IrO2 Supported on Hydrogenated TiO2 Nanotube Array as OER Electro-Catalyst for Water Electrolysis. Int J Hydrogen Energy 2017, 42, 3572–3578. [Google Scholar] [CrossRef]
- Schlicht, S.; Büttner, P.; Bachmann, J. Highly Active Ir/TiO2 Electrodes for the Oxygen Evolution Reaction Using Atomic Layer Deposition on Ordered Porous Substrates. ACS Appl Energy Mater 2019, 2, 2344–2349. [Google Scholar] [CrossRef]
- Suhadolnik, L.; Bele, M.; Čekada, M.; Jovanovič, P.; Maselj, N.; Lončar, A.; Dražić, G.; Šala, M.; Hodnik, N.; Kovač, J.; et al. Nanotubular TiOxNy-Supported Ir Single Atoms and Clusters as Thin-Film Electrocatalysts for Oxygen Evolution in Acid Media. Chemistry of Materials 2023, 35, 2612–2623. [Google Scholar] [CrossRef]
- Chatzitakis, A.; Grandcolas, M.; Xu, K.; Mei, S.; Yang, J.; Jensen, I.J.T.; Simon, C.; Norby, T. Assessing the Photoelectrochemical Properties of C, N, F Codoped TiO2 Nanotubes of Different Lengths. Catal Today 2017, 287, 161–168. [Google Scholar] [CrossRef]
- Liu, X.; Risbakk, S.; Almeida Carvalho, P.; Yang, M.; Hoff Backe, P.; Bjørås, M.; Norby, T.; Chatzitakis, A. Immobilization of FeFe-Hydrogenase on Black TiO2 Nanotubes as Biocathodes for the Hydrogen Evolution Reaction. Electrochem commun 2022, 135, 107221. [Google Scholar] [CrossRef]
- Liang, F.; Zhang, J.; Zheng, L.; Tsang, C.K.; Li, H.; Shu, S.; Cheng, H.; Li, Y.Y. Selective Electrodeposition of Ni into the Intertubular Voids of Anodic TiO2 Nanotubes for Improved Photocatalytic Properties. J Mater Res 2013, 28, 405–410. [Google Scholar] [CrossRef]
- Xu, B.; He, Y.; Zhang, Y.; Ma, Z.; Zhang, Y.; Song, W. In Situ Growth of Tunable Gold Nanoparticles by Titania Nanotubes Templated Electrodeposition for Improving Osteogenesis through Modulating Macrophages Polarization. ACS Applied Materials & Interfaces 2022, 14, 50520–50533. [Google Scholar] [CrossRef]
- Macak, J.; Gong, B.; Hueppe, M.; Schmuki, P. Filling of TiO2 Nanotubes by Self-Doping and Electrodeposition. Adv. Mater. 2007, 19, 3027–3031. [Google Scholar] [CrossRef]

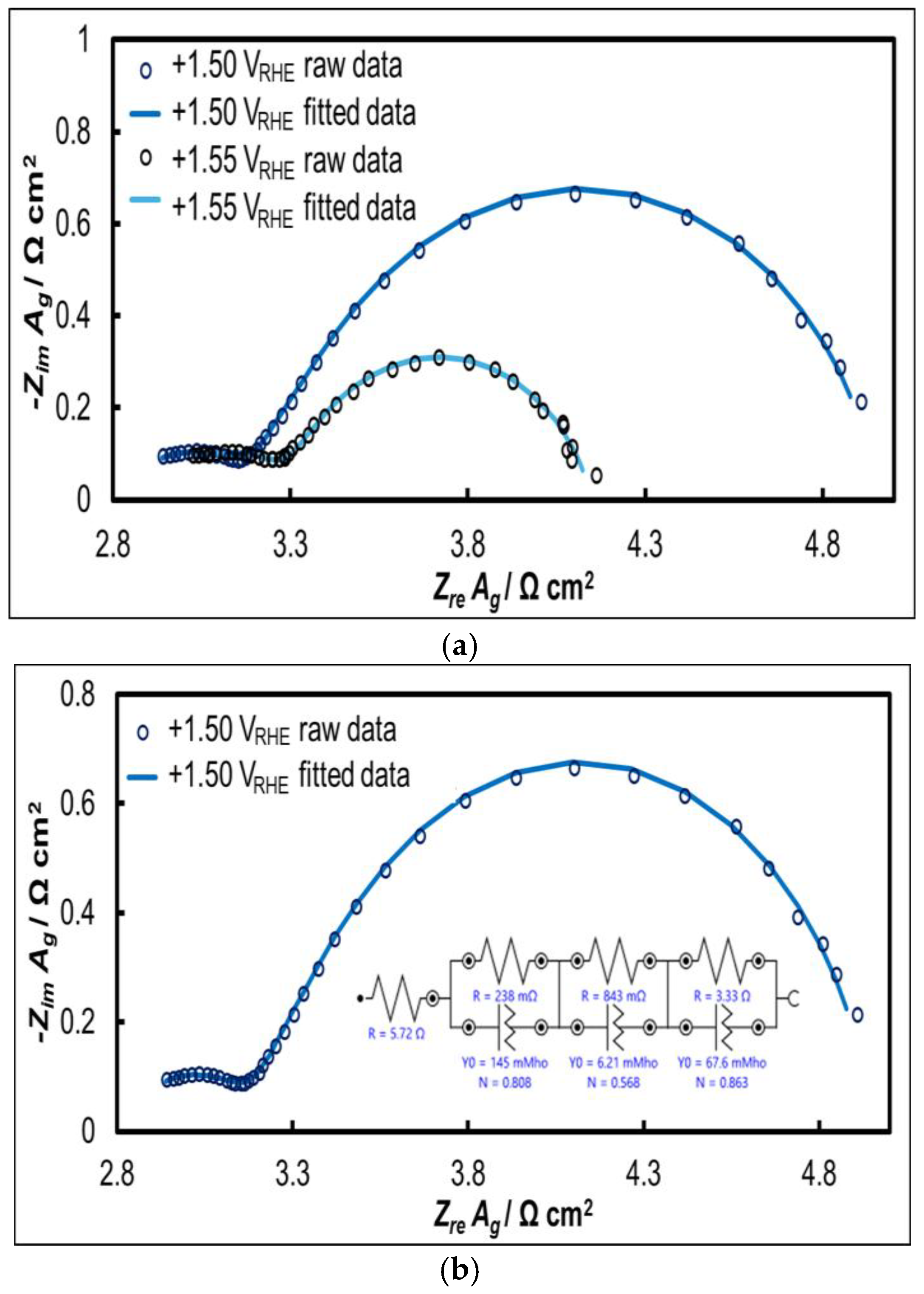
| Parameters | +1.50 VRHE | +1.55 VRHE | |
|---|---|---|---|
| Rsolution (Rsol) | rs/ Ω cm2 | 2.800 | 2.714 |
| Rnanotubes (Rt) | rt/ Ω cm2 | 0.117 | 0.290 |
| CPEnanotubes(Qt) | yt/ S sn cm-2 | 0.296 | 0.001 |
| nt | 0.808 | 0.635 | |
| Rpores (Rp) | rp/ Ω cm2 | 0.413 | 0.298 |
| CPEpores(Qp) | yp/ S sn cm-2 | 0.013 | 0.012 |
| np | 0.568 | 0.643 | |
| Rchargetransfer (Rct) | rct/ Ω cm2 | 1.632 | 0.851 |
| CPEdouble layer (Qdl) | ydl/ S sn cm-2 | 0.138 | 0.144 |
| ndl | 0.863 | 0.792 | |
| χ2 | 0.00019 1 | 0.00033 1 | |
| Cdl/ mF cm-2 | 108.8 | 83.1 | |
| RctCdl/ Ω F (s) | 0.178 | 0.071 | |
| Catalyst | Support |
η @ 10 mA cm-2 (mV) |
Jgeom @ 300 mV (mA cm-2) |
Jmass @ 300 mV (A gIr-1) |
Ref. |
|---|---|---|---|---|---|
| IrOx | bTNTs | 240 | 70 | 258 | This work |
| IrO2 | TNTs | 360 | 3.9 | – | [84] |
| IrO2 | Self-doped TNTs |
– | – | 116 | [85] |
| IrO2 | Hydrogenated TNTs |
– | 0.68 | 29.5 | [86] |
| Ir | TNTs | 240 | 23 | 143 | [87] |
| Ir | TiOxNy NTs | – | – | 286 | [88] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





