Submitted:
29 April 2025
Posted:
29 April 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results and Discussion
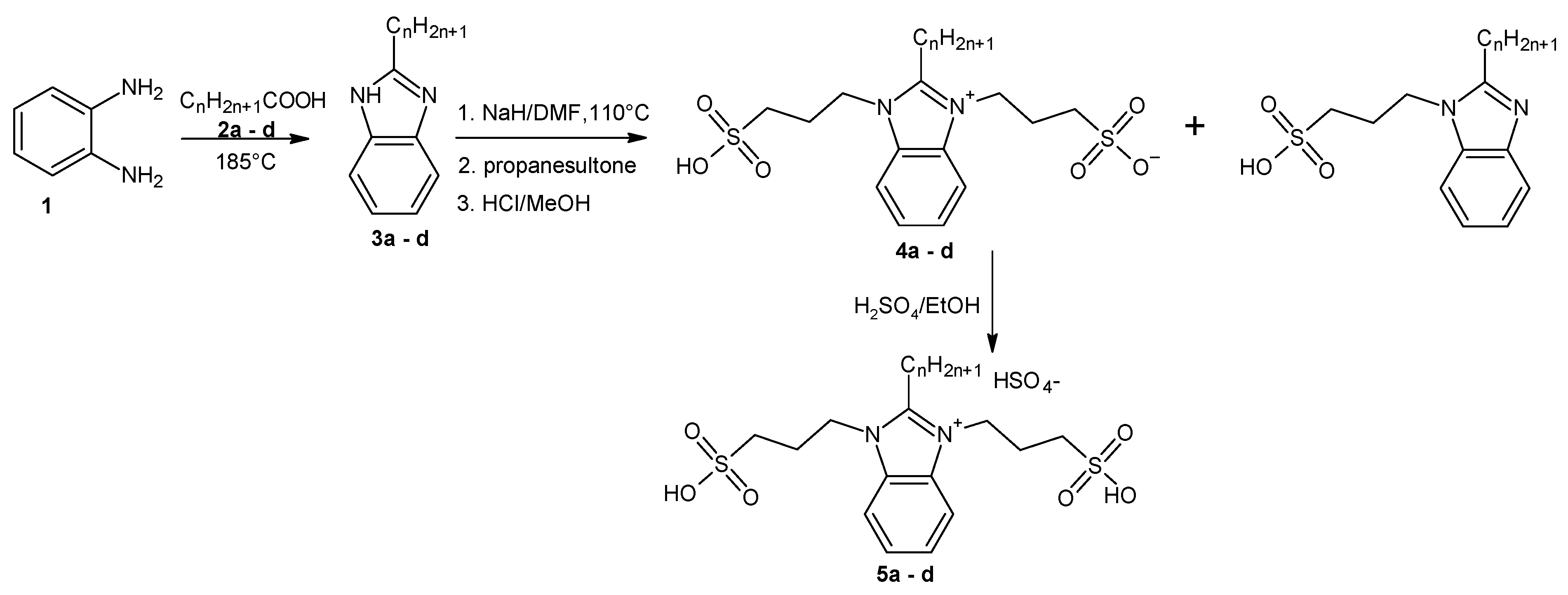
2.1. Synthesis and Characterization of ASBIL Catalysts 5a - d
2.2. Catalytic Performance of the Prepared ASBILs
2.2.1. Development and Validation of the NMR Analysis Methodology
2.2.2. Optimization of Catalyst Dosage and Reaction Conditions
| Amount of catalyst C15-ASBIL [mol% based on oleic acid mass] |
Conversion | |||||
|---|---|---|---|---|---|---|
| 1 h | 2 h | 3 h | 4 h | 5 h | 6 h | |
| 10 | 0.88 | 0.92 | 0.94 | 0.94 | 0.97 | 0.97 |
| 15 20 |
0.945 0.975 |
0.95 0.97 |
0.955 0.97 |
0.96 0.97 |
0.96 0.97 |
0.96 0.97 |
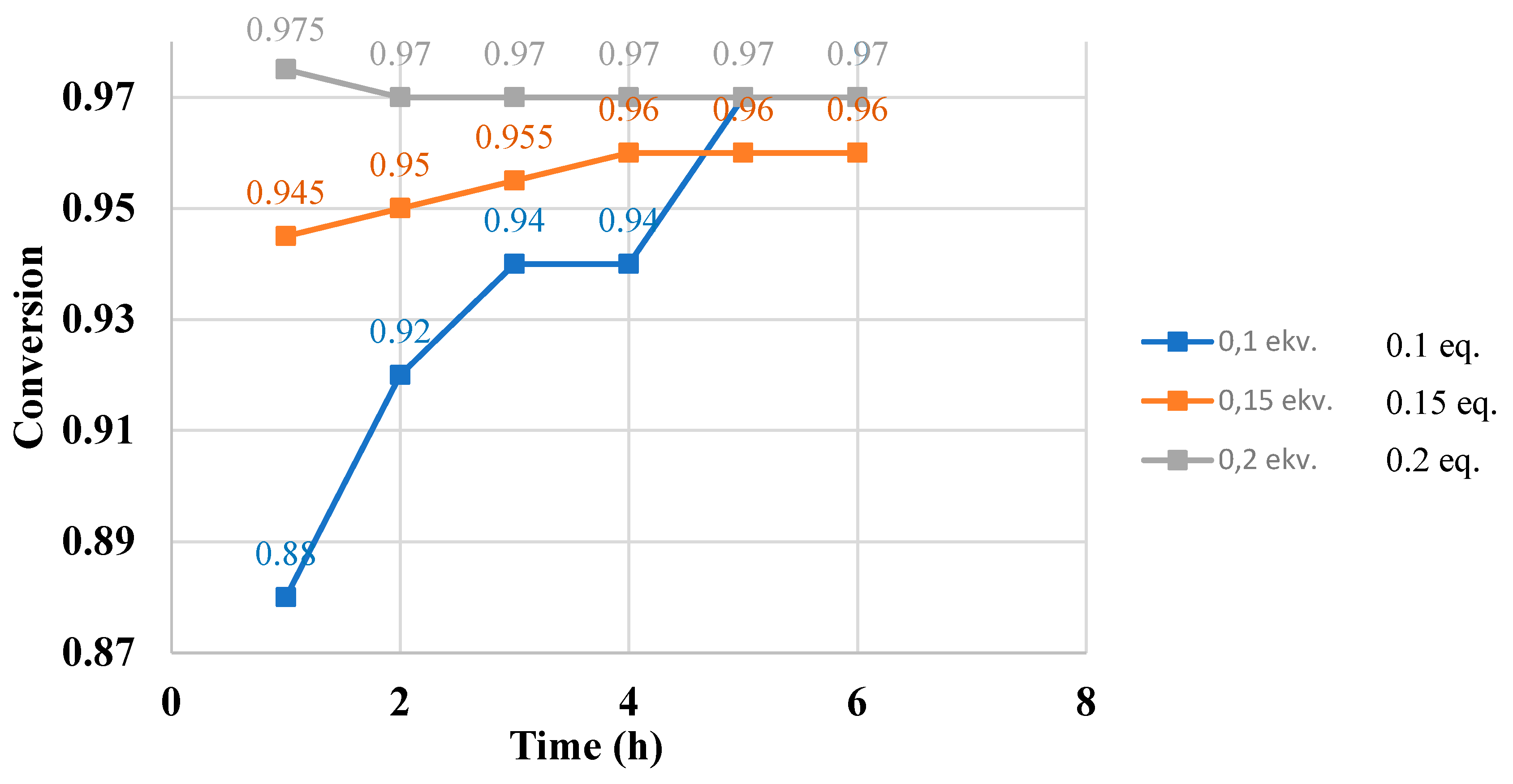
2.2.3. Catalytic Activity of the Prepared ASBILs
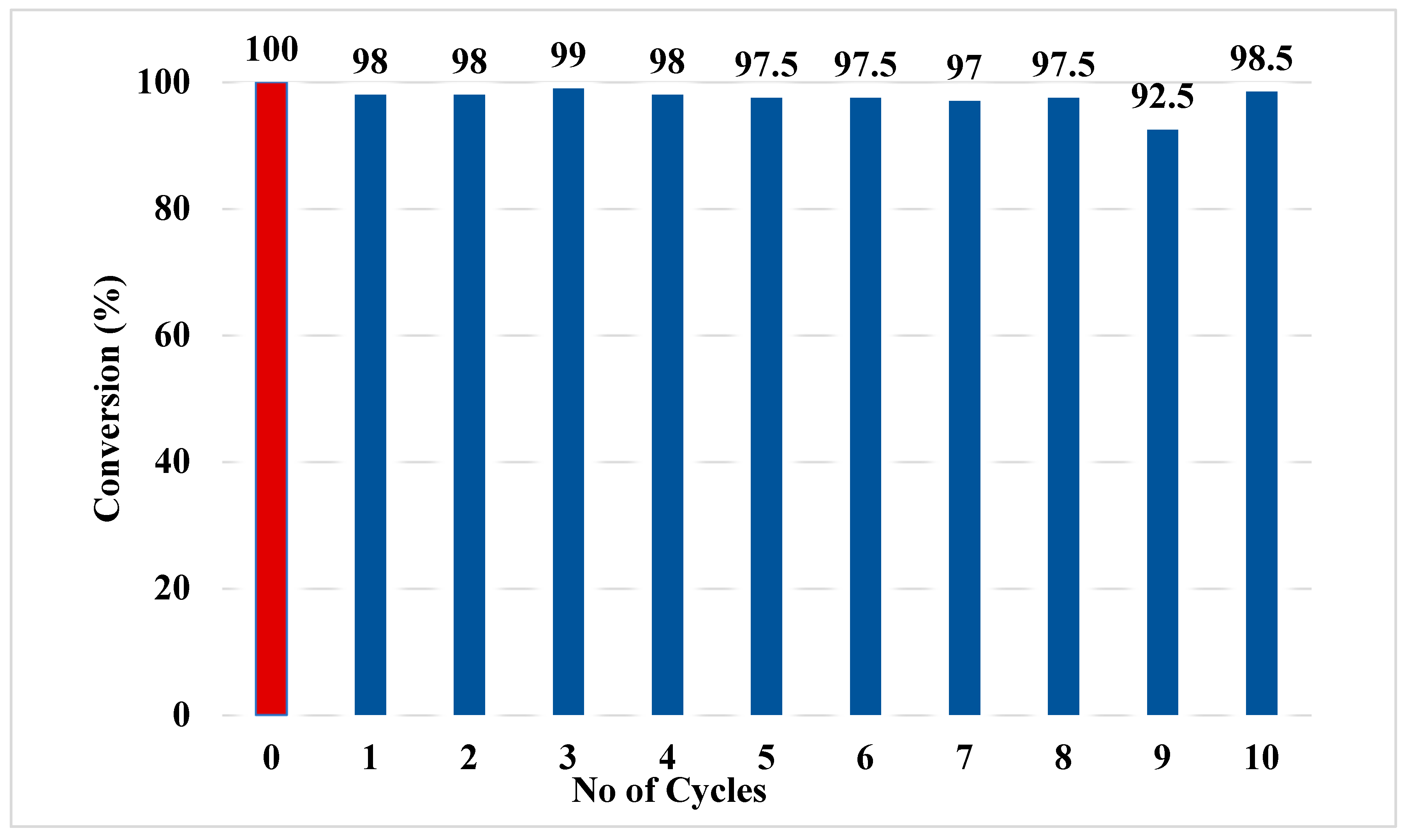
2.2.4. Catalyst Recyclability
3. Materials and Methods
3.1. Materials and Reagents
3.2. Characterization Methods
3.3. Synthesis of the Catalysts
- 2-Alkyl-1H-benzo[d]imidazoles 3a - d
- 2-Nonyl-1H-benzo[d]imidazole 3a
- 2-Undecyl-1H-benzo[d]imidazole 3b
- 2-Tridecyl-1H-benzo[d]imidazole 3c
- 2-Heptadecyl-1H-benzo[d]imidazole 3d
- 3-(2-Alkyl-1-(3-sulfopropyl)-1H-benzo[d]imidazol-3-ium-3-yl)propane-1-sulfonates 4a - d
- 3-(2-Nonyl-1-(3-sulfopropyl)-1H-benzo[d]imidazol-3-ium-3-yl)propane-1-sulfonate 4a
- 3-(2-Undecyl-1-(3-sulfopropyl)-1H-benzo[d]imidazol-3-ium-3-yl)propane-1-sulfonate 4b
- 3-(2-Tridecyl-1-(3-sulfopropyl)-1H-benzo[d]imidazol-3-ium-3-yl)propane-1-sulfonate 4c
- 3-(2-Heptadecyl-1-(3-sulfopropyl)-1H-benzo[d]imidazol-3-ium-3-yl)propane-1-sulfonate 4d
- 2-Alkyl-1,3-di(3-sulfopropyl)-1H-benzo[d]imidazol-3-ium hydrogen sulfates 5a - d
- 2-Nonyl-1,3-di(3-sulfopropyl)-1H-benzo[d]imidazol-3-ium hydrogen sulfate 5a
- 2-Undecyl-1,3-di(3-sulfopropyl)-1H-benzo[d]imidazol-3-ium hydrogen sulfate 5b
- 2-Tridecyl-1,3-di(3-sulfopropyl)-1H-benzo[d]imidazol-3-ium hydrogen sulfate 5c
- 2-Heptadecyl-1,3-di(3-sulfopropyl)-1H-benzo[d]imidazol-3-ium hydrogen sulfate 5d
3.4. Evaluation of Catalytic Performance
3.4.1. General Procedure for the Esterification of Fatty Acids with Ethanol and Sampling
3.4.2. Optimization of Catalyst Quantity and Reaction Time
3.4.3. Evaluation of Catalytic Performance
3.4.4. Recycling of the Catalyst 5b and Evaluation of Its Stability
3.4.5. Determination of the Conversion by 1H NMR
3.4.6. NMR Sensitivity Test
4. Conclusions
Supplementary Materials
Author Contributions
Data Availability Statement
Conflicts of Interest
References
- S. Prasad, K.K. Yadav, S. Kumar, P. Pandita, J.K. Bhutto, M.A. Alreshidi, B. Ravindran, Z.M. Yaseen, S.M. Osman, M.M.S. Cabral-Pinto, Review on biofuel production: Sustainable development scenario, environment, and climate change perspectives − A sustainable approach, Journal of Environmental Chemical Engineering 12 (2024) 111996. [CrossRef]
- K. Srikumar, Y.H. Tan, J. Kansedo, I.S. Tan, N.M. Mubarak, M.L. Ibrahim, P.N.Y. Yek, H.C.Y. Foo, R.R. Karri, M. Khalid, A review on the environmental life cycle assessment of biodiesel production: Selection of catalyst and oil feedstock, Biomass and Bioenergy 185 (2024) 107239. [CrossRef]
- S.M. Farouk, A.M. Tayeb, S.M.S. Abdel-Hamid, R.M. Osman, Recent advances in transesterification for sustainable biodiesel production, challenges, and prospects: a comprehensive review, Environ Sci Pollut Res 31 (2024) 12722–12747. [CrossRef]
- S. Pandey, I. Narayanan, R. Selvaraj, T. Varadavenkatesan, R. Vinayagam, Biodiesel production from microalgae: A comprehensive review on influential factors, transesterification processes, and challenges, Fuel 367 (2024) 131547. [CrossRef]
- J. Julkipli, S. Babel, A.M. Bilyaminu, E.R. Rene, Hydrogen and biodiesel production from food waste: a review, Environ Chem Lett 22 (2024) 585–607. [CrossRef]
- T.M.I. Mahlia, Z.A.H.S. Syazmi, M. Mofijur, A.E.P. Abas, M.R. Bilad, H.C. Ong, A.S. Silitonga, Patent landscape review on biodiesel production: Technology updates, Renewable and Sustainable Energy Reviews 118 (2020) 109526. [CrossRef]
- N. Anil, P.K. Rao, A. Sarkar, J. Kubavat, S. Vadivel, N.R. Manwar, B. Paul, Advancements in sustainable biodiesel production: A comprehensive review of bio-waste derived catalysts, Energy Conversion and Management 318 (2024) 118884. [CrossRef]
- V.G. Nguyen, P. Sharma, M. Dzida, V.H. Bui, H.S. Le, A.S. El-Shafay, H.C. Le, D.T.N. Le, V.D. Tran, A Review on Metal–Organic Framework as a Promising Catalyst for Biodiesel Production, Energy Fuels 38 (2024) 2654–2689. [CrossRef]
- Y. Zhang, S. Sun, A review on biodiesel production using basic ionic liquids as catalysts, Industrial Crops and Products 202 (2023) 117099. [CrossRef]
- R. D., N. Ghosh, S. Lalthazuala Rokhum, G. Halder, Current progress and future outlooks of microwave-irradiated biodiesel production: A holistic review, Chemical Engineering Journal 482 (2024) 149033. [CrossRef]
- P.R. Costa Neto, M.S. Balparda Caro, L.M. Mazzuco, M. da G. Nascimento, Quantification of soybean oil ethanolysis with1 H NMR, J Americ Oil Chem Soc 81 (2004) 1111–1114. [CrossRef]
- S.K. Bharti, R. Roy, Quantitative 1H NMR spectroscopy, TrAC Trends in Analytical Chemistry 35 (2012) 5–26. [CrossRef]
- G.F. Pauli, B.U. Jaki, D.C. Lankin, Quantitative1 H NMR: Development and Potential of a Method for Natural Products Analysis, J. Nat. Prod. 68 (2005) 133–149. [CrossRef]
- G.F. Pauli, T. Gödecke, B.U. Jaki, D.C. Lankin, Quantitative1 H NMR. Development and Potential of an Analytical Method: An Update, J. Nat. Prod. 75 (2012) 834–851. [CrossRef]
- G.F. Pauli, S.-N. Chen, C. Simmler, D.C. Lankin, T. Gödecke, B.U. Jaki, J.B. Friesen, J.B. McAlpine, J.G. Napolitano, Importance of Purity Evaluation and the Potential of Quantitative1 H NMR as a Purity Assay: Miniperspective, J. Med. Chem. 57 (2014) 9220–9231. [CrossRef]
- U. Holzgrabe, Quantitative NMR spectroscopy in pharmaceutical applications, Progress in Nuclear Magnetic Resonance Spectroscopy 57 (2010) 229–240. [CrossRef]
- A. Barison, C.W. Pereira da Silva, F.R. Campos, F. Simonelli, C.A. Lenz, A.G. Ferreira, A simple methodology for the determination of fatty acid composition in edible oils through1 H NMR spectroscopy, Magnetic Reson in Chemistry 48 (2010) 642–650. [CrossRef]
- J.K. Satyarthi, D. Srinivas, P. Ratnasamy, Estimation of Free Fatty Acid Content in Oils, Fats, and Biodiesel by1 H NMR Spectroscopy, Energy Fuels 23 (2009) 2273–2277. [CrossRef]
- V.M. Mello, F.C.C. Oliveira, W.G. Fraga, C.J. do Nascimento, P.A.Z. Suarez, Determination of the content of fatty acid methyl esters (FAME) in biodiesel samples obtained by esterification using1 H-NMR spectroscopy, Magnetic Reson in Chemistry 46 (2008) 1051–1054. [CrossRef]
- G. Knothe, J.A. Kenar, Determination of the fatty acid profile by1 H-NMR spectroscopy, Euro J Lipid Sci & Tech 106 (2004) 88–96. [CrossRef]
- C. Siciliano, E. Belsito, R. De Marco, M.L. Di Gioia, A. Leggio, A. Liguori, Quantitative determination of fatty acid chain composition in pork meat products by high resolution 1H NMR spectroscopy, Food Chemistry 136 (2013) 546–554. [CrossRef]
- P. Siudem, A. Zielińska, K. Paradowska, Application of 1H NMR in the study of fatty acids composition of vegetable oils, Journal of Pharmaceutical and Biomedical Analysis 212 (2022) 114658. [CrossRef]
- M. ter Horst, S. Urbin, R. Burton, C. McMillan, Using proton nuclear magnetic resonance as a rapid response research tool for methyl ester characterization in biodiesel, Lipid Technology 21 (2009) 39–41. [CrossRef]
- G.P. Mambrini, C. Ribeiro, L.A. Colnago, Nuclear magnetic resonance spectroscopic analysis of ethyl ester yield in the transesterification of vegetable oil: an accurate method for a truly quantitative analysis, Magnetic Reson in Chemistry 50 (2012) 1–4. [CrossRef]
- I.G. Rosset, M.C.H. Tavares, E.M. Assaf, A.L.M. Porto, Catalytic ethanolysis of soybean oil with immobilized lipase from Candida antarctica and 1H NMR and GC quantification of the ethyl esters (biodiesel) produced, Applied Catalysis A: General 392 (2011) 136–142. [CrossRef]
- R. Guzatto, D. Defferrari, Q.B. Reiznautt, Í.R. Cadore, D. Samios, Transesterification double step process modification for ethyl ester biodiesel production from vegetable and waste oils, Fuel 92 (2012) 197–203. [CrossRef]
- R.C.M. dos Santos, P.C. Gurgel, N.S. Pereira, R.A. Breves, P.R.R. de Matos, L.P. Silva, M.J.A. Sales, R. de V.V. Lopes, Ethyl esters obtained from pequi and macaúba oils by transesterification with homogeneous acid catalysis, Fuel 259 (2020) 116206. [CrossRef]
- F. Faraguna, M. Racar, Z. Glasovac, A. Jukić, Correlation Method for Conversion Determination of Biodiesel Obtained from Different Alcohols by1 H NMR Spectroscopy, Energy Fuels 31 (2017) 3943–3948. [CrossRef]
- M. dos P.M. de Jesus, L.N. de Melo, J.P.V. da Silva, A.C. Crispim, I.M. Figueiredo, J.H. Bortoluzzi, S.M.P. Meneghetti, Evaluation of Proton Nuclear Magnetic Resonance Spectroscopy for Determining the Yield of Fatty Acid Ethyl Esters Obtained by Transesterification, Energy Fuels 29 (2015) 7343–7349. [CrossRef]
- K.I. Doudin, Quantitative and qualitative analysis of biodiesel by NMR spectroscopic methods, Fuel 284 (2021) 119114. [CrossRef]
- G.G. Shimamoto, L.F. Bianchessi, M. Tubino, Alternative method to quantify biodiesel and vegetable oil in diesel-biodiesel blends through 1 H NMR spectroscopy, Talanta 168 (2017) 121–125. [CrossRef]
- V.M. Mello, F.C.C. Oliveira, W.G. Fraga, C.J. do Nascimento, P.A.Z. Suarez, Determination of the content of fatty acid methyl esters (FAME) in biodiesel samples obtained by esterification using1 H-NMR spectroscopy, Magnetic Reson in Chemistry 46 (2008) 1051–1054. [CrossRef]
- M. Morgenstern, J. Cline, S. Meyer, S. Cataldo, Determination of the Kinetics of Biodiesel Production Using Proton Nuclear Magnetic Resonance Spectroscopy (1 H NMR), Energy Fuels 20 (2006) 1350–1353. [CrossRef]
- L.A. Anderson, A.K. Franz, Real-Time Monitoring of Transesterification by1 H NMR Spectroscopy: Catalyst Comparison and Improved Calculation for Biodiesel Conversion, Energy Fuels 26 (2012) 6404–6410. [CrossRef]
- G.F. Ghesti, J.L. de Macedo, I.S. Resck, J.A. Dias, S.C.L. Dias, FT-Raman Spectroscopy Quantification of Biodiesel in a Progressive Soybean Oil Transesterification Reaction and Its Correlation with1 H NMR Spectroscopy Methods, Energy Fuels 21 (2007) 2475–2480. [CrossRef]
- T.H. Nguyen Thi, J. Koutecká, P. Kaule, L. Vrtoch, V. Šícha, J. Čermák, Design and Synthesis of New Sulfonic Acid Functionalized Ionic Liquids as Catalysts for Esterification of Fatty Acids with Bioethanol, Molecules 28 (2023) 5231. [CrossRef]
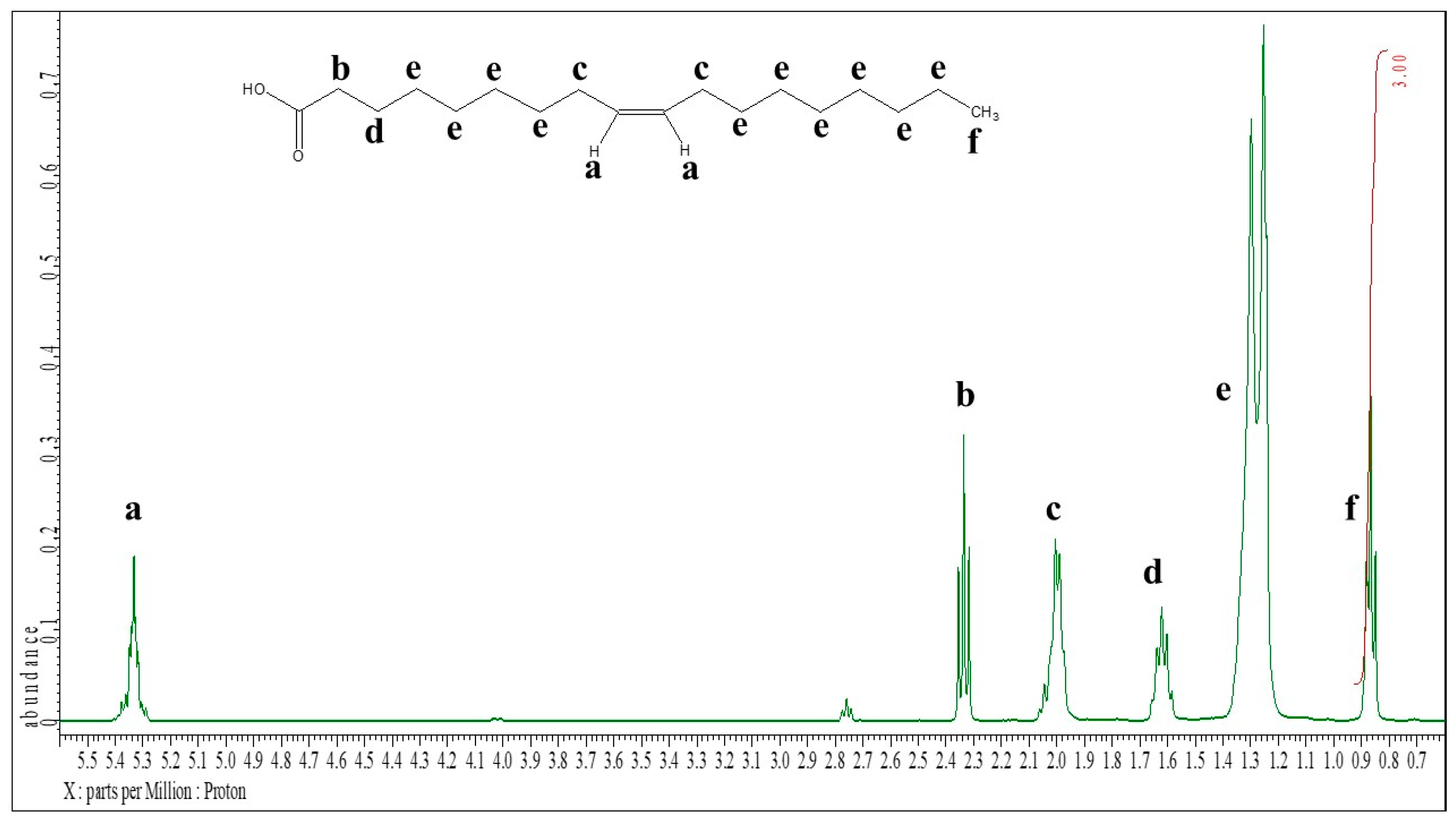
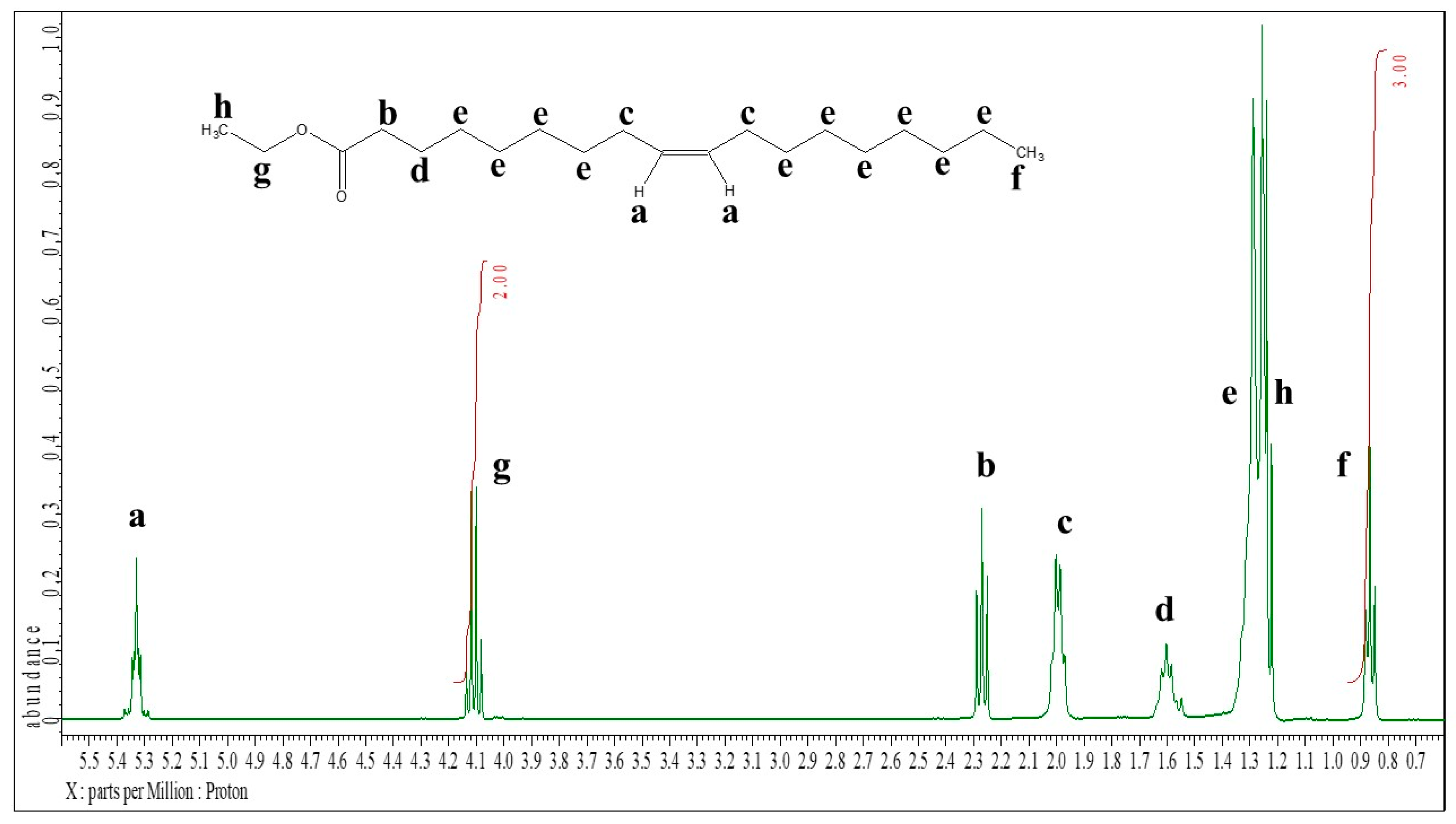
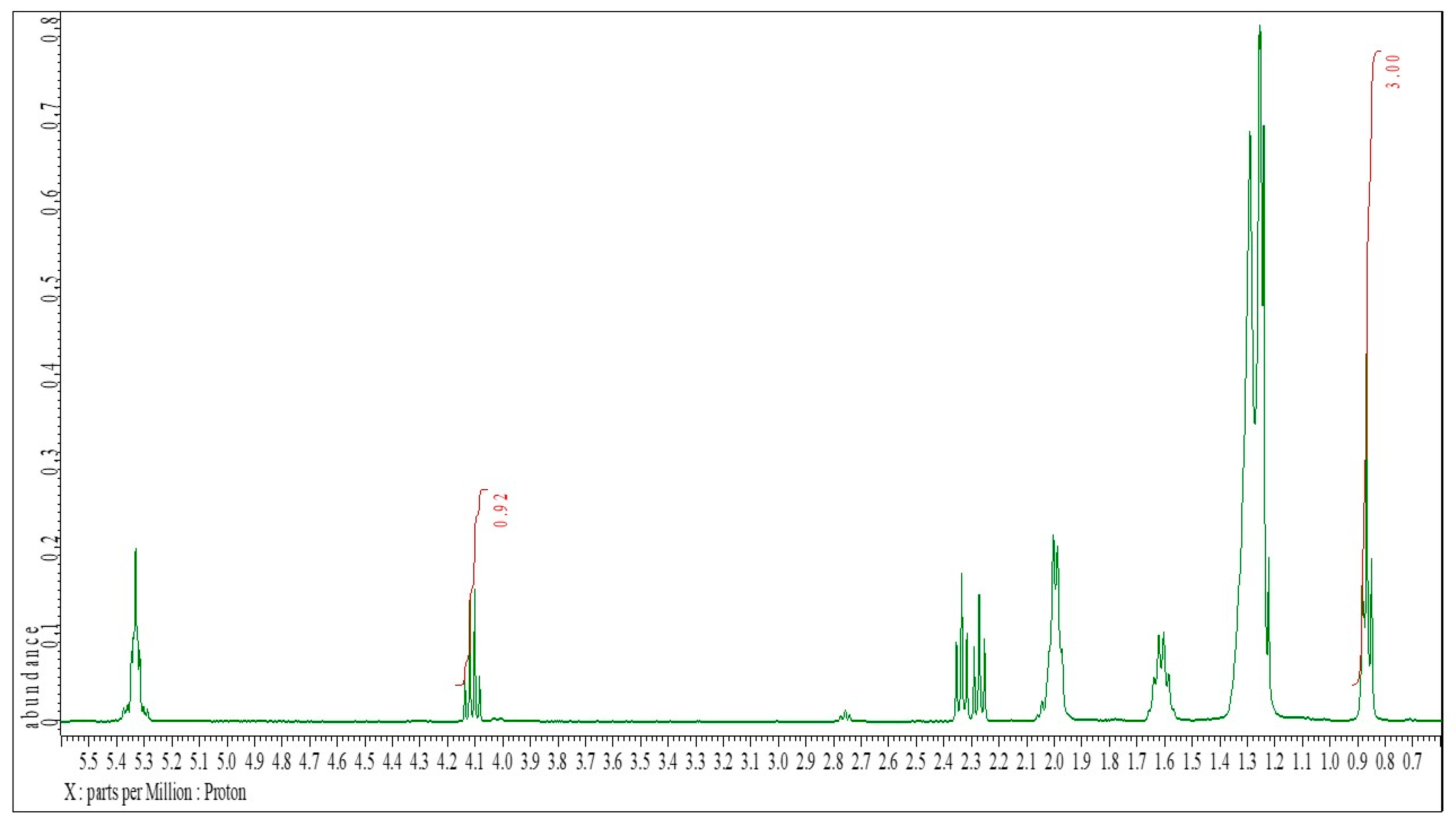
| Sample | n(OA) [mmol] |
n(EO) [mmol] |
inaccuracy of qNMR [%] |
||
|---|---|---|---|---|---|
|
1 2 3 4 5 |
0.03215484516 0.03016903525 0.02016360837 |
0.0000000000 0,008081617983 0,01855782901 |
0.0000000000 0,2112805220 0,4792649825 |
0.000 0,205 0,460 |
0.00 2,97 4.02 |
| 0.009974653801 | 0,03034354703 | 0,7526017135 | 0,740 | 1.67 | |
| 0.00000000000 | 0,03894899193 | 1.0000000000 | 1.000 | 0.00 |
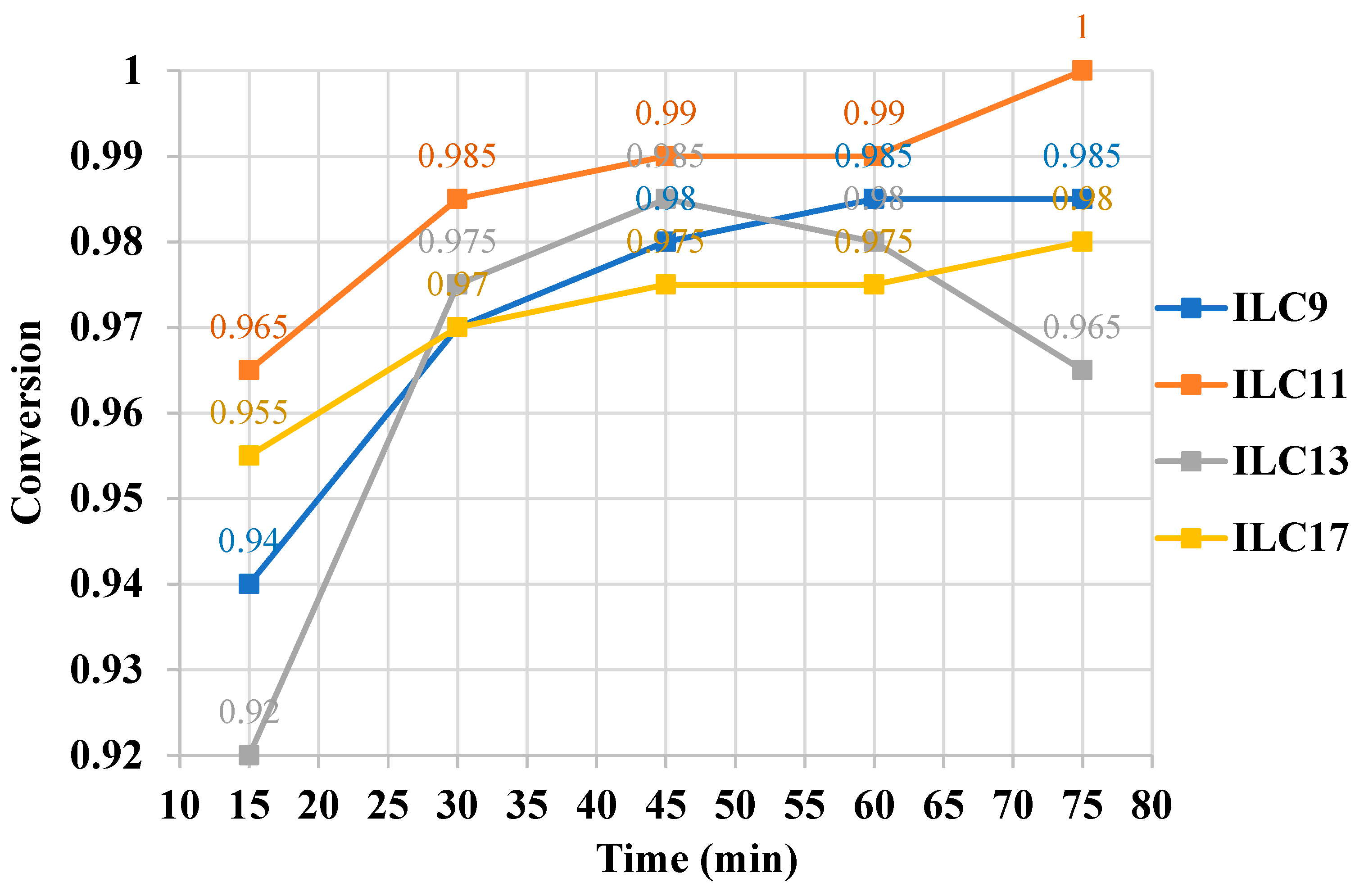
| Catalysts | Conversion | Times required to reach reaction equilibrium [min] |
||||
|---|---|---|---|---|---|---|
| 1 5 min | 30 min | 4 5 min | 60 min | 7 5 min | ||
| 5a (C9-ASBIL) | 0.940 0.965 0.920 0.955 |
0.970 0.985 0.975 0.970 |
0.980 0.990 0.985 0.975 |
0.985 0.990 0.980 0.975 |
0.985 1.000 0.965 0.980 |
60 45 45 75 |
|
5b (C11-ASBIL) 5c (C13-ASBIL) 5d (C17-ASBIL) | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





