1. Introduction
The Eph receptor-ephrin system has fourteen tyrosine kinase receptors (EphA1–A8, A10, and EphB1–B4, B6) and eight ephrin ligands—five GPI-anchored (ephrin-A1 to A5) and three transmembrane types (ephrin-B1 to B3) [
1]. EphA receptors mainly interact with ephrin-As, while EphB receptors bind to ephrin-Bs, but EphA4 can bind all ephrins, and EphB2 can bind ephrin-A5 [
1]. The interaction between Eph receptors and ephrins at intercellular junctions triggers bidirectional signaling: forward and reverse signaling, respectively. This process involves the dimerization and higher-order oligomerization of Eph receptors and ephrins, the tyrosine phosphorylation of both Eph receptors and ephrin-Bs, and the recruitment of cytoplasmic effectors that contain SH2, PDZ, and other signaling domains [
2].
The Eph receptor-ephrin system is essential for embryonic [
3,
4], neural [
5,
6], and vascular development [
7,
8]. Furthermore, a growing number of evidence implicates the critical roles in cancers [
9,
10]. The expression levels of Eph receptors and ephrins are often altered in tumors, increasing or decreasing compared to normal tissues. Therefore, they have dual roles in tumor promotion or suppression. In some tumors, Eph receptors are upregulated during the early stages but become downregulated during the tumor progression, indicating that they may play distinct roles in tumor initiation and progression [
1].
EphA7 was first cloned as a member of Eph homologous kinase, EHK-3, and the expression was restricted to the adult nervous system and more widely expressed in the embryo [
11]. In neural stem cells, the EphA7–ephrin-A5 complex is associated with tumor necrosis factor receptor superfamily member 1A, which resulted in the induction of apoptotic cell death [
12]. EphA7 forward signaling can cause apoptosis in prostate cancer cells through inhibition of AKT-mediated cell survival signaling [
13,
14]. The EphA7 phosphorylation was positively related with ephrin-A5 expression in human prostate tissue, suggesting the tumor suppressive function of EphA7-ephrin-A5 axis in prostate cancer. Moreover, loss of EphA7 is frequently observed in lymphomas [
15].
EphA7 mutations have been suggested to be a driver gene in small-cell lung cancer [
16]. However, detailed molecular mechanism has not been clarified. EphA7 mutations were observed in various tumor types and show a strong association with reduced patient survival [
1]. Furthermore, EphA7 mutation in multiple cancers has been implied as a predictive biomarker for immune checkpoint inhibitors. Among fourteen Eph receptors, EphA7-mutant was enriched in patients responding to immune checkpoint inhibitor therapy [
17].
A study showed that Eph7 bound to the immune inhibitory receptor leukocyte immunoglobin like receptor family B5 (LILRB5), which activated LILRB5 signaling. LILRB5 plays a critical role in supporting immunosuppressive myeloid cells, which are critical obstacles to checkpoint inhibitor therapies [
18].
Although several anti-EphA7 mAbs have been developed for western blotting and immunohistochemistry (IHC) [
14], there is no anti-EphA7 mAb suitable for flow cytometry. We have developed various mAbs against receptor tyrosine kinases such as epidermal growth factor receptor family [
19,
20,
21] and Eph family [
22,
23,
24] using the Cell-Based Immunization and Screening (CBIS) method. The CBIS method includes immunizing antigen-overexpressed cells and flow cytometry-based high-throughput screening. Therefore, mAbs obtained by the CBIS method tend to recognize conformational epitopes and are suitable for flow cytometry. Furthermore, some of these mAbs also apply to western blotting and IHC. This study employed the CBIS method to generate highly versatile anti-EphA7 mAb.
2. Materials and Methods
2.1 Cell Lines and Plasmids
Cell lines, including Chinese hamster ovary (CHO)-K1, LN229 glioblastoma, P3X63Ag8U.1 (P3U1) myeloma, were obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA). The complementary DNA of EphA7 (Catalog No.: RC226293, Accession No.: NM_004440) plus an N-terminal MAP16 tag [
25] and an N-terminal PA16 tag [
26], which are respectively recognized by an anti-MAP16 tag mAb (PMab-1) and an anti-PA16 tag mAb (NZ-1), were subcloned into a pCAG-Ble vector [FUJIFILM Wako Pure Chemical Corporation (Wako), Osaka, Japan]. Afterward, plasmids were transfected into CHO-K1 and LN229 cells using the Neon transfection system (Thermo Fisher Scientific, Inc., Waltham, MA). Stable transfectants [CHO/PA16-EphA7 (CHO/EphA7) and LN229/MAP16-EphA7] were subsequently selected by a cell sorter (SH800, Sony Corp., Tokyo, Japan) using anti-tag mAbs, PMab-1 and NZ-1, respectively. After sorting, cultivation in a medium containing 0.5 mg/mL of Zeocin (InvivoGen, San Diego, CA, USA) was conducted.
Other Eph receptor-expressing CHO-K1 cells (e.g., CHO/EphA2) were established as previously reported [
22].
2.2. Antibodies
An anti-isocitrate dehydrogenase 1 (IDH1) mAb (clone RcMab-1) was developed previously [
27]. Alexa Fluor 488-conjugated anti-mouse IgG and Alexa Fluor 488-conjugated anti-rat IgG were purchased from Cell Signaling Technology, Inc. (Danvers, MA, USA). Secondary horseradish peroxidase-conjugated anti-mouse IgG and anti-rat IgG were obtained from Agilent Technologies Inc. (Santa Clara, CA, USA) and Merck KGaA (Darmstadt, Germany), respectively.
2.3. Hybridoma Production
For developing anti-EphA7 mAbs, two 5-week-old female BALB/cAJcl mice purchased from CLEA Japan (Tokyo, Japan) were immunized with LN229/MAP16-EphA7 (1 × 108 cells/mouse) via the intraperitoneal route starting at 6-week-old. Alhydrogel adjuvant 2% (InvivoGen, San Diego, CA, USA) was added to the immunogen cells in the first immunization. Three additional injections of LN229/MAP16-EphA7 (1 × 108 cells/mouse) were conducted intraperitoneally without an adjuvant addition every week. A last booster injection was also performed with 1 × 108 cells/mouse of LN229/MAP16-EphA7 via intraperitoneal route two days before harvesting spleen cells from mice.
We gently executed cell-fusion of P3U1 myeloma cells with the harvested splenocytes. The hybridoma supernatants were screened by flow cytometry using CHO/EphA7 and parental CHO-K1 cells. Anti-EphA7 mAbs were purified from the hybridoma supernatants, as described previously [
22].
2.4. Production of Recombinant Antibodies
Variable (VH) and constant (CH) regions of heavy chain cDNAs of Ea7Mab-10 were subcloned into the pCAG-Neo vector (Wako). Variable (VL) and constant (CL) regions of light chain cDNAs of Ea7Mab-10 were subcloned into the pCAG-Ble vector (Wako). NZ-33 was produced using the VH of NZ-1 and constant (CH) region of mouse IgG2a, and the light chain of NZ-1. Variable (VH) region of NZ-1 and CH region of mouse IgG2a were subcloned into the pCAG-Neo vector (Wako). Variable (VL) and constant (CL) regions of light chain cDNAs of NZ-1 were subcloned into the pCAG-Ble vector (Wako). These vectors were transfected into ExpiCHO-S cells using the ExpiCHO Expression System (Thermo Fisher Scientific Inc.). Ea7Mab-10 and NZ-33 were purified using Ab-Capcher (ProteNova, Kagawa, Japan).
2.5. Flow Cytometry
Cells were harvested using 1 mM EDTA (Nacalai Tesque, Inc.) to prevent enzymatic degradation. Following collection, the cells were washed gently with PBS containing 0.1% bovine serum albumin (BSA) and incubated with primary monoclonal antibodies (mAbs) for 30 minutes at 4°C. Subsequently, they were stained with Alexa Fluor 488-conjugated anti-mouse (diluted 1:1000) before fluorescence analysis using the SA3800 Cell Analyzer (Sony Corp.).
2.6. Determination of the Binding Affinity by Flow Cytometry
CHO/EphA7 cells were suspended in 100 μL serially diluted Ea
7Mab-10 (100 µg/mL to 0.006 µg/mL), after which Alexa Fluor 488-conjugated anti-mouse IgG (dilution rate; 1:200) was treated. The dissociation constant (K
D) was determined as described previously [
24].
2.7. Western Blotting
Cells were lysed and boiled in sodium dodecyl sulfate (SDS) sample buffer (Nacalai Tesque, Inc.). Western blotting was performed as described previously [
24].
2.8. Immunohistochemistry
Formalin-fixed paraffin-embedded (FFPE) CHO/EphA7 and CHO-K1 cell blocks were prepared using iPGell (Genostaff Co., Ltd., Tokyo, Japan). The FFPE cell sections were stained with Ea7Mab-10 (0.1 μg/mL) or NZ-33 (0.1 μg/mL) using BenchMark ULTRA PLUS with the ultraView Universal DAB Detection Kit (Roche Diagnostics, Indianapolis, IN, USA).
3. Results
3.1. Development of an Anti-EphA7 mAb, Ea7Mab-10 Using the CBIS Method
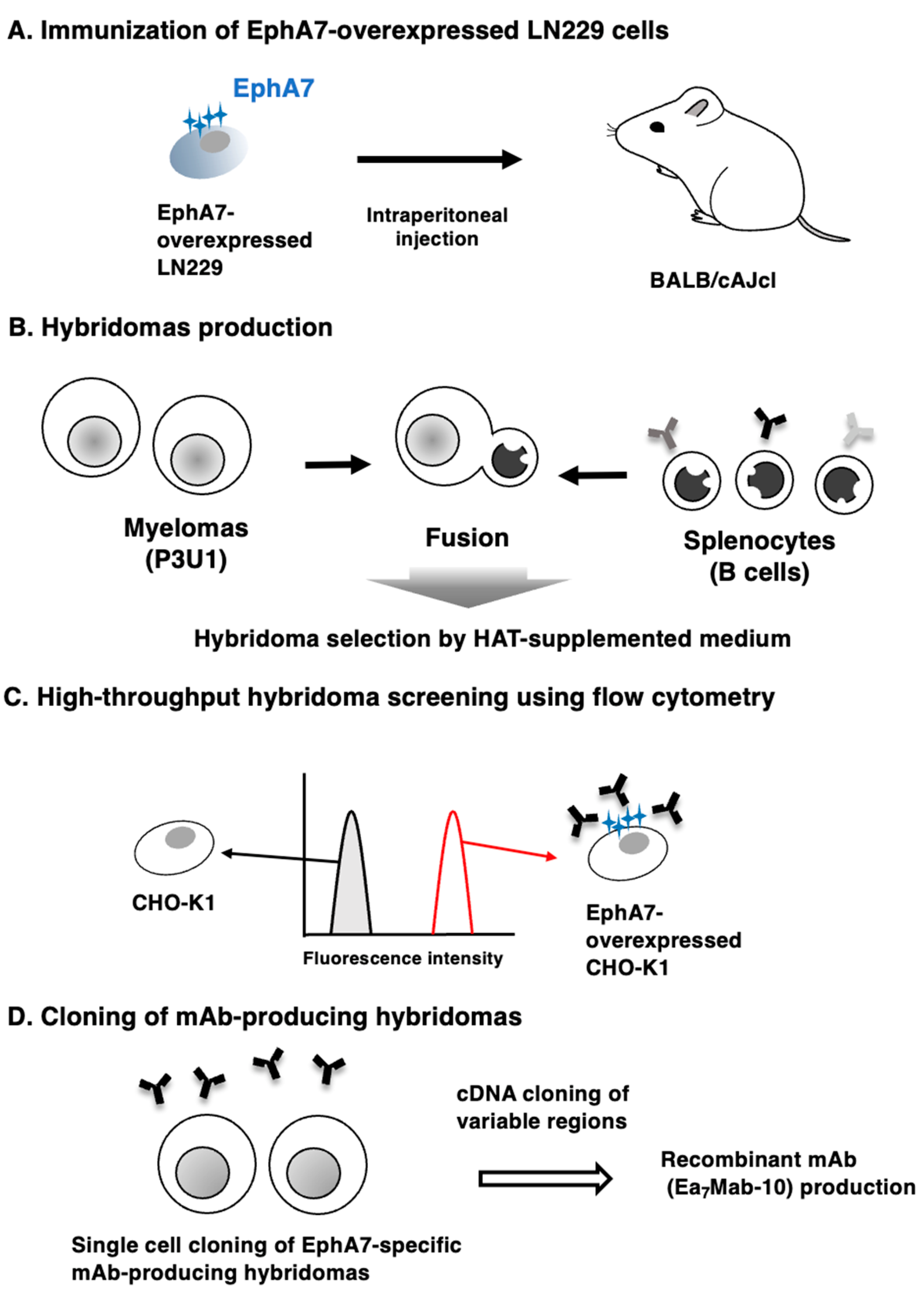
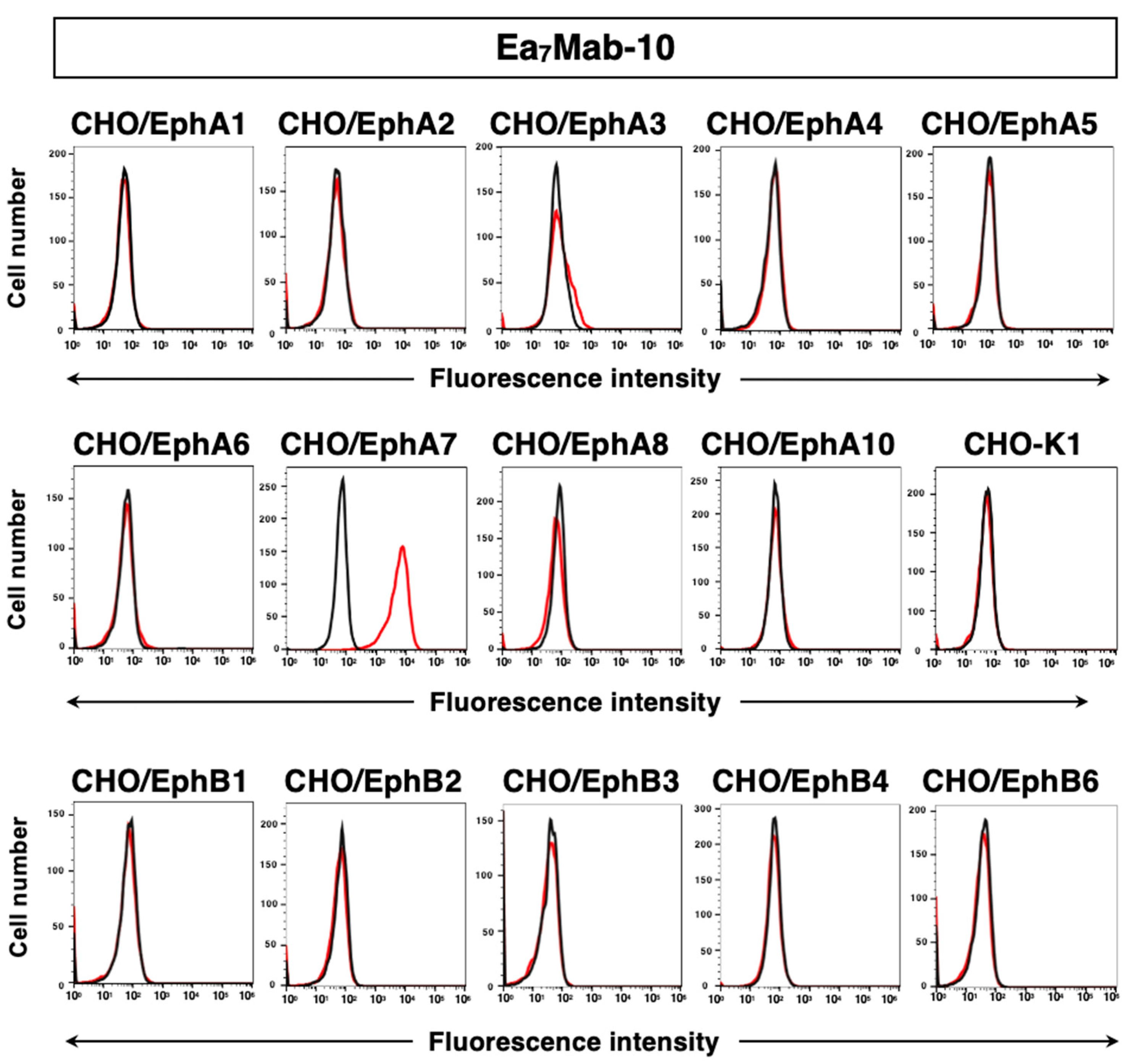
To establish mAbs targeting EphA7, we employed the CBIS method using EphA7-overexpressed cells. Anti-EphA7 mAbs-producing hybridoma were screened by flow cytometric analysis (
Figure 1). Two female BALB/cAJcl mice were immunized with LN229/MAP16-EphA7 for 5 times. Subsequently, splenocytes removed from immunized mice and fused with P3U1 cells. After confirming hybridoma formation, flow cytometric high throughput screening was conducted to select CHO/EphA7 reactive and parental CHO-K1 nonreactive supernatants of hybridomas. After limiting dilution and additional analysis, we established thirteen clones of anti-EphA7 mAbs (
http://www.med-tohoku-antibody.com/topics/001_paper_antibody_PDIS.htm#EphA7). Among them, we established and selected a clone Ea
7Mab-10 (mouse IgG
1, kappa) by the reactivity and specificity. As shown in
Figure 2, Ea
7Mab-10 recognized CHO/EphA7. Importantly, Ea
7Mab-10 never reacted with other Eph receptors (EphA1 to A6, A8, A10, B1 to B4, and B6)-overexpressed CHO-K1. This result indicates that Ea
7Mab-10 is a EphA7 specific mAb.
3.2. Investigation of the Reactivity of Ea7Mab-10 Using Flow Cytometry
We cloned cDNAs of V
H and V
L regions of Ea
7Mab-10 and produced recombinant Ea
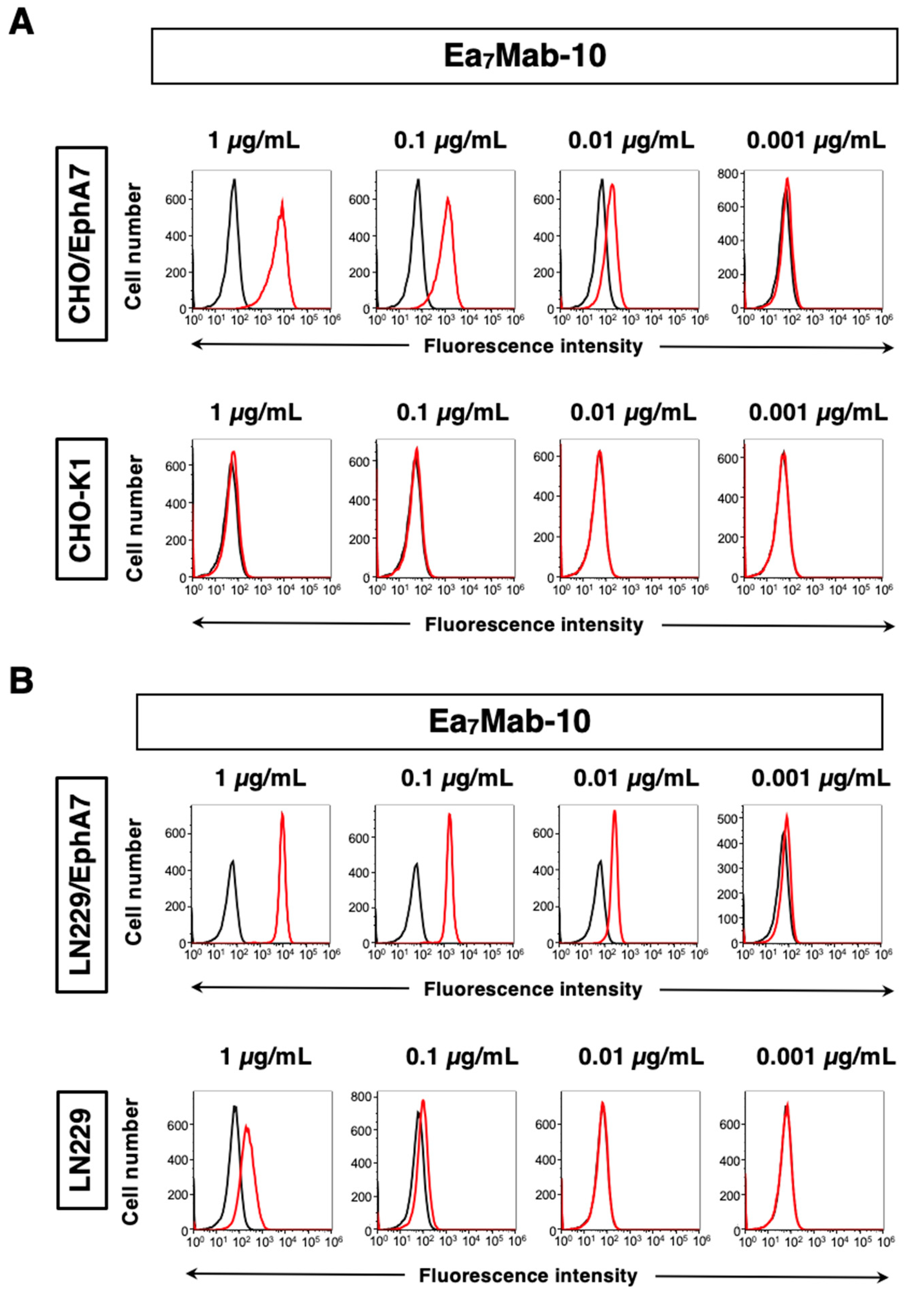
7Mab-10 for the further validation. We first checked the reactivity of Ea
7Mab-10 by flow cytometric analysis. Results showed that Ea
7Mab-10 recognized CHO/EphA7 dose-dependently (
Figure 3A). Ea
7Mab-10 did not react with parental CHO-K1 cells (
Figure 3A). Furthermore, Ea
7Mab-10 exhibited the dose-dependent reaction to LN229/EphA7 cells (
Figure 3B). Ea
7Mab-10 recognized parental LN229 cells at 1 µg/ml and 0.1 µg/ml (
Figure 3B). These results indicate that Ea
7Mab-10 can detect EphA7 in flow cytometry.
3.3. Calculation of the Binding Affinity of Ea7Mab-10 Using Flow Cytometry
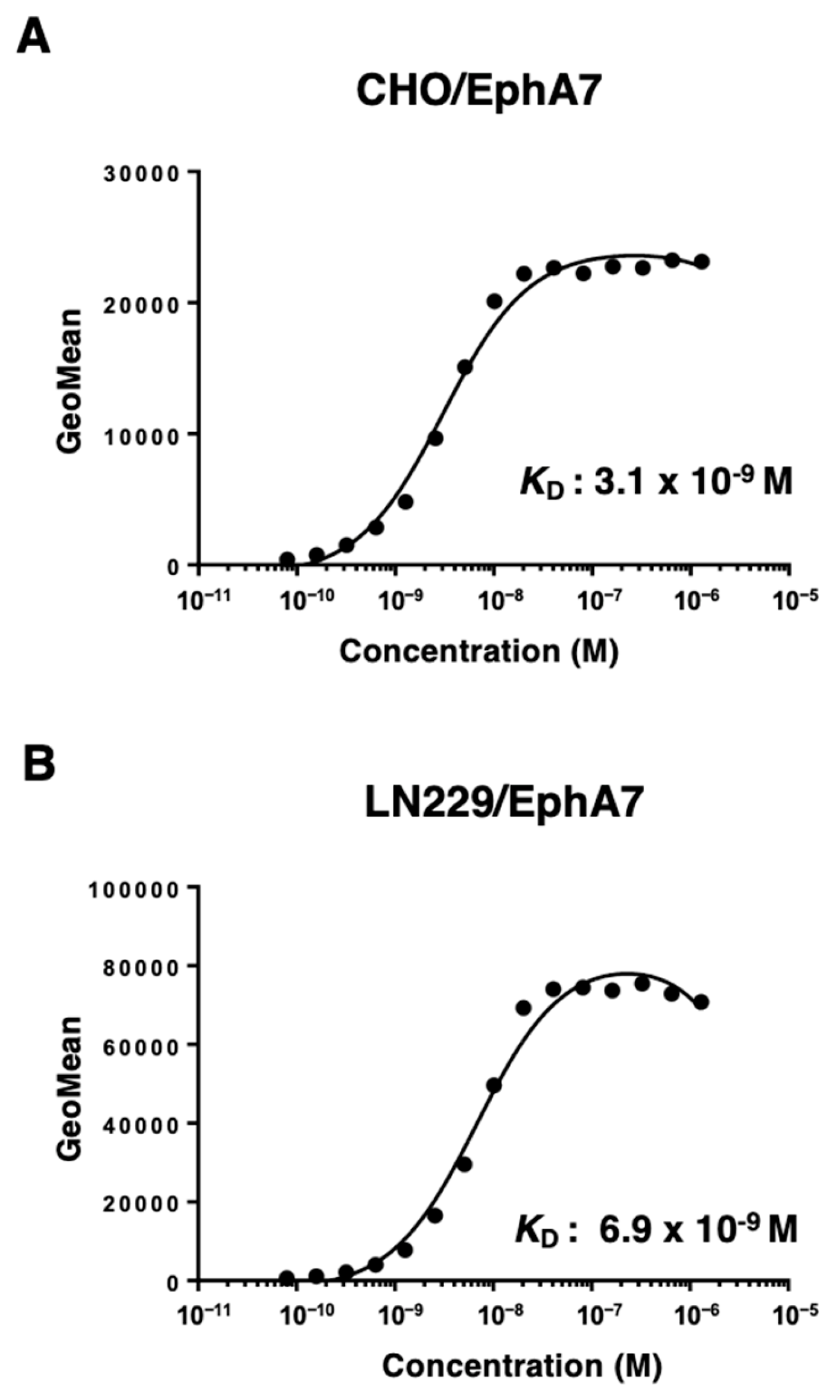
To evaluate the binding affinity of Ea
7Mab-10, flow cytometry was performed using CHO/EphA7 and LN229/EphA7 cells. The K
D values of Ea
7Mab-10 for CHO/EphA7 and LN229/EphA7 were 3.1×10
-9 M and 6.9×10
-9 M, respectively (
Figure 4). These results demonstrate that Ea
7Mab-10 has high affinity to EphA7-overexpressed cells.
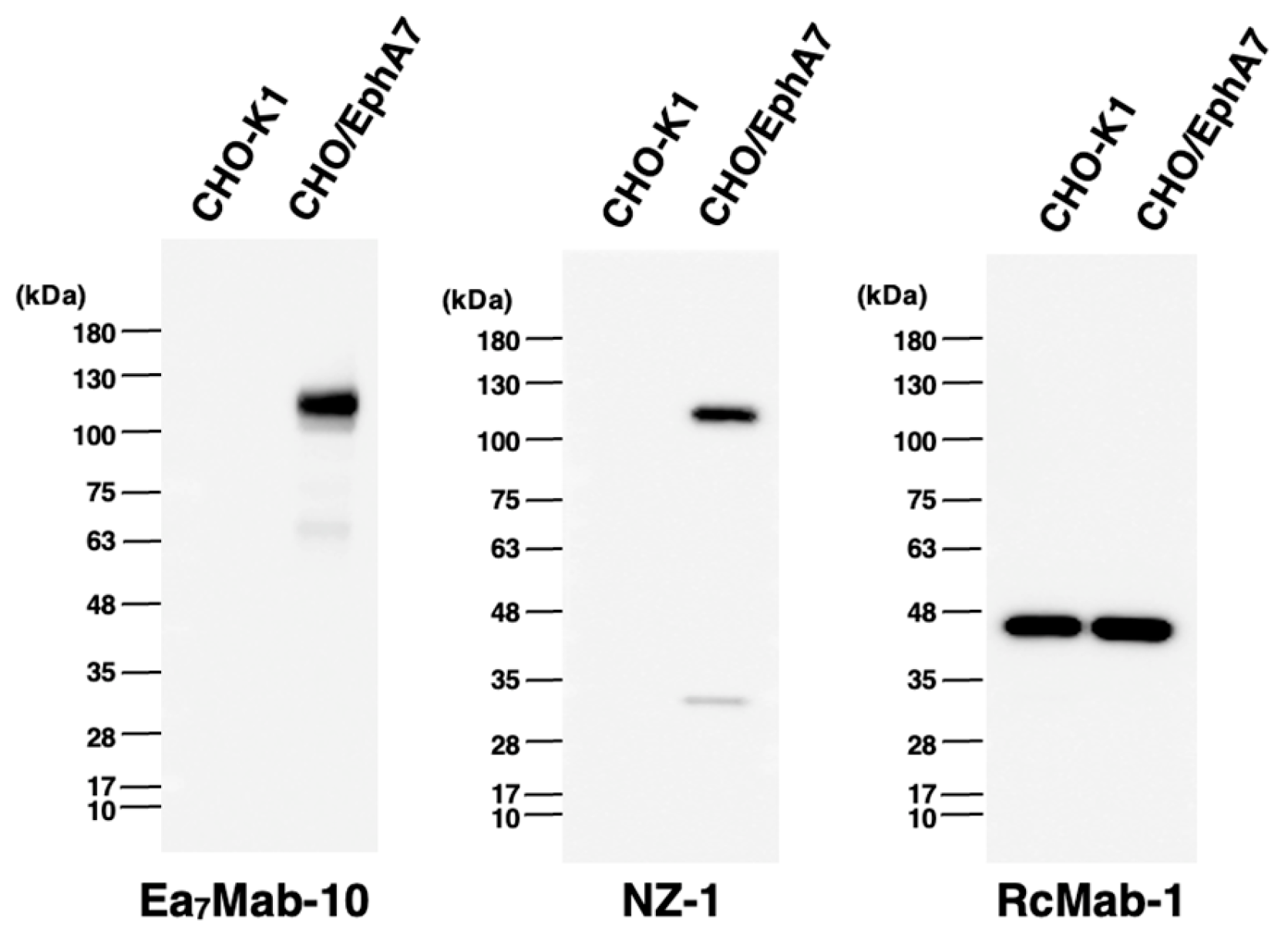
3.4. Western Blotting Using Ea7Mab-10
We investigated whether Ea
7Mab-10 can be used for western blot analysis by analyzing CHO-K1 and CHO/EphA7 cell lysates. As shown in
Figure 5, Ea
7Mab-10 could clearly detect exogenously expressed-EphA7 as the band around 110 kDa in CHO/EphA7 cell lysates, while no band was detected in parental CHO-K1 cells. An anti-PA16 tag mAb NZ-1 was used as a positive control and could also detect a band at the same position in CHO/EphA7 cell lysates. An anti-IDH1 mAb (clone RcMab-1) was used for internal control. These results demonstrate that Ea
7Mab-10 can detect EphA7 in western blotting.
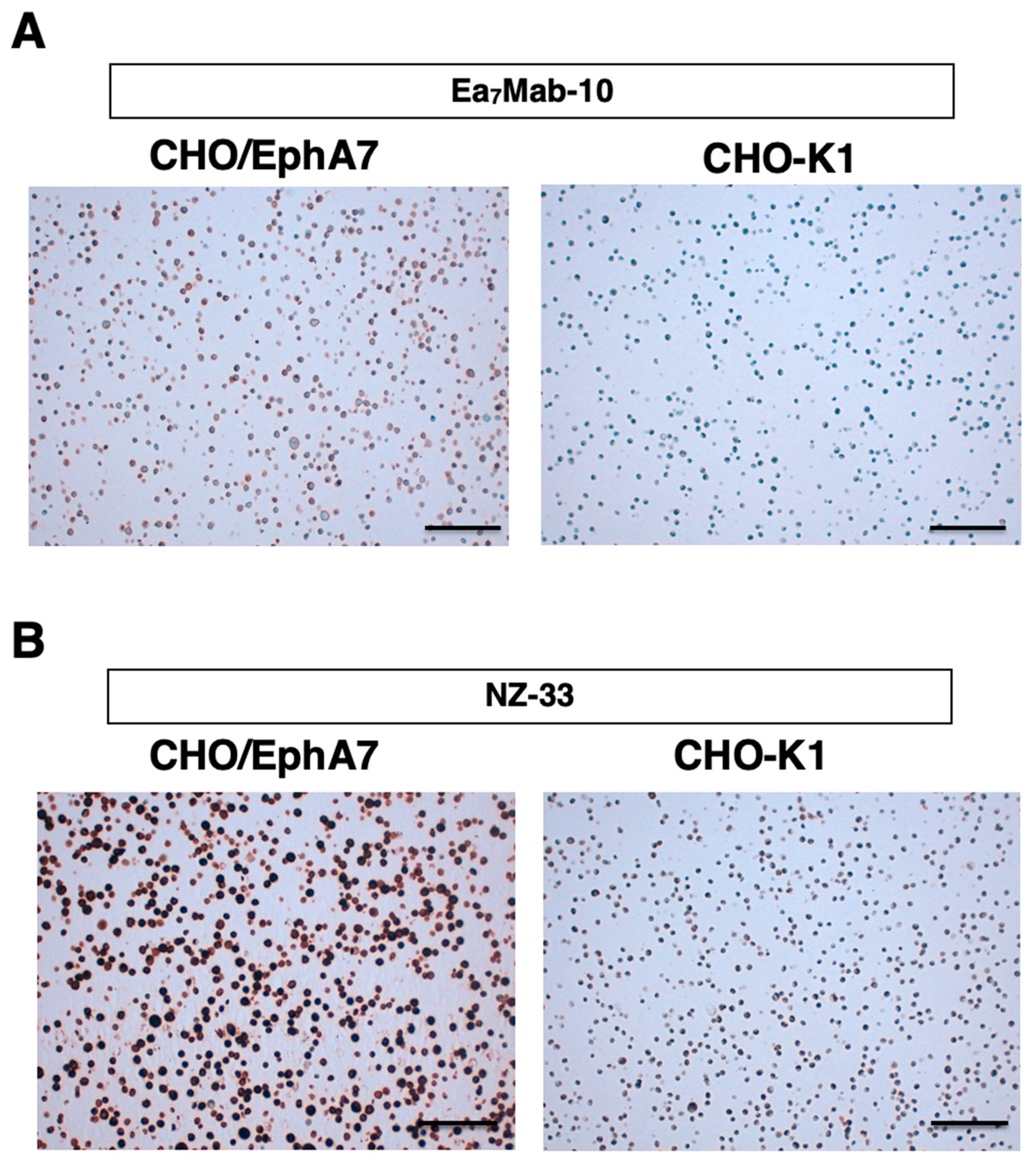
3.5. IHC Using Ea7Mab-10
To evaluate whether Ea
7Mab-10 can be used for IHC, FFPE CHO-K1 and CHO/EphA7 sections were stained with Ea
7Mab-10. Apparent membranous staining by Ea
7Mab-10 was observed in CHO/EphA7 (
Figure 6A). An anti-PA16 tag mAb, NZ-33 also showed potent reactivity to CHO/EphA7 (
Figure 6B). These results indicate that Ea
7Mab-10 applies to IHC for detecting EphA7-positive cells in FFPE cell samples
4. Discussion
This study demonstrated a novel anti-human EphA7 mAb, Ea
7Mab-10, which is applicable for various experiments. Ea
7Mab-10 can specifically detect EphA7 in flow cytometry (
Figure 2,
Figure 3 and
Figure 4), western blotting (
Figure 5), and IHC (
Figure 6).
Studies have demonstrated that EphA7 shows context-dependent roles in tumor progression and suppression [
28]. EphA7 is inactivated in 72% of follicular lymphomas (FLs) [
15]. The EphA7 knockdown drove lymphoma development in a murine model. Furthermore, a soluble EphA7 splice variant interfered with another Eph-receptor and inhibited tumorigenic signaling in FL cells [
15]. In prostate cancer specimens, the EphA7 was significantly decreased compared to paired normal tissues [
14]. In esophageal squamous cell carcinoma, low EphA7 expression significantly correlates with lymph node metastases, poor tumor differentiation, and advanced pTNM staging [
29]. Consequently, patients with a low EphA7 expression have a poorer prognosis compared with those with high expression [
29].
Conversely, increased EphA7 expression correlates with adverse outcomes and increased tumor vascularity in glioblastoma multiforme [
30]. Since Ea
7Mab-10 recognizes glioblastoma LN229 without exogenous EphA7 expression (
Figure 3B), Ea
7Mab-10 can be used for thr detection of endogenous EphA7. Glioblastoma patients with positive EphA7 expression showed reduced median survival compared to those with negative expression, with EphA7 protein expression inversely correlating with overall survival [
30]. These IHC analyses were performed by a rabbit anti-EphA7 polyclonal antibody. Further studies are needed to show the usefulness of Ea
7Mab-10 for IHC of FFPE tumor tissues.
The EphA7 mutation has been implicating a key driver in small cell lung cancer [
16]. Although the involvement of EphA7 with the regulation of the actin cytoskeleton was suggested, detailed analyses have not been conducted. EphA7 mutations were also found in various tumors and exhibited a strong association with reduced patient survival [
1]. In contrast, a study demonstrated the robust link between EphA7 mutations and better clinical outcomes in immune checkpoint inhibitors-treated patients. Notably, EphA7 mutant tumor patients without immune checkpoint inhibitor therapy had significantly worse overall survival [
17]. Although further studies are essential to determine whether EphA7 in tumors should be targeted, Ea
7Mab-10 may be useful as therapeutic and/or diagnostic mAb in the future.
Eph7 is reported to be involved in immunosuppression through interaction with LILRB5 on immunosuppressive myeloid cells. The Eph7-binding to LILRB5 stimulates the expression of immunosuppressive markers on myeloid cells from cancer patients [
18]. In a transgenic mouse model of myeloid cell-specific LILRB5 expression, the Eph receptor on tumor cells bound to LILRB5 on myeloid cells, which resulted in increased immunosuppressive myeloid cells, decreased functional T cells, and increased tumor growth compared to control [
18]. Therefore, the blockade of EphA7-induced LILRB5 signaling is thought to be important for the potentiation of immune checkpoint inhibitor therapies. In addition to Ea
7Mab-10, we have been cloned several anti-Eph7 mAb clones. It is essential to evaluate the neutralization activity of Eph7-LILRB5 interaction to develop novel tumor immunotherapy.
Author Contributions
Shiori Fujisawa: Writing – original draft, Investigation; Haruto Yamamoto: Investigation; Tomohiro Tanaka: Investigation, Funding acquisition; Mika K. Kaneko: Conceptualization; Hiroyuki Suzuki: Investigation, Writing – review and editing; Yukinari Kato: Conceptualization, Funding acquisition, Project administration, Writing – review and editing.
Funding
This research was supported in part by Japan Agency for Medical Research and Development (AMED) under Grant Numbers: JP25am0521010 (to Y.K.), JP25ama121008 (to Y.K.), JP25ama221339 (to Y.K.), and (to Y.K.), and by the Japan Society for the Promotion of Science (JSPS) Grants-in-Aid for Scientific Research (KAKENHI) grant nos. 24K18268 (to T.T.) and 25K10553 (to Y.K.).
Institutional Review Board Statement
The animal study protocol was approved by the Animal Care and Use Committee of Tohoku University (Permit number: 2022MdA-001) for studies involving animals.
Informed Consent Statement
Not applicable.
Data Availability Statement
All related data and methods are presented in this paper. Additional inquiries should be addressed to the corresponding authors.
Conflicts of Interest
The authors declare no conflict of interest involving this article.
References
- Pasquale, E.B. Eph receptors and ephrins in cancer progression. Nat. Rev. Cancer 2024, 24, 5–27. [Google Scholar] [CrossRef] [PubMed]
- Pasquale, E.B. Eph receptor signaling complexes in the plasma membrane. Trends Biochem. Sci. 2024, 49, 1079–1096. [Google Scholar] [CrossRef] [PubMed]
- Bush, J.O. Cellular and molecular mechanisms of EPH/EPHRIN signaling in evolution and development. Curr Top Dev Biol 2022, 149, 153–201. [Google Scholar] [PubMed]
- Cayuso, J.; Xu, Q.; Wilkinson, D.G. Mechanisms of boundary formation by Eph receptor and ephrin signaling. Dev. Biol. 2015, 401, 122–131. [Google Scholar] [CrossRef]
- Rasool, D.; Jahani-Asl, A. Master regulators of neurogenesis: the dynamic roles of Ephrin receptors across diverse cellular niches. Transl. Psychiatry 2024, 14, 462. [Google Scholar] [CrossRef]
- Kania, A.; Klein, R. Mechanisms of ephrin-Eph signalling in development, physiology and disease. Nat. Rev. Mol. Cell Biol. 2016, 17, 240–256. [Google Scholar] [CrossRef]
- Zhu, Y.; Su, S.A.; Shen, J.; et al. Recent advances of the Ephrin and Eph family in cardiovascular development and pathologies. iScience 2024, 27, 110556. [Google Scholar] [CrossRef]
- Rudno-Rudzińska, J.; Kielan, W.; Frejlich, E.; et al. A review on Eph/ephrin, angiogenesis and lymphangiogenesis in gastric, colorectal and pancreatic cancers. Chin. J. Cancer Res. 2017, 29, 303–312. [Google Scholar] [CrossRef]
- Guo, X.; Yang, Y.; Tang, J.; Xiang, J. Ephs in cancer progression: complexity and context-dependent nature in signaling, angiogenesis and immunity. Cell Commun. Signal 2024, 22, 299. [Google Scholar] [CrossRef]
- Arora, S.; Scott, A.M.; Janes, P.W. Eph Receptors in Cancer. Biomedicines 2023, 11(2). [Google Scholar] [CrossRef]
- Valenzuela, D.M.; Rojas, E.; Griffiths, J.A.; et al. Identification of full-length and truncated forms of Ehk-3, a novel member of the Eph receptor tyrosine kinase family. Oncogene 1995, 10, 1573–1580. [Google Scholar] [PubMed]
- Lee, H.; Park, E.; Kim, Y.; Park, S. EphrinA5-EphA7 complex induces apoptotic cell death via TNFR1. Mol. Cells 2013, 35, 450–455. [Google Scholar] [CrossRef] [PubMed]
- Cai, C.; Zhang, M.; Liu, L.; et al. ADAM10-cleaved ephrin-A5 contributes to prostate cancer metastasis. Cell Death Dis. 2022, 13, 453. [Google Scholar] [CrossRef]
- Li, S.; Wu, Z.; Ma, P.; et al. Ligand-dependent EphA7 signaling inhibits prostate tumor growth and progression. Cell Death Dis. 2017, 8, e3122. [Google Scholar] [CrossRef]
- Oricchio, E.; Nanjangud, G.; Wolfe, A.L.; et al. The Eph-receptor A7 is a soluble tumor suppressor for follicular lymphoma. Cell 2011, 147, 554–564. [Google Scholar] [CrossRef]
- Peifer, M.; Fernández-Cuesta, L.; Sos, M.L.; et al. Integrative genome analyses identify key somatic driver mutations of small-cell lung cancer. Nat. Genet. 2012, 44, 1104–1110. [Google Scholar] [CrossRef]
- Zhang, Z.; Wu, H.X.; Lin, W.H.; et al. EPHA7 mutation as a predictive biomarker for immune checkpoint inhibitors in multiple cancers. BMC Med. 2021, 19, 26. [Google Scholar] [CrossRef]
- He, Y.; Zhang, C.; Tan, L.; et al. Eph receptors activate myeloid checkpoint receptor LILRB5 to support tumor development. Cancer Immunol Res 2025. [CrossRef]
- Asano, T.; Ohishi, T.; Takei, J.; et al. Anti-HER3 monoclonal antibody exerts antitumor activity in a mouse model of colorectal adenocarcinoma. Oncol Rep 2021, 46(2). [Google Scholar] [CrossRef]
- Itai, S.; Yamada, S.; Kaneko, M.K.; et al. Establishment of EMab-134, a Sensitive and Specific Anti-Epidermal Growth Factor Receptor Monoclonal Antibody for Detecting Squamous Cell Carcinoma Cells of the Oral Cavity. Monoclon. Antib. Immunodiagn. Immunother. 2017, 36, 272–281. [Google Scholar] [CrossRef]
- Itai, S.; Fujii, Y.; Kaneko, M.K.; et al. H(2)Mab-77 is a Sensitive and Specific Anti-HER2 Monoclonal Antibody Against Breast Cancer. Monoclon. Antib. Immunodiagn. Immunother. 2017, 36, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Ubukata, R.; Suzuki, H.; Hirose, M.; et al. Establishment of a highly sensitive and specific anti-EphB2 monoclonal antibody (Eb2Mab-12) for flow cytometry. MI 2025. [CrossRef]
- Tanaka, T.; Kaneko, Y.; Yamamoto, H.; et al. Development of a novel anti-erythropoietin-producing hepatocellular receptor B6 monoclonal antibody Eb(6)Mab-3 for flow cytometry. Biochem Biophys Rep 2025, 41, 101960. [Google Scholar] [PubMed]
- Satofuka, H.; Suzuki, H.; Tanaka, T.; et al. Development of an anti-human EphA2 monoclonal antibody Ea2Mab-7 for multiple applications. Biochemistry and Biophysics Reports 2025, 42, 10998. [Google Scholar] [CrossRef]
- Fujii, Y.; Kaneko, M.K.; Kato, Y. MAP Tag: A Novel Tagging System for Protein Purification and Detection. Monoclon. Antib. Immunodiagn. Immunother. 2016, 35, 293–299. [Google Scholar] [CrossRef]
- Fujii, Y.; Kaneko, M.; Neyazaki, M.; et al. PA tag: a versatile protein tagging system using a super high affinity antibody against a dodecapeptide derived from human podoplanin. Protein Expr Purif 2014, 95, 240–247. [Google Scholar] [CrossRef]
- Ikota, H.; Nobusawa, S.; Arai, H.; et al. Evaluation of IDH1 status in diffusely infiltrating gliomas by immunohistochemistry using anti-mutant and wild type IDH1 antibodies. Brain Tumor Pathol. 2015, 32, 237–244. [Google Scholar] [CrossRef]
- Chen, X.; Yu, D.; Zhou, H.; et al. The role of EphA7 in different tumors. Clin. Transl. Oncol. 2022, 24, 1274–1289. [Google Scholar] [CrossRef]
- Bai, Y.Q.; Zhang, J.Y.; Bai, C.Y.; et al. Low EphA7 Expression Correlated with Lymph Node Metastasis and Poor Prognosis of Patients with Esophageal Squamous Cell Carcinoma. Acta Histochem. Cytochem. 2015, 48, 75–81. [Google Scholar] [CrossRef]
- Wang, L.F.; Fokas, E.; Juricko, J.; et al. Increased expression of EphA7 correlates with adverse outcome in primary and recurrent glioblastoma multiforme patients. BMC Cancer 2008, 8, 79. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).