Submitted:
25 April 2025
Posted:
28 April 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
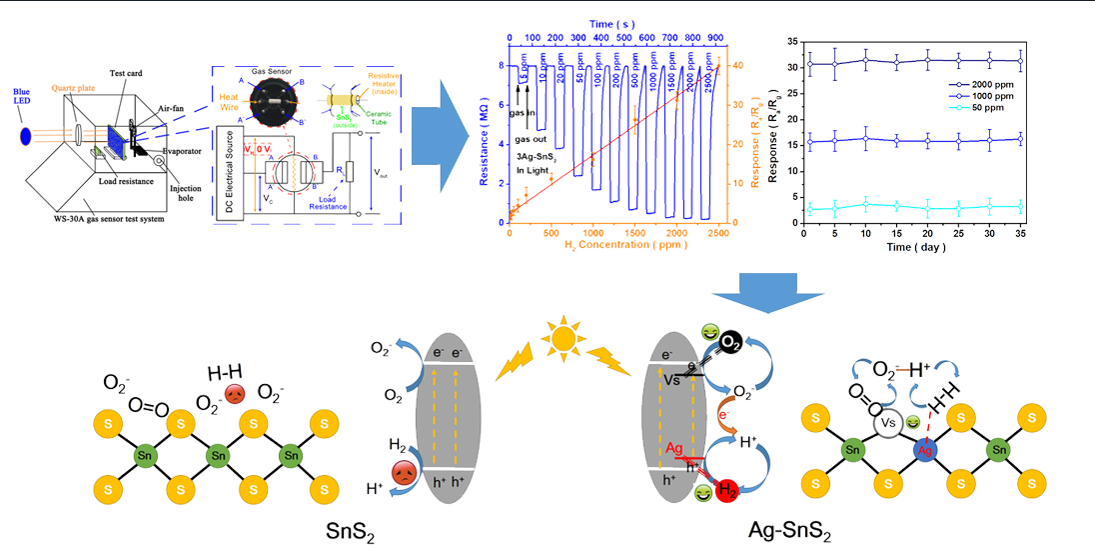
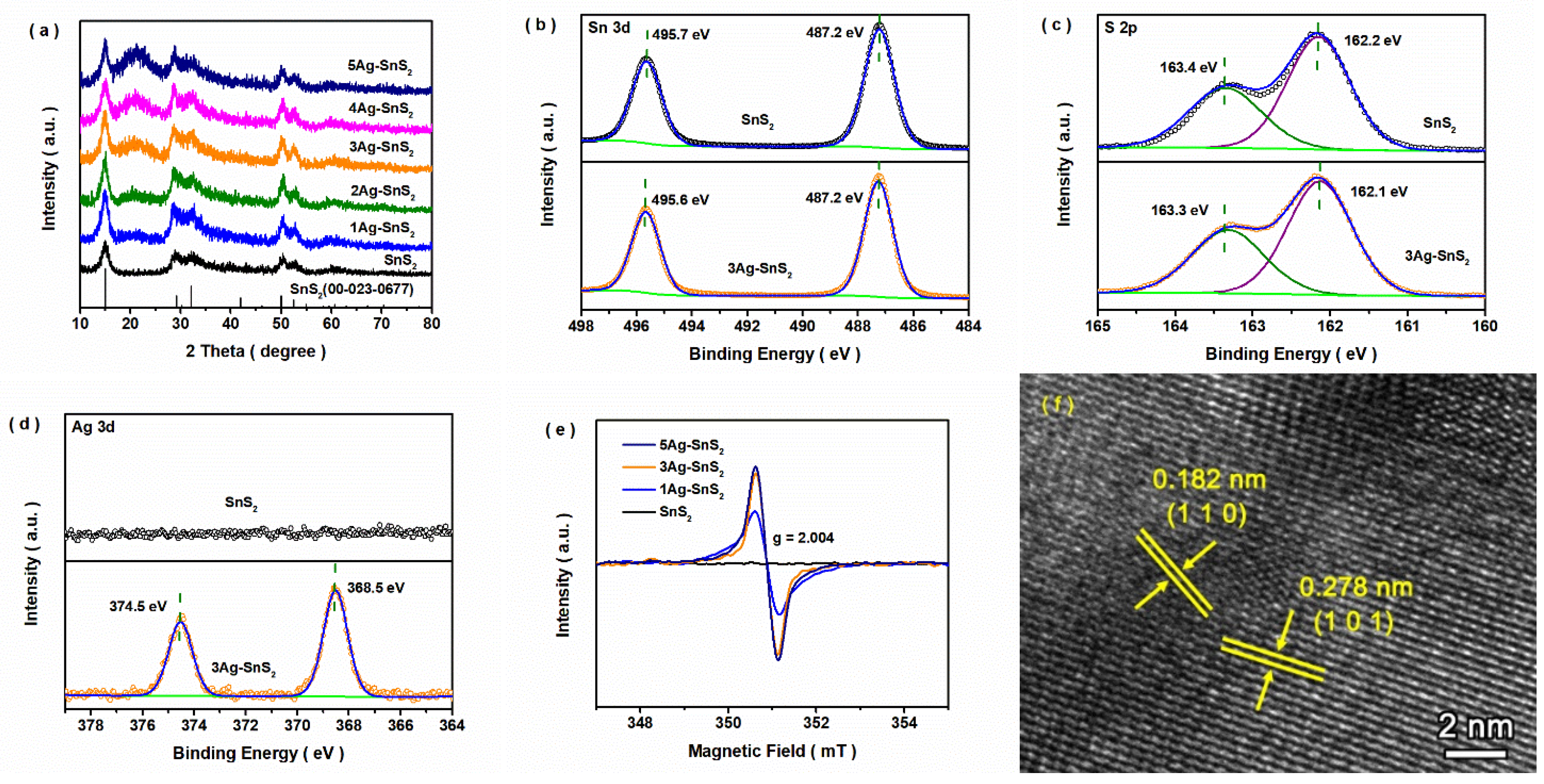
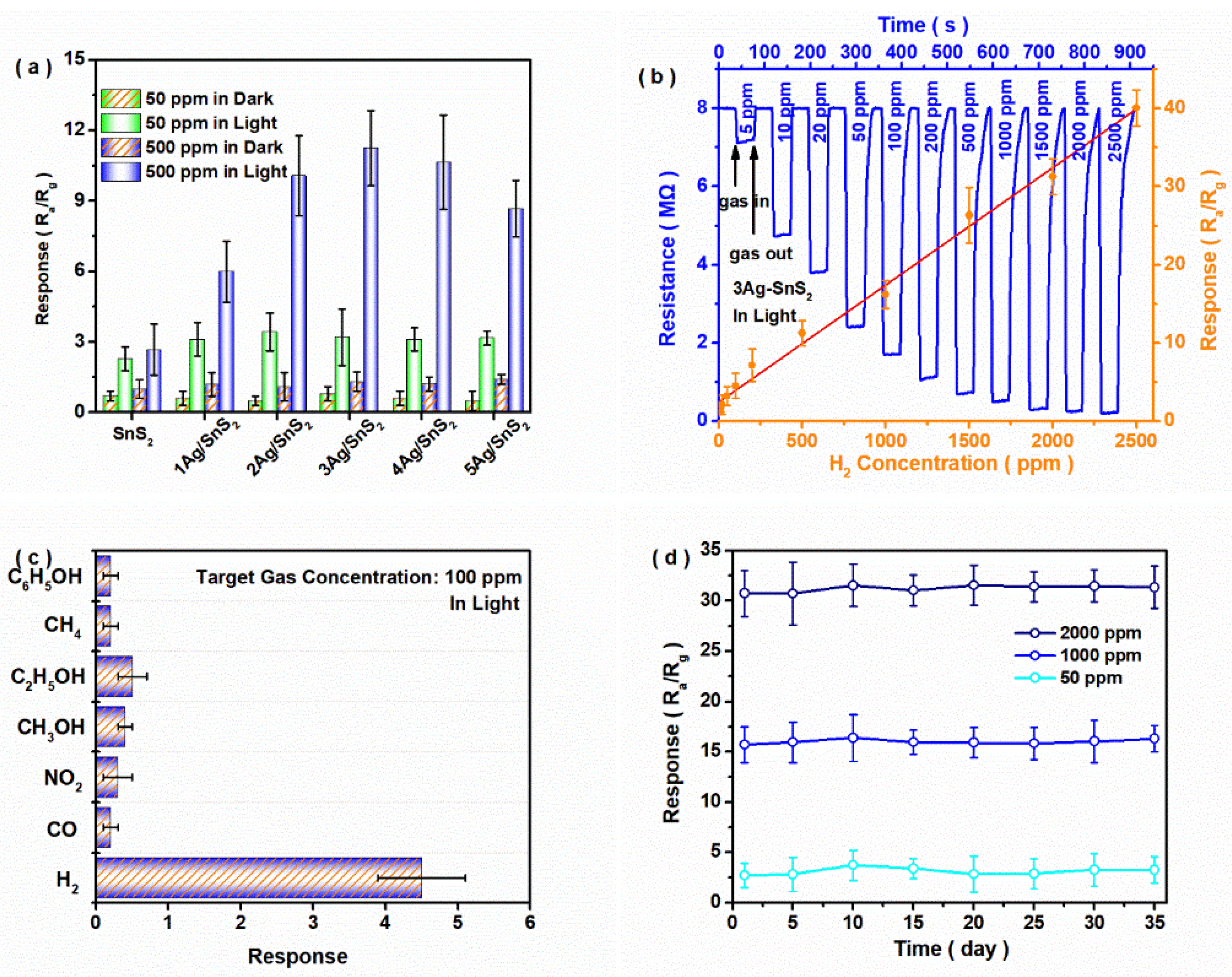
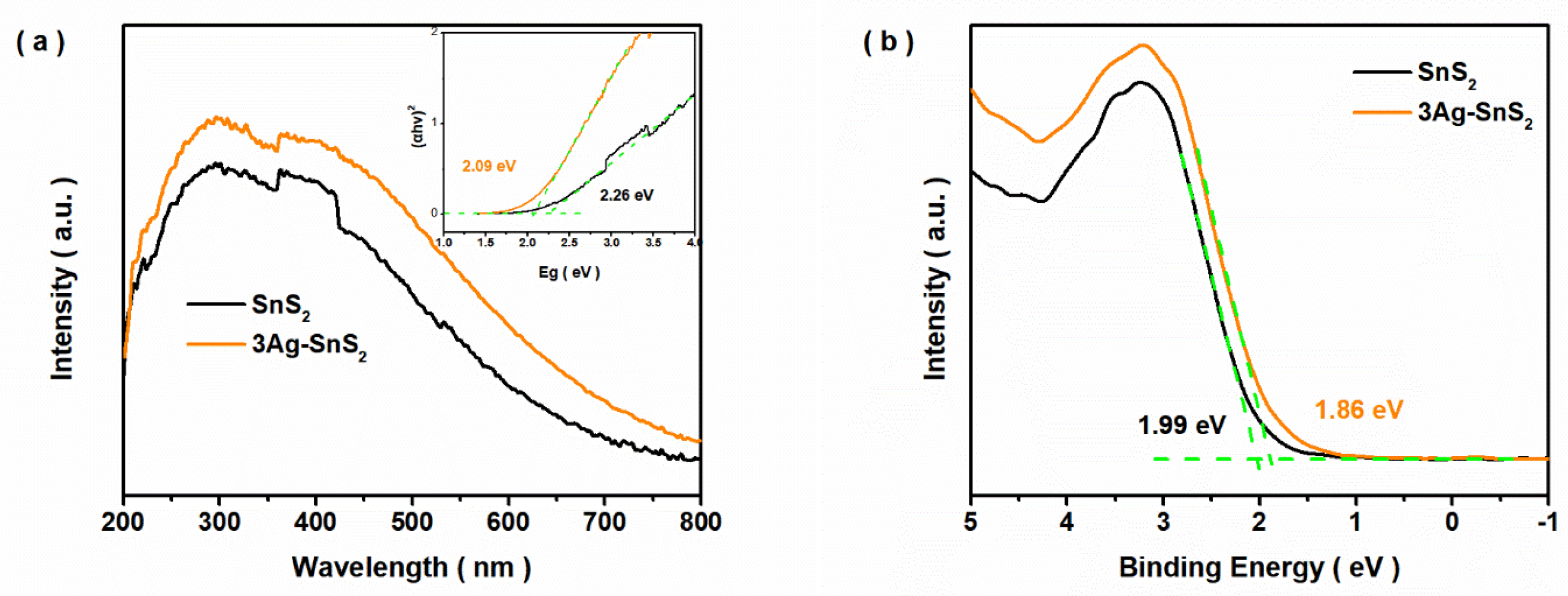
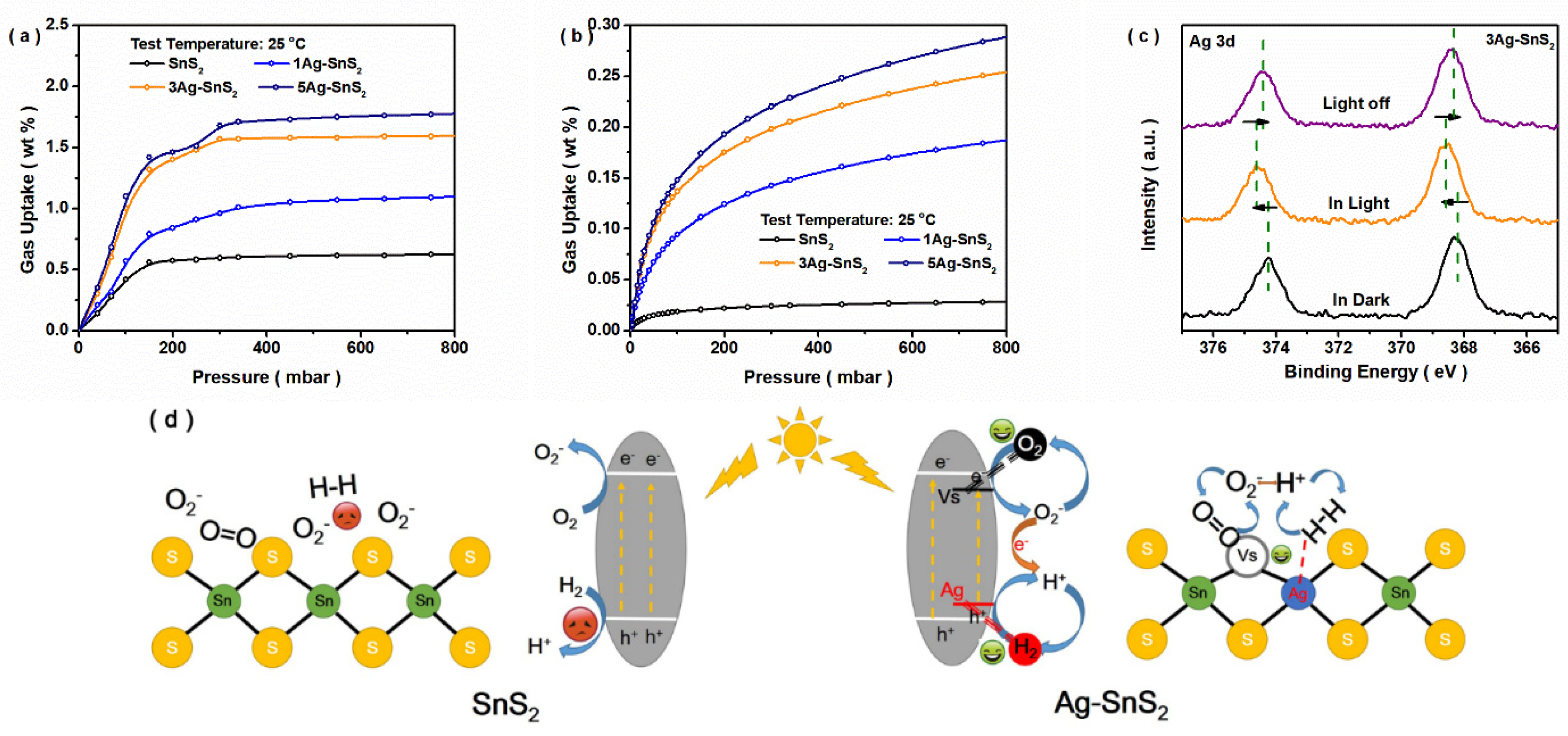
3. Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Hjiri, M.; Dhahri, R.; Benmansour, N.; Neri, G. Laser irradiated gas sensors: A review. Micro Nanostruct. 2025, 204, 208157. [CrossRef]
- Liu, Y.; Chen, S.; Xiao, B.; Chu, J.; Wang, H.; Chen, Y.; Yao, T.; Yang, A.; Han, X.; Rong, M.; Wang, X. Ultra-large Sn3O4 nanosheets with Sn2+ defect for highly efficient hydrogen sensing. Sens. Actuators, B 2024, 401, 135025. [CrossRef]
- Yin, X.-T.; Wu, S.-S.; Dastan, D.; Nie, S.; Liu, Y.; Li, Z.-G.; Zhou, Y.-W.; Li, J.; Faik, A.; Shan, K.; Shi, Z.; Tarighat, M. A.; Ma, X.-G. Sensing selectivity of SnO2-Mn3O4 nanocomposite sensors for the detection of H2 and CO gases. Surf. Interfaces 2021, 25, 101190. [CrossRef]
- Meng, X.; Bi, M.; Xiao, Q.; Gao, W. Ultrasensitive gas sensor based on Pd/SnS2/SnO2 nanocomposites for rapid detection of H2. Sens. Actuators, B 2022, 359, 131612. [CrossRef]
- Zhu, M.; Zhang, H.; Zhang, S.; Yao, H.; Shi, X.; Xu, S. Chemoresistive gas sensors based on noble-metal-decorated metal oxide semiconductors for H2 detection. Materials, 2025, 18, 451. [CrossRef]
- Mishra, R. K.; Choi, H. J.; Ryu, J. W.; Choi, G. J.; Kumar, V.; Kumar, P.; Singh, J.; Kumar, S.; Gwag, J. S. Recent progress in gas sensing based on 2D SnS2 and its heterostructure platforms: A review. Sens. Actuators, A 2024, 365, 114860. [CrossRef]
- Saggu, I. S.; Singh, S.; Chen, K.; Xuan, Z.; Swihart, M. T.; Sharma, S. Ultrasensitive room-temperature NO2 detection using SnS2/MWCNT composites and accelerated recovery kinetics by UV activation. ACS Sensors 2023, 8, 243-253. [CrossRef]
- Lee, S. M.; Kim, Y. J.; Park, S. J.; Cheon, W. S.; Kim, J.; Nam, G. B.; Kim, Y.; Jang, H. W. In-situ growth of 2D MOFs as a molecular sieving layer on SnS2 nanoflakes for realizing ultraselective H2S detection. Adv. Funct. Mater. 2025, 35, 2417019. [CrossRef]
- Qiu, P.; Qin, Y.; Wang, X. S-vacancies and Ag nanoparticles in SnS2 nanoflakes for ethanol sensing: A combined experimental and theoretical investigation. ACS Appl. Nano Mater. 2022, 5, 10839-10847. [CrossRef]
- Lu, Y.; Zhang, J.; Wang, W.; Fan, Y.; Liu, C.; Zhou, J.; Liu, D.; Ruan, S. Au-Pd modified SnS2 nanosheets for conductometric detection of xylene gas. Sens. Actuators, B 2022, 351, 130907. [CrossRef]
- Maria, K. H.; Sakhuja, N.; Jha, R. K.; Bhat, N. Ultra-sonication assisted synthesis of 2D SnS2 nanoflakes for room-temperature NO gas detection. IEEE Sens. J. 2021, 21, 10420-10427. [CrossRef]
- Eom, T. H.; Cho, S. H.; Suh, J. M.; Kim, T.; Lee, T. H.; Jun, S. E.; Yang, J. W.; Lee, J.; Hong, S.-H.; Jang, H. W. Substantially improved room temperature NO2 sensing in 2-dimensional SnS2 nanoflowers enabled by visible light illumination. J. Mater. Chem. A 2021, 9, 11168-11178. [CrossRef]
- Ramakrishnan, K.; Ajitha, B.; Ashok Kumar Reddy, Y. Review on metal sulfide-based nanostructures for photodetectors: From ultraviolet to infrared regions. Sens. Actuators, A 2023, 349, 114051. [CrossRef]
- Li, Q.; Wang, X.; Li, H.; Guo, X. Light-activated gas sensors. Chin. Sci. Bull. 2022, 67, 1837-1850. [CrossRef]
- Arain, S.; Usman, M.; Saeed, F.; Feng, S.; Rehman, W.; Liu, X.; Dai, H. Microemulsion-based synthesis of highly efficient Ag-doped fibrous SiO2-TiO2 photoanodes for photoelectrochemical water splitting. Catalysts, 2025, 15, 66. [CrossRef]
- An, Q.; Li, J.; Peng, J.; Hu, L. DFT study of small gas molecules (C2H2, CH4, CO and H2) adsorbed on Au, Ag-doped ZnO monolayer. Chem. Phys. Lett. 2025, 869, 142043. [CrossRef]
- Yang, H.; Du, Z.; Yang, Y.; Li, X.; Wu, Q.; Tang, J.; Wang, X.; Zeng, D. Ag intercalated SnS2 with S vacancy and expanded interlayer for enhancing NO2 sensing. Sens. Actuators, B 2023, 393, 134140. [CrossRef]
- Wang, J.-C.; Ma, H.; Shi, W.; Li, W.; Zhang, Z.; Hou, Y.; Zhang, W.; Chen, J. Designed synthesized step-scheme heterojunction of Bi2WO6 nanosheet supported on CuBi2O4 nanorod with remarkable photo-assisted gas sensing for N-butyl alcohol. J. Environ. Chem. Eng. 2024, 12, 112698. [CrossRef]
- Wang, J.-C.; Shi, W.; Sun, X.-Q.; Wu, F.-Y.; Li, Y.; Hou, Y. Enhanced photo-assisted acetone gas sensor and efficient photocatalytic degradation ssing Fe-doped hexagonal and monoclinic WO3 phase−junction. Nanomaterials, 2020, 10, 398. [CrossRef]
- Tian, W.; Han, J.; Wan, L.; Li, N.; Chen, D.; Xu, Q.; Li, H.; Lu, J. Enhanced piezocatalytic activity in ion-doped SnS2 via lattice distortion engineering for BPA degradation and hydrogen production. Nano Energy 2023, 107, 108165. [CrossRef]
- Lv, Y.-R.; Wang, Z.-L.; Yang, Y.-X.; Luo, Y.; Yang, S.-Y.; Xu, Y.-H. Tin bisulfide nanoplates anchored onto flower-like bismuth tungstate nanosheets for enhancement in the photocatalytic degradation of organic pollutant. J. Hazard. Mater. 2022, 432, 128665. [CrossRef]
- Lei, Z.; Wang, W.; Sun, T.; Liu, E.; Gao, T. Efficient photocatalytic H2 evolution over SnS2/twinned Mn0.5Cd0.5S hetero-homojunction with double S-scheme charge transfer routes. J. Mater. Sci. Technol. 2025, 216, 81-92. [CrossRef]
- Mamo, T. T.; Qorbani, M.; Hailemariam, A. G.; Putikam, R.; Chu, C.-M.; Ko, T.-R.; Sabbah, A.; Huang, C.-Y.; Kholimatussadiah, S.; Billo, T.; Hussien, M. K.; Chang, S.-Y.; Lin, M.-C.; Woon, W.-Y.; Wu, H.-L.; Wong, K.-T.; Chen, L.-C.; Chen, K.-H. Enhanced CO2 photoreduction to CH4 via *COOH and *CHO intermediates stabilization by synergistic effect of implanted P and S vacancy in thin-film SnS2. Nano Energy 2024, 128, 109863. [CrossRef]
- Qin, Y.; Zhang, Y.; Qiu, P.; Lei, S. SnO2-Co3O4 nanocomposite sensor: Achieving ultra-selective hydrogen detection in mixed gas environments. Sens. Actuators, B 2025, 422, 136521. [CrossRef]
- Li, A.; Zhao, S.; Bai, J.; Xiao, H.; Gao, S.; Shen, Y.; Yuan, Z.; Meng, F. The role of AuSn alloys in optimizing SnO2 nanospheres for chemoresistive hydrogen sensing. Sens. Actuators, B 2025, 427, 137214. [CrossRef]
- Liu, Y.; Chen, S.; Xiao, B.; Chu, J.; Wang, H.; Chen, Y.; Yao, T.; Yang, A.; Han, X.; Rong, M.; Wang, X. Ultra-large Sn3O4 nanosheets with Sn2+ defect for highly efficient hydrogen sensing. Sens. Actuators, B 2024, 401, 135025. [CrossRef]
- Liu, W.; Zou, J.; Li, S.; Li, J.; Li, F.; Zhan, Z.; Zhang, Y. Pd/In2O3-based bilayer H2 sensor with high resistance to silicone toxicity and ultra-fast response. Int. J. Hydrogen Energy 2023, 48, 5743-5753. [CrossRef]
- Meng, X.; Bi, M.; Xiao, Q.; Gao, W. Ultrasensitive gas sensor based on Pd/SnS2/SnO2 nanocomposites for rapid detection of H2. Sens. Actuators, B 2022, 359, 131612. [CrossRef]
- Lu, S.; Zhang, Y.; Liu, J.; Li, H.-Y.; Hu, Z.; Luo, X.; Gao, N.; Zhang, B.; Jiang, J.; Zhong, A.; Luo, J.; Liu, H. Sensitive H2 gas sensors based on SnO2 nanowires. Sens. Actuators, B 2021, 345, 130334. [CrossRef]
- Tang, C.; Jin, W.; Xiao, X.; Qi, X.; Ma, Y.; Ma, L. Graphene-based chemiresistive hydrogen sensor for room temperature operation. Sens. Actuators, B 2025, 424, 136889. [CrossRef]
- Kumar, G.; Li, X.; Du, Y.; Geng, Y.; Hong, X. UV-light enhanced high sensitive hydrogen (H2) sensor based on spherical Au nanoparticles on ZnO nano-structured thin films. J. Alloys Compd. 2019, 798, 467-477. [CrossRef]
- Wang, F.; Hu, K.; Liu, H.; Zhao, Q.; Wang, K.; Zhang, Y. Low temperature and fast response hydrogen gas sensor with Pd coated SnO2 nanofiber rods. Int. J. Hydrogen Energy 2020, 45, 7234-7242. [CrossRef]
- Pandey, G.; Lawaniya, S. D.; Kumar, S.; Dwivedi, P. K.; Awasthi, K. A highly selective, efficient hydrogen gas sensor based on bimetallic (Pd–Au) alloy nanoparticle (NP)-decorated SnO2 nanorods. J. Mater. Chem. A 2023, 11, 26687-26697. [CrossRef]
- Wang, Z.; Cheng, B.; Zhang, L.; Yu, J.; Tan, H. BiOBr/NiO S-scheme heterojunction photocatalyst for CO2 photoreduction. Sol. RRL 2022, 6, 2100587. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




