1. Introduction
Chronic coronary syndrome (CCS) and atrial fibrillation (AF) are intricately linked cardiovascular conditions that share common pathophysiological mechanisms, contributing to increased morbidity and mortality. The first one, characterized by the persistent effects of atherosclerotic cardiovascular disease, results from a complex interplay of chronic inflammation, ischemia, and myocardial remodeling. This persistent ischemic burden can lead to structural and electrical remodeling, paving the way for the development of AF. The presence of atherosclerosis can disrupt normal myocardial function, creating an environment conducive to electrical instability and arrhythmogenesis. Concurrently, AF itself can exacerbate coronary artery disease (CAD) through mechanisms such as increased heart rate, impaired hemodynamics, and elevated myocardial oxygen demand. Considering the complexities of managing patients with both CCS and AF, identifying reliable biomarkers becomes essential for early diagnosis, risk stratification, and tailored therapeutic interventions. Among the most promising candidates in this arena are galectin-3 and pentraxin-3, both of which have garnered significant attention for their roles in inflammation and atherosclerosis—two pivotal processes that underpin the pathophysiology of both CCS and AF [
1,
2,
3].
Galectin-3, a member of the galectin family of glycan-binding proteins, is primarily expressed in activated macrophages, endothelial cells, and fibroblasts. Its role in inflammation is multifaceted, as it participates in various cellular processes, including cell adhesion, migration, and apoptosis. In the context of cardiovascular disease, galectin-3 is produced in response to myocardial injury and stress, often serving as a marker of adverse cardiac remodeling and heart failure. Elevated levels of galectin-3 have been linked to increased inflammation, fibrosis, and the progression of atherosclerosis. A study by Li et al., found that elevated galectin-3 levels were associated with the presence of CAD as well as coronary stability and complexity [
4]. This study found that higher levels of galectin-3 were associated with worse clinical outcomes in patients with CAD, this suggesting that galectin-3 could be a valuable biomarker for identifying patients at increased risk for adverse events as elevated galectin-3 levels may indicate increased myocardial stress and remodeling, which are critical factors in the progression of heart disease. By promoting the accumulation of immune cells in atherosclerotic plaques, galectin-3 contributes to the inflammatory milieu that underpins plaque instability, thereby heightening the risk of acute coronary events. Furthermore, its role in mediating fibroblast activation and collagen deposition underscores its significance in the development of myocardial fibrosis, a common consequence of chronic coronary ischemia. Regarding AF, Procyk et al claimed that galectin-3 could be an useful biomarker in AF diagnostic, that its concentration is different between the types of AF (paroxysmal, persistent, and permanent), and finaly, that it can predict the recurrence of AF in patients after ablation, but also postoperative atrial fibrillation (POAF) after cardiac surgery [
5,
6].
Pentraxin-3, another key player in the inflammatory cascade, is an acute-phase protein produced in response to pro-inflammatory stimuli such as cytokines and pathogen-associated molecular patterns. Unlike the liver-produced C-reactive protein (CRP), pentraxin-3 is secreted by various immune and non-immune cells, including endothelial cells and adipocytes, and plays a crucial role in the regulation of innate immunity and the resolution of inflammation. Clinical studies have so far provided contrasting results, highlighting a debated role of pentraxin-3 as an active mediator of endothelial dysfunction, atherosclerotic plaque vulnerability and worse outcome after ischemic events [
7]. Therefore, substantial evidence suggests a dual role of pentraxin-3 as modulator or amplifiers of the innate immune response. A metanalysis noted that CAD patients with the highest pentraxin-3 level had an increased risk of all-cause mortality, cardiac death, and cardiac events, but elevated pentraxin-3 level appeared to not significantly increase the risk of cardiac events in stable CAD subgroup [
8]. On the other hand, pentraxin-3 may be involved in the stabilization of atherosclerotic plaques, potentially reducing the risk of rupture and subsequent thromboembolic events as Ristagno el al claimed that pentraxin-3 can have cardioprotective and atheroprotective roles regulating inflammation. Meanwhile, pentraxin-3 has also been implicated in AF; for instance, a study by Soeki et al, showed that local pentraxin-3 production in the left atrium might reflect the local inflammation of AF [
9,
10,
11].
The balance between these two molecules may serve as a critical indicator of disease severity and progression. A predominance of galectin-3 over pentraxin-3 may suggest a shift toward a more inflammatory and destructive process, while a relative increase in pentraxin-3 could imply a protective response aimed at resolving inflammation. This nuanced interplay not only enhances our understanding of the pathophysiological mechanisms underlying CCS and AF but also opens avenues for exploring novel therapeutic strategies that target these biomarkers to modulate inflammation and improve clinical outcomes [
5,
12,
13].
As systemic inflammation is a key contributor to disease progression and adverse cardiovascular events in CCS and/or AF, inflammatory parameters such as the Pan-Immune-Inflammation Value (PIV) and the Systemic Immune-Inflammation Index (SII) have emerged as useful indicators for assessing the immune-inflammatory status of patients. Elevated PIV, calculated as (neutrophils × thrombocytes × monocytes)/lymphocytes, reflects a heightened pro-inflammatory state that may promote endothelial dysfunction, plaque instability, and myocardial remodeling. Research focusing on NSTEMI patients found that a PIV cut-off of 568.2 predicted severe coronary lesions, with a sensitivity of 91% and specificity of 81.1% [
14]. Similarly, a high SII value, derived from (neutrophils × platelets)/lymphocytes, has been associated with worse clinical outcomes in cardiovascular conditions [
15]. These indices provide insight into the complex interplay between immune activation and thrombogenic pathways, making them valuable tools in the risk stratification and management of CCS and AF patients. A study involving CCS patients determined that an SII cut-off value of 620 was associated with 78.4% sensitivity and 64.0% specificity in predicting hemodynamically significant coronary artery stenosis [
16].
Additionally, simpler ratios like the lymphocyte-to-neutrophil ratio (Ly/N) and the lymphocyte-to-leukocyte ratio (Ly/WBC) offer clinically relevant information regarding the systemic immune balance. A low Ly/N ratio signifies neutrophil predominance and suppressed lymphocyte-mediated immunity, both of which are hallmarks of sustained inflammation that contribute to atherosclerosis progression and atrial structural changes seen in AF. Likewise, a decreased Ly/WBC ratio may signal a systemic pro-inflammatory milieu, associated with plaque destabilization, increased arrhythmogenic potential, and a higher risk of cardiovascular events. A meta-analysis indicated that patients with a lower Ly/N had a notably higher rate of AF recurrence; in patients undergoing catheter ablation for AF, the rapport between neutrophils and lymphocytes predicted AF recurrence with a sensitivity of 73% and specificity of 67% [
17,
18].
In this cohort of 131 patients, we aimed to explore the association between galectin-3, pentraxin-3, the severity of coronary stenosis, and the presence of AF. Furthermore, we performed a comparative analysis of inflammatory parameters—including PIV, SII, Ly/N, and Ly/WBC—between patients with S-CCS and N-CCS. This approach allowed us to evaluate the potential association between systemic inflammation, biomarker expression, and the clinical presentation of coronary artery disease.
2. Materials and Methods
2.1. Study Design, Patients and Investigation
We conducted a prospective study that included 131 consecutively enrolled patients admitted with an indication of coronarography exploration between January and June 2024. Patients over the age of 18, with or without AF, who presented an indication of coronarography, presenting signs of stable CAD (due to high risk features such as strong family history of CAD, hypertension, dyslipidemia, diabetes, obesity or smoking and who presented angina or positive non-invasive tests such as exercise stress test, echocardiography stress and myocardial scintigraphy or who were identified with coronary stenosis detected at coronary computed tomography angiography), and who were with the ability to comprehend and accept an informed consent form were included in this research.
Patients under the age of 18 years, patients without signed informed consent, patients with acute myocardial infarction, chronic kidney disease with creatinine clearance below 30 mL/min/1.73 m2, hemodynamically significant valve diseases (more than mild severity), heart failure (HF) with a NYHA class 3 and 4, patients with thyroid and psychiatric disorders were excluded from the study.
2.2. Clinical Investigation and Data Collection
For the patients who were enrolled, we gathered clinical, biological, electrocardiographic (ECG), and echocardiographic data. Sex, age, body mass index (BMI) and abdominal circumference were included in the demographic characteristics. The patients’ smoking or non-smoking status was also assessed. We noted patient-associated comorbidities such as a history of AF and its type, hypertension (HTN), HF, diabetes mellitus (DM), chronic kidney disease (CKD) with clearance of creatinine between 60 and 30 mL/min/1.73 m2, chronic pulmonary obstructive disease (COPD), aortic atherosclerosis plaques, dyslipidemia, peripheral arterial disease (PAD), transient ischemic attack (TIA) and stroke. The coronary angiography was done using an Azurion 7 Philips machine and the severity of coronary stenosis was assed visually or using FFR/IFR (Fractional Flow Reserve /Instantaneous wave Free Ratio) when the situation required. Chronic treatment with statins, betablockers, angiotensin-converting-enzyme (ACE) inhibitors, antidiabetic medication, oral anticoagulants, antiplatelet agents or proton pump inhibitors (PPIs) in all patients was noted.
For the patients’ biological profiles, we gathered information on their galectin-3, pentraxin-3, total cholesterol, low-density lipoprotein-cholesterol (LDLc), high-density lipoprotein-cholesterol (HDLc), triglyceride levels and conducted liver function tests, including alanine aspartate transferase (AST), alanine aminotransferase (ALT), gamma-glutamyl-transferase (GGT), renal function and fasting blood sugar levels. Moreover, regarding inflammation status, c-reactive protein (CRP) was dosed; PIV, SII, Ly/N and Ly/WBC were calculated for each patient from hemoleucogram components.
2.3. Statistical Analysis
Statistical analysis was performed using the SPSS version 29.0 software package (SPSS Inc., Chicago, IL, USA). For the numerical data, standard descriptive statistics parameters were calculated (mean, standard deviation, minimum, maximum values, and the median). To compare the arithmetic means of quantitative variables in two samples, we used the students’ t-test, under the hypothesis that the values of the analyzed parameter follow a normal distribution (a fact that was tested beforehand using a goodness-of-fit test, such as the Kolmogorov-Smirnov test). When comparing the arithmetic means across multiple samples, which also adhere to normality, we employed the ANOVA (analysis of variance) test, after first testing for homogeneity of variances using Levene's test. In cases where the variances were not homogeneous, we used the Welch correction for the ANOVA test, which is known to be more robust. If the normal distribution of values is not met, we used the non-parametric Mann-Whitney test to compare a parameter between two samples, and the non-parametric Kruskal-Wallis test for comparing the same parameter across three or more samples; both tests are based on the analysis of ranks assigned to the values of the parameter being compared. In the case of comparisons between multiple samples, when we identified statistically significant differences, we also employed post-hoc tests to locate the differences between the samples taken two at a time. For the ANOVA test, we used the post-hoc Tukey test (specifically its Tukey-Kramer correction for unbalanced samples of different sizes); for the Welch ANOVA test, we employed the Games-Howell post-hoc test, and for the non-parametric Kruskal-Wallis alternative, we used the Dunn post-hoc procedure with Bonferroni adjustment.
The simplest method for testing differences between the values of a qualitative variable across different groups is the Chi-squared test, which we also employed in this study. To investigate the association between two quantitative variables, we calculated the Pearson correlation coefficient r, along with its significance level and the corresponding 95% confidence interval, which allowed for characterizing the direction and strength of this association. The significance level we used was the standard 0.05. Values of the significance coefficient p < 0.05 were characterized as statistically significant, while values of p < 0.01 were characterized as highly statistically significant. In order to determine the diagnostic performance of the included parameters, we compared the areas under the curve (AUC) that resulted from receiver operating characteristic (ROC) analysis.
2.3. Ethics
We conducted this study according to the ethical principles mentioned in the Declaration of Helsinki (revised in 2013). At admission, all patients signed a standard written informed consent in order to participate in the study. The research protocol was validated by the local Ethics Committees of both the University of Medicine and Pharmacy “Gr. T. Popa” (no. 352/09.10.2023), and of the St. Spiridon Emergency Clinical Hospital (no. 75/11.09.2023).
3. Results
3.1. Baseline Characteristics
We enrolled 131 patients, further divided into two subgroups: 65 patients with S-CCS (significant chronic coronary syndrome defined as stenosis ≥ 70% for one of the coronary arteries, except for left main where stenosis ≥ 50%) at coronarography examination and 66 patients with N-CCS (nonsignificant chronic coronary syndrome defined as stenosis < 70% for coronary arteries, except for left main where stenosis < 50%). In
Table 1, the general characteristics, comorbidities, treatment, echocardiographic and laboratory parameters of the patients included are summarized. We found a significantly higher prevalence of important cardiovascular risk factors among patients S-CCS, such as dyslipidemia, diabetes mellitus, and a low level of HDL-cholesterol, compared to the baseline characteristics of the patients from the control group. However, regarding other traditional cardiovascular risk factors (age, gender, family history of ischemic coronary disease, smoking status, hypertension, obesity), there were no noteworthy differences between the two groups.
There was a significant difference (p = 0.026), with a higher percentage of sinus rhythm in the S-CCS group (53.8%) compared to the N-CCS group (37.9%) and moreover, AF was more prevalent in the N-CCS group (62.1%) compared to the S-CCS group (46.2%), with a p value closed to statistically significant threshold (p=0.067). The overall prevalence of PAD in this cohort was 9.9%, with a significant difference between the N-CCS group (4.5%) and the S-CCS group (15.4%), as indicated by a p-value of 0.038.
The overall mean of BMI (body mass index) is 30.35 kg/m², with a statistically significant difference observed between the N-CCS group (31.27 kg/m²) and the S-CCS group (29.40 kg/m²), with a p-value of 0.031. However, despite the fact that overall prevalence of obesity is high in both groups (77.1%), there is no significant difference between the 2 groups (p = 0.195). Smoking prevalence is similar across both groups (58.0% overall), with no significant difference (p = 0.648). There is a trend toward higher abdominal circumference in the N-CCS group (110.64 cm) compared to the S-CCS group (106.40 cm), but the difference is not statistically significant (p = 0.087).
An echocardiographic assessment of the LV systolic function showed a mean LVEF of 48.91%, with a standard deviation of 11.4%; there is no statistical differences between the two groups. Other mean values calculated based on echocardiographic data were as follows:
Interventricular septum: 11.21 ± 1.663 mm;
LV posterior wall: 10.96 ± 1.459 mm;
LV end-diastolic diameter: 51.21 ± 7.081 mm.
Left atrial indexed volume: 38.93 ± 14.292 mL/m2
Right atrial indexed volume: 31.02 ± 11.715 mL/m2
A borderline significant difference (p = 0.050) in tricuspid regurgitation velocity suggests potential differences in right heart function between groups, but there are no differences between right ventricular longitudinal contractile function as there is no significant difference in tricuspid annular plane systolic excursion (p = 0.735).
Regarding biological parameters, there is a significant statistical difference (p = 0.004) for only high-density lipoprotein-cholesterol (HDLc), its levels being lower in the S-CCS group (40.88 mg/dL) compared to N-CCS (46.06 mg/dL), suggesting that this might be the most accurate parameter from lipid profile linked to coronary stenosis severity.
Marked differences in pharmacologic treatment were observed between the two groups. Among S-CCS patients, 60 out of 65 received dual antiplatelet therapy (aspirin and clopidogrel) following percutaneous coronary intervention (PCI), while the remaining 5 patients underwent coronary artery bypass grafting (CABG) and required SAPT. All patients who received DAPT were also prescribed PPIs, in accordance with recommendations for gastroprotection during dual antiplatelet therapy. The consistent use of nitrates and trimetazidine in the S-CCS group further reflects comprehensive, guideline-directed management for patients with established CAD. The broader use of lipid-lowering agents in the S-CCS group underscores the emphasis on secondary prevention and the recognition of higher atherosclerotic risk in these patients.
3.2. Inflammatory Biomarkers and Parameters According to the Severity of Coronary Stenosis and the Presence/Type of AF
To assess the relationship between inflammation, AF and coronary stenosis, patients were divided depending on CCS (N-CSS vs S-CCS) and AF (sinusal rhythm vs the type of AF). Not only galectin-3 and pentraxin-3 were analyzed, but also CRP (C- reactive protein) and others inflammatory markers were included in the study such as PIV, SII, Ly/N and Ly/WBC (
Table 2).
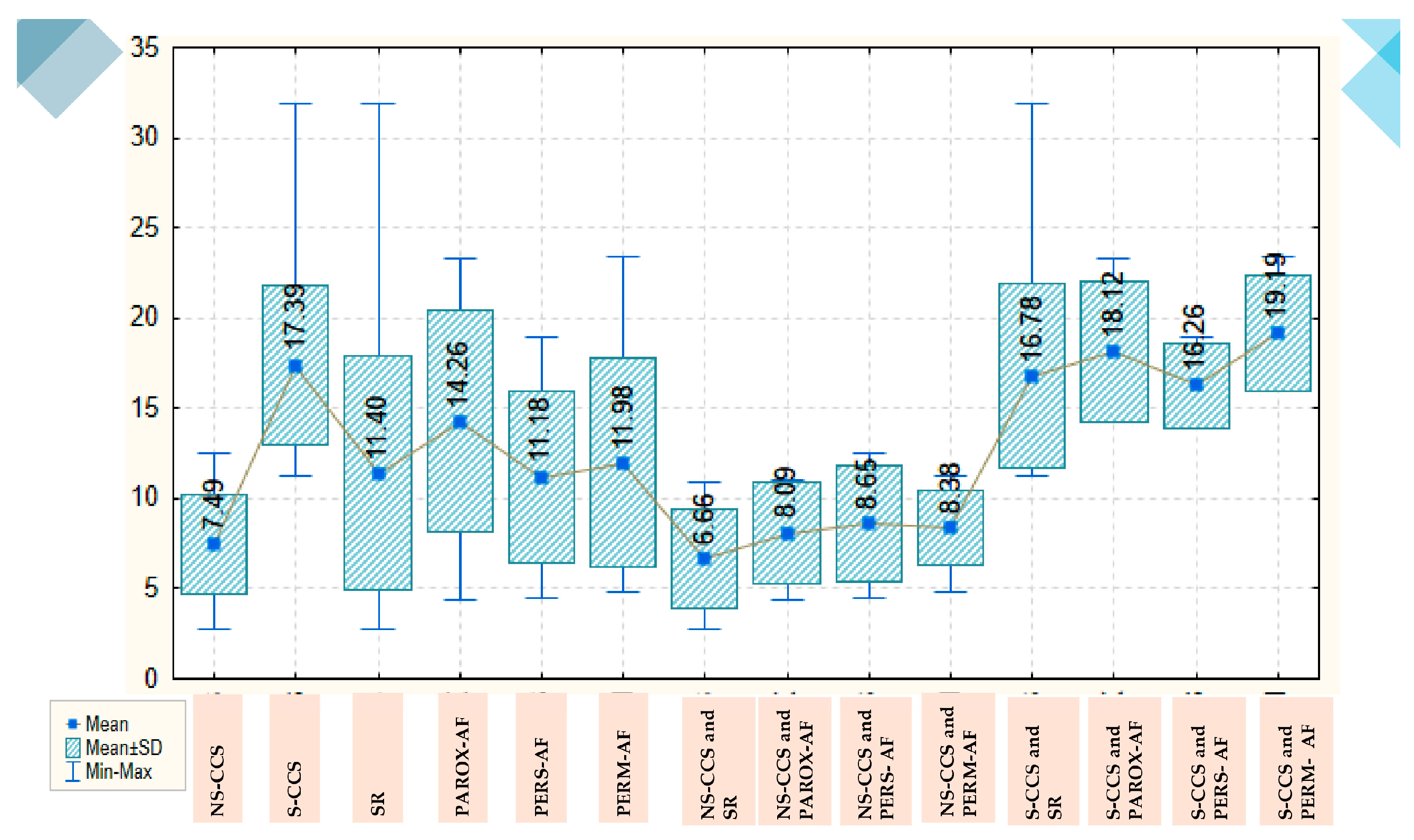
The galectin-3 value is more than double in patients with S-CCS compared with those N-CCS, with a mean of 17.39 ± 4,459 versus 7.49 ± 2,732, which is an important and statistically significant difference (p<0.001). AF, however, is not associated with statistically significant variations in galectin-3 values, neither globally nor separately in the group of patients with S-CCS or N-CCS. Overall, patients with SR have a mean galectin-3 level of 11.40 ± 6,482, which remains similar in those with persistent or permanent AF and increases by about 3 units in those with paroxysmal AF (to a mean of 14. 26 ± 6.111). In the group of N-CCS, galectin-3 values are decreased: thus, in those with SR the mean value recorded is 6.66 ± 2.777 and increases by about 2 units in those with various types of AF. In the group of S-CCS, the mean value of galectin-3 in those with SR is significantly higher (16.78 ± 5.111) and also increases by about 2 units in patients with various types of AF (
Figure 1).
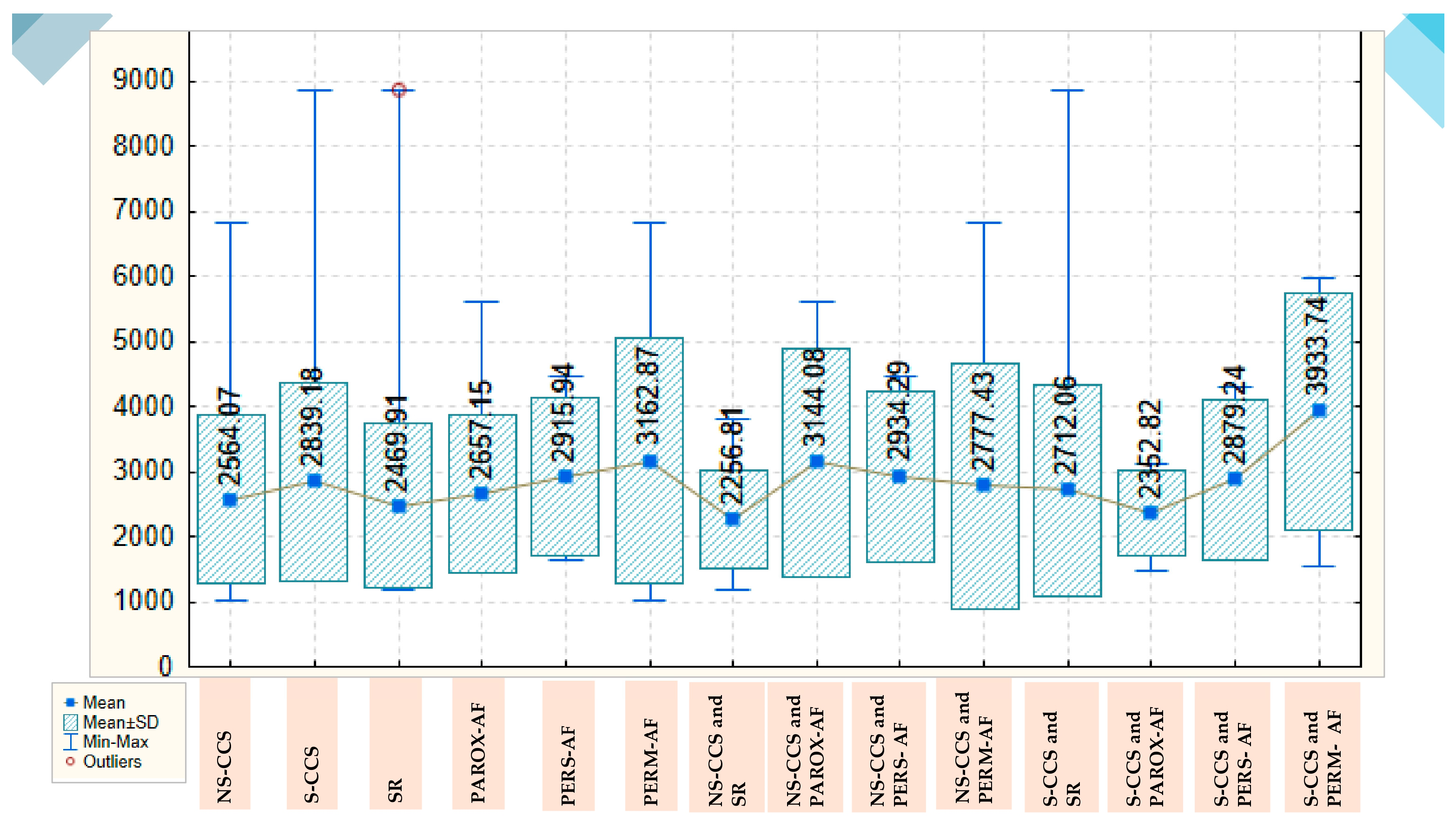
Pentraxin-3 values are also higher in S-CCS compared to N-CCS, with a mean of 2839.18 ± 1521.639 versus 2564.07 ± 1299.055. These values are also lower in patients with SR, with a mean of 2469.91 ± 1253.782, and steadily increase in those with paroxysmal, persistent and permanent AF, for whom they reach a mean of 3162.87 ± 1893.068. In the group of N-CCS, the lowest values for pentraxin-3 are also found in those with SR, with a mean of 2256.81 ± 763.433, the highest values being observed in those with paroxysmal AF, with a mean of 3144.08 ± 1751.972. In the group of S-CCS, the mean value of pentraxin-3 in those with SR is 2712.06 ± 1631.495, decreases slightly in those with paroxysmal AF, to a mean of 2352.82 ± 659.705 and reaches a maximum for patients with permanent AF (3933.74 ± 1819.380); there are clear variations, but the statistical significance threshold is not reached (
Figure 2).
PIV values are also higher in S-CCS, with a mean of 44.76 ± 30.775, compared to those with N-CCS, with a mean of 37.40 ± 24.584, being a difference in the immediate vicinity of the statistical significance threshold (p=0.057). Also, the mean PIV values in patients with SR is 37.95 ± 29.637 and increases in those with various types of AF, reaching a maximum of 45.00 ± 28.786 in those with permanent AF, but differences that this time are not statistically significant. SII values are visibly higher in patients with S-CCS, with a mean of 681.14 ± 352.469, compared to those with N-CCS, with a mean of 621.13 ± 341.148. The highest values are also found in patients with paroxysmal AF, with a mean of 715.17 ± 359.110, and the lowest in those with SR, with a mean of 602.89 ± 355.585, while patients with persistent or permanent AF have mean values for SII intermediate between the extremes already highlighted. None of these differences, however, are statistically significant.
On the other hand, the Ly/N ratio is slightly higher in N-CCS, with a mean of 0.46 ± 0.205, compared to S-CCS (0.41 ± 0.185). Patients with SR have slightly higher values of this ratio than those with various types of AF, with a mean of 0.47 ± 0.194. In the group of N-CCS, the highest values for the Ly/N ratio are also observed in patients with SR, with a mean of 0.51 ± 0.190, and the lowest are found in patients with paroxysmal AF, with a mean of 0.32 ± 0.103. The variations thus found are in the neighborhood of the statistical significance threshold (p=0.060). The Ly/WBC ratio is also higher in patients with N-CCS, with a mean of 0.29 ± 0.088, compared to those with S-CCS (0.26 ± 0.079), being a difference close to the statistical significance threshold (p=0.063), and also in patients with SR, with a mean of 0.28 ± 0.083, compared to those with various types of AF, with a mean of about 0.26. The highest values of this ratio are found in patients with N-CCS and SR, with a mean of 0.31 ± 0.091, and the lowest in patients with S-CCS and permanent AF, with a mean of 0.22 ± 0.078.
Regarding CRP, mean values are slightly lower in patients with N-CCS (0.53 ± 0.772) compared to those with S- CCS (0.75 ± 1.105). These values are also lower in patients with SR, with a mean of 0.50 ± 0.717, or persistent AF, with a mean of 0.37 ± 0.211. The highest CRP values were found in patients with S-CCS and permanent AF (1.15 ± 1.898).
3.3. Diagnostic Performance of Galectin-3 in CCS
We introduced the parameters with statistically significant differences between the S-CCS and N-CCS (galectin-3, BMI, TRV and HDLc) into a binary logistic regression model. The model was constructed using the Enter method. The Hosmer-Lemeshow split test indicates that the constructed model is viable, as its results are statistically insignificant (p = 1.000). Initially, the prediction accuracy for S-CCS was 55.2%, and at the end it reached 97.7%, indicating that the identified predictors are useful. The model explains 95.8% of the variance in the diagnosis of S-CCS (Nagelkerke R² = 0.958), thus confirming its usefulness, and has a sensitivity of 97.4% and a specificity of 97.9%. Of all predictors tested, only galectin-3 has a statistically significant role, the rest did not contribute significantly to the model. Increasing the level of galectin-3 by one unit increases the odds of significant lesion by 14.003-fold, when the other predictors are held constant. These results suggest that galectin-3 may be a relevant biomarker for identifying patients with significant coronary stenosis (
Table 3).
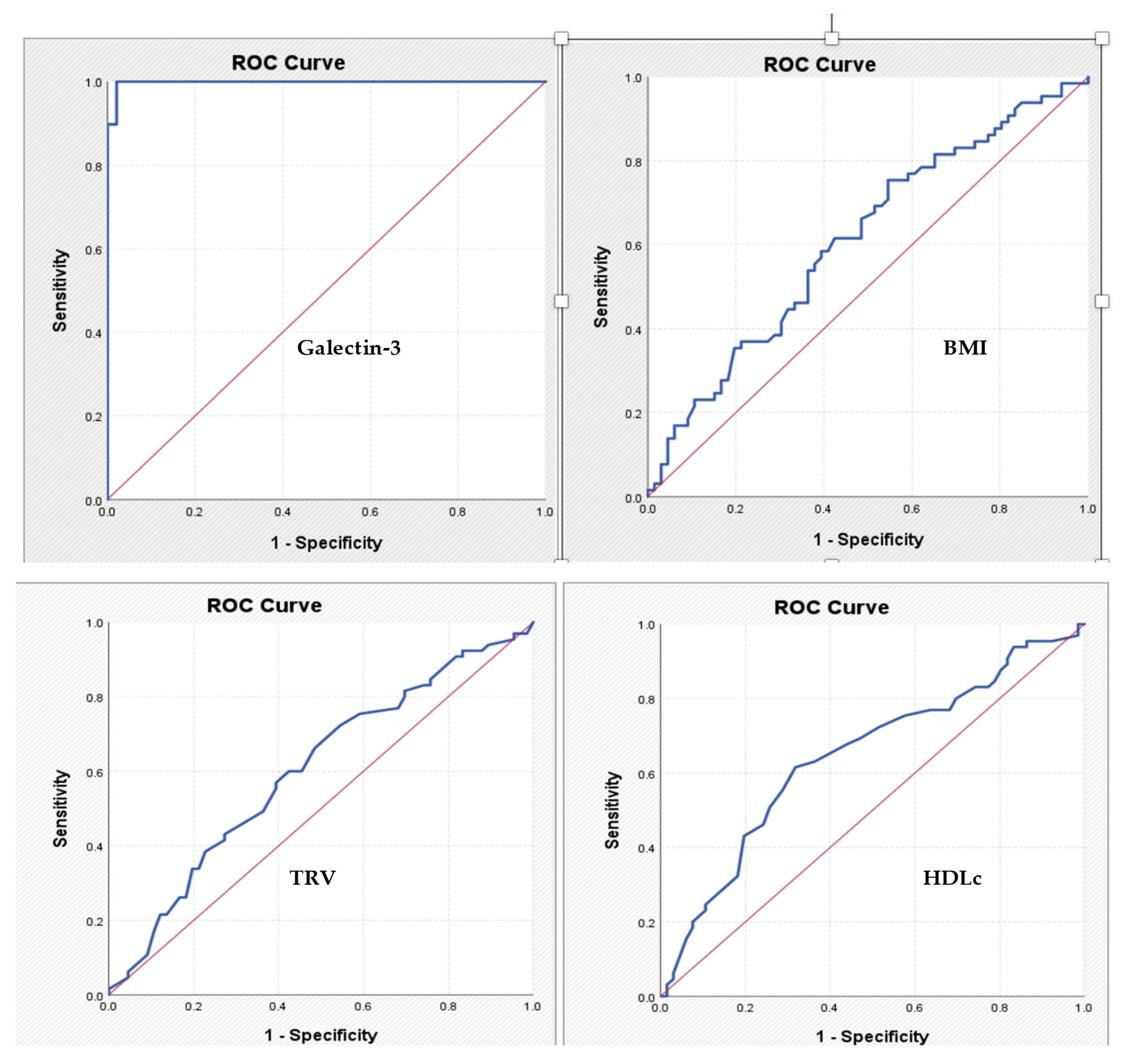
For galectin-3, the cut-off value identified is 11.63 - the corresponding AUC coefficient is 0.998, which means an excellent discriminatory power, with a sensitivity of 97.4% and a specificity of 97.9%. For the other tested parameters, the power of discrimination is much lower and the identified cut-off values have modest sensitivity and specificity coefficients (
Table 4,
Figure 3).
3.4. Galectin-3: Correlations with the Other Inflammatory Parameters
We analyzed the Pearson correlation for the parameters with statistically significant differences between the S-CCS and N-CCS (galectin-3, BMI, TRV and HDLc) and also between galectin-3 and other inflammatory parameters. Galectin-3 is statistically significantly inversely correlated, albeit weakly, with HDLc, which supports the protective role of HDLc in atherosclerosis. There is also a moderate positive correlation between Galectin 3 and PIV, which is statistically significant (p-value = 0.009) with a Confidence Interval of [-0.459, -0.068]. Moreover, there is a weak negative correlation between Galectin-3, Ly/N and respectively Ly/WBC; although the relationship is not very strong, it is statistically significant, suggesting again the protective role of lymphocytes against inflammation and atherosclerosis (
Table 5).
4. Discussion
This study aimed to explore the role of galectin-3 and pentraxin-3 as biomarkers in patients with CCS and AF, highlighting their potential for improving diagnostic accuracy in these overlapping conditions. Moreover, the study offers a comprehensive comparison between patients with N-CCS and S-CCS, highlighting notable differences in clinical characteristics, comorbidities, echocardiographic findings, biochemical profiles, and treatment approaches.
Interestingly, patients with S-CCS exhibited a significantly lower BMI compared to those with N-CCS. This observation aligns with the "obesity paradox", wherein overweight or mildly obese individuals with established cardiovascular disease (CVD) may experience better prognoses than their normal-weight counterparts. Several studies have documented this phenomenon, suggesting that excess adiposity might confer protective benefits in certain CVD contexts. However, the underlying mechanisms remain a topic of ongoing research. This underscores the complexity of cardiovascular risk factors, where traditional metrics like BMI may not fully capture the nuances of disease progression. Despite the lower BMI, S-CCS patients demonstrated a higher prevalence of dyslipidemia and PAD, both indicative of advanced systemic atherosclerosis [
19,
20].
In this study, SR was observed in 45.8% of patients, with a statistically significant difference between the S-CCS group (53.8%) and the N-CCS group (37.9%) (p = 0.026). This suggests also a higher prevalence of AF among patients with N-CCS, an observation that may initially appear counterintuitive. However, emerging evidence suggests that even non-obstructive coronary lesions can contribute to the development of AF. The LIFE-Heart Study demonstrated that patients with coronary artery sclerosis—defined as non-critical plaques with stenosis <75%—had a higher prevalence of AF compared to those with obstructive CAD. Specifically, AF was present in 11.3% of patients with coronary artery sclerosis versus 4.7% in those with obstructive CAD. This counterintuitive finding may be explained by the role of atrial ischemia in AF pathogenesis. Non-obstructive plaques can still impair atrial perfusion, leading to structural and electrical remodeling that predisposes to AF. Additionally, patients with N-CCS may have a higher burden of comorbidities such as hypertension and diabetes, which are known risk factors for AF [
21,
22]. Moreover, the presence of AF in patients with less severe coronary lesions may be underrecognized. The CASS registry reported a low prevalence of AF (0.6%) among patients with angiographically proven CAD, suggesting potential underdiagnosis or underreporting. This underestimation underscores the need for vigilant screening for AF in patients with N-CCS, as early detection and management are crucial for preventing adverse outcomes [
23].
Echocardiographic parameters were largely comparable between the N-CCS and S-CCS groups, with no statistically significant differences observed in LVDD, IVS, LVPW or LVEF. Similarly, indexed left and right atrial volumes were not significantly different between groups, suggesting a comparable degree of atrial structural remodeling. Of note, TRV was marginally lower in the S-CCS group (2.12 ± 0.616 m/s) compared to the N-CCS group (2.33 ± 0.613 m/s), with a p-value of 0.050, nearing statistical significance. TRV is commonly used as a surrogate for estimating PAPs, and elevated values are associated with pulmonary hypertension and right-sided cardiac pressure overload. The slightly lower TRV in S-CCS patients may reflect more preserved right ventricular-pulmonary circulation dynamics or potentially a difference in left-sided filling pressures and pulmonary venous backflow. However, as PAPs and TAPSE did not differ significantly, the clinical relevance of this finding remains unclear. It is possible that patients with N-CCS may have subclinical diastolic dysfunction or occult pulmonary vascular disease contributing to this observation. Future studies with comprehensive hemodynamic assessments or advanced imaging techniques such as right heart catheterization or speckle-tracking echocardiography may help clarify this association.
Analysis of biochemical markers revealed that most parameters—including glycemic indices, renal function (creatinine, estimated glomerular filtration rate), hepatic enzymes (AST, ALT, GGT), and other lipid fractions (LDLc, total cholesterol, triglycerides)—did not significantly differ between N-CCS and S-CCS groups. However, a notable exception was observed in HDLc levels, which were significantly lower in S-CCS patients (40.88 ± 10.848 mg/dL vs. 46.06 ± 11.395 mg/dL in N-CCS, p = 0.004). HDLc plays a critical anti-atherogenic role by promoting reverse cholesterol transport, exerting antioxidant and anti-inflammatory effects, and improving endothelial function. Reduced levels of HDLc have been consistently linked to increased cardiovascular risk and more extensive coronary atherosclerosis. In this study, the lower HDLc in the S-CCS group may reflect an impaired atheroprotective lipid profile contributing to the severity of coronary lesions. Additionally, HDLc is sensitive to systemic inflammation, oxidative stress, and metabolic dysfunction—all of which are frequently present in patients with significant CAD. These findings suggest that HDLc may serve as a particularly sensitive biomarker in differentiating patients at higher risk for significant coronary pathology, even when other lipid or metabolic markers appear within acceptable ranges. This emphasizes the importance of evaluating not only LDLc levels but also the qualitative aspects of the lipid profile when assessing cardiovascular risk and planning lipid-lowering therapy [
24,
25].
Our findings strongly support the clinical relevance of galectin-3 as a biomarker in CCS. Serum levels of galectin-3 were more than double in patients with S-CCS compared to those with N-CCS, with a high statistical significance (p < 0.001). This aligns with previous findings, such as those by Li et al., who demonstrated galectin-3’s association with the presence, complexity, and severity of CAD, as well as its potential to predict adverse events due to its involvement in myocardial fibrosis and inflammation. In addition, galectin-3 has been identified as a marker of cardiac remodeling and progression to heart failure in various clinical settings, further reinforcing its diagnostic and therapeutic relevance in chronic ischemic conditions [
4].
Interestingly, while galectin-3 correlated robustly with the severity of coronary lesions, it did not vary significantly between patients with and without AF. This finding diverges slightly from other studies, such as those by Procyk et al, which suggest galectin-3 levels may help distinguish AF subtypes and predict recurrence post-ablation or postoperatively. However, the absence of a significant trend in our cohort might be attributed to sample distribution or the underlying predominance of ischemic pathology over arrhythmogenic remodeling [
6].
In contrast, pentraxin-3, an acute-phase protein with regulatory roles in inflammation and endothelial function, exhibited a progressive increase across AF subtypes, with the highest levels in patients with permanent AF. These trends, while not statistically significant, mirror the findings of Soeki et al., who described localized pentraxin-3 elevation in the left atrium during AF as a reflection of local inflammatory processes. Additionally, although pentraxin-3 levels were higher in the S-CCS group, the variation was modest and non-significant, which is consistent with meta-analyses reporting a stronger association of pentraxin-3 with acute coronary syndromes rather than stable CAD [
8,
11].
The interplay between galectin-3 and pentraxin-3 observed in our study underscores their distinct yet complementary roles in cardiovascular pathophysiology. Galectin-3 appears to predominantly reflect pro-inflammatory and profibrotic activity, contributing to coronary plaque progression and myocardial remodeling. In contrast, pentraxin-3 may signify a more nuanced immune response, potentially serving as a regulatory factor that dampens excessive inflammation and promotes plaque stabilization. This dual behavior is consistent with findings by Melvin et al., who demonstrated that elevated levels of both galectin-3 and pentraxin-3 in patients with stable CAD were associated with increased cardiovascular risk. However, they also highlighted that the biomarkers act through non-redundant pathways, with galectin-3 reflecting ongoing fibrotic stress and pentraxin-3 possibly indicating an adaptive or protective immune response [
12,
26].
Composite inflammatory indices, including the PIV, SII, Ly/N and Ly/WBC, provide a comprehensive reflection of systemic immune activation and inflammatory burden. In the present study, elevated levels of PIV and SII were noted in S-CCS and AF subgroups, with values approaching statistical significance. These observations are consistent with the expanding body of literature supporting the prognostic relevance of such composite inflammatory markers in cardiovascular risk stratification. Prior investigations in both NSTEMI and stable CAD cohorts have demonstrated that heightened systemic inflammatory activity, as captured by these indices, correlates with adverse cardiovascular outcomes. Moreover, the observed inverse correlations between galectin-3 and HDLc, Ly/N, and Ly/WBC ratios further underscore the complex interplay between lipid metabolism, immune modulation, and vascular inflammation. These findings reinforce the clinical relevance of integrating systemic inflammatory biomarkers and lipid parameters into a multidimensional approach for cardiovascular risk assessment and underscore the pathophysiological interdependence of metabolic and immune pathways in the progression of atherosclerotic disease [
14,
16,
27,
28].
Finally, the diagnostic performance of galectin-3 was remarkable. A cut-off value of 11.63 ng/mL yielded both sensitivity and specificity above 97%, as confirmed by ROC analysis (AUC = 0.998). These results underscore its potential as a robust non-invasive tool for identifying patients with significant coronary lesions and might aid in optimizing the use of coronary angiography.
Study Limitations
This study has several limitations that must be acknowledged when interpreting the results. Firstly, the research was conducted in a single-center setting, which inherently limits the generalizability of the findings. Patient populations, clinical practices, and resource availability may vary significantly across institutions and geographic regions, potentially affecting the external validity of the conclusions drawn. Secondly, the sample size, although adequate for initial exploratory analyses, remains relatively modest. With a total of 131 patients divided into two nearly equal groups (N-CCS and S-CCS), the statistical power to detect subtle but clinically meaningful differences may have been limited, particularly for parameters that approached significance. A larger cohort would enhance the robustness of the statistical analyses and allow for multivariable modeling to control for potential confounding variables.
Another notable limitation pertains to the measurement of emerging inflammatory and fibrosis biomarkers, specifically galectin-3 and pentraxin-3. Due to resource constraints, only a single determination of each biomarker was performed per patient, exclusively at the time of admission. This restricted sampling approach precludes assessment of temporal dynamics, treatment response, or correlation with long-term outcomes. Given the evolving role of galectin-3 and pentraxin-3 in cardiovascular pathophysiology—especially their associations with myocardial fibrosis, vascular inflammation, and prognosis in CAD—the lack of serial measurements represents a missed opportunity for a more nuanced analysis. However, we consider this small study as a pilot one. Therefore, based on these results we can now develop a larger one.
5. Conclusions
This study provides compelling evidence that galectin-3 is a powerful biomarker associated with the severity of CCS. Its high diagnostic accuracy and strong correlation with coronary stenosis severity might position it as a possible valuable tool in clinical decision-making, particularly in stratifying patients for invasive diagnostic procedures.
Pentraxin-3, while less specific for coronary artery disease, may provide complementary insights in the context of AF, reflecting underlying atrial inflammation and disease chronicity. When combined, these biomarkers may offer a nuanced understanding of the inflammatory burden in patients with CCS and AF.
The integration of inflammatory indices (PIV, SII, Ly/N, Ly/WBC) further enhances patient profiling by capturing systemic immune dynamics. These indices, alongside biomarker data, may contribute to a more personalized and predictive cardiovascular care model.
Future multicenter studies with larger cohorts and longitudinal follow-up are warranted to validate these findings and explore their prognostic implications. Moreover, therapeutic targeting of the galectin-3 and pentraxin-3 pathways could open new avenues in the management of inflammation-driven cardiovascular disease.
Author Contributions
Conceptualization, A.-F.O. and M.F.; methodology, P.C.M., M.G. and S.D.D.; software, P.C.M., M.G. and S.D.D.; validation, A.J., M.M. and D.I.; formal analysis, A.M.B., R.S.M. and D.I.; investigation, A.M.B., R.S.M. and D.I.; resources A.J., M.M. and D.I.; data curation, C.D. and R.M.; writing—original draft preparation, A.-F.O.; writing—review and editing, A.-F.O. and M.F.; visualization, A.-F.O. and M.F.; supervision, I.I.C.-E., M.F. and D.M.T.; project administration, I.I.C.-E., R.M. and D.M.T.; funding acquisition, C.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research is a study from a PhD thesis, and it received research funding from University of Medicine and Pharmacy “Gr. T. Popa”.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board (or Ethics Committee) of University of Medicine and Pharmacy “Gr. T. Popa” (no. 352/09.10.2023) and of the St. Spiridon Emergency Clinical Hospital (no. 75/11.09.2023).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study. Written informed consent has been obtained from the patient(s) to publish this paper.
Data Availability Statement
All the data presented in this study are available within the article. The first author has all data used in this study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Hu, H.-L.; Wang, Y.-Y.; Jing, D.-T.; Zhu, F. Galectin-3 as a Novel Biomarker of Predicting Prognosis in Patients with Coronary Artery Disease. Angiology 2024, 75, 598–599. [Google Scholar] [CrossRef] [PubMed]
- Oancea, A.F.; Jigoranu, R.A.; Morariu, P.C.; Miftode, R.-S.; Trandabat, B.A.; Iov, D.E.; Cojocaru, E.; Costache, I.I.; Baroi, L.G.; Timofte, D.V.; et al. Atrial Fibrillation and Chronic Coronary Ischemia: A Challenging Vicious Circle. Life 2023, 13, 1370. [Google Scholar] [CrossRef]
- Oancea, A.-F.; Morariu, P.; Buburuz, A.; Miftode, I.-L.; Miftode, R.; Mitu, O.; Jigoranu, A.; Floria, D.-E.; Timpau, A.; Vata, A.; et al. Spectrum of Non-Obstructive Coronary Artery Disease and Its Relationship with Atrial Fibrillation. JCM 2024, 13, 4921. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Guo, K.; Huang, X.; Feng, L.; Yuan, Y.; Li, J.; Lao, Y.; Guo, Z. Association Between Serum Galectin-3 Levels and Coronary Stenosis Severity in Patients With Coronary Artery Disease. Front. Cardiovasc. Med. 2022, 9, 818162. [Google Scholar] [CrossRef]
- Blanda, V.; Bracale, U.M.; Di Taranto, M.D.; Fortunato, G. Galectin-3 in Cardiovascular Diseases. IJMS 2020, 21, 9232. [Google Scholar] [CrossRef] [PubMed]
- Procyk, G.; Czapla, A.; Jałocha, K.; Tymińska, A.; Grabowski, M.; Gąsecka, A. The Role of Galectin-3 in Atrial Fibrillation. J Mol Med 2023, 101, 1481–1492. [Google Scholar] [CrossRef]
- Casula, M.; Montecucco, F.; Bonaventura, A.; Liberale, L.; Vecchié, A.; Dallegri, F.; Carbone, F. Update on the Role of Pentraxin 3 in Atherosclerosis and Cardiovascular Diseases. Vascular Pharmacology 2017, 99, 1–12. [Google Scholar] [CrossRef]
- Chu, Y.; Teng, J.; Feng, P.; Liu, H.; Wang, F.; Li, X. Pentraxin-3 in Coronary Artery Disease: A Meta-Analysis. Cytokine 2019, 119, 197–201. [Google Scholar] [CrossRef]
- Kamarullah, W.; Nurcahyani; Multazam, R.B.; Josephine, C.M. Pentraxin 3 Concentration Is Associated with Poor Outcomes in Patients with Coronary Artery Disease: A Systematic Review and Dose–Response Meta-Analysis. Acta Cardiologica 2022, 77, 385–394. [Google Scholar] [CrossRef]
- Ristagno, G.; Fumagalli, F.; Bottazzi, B.; Mantovani, A.; Olivari, D.; Novelli, D.; Latini, R. Pentraxin 3 in Cardiovascular Disease. Front. Immunol. 2019, 10, 823. [Google Scholar] [CrossRef]
- Soeki, T.; Bando, S.; Uematsu, E.; Matsuura, T.; Niki, T.; Ise, T.; Kusunose, K.; Hotchi, J.; Ueda, Y.; Tomita, N.; et al. Pentraxin 3 Is a Local Inflammatory Marker in Atrial Fibrillation. Heart Vessels 2014, 29, 653–658. [Google Scholar] [CrossRef]
- George, M.; Shanmugam, E.; Srivatsan, V.; Vasanth, K.; Ramraj, B.; Rajaram, M.; Jena, A.; Sridhar, A.; Chaudhury, M.; Kaliappan, I. Value of Pentraxin-3 and Galectin-3 in Acute Coronary Syndrome: A Short-Term Prospective Cohort Study. Therapeutic Advances in Cardiovascular Disease 2015, 9, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Floria, M.; Oancea, A.F.; Morariu, P.C.; Burlacu, A.; Iov, D.E.; Chiriac, C.P.; Baroi, G.L.; Stafie, C.S.; Cuciureanu, M.; Scripcariu, V.; et al. An Overview of the Pharmacokinetics and Pharmacodynamics of Landiolol (an Ultra-Short Acting Β1 Selective Antagonist) in Atrial Fibrillation. Pharmaceutics 2024, 16, 517. [Google Scholar] [CrossRef]
- Cetinkaya, Z.; Kelesoglu, S.; Tuncay, A.; Yilmaz, Y.; Karaca, Y.; Karasu, M.; Secen, O.; Cinar, A.; Harman, M.; Sahin, S.; et al. The Role of Pan-Immune-Inflammation Value in Determining the Severity of Coronary Artery Disease in NSTEMI Patients. JCM 2024, 13, 1295. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Nie, H.; Bu, G.; Tong, X.; Bai, X. Systemic Immune-Inflammation Index (SII) and the Risk of All-Cause, Cardiovascular, and Cardio-Cerebrovascular Mortality in the General Population. Eur J Med Res 2023, 28, 575. [Google Scholar] [CrossRef]
- Erdoğan, M.; Erdöl, M.A.; Öztürk, S.; Durmaz, T. Systemic Immune-Inflammation Index Is A Novel Marker to Predict Functionally Significant Coronary Artery Stenosis. Biomark. Med. 2020, 14, 1553–1561. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Liu, L.; Chai, M.; Cai, Z.; Wang, D. Predictive Value of Neutrophil to Lymphocyte Ratio for Clinical Outcome in Patients with Atrial Fibrillation: A Systematic Review and Meta-Analysis. Front Cardiovasc Med 2024, 11, 1461923. [Google Scholar] [CrossRef]
- Guo, X.; Zhang, S.; Yan, X.; Chen, Y.; Yu, R.; Long, D.; Sang, C.; Du, X.; Dong, J.; Ma, C. Postablation Neutrophil/Lymphocyte Ratio Correlates with Arrhythmia Recurrence after Catheter Ablation of Lone Atrial Fibrillation. Chin Med J (Engl) 2014, 127, 1033–1038. [Google Scholar] [CrossRef]
- Oga, E.A.; Eseyin, O.R. The Obesity Paradox and Heart Failure: A Systematic Review of a Decade of Evidence. Journal of Obesity 2016, 2016, 1–9. [Google Scholar] [CrossRef]
- Bundhun, P.K.; Li, N.; Chen, M.-H. Does an Obesity Paradox Really Exist After Cardiovascular Intervention?: A Systematic Review and Meta-Analysis of Randomized Controlled Trials and Observational Studies. Medicine (Baltimore) 2015, 94, e1910. [Google Scholar] [CrossRef]
- Kornej, J.; Henger, S.; Seewöster, T.; Teren, A.; Burkhardt, R.; Thiele, H.; Thiery, J.; Scholz, M. Prevalence of Atrial Fibrillation Dependent on Coronary Artery Status: Insights from the LIFE-Heart Study. Clinical Cardiology 2020, 43, 1616–1623. [Google Scholar] [CrossRef] [PubMed]
- Nucifora, G.; Schuijf, J.D.; Tops, L.F.; Van Werkhoven, J.M.; Kajander, S.; Jukema, J.W.; Schreur, J.H.M.; Heijenbrok, M.W.; Trines, S.A.; Gaemperli, O.; et al. Prevalence of Coronary Artery Disease Assessed by Multislice Computed Tomography Coronary Angiography in Patients With Paroxysmal or Persistent Atrial Fibrillation. Circ: Cardiovascular Imaging 2009, 2, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Cameron, A.; Schwartz, M.J.; Kronmal, R.A.; Kosinski, A.S. Prevalence and Significance of Atrial Fibrillation in Coronary Artery Disease (CASS Registry). The American Journal of Cardiology 1988, 61, 714–717. [Google Scholar] [CrossRef] [PubMed]
- Bagheri, B.; Alikhani, A.; Mokhtari, H.; Rasouli, M. Esterification of HDL-Cholesterol Is Decreased in Diabetes Mellitus and CAD and Enhanced Following Treatment with Statins. Med Arch 2018, 72, 197–201. [Google Scholar] [CrossRef]
- Guo, X.; Ma, L. Inflammation in Coronary Artery Disease-Clinical Implications of Novel HDL-Cholesterol-Related Inflammatory Parameters as Predictors. Coron Artery Dis 2023, 34, 66–77. [Google Scholar] [CrossRef]
- Maiolino, G.; Rossitto, G.; Pedon, L.; Cesari, M.; Frigo, A.C.; Azzolini, M.; Plebani, M.; Rossi, G.P. Galectin-3 Predicts Long-Term Cardiovascular Death in High-Risk Patients With Coronary Artery Disease. ATVB 2015, 35, 725–732. [Google Scholar] [CrossRef]
- Prajapati, J.H.; Sahoo, S.; Nikam, T.; Shah, K.H.; Maheriya, B.; Parmar, M. Association of High Density Lipoprotein with Platelet to Lymphocyte and Neutrophil to Lymphocyte Ratios in Coronary Artery Disease Patients. Journal of Lipids 2014, 2014, 1–8. [Google Scholar] [CrossRef]
- Varol, E.; Bas, H.A.; Aksoy, F.; Ari, H.; Ozaydin, M. Relationship Between Neutrophil–Lymphocyte Ratio and Isolated Low High-Density Lipoprotein Cholesterol. Angiology 2014, 65, 630–633. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).