Submitted:
19 July 2024
Posted:
20 July 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Echocardiographic Examination
2.3. Measurement of Serum Galectin-3 Concentration
2.4. Statistical Analysis
3. Results
3.1. Study Population and Echocardiographic Parameters
3.2. Serum Galectin-3 Concentration
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Blanda, V.; Bracale, U.M.; Di Taranto, M.D.; Fortunato, G. Galectin-3 in Cardiovascular Diseases. International Journal of Molecular Sciences 2020, 21, 9232. [Google Scholar] [CrossRef]
- Chen, S.-C.; Kuo, P.-L. The Role of Galectin-3 in the Kidneys. International Journal of Molecular Sciences 2016, 17, 565. [Google Scholar] [CrossRef]
- Vora, A.; de Lemos, J.A.; Ayers, C.; Grodin, J.L.; Lingvay, I. Association of Galectin-3 With Diabetes Mellitus in the Dallas Heart Study. The Journal of Clinical Endocrinology & Metabolism 2019, 104, 4449–4458. [Google Scholar] [CrossRef]
- Cheng, D.; Liang, B.; Li, Y. Serum Galectin-3 as a Potential Marker for Gastric Cancer. Med Sci Monit 2015, 21, 755–760. [Google Scholar] [CrossRef]
- Ho, J.E.; Liu, C.; Lyass, A.; Courchesne, P.; Pencina, M.J.; Vasan, R.S.; Larson, M.G.; Levy, D. Galectin-3, a Marker of Cardiac Fibrosis, Predicts Incident Heart Failure in the Community. Journal of the American College of Cardiology 2012, 60, 1249–1256. [Google Scholar] [CrossRef]
- de Boer, R.A.; Voors, A.A.; Muntendam, P.; van Gilst, W.H.; van Veldhuisen, D.J. Galectin-3: A Novel Mediator of Heart Failure Development and Progression. European Journal of Heart Failure 2009, 11, 811–817. [Google Scholar] [CrossRef]
- Lok, D.J.A.; Van Der Meer, P.; de la Porte, P.W.B.-A.; Lipsic, E.; Van Wijngaarden, J.; Hillege, H.L.; van Veldhuisen, D.J. Prognostic Value of Galectin-3, a Novel Marker of Fibrosis, in Patients with Chronic Heart Failure: Data from the DEAL-HF Study. Clin Res Cardiol 2010, 99, 323–328. [Google Scholar] [CrossRef]
- de Boer, R.A.; Yu, L.; van Veldhuisen, D.J. Galectin-3 in Cardiac Remodeling and Heart Failure. Curr Heart Fail Rep 2010, 7, 1–8. [Google Scholar] [CrossRef]
- Gehlken, C.; Suthahar, N.; Meijers, W.C.; de Boer, R.A. Galectin-3 in Heart Failure: An Update of the Last 3 Years. Heart Failure Clinics 2018, 14, 75–92. [Google Scholar] [CrossRef]
- Yancy, C.W.; Jessup, M.; Bozkurt, B.; Butler, J.; Casey, D.E.; Colvin, M.M.; Drazner, M.H.; Filippatos, G.S.; Fonarow, G.C.; Givertz, M.M.; et al. 2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Circulation 2017, 136, e137–e161. [Google Scholar] [CrossRef]
- Lok, D.J.; Lok, S.I.; Bruggink-André de la Porte, P.W.; Badings, E.; Lipsic, E.; van Wijngaarden, J.; de Boer, R.A.; van Veldhuisen, D.J.; van der Meer, P. Galectin-3 Is an Independent Marker for Ventricular Remodeling and Mortality in Patients with Chronic Heart Failure. Clin Res Cardiol 2013, 102, 103–110. [Google Scholar] [CrossRef]
- de Boer, R.A.; Lok, D.J.A.; Jaarsma, T.; van der Meer, P.; Voors, A.A.; Hillege, H.L.; van Veldhuisen, D.J. Predictive Value of Plasma Galectin-3 Levels in Heart Failure with Reduced and Preserved Ejection Fraction. Annals of Medicine 2011, 43, 60–68. [Google Scholar] [CrossRef]
- Joglar, J.A.; Chung, M.K.; Armbruster, A.L.; Benjamin, E.J.; Chyou, J.Y.; Cronin, E.M.; Deswal, A.; Eckhardt, L.L.; Goldberger, Z.D.; Gopinathannair, R.; et al. 2023 ACC/AHA/ACCP/HRS Guideline for the Diagnosis and Management of Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2024, 149. [Google Scholar] [CrossRef]
- Pauklin, P.; Zilmer, M.; Eha, J.; Tootsi, K.; Kals, M.; Kampus, P. Markers of Inflammation, Oxidative Stress, and Fibrosis in Patients with Atrial Fibrillation. Oxidative Medicine and Cellular Longevity 2022, 2022, e4556671. [Google Scholar] [CrossRef]
- Burstein, B.; Nattel, S. Atrial Fibrosis: Mechanisms and Clinical Relevance in Atrial Fibrillation. Journal of the American College of Cardiology 2008, 51, 802–809. [Google Scholar] [CrossRef]
- Chen, D.; Procter, N.; Goh, V.; Liu, S.; Chua, S.J.; Assadi-Khansari, B.; Stewart, S.; Horowitz, J.D.; Sverdlov, A.L.; Ngo, D.T. New Onset Atrial Fibrillation Is Associated with Elevated Galectin-3 Levels. International Journal of Cardiology 2016, 223, 48–49. [Google Scholar] [CrossRef]
- Fashanu, O.E.; Norby, F.L.; Aguilar, D.; Ballantyne, C.M.; Hoogeveen, R.C.; Chen, L.Y.; Soliman, E.Z.; Alonso, A.; Folsom, A.R. Galectin-3 and Incidence of Atrial Fibrillation: The Atherosclerosis Risk in Communities (ARIC) Study. American Heart Journal 2017, 192, 19–25. [Google Scholar] [CrossRef]
- Gong, M.; Cheung, A.; Wang, Q.; Li, G.; Goudis, C.A.; Bazoukis, G.; Lip, G.Y.H.; Baranchuk, A.; Korantzopoulos, P.; Letsas, K.P.; et al. Galectin-3 and Risk of Atrial Fibrillation: A Systematic Review and Meta-analysis. Clinical Laboratory Analysis 2020, 34, e23104. [Google Scholar] [CrossRef]
- Procyk, G.; Czapla, A.; Jałocha, K.; Tymińska, A.; Grabowski, M.; Gąsecka, A. The Role of Galectin-3 in Atrial Fibrillation. J Mol Med 2023, 101, 1481–1492. [Google Scholar] [CrossRef]
- Lee, G.-W.; Kang, M.-H.; Ro, W.-B.; Song, D.-W.; Park, H.-M. Circulating Galectin-3 Evaluation in Dogs With Cardiac and Non-Cardiac Diseases. Front. Vet. Sci. 2021, 8, 741210. [Google Scholar] [CrossRef]
- Vichit, P.; Rungsipipat, A.; Surachetpong, S.D. Changes of Cardiac Function in Diabetic Dogs. Journal of Veterinary Cardiology 2018, 20, 438–450. [Google Scholar] [CrossRef]
- Ribeiro, C.; Santos, M.S.; DE Matos, A.J.; Barros, R.; Gärtner, F.; Rutteman, G.R.; DE Oliveira, J.T. Serum Galectin-3 Levels in Dogs with Metastatic and Non-Metastatic Mammary Tumors. In Vivo 2016, 30, 13–16. [Google Scholar]
- Stack, J.P.; Fries, R.C.; Kruckman, L.; Kadotani, S.; Wallace, G. Galectin-3 as a Novel Biomarker in Cats with Hypertrophic Cardiomyopathy. J Vet Cardiol 2023, 48, 54–62. [Google Scholar] [CrossRef]
- Sakarin, S.; Rungsipipat, A.; Surachetpong, S.D. Galectin-3 in Cardiac Muscle and Circulation of Dogs with Degenerative Mitral Valve Disease. Journal of Veterinary Cardiology 2016, 18, 34–46. [Google Scholar] [CrossRef]
- Rešetar Maslov, D.; Farkaš, V.; Rubić, I.; Kuleš, J.; Beletić, A.; Beer Ljubić, B.; Šmit, I.; Mrljak, V.; Torti, M. Serum Proteomic Profiles Reflect the Stages of Myxomatous Mitral Valve Disease in Dogs. International Journal of Molecular Sciences 2023, 24, 7142. [Google Scholar] [CrossRef]
- Kim, Y.-M.; Kim, S.-W.; Kim, J.-H. Galectin-3 Is Able to Differentiate Dogs with Myxomatous Mitral Valve Disease from Healthy Control Dogs. Am J Vet Res 2023. [Google Scholar] [CrossRef]
- Winter, R.L.; Maneval, K.L.; Ferrel, C.S.; Clark, W.A.; Herrold, E.J.; Rhinehart, J.D. Evaluation of Right Ventricular Diastolic Function, Systolic Function, and Circulating Galectin-3 Concentrations in Dogs with Pulmonary Stenosis. Veterinary Internal Medicne 2023, 37, 2030–2038. [Google Scholar] [CrossRef]
- Klein, S.; Nolte, I.; Granados-Soler, J.L.; Lietz, P.; Sehn, M.; Raue, J.F.; Rohn, K.; Packeiser, E.-M.; Bach, J.-P. Evaluation of New and Old Biomarkers in Dogs with Degenerative Mitral Valve Disease. BMC Vet Res 2022, 18, 256. [Google Scholar] [CrossRef]
- Buchanan, J.W. Spontaneous Arrhythmias and Conduction Disturbances in Domestic Animals. Ann N Y Acad Sci 1965, 127, 224–238. [Google Scholar] [CrossRef]
- Noszczyk-Nowak, A.; Michałek, M.; Kałuża, E.; Cepiel, A.; Pasławska, U. Prevalence of Arrhythmias in Dogs Examined between 2008 and 2014. J Vet Res 2017, 61, 103–110. [Google Scholar] [CrossRef]
- Hellemans, A.; Schittekatte, M.; Covents, M.; Smets, P. Diagnosis and Management of Arrhythmias in Dogs: A Cross-Sectional Online Survey among Flemish Veterinary Practitioners. Vet Rec Open 2022, 9, e35. [Google Scholar] [CrossRef]
- Romito, G.; Guglielmini, C.; Poser, H.; Baron Toaldo, M. Lorenz Plot Analysis in Dogs with Sinus Rhythm and Tachyarrhythmias. Animals 2021, 11, 1645. [Google Scholar] [CrossRef]
- Guglielmini, C.; Chetboul, V.; Pietra, M.; Pouchelon, J.L.; Capucci, A.; Cipone, M. Influence of Left Atrial Enlargement and Body Weight on the Development of Atrial Fibrillation: Retrospective Study on 205 Dogs. The Veterinary Journal 2000, 160, 235–241. [Google Scholar] [CrossRef]
- Arcuri, G.; Valente, C.; Perini, C.; Guglielmini, C. Risk Factors for Atrial Fibrillation in the Dog: A Systematic Review. Vet Sci 2024, 11, 47. [Google Scholar] [CrossRef]
- Friederich, J.; Seuß, A.C.; Wess, G. The Role of Atrial Fibrillation as a Prognostic Factor in Doberman Pinschers with Dilated Cardiomyopathy and Congestive Heart Failure. The Veterinary Journal 2020, 264, 105535. [Google Scholar] [CrossRef]
- Jung, S.W.; Sun, W.; Griffiths, L.G.; Kittleson, M.D. Atrial Fibrillation as a Prognostic Indicator in Medium to Large-Sized Dogs with Myxomatous Mitral Valvular Degeneration and Congestive Heart Failure. J Vet Intern Med 2016, 30, 51–57. [Google Scholar] [CrossRef]
- Borgeat, K.; Pack, M.; Harris, J.; Laver, A.; Seo, J.; Belachsen, O.; Hannabuss, J.; Todd, J.; Ferasin, L.; Payne, J.R. Prevalence of Sudden Cardiac Death in Dogs with Atrial Fibrillation. J Vet Intern Med 2021, 35, 2588–2595. [Google Scholar] [CrossRef]
- Chetboul, V.; Tissier, R. Echocardiographic Assessment of Canine Degenerative Mitral Valve Disease. J Vet Cardiol 2012, 14, 127–148. [Google Scholar] [CrossRef]
- Keene, B.W.; Atkins, C.E.; Bonagura, J.D.; Fox, P.R.; Häggström, J.; Fuentes, V.L.; Oyama, M.A.; Rush, J.E.; Stepien, R.; Uechi, M. ACVIM Consensus Guidelines for the Diagnosis and Treatment of Myxomatous Mitral Valve Disease in Dogs. Journal of Veterinary Internal Medicine 2019, 33, 1127–1140. [Google Scholar] [CrossRef]
- Bonagura, J.D.; Visser, L.C. Echocardiographic Assessment of Dilated Cardiomyopathy in Dogs. J Vet Cardiol 2022, 40, 15–50. [Google Scholar] [CrossRef]
- Wess, G. Screening for Dilated Cardiomyopathy in Dogs. J Vet Cardiol 2022, 40, 51–68. [Google Scholar] [CrossRef]
- Wess, G.; Domenech, O.; Dukes-McEwan, J.; Häggström, J.; Gordon, S. European Society of Veterinary Cardiology Screening Guidelines for Dilated Cardiomyopathy in Doberman Pinschers. J Vet Cardiol 2017, 19, 405–415. [Google Scholar] [CrossRef]
- Meurs, K.M.; Stern, J.A.; Sisson, D.D.; Kittleson, M.D.; Cunningham, S.M.; Ames, M.K.; Atkins, C.E.; DeFrancesco, T.; Hodge, T.E.; Keene, B.W.; et al. Association of Dilated Cardiomyopathy with the Striatin Mutation Genotype in Boxer Dogs. J Vet Intern Med 2013, 27, 1437–1440. [Google Scholar] [CrossRef]
- Hindricks, G.; Potpara, T.; Dagres, N.; Arbelo, E.; Bax, J.J.; Blomström-Lundqvist, C.; Boriani, G.; Castella, M.; Dan, G.-A.; Dilaveris, P.E.; et al. 2020 ESC Guidelines for the Diagnosis and Management of Atrial Fibrillation Developed in Collaboration with the European Association for Cardio-Thoracic Surgery (EACTS). European Heart Journal 2021, 42, 373–498. [Google Scholar] [CrossRef] [PubMed]
- Pedro, B.; Fontes-Sousa, A.P.; Gelzer, A.R. Diagnosis and Management of Canine Atrial Fibrillation. Vet J 2020, 265, 105549. [Google Scholar] [CrossRef]
- Cornell, C.C.; Kittleson, M.D.; Della Torre, P.; Häggström, J.; Lombard, C.W.; Pedersen, H.D.; Vollmar, A.; Wey, A. Allometric Scaling of M-Mode Cardiac Measurements in Normal Adult Dogs. J Vet Intern Med 2004, 18, 311–321. [Google Scholar] [CrossRef] [PubMed]
- Rishniw, M.; Erb, H.N. Evaluation of Four 2-Dimensional Echocardiographic Methods of Assessing Left Atrial Size in Dogs. J Vet Intern Med 2000, 14, 429–435. [Google Scholar] [CrossRef]
- Guglielmini, C.; Goncalves Sousa, M.; Baron Toaldo, M.; Valente, C.; Bentivoglio, V.; Mazzoldi, C.; Bergamin, I.; Drigo, M.; Poser, H. Prevalence and Risk Factors for Atrial Fibrillation in Dogs with Myxomatous Mitral Valve Disease. J Vet Intern Med 2020, 34, 2223–2231. [Google Scholar] [CrossRef]
- Guglielmini, C.; Valente, C.; Romito, G.; Mazzoldi, C.; Baron Toaldo, M.; Goncalves Sousa, M.; Wolf, M.; Beluque, T.; Domenech, O.; Patata, V.; et al. Risk Factors for Atrial Fibrillation in Dogs with Dilated Cardiomyopathy. Front Vet Sci 2023, 10, 1183689. [Google Scholar] [CrossRef] [PubMed]
- Romito, G.; Darida, S.; Valente, C.; Poser, H.; Contiero, B.; Cipone, M.; Guglielmini, C. Prevalence and Prognostic Role of L Wave and Selected Clinical and Echocardiographic Variables in Dogs with Atrial Fibrillation. J Vet Intern Med 2023, 37, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Pedro, B.; Fontes-Sousa, A.P.; Gelzer, A.R. Canine Atrial Fibrillation: Pathophysiology, Epidemiology and Classification. The Veterinary Journal 2020, 265, 105548. [Google Scholar] [CrossRef] [PubMed]
- Pathak, R.; Lau, D.H.; Mahajan, R.; Sanders, P. Structural and Functional Remodeling of the Left Atrium: Clinical and Therapeutic Implications for Atrial Fibrillation. J Atr Fibrillation 2013, 6, 986. [Google Scholar] [PubMed]
- Brundel, B.J.J.M.; Melnyk, P.; Rivard, L.; Nattel, S. The Pathology of Atrial Fibrillation in Dogs. J Vet Cardiol 2005, 7, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Goette, A.; Kalman, J.M.; Aguinaga, L.; Akar, J.; Cabrera, J.A.; Chen, S.A.; Chugh, S.S.; Corradi, D.; D’Avila, A.; Dobrev, D.; et al. EHRA/HRS/APHRS/SOLAECE Expert Consensus on Atrial Cardiomyopathies: Definition, Characterisation, and Clinical Implication. Journal of Arrhythmia 2016, 32, 247–278. [Google Scholar] [CrossRef] [PubMed]
- Vollmar, A.C.; Aupperle, H. Cardiac Pathology in Irish Wolfhounds with Heart Disease. Journal of Veterinary Cardiology 2016, 18, 57–70. [Google Scholar] [CrossRef] [PubMed]
- Janus, I.; Noszczyk-Nowak, A.; Nowak, M.; Ciaputa, R.; Kandefer-Gola, M.; Pasławska, U. A Comparison of the Histopathologic Pattern of the Left Atrium in Canine Dilated Cardiomyopathy and Chronic Mitral Valve Disease. BMC Veterinary Research 2016, 12, 3. [Google Scholar] [CrossRef] [PubMed]
- Tursi, M.; Mazzotta, E.; Biasato, I.; Poser, H.; Guglielmini, C. Pathology in Practice. J Am Vet Med Assoc 2016, 248, 1359–1361. [Google Scholar] [CrossRef] [PubMed]
- Baron Toaldo, M.; Romito, G.; Guglielmini, C.; Diana, A.; Pelle, N.G.; Contiero, B.; Cipone, M. Assessment of Left Atrial Deformation and Function by 2-Dimensional Speckle Tracking Echocardiography in Healthy Dogs and Dogs With Myxomatous Mitral Valve Disease. J Vet Intern Med 2017, 31, 641–649. [Google Scholar] [CrossRef]
- Caivano, D.; Rishniw, M.; Birettoni, F.; Patata, V.; Giorgi, M.E.; Porciello, F. Left Atrial Deformation and Phasic Function Determined by Two-Dimensional Speckle-Tracking Echocardiography in Dogs with Myxomatous Mitral Valve Disease. J Vet Cardiol 2018, 20, 102–114. [Google Scholar] [CrossRef]
- Baron Toaldo, M.; Mazzoldi, C.; Romito, G.; Poser, H.; Contiero, B.; Cipone, M.; Guglielmini, C. Echocardiographic Predictors of First Onset of Atrial Fibrillation in Dogs with Myxomatous Mitral Valve Disease. J Vet Intern Med 2020, 34, 1787–1793. [Google Scholar] [CrossRef]
- Guglielmo, M.; Pontone, G. Clinical Implications of Cardiac Magnetic Resonance Imaging Fibrosis. European Heart Journal Supplements 2022, 24, I123–I126. [Google Scholar] [CrossRef] [PubMed]
- Mewton, N.; Liu, C.Y.; Croisille, P.; Bluemke, D.; Lima, J.A.C. Assessment of Myocardial Fibrosis With Cardiovascular Magnetic Resonance. Journal of the American College of Cardiology 2011, 57, 891–903. [Google Scholar] [CrossRef] [PubMed]
- Ravassa, S.; López, B.; Treibel, T.A.; San José, G.; Losada-Fuentenebro, B.; Tapia, L.; Bayés-Genís, A.; Díez, J.; González, A. Cardiac Fibrosis in Heart Failure: Focus on Non-Invasive Diagnosis and Emerging Therapeutic Strategies. Molecular Aspects of Medicine 2023, 93, 101194. [Google Scholar] [CrossRef] [PubMed]
- Pison, L.; Hocini, M.; Potpara, T.S.; Todd, D.; Chen, J.; Blomstrom-Lundqvist, C.; Blomstrom-Lundqvist, C.; Bongiorni, M.G.; Pison, L.; Proclemer, A.; et al. Work-up and Management of Lone Atrial Fibrillation: Results of the European Heart Rhythm Association Survey. Europace 2014, 16, 1521–1523. [Google Scholar] [CrossRef] [PubMed]
- Frustaci, A.; Caldarulo, M.; Buffon, A.; Bellocci, F.; Fenici, R.; Melina, D. Cardiac Biopsy in Patients with “Primary” Atrial Fibrillation. Histologic Evidence of Occult Myocardial Diseases. Chest 1991, 100, 303–306. [Google Scholar] [CrossRef] [PubMed]
- Mahnkopf, C.; Badger, T.J.; Burgon, N.S.; Daccarett, M.; Haslam, T.S.; Badger, C.T.; McGann, C.J.; Akoum, N.; Kholmovski, E.; Macleod, R.S.; et al. Evaluation of the Left Atrial Substrate in Patients with Lone Atrial Fibrillation Using Delayed-Enhanced MRI: Implications for Disease Progression and Response to Catheter Ablation. Heart Rhythm 2010, 7, 1475–1481. [Google Scholar] [CrossRef] [PubMed]
- Falk, T.; Jönsson, L.; Olsen, L.H.; Pedersen, H.D. Arteriosclerotic Changes in the Myocardium, Lung, and Kidney in Dogs with Chronic Congestive Heart Failure and Myxomatous Mitral Valve Disease. Cardiovasc Pathol 2006, 15, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Falk, T.; Jönsson, L. Ischaemic Heart Disease in the Dog: A Review of 65 Cases. J Small Anim Pract 2000, 41, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Tidholm, A.; Jönsson, L. Histologic Characterization of Canine Dilated Cardiomyopathy. Vet Pathol 2005, 42, 1–8. [Google Scholar] [CrossRef]
- de Boer, R.A.; van Veldhuisen, D.J.; Gansevoort, R.T.; Muller Kobold, A.C.; van Gilst, W.H.; Hillege, H.L.; Bakker, S.J.L.; van der Harst, P. The Fibrosis Marker Galectin-3 and Outcome in the General Population. Journal of Internal Medicine 2012, 272, 55–64. [Google Scholar] [CrossRef]
- Seropian, I.M.; Cassaglia, P.; Miksztowicz, V.; González, G.E. Unraveling the Role of Galectin-3 in Cardiac Pathology and Physiology. Front Physiol 2023, 14, 1304735. [Google Scholar] [CrossRef]
- Loffredo, F.S.; Nikolova, A.P.; Pancoast, J.R.; Lee, R.T. Heart Failure with Preserved Ejection Fraction: Molecular Pathways of the Aging Myocardium. Circ Res 2014, 115, 97–107. [Google Scholar] [CrossRef]
- Frangogiannis, N.G. Cardiac Fibrosis: Cell Biological Mechanisms, Molecular Pathways and Therapeutic Opportunities. Mol Aspects Med 2019, 65, 70–99. [Google Scholar] [CrossRef]
- Biernacka, A.; Frangogiannis, N.G. Aging and Cardiac Fibrosis. Aging and Disease 2011, 2, 158–173. [Google Scholar]
- da Silveira, J.S.; Scansen, B.A.; Wassenaar, P.A.; Raterman, B.; Eleswarpu, C.; Jin, N.; Mo, X.; White, R.D.; Bonagura, J.D.; Kolipaka, A. Quantification of Myocardial Stiffness Using Magnetic Resonance Elastography in Right Ventricular Hypertrophy: Initial Feasibility in Dogs. Magn Reson Imaging 2016, 34, 26–34. [Google Scholar] [CrossRef]
- Horiuchi, Y.U.; Wettersten, N.; Vanveldhuisen, D.J.; Mueller, C.; Nowak, R.; Hogan, C.; Kontos, M.C.; Cannon, C.M.; Birkhahn, R.; Vilke, G.M.; et al. The Influence of Body Mass Index on Clinical Interpretation of Established and Novel Biomarkers in Acute Heart Failure. J Card Fail 2023, 29, 1121–1131. [Google Scholar] [CrossRef]
- Mariana Barros Melo Da Silveira, M.; Victor Batista Cabral, J.; Tavares Xavier, A.; Palmeira Do Ó, K.; Francisco De Moura Junior, J.; Tavares De Carvalho, O.; Bezerra Mendes Filho, E.; Furtado De Mendonça Belmont, T.; Maria Del Castillo, J.; Jesus Barreto De Melo Rêgo, M.; et al. The Role of Galectin-3 in Patients with Permanent and Paroxysmal Atrial Fibrillation and Echocardiographic Parameters of Left Atrial Fibrosis. Mol Biol Rep 2023, 50, 9019–9027. [Google Scholar] [CrossRef]
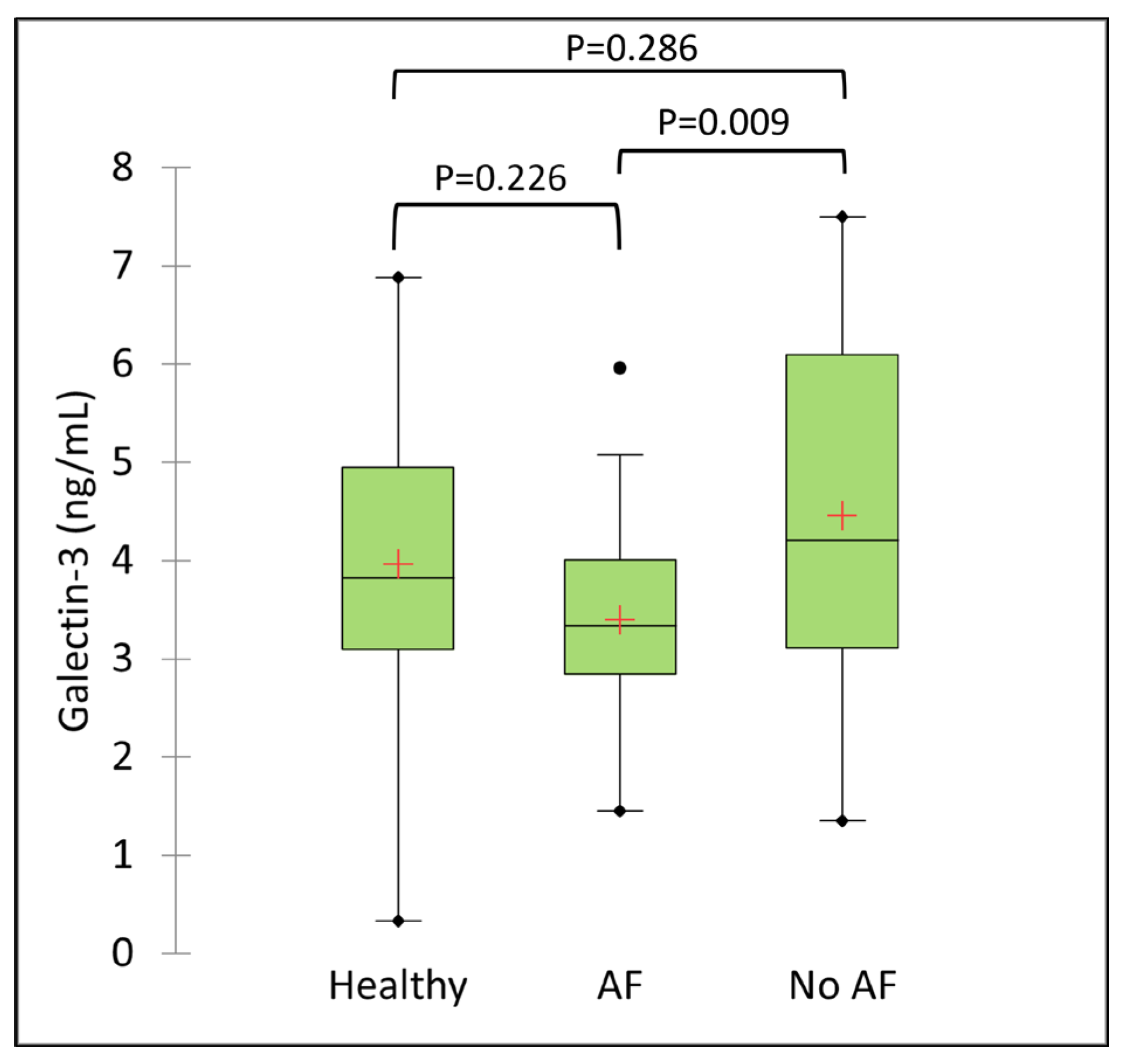
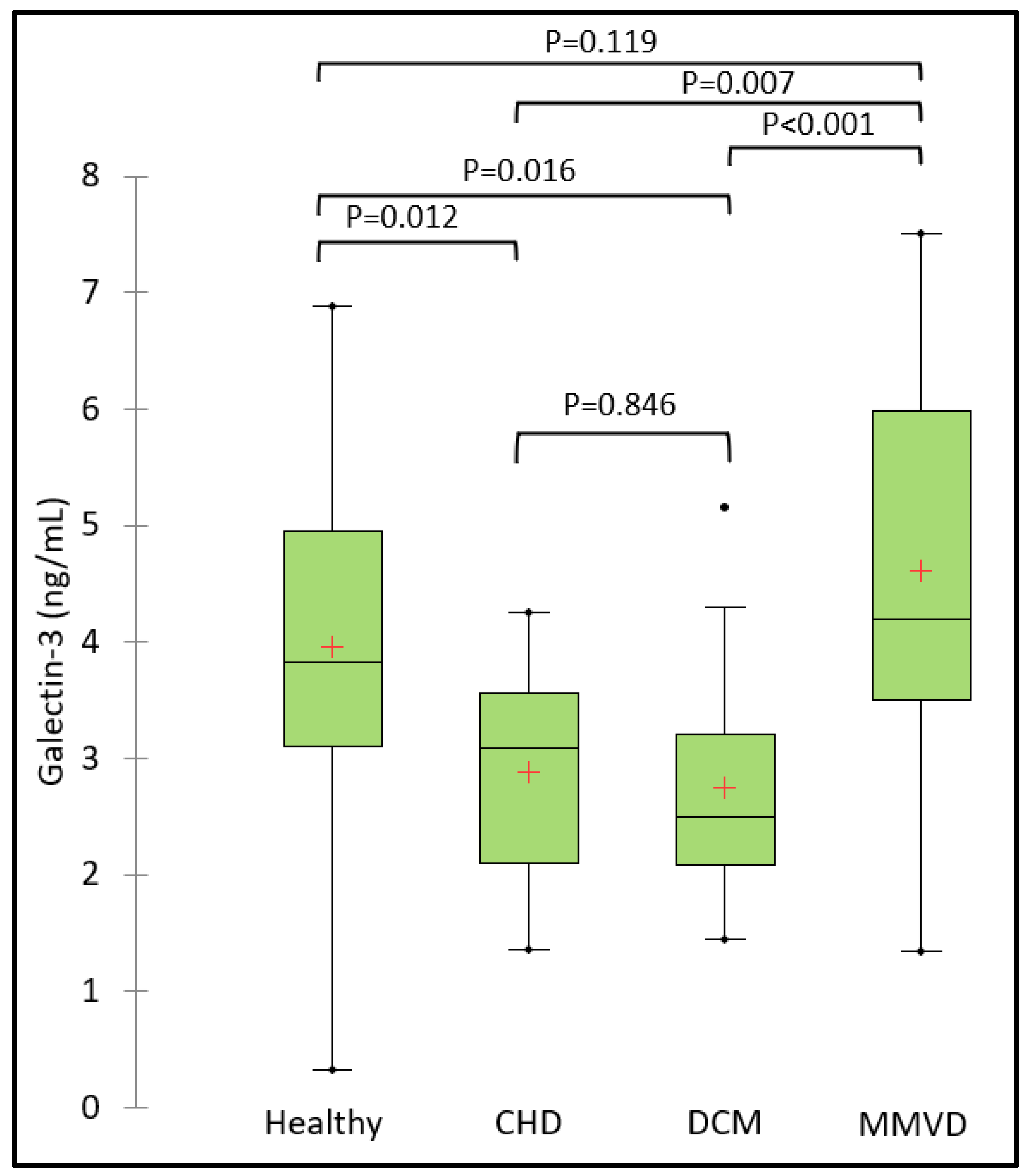
| Variable | Category | Healthy group (N=17) | AF group (N=30) | No AF group (N=33) | Overall P-value |
|---|---|---|---|---|---|
| Breed | Purebred, N (%) | 14/18 (78%) | 25/30 (83%) | 25/33 (76%) | 0.754 |
| Sex | Male/Female | 7/11 | 19/11 | 21/12 | 0.176 |
| Age (years) | Mean ± SD | 10 ± 3 | 8±3 | 10±4 | 0.123 |
| Body weight (Kg) | Median (min-max) | 13.7 (2.2-48.5)b | 30 (4-120)a | 12 (2.4-48.2)b | 0.001 |
| Concurrent disease | Yes, N (%) | - | 4/30 (13%) | 9/33 (27%) | 0.279 |
| ACVIM stages | C+D, N (%) | - | 28/30 (93%) | 12/33 (36%) | <0.001 |
| Treatment ad admission | CT, N (%) | - | 19 (63%) | 22 (67%) | 0.99 |
| CT + OT, N (%) | - | 5 (17%) | 3 (9%) | 0.603 | |
| NT, N (%) | - | 6 (20%) | 8 (24%) | 0.919 | |
| LA (cm) | Median (min-max) | 2.3 (1.4-3.6)c | 5.6 (3.3-8.5)a | 3.7 (2.6-6.7)b | <0.001 |
| Ao (cm) | Median (min-max) | 1.7 (1.0-2.7)b | 2.5 (1.1-3.1)a | 1.97 (1.2-3.2)ab | <0.001 |
| LA:Ao | Median (min-max) | 1.4 (1.2-1.9)b | 2.2 (1.7-4.2)a | 2.0 (1.5-3.2)a | <0.001 |
| LVDDn | Mean ± SD | 1.38 ± 0.07b | 2.09 ± 0.05a | 2.08 ± 0.05a | <0.001 |
| LVSDn | Mean ± SD | 0.86 ± 0.08b | 1.34 ± 0.06a | 1.25 ± 0.05a | <0.001 |
| FS (%) | Mean ± SD | 34.9 ± 3.3 | 29.6 ± 2.5 | 36 ± 2.4 | 0.159 |
| E Mitral (m/s) | Mean ± SD | 0.67 ± 0.08c | 1.48 ± 0.06a | 1.15 ± 0.06b | <0.001 |
| Variable | Category | Healthy group (N=17) | CHD group (N=7) | DCM group (N=16) | MMVD group (N=40) | Overall P-value |
|---|---|---|---|---|---|---|
| Breed | Purebred, N (%) | 14/18 (78%)ab | 7/7 (100%)a | 16/16 (100%)a | 27/40 (68%)b | 0.025 |
| Sex | Male/Female | 7/11b | 0/7c | 13/3a | 27/13ab | 0.001 |
| Age (years) | Mean ± SD | 10±3a | 3±3c | 7±2b | 11±3a | <0.001 |
| Body weight (kg) | Median (min-max) | 13.7 (2.2-48.5)b | 23 (2.4-37.1)b | 42 (32.6-120)a | 11 (4-72)b | <0.001 |
| Concurrent disease | Yes, N (%) | - | 0 | 4/16 (25%) | 9/40 (23%) | 0.351 |
| ACVIM stages | C+D, N (%) | - | 4/7 (57%)ab | 6/16 (38%)b | 30/40 (75%)a | 0.029 |
| Treatment ad admission | CT | - | 5/7 (71%) | 10/16 (63%) | 26/40 (65%) | 0.918 |
| CT + OT | - | 0 | 2/16 (13%) | 6/40 (15%) | 0.546 | |
| NT | - | 2/7 (29%) | 4/16 (25%) | 8/40 (20%) | 0.84 | |
| LA (cm) | Median (min-max) | 2.3 (1.4-3.6)b | 5.8 (2.6-6.8)a | 4.9 (3.7-6.6)a | 4.4 (2.82-8.5)a | <0.001 |
| Ao (cm) | Median (min-max) | 1.7 (1.0-2.7)b | 2.4 (1.4-3.0)a | 2.6 (2.2-3.1)a | 1.7 (1.1-3.2)b | <0.001 |
| LA:Ao | Median (min-max) | 1.4 (1.2-1.9)c | 2.2 (1.6-2.4)ab | 1.8 (1.5-3.0)b | 2.3 (1.63-4.2)a | <0.001 |
| LVDDn | Mean ± SD | 1.38 ± 0.07c | 2.38 ± 0.11a | 1.93 ± 0.08b | 2.09 ± 0.05ab | <0.001 |
| LVSDn | Mean ± SD | 0.86 ± 0.08c | 1.59 ± 0.11a | 1.55 ± 0.07a | 1.13 ± 0.04b | <0.001 |
| FS (%) | Mean ± SD | 34.9 ± 3.3b | 28.9 ± 3.5b | 13.4 ± 2.3c | 41.5 ± 1.5a | <0.001 |
| E Mitral (m/s) | Mean ± SD | 0.67 ± 0.08c | 1.57 ± 0.13a | 1.05 ± 0.09b | 1.36 ± 0.06a | <0.001 |
| Variable | r | P-value |
|---|---|---|
| Age (years) | 0.46 | <0.001 |
| BW (Kg) | -0.40 | <0.001 |
| LA (cm) | -0.19 | 0.087 |
| Ao (cm) | -0.35 | 0.002 |
| LA:Ao | 0.18 | 0.118 |
| LVDDn | 0.19 | 0.092 |
| LVSDn | -0.18 | 0.101 |
| FS (%) | 0.41 | <0.001 |
| E Mitral (m/s) | 0.10 | 0.357 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





